Abstract
BACKGROUND:
Gait speed is an important measure of health status for older adults and individuals with neurological conditions. Literature reports that measurements made by people are not as accurate as automatic timers.
RESEARCH QUESTION:
Is the GaitBox (GB), a device to measure walking speed (WS) automatically and accurately, a valid approach to walking speed measurement in a clinical setting?
METHODS:
Two prospective validation studies were completed comparing the GB to human timers (HT) and the Sprint Timing System (STS). Subjects were recruited from convenience samples of healthy older adults (S1, N=35, 72.4 ± 7.4 years of age) and individuals with Spinal Cord Injury (SCI), Traumatic Brain Injury (TBI), or unknown / no diagnosis (S2, N=44, 35.3 ± 13.5 years of age). Subjects completed 4 timed walks. The GB, HT, and STS simultaneously measured WS across a 4 m or 10 m course. Protocol followed an adapted version of the NIH Walk Test. Subjects were instructed to walk at a normal pace. Validity and reliability were determined using Pearson correlations, absolute mean differences, Intraclass Correlation Coefficients (ICC’s) and Bland-Altman plots.
RESULTS:
WS measured in both studies demonstrated strong correlations between GB and STS (r=0.98-0.99, p<0.0001), excellent test-retest reliability GB ICC’s (0.93-0.94), no systematic bias, and good precision. In S1 and S2, ICC’s between GB and STS were excellent at 0.91 and 0.93, respectively.
SIGNIFICANCE:
Considering the increased use of WS as a clinically relevant measure of mobility, functional decline, and recovery, accurate measurement of WS are important. These studies show the GB is a valid and reliable measurement tool within various populations completing the 4 m and 10 m walk tests at a usual speed. Additional populations and walking distances should be evaluated further. Due to its accuracy, the GaitBox is a valid alternative to HT in the clinic setting.
Keywords: Walking speed, gait, technology, functional status, older adults
INTRODUCTION
Walking speed is an indicator of functional status in older adults [1] and change in health status for a variety of clinical populations such as people diagnosed with hip fracture, stroke, incomplete spinal cord injury (SCI), Parkinson’s disease [2], Multiple Sclerosis (MS) [3] and others. There is a coordinated effort to include gait speed measurements as a part of standard clinical care, with an increasing number of researchers and clinicians indicating it is the 6th vital sign [2, 4].
Various gait speed measurement protocols and methodologies have been used in research settings, making comparison of research difficult between populations and studies [5]. The Short Physical Performance Battery (SPPB) [6] and the NIH Toolbox Walk Test (NIH-WT) [7] guides provide guidelines / protocols for conducting gait speed measurements. Even with these guides, research supports that there are inconsistencies in measuring gait speed regarding walking distance, acceleration zone inclusion / distance, and instrumentation [8].
It is especially important to measure walking speed accurately considering that gait speed is an increasingly used metric in clinics and research studies. Hardy et al. found that an increase in usual gait speed in older adults over a one-year period, strongly predicted survival over an eight-year follow-up period. Specifically, these individuals had a 58% decrease in relative risk and a 17.7% decrease in absolute risk of death [4]. Other studies have shown that, for older adults, a 0.04 to 0.06 m/s change in walking speed has been found to show a small meaningful change in survival, while a 0.10 to 0.14 m/s change has been associated with a moderate change [9]. Additionally, a higher overall gait speed for older adults has been connected to increased life expectancy [10]. Studenski et al. found that age, sex, and gait speed taken together was as accurate a predictor of survival compared to other traditional measures of health (i.e. age, sex, chronic conditions, smoking history, blood pressure, body mass index, and hospitalization) [10]. Studies of individuals with MS have linked walking speed with benchmarks associated with functional limitations and impairments [11]. In individuals with SCI, walking speed is an important factor in regaining ambulation [12]. While more research is needed to identify mechanistic correlations between walking speed and disease progression [11], these studies indicate a need to have accurate gait speed measurements. Currently, the standard clinical measurement technique is made by a human timer with a stopwatch over a known distance [13, 14]. While distance, pace, and static or dynamic start have been shown to potentially influence meaningfulness of results [15], a study by Maggio et al. indicates that measurements of gait speed using a stopwatch may lead to misclassification of health status when compared to instrumented techniques (accelerometers) for older adults, but additional studies are needed to further assess this claim [13]. Youdas et al. found that gait parameters were more responsive to change when measured by an instrumented GAITRite system (CIR Systems, Franklin, NJ), a portable electronic walkway designed to capture gait parameters with pressure sensors, compared to manual timing for older adults [16]. Additionally, studies have shown variations in walking speeds depending on the measurement technique (e.g., manual timers versus instrumented measurement techniques) for individuals with respiratory disease (maximal walking speed) [17], older adults (higher average speeds) [16], and stroke survivors [18]. Others have reported strong agreement between manual and instrumented techniques [14]. These instrumented systems often come at a high cost to clinics based on cost to purchase, time to setup, training, and space limitations. Research supporting the importance of walking speed for older adult and clinical populations along with difficulty comparing results of various research studies and clinical visits, indicates a need for low cost and reliable instrumented system to measure walking speed.
The GaitBox was developed as a low-cost instrumented gait speed measurement tool. The purpose of these prospective validation and reliability studies was (1) to show the GaitBox is an accurate alternative to the typical clinical human timer measurement, (2) to assess if differences between the GaitBox and Sprint Timing System are small enough to detect clinically meaningful differences in walking speed (> 0.05 m/s [9]), and, (3) to determine the test-retest reliability of the GaitBox system in two heterogeneous samples.
METHODS
NIH Toolbox Walk Gait Speed Test
Adapted from the SPPB Guide, the NIH-WT proposes a consistent methodology to measure walking speed for people of various ages and populations [7]. In the NIH-WT, the walking path is setup with marks placed at the start and stop timing distances. Subjects are instructed to walk at a comfortable, usual pace from the start mark past the stop mark when instructed by the human timer. Trained timers walk to the side of and slightly behind the subject and start and stop timing when the subject’s foot crosses the timing marks. Human timers (HT) use stopwatches to record the elapsed time between the marks and walking speed is calculated by dividing the distance by the elapsed time [6]. While not called for in the SPPB Guide or NIH Toolbox, many research studies support the need for the inclusion of an untimed acceleration and deceleration zone at the beginning and end of the path, allowing for measurements to be taken irrespective of acceleration and deceleration speeds [17]. For this study, we include these acceleration and deceleration zones because, 1) both acceleration and deceleration zones are used within the research team’s University Health System for older adult gait speed monitoring, 2) greater measurement variability has been seen when excluding an acceleration zone [8], 3) a deceleration zone allowed for consistent instrumented measurements since we could ensure subjects completely passed the end timing mark and ensured subjects did not ‘hard stop’ at the end line, potentially offering challenging timing to the human timers, and 4) it was decided that the acceleration zone would allow for the human timers to more accurately start timing as opposed to a quick reaction on ‘go’ or when a subject took their first step.
The NIH Toolbox 4-meter walk test has been adapted over various distances and populations. In a systematic review of walking speed tests, Graham et. al found walking tests at 10, 6, and 4 m were most common [5]. Additionally, there have been population specific walking tests that have gained traction in specific communities, such as the timed 25 ft (7.62 m) walk test as part of the Multiple Sclerosis Functional Composite [19].
Device Design
We developed the GaitBox (GB) (Figure 1) to be a low cost and accurate alternative to clinical gait speed measurements made by human timers. In its current iteration, the device employs an Arduino Uno microcontroller and a Garmin LIDAR-Lite V3 Laser Ranging Module. Gait speed is automatically measured and displayed on an LCD screen using data analyzed from the LIDAR sensor. Data is displayed but not stored on the device and the display is erased after each test for data privacy and ease of use. The device is setup to help facilitate the NIT-WT procedure and can be setup to measure speed over any distance up to 40 meters. For testing described here, the device was set up to measure a 4 m walk test (meter walk test: MWT) and 10 MWT track based on the NIH-WT protocol.
Figure 1.
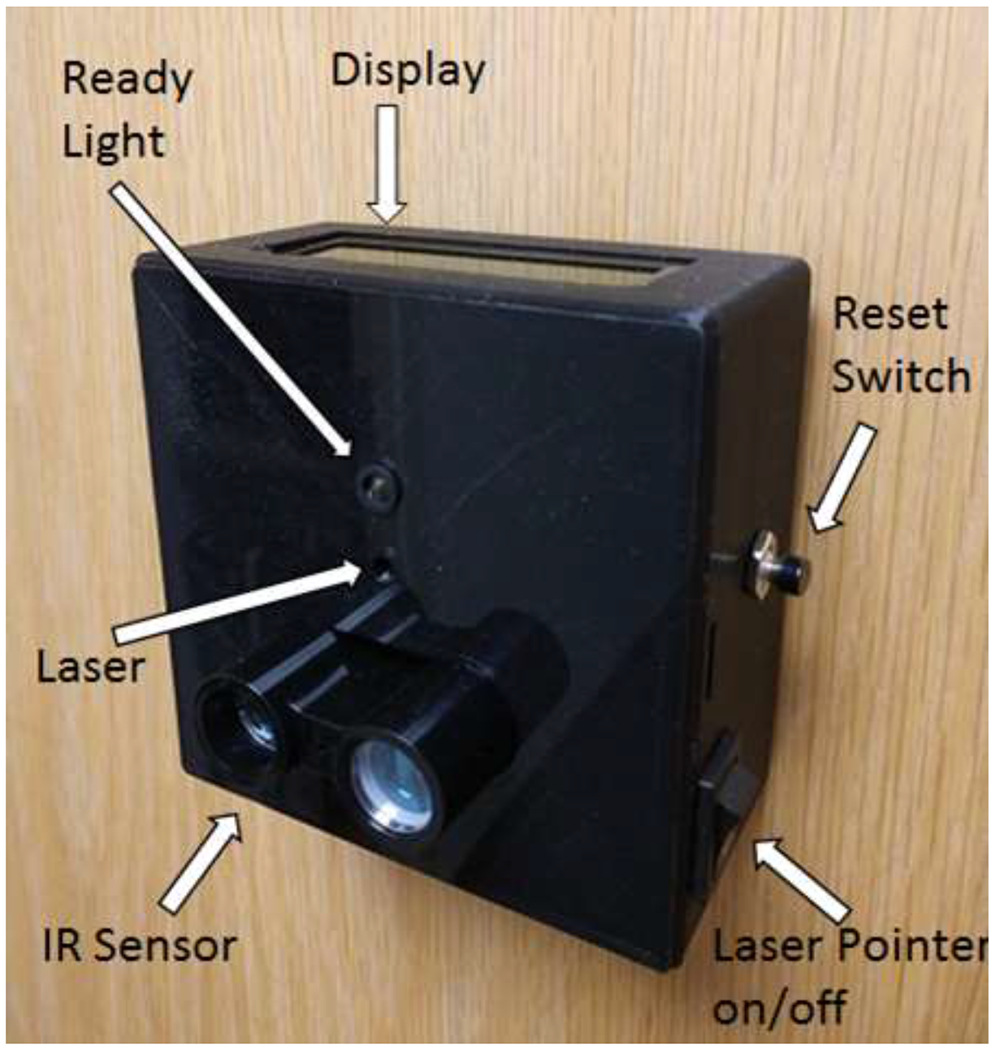
GaitBox device showing components. Figure 1 shows an image of the GaitBox device attached to a door.
To measure walking speed, the device is positioned approximately 1.2 m off the ground and pointed down the center of the walking path at the subject’s torso. The GB can be set up so the subject walks towards or away from the device. It can be mounted a variety of ways. For testing and clinical use, we have attached it to an IV pole, and to a wall or door using Velcro. The device is powered via wall power or a battery pack. When on, the device indicates it is ready to collect data with a welcome message displayed on the LCD screen and by blinking the built in LED. When the walking path is clear the LED will turn on, indicating that the device is ready to take a measurement. When the device is ready, the clinician instructs the subject to walk to the end of the walking path. The velocity of the subject is automatically calculated in m/sec and displayed on the LCD screen. Pressing the reset button clears the display and readies the device for another measurement.
Previous work done by Clark et al. found that LIDAR is an appropriate sensor to measure walking speed across short distances (0.5 m and 4 m). The device used for their study was similar to our GB in hardware design and is available open source online. While our devices are similar, we did not use Clark et al.’s designs, but instead created our own device around the same time. At the time of completing this study, their website with open source code was not available, therefore we could not compare our software design to their design. Regardless, Clark et al. showed that LIDAR is accurate for short distances in healthy populations [20]. This study aims to show that our LIDAR based device and our custom software is an accurate way to measure walking speed across 4 m in older adults and 10 m in diverse clinical and healthy populations.
Study Design
We completed two separate prospective validation studies comparing the GB to a commercially available automatic timing system (Sprint Timing System, Brower Timing Systems, LLC Draper, UT [21]) and human timers (HT) following the adapted NIH-WT procedure. In both studies, subjects completed four (4) timed walking tests: one practice and three official measurements. The researcher instructed the subjects to begin walking after a countdown from three (e.g. 3, 2, 1, go) at a comfortable, normal pace, from a start mark at the beginning of the acceleration zone to another mark indicating the end of the deceleration zone. The studies were conducted under the supervision of the appropriate institutions IRB (Study 1: reference ID 344453, Protocol ID Pro00089655, Study 2: reference ID 1319802, Protocol ID Pro00089655). Both studies received a waiver of documentation of consent. This was approved due to not collecting personal health information, minimal risk, and subjects not members of a culturally distinct group.
Inclusion criteria was met if subjects could: (1) complete the four walks at the respective study distances (4 m or 10 m) with or without assistive devices (e.g. canes, walkers, braces) and (2) had no physical, cognitive, or behavioral impairment that would prevent safe completion of the trials. Both male and female subjects were recruited. Convenience samples of 35 community dwelling older adults 55 years or older (mean 72.4 ± 7.4 years of age) in Durham, NC, completed Study 1, while 44 subjects over the age of 18 representing subjects with diagnoses of SCI, TBI, and unknown populations (mean 35.3 ± 13.5 years of age) completed the Study 2 at the ProMotion Fitness Center and the MS Institute at the Shepherd Center in Atlanta, GA.
Technology and Walking Path Layout
The Sprint Timing System (STS) uses two pairs of break beam sensors. Timing starts when the user interrupts the light beams from the first pair of sensors and stops when the user interrupts the second beam. The STS displays the elapsed time which the investigator uses to manually calculate velocity based on the distance traveled between the break beam sensor pairs. The GB was mounted on an inexpensive, adjustable height camera tripod. Both the STS and GB automatically captured data over these distances.
We set up the testing course per the NIH-WT guidelines with the addition of untimed acceleration and deceleration zones. The course included an unmarked measurement zone (4 m for Study 1, 10 m for Study 2) and acceleration and deceleration zones with start and end lines marked with tape. These zones were added to the walking path as is common in research protocols. The NIH-Toolbox protocol provides no guidance on the use of acceleration / deceleration zones. The first STS module pair was placed across the walking path at the start of the measurement zone 0.7 m from start. The second STS module pair was placed at the end of the measurement zone (either 4 m or 10 m). A 2 m deceleration zone was used for both studies, indicated by a stop tape mark (Figure 2). The 0.7 m acceleration zone was shorter than the deceleration zone due to due to space limitations. A one (1) inch piece of tape was used by the human timers to start and end their timing. The STS timing gates were 2 feet on either side of the walking path. The beginning of the acceleration and end of the deceleration zones were clearly marked with a strip of tape to give the subjects start and end targets. The locations of the module and a small piece of tape indicated to the human timer when to start and stop timing.
Figure 2.
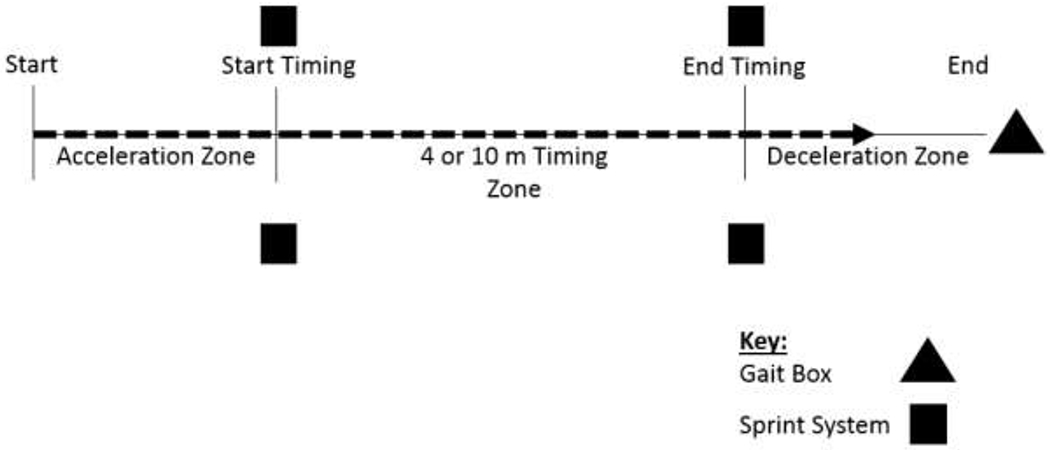
Gait speed measurement setup, 4MWT and 10MWT.
The GB was pointed at the center of the torso, 1.2 m from the ground. The Sprint System was positioned at this height, in line with the GB sensor location. Both the Toolbox and SPPB Guide indicate to start and stop measurement when the subject’s foot is completely past the timing line [6, 7]. For consistency in measurement between the STS and GB, the HT were instructed to take measurements when the subject’s waist crossed the start / stop timing indicators.
Statistical Analysis
Univariate and descriptive statistics included mean (±standard deviation) for normally distributed data, or proportions for count data. The test for normality for continuous variables was performed using the Shapiro-Wilk test. We conducted all validity tests using STS as the standard, reference measure. The mean of the three official timed walks was calculated and used as subjects’ data points for the majority of analyses. Associations between measures was determined using Pearson correlation coefficients. Precision was assessed using a paired t-test of absolute mean differences, and associated p-values, between GB and STS. Bias in the GB measures was assessed using Bland-Altman plots (difference between GB and STS plotted against the mean of GB and STS) [22]. All three walking trials were used to determine test-reliability by calculating the intraclass correlation coefficient (ICC), and to determine validity by calculating the ICC between devices using a mixed effects model (PROC MIXED), accounting for the correlated repeated measures. In one Study 2 participant, a HT data point was not plausible (gait speed of 7.36 m/s) and was removed from the dataset. All analyses were conducted using SAS v9.4 (SAS Institute, Cary, NC).
RESULTS
Study 1, 4 MWT
The study sample was predominately male (74%) and mobility intact with a mean gait speed of 1.18 m/s (Table 1). Results showed strong associations between the GB and HT (r=0.99) and the GB and STS (r=0.98, p<0.0001; Figure 3a). Validity was further tested with the ICC among all three walking trials between GB and STS, which demonstrated excellent validity (ICC = 0.91). Test-retest reliability of GB was excellent with an ICC of 0.94. Absolute mean gait speed differences between the GB and HT (0.02 ± 0.03 m/s; P = 0.01) and GB and STS (0.00007 ± 0.03 m/s; P = 0.98) were clinically negligible. The Bland Altman plot between GB and STS (Figure 4a) demonstrated no systematic bias across all gait speeds, with a plot correlation coefficient of 0.07 (P = 0.71).
Table 1.
Characteristics of Study 1 and Study 2 subjects.
| Study Group | N | Diagnosis | Age (Mean ± SD) | Male N (%) | Usual Gait Speed (GB) (Mean ± SD) | Walked with Assistive Device N (%) [walker, cane/crutch, other] * |
|---|---|---|---|---|---|---|
| Study 1 | 35 | NA | 72.4 ± 7.2 | 26 (74%) | 1.18 ± 0.19 m/s | 4 (11%) [1,1,2] |
| Study 2 | 44 | SCI - 20, TBI - 15, Unknown - 9 | 35.3 ± 13.5 | 19 (43%) | 1.39 ± 0.25 m/s | 18 (41%) [5, 11, 2] |
m/s = meters per second
SCI = spinal cord injury
TBI = traumatic brain injury
GB = GaitBox measurement data
some participants used multiple assistive devices
Figure 3.
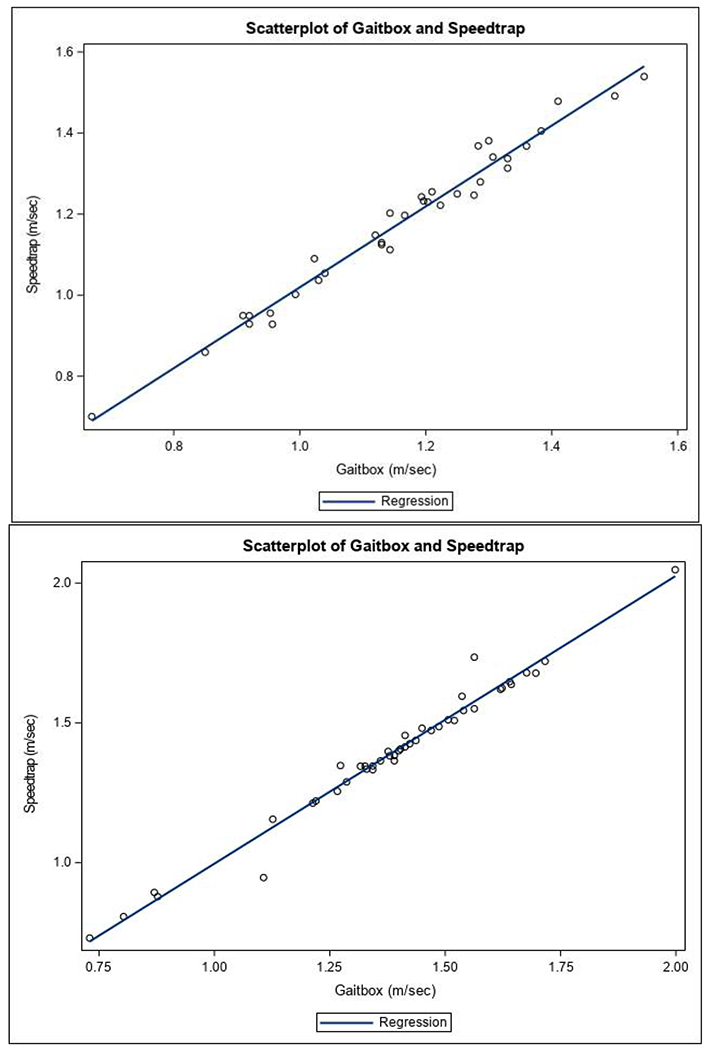
Scatterplot of GB and STS (Speedtrap) in Study 1 (Figure 3a) and Study 2 (Figure 3b).
Figure 4.
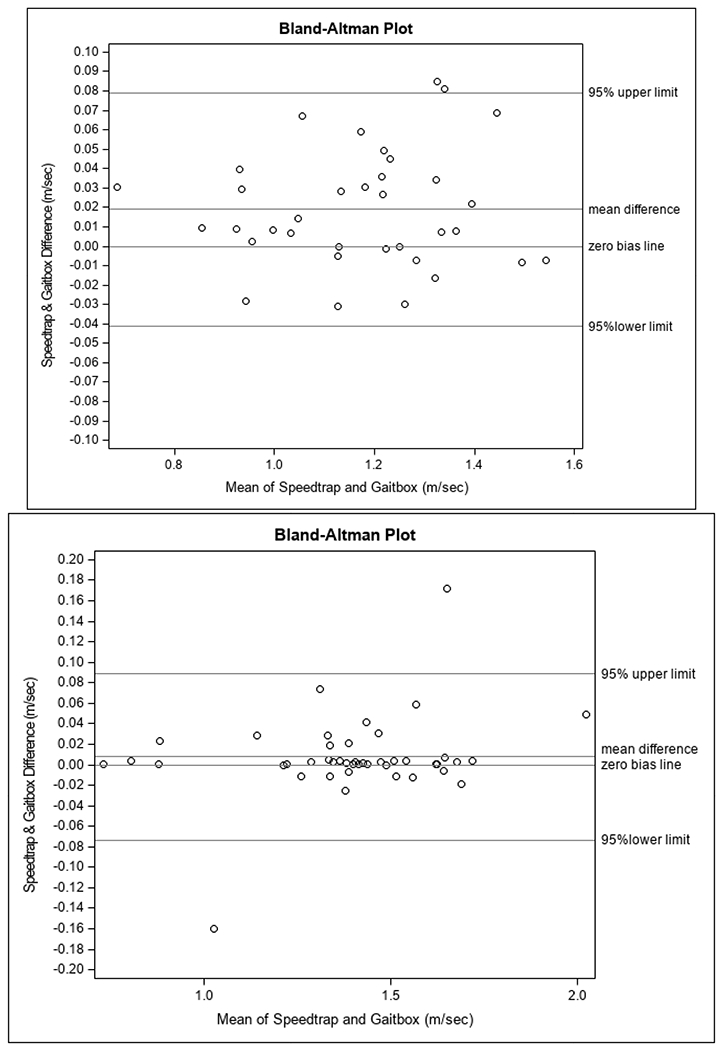
Bland-Altman plot of GB and STS in Study 1 (Figure 4a) and Study 2 (Figure 4b).
Study 2, 10 MWT
In contrast to Study 1, this sample was predominantly female (57%) with a mean usual gait speed of 1.39 m/s, in-line with the younger mean age of 35.3 years. Many individuals in this group (41%) also used an assistive device (6 forearm crutches, 2 underarm crutches, 3 canes, 5 walkers, 1 prosthetic leg, and 1 walking stick), which reflects the population from which they were drawn (Table 1). Results from this group also demonstrated a strong correlation between the GB and HT (r=0.99) and between GB and STS (r=0.99, p<0.0001; Figure 3b). The validity of GB compared to STS among all three walking trials in this sample was also excellent (ICC = 0.93). Test-retest reliability of the GB was also excellent in Study 2, with an ICC of 0.93. The absolute mean difference between GB and STS demonstrated very good precision, with a difference in mean gait speeds of 0.008±0.041 m/s (P = 0.22). The absolute mean difference between GB and HT was similar, at 0.006±0.040 m/s (P = 0.28). Bland Altman plot (Figure 4b) analysis demonstrated no systematic bias across all gait speeds when comparing the GB to STS, with a plot correlation coefficient of 0.26 (P = 0.09).
DISCUSSION
The findings from this study demonstrate excellent validity and reliability of the GB. Correlations among GB, HT, and STS were very strong in both study groups (r = 0.98-0.99). ICC’s (0.91-0.93) between GB and STS among all three walking trials were excellent in both study groups. The test-retest reliability ICC’s of GB (0.93-0.94) were also excellent in both study groups. The precision of the GB, as compared to STS, was within acceptable clinically detectable absolute mean differences (>0.02 m/s), indicating that the GB is precise enough to detect variability in gait speeds that would likely trigger an intervention. Our last method of validation included examination of bias. Bland-Altman plots indicated no systematic bias in the GB compared to STS in both study groups across their respective ranges of gait speeds.
The reported and observed differences in measurement techniques and distances have shown a need for a consistent methodology and an instrumented device. This is especially important when considering a 0.11 - 0.14 m/s change in older adults, 0.17 m/s change in incomplete SCI, and a 0.26 m/s change in MS are considered meaningful changes in walking speed [8]. While these values are the minimum detectable change (MDC) [8] and therefore considered corrected for variation in measurements due to random error and protocol changes [23], the differences in measurement protocols can be quite vast and it is not clear what amount of variation is accounted for in these measurements. Other studies indicate, on average, as low as a 0.05 m/s change in older adults is meaningful [9]. These changes are associated with adverse outcomes [24] and falls risk, in older adults [25] as well as used to predict recovery in other populations [11, 12]. The GB provides a consistent methodology tool for providers and clinics. Additionally, research supports that while a 4 m walk test may be an accurate measure for older adults, it cannot be compared to a 10 m walk test [26]. Therefore, between clinic visits or clinical locations, if providers are not using a consistent measurement path distance there may be issues with validity of speed changes over time. While within a clinic the protocol is similar, we have observed provider differences in timing distances within the same healthcare organization. Clinics conduct a 4 m, 6 m, or 10 m walk test, making comparison to past values difficult between locations. The GB allows for a consistent measurement between providers and clinics. Due to the device accuracy across both 4 m, 10 m, and different population groups, it is accurate to say the GB shows it is a valid measure of gait speed.
Some limitations of this study include the small sample size within Study 2 for each demographic area of SCI and TBI. Additionally, we only collected subject speed at a single, usual walking speed with an acceleration / deceleration zone. Additional studies observing each of these populations individually, walking speed without acceleration and deceleration zones, or at maximum speed would offer a more complete representation of the GB accuracy within these populations and measurement protocols.
There are several advantages of this device and approach to measuring walking speed over measurement by human timers; (1) the device is accurate and reliable, (2) the device requires minimal training to use and no changes to the walking path, and (3) the use of a hands-free measurement tool allows the human timer to act as a spotter for subjects who are unstable or need extra support. The spot is also able to walk next to the subject rather than having to walk slightly behind, as called for in the NIH-WT [7], increasing subject safety without affecting the measurement.
In addition to validation and reliability studies, the GB has been placed in a variety of clinics throughout the University Health system as part of standard of care. Most notably, the device has been placed in a geriatric clinic, retirement community, and cardiac rehabilitation clinic. These locations have helped in the design process by providing valuable feedback on device ease of use as well as data reporting. The authors are working with a product development company to produce a commercially available version of the device for sale to clinics and researchers.
As described earlier, we have observed and research supports, that inconsistencies with measurement techniques between the SPPB Guide and the NIH_WT and people administering the procedures can contribute to measurement errors in walking speed. Existing technology solutions are expensive and can be difficult to use and may not be able to be fit to the available space in the clinic. These factors support and the results of this validation study demonstrate that the GaitBox can serve as a low cost, easy to use device to measure walking speed.
CONCLUSION
These results indicate the GB is a reliable and valid measure of clinical gait speed as compared to the research grade measurement system (STS) for clinical populations and healthy older adults. This device can be used as an alternate to high cost instrumented devices or the human timer, with the goal of creating a consistent, accurate, and fast measurement methodology for use in clinical and research environments.
This device also has the potential to have an impact as a passive gait speed monitor observing changes in walking speed in a home setting. We are in the process of adapting the system to act as a passive monitor at home which will allow for additional data points for at risk and recovering populations. As gait speed measurements continue to become a vital part of the standard of care, an accurate, simple, consistent, low cost alternative to gait speed measurements by HT or research grade devices becomes increasingly important.
Highlights.
GaitBox is a clinically accurate measurement tool for walking speed over 4 and 10 m
GaitBox reduces potential human error associated with measurement
GaitBox can be produced at a much lower price than other instrumented techniques
ACKNOWLEDGEMENTS:
The authors would like to that Erin Radcliffe, Shepherd Center, Atlanta, GA for collecting data related to Study 2 and Dr. Miriam Morey, Duke University for her support throughout this endeavor.
FUNDING ACKNOWLEDGEMENTS:
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the National Institute on Disability, Independent Living and Rehabilitation Research in the U.S. Department of Health and Human Services [grant number 90RE5028]; and the National Institutes of Health [grant number P30AG028716].
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT:
The Author(s) declare that there are no conflicts of interest.
IRB:
Study 1 was conducted under the Duke Health, Durham, NC IRB, reference number 344453, protocol ID Pro00089655. Study 2 was conducted under the Shepherd Center, Atlanta, GA IRB, reference number 1319802, protocol ID Pro00089655.
REFERENCES
- [1].Abelian Van Kan G, Rolland Y, Andrieu S, Bauer J, Beachet O, et al. , Gait Speed at Usual Pace as a Predictor of Adverse Outcomes in Community-Dwelling Older People an International Academy on Nutrition and Aging (IANA) Task Force, The Journal of Nutrition, Health & Aging. 13(10) (2009) 881–889. [DOI] [PubMed] [Google Scholar]
- [2].Lusardi MM, Is walking speed a vital sign? Absolutely, Top Geriatr Rehabil. 28(2) (2012) 67–76. doi: 10.1097/TGR.0b013e31824385a4. [DOI] [Google Scholar]
- [3].Fischer JS, Rudick RA, Cutter GR, Reingold SC, The Multiple Sclerosis Functional Composite measure (MSFC): an integrated approach to MS clinical outcome assessment, Multiple Sclerosis Journal. 5(4) (1999) 244–250. 10.1177/135245859900500409. [DOI] [PubMed] [Google Scholar]
- [4].Hardy SE, Perera S, Roumani YF, Chandler JM, Studenski SA, Improvement in usual gait speed predicts better survival in older adults, J Am Geriatr Soc. 55(11) (2007) 1727–1734. Doi: 10.1111/j.1532-5415.2007.01413.x. [DOI] [PubMed] [Google Scholar]
- [5].Graham JE, Ostir GV, Fisher SR, Ottenbacher KJ, Assessing walking speed in clinical research: a systematic review, J Eval Clin Pract. 14(4) (2008) 552–562. doi: 10.1111/j.1365-2753.2007.00917.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Guralnik J, Short Physical Performance Battery (SPPB), NIH National Institute on Aging. https://www.nia.nih.gov/research/labs/leps/short-physical-performance-battery-sppb. (accessed January 24, 2020).
- [7].Kallen M, Slotkin J, Griffith J, Magasi S, Salsman J, Nowinski C, et al. , NIH Toolbox Technical Manual Domain: MOTOR Subdomain: LOCOMOTION Measure: NIH Toolbox 4-Meter Walk Gait Speed Test. http://www.healthmeasures.net/images/nihtoolbox/Technical_Manuals/Motor/Toolbox_4-Meter_Walk_Gait_Speed_Test_Technical_Manual.pdf, 2012. (accessed January 24, 2020).
- [8].Middleton A, Fritz SL, Lusardi M, Walking Speed: The Functional Vital Sign, J Aging Phys Act. 23(2) (2015) 314–322. doi: 10.1123/japa.2013-0236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Perera S, Mody SH, Woodman RC, Studenski SA, Meaningful Change and Responsiveness in Common Physical Performance Measures in Older Adults, J Am Geriatr Soc. 54(5) (2006) 743–9. Doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- [10].Studenski S, Perera S, Patel K, Rosano C, Faulkner K, Inzitari M, et al. , Gait Speed and Survival in Older Adults, Journal of the American Medical Association. 305(1) (2011) 50–58. Doi: 10.1001/jama.2010.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Goldman MD, Motl RW, Scagnelli J, Pula JH, Sosnoff JJ, Cadavid D, Clinically meaningful performance benchmarks in MS, Neurology. 81(21) (2013) 1856–1863. Doi: 10.1212/01.wnl.0000436065.97642.d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].van Silfhout L, Hosman AJF, A Bartels RHM, Edwards MJR, Abel R, Curt A, et al. , Ten Meters Walking Speed in Spinal Cord-Injured Patients: Does Speed Predict Who Walks and Who Rolls?, Neurorehabil Neural Repair. 31(9) (2017) 842–850. DOI: 10.1177/1545968317723751. [DOI] [PubMed] [Google Scholar]
- [13].Maggio M, Ceda GP, Ticinesi A, De Vita F, Gelmini G, Costantino C, et al. , 2016. Instrumental and Non-Instrumental Evaluation of 4-Meter Walking Speed in Older Individuals, PLoS One. 11(4), e0153583. doi: 10.1371/journal.pone.0153583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Bethoux F, Varsanik JS, Chevalier TW, Halpern EF, Stough D, Kimmel ZM, Walking speed measurement with an Ambient Measurement System (AMS) in patients with multiple sclerosis and walking impairment, Gait & Posture. 61 (2018) 393–397. doi: 10.1016/j.gaitpost.2018.01.033. [DOI] [PubMed] [Google Scholar]
- [15].Graham JE, Ostir GV, Kuo YF, Fisher SR, Ottencacher KJ, Relationship between test methodology and mean velocity in timed walk tests: a review, Arch Phys Med Rehabil. 89 (2007) 865–872. DOI: 10.1016/j.apmr.2007.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Youdas JW, Childs KB, McNeil ML, Mueller AC, Quilter CM, Hollman JH, Responsiveness of 2 procedures for measurement of temporal and spatial gait parameters in older adults, PM R. 2(6) (2010) 537–43. doi: 10.1016/j.pmrj.2010.02.008. [DOI] [PubMed] [Google Scholar]
- [17].Karpman C, Lebrasseur NK, Depew ZS, Novotny PJ, Benzo RP, Measuring gait speed in the out-patient clinic: methodology and feasibility, Respir Care. 59(4) (2014) 531–7. doi: 10.4187/respcare.02688. [DOI] [PubMed] [Google Scholar]
- [18].Peters DM, Middleton A, Donley JW, Blanck EL, Fritz SL, Concurrent validity of walking speed values calculated via the GAITRite electronic walkway and 3 meter walk test in the chronic stroke population, Physiother Theory Pract. 30(3) (2014) 183–8. doi: 10.3109/09593985.2013.845805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Multiple Sclerosis Functional Composite (MSFC), MS Society. https://www.nationalmssociety.org/For-Professionals/Researchers/Resources-for-Researchers/Clinical-Study-Measures/Multiple-Sclerosis-Functional-Composite-(MSFC). (accessed July 8, 2019).
- [20].Clark RA, Pua YH, Bower KJ, Bechard L, Hough E, Charlton PC, et al. , Validity of a low-cost laser with freely available software for improving measurement of walking and running speed, Journal of Science and Medicine in Sport. 22(2) (2019) 212–216. [DOI] [PubMed] [Google Scholar]
- [21].Sprint system. https://browertiming.com/tci-timing-system. (accessed 8 July 2019). [Google Scholar]
- [22].Bland JM, Altman DG, Statistical methods for assessing agreement between two methods of clinical measurement, Lancet. 1(8476) (1986) 307–310. [PubMed] [Google Scholar]
- [23].Goldberg A, Schepens S, Measurement error and minimum detectable change in 4-meter gait speed in older adults, Aging Clin Exp Res. 23(5-6) (2011) 406–412. doi: 10.1007/bf03325236. [DOI] [PubMed] [Google Scholar]
- [24].Cesari M, Kritchevsky S, J Penninx BWH,Nicklas BJ, Simonsick EM, Newman AB, et al. , Prognostic Value of Usual Gait Speed in Well-Functioning Older People – Results from the Health, Aging and Body Composition Study, J Am Geriatr Soc. 53(10) (2005) 1675–80. doi: 10.1111/j.1532-5415.2005.53501.x. [DOI] [PubMed] [Google Scholar]
- [25].Montero-Odasso M, Schapira M, Soriano ER, Varela M, Kaplan R, Camera LA, et al. , Gait Velocity as a Single Predictor of Adverse Events in Healthy Seniors Aged 75 Years and Older, J Gerontol A Biol Scie Med Sci. 60A(10) (2005) 1304–1309. doi: 10.1093/gerona/60.10.1304. [DOI] [PubMed] [Google Scholar]
- [26].Peters DM, Fritz SL, Krotish DE, Assessing the reliability and validity of a shorter walk test compared with the 10-Meter Walk Test for measurements of gait speed in healthy, older adults, J. Geriatr Phys Ther. 36(1) (2013) 24–30. 10.1519/JPT.0b013e318248e20d. [DOI] [PubMed] [Google Scholar]


