Abstract
Introduction:
We evaluated whether competing risk of death or selective survival could explain the reported inverse association between cancer history and dementia incidence (incidence rate ratio [IRR] ≈ 0.62–0.85).
Methods:
A multistate simulation model of a cancer- and dementia-free cohort of 65-year-olds was parameterized with real-world data (cancer and dementia incidence, mortality), assuming no effect of cancer on dementia (true IRR = 1.00). To introduce competing risk of death, cancer history increased mortality. To introduce selective survival, we included a factor (prevalence ranging from 10% to 50%) that reduced cancer mortality and dementia incidence (IRRs ranged from 0.30 to 0.90). We calculated IRRs for cancer history on dementia incidence in the simulated cohorts.
Results:
Competing risk of death yielded unbiased cancer-dementia IRRs. With selective survival, bias was small (IRRs = 0.89 to 0.99), even under extreme scenarios.
Discussion:
The bias induced by selective survival in simulations was too small to explain the observed inverse cancer-dementia link, suggesting other mechanisms drive this association.
Keywords: Alzheimer’s disease, cancer, competing risks, dementia, selection bias, simulation
1. INTRODUCTION
Robust literature supports an inverse association between history of cancer and dementia incidence.1–12 Meta-analyses indicate dementia incidence rates are 15% to 38% lower among those with history of cancer compared to those without; this inverse association persists across multiple cancer types.1,3,4,8,9,11,12 Some explanations for this association hold exciting potential for understanding dementia etiology. For example, identification of a shared cause with opposing effects (eg, a genetic or environmental factor that increases cancer risk but reduces dementia risk) would open new avenues of dementia prevention and treatment research.13–15 However, two important artefactual mechanisms have been proposed to explain the observed cancer-dementia inverse association: competing risk of death and selective survival.10,13–16 Whether the cancer-dementia association helps elucidate dementia etiology depends on whether it is explained by these mechanisms.
The competing risk of death is the phenomenon wherein mortality precludes dementia onset.17,18 Because cancer raises mortality, cumulative dementia incidence could appear lower in those with cancer history.10 In addition to the competing risk of death, selective survival (also known in epidemiologic literature as a type of selection or collider bias19–21) would occur if individuals who are more likely to survive cancer are also different in ways that protect them from dementia;13,16 that is, if any unmeasured factors influence who survives cancer and, independently, dementia onset. Although sometimes conflated, the competing risk of death and selective survival are distinct and either mechanism could result in lower observed dementia incidence among individuals with cancer history than without. If the observed inverse cancer-dementia association cannot be explained by these mechanisms, this would support other possible explanations, such as shared biological processes or effects of cancer or cancer treatment on dementia risk. We used simulations to evaluate the plausibility that the competing risk of death or selective survival explain the inverse cancer-dementia association.
2 |. METHODS
2.1 |. Multistate Markov simulation model structure
We developed a continuous-time multistate Markov model of a cohort of 65-year-olds. Multistate models, which model how individuals or groups transition across possible states (eg, disease progression),22,23 have been used in a variety of applications in dementia,24–28 including forecasting prevalence of preclinical and clinical Alzheimer’s disease.29 Our model included nine states (Figure 1): eight states corresponded to all combinations of cancer history (no/yes), dementia (no/yes), and U (a binary characteristic, representing selection, that reduced mortality rates among those with cancer history and reduced dementia incidence rates regardless of cancer history), and one state corresponded to death. State transitions in the model denoted incidence of cancer, dementia, or mortality (eg, transition from State A to State B in Figure 1 indicated dementia incidence in those without cancer).
FIGURE 1.

Schematic of multistate simulation model. Arrow thicknesses qualitatively represent relative magnitude of incidence rates. Blue arrows, transition from no dementia to dementia. Orange arrows, transition from no history of cancer to history of cancer. Green arrows, transition to death. Transition rates were obtained from real-world data. For example, for the age band 65 to 70, the dementia incidence rate is 5.4/1000 person-years (PY) (ACT study), cancer (all types) incidence rate is 162.2/1,000PY (SEER data). The overall mortality rate is 413.3/1000 PY (U.S. life tables); cancer increases the mortality rate 2.92-fold (SEER), dementia increases the mortality rate 7.45-fold,34 and the effects of cancer and dementia on mortality are multiplicative. In the base case selective survival model, U reduces both cancer mortality and dementia incidence rates by 30%. Abbreviations: ACT = Adult Changes in Thought; SEER = Surveillance, Epidemiology, and End Results
Transitions across states were unidirectional (shown by arrows in Figure 1), reflecting that a person could not revert to a disease-free state after cancer or dementia incidence, or to being alive after death. The rationale for this is that history of cancer, the exposure used in published studies of the cancer-dementia association, does not change even if cancer is cured, and that dementia and death are irreversible. Transition rates between states (eg, from “no cancer, no dementia,” to “cancer, no dementia”) were allowed to vary by age (detailed below), but not by duration in a state.
We simulated only characteristics needed to define the nine states in the model and analyze the data to obtain effect estimates of interest (see Section 2.4); these were age, cancer status, dementia status, the selective survival variable U, and death. For sex-specific cancer (breast and prostate) models, we simulated the cohort as all female or all male. Because data were simulated, informed consent was not required. All analyses were conducted in R version 3.6.1. The model was specified using differential equations corresponding to states in Figure 1, and solved using the Livermore solver in the deSolve package.30 The code is available at https://github.com/Mayeda-Research-Group/CancerAD-survivalsims.
2.2 |. Causal scenarios
We conducted simulations for two causal scenarios: one with only the competing risk of death and one with the competing risk of death and selective survival. For each scenario, the cohort was free of dementia and history of cancer at baseline, and we simulated data at 1-month intervals for 40 years of follow-up (ie, until the cohort was age 105). In the causal scenario with only the competing risk of death, the entire cohort was designated U = 0 (State A in Figure 1), and progressed through states B, C, D, and I over time. For the causal scenario including selective survival in addition to the competing risk of death, we set U = 1 for a proportion of the cohort (described in Section 2.3). Thus, at baseline, the cohort was split between states A and E, and progressed through other states over time. Note that we could not simulate a scenario that included selective survival but not the competing risk of death because selective survival occurs in a cohort in which there is selection (loss from the cohort over time) due to mortality, and mortality is a competing risk for dementia. Thus, any time selective survival occurs, the competing risk of death will also occur.
2.3 |. Model parameters
Transition (ie, incidence) rates were age dependent (specific for 5-year age bands) and the model was parameterized using real-world data. Here, we give an overview of transition rates; a complete list of transition rate sources is in the supporting information.
We developed versions of the model for all types of cancer combined and for three specific cancer types: lung, breast (female cohort), and prostate (male cohort). These specific cancer types are the most common types of cancer in the United States and represent varying fatality rates, which could affect our estimates of cancer-dementia associations.31 Lung cancer has poor 5-year survival, while breast and prostate cancers have high 5-year survival.31,32 Each version of the model retains the same state transition structure, but has cancer type–specific transition rates.
Age-specific cancer incidence rates (all cancers and site-specific lung, breast, and prostate) were obtained from the Surveillance, Epidemiology, and End Results (SEER) Program; we used sex-specific incidence rates for the breast and prostate cancer models.32 Age-specific dementia incidence rates were calibrated to the Adult Changes in Thought (ACT) study; for the breast and prostate cancer models, sex-specific dementia incidence rates were used.33 Dementia was ascertained in ACT as follows: all participants were screened biannually with the Cognitive Abilities Screening Instrument; those with scores <86 received diagnostic evaluations, including physical, neurological, and neuropsychological examinations, and laboratory and imaging studies.33 Diagnosis was determined by a consensus conference of clinicians and neuropsychologists. Because our goal was to evaluate the plausibility that the inverse cancer-dementia association could be fully explained by the competing risk of death or selective survival, we specified that at all ages, the true dementia incidence rate was the same in those with history of cancer versus without.
Cancer mortality rate ratios were calculated using SEER age-specific (65–74 vs 75+) and cancer type–specific 5-year relative survival data, assuming a constant rate ratio during each age interval32 (details available in supporting information). Dementia mortality rate ratios were taken directly from published data.34 Cancer and dementia were assumed to have multiplicative effects on mortality rates. For lung and all cancer type models, we calibrated cumulative survival in the simulated cohort to match U.S. life tables for birth years 1919 to 1921;35 sex-specific models were calibrated to match sex-specific U.S. life tables for this birth cohort.
As described above, we specified a binary variable U (Figure 1) to represent characteristics that reduced mortality rates in those with cancer history and reduced dementia incidence rates regardless of cancer history, which could lead to selective survival and potentially induce an artefactual inverse cancer-dementia association.19–21 In the model with only the competing risk of death, we set prevalence of this selection variable U to 0% (no selective survival). In the model including selective survival, we initially set prevalence of U to 30%, and specified that U reduced cancer mortality and dementia incidence rates each by 30% (incidence rate ratio [IRR] = 0.70). We selected these inputs because they represented plausible values for an unknown variable with moderate effects on cancer mortality and dementia incidence.
To determine conditions under which the competing risk of death and bias due to selective survival would produce the effect sizes consistent with those observed in the literature, we varied the three input parameters of the simulation model that relate to the selective survival variable U, and therefore affect the magnitude of potential bias: prevalence of U and effects of U on cancer mortality and dementia incidence. We considered a range of values, ranging from low to high prevalence and null to large effect sizes. Specifically, we varied prevalence of U in the cohort at baseline (0% to 50% in 10% increments) and strength of effects of U on cancer mortality and dementia incidence (IRRs of 0.30, 0.50, 0.70, 0.90, 1.00 [no effect]).
2.4 |. Quantification of bias
To compare results to published estimates of the cancer-dementia relationship, for each simulation we estimated the ratio of true instantaneous dementia incidence rate among living individuals with history of cancer to the true instantaneous dementia incidence rate among living individuals without history of cancer. This is consistent with a cause-specific hazard ratio, such as those estimated from the Cox proportional hazards models, that are typically reported in the literature.1,5–7,9,36 In all scenarios, recall that we specified no true effect of cancer on dementia incidence (ie, the true causal IRRcancer-dementia was specified to be 1.00 [null]).
For each simulated cohort, we estimated the observed IRRcancer-dementia and quantified the bias as the difference between the observed IRRcancer-dementia and the truth (1.00). The observed IRRcancer-dementia for each month of follow-up was calculated as the ratio of the instantaneous rates of dementia incidence in those with history of cancer versus without in that month. To summarize these monthly measures, an overall IRRcancer-dementia and age-specific IRRcancer-dementia by decade (65–74, 75–84, 85–94, 95–104) were calculated as the exponentiated averages of the natural log of the monthly IRRs, weighted according to the proportion of the cohort still at risk for dementia at the beginning of each month. Because the true IRRcancer-dementia was 1.00, any deviation from 1.00 observed in simulations was due to bias. The magnitude of bias was taken as the difference between observed overall IRRcancer-dementia or age-specific IRRcancer-dementia by decade and the true value of 1.00.
2.5 |. Additional explanatory analyses
In the causal scenario with selective survival in addition to the competing risk of death, bias was expected to occur through U, the characteristic that reduced cancer mortality and dementia incidence. Although cancer history and U were independent at baseline in our simulations, because U decreased mortality in those with history of cancer, we expected higher prevalence of U among surviving, dementia-free individuals with history of cancer than in those without at older ages. Because U also reduced dementia incidence, this was expected to induce an inverse cancer-dementia association. To clarify this selective survival mechanism of inducing bias, we examined the cancer-U association by plotting prevalence of U in the surviving, dementia-free cohort, stratified by cancer history, over time.
As an additional demonstration of the potential impact of the competing risk of death and selective survival, we estimated the cancer-dementia cumulative incidence ratio (CIRcancer-dementia) in our simulations. The CIRcancer-dementia was calculated as the ratio of lifetime dementia risk in individuals with history of cancer prior to dementia incidence versus individuals with no cancer incidence or with cancer incidence after dementia incidence. We examined the CIRcancer-dementia from models with competing risk of death only and from models that included selective survival.
3 |. RESULTS
For all simulations, simulated mortality, cancer, and dementia incidence rates were well calibrated to corresponding U.S. life tables, SEER, and ACT data. Figure 2 gives an example of how simulated cohorts transitioned through states over time, using the all-cancers model for the scenario with only the competing risk of death. The entire cohort starts cancer- and dementia-free (light blue), and over time, prevalence of cancer and dementia initially increase as some of the cohort transitions to these states, and subsequently decrease as the cohort transitions into the death state (dark gray). After 40 years of follow-up (age 105), nearly 100% of the cohort had died.
FIGURE 2.

Proportion of cohort in each state in the all-cancers model for the scenario with competing risk of death only
In the causal scenario that included the competing risk of death, but not selective survival, observed IRRs for the effect of cancer on dementia were unbiased (null) for all modeled cancer types and ages (Figure 3A). In contrast, the scenario with selective survival, in which prevalence of U was 30% at baseline (age 65) and U reduced both cancer mortality and dementia incidence by 30% (IRRU-cancer mortality = 0.70, IRRU-dementia = 0.70), induced a small inverse association. For example, in the all-cancers model, the overall IRRcancer-dementia across all ages was 0.99. Age-specific cancer-dementia IRRs were more biased at older ages: the observed IRRcancer-dementia for ages 65 to 74 was 0.99, while the observed IRRcancer-dementia for ages 95 to 104 was 0.94 (Figure 3B). Bias in overall and age-specific IRRcancer-dementia were similar in the models for site-specific cancers.
FIGURE 3.

Observed incidence rate ratios (IRR) from simulation scenarios with (A) competing risk of death only, and (B) competing risk of death and selective survival
Observed bias in the overall IRRcancer-dementia depended on the baseline prevalence of U, the characteristic that reduced cancer mortality and dementia incidence rates, and on the strength of the effects of U on cancer mortality and dementia incidence. Figure 4 shows results for the all-cancers model. Panels left to right correspond to increasing baseline prevalence of U (10% to 50%). Within each panel, moving left to right on the x-axis corresponds to decreasing strength of effect of U on dementia incidence (strongest effect, IRRU-dementia = 0.30 to no effect, IRRU-dementia = 1.00). The shade of each gray line corresponds to strength of the effect of U on cancer mortality (darkest is strongest effect, IRRU-cancer mortality = 0.30 to lightest, null effect, IRRU-cancer mortality = 1.00). In most scenarios, observed IRRcancer-dementia was close to null (ie, 0.95 > = IRRcancer-dementia > = 1.00). When U had no effect on either cancer mortality or dementia incidence (IRRU-cancer mortality = 1.00 or IRRU-dementia = 1.00), observed IRRcancer-dementia was also null (unbiased). The most biased IRRcancer-dementia was 0.89, observed in the most extreme selective survival scenario (IRRU-cancer mortality = 0.30, IRRU-dementia = 0.30, and p[U] = 0.50). Bias was marginally larger in lung cancer models and smaller in breast and prostate models (supporting information).
FIGURE 4.
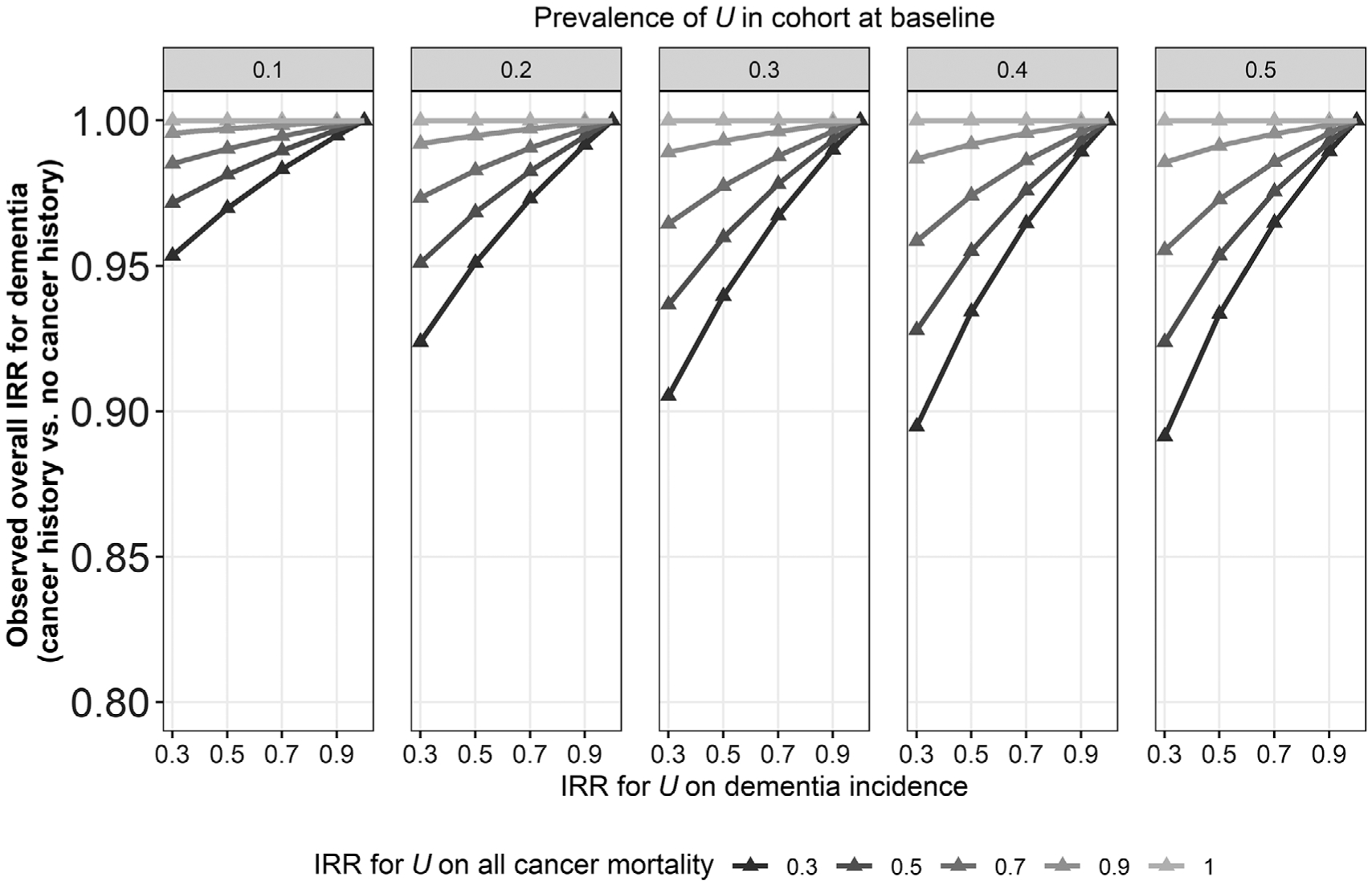
Observed incidence rate ratios (IRR) from simulation models for all cancers in the scenario with both the competing risk of death and selective survival across varying values for U (prevalence, effect on cancer mortality, and effect on dementia incidence). Effect of cancer on mortality was defined by age-specific relative survival (SEER)
Results described above can be explained by the selection processes that occurred in simulated cohorts. Figure 5 shows prevalence of U over time in the simulated cohort surviving dementia free, stratified by history of cancer, in the all-cancers model that included selective survival (IRRU-cancermortality = 0.70, IRRU-dementia = 0.70, and p[U] = 0.30). Early in follow-up, those with and without history of cancer had similar prevalence of U. Over time, prevalence of U among those surviving dementia free increased in both groups but increased faster in those with history of cancer. Thus, the increasing bias in the IRRs at older ages described above occurred because those with cancer history became relatively more selected (enriched) for U at older ages, and U protected against dementia.
FIGURE 5.
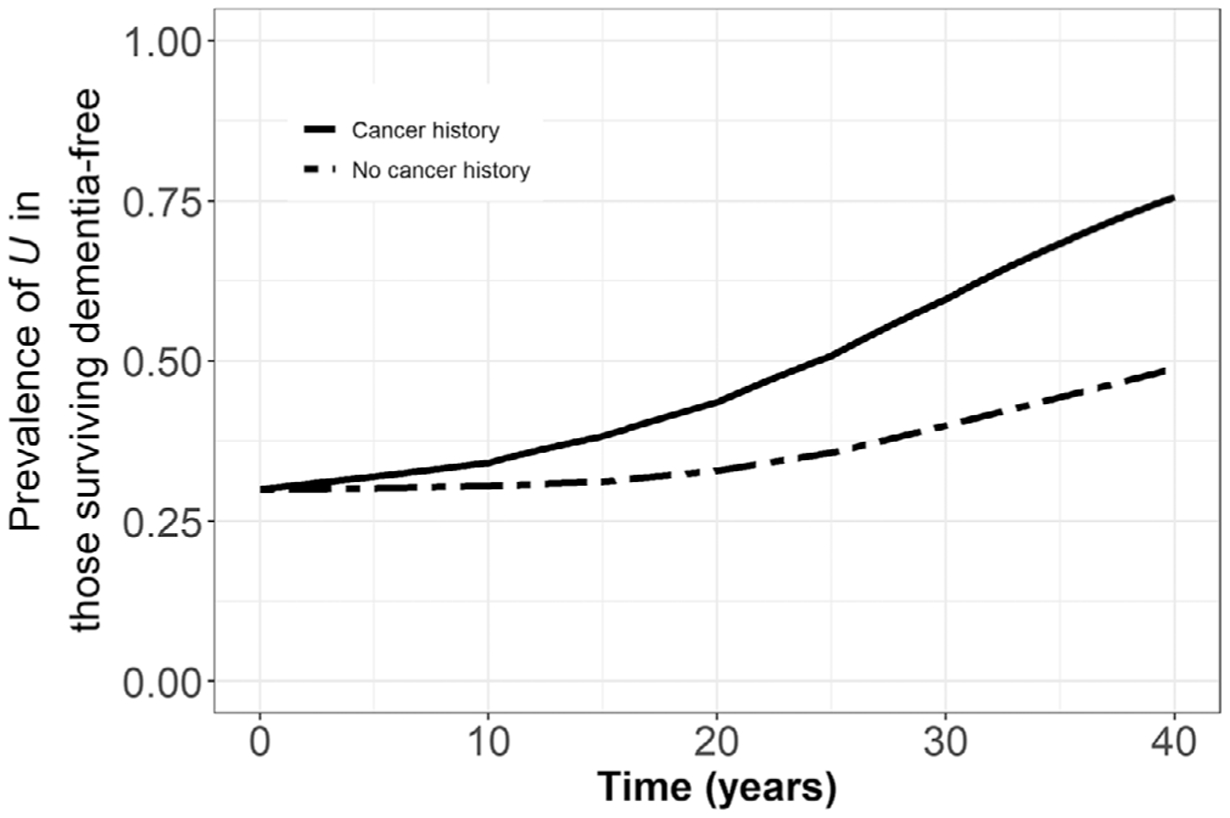
Prevalence of U among those surviving dementia free, stratified by history of cancer in the all-cancers model in the scenario with both the competing risk of death and selective survival (prevalence of U = 0.30, IRRU-cancer mortality = 0.70, IRRU-dementia = 0.70)
Finally, we examined lifetime risk of dementia among those with history of cancer versus without. In the scenario with only competing risk of death, the cancer-dementia CIR was 0.53, meaning that those with history of cancer have 47% lower lifetime risk of dementia than those without. The effect of lung cancer on dementia was more extreme (CIR = 0.23), while the CIRs for less fatal cancers (breast and prostate) were 1.00 and 1.04, respectively (Figure 6A). In the causal scenario that incorporated selective survival, CIRs were increased relative to the scenario with only competing risk of death (CIRallcancers = 0.60, CIRlung = 0.26, CIRbreast = 1.11, CIRprostate = 1.14, Figure 6B). This occurred because U reduced cancer mortality, meaning more individuals with cancer survived to older ages when dementia incidence is higher. Positive lifetime associations for breast and prostate cancer are due to the fact that both cancer and dementia are correlated with older age; high survival in these cancers led to positive associations in lifetime risk of dementia (CIRs > 1.00), while in the lung and all-cancer models this age-based positive association was reversed by the high mortality in those with history of cancer (derivation in supporting information).
FIGURE 6.
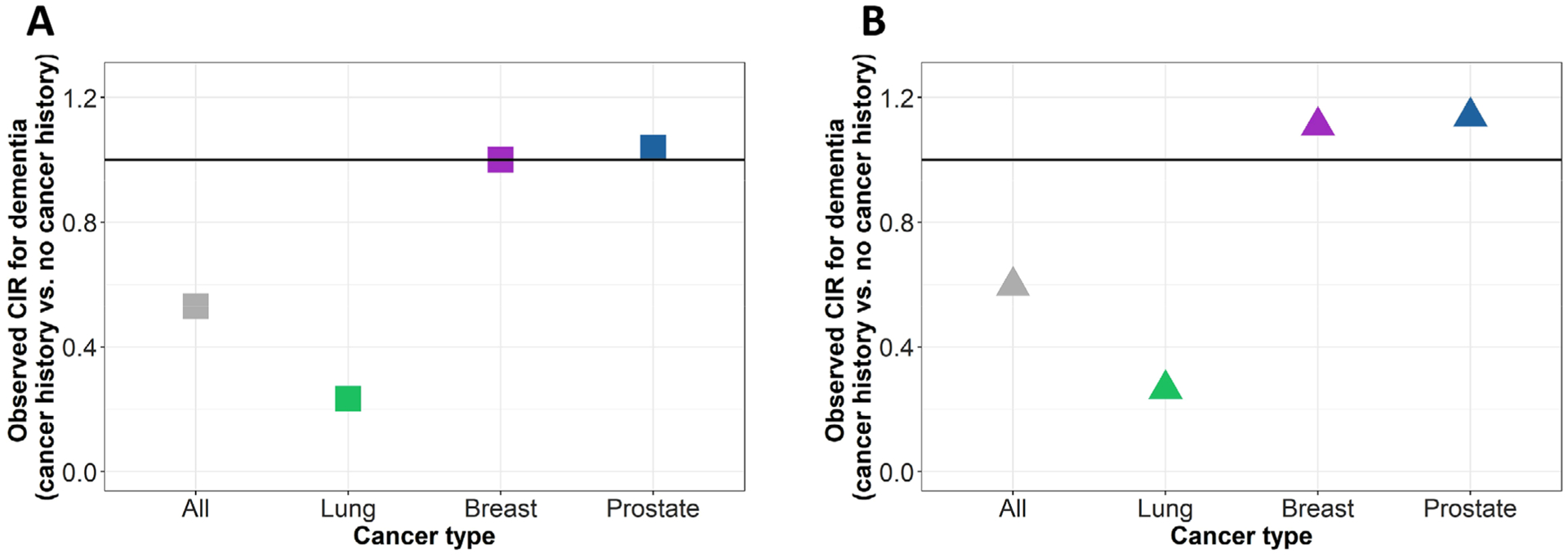
Observed cumulative incidence ratios (CIR) in the simulation model with (A) competing risk of death only, and (B) competing risk of death and selective survival, showing reduced lifetime risk, rather than rate, of dementia in cancers with higher mortality (all cancers and lung cancer)
4 |. DISCUSSION
We aimed to determine whether the competing risk of death or selective survival could explain the inverse cancer-dementia association observed in the literature. We simulated cohorts of cancer- and dementia-free 65-year-olds, calibrating with real-world data and specifying no true effect of cancer on dementia incidence. In our simulated cohorts, the competing risk of death did not bias the observed IRRcancer-dementia, and bias induced after addition of selective survival was modest.
There is substantial literature on the inverse cancer-dementia association.1–10 Although some recent studies report smaller effect estimates, meta-analyses show that dementia incidence is 15% to 38% lower among those with history of cancer compared to those without.1,3–7,37 Some studies examining diverse site-specific cancers, including breast, colorectal, lung, and non-melanoma skin cancers, show similar results.1,8,9,11,12 Several explanations, including both etiologically informative and artefactual, have been offered for these findings. For example, the inverse association could be produced if either cancer itself or cancer treatment truly prevent dementia or if an unknown factor both causes cancer and prevents dementia;15 some work supports such a shared biological basis.13–15,38,39 If any of these processes contribute substantially to the inverse cancer-dementia association, this could provide important insights into dementia etiology and potential prevention strategies. Although the inverse association appears in non-lethal cancers such as non-melanoma skin cancer,9,12 artefactual mechanisms related to survival or differential diagnostic practices (ie, lower dementia diagnosis rates among people with cancer history) have not been definitively ruled out.
In this study, we examined two artefactual explanations for the inverse cancer-dementia association: the competing risk of death and selective survival. The observed cancer-dementia IRRs in our simulations were not as protective as those in the literature. The competing risk of death induced no bias in the IRRcancer-dementia, and the addition of selective survival with plausible parameter values yielded cancer-dementia IRRs close to null (eg, 0.99), far from the estimates reported in meta-analyses.1,3,4 More biased effect estimates (eg, IRR = 0.89) were observed only in selective survival scenarios with unrealistic selective survival parameter values (eg, P[U] = 40%−50% at baseline, U reduced cancer mortality and dementia incidence by 70%). These biases were still not large enough to explain the empirically observed associations. Slightly more bias was observed in models for lung cancer, due to its higher mortality rate, while less bias was observed in the breast and prostate models, which have lower mortality rates. Scenarios that could introduce substantial bias would require an unknown risk factor (or factors) with high prevalence and large protective effects on cancer mortality and dementia incidence. Although it is possible that some rare unknown factors have such effects, it is unlikely that these factors could, in aggregate, have such high prevalence and large protective effects across the population.
Simulation studies offer the opportunity to conduct analyses when the true effect is specified; simulation therefore offered the optimal approach to determine whether, assuming no true effect of cancer history on dementia incidence, an inverse association could be induced via artefactual survival mechanisms. Limitations of our study include simplifications necessary to define states in the simulation model, and limitations of data available to parameterize the model. However, we do not think these limitations could substantively alter our conclusions. For example, dementia incidence rates in our model came from a predominantly non-Latino white cohort.33 Differences in dementia incidence rates by race/ethnicity are well documented;40 among racial/ethnic groups with higher dementia incidence (eg, Black Americans and American Indians/Alaska natives), the higher dementia rates in the cancer-free group (denominator of IRRcancer-dementia) would require even larger selective survival bias to obtain an IRRcancer-dementia similar to those observed in the literature. In addition, we modeled incident dementia dichotomously rather than modeling underlying continuous cognitive decline, and we did not model etiologic subtypes of dementia. To date, studies have not examined the potential for different impact of cancer on Alzheimer’s disease versus vascular dementia, although the simulation models could be adapted to account for this if data become available to parameterize models for dementia subtypes. Finally, our model assumed that increased mortality rates after cancer incidence persisted indefinitely. If mortality rates do not remain elevated >5 years after cancer diagnosis (eg, in remission), this assumption likely overestimated the strength of selective survival, making our estimates of the magnitude of bias likely overestimates; true bias induced by the competing risk of death and selective survival may be smaller than our simulations suggested. However, it is possible that recurrent disease or other consequences of cancer or cancer treatment could result in persistent, or even increasingly elevated mortality among those with cancer history.
Understanding the inverse cancer-dementia association offers a potentially rich opportunity to advance knowledge about dementia etiology and effective prevention strategies, provided the association is not artefactual. Our simulations illustrate that neither moderate nor extreme selective survival scenarios induced large enough bias to account for the cancer-dementia association observed in the literature. Future work, including empirical analyses and simulation studies, should explore other explanations, such as differential diagnostic practices, a shared biological basis, or true causal effect of cancer or cancer treatment on dementia, and examine dementia subtypes.
Supplementary Material
RESEARCH IN CONTEXT.
Systematic review: The authors reviewed the literature on the association between cancer history and dementia incidence using PubMed and Google Scholar. Prior meta-analyses of the literature on this topic are cited, along with several papers published after the publication of the meta-analyses.
Interpretation: Our findings indicated that the competing risk of death and selective survival do not induce sufficient bias in the cancer-dementia incidence rate ratio to account for the magnitude of the association observed in the literature.
Future directions: Future work should explore the plausibility of other explanations for this observed inverse cancer-dementia association, such as differential diagnostic practices, a shared biological basis, or true causal effect of cancer or cancer treatment on dementia incidence.
Funding information
National Institutes of Health, Grant/Award Numbers: NIA RF1AG059872, NCI R03CA241841
Footnotes
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of the article.
REFERENCES
- 1.Ma LL, Yu JT, Wang HF, et al. Association between cancer and Alzheimer’s disease: systematic review and meta-analysis. J Alzheimer’s Dis. 2014;42:565–573. [DOI] [PubMed] [Google Scholar]
- 2.Nudelman KNH, Risacher SL, West JD, McDonald BC, Gao S, Saykin AJ. Association of cancer history with Alzheimer’s disease onset and structural brain changes. Front Physiol. 2014;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang Q, Guo S, Zhang X, et al. Inverse relationship between cancer and Alzheimer’s disease: a systemic review meta-analysis. Neurol Sci. 2015;36:1987–1994. [DOI] [PubMed] [Google Scholar]
- 4.Papageorgakopoulos TN, Moraitou D, Papanikolaou M, Tsolaki M. The association between Alzheimer’s disease and cancer: systematic review—meta-analysis. Hell J Nucl Med. 2017;20: 45–57. [PubMed] [Google Scholar]
- 5.Aiello Bowles EJ, Walker RL, Anderson ML, Dublin S, Crane PK, Larson EB. Risk of Alzheimer’s disease or dementia following a cancer diagnosis. PLoS One. 2017;12(6):e0179857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Frain L, Swanson D, Cho K, et al. Association of cancer and Alzheimer’s disease risk in a national cohort of veterans. Alzheimer’s Dement. 2017;13:1364–1370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Freedman DM, Wu J, Chen H, et al. Associations between cancer and Alzheimer’s disease in a U.S. Medicare population. Cancer Med. 2016;5:2965–2976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Driver JA, Beiser A, Au R, et al. Inverse association between cancer and Alzheimer’s disease: results from the Framingham Heart Study. BMJ. 2012;344:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidt SAJ, Ording AG, Horváth-Puhó E, Sørensen HT, Henderson VW. Non-melanoma skin cancer and risk of Alzheimer’s disease and all-cause dementia. PLoS One. 2017;12(2):e0171527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cancer Ganguli M. and dementia: it’s complicated. Alzheimer Dis Assoc Disord. 2015;29:177–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Musicco M, Adorni F, Di Santo S, et al. Inverse occurrence of cancer and Alzheimer disease: a population-based incidence study. Neurology. 2013;81:322–328. [DOI] [PubMed] [Google Scholar]
- 12.White RS, Lipton RB, Hall CB, Steinerman JR. Nonmelanoma skin cancer is associated with reduced Alzheimer disease risk. Neurology. 2013;80:1966–1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ospina-Romero M, Abdiwahab E, Kobayashi L, et al. Rate of memory change before and after cancer diagnosis. JAMA Netw Open. 2019;2(6):e196160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma SL, Tang NLS, Tam CWC, et al. A PIN1 polymorphism that prevents its suppression by AP4 associates with delayed onset of Alzheimer’s disease. Neurobiol Aging. 2012;33:804–813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Driver JA. Inverse association between cancer and neurodegenerative disease: review of the epidemiologic and biological evidence. Biogerontology. 2014;15:547–557. [DOI] [PubMed] [Google Scholar]
- 16.van der Willik KD, Schagen SB, Ikram MA. Cancer and dementia: two sides of the same coin. Eur J Clin Invest. 2018;48(11):e13019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen PK, Geskus RB, De witteT, Putter H. Competing risks in epidemiology: possibilities and pitfalls. Int J Epidemiol. 2012;41:861–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang CCH, Zhao Y, Lee CW, Ganguli M. Smoking, death, and Alzheimer disease: a case of competing risks. Alzheimer Dis Assoc Disord. 2012;26:300–306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hernán MA, Hernández-Díaz S, Robins JM. A structural approach to selection bias. Epidemiology. 2004;15:615–625. [DOI] [PubMed] [Google Scholar]
- 20.Mayeda ER, Maria Glymour M. The obesity paradox in survival after cancer diagnosis: tools for evaluation of potential bias. Cancer Epidemiol Biomarkers Prev. 2017;26:17–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mayeda ER, Banack HR, Bibbins-Domingo K, et al. Can survival bias explain the age attenuation of racial inequalities in stroke incidence?. Epidemiology. 2018;29:525–532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Andersen PK, Keiding N. Multi-state models for event history analysis. Stat Methods Med Res. 2002;11:91–115. [DOI] [PubMed] [Google Scholar]
- 23.Ackley SF, Mayeda ER, Worden L, Enanoria WTA, Glymour MM, Porco TC. Compartmental model diagrams as causal representations in relation to DAGs. Epidemiol Method. 2017;6(1):20060007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brookmeyer R, Abdalla N. Estimation of lifetime risks of Alzheimer’s disease dementia using biomarkers for preclinical disease. Alzheimer’s Dement. 2018;14:981–988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Binder N, Balmford J, Schumacher M. A multi-state model based reanalysis of the Framingham Heart Study: is dementia incidence really declining. Eur J Epidemiol. 2019;34:1075. [DOI] [PubMed] [Google Scholar]
- 26.Vermunt L, Sikkes SAM, van den Hout A, et al. Duration of preclinical, prodromal, and dementia stages of Alzheimer’s disease in relation to age, sex, and APOE genotype. Alzheimer’s Dement. 2019;15: 888–898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wei S, Xu L, Kryscio RJ. Markov transition model to dementia with death as a competing event. Comput Stat Data Anal. 2014;80:78–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Robitaille A, van den Hout A, Machado RJM, Bennett DA, Čukić I, Deary IJ, et al. Transitions across cognitive states and death among older adults in relation to education: a multistate survival model using data from six longitudinal studies. Alzheimer’s Dement. 2018;14:462–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dement. 2018;14:121–129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Soetaert K, Petzoldt T, Setzer RW. Solving Differential Equations in R : package deSolve. J Stat Softw. 2010;33(9), 1–25.20808728 [Google Scholar]
- 31.Surveillance Research Program, National Cancer Institute. Cancer Stat Facts: Common Cancer Sites [Internet]. n.d. https://seer.cancer.gov/statfacts/html/common.html. (accessed February 14, 2020).
- 32.Surveillance Research Program NCI. SEER*Explorer: An interactive website for SEER cancer statistics [Internet]. n.d. https://seer.cancer.gov/explorer/.
- 33.Tom SE, Hubbard RA, Crane PK, et al. Characterization of dementia and Alzheimer’s disease in an older population: updated incidence and life expectancy with and without dementia. Am J Public Health. 2015;105:408–413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mayeda ER, Glymour MM, Quesenberry CP, Johnson JK, Pérez-Stable EJ, Whitmer RA. Survival after dementia diagnosis in five racial/ethnic groups. Alzheimer’s Dement. 2017;13:761–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Division of vital statistics. National Vital Statistics Reports 2019;68:49–52. [Google Scholar]
- 36.Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009;170:244–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ording AG, Horváth-Puhó E, Veres K, et al. Cancer and risk of Alzheimer’s disease: small association in a nationwide cohort study. Alzheimer’s Dement. 2020;16(7):953–964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seddighi S, Houck AL, Rowe JB, Pharoah PDP. Evidence of a causal association between cancer and Alzheimer’s disease: a mendelian randomization analysis. Sci Rep. 2019;9:13548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yarchoan M, James BD, Shah RC, et al. Association of cancer history with Alzheimer’s disease dementia and neuropathology. J Alzheimer’s Dis. 2017;56:699–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mayeda ER, Glymour MM, Quesenberry CP, Whitmer RA. Inequalities in dementia incidence between six racial and ethnic groups over 14 years. Alzheimer’s Dement. 2016;12:216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


