Abstract
Introduction:
The learning curve associated with robotic pancreatoduodenectomy (RPD) is a hurdle for new programs to achieve optimal results. Since early analysis, robotic training has recently expanded, and the RPD approach has been refined. The purpose of this study is to examine RPD outcomes for surgeons who implemented a new program after receiving formal RPD training to determine if such training reduces the learning curve.
Methods:
Outcomes for consecutive patients undergoing RPD at a single tertiary institution were compared to optimal RPD benchmarks from a previously reported learning curve analysis. Two surgical oncologists with formal RPD training performed all operations with one surgeon as bedside assistant and the other at the console.
Results:
40 consecutive RPD operations were evaluated. Mean operative time was 354±54 minutes, and blood loss was 300 ml. Length of stay was 7 days. Three patients (7.5%) underwent conversion to open. Pancreatic fistula affected 5 patients (12.5%). Operative time was stable over the study and lower than the reported benchmark. These RPD operative outcomes were similar to reported surgeon outcomes after the learning curve.
Conclusion:
This study suggests formal robotic training facilitates safe and efficient adoption of RPD for new programs, reducing or eliminating the learning curve.
Keywords: robotic training, robotic pancreatectomy, robotic curriculum, learning curve
Introduction
Robotic pancreatectomy has emerged as a safe and feasible alternative approach to open surgery (1-3), with comparative effectiveness studies suggesting several benefits (4, 5). The experience required to optimize peri-operative outcomes or “the learning curve,” particularly for PD, is an important hurdle to implementation of new programs and wider dissemination of the robotic approach to pancreatic surgery (6, 7).
Analyses from early adopters of robotic pancreatoduodenectomy (RPD) suggest that 40 to 250 procedures are required to optimize outcomes and overcome the learning curve (8-12). Many of these studies examined results for surgeons who did not have prior robotic training and during a period when the technical conduct of the operation was still being refined. Since these reports, robotic training has expanded dramatically and rigorous curricula developed specific to robotic pancreatic surgery (13-18).
In the current study, we evaluated the early experience of a new program for RPD implemented by two surgeons, one of whom received formal robotic pancreas specific training during residency and fellowship. Outcomes after the first 40 consecutive RPD cases were evaluated and compared to previously reported benchmarks of optimized results. This analysis answers critical questions about the optimization of robotic pancreatic surgery for new programs adopting the approach in the era of comprehensive robotic training programs.
Methods
Patient Selection
After Institutional Review Board approval (#1907626903), a prospectively maintained database of pancreatectomy operations was retrospectively reviewed to identify patients undergoing pancreatoduodenectomy (PD) at a single academic institution between October 2018 and June 2020. Patients underwent a multi-disciplinary evaluation including high quality cross sectional imaging to evaluate for vascular involvement or evidence of metastatic disease. All patients were initially considered for the robotic approach unless there was anticipated need for vein resection, extensive prior abdominal surgery or need for concomitant open procedure.
Formal Robotic Training
Formal robotic training for the two primary surgeons involved two pathways. One surgeon had extensive robotic training and utilization as a resident and fellow (16), with 9 months of fellowship spent on a robotic pancreatic surgery service. As a result, this surgeon participated in over 65 robotic cases including 23 pancreatoduodenectomies and 22 distal pancreatectomies. The other surgeon began using the robotic approach for upper abdominal operations after basic robotic skills training, case observations and proctored cases. For the purposes of learning RPD, the surgeon completed an intensive training involving several weeks of video-based simulation and biotissue drills culminating in a two-day on-site course with case observation, RPD-specific didactic lectures, coaching and completion of RPD using a frozen cadaver. Both surgeons’ video and biotissue drills were reviewed with direct feedback (17).
Robotic Technique
The conduct of RPD has been previously reported with minor modifications outlined below (19). Each case used the Da Vinci Xi platform docked on the patient’s right side with a split leg table. Patient position is reverse Trendelenburg, arms out and slight right side up. Four upper abdominal robotic 8 mm ports are placed with a 5 mm assistant port in the right lower quadrant and a 15 mm assistant port in the left lower quadrant (LLQ). Diagnostic laparoscopy is done and liver retractor placed. The operation follows previously reported steps (20), beginning with opening the lesser sac, mobilization of colon, hepatic flexure, wide Kocher maneuver, division of ligament of Treitz and proximal jejunum after pulling it through the retroperitoneal defect. The distal stomach, gastroduodenal artery and bile duct are divided using staplers. The neck of the pancreas is divided using monopolar cautery scissors and uncinate process attachments to retroperitoneum are divided with a bipolar energy device. The specimen is extracted in a bag through extension of the LLQ incision and anastomoses are a two-layer neoduodenal modified Blumgart duct to mucosa pancreatojejunostomy with 4 or 5 Fr Hobbs stent, single layer running or interrupted hepaticojejunostomy and antecolic, retrogastric, isoperistaltic gastrojejunostomy. A vascularized falciform flap is placed over the gastroduodenal artery stump and a drain is placed through the right flank robotic port positioned anterior to anastomoses. Both surgeons are present for all RPD operations until safe specimen extraction. After completion of approximately 3-5 RPD cases, we made the following modifications to the procedure: 1) less mobilization of hepatic flexure when ligament of Treitz dissection is uncomplicated and 2) stapled side-to-side gastrojejunostomy.
Analysis of Peri-operative Outcomes
All patients undergoing RPD followed the WVU Surgical Oncology Enhanced Recovery after Surgery (ERAS) protocol. The ERAS major components are formal Preoperative Evaluation Clinic visit, preoperative pro-immune formula supplementation, pain team consultation with placement of paravertebral catheters when feasible or transversus abdominus plane block, goal-directed fluid therapy, no nasogastric tube use, early mobilization, removal of Foley catheter on day 1 and routine drain amylase studies on days 1 and 3 after operation. Patients with procedures initiated robotically were included in the robotic cohort as an intention to treat analysis unless otherwise specified. 90-day post-operative outcomes were evaluated. Operative time was assessed from skin incision to skin closure. Complications were scored according to the Clavien-Dindo classification (21). International Study Group on Pancreatic Surgery (ISGPS) definitions were utilized for scoring delayed gastric emptying (DGE) (22) and pancreatic fistula (23).
Statistical Analysis
Continuous data are reported as mean ± standard deviation or median (Interquartile Range (IQR)) based on distribution of data. Categorical data are reported as frequency (%). Clinical, pathologic and outcome data were compared to the previously reported optimized benchmark outcomes (8). Unpaired two-tailed t test was used to evaluate normally distributed continuous data, while Mann Whitney test was used for non-normally distributed continuous data. Fischer’s exact test was used for frequency data. p <0.05 was considered statistically significant.
Results
Patient demographics and clinical characteristics
During the study period, 52 PD cases were done with 40 RPD (77%) and 12 open (23%) (Figure 1). Open PD was chosen due to extensive prior abdominal surgery (n=6), portal/superior mesenteric vein involvement (n=4), duodenal perforation from ERCP resulting in large abscess in the porta hepatis (n=1) or need for concomitant liver resection (n=1).Patient demographic and clinical data are summarized in Table 1. The mean age was 64 and 53% of patients were female. Twenty-seven procedures were performed for malignancy (68%) of which 18 were for pancreatic adenocarcinoma (45%).
Figure 1: Distribution in approach to pancreatoduodenectomy from October 2018 to June 2020.
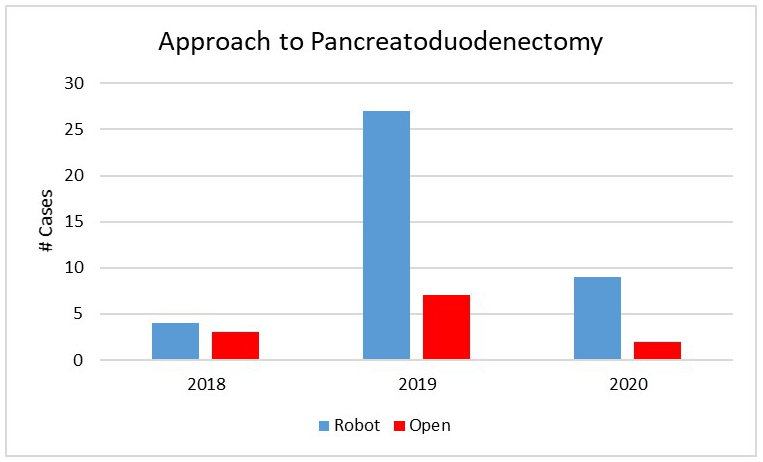
Nearly 80% of cases throughout the experience were approached robotically with contraindications to robotic surgery including the need for venous resection, extensive prior abdominal surgery or the need for concomitant procedures such as liver resection.
Table 1: Patient demographics and characteristics for consecutive robotic pancreatoduodenectomy procedures.
BMI= body mass index, AA-CCI= age-adjusted Charleson comorbidity index, PDAC=pancreatic adenocarcinoma, NET= neuroendocrine tumor
| Patient Demographics | ||
|---|---|---|
| Age | 64 ± 13 | |
| Sex | ||
| Male | 19 (47%) | |
| Female | 21 (53%) | |
| BMI | 26.9 ± 4.7 | |
| AA-CCI | 5 (4-7) | |
| Prior Abdominal Surgery | 25 (63%) | |
| Smoking | ||
| Active | 7 (18%) | |
| Former | 15 (38%) | |
| Neoadjuvant treatment | 9 (23%) | |
| Pathologic Characteristics | ||
| Diagnosis | ||
| PDAC | 18 (45%) | |
| Ampullary adenocarcinoma | 4 (10%) | |
| Cholangiocarcinoma | 1 (3%) | |
| NET | 3 (8%) | |
| Pre-malignanty | 8 (20%) | |
| Metastatic | 1 (3%) | |
| Benign | 5 (13%) | |
| Tumor Size (cm) | 3.2 ± 1.2 | |
| Lymph Node Positive | 23 (56%) | |
| # Positive lymph nodes | 1 (0 - 2.5) | |
| Lymphovascular invasion | 15 (38%) | |
| Perineural invasion | 17 (43%) | |
| Grade | ||
| 1 | 7 (18%) | |
| 2 | 18 (45%) | |
| 3 | 5 (13%) | |
| n/a | 10 (25%) | |
Data reported as frequency, n(%), mean ± standard deviation, or median (IQR)
Peri-operative outcomes
Clinical outcomes following RPD are reported in Table 2. Three patients underwent conversion to an open procedure (7.5%). Reasons for conversion included failure to progress due to adhesions (n=1), portal vein involvement requiring segmental resection (n=1) and bleeding from a replaced right hepatic artery (n=1). Mean operative time was 354 minutes. There was a gradual downtrend in operative time over the course of 40 procedures without a significant decline at any point during the experience (Figure 2). There were zero 90-day mortalities. The median length of stay was 7 days. The pancreatic fistula rate was 12.5% with 4 ISGPS Grade B leaks and 1 Grade C leak. One fistula occurred in a case converted to open; therefore, the rate of pancreatic fistula after completed RPD was 10.8%.
Table 2: Clinical outcomes of 40 consecutive RPDs for surgeons with formal robotic training.
| Intra-Operative Outcomes | ||
|---|---|---|
| Operative time, min | 354 ± 54 | |
| Estimated blood loss, mL | 300 (160-500) | |
| Conversion to open | 3 (7.5%) | |
| Bleeding | 1 (33%) | |
| Need for vein resection | 1 (33%) | |
| Adhesions | 1 (33%) | |
| Pathologic Outcomes | ||
| Lymph node harvest | 26 (16-28) | |
| R0 Resection Rate | 37 (92.5%) | |
| Post-Operative Outcomes | ||
| Transfusion | 5 (13%) | |
| Length of stay | 7 (6-10) | |
| Readmission | 12 (30%) | |
| Discharge Disposition | ||
| Home | 38 (95%) | |
| SNF/Rehab | 2 (5%) | |
| Morbidity | 22 (55%) | |
| Clavien-Dindo | ||
| 1 | 4 (10%) | |
| 2 | 8 (20%) | |
| 3 | 6 (15%) | |
| 4 | 4 (10%) | |
| 5 | 0 (0%) | |
| Pancreatic Fistula | 5 (12.5%) | |
| B | 4 (10%) | |
| C | 1 (2.5%) | |
| Delayed Gastric Emptying | 6 (15%) | |
| A | 1 (2.5%) | |
| B | 3 (7.5%) | |
| C | 2 (6.7%) | |
| Post-Operative Hemorrhage | 1 (2.5%) | |
| 30 Day Mortality | 0 (0%) | |
| 90 Day Mortality | 0 (0%) | |
Figure 2: Gradual improvement in operative time for robotic pancreatoduodenectomy for surgeons with formal robotic training.
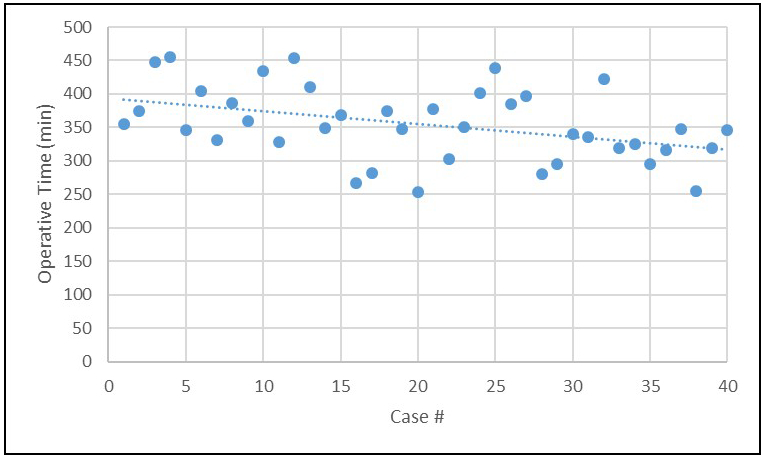
The lack of a plateau followed by a steep decline suggests that absence of a learning curve in this initial experience. Importantly, the mean operative time and significant majority of cases were well below the previously reported benchmark of 417 minutes.
Comparison to previously reported benchmark outcomes after the learning curve
A prior study identified a learning curve of 80 cases to optimize perioperative outcomes for RPD including benchmark goals which were achieved after 120 RPD procedures (3). Perioperative outcomes in this study of 40 cases are comparable to the benchmark outcomes rather than the outcomes representing initial learning curve experience (Table 3). There were no significant differences between any of the patient demographics or characteristics in the previously reported optimized robotic experience and the current cohort (Supplemental table 1). All clinical outcomes were comparable to the post-learning curve results, with significant improvements in operative time (354 ± 54 versus 417 ± 78 minutes, p <0.0001) and length of stay (7 versus 9 days, p<0.0001).
Table 3: Comparison of RPD outcomes of formally trained surgeons with previously reported post-learning curve optimized outcomes.
| Post-Learning Curve Benchmarks* (n=120) |
Initial RPDs w/ Formal Training (n=40) |
p | |
|---|---|---|---|
| Operative time, min | 417 ± 78 | 354 ± 54 | <0.0001 |
| Estimated blood loss,ml | 250 (150-400) | 300 (160-500) | 0.10 |
| Rate No. (%) | |||
| Conversion | 3.3 | 7.5 | 0.37 |
| Transfusion | 21.7 | 12.5 | 0.35 |
| Pancreatic Fistula (ISGPF grade B/C) | 6.9 | 12.5 | 0.32 |
| Readmission | 29.2 | 30 | 0.99 |
| 90-Day Mortality | 3.3 | 0 | 0.57 |
| R0 resection | 91.4 | 92.5 | 0.99 |
| Clavien-Dindo classification rate (%) | |||
| <3 | 43.2 | 32.5 | 0.26 |
| >3 | 23.3 | 25 | 0.83 |
| Length of stay, days | 9 (7-14) | 7 (6-10) | <0.0001 |
| Lymph node harvest | 26 (19-32) | 26 (16-28) | 0.11 |
Boone, BA, Zenati M, Hogg ME. Assessment of Quality Outcomes for Robotic Pancreaticoduodenectomy. JAMA Surg. 2015; 150(5):416-422.
Discussion
Robotic pancreatic resection is safe and feasible and non-randomized evidence suggests possible clinical advantages over open surgery (5). If the learning curve, or number of cases needed to achieve optimal surgical outcomes, is too high then wider adoption of the robotic approach may not occur. Initial studies examining the learning curve are based on the experience of surgeons without formal robotic training. Indeed, these surgeons are the pioneers of RPD and other operations. At present, the steps and technical aspects of RPD have been refined and formal training programs implemented. In the current study, we demonstrate that formal training in RPD reduces or eliminates the learning curve for implementation of a new program. Importantly, because RPD was new for the institution, these outcomes are reflective of not only the surgeons’ learning curve but also the operating room and post-operative care teams. These findings highlight the necessity of formal robotic training programs in pancreatic surgery to ensure safe implementation. Additionally, these data are critically important to facilitate dissemination of the robotic approach, as pancreatic surgeons are eager to participate in formal training if the learning curve is surmountable (24).
Other recently published RPD experiences confirm our findings with a short, or absent learning curve (25). The prior robotic experience of the surgeons is not specified in some studies, and one can speculate surgeons reporting more recent series have had broader exposure to robotic training than early adopters did. Besides formal training, ongoing mentorship positively impact the early outcomes of on outcomes with each generation of surgeons demonstrating improved outcomes in their early experience (26). These studies, when combined with the current analysis, suggest that formal robotic training and access to expert robotic surgeons facilitates a smoother transition with improved outcomes for new robotic pancreatic surgeons.
The reported optimization for RPD suggests it may be shorter than the learning curve associated with open PD (27-29). Formal robotic training is enhanced with virtual simulation and high quality videos of robotic procedures. These help the learner with the technical conduct of the operation and steps to facilitate adoption (30). It is possible that virtual training, when combined with the theoretical technical advantages of robotic surgery (enhanced binocular vision, instrument articulation and ability to control multiple instruments and camera simultaneously), may be the critical aspect reducing the learning curve for robotic pancreatic surgery after formal training. Further study is needed to explore this concept, and if confirmed, it may also have implications for use of virtual and formal training for any complex procedure, whether robotic, laparoscopic, hybrid or open.
Operative time, representing the ability to move efficiently through a procedure, is an important metric of proficiency. The current study shows steady improvement in operative time over the course of the experience. However, compared to other studies, operative time in this analysis does not have a plateau or significant decline suggesting the absence of a true learning curve. Moreover, the operative time is below the optimized benchmark previously reported of 417 minutes (8) and a more recent experience reporting outcomes of 500 RPD (2). Formal training is likely the primary explanation for this finding, and our modifications to the procedure may have also had a modest effect. Quality of outcomes is the other equally important metric of proficiency. The rate of complications in the current study is on par with other series as is the length of stay (LOS). It is important to note that the LOS reported was also likely influenced by our immediate use of an ERAS protocol (31), which was not widely utilized during early implementation of robotic pancreatic surgery.
There are several limitations to the study. The current analysis is an isolated retrospective experience of two surgeons at a single institution. As such, the findings need to be replicated by other surgeons and centers to establish the true impact of formal robotic training on early RPD outcomes. One of the surgeons had extensive experience with open PD prior to initiating robotic training while the other surgeon had more extensive robotic pancreatic surgery experience than open. It is unclear how the inclusion of an experienced open pancreatic surgeon on the team influences results. Additionally, we utilized a two-surgeon model for implementation of our program, meaning two faculty surgeons are conducting the majority of the operation. While we believe this is critically important to the safe implantation of a new RPD program, this certainly influenced our results and the demonstrated outcomes may not be reproduced with a single faculty surgeon conducting the operation. Despite these limitations, the current experience suggests formal robotic training provides earlier optimization of RPD outcomes for new adopters. Further study of the influence of robotic training on the learning curve and dissemination of RPD and other complex robotic procedures is needed. If validated, these studies will support a requirement for formal training for complex pancreatic and other operations for new programs.
Supplementary Material
Synopsis:
This study examines outcomes of robotic pancreatoduodenectomy for surgeons with robotic pancreatectomy training, suggesting that formal robotic training reduces the previously established learning curve.
Acknowledgements
Research reported in this publication was supported by the National Institute of General Medical Sciences of the National Institutes of Health under Award Number 5U54GM104942-04 (BB). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The data that support the findings of this study are available from the corresponding author upon reasonable request.
Footnotes
Disclosures: None
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- 1.Zureikat AH, Moser AJ, Boone BA, Bartlett DL, et al. 250 robotic pancreatic resections: safety and feasibility. Ann Surg. 2013;258(4):554–9; discussion 9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zureikat AH, Beane JD, Zenati MS, Al Abbas AI, et al. 500 Minimally Invasive Robotic Pancreatoduodenectomies: One Decade of Optimizing Performance. Ann Surg. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Girgis MD, Zenati MS, King JC, Hamad A, et al. Oncologic Outcomes After Robotic Pancreatic Resections Are Not Inferior to Open Surgery. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 4.McMillan MT, Zureikat AH, Hogg ME, Kowalsky SJ, et al. A Propensity Score-Matched Analysis of Robotic vs Open Pancreatoduodenectomy on Incidence of Pancreatic Fistula. JAMA Surg. 2017;152(4):327–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zureikat AH, Postlewait LM, Liu Y, Gillespie TW, et al. A Multi-institutional Comparison of Perioperative Outcomes of Robotic and Open Pancreaticoduodenectomy. Ann Surg. 2016;264(4):640–9. [DOI] [PubMed] [Google Scholar]
- 6.Edil BH, Schulick RD. Challenges of minimally invasive pancreas surgery growth with such a high learning curve. JAMA Surg. 2015;150(5):423. [DOI] [PubMed] [Google Scholar]
- 7.Hoehn RS, Nassour I, Adam MA, Winters S, et al. National Trends in Robotic Pancreas Surgery. J Gastrointest Surg. 2020. [DOI] [PubMed] [Google Scholar]
- 8.Boone BA, Zenati M, Hogg ME, Steve J, et al. Assessment of quality outcomes for robotic pancreaticoduodenectomy: identification of the learning curve. JAMA Surg. 2015;150(5):416–22. [DOI] [PubMed] [Google Scholar]
- 9.Zhang T, Zhao ZM, Gao YX, Lau WY, et al. The learning curve for a surgeon in robot-assisted laparoscopic pancreaticoduodenectomy: a retrospective study in a high-volume pancreatic center. Surg Endosc. 2019;33(9):2927–33. [DOI] [PubMed] [Google Scholar]
- 10.Watkins AA, Kent TS, Gooding WE, Boggi U, et al. Multicenter outcomes of robotic reconstruction during the early learning curve for minimally-invasive pancreaticoduodenectomy. HPB (Oxford). 2018;20(2):155–65. [DOI] [PubMed] [Google Scholar]
- 11.Napoli N, Kauffmann EF, Perrone VG, Miccoli M, et al. The learning curve in robotic distal pancreatectomy. Updates Surg. 2015;67(3):257–64. [DOI] [PubMed] [Google Scholar]
- 12.Shi Y, Wang W, Qiu W, Zhao S, et al. Learning Curve From 450 Cases of Robot-Assisted Pancreaticoduocectomy in a High-Volume Pancreatic Center: Optimization of Operative Procedure and a Retrospective Study. Ann Surg. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Dulan G, Rege RV, Hogg DC, Gilberg-Fisher KM, et al. Developing a comprehensive, proficiency-based training program for robotic surgery. Surgery. 2012;152(3):477–88. [DOI] [PubMed] [Google Scholar]
- 14.Hogg ME, Besselink MG, Clavien PA, Fingerhut A, et al. Training in Minimally Invasive Pancreatic Resections: a paradigm shift away from "See one, Do one, Teach one". HPB (Oxford). 2017;19(3):234–45. [DOI] [PubMed] [Google Scholar]
- 15.Knab LM, Zureikat AH, Zeh HJ 3rd, Hogg ME. Towards standardized robotic surgery in gastrointestinal oncology. Langenbecks Arch Surg. 2017;402(7):1003–14. [DOI] [PubMed] [Google Scholar]
- 16.Mark Knab L, Zenati MS, Khodakov A, Rice M, et al. Evolution of a Novel Robotic Training Curriculum in a Complex General Surgical Oncology Fellowship. Ann Surg Oncol. 2018;25(12):3445–52. [DOI] [PubMed] [Google Scholar]
- 17.Tam V, Zenati M, Novak S, Chen Y, et al. Robotic Pancreatoduodenectomy Biotissue Curriculum has Validity and Improves Technical Performance for Surgical Oncology Fellows. J Surg Educ. 2017;74(6):1057–65. [DOI] [PubMed] [Google Scholar]
- 18.Vining CC, Hogg ME. How to train and evaluate minimally invasive pancreas surgery. J Surg Oncol. 2020;122(1):41–8. [DOI] [PubMed] [Google Scholar]
- 19.Wilson GC, Zeh HJ 3rd, Zureikat AH. How I Do It: Robotic Pancreaticoduodenectomy. J Gastrointest Surg. 2019;23(8):1661–71. [DOI] [PubMed] [Google Scholar]
- 20.Nguyen KT, Zureikat AH, Chalikonda S, Bartlett DL, et al. Technical aspects of robotic-assisted pancreaticoduodenectomy (RAPD). J Gastrointest Surg. 2011;15(5):870–5. [DOI] [PubMed] [Google Scholar]
- 21.Clavien PA, Barkun J, de Oliveira ML, Vauthey JN, et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009;250(2):187–96. [DOI] [PubMed] [Google Scholar]
- 22.Wente MN, Bassi C, Dervenis C, Fingerhut A, et al. Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery. 2007;142(5):761–8. [DOI] [PubMed] [Google Scholar]
- 23.Bassi C, Marchegiani G, Dervenis C, Sarr M, et al. The 2016 update of the International Study Group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 Years After. Surgery. 2017;161(3):584–91. [DOI] [PubMed] [Google Scholar]
- 24.van Hilst J, de Rooij T, Abu Hilal M, Asbun HJ, et al. Worldwide survey on opinions and use of minimally invasive pancreatic resection. HPB (Oxford). 2017;19(3):190–204. [DOI] [PubMed] [Google Scholar]
- 25.Guerra F, Checcacci P, Vegni A, di Marino M, et al. Surgical and oncological outcomes of our first 59 cases of robotic pancreaticoduodenectomy. J Visc Surg. 2019;156(3):185–90. [DOI] [PubMed] [Google Scholar]
- 26.Rice MK, Hodges JC, Bellon J, Borrebach J, et al. Association of Mentorship and a Formal Robotic Proficiency Skills Curriculum With Subsequent Generations' Learning Curve and Safety for Robotic Pancreaticoduodenectomy. JAMA Surg. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tseng JF, Pisters PW, Lee JE, Wang H, et al. The learning curve in pancreatic surgery. Surgery. 2007;141(5):694–701. [DOI] [PubMed] [Google Scholar]
- 28.Schmidt CM, Turrini O, Parikh P, House MG, et al. Effect of hospital volume, surgeon experience, and surgeon volume on patient outcomes after pancreaticoduodenectomy: a single-institution experience. Arch Surg. 2010;145(7):634–40. [DOI] [PubMed] [Google Scholar]
- 29.Fisher WE, Hodges SE, Wu MF, Hilsenbeck SG, et al. Assessment of the learning curve for pancreaticoduodenectomy. Am J Surg. 2012;203(6):684–90. [DOI] [PubMed] [Google Scholar]
- 30.Al Abbas AI, Jung JP, Rice MK, Zureikat AH, et al. Methodology for Developing an Educational and Research Video Library in Minimally Invasive Surgery. J Surg Educ. 2019;76(3):745–55. [DOI] [PubMed] [Google Scholar]
- 31.Kowalsky SJ, Zenati MS, Steve J, Esper SA, et al. A Combination of Robotic Approach and ERAS Pathway Optimizes Outcomes and Cost for Pancreatoduodenectomy. Ann Surg. 2019;269(6):1138–45. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.


