Abstract
Background and aim.
Muscularis macrophages (MMs) are not only mediators of innate immunity, but, also, functionally interact with cells important for gastrointestinal motility. The aim of this study is to determine the spatial relationship and types of contacts between the MMs and neighbouring cells in the muscularis propria of human and mouse stomach, small and large intestine.
Methods.
The distribution and morphology of MMs and their contacts with other cells were investigated by immunohistochemistry and transmission electron microscopy.
Key results.
Immunohistochemistry showed variable shape and number of MMs according to their location in different portions of the muscle coat. By double labeling, a close association between MMs and neighbouring cells, i.e. neurons, smooth muscle cells, interstitial cells of Cajal (ICCs), telocytes (TCs)/PDGFRα-positive cells, was seen. Electron microscopy demonstrated that in the muscle layers of both animal species, MMs have similar ultrastructural features and have specialised cell-to-cell contacts with smooth muscle cells and TCs/PDGFRα-positive cells but not with ICCs and enteric neurons.
Conclusion & Inferences.
This study describes varying patterns of distribution of MMs between different regions of the gut, and reports the presence of distinct and extended cell-to-cell contacts between MMs and smooth muscle cells and between MMs and TCs/PDGFRα-positive cells. In contrast, MMs, although close to ICCs and nerve elements did not make contact with them. These findings indicate specialized and variable roles for MMs in the modulation of gastrointestinal motility whose significance should be more closely investigated in normal and pathological conditions.
Keywords: Innate immunity, Telocytes-muscularis macrophages contact, smooth muscle cells-muscularis macrophages contact, fibroblast-like cells, immunohistochemistry, electron microscopy
Introduction
Muscularis macrophages (MMs) are immune cells residing within the gut muscle coat1-7. Besides their well-established role in the modulation of innate immunity, MMs are emerging as key players in the maintenance of gut homeostasis by interacting with enteric neurons, smooth muscle cells and the gut pacemaker cells, interstitial cells of Cajal (ICCs)8-16. These interactions are partly dependent on the productions of factors by neurons that have known effects on the survival and differentiation of myeloid cells. For example, intestinal myenteric neurons express colony stimulating factor-1 (CSF1)13, which is required for normal maintenance and development of MMs. Csf1op/op mice that, due to a point mutation, do not produce CSF1 and lack most MMs12, have disorganised myenteric ganglia and a higher density of gastric myenteric neurons compared to age-matched control mice13,14. In addition, Csf1op/op mice have a higher proportion of nitrergic myenteric neurons but no differences in cholinergic neurons compared to wild type controls, suggesting the possibility that MMs preferentially modulates certain populations of myenteric neurons15. A close relationship between MMs and nerves has been described by immunohistochemistry8 but cell-to-cell contacts, i.e. with adhesions of the glycocalyx of the plasma membranes of the two contiguous cells (that appears as an electron-transparent gap of 20 nm), have not yet been confirmed at the ultrastructural level6. Neither immunohistochemical nor ultrastructural reports are available to support the existence of cell-to-contacts between smooth muscle cells and MMs although these two cell types were seen to functionally interact through the TRPV4 channel16. In contrast, immunohistochemical studies indicate that macrophages are “enveloped” by long cell processes considered to belong to ICCs17 and, in two studies performed by transmission electron microscopy (TEM), “macrophage-like cells”18 and “gut resident macrophages”1,6 are described making close contacts with these presumed ICCs, in both humans and mice.
Recently, a cell type with an unusual ultrastructural morphology was identified under TEM and initially named “interstitial-Cajal-like-cell (ICLC)”; however, soon after it was named “telocyte (TC)” because of its morphology characterized by the presence of long and thin processes (= telo-podes)19-21. Immunohistochemically, the TCs showed PDGFRα-, CD34- and SK3-positivity22-24. In parallel, cells having the same morphology, immunohistochemical properties and sharing the same location as TCs, were at first named as “fibroblasts”, then “fibroblast-like cells” and finally referred as “PDGFRα-positive cells”25-28. Presently, TCs and PDGFRα-positive cells are generally considered to be the same cell type (indicated in this paper as TCs/PDGFRα-positive cells) and diverse functional roles have been hypothesized for them29. In the gut muscle coat, these cells appear to be involved in gastrointestinal motility i) performing a supporting role due to the 3-D network they form that is, at the same time, resistant and deformable following gastrointestinal stretching as the organ moves; ii) in contributing to purinergic motor neurotransmission22,23,30-32 .
The enteric nervous system provides the environment in which MMs differentiate and acquire their mature phenotype and, as a consequence of their location, MMs establish interactions not only with myenteric neurons but also with ICCs and smooth muscle cells, thus contributing to gastrointestinal physiology. Since spatial associations in biological systems can indicate functional interactions, it is important to determine the relationships between MMs and adjacent cells in different regions of the gastrointestinal muscularis propria. The relationship includes a morphological description and determination of the topographical distribution of subtypes of MMs and their relationship to other cells in the tissue. At the ultrastructural level, phagocytic activity and antigen presentation require direct contact between MMs and adjacent cells, whereas diffusible signaling molecules can be effective when the two cells are close but not touching. The nature and distance of contacts with other cells is therefore an indication of the type of interaction between MMs and other cells. Based on the evidence that MMs modulate gastrointestinal motility, the aim of this study was to first determine in stomach and small and large intestine from mice and humans, patterns of association between MMs and other cell types and secondly to understand the nature of this close association. To reach this aim, we used immunohistochemistry and electron microscopy
Our study shows in both mouse and human tissue, a heterogeneous distribution of MMs in different regions of the muscularis propria and the presence of cell-to-cell contacts between both MMs and smooth muscle cells and TCs/PDGFRα-positive cells, but no direct cell-to-cell contacts with myenteric neurons and ICCs.
Materials and Methods
All human and mouse tissues were taken avoiding possible regions of inflammation. All mouse tissues were taken from healthy, wild type C57Bl6 mice. All human tissues were from apparently normal margins of the resected biopsy.
Immunohistochemistry
This study, approved by the Mayo Clinic Institutional Animal Care and Use Committee, was conducted on 12-14 weeks old C57BL/6 mice (Jackson#000664) humanely killed by carbon dioxide exposure followed by cervical dislocation. The stomachs and small intestines were removed and the smooth muscle layers separated from the mucosa. The resulting muscularis propria was fixed with 4% paraformaldehyde in 0.1 M phosphate buffer for 4 hrs. After fixation, smooth muscle whole mounts were rinsed in 0.1 M phosphate buffered saline (PBS) and blocked by incubation in 10% normal donkey serum in PBS and 0.3% Triton overnight at 4°C. After blocking, muscularis propria was labeled in the presence of primary antibodies overnight at 4°C. After washing, the tissue was incubated with the appropriate secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA), washed and incubated with 4’,6-diamidino-2-phenylindole dilactate (DAPI dilactate, Invitrogen, Carlsbad, CA) for 30 min to label the nuclei. For all immunohistochemical studies, controls omitting the primary antibody and controls in double labeling experiments with a mis-matched secondary antibody were done. The list of antibodies used for the experiments is shown in Supplementary Table 1. Labeled tissues were examined with a laser scanning confocal microscope using a 20X (NA 0.95) XLUMPlanFl objective (Olympus Japan) in Fluoview (Olympus Japan) using the optimal confocal aperture to give a resolution of 0.994 × 0.994 × 1.13 μm (X x Y x Z). Stacks of confocal images across the full thickness of the muscularis propria were collected from 4 different mice (n=4). For the quantification of cells in the intestine, 6 different fields of view that were counted from 6 non-adjacent fields of view selected at random from each tissue. Data represent means of means. For quantification of the labeling, all of the confocal image stacks were flattened into projections using the FV10-ASW Viewer (Olympus). For the quantification of cells in the stomach, three different fields were taken from the corpus and 3 from the antrum. The flattened images were renumbered in random order and macrophage numbers determined by an observer blinded to the source. All cells were counted from fields with the dimensions 636 μm x 636 μm. For 3D reconstruction and volume rendering, to generate orthogonal views, images were collected on the FV1000 Olympus LSM with a 60X, water immersion 1.3NA objective at a XxYxZ resolution of 0.331×0.331×0.57 μm. Image stacks were volume rendered, rotated and cropped in Analyze™ (Mayo Foundation, Rochester MN).
Transmission electron microscopy (TEM)
This study was conducted on both mouse and human specimens at the Research Unit of Histology and Embryology, Dept. of Experimental and Clinical Medicine, University of Florence, Italy. Full-thickness samples of gastric (corpus) and intestinal (ileum and descending colon) wall were obtained from 4 adult male mice weighing 25 ± 1.5 g (C57BL/10, Harlan, Udine, Italy). Human full-thickness gastric samples, taken from the greater curvature at least 7 cm far away from the neoplasm, were obtained from 4 patients (3 females, 1 male, mean age 61 years) undergoing total gastrectomy for gastric cancer. Full thickness intestinal (ileum, 4 females, mean age 48 years; descending colon, 1 female, 3 males, mean age 59 years) specimens were obtained from patients undergoing surgery for cancer and care was taken in cutting the specimens far from the tumour and in choosing the areas devoid of inflammation and appearing normal at macroscopic evaluation. All the patients gave written consent and the local ethical committee approved the study. Then, both mouse and human samples were processed for TEM investigation. Immediately after excision, specimens comprising the lamina propria and muscle coat were fixed overnight at 4°C in Karnowsky (8% paraformaldehyde in distilled water and 0.2 M PBS containing 0.055 g L−1 NaPO4 and 0.04 M Lysine, added with 0.5% glutaraldehyde). Then, specimens were post-fixed with 1% osmium tetroxide in 0.1 M PBS for 2 hrs at 4°C, dehydrated in graded series of acetone and embedded in Epon by using flat moulds. Semi-thin sections were obtained with an LKB NOVA ultra-microtome (Stockholm, Sweden), taking care to avoid those areas presenting with signs of inflammation and stained with a solution of toluidine blue in 0.1 M borate buffer and observed under a light microscope to check the area of interest. Then, ultra-thin sections (50 or 60 nm thick) of the selected areas were cut by using a diamond knife, stained with an alcoholic solution of uranyl acetate in methanol (50:50) for 12 min at 45°C followed by an aqueous solution of concentrated bismuth subnitrate for 10 min at RT, examined under a JEOL 1010 electron microscope (Tokyo, Japan) and photographed.
Characterization of F4/80 positive cells in the gastric muscularis propria
Flow cytometry of gastric muscularis propria was completed using a LSR II analytical flow cytometer (Becton Dickinson, San Jose, CA, USA) located in the Mayo Clinic Flow Cytometry Core Facility. Aliquots of cells were either unstained or stained with individual fluorescently labeled antibodies (SupplTable2) to establish instrument voltages, compensation and appropriate gates. Briefly, forward and side scatter parameters were established using the unstained cell populations and the baseline voltage levels for each fluorochrome were set between the 1st and 2nd logs on the fluorescence scale. Each positive control tube was initially run without storing the data to ensure that the positive signals were on scale. Data were analyzed using FlowJo X software (Tree Star Inc., Ashland, OR).
Results
Density and morphology of MMs change across the different regions of the muscularis propria in mice.
In both mouse and human tissues, MMs were found in the myenteric plexus region and in the intramuscular stroma between the smooth muscle cells of both muscle layers.
For this study, we used antibodies against the type II, major histocompatibility complex (MHC-II) as a marker for MMs. The MHC-II antigen is a complex of proteins that mark antigen-presenting cells including the majority of MMs in gastrointestinal tissue10,13. We used MHC-II as a marker for MMs in immunohistochemical studies in preference to F4/80, which we and others used previously2,14. We are aware that MHC-II antigens are present on other antigen-presenting cells as well as MMs, which includes dendritic cells, B cells, endothelial cells so we tested by flow cytometry the overlap of F4/80 with MHC-II in MMs. The results indicate that 72% of F4/80 positive cells are also positive for MHC-II (Suppl. Fig.1), therefore we considered that the majority of MHC-II-positive cells identified as adjacent to other cell types by immuno-fluorescent labeling were MMs (F4/80 positive). However it is possible that other antigen-presenting cells are also adjacent to, and functionally affect, these other cell types, which indicates that a thorough characterization of gastrointestinal immune cells in the tunica muscularis is still needed.
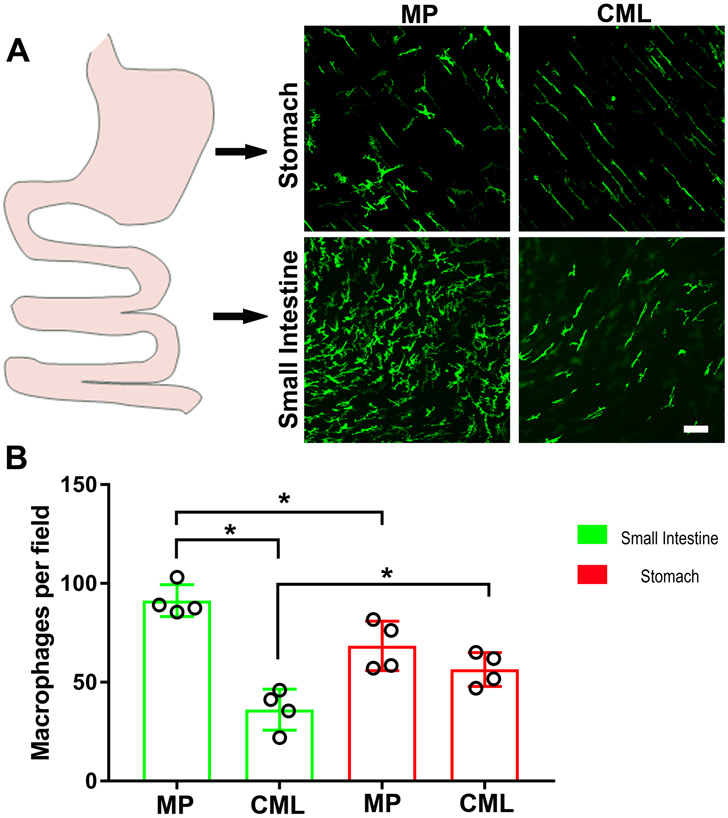
By immunohistochemistry, MHC-II-positive MMs were quantified in the different regions to determine their density across the muscularis propria in both mouse stomach and small intestine (Fig.1A). The number of MMs in the myenteric plexus region was higher in comparison with the number of MMs present in the circular muscle layers in the small intestine (Fig.1B), whereas no difference between the two cell populations was observed in the stomach.
Figure 1. Density and morphology of muscularis macrophages differ between the smooth muscle layers and the myenteric regions of the stomach and the small intestine.
A) Distribution of MHC-II+ macrophages in the different regions of the stomach and the small intestine. B) Quantification of MHC-II+ macrophages in the different regions of the stomach and the small intestine. CML = circular muscle layer; MP = myenteric plexus.N = 4 mice. T test. *P< 0.05.Scale bar: A: 20μm.
The number of MMs in the myenteric plexus of the small intestine was higher than the number of MMs in the myenteric plexus of the stomach (n=4, p<0.01). On the contrary, the number of MMs in the smooth muscle layers of the small intestine was less than the number of MMs in the smooth muscle layers of the stomach (n=4, p<0.05). As previously described, most of the intramuscular MMs had a spindle shape which follows the smooth muscle cell orientation and typically two or at most three ramified processes at their opposite poles; conversely, most of the MMs surrounding the myenteric plexus are characterized by several richly ramified branches developing from a large, oval body (Fig.1A).
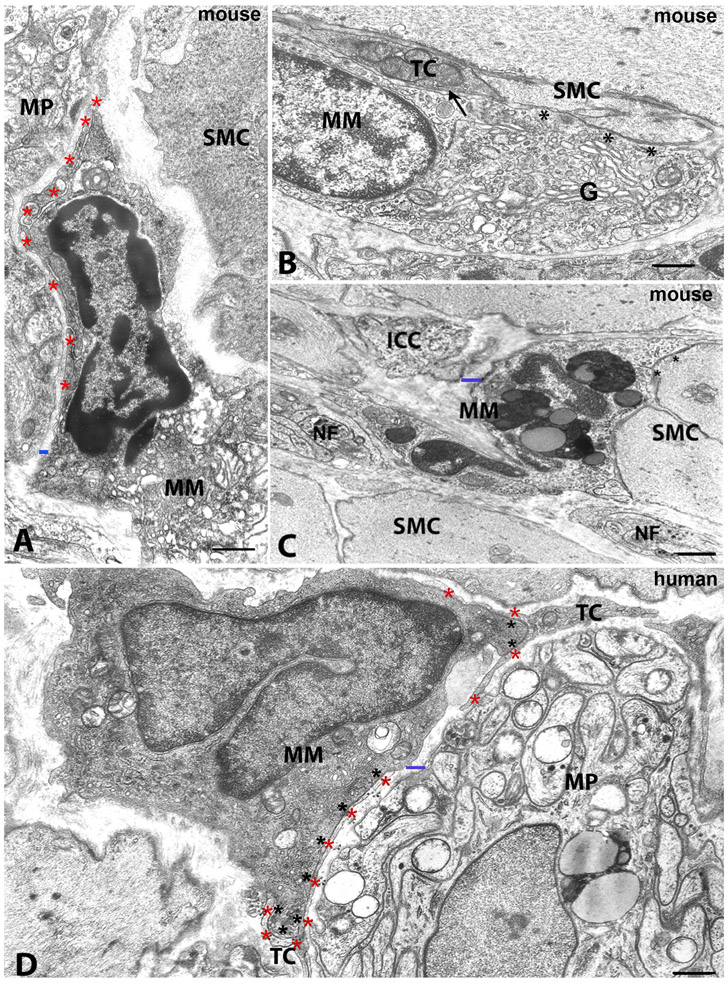
The ultrastructural characteristics of MMs were found to be similar among the gut regions and between humans and mice. Many of the MMs located close to the ganglia had a large, oval body and several processes starting from every side of their body (Fig.2). In contrast, most of the MMs intermingled with nerve fibers or the intramuscular MMs, had a thin and elongated cell body with few skinny processes that sometimes had spines and whose cytoplasm was devoid of organelles (Fig.3B,C, 4B,C). In most of the MMs, the nucleus had an irregular contour with one or more indentations and sometimes had the kidney shape that is typical of macrophage-precursors i.e. monocytes (Fig.2D, 3C, 4B,C). Clumps of heterochromatin were accumulated along the nuclear envelope and close to the nucleolus (Fig.2A, 4A,B). The cytoplasm was either clear (Fig.2B,D, 3B, 4C), especially in the intramuscular MMs, or electron dense (Fig.2A,D, 4A,B), but always rich in organelles and appeared to be functionally active. The most typical organelles in MMs were: free ribosomes, cisternae of rough endoplasmic reticulum and numerous tubules and vesicles of smooth endoplasmic reticulum, an extended Golgi apparatus, numerous mitochondria often round-shaped and with a clear matrix, many primary lysosomes (empty vesicles or containing a dense granule), few residual bodies, and numerous coated vesicles (Fig.2A,B,D, 3A,B, 4A-C). There was one significant difference between human and mouse MMs. Unlike human MMs, mouse MMs sometimes also contained secondary lysosomes and residual bodies (Fig.2C).
Figure 2. Transmission electron microscopic image. Morphology of macrophages and their relationships with nerve elements, ICCs and TCs/PDGFRα-positive cells in mouse and human tissue.
A-C. Mouse. A: small intestine, myenteric plexus region. MM: macrophage with a nucleus with dark chromatin and a cytoplasm rich in organelles The macrophage has an extended cell-to-cell contact with a thin process of a telocyte (red asterisks). MP: ganglion of the myenteric plexus. SMC: smooth muscle cell. The blue bar indicates a gap of 200 nm between the macrophage and the ganglion. B: stomach, circular muscle layer. MM: macrophage in cell to cell contact with a telocyte (TC, arrow) and a smooth muscle cell (SMC, asterisks). The macrophage has a clear cytoplasm and a large Golgi apparatus (G). C: stomach, circular muscle layer. MM: macrophage with several big phagosomes within the cytoplasm. This cell is near an interstitial cell of Cajal (ICC), nerve fibers (NF) and smooth muscle cells (SMC), one of which has cell-to-cell contacts (asterisks) with the macrophage. The blue bar indicates a gap of 200 nm between the macrophage and the ICC. D: human colon, myenteric plexus region. MM: a macrophage near to a ganglion of the myenteric plexus (MP) and with a dark cytoplasm rich in organelles and primary lysosomes. The nucleus has a kidney shape. The black asterisks indicate the extended cell-to cell contact with a telocyte (TC, red asterisk) process. Scale bar: A,B,C,D: 0.6μm; C: 0.10μm.
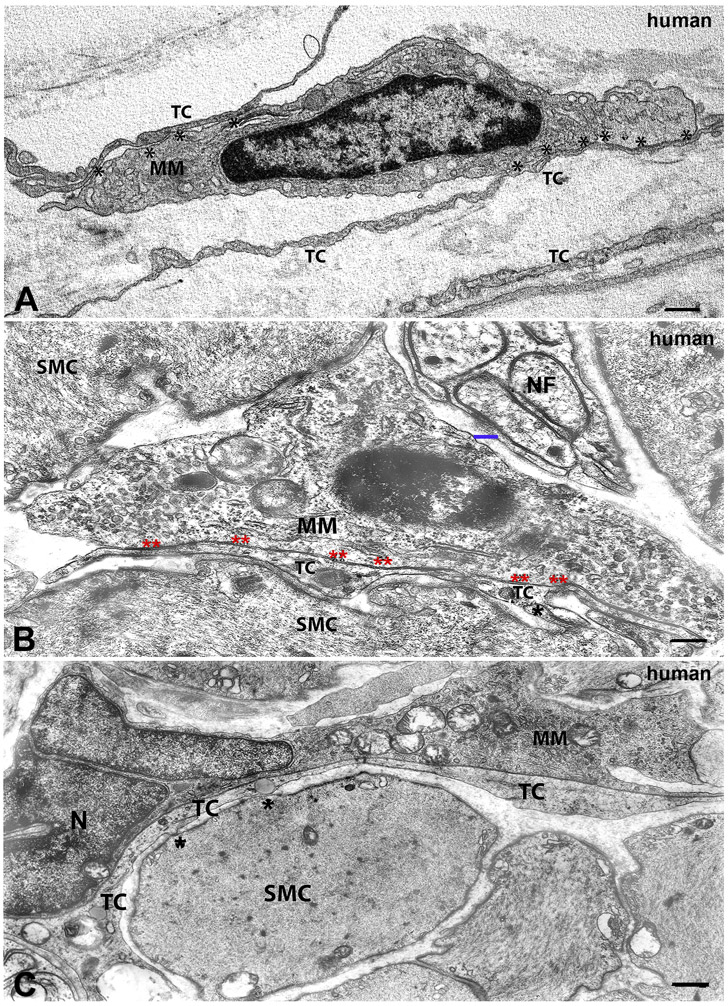
Figure 3. Transmission electron microscopic image. Morphology of macrophages and their cell-to cell contacts with TCs/PDGFRα-positive cells in human tissue.
A. Human stomach, myenteric plexus region. Thin and long telocyte (TC) processes, two of which establish extended cell-to cell contacts (asterisks) with a macrophage (MM). The MM has a dark cytoplasm rich in small vesicles. B, human stomach and C, human colon, circular muscle layer. MM: macrophage with a clear cytoplasm near to a nerve fiber (nerve) in B. The blue bar indicates a gap of 200 nm between the macrophage and the nerve fiber. In B and C, the smooth muscle cells (SMC) are in contact (black asterisks) with long telocyte (TC) processes, which, in turn are in cell-to cell contact (double asterisks) with a macrophage (MM). No basal lamina is interposed in these areas of contact. In C, the macrophage has an indented nucleus (N). Scale bar: A: 0.4μm; B: 0.5μm; C: 0.10μm.
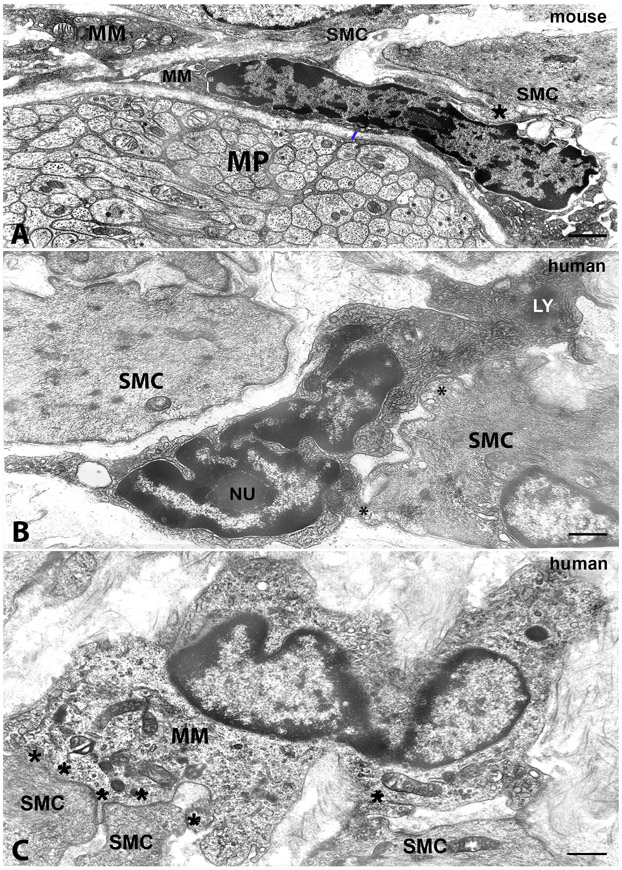
Figure 4. Transmission electron microscopic image. Morphology of macrophages and their cell-to cell contacts with smooth muscle cells.
A, mouse colon, myenteric plexus region. B, human stomach and C, human colon, circular muscle layer. A: MM: macrophage near a myenteric plexus ganglion (MP) and in close contact with smooth muscle cells (SMC). The asterisk indicates a MM-SMC contact of a peg-and socket type. The blue bar indicates a gap of 200 nm between the macrophage and the ganglion. MM has a dark cytoplasm and dark clumps of heterochromatin are accumulated along the nuclear envelope and close to the nucleolus. B and C: MM: macrophages in cell-to cell contacts (asterisks) with smooth muscle cells (SMC). In B, the MM has clumps of dark granular material, heterochromatin, adhering to the nuclear envelope all along the nucleus contour and around the nucleolus (Nu), a dark cytoplasm rich in coated vesicles and with several primary lysosomes and two dense bodies (LY). In C, the MM has a clear cytoplasm with several primary lysosomes and an indented nucleus. Scale bar: A,B: 0.6μm; C: 0.8μm.
As a consequence of their location within the gut muscle coat, MMs share the environment with multiple cell types. These relationships were similar among the gut regions and between the two species. In humans, however, they were more easily identifiable than in mice due to the wideness of the intermuscular stromal space.
Macrophages, enteric neurons and ICCs.
Near myenteric ganglia, the multiple branches of the MMs seemed to touch (Fig.5, Supplementary Videos1-2) or be closely associated with neuronal bodies in the stomach and small intestine of the mouse. Within the muscle layers, MMs were closely associated with PGP9.5-positive (Fig.5A-B) and NOS1-positive nerve fibers (Fig.5C). The spatial resolution of these images is such that fluorescent structures cannot be distinguished if they are closer than approximately 400 nm. The significant overlap between the signals suggests that MMs and myenteric neurons are very close together. Similarly, MMs are closely associated with either ICCs located in the myenteric plexus region or those within the muscle layers (Fig.6, Supplementary Videos 3-4). However, by TEM, MMs were found distant to either the ICCs (Fig.2C) or the myenteric neurons and intramuscular nerves (Fig.2A,C,D, 3B,4A) with a gap of a minimum of 200-300nm. The typical cell-to-cell contacts having a gap of 20 nm, devoid of intercellular material but occupied by the adherent glycocalyces of the plasma membranes of the two contiguous cells, were never observed.
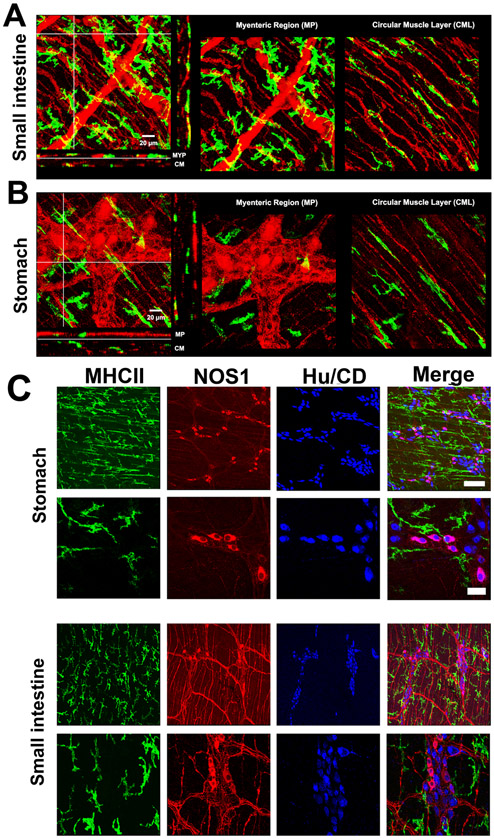
Figure 5. Muscularis macrophages and myenteric neurons in mouse.
A-B) 3D volume rendered images of MHC-II+ immunolabeling (green, macrophages) and PGP9.5 immunolabeling (red, neuronal structures) in small intestine and stomach from a 14-16 week old mouse. Scale bar: 20μm. Note that MHC-II+ cells are closely associated with PGP9.5 positive neurons in the myenteric ganglia and with nerve fibres at the sub-mucosal surface. In the mouse gastric body (B), MHC-II+ cells are also closely associated with PGP9.5 positive neurons in the myenteric ganglia but also observed within the circular muscle layer. C) Distribution of MHC-II+ macrophages in the stomach and small intestine in relation to NOS1 and HuC/D positive cells: low magnification, scale bar: 50 μm; high magnification, scale bar: 15 μm.
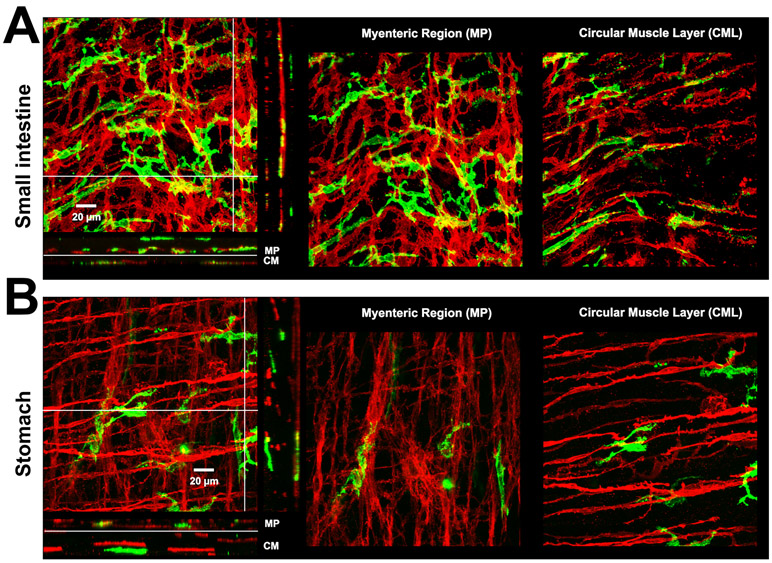
Figure 6. Muscularis macrophages and interstitial cells of Cajal.
A-B) 3D volume rendered images of MHC-II+ immunolabeling (green, macrophages) and Kit immunolabeling (red, ICCs) in small intestine and stomach of a 14-16 week old mouse. Scale bar:20 μm. 1:500
Macrophages, TCs/PDGFRα-positive cells and Smooth Muscle Cells.
By immunohistochemistry, in reconstructed 3D images, PDGFRα-positive cells were organized in a 3-D network (Fig.7, Supplementary Videos 5-6). In both the myenteric plexus region and within the muscle layers, MMs were located at the inner border of these meshes closely associated with PDGFRα-positive cells. With the higher resolution of TEM, MMs were revealed to establish cell-to-cell contacts with TCs/PDGFRα+ cells. MMs were also found to have direct membrane to membrane contacts with the smooth muscle cells. The MMs-TCs/PDGFRα+ cell contact consisted of extended plasma membrane apposition with a gap of 200-300 nm involving the long and thin processes of TCs/PDGFRα+ cells and the MM cell body (Fig.2A,D, 3A,C), while MM-smooth muscle cell contacts were usually short (Fig.2B, 4A-C) and, frequently, MMs embrace smooth muscle cell projections forming structures characteristic of peg-and-socket junctions (Fig.4A). No membrane-specializations were appreciable at the level of these contact areas. It is noteworthy that the long processes of TCs/PDGFRα+ cells were also seen to be intercalated between MMs and smooth muscle cells making simultaneous contacts with both cell types (Fig. 3B,C).
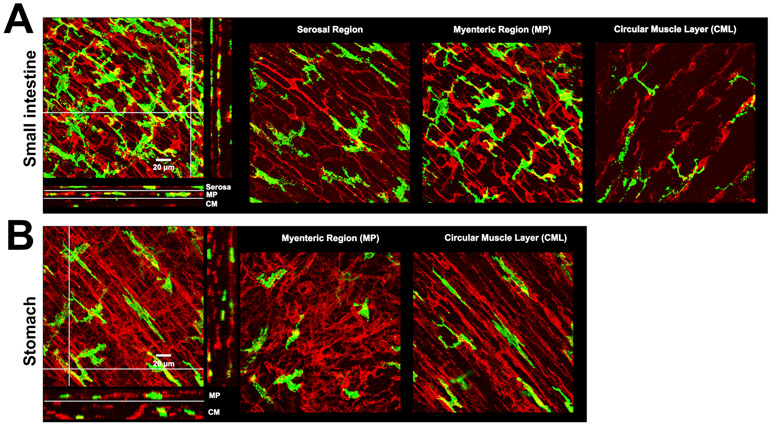
Figure 7. Muscularis macrophages and TCs/PDGFRα-positive cells cells.
A-B) 3D volume rendered images of MHC-II+ immunolabeling (green, macrophages) and PDGFRα immunolabeling (red, TCs/PDGFRα-positive cells) in small intestine and stomach of a 14-16 week old mouse. Scale bar:20 μm.
Discussion
The emerging role of MMs in the maintenance of gut homeostasis and GI motility makes it important to understand whether and how MMs interact with neighbouring cells sharing the same environment. Previous studies, mainly conducted in mice, reported the existence of an interaction between MMs and myenteric neurons8,10 and, in a small number of studies, between MMs and ICCs or smooth muscle cells6,16. Importantly, no study has ever described the interaction between MMs and PDGFRα-positive cells, also known as telocytes. Moreover, most of the published studies report functional interactions and histochemistry at the resolution of light microscopy while only sporadic reports are available on the ultrastructural characteristics of MMs, with few studies on human tissues.
In the present study, we report by a combination of immunohistochemistry and TEM, that MMs establish cell-to-cell contacts with TCs/PDGFRα-positive cells and smooth muscle cells, whereas they do not establish this type of contacts with neuronal structures and ICCs. This study also describes a different pattern of distribution of MMs between different regions of the gut, the small intestine and the stomach, as well as variability in distribution of MMs across the different regions. Electron microscopy shows no differences in ultrastructure of MMs from the different regions of the muscularis propria and confirms the morphology of MMs previously described in mouse and humans6,17,33. However, some differences in the body shape or number of processes were observed between the MMs located in the diverse portions of the muscle wall.
Macrophages play a central role in innate immunity and to fulfil their function they polarize to different phenotypes beyond the original binary classification of classical M1, pro-inflammatory, and the alternatively activated M2, anti-inflammatory ones. Resident intestinal MMs in C57Bl6J mice, under basal conditions, have an anti-inflammatory phenotype10 as indicated by their ability to protect the tissue in case of injury and/or viral infection. This is in line with the ultrastructural evidence present in this study. In fact, the cytoplasm of MMs is filled with primary lysosomes, coated vesicles and a large Golgi apparatus indicating continuous degradation/production of small molecules to constantly clean the site where they reside, and, only rarely and only in the mouse, phagosomes and residual bodies are present. It has been suggested that MMs clear neuronal fragments following apoptosis through phagocytosis34, we detected sporadic MMs with a phagocytic morphology possibly consistent with a very small number of myenteric neurons undergoing apoptosis in healthy tissues. As shown previously10, MMs often have a different and distinct shape between the myenteric plexus region and the muscle layers in the mouse small intestine. Cell shape changes have been associated with different states of activation35. Bone marrow-derived cells following polarization in vitro acquire two distinct morphologies indicative of different activation states35. In addition, macrophage elongation is associated with the expression of M2 markers35. However, despite evidence suggesting an association between macrophage shape and activation state, it is possible that the spindle shapes of intramuscular MMs and the more ramified morphologies of MMs in the myenteric regions of the gut are dictated, under basal conditions, more by the extracellular space and physical forces on the MMs than by functional changes.
A functional interaction between MMs and myenteric neurons needs still to be proven but a close proximity between the 2 cells type has been described8,11,13,15. Interestingly, despite reports suggesting a cell-to-cell contact between MMs and myenteric neurons by using light microscopy techniques8,10,15, by TEM examination we demonstrate that these cells, although close to each other, never establish these membrane to membrane contacts. However, the MMs and neuronal structures in the muscularis propria were found to be close enough, i.e. with a gap of 200-300 nm, to support previously described functional interactions10 via exchange of factors released from both cell types. In support of this possibility, it is known that a similar gap occurring between gastrointestinal nerve endings and smooth muscle cells is adequate to guarantee the neurotransmission. Notably, and in agreement with evidence that gastric MMs regulate nitrergic neurons15, we observed close association of MMs with NOS1-positive neurons and fibres in the stomach and the small intestine.
In animal models of diabetic gastroparesis, the depletion of ICC associated with delayed gastric emptying has been directly linked to changes in macrophage phenotype36. In patients with diabetic gastroparesis, delayed gastric emptying is linked to loss of ICCs that is correlated with a loss of CD206-positive MMs in the gastric muscularis propria36. These studies indicate a role for MMs in the maintenance of ICCs and that delayed gastric emptying in diabetic gastroparetic patients is linked to changes in the balance of MMs with opposing cytoprotective and injurious functions36. Morphological reports showing interactions between MMs and ICC are few. MMs have been described in proximity to ICCs in humans and during the early stage of development in mice37. In this study, although immunohistochemistry shows MMs closely associated with ICCs, our ultrastructural findings clearly demonstrate that, similarly to nerve elements, MMs and ICCs do not appear to form cell-to-cell contacts in healthy tissue.
The observation that some of the cell-to-cell contacts between MMs and smooth muscle are similar to peg-and-socket type junctions is interesting since previous reports observed peg-and-socket-like junctions between MMs and both ICC and longitudinal smooth muscle cells following parasitic infection but not in healthy tissue38. These studies on tissues from infected mice also reported the presence of MMs with clear ultra-structural markers of activation and evidence of phagocytic activity in the days after recovery from T. Spiralis infection, which we did not observe in our work on healthy tissues. Peg-and-socket junctions allow direct presentation of membrane restricted signalling molecules including interleukins and cytokines and may help preserve and maintain contact between the two cell types during muscle coat movements. The interaction of MMs with smooth muscle cells in healthy tissue may contribute to maintenance of normal function and structure, especially since the MMs that we studied did not have activated phenotypes.
Extended cell to cell contacts between MMs and TCs/PDGFRα-positive cells have not been previously described but were indicated by immunofluorescence labeling and reproducibly seen by electron microscopy in this study, where the long and thin processes of the TCs/PDGFRα+ cells envelope the cell bodies of MMs. In the human colon muscle coat, cells called fibroblast-like-cells, enveloping macrophages have been previously described17. We propose that these extended contacts between MMs and TC/PDGFRα-positive cell processes, are analogous to microglia in the CNS, which are long-lived and play a central role in monitoring and modifying the environment in the brain in health and disease39,40. Moreover, the presence of concomitant and extended MM-TC-smooth muscle cell contacts with TC processes between the MM and the smooth muscle cell indicates a potential contribution of MMs to the maintenance or establishment of normal gastrointestinal motility32. Functional studies are needed to compliment these morphological observations and determine how these connections between TCs/PDGFRα+ cells, MMs and smooth muscle cells can modify cell-to-cell communication and signaling29 in gastrointestinal motility. This paper reports clear differences in the morphology of cell-to-cell contact between MMs and other cell types in the intestinal tunica muscularis from normal tissue. We propose that these represent functionally different types of interactions between MMs and other cell types. For example, direct interactions of membrane-restricted molecules presented at direct contacts between plasma membranes that can alter MM activation status, or exchange of soluble signaling molecules across the 200 nm gaps between plasma membranes in the other types of associations between MMs and adjacent cells. This work cannot address the phenotype of the MMs in the muscular layers but it opens the door to future studies aimed at understanding the dynamic, cellular biology of these interactions.
In summary, both in mice and humans, immunofluorescence labeling showed variable shapes and numbers of MMs according to their location. Immunofluorescence labeling also showed close association with the neighboring cells, namely myenteric neurons, smooth muscle cells, ICCs and TCs/PDGFRα-positive cells. Using TEM, we showed that the MMs are located within the 3D network of TCs/PDGFRα-positive cells and form specialised contacts with neighbouring cells. . We would stress that the modality these cells use to cross-talk to each other are different since MMs are adjacent to myenteric neurons and ICCs (with a minimum gap of 200-300 nm), whereas they form cell-to-cell contacts (with adhesion of the contiguous plasma membranes) with TCs/PDGFRα-positive cells and the smooth muscle cells, which, in turn, are in cell-to-cell-contact with both ICCs and neuronal structures. Briefly, MMs, ICCs, nerve elements, TCs/PDGFRα+ cells and smooth muscle cells are inter-connected, although through different modalities, and their interactions likely support a variety of roles for MMs in gastrointestinal motility in both normal and pathological conditions.
Supplementary Material
Supplementary Figure1.
Distribution of F4/80 positive MMs in relation to MHC-II positive MMs in the gastric muscularis propria by flow cytometry.
Supplementary Video 1.
Visualization of macrophages (green) and nerve fibers (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 2.
Visualization of macrophages (green) and nerve fibers (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 3.
Visualization of macrophages (green) and ICCs (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 4.
Visualization of macrophages (green) and ICCs (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 5.
Visualization of macrophages (green) and PDGFRα-positive cells (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 6.
Visualization of macrophages (green) and PDGFRα-positive cells (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.
ACKNOWLEDGEMENTS:
We thank Mrs. Kristy Zodrow for her excellent assistance with this work. We thank the Mayo Microscopy and Cell Analysis Core for the assistance. We also thank Dr. Vanda Lennon for supplying the ANNA-1, HuC/D antibody used for the immunohistochemistry study.
Grant support: This work was supported by NIH P01 DK 68055, P30DK084567 (Mayo Clinic Center for Cell Signaling in Gastroenterology), NIH R01 DK057061, AGA Rome Foundation award # 36 and ANMS Young Investigator Award, The National Natural Science Foundation of China # 81670492.
Footnotes
CONFLICTS OF INTEREST
No conflict of interest.
REFERENCES
- 1.Mikkelsen HB, Thuneberg L, Rumessen JJ, Thorball N. Macrophage-like cells in the muscularis externa of mouse small intestine. Anat Rec 1985;213(1):77–86. [DOI] [PubMed] [Google Scholar]
- 2.Mikkelsen HB, Mirsky R, Jessen KR, Thuneberg L. Macrophage-like cells in muscularis externa of mouse small intestine: immunohistochemical localization of F4/80, M1/70, and Ia-antigen. Cell Tissue Res 1988;252(2):301–6. [DOI] [PubMed] [Google Scholar]
- 3.Mikkelsen HB, Rumessen JJ. Characterization of macrophage-like cells in the external layers of human small and large intestine. Cell Tissue Res 1992;270:273–9. [DOI] [PubMed] [Google Scholar]
- 4.Mikkelsen HB. Macrophages in the external muscle layers of mammalian intestines. Histol Histopathol 1995;10(3):719–36. [PubMed] [Google Scholar]
- 5.Mikkelsen HB, Garbarsch C, Tranum-Jensen J, Thuneberg L. Macrophages in the small intestinal muscularis externa of embryos, newborn and adult germ-free mice. J Mol Histol 2004;35(4):377–87. [DOI] [PubMed] [Google Scholar]
- 6.Mikkelsen HB. Interstitial cells of Cajal, macrophages and mast cells in the gut musculature: morphology, distribution, spatial and possible functional interactions. J Cell Mol Med 2010;14(4):818–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mikkelsen HB, Larsen JO, Froh P, Nguyen TH. Quantitative assessment of macrophages in the muscularis externa of mouse intestines. Anat Rec (Hoboken) 2011;294(9):1557–65. [DOI] [PubMed] [Google Scholar]
- 8.Phillips RJ, Powley TL. Macrophages associated with the intrinsic and extrinsic autonomic innervation of the rat gastrointestinal tract. Auton Neurosci: Basic and Clinical 2012;169:12–27.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pierce JH, Di Marco E, Cox GW, Lombardi D, Ruggiero M, Varesio L, Wang LM,Choudhury GG, Sakaguchi AY, Di Fiore PP, et al. Macrophage-colony-stimulating factor (CSF-1) induces proliferation, chemotaxis, and reversible monocytic differentiation in myeloid progenitor cells transfected with the human c-fms/CSF-1 receptor cDNA. Proc Natl Acad Sci U S A 1990;87(15):5613–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gabanyi I, Muller PA, Feighery L, Oliveira TY, Costa-Pinto FA, Mucida D. Neuro-immune interactions drive tissue programming in intestinal macrophages. Cell 2016;164(3):378–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.De Schepper S, Verheijden S, Aguilera-Lizarraga J, Viola MF, Boesmans W, Stakenborg N, Voytyuk I, Schmidt I, Boeckx B, Dierckx de Casterlé I, Baekelandt V, Gonzalez Dominguez E, Mack M, Depoortere I, De Strooper B, Sprangers B, Himmelreich U, Soenen S, Guilliams M, Vanden Berghe P, Jones E, Lambrechts D, Boeckxstaens G. Self-maintaining gut macrophages are essential for intestinal homeostasis. Cell 2019;176(3):676. [DOI] [PubMed] [Google Scholar]
- 12.Mikkelsen HB, Thuneberg L. Op /op mice defective in production of functional colony-stimulating factor-1 lack macrophages in muscularis externa of the small intestine. Cell Tissue Res 1999;295(3):485–93. [DOI] [PubMed] [Google Scholar]
- 13.Muller PA, Koscsó B, Rajani GM, Stevanovic K, Berres ML, Hashimoto D, Mortha A, Leboeuf M, Li XM, Mucida D, Stanley ER, Dahan S, Margolis KG, Gershon MD, Merad M, Bogunovic M. Crosstalk between muscularis macrophages and enteric neurons regulates gastrointestinal motility. Cell 2014;158(5):1210. [DOI] [PubMed] [Google Scholar]
- 14.Cipriani G, Gibbons SJ, Miller KE, Yang DS, Terhaar ML, Eisenman ST, Ördög T, Linden DR, Gajdos GB, Szurszewski JH, Farrugia G. Change in populations of macrophages promotes development of delayed gastric emptying in Mice. Gastroenterology 2018;154(8):2122–2136.e12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cipriani G, Terhaar ML, Eisenman ST, Ji S, Linden DR, Wright AM, Sha L, Ordog T, Szurszewski JH, Gibbons SJ, Farrugia G. Muscularis propria macrophages alter the proportion of nitrergic but not cholinergic gastric myenteric neurons. Cell Mol Gastroenterol Hepatol 2019;7(3):689–91.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J, Qian A, Oetjen LK, Yu W, Yang P, Feng J, Xie Z, Liu S, Yin S, Dryn D, Cheng J, Riehl TE, Zholos AV, Stenson WF, Kim BS, Hu H. TRPV4 Channel signaling in macrophages promotes gastrointestinal motility via direct effects on smooth muscle cells. Immunity 2018;49(1):107–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rumessen JJ, Vandewinden JM, Rasmussen H, Hansen A, Horn T. Ultrastructure of interstitial cells of Cajal in myenteric plexus of human colon. Cell Tissue Res 2009;337(2):197–212. [DOI] [PubMed] [Google Scholar]
- 18.Faussone-Pellegrini MS, Pantalone D, Cortesini C. Smooth muscle cells, interstitial cells of Cajal and myenteric plexus interrelationships in the human colon. Acta Anat (Basel) 1990;139(1):31–44. [DOI] [PubMed] [Google Scholar]
- 19.Popescu LM, Faussone-Pellegrini MS. TELOCYTES – a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to TELOCYTES. J Cell Mol Med 2010;14:729–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Faussone-Pellegrini MS, Gherghiceanu M. Telocyte’s contacts. Semin Cell Dev Biol 2016; 201;55:3–8. [DOI] [PubMed] [Google Scholar]
- 21.Cretoiu D, Cretoiu SM, Simionescu AA, Popescu LM. Telocytes, a distinct type of cell among the stromal cells present in the lamina propria of jejunum. Histol Histopathol 2012;27(8):1067–78. [DOI] [PubMed] [Google Scholar]
- 22.Pieri L, Vannucchi MG, Faussone-Pellegrini MS. Histochemical and ultrastructural characteristics of an interstitial cell type different from ICC and resident in the muscle coat of human gut. J Cell Mol Med 2008;12:1944–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vannucchi MG, Traini C, Manetti M, Ibba-Manneschi L. Faussone-Pellegrini MS. Telocytes express PDGFRα in the human gastrointestinal tract. J Cell Mol Med 2013;17(9):1099–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosenbaum ST, Svalø J, Nielsen K, Larsen T, Jørgensen JC, Bouchelouche P. Immunolocalization and expression of small-conductance calcium-activated potassium channels in human myometrium. J Cell Mol Med 2012;16(12):3001–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kurahashi M, Nakano Y, Hennig GW, Ward SM, Sanders KM. Platelet-derived growth factor receptor α-positive cells in the tunica muscularis of human colon. J Cell Mol Med 2012;16(7):1397–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Vanderwinden JM, Rumessen JJ, Kerchove d’Exaerde A de, Gillard K, Panthier JJ, De Laet MH, Schiffmann SN. Kit-negative fibroblast-like cells expressing SK3, a Ca2+-activated K+ channel, in the gut musculature in health and diseases. Cell Tissue Res 2002;310:349–58. [DOI] [PubMed] [Google Scholar]
- 27.Fujita A, Takeuchi T, Jun H, Hata F. Localization of Ca2+-activated K+ channel, SK3, in fibroblast-like cells forming gap junctions with smooth muscle cells in the mouse small intestine. J Pharmacol Sci 2003;92(1):35–42. [DOI] [PubMed] [Google Scholar]
- 28.Iino S, Horiguchi K, Horiguchi S, Nojyo Y. c-Kit-negative fibroblast-like cells express platelet-derived growth factor receptor alpha in the murine gastrointestinal musculature. Histochem Cell Biol 2009;131:691–702. [DOI] [PubMed] [Google Scholar]
- 29.Kondo A, Kaestner KH. Emerging diverse roles of Telocytes. Development 2019; 146, dev175018. doi: 10.1242/dev.175018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kurahashi M, Zheng H, Dwyer L, Koh SD, Sanders KM. A functional role for the ‘fibroblast-like cells’ in gastrointestinal smooth muscles. J Physiol 2011;589:697–710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cobine CA, Hennig GW, Kurahashi M, Sanders KM, Ward SM, Keef KD. Relationship between interstitial cells of Cajal, fibroblast-like cells and inhibitory motor nerves in the internal anal sphincter. Cell Tissue Res 2011;344:17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanders KM, Ward SM, Koh SD. Interstitial cells: regulators of smooth muscle function. Physiol Rev 2014;94(3):859–907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Chen X, Zheng Y, Manole CG, Wang X, Wang Q. Telocytes in human oesophagus. J Cell Mol Med 2013;17(11):1506–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kulkarni S, Micci MA, Leser J, Shin C, Tang SC, Fu YY, Liu L, Li Q, Saha M, Li C, Enikolopov G, Becker L, Rakhilin N, Anderson M, Shen X, Dong X, Butte MJ, Song H, Southard-Smith EM, Kapur RP, Bogunovic M, Pasricha PJ. Adult enteric nervous system in health is maintained by a dynamic balance between neuronal apoptosis and neurogenesis. Proc Natl Acad Sci U S A. 2017;114(18):E3709–E3718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.McWhorter FY, Wang T, Nguyen P, Chung T, Liu WF. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci U S A 2013;110(43):17253–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grover M, Farrugia G, Lurken MS, Bernard CE, Faussone-Pellegrini MS, Smyrk TC,Parkman HP, Abell TL, Snape WJ, Hasler WL, Ünalp-Arida A, Nguyen L, Koch KL, Calles J, Lee L, Tonascia J, Hamilton FA, Pasricha PJ; NIDDK Gastroparesi Clinical Research Consortium. Cellular changes in diabetic and idiopathic gastroparesis. Gastroenterology 2011;140(5):1575–85.e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Avetisyan M, Rood JE, Huerta Lopez S, Sengupta R, Wright-Jin E, Dougherty JD, Behrens EM, Heuckeroth RO. Muscularis macrophage development in the absence of an enteric nervous system. Proc Natl Acad Sci U S A 2018;115(18):4696–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang XY, Berezin I, Mikkelsen HB, Der T, Bercik P, Collins SM, Huizinga JD. Pathology of interstitial cells of Cajal in relation to inflammation revealed by ultrastructure but not immunohistochemistry. Am J Pathol. 2002. April;160(4):1529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]; Erratum in: Am J Pathol 2003. January;162(1):359. Huizinga Jan D [corrected to Huizinga Jan D]. PubMed PMID: 11943737; PubMed Central PMCID:PMC1867230. [Google Scholar]
- 39.Verheijden S, De Schepper S, Boeckxstaens GE. Neuron-macrophage crosstalk in the intestine: a “microglia” perspective. Front Cell Neurosci 2015;9:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Butovsky O, Weiner HL. Microglial signatures and their role in health and disease. Nat Rev Neurosci. 2018. October;19(10):622–635. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure1.
Distribution of F4/80 positive MMs in relation to MHC-II positive MMs in the gastric muscularis propria by flow cytometry.
Supplementary Video 1.
Visualization of macrophages (green) and nerve fibers (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 2.
Visualization of macrophages (green) and nerve fibers (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 3.
Visualization of macrophages (green) and ICCs (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 4.
Visualization of macrophages (green) and ICCs (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 5.
Visualization of macrophages (green) and PDGFRα-positive cells (red) distribution in the gastric muscularis propria. Overlap of fluorescence signal is indicated in white.
Supplementary Video 6.
Visualization of macrophages (green) and PDGFRα-positive cells (red) distribution in the small intestine muscularis propria. Overlap of fluorescence signal is indicated in white.