Abstract
OBJECTIVE:
We obtained preliminary evidence on the efficacy of early prophylaxis on the risk of central venous catheter (CVC)-associated deep venous thrombosis (CADVT) and its effect on thrombin generation in critically ill children.
DESIGN:
Bayesian phase 2b randomized clinical trial (RCT)
SETTING:
7 pediatric intensive care units (ICU)
PATIENTS:
Children <18 years old with newly inserted CVC and at low risk of bleeding
INTERVENTION:
Enoxaparin adjusted to anti-Xa level of 0.2–0.5 IU/mL started at <24 hours after insertion of CVC (enoxaparin arm) vs usual care without placebo (usual care arm)
MEASUREMENTS AND MAIN RESULTS:
At the interim analysis, the proportion of CADVT on ultrasonography in the usual care arm, which was 54.2% of 24 children, was significantly higher than previously reported. This resulted in misspecification of the pre-approved Bayesian analysis, reversal of direction of treatment effect and early termination of the RCT. Nevertheless, with 30.4% of 23 children with CADVT on ultrasonography in the enoxaparin arm, risk ratio of CADVT was 0.55 (95% credible interval: 0.24, 1.11). Including children without ultrasonography, clinically relevant CADVT developed in 1 (3.7%) of 27 children in the enoxaparin arm and 7 (29.2%) of 24 in the usual care arm (p=0.02). Clinically relevant bleeding developed in 1 child randomized to the enoxaparin arm. Response profile of endogenous thrombin potential, a measure of thrombin generation, was not statistically different between trial arms.
CONCLUSIONS:
These findings suggest the efficacy and safety of early prophylaxis that should be validated in a pivotal RCT.
CLINICALTRIALS.GOV IDENTIFIER:
Keywords: Bayesian statistics, bleeding, central venous catheter, deep venous thrombosis, enoxaparin, pediatric intensive care unit
ARTICLE TWEET:
Early prophylaxis with enoxaparin may safely reduce the risk of catheter-associated thrombosis in critically ill children.
INTRODUCTION
Venous thromboembolism (VTE) significantly impacts the health of children (1, 2). The annual rate of VTE in hospitalized children increased by 70% over nearly a decade (3). Pediatric VTE, which is predominantly deep venous thrombosis (DVT), is the 2nd largest contributor to harm accounting for 16% of all serious safety events in hospitalized children (4). Critical illness and central venous catheters (CVC) are the most important risk factors for DVT in children (5). A meta-analysis of 676 children showed that the absolute risk of CVC-associated DVT (CADVT) diagnosed using systematic radiologic screening in critically ill children with untunneled CVC was 0.18 (95% confidence interval, CI: 0.12–0.25) (6).
Pediatric VTE is potentially preventable (7, 8). However, pharmacologic prophylaxis against CADVT is not recommended in hospitalized children because of paucity of pediatric randomized clinical trials (RCT) (9). Prophylaxis of Thrombo-Embolism in Kids Trial (PROTEKT), the largest RCT of prophylaxis against CADVT in hospitalized children, was stopped early for poor enrollment (10). In PROTEKT, risk ratio of radiologically confirmed CADVT with reviparin vs placebo was 1.2 (95% CI: 0.4–3.2). Reviparin was started late at mean of 2.6 days after insertion of CVC when nearly 50% of CADVT would have already developed and only achieved mean anti-Xa level of 0.1 IU/mL (10, 11). In preparation for a pivotal RCT of pharmacologic prophylaxis against CADVT, our first aim was to obtain preliminary evidence on the efficacy of early prophylaxis started within 24 hours after insertion of the CVC on the risk of CADVT in critically ill children. Our second aim was to evaluate the effect of a prophylactic strategy that targeted the typically used anti-Xa level of 0.2–0.5 IU/mL on thrombin generation, the key event in thrombus formation, in critically ill children.
METHODS
Study Design and Oversight
The Catheter-Related Early Thromboprophylaxis with Enoxaparin (CRETE) Trial was an open-label blinded endpoint Bayesian phase 2b RCT conducted in 7 pediatric intensive care units (ICU) in the United States from November 2017 to August 2019. Children were randomized 1:1 to enoxaparin or usual care with permuted block design and variable block sizes. Computerized Randomization was stratified by ICU and age, i.e., <1 year, 1–13 years and >13 years (12). Yale Human Investigations Committee (HIC#1302011506) and local institutional review boards approved the CRETE Trial. Food and Drug Administration (FDA) approved the use of enoxaparin. Parental permission and assent, as appropriate, were obtained on enrollment. A data and safety monitoring board (DSMB) and safety monitor oversaw the CRETE Trial and reviewed the data.
Subjects
We enrolled children admitted to the ICU who were <24 hours after insertion of an untunneled CVC in the internal jugular or femoral vein, with CVC required for ≥24 hours, >36 weeks corrected gestational age to <18 years old, and with anticipated stay in the ICU ≥48 hours. Ultrasonography is inaccurate in diagnosing subclavian CADVT (13). We excluded those with coagulopathy, clinically relevant bleeding as defined by the International Society on Thrombosis and Haemostasis (ISTH), <60 days from a clinically relevant bleed, <7 days after trauma or surgery, on concurrent antithrombotic agent, DVT at site of insertion of the CVC in prior 6 weeks, allergy to heparin or history of heparin-induced thrombocytopenia (14–17). Coagulopathy was defined as international normalized ratio >2.0, activated partial thromboplastin time >50 seconds or platelet count <50,000/mm3 (16).
Procedures
Children randomized to enoxaparin received enoxaparin subcutaneously every 12 hours at 0.75 mg/kg for children ≤2 months old or 0.5 mg/kg (maximum of 30 mg) for others, which was adjusted for obesity and renal insufficiency (9). First dose was administered <24 hours after insertion of CVC and adjusted to achieve an anti-Xa level of 0.2–0.5 IU/mL. Anti-Xa level was measured locally 4–6 hours after every third dose until target was reached, then weekly. Enoxaparin continued until end of study, which was removal of CVC, or earlier upon discharge from ICU, radiologic diagnosis of CADVT, start of therapeutic anticoagulation, or 28 days after insertion of CVC. Enoxaparin was discontinued after a clinically relevant bleed, and held 12 hours before surgery or invasive procedure, for new coagulopathy, or suspected heparin induced thrombocytopenia (HIT). Enoxaparin was resumed 24 hours after coagulopathy was corrected, 24 hours after surgery or procedure, and after HIT was excluded. Children randomized to usual care did not receive placebo. Ultrasonography of the vein proximal and distal to site of insertion of the CVC was performed by blinded technicians using standard procedures within 24 hours of end of study (13). Children were monitored daily for bleeding (18).
Blood was drawn into citrated tubes (BD Vacutainer Plus Plastic Citrate Tubes, Becton and Dickinson Company, Franklin Lakes, NJ, USA) on the day of, day after and day 4 after insertion of CVC, processed locally for platelet poor plasma, then frozen at −70°C until analyzed. Endogenous thrombin potential (ETP), a measure of thrombin generation, was measured from plasma using Calibrated Automated Thrombogram (Thrombinoscope, Maastricht, Netherlands) and 1 pM of tissue factor (PPP-Reagent LOW, Stago Diagnsotica, Parsippany, NJ, USA) per manufacturer’s protocol. Factor VIII activity and D-dimer level were measured using Siemens Factor VIII Chromogenic Assay and Stratus CS Acute Care DDMR Testpak (Siemens Healthcare Diagnostics Inc., Newark, DE), respectively.
Outcome Measures
Primary outcome for the first aim was presence of CADVT, defined as DVT in the site of insertion of the CVC confirmed by ultrasonography. A committee of 3 pediatric radiologists blindly and independently diagnosed CADVT (13). CADVT was diagnosed if ≥2 were present: intravenous echogenic material adherent to the venous wall, non-compressibility of the vein, or abnormal venous Doppler (19). At least 2 concurring radiologists were needed to diagnose CADVT. Their diagnosis was not relayed to the clinical team. However, a local radiologist read the images and provided results to the clinical team who decided on treatment. Complete or partial occlusion of flow defined an occlusive or nonocclusive CADVT, respectively (20). Clinically relevant CADVT was defined as a radiologically diagnosed CADVT that had signs of DVT, including dysfunction of the CVC, as detected by the clinical team, or that was treated by the clinical team (21, 22). Secondary outcomes included measures of efficacy, safety, feasibility and compliance. Primary outcome for the second aim was ETP with factor VIII activity and D-dimer level as secondary outcomes.
Statistical Considerations
The CRETE Trial was designed and approved by the FDA and DSMB to use Bayesian inference for primary analysis of the first aim. Statistical details are in the Supplemental Material. Bayesian inference proceeds by weighted averaging of previous information, i.e., prior, and likelihood function of observed data to produce updated information, i.e., posterior (23). In the presence of pertinent information, informative priors can be used. In its absence, minimally informative priors are used. Borrowing of information with informative priors can mitigate the effect of limited sample size (24). Estimates of parameters of interest, e.g., risk of CADVT and risk ratio with enoxaparin, are reported from the posteriors.
We planned to enroll 100 children. For the primary analysis comparing the risk of CADVT between trial arms, risk of CADVT without prophylaxis from the meta-analysis was used as informative prior for the usual care arm (Supplemental Figure) (6). Minimally informative prior was used for the enoxaparin arm because information was not available. We planned to test the absolute difference in the risks of CADVT to leverage the available information. Our statistical hypothesis was that there was >60% probability that the difference in the risk of CADVT was ≥0.06 points, in terms of risk, lower with prophylaxis. This represented a clinically significant relative risk reduction with enoxaparin of ≥33%, assuming the risk of CADVT without prophylaxis was 0.18 (6). In the supportive analysis, we chose an approach that was free of assumptions on the risks of CADVT and estimated the risk ratio of CADVT with enoxaparin using minimally informative prior given the absence of pertinent information on treatment effect. The risks and ratio were reported as medians of the posteriors with 95% equal-tailed credible intervals (CrI). FDA requested an interim efficacy analysis after 50 children were randomized. Data from these children were used to determine the probability of proving our hypothesis using predictive probability (25). The CRETE Trial would be terminated for futility if this predictive probability was <10%.
Frequentist analyses were performed for comparison and in the absence of pre-specified analysis for the second aim and secondary outcome measures (26). Chi-squared or Fisher’s exact tests were used to compare categorical variables between trial arms, while Mann-Whitney U test was used to compare continuous variables. Linear mixed effects model that included the interaction between trial arm and sampling day regarded as a categorical variable assessed the associations of ETP, factor VIII activity and D-dimer level with treatment with random intercept per child. Statistical test of the null hypothesis of no interaction compared mean response profiles, defined as patterns of change in biomarker level from day of insertion of the CVC, between trial arms (27). A p value <0.05 was considered statistically significant.
Data was presented as counts (percentage) or median (interquartile range, IQR). Intention to treat principle was applied to all analyses. All statistical analyses were conducted using Stata 16.1 (StataCorp, College Station, TX).
RESULTS
Subjects
The DSMB recommended termination after interim analysis. The predictive probability was 0.9% (Supplemental Material).
A total of 51 (31.1%) of 164 eligible children were randomized to enoxaparin (n=27) or usual care (n=24; Figure 1). Median age was 2.8 years (IQR: 0.4, 8.9 years) in the enoxaparin arm and 1.0 years (IQR: 0.3, 8.9 years) in the usual care arm (Table 1). CVC was most commonly inserted in the right internal jugular vein in the enoxaparin (55.6%) and usual care (54.2%) arms. Co-interventions were comparable, except for use of neuromuscular blockade, which was more common in the enoxaparin arm (77.8% vs 37.5% in the usual care arm). None received additional anticoagulants or aspirin. Use of mechanical thromboprophylaxis was comparable.
Figure 1.
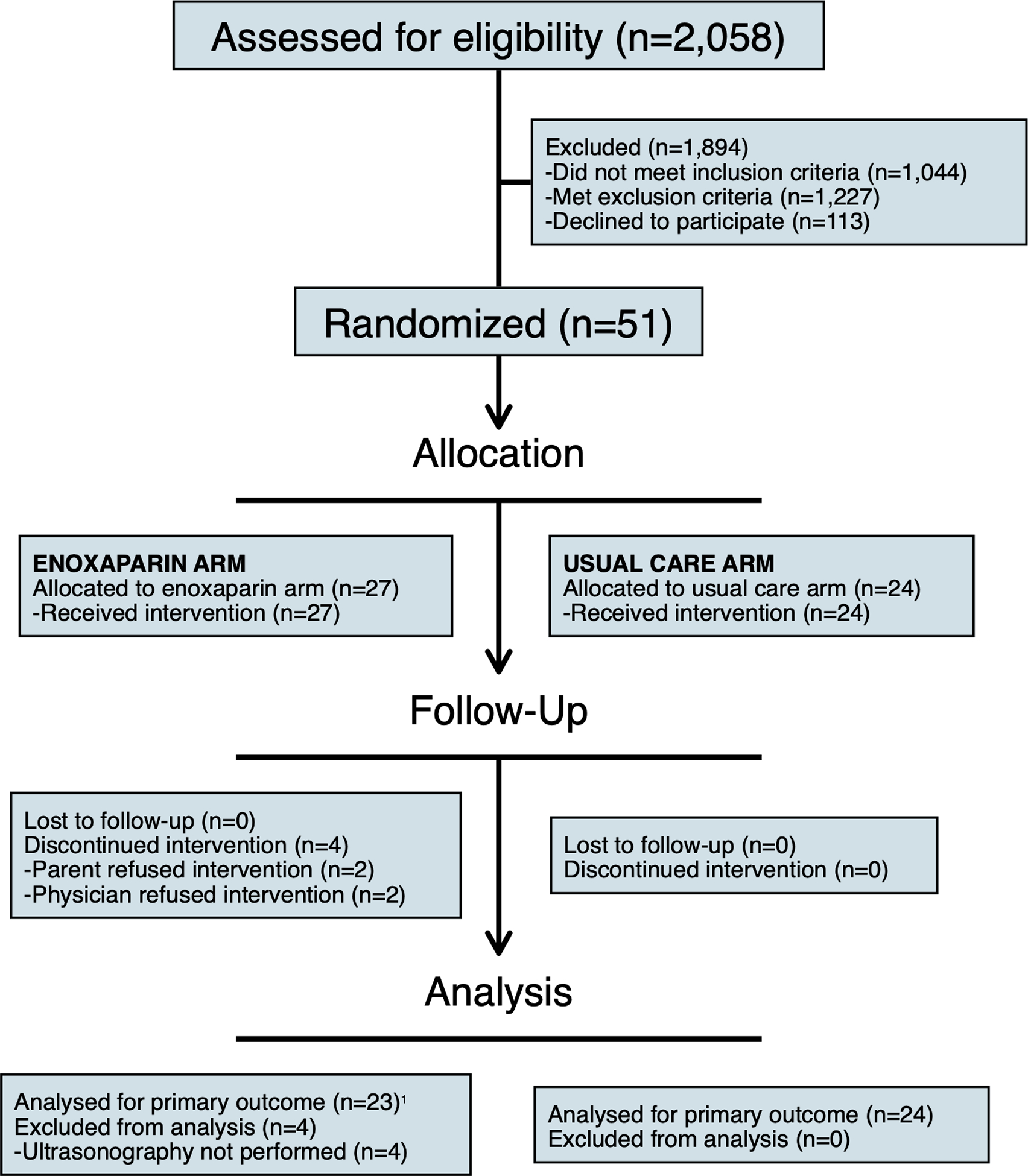
CONSORT diagram. 1All children, regardless of the presence of ultrasonography, were analyzed for the other outcome measures.
Table 1.
Baseline characteristics and co-interventions of children in the enoxaparin and usual care arms.
| Enoxaparin Arm (n=27) | Usual Care Arm (n=24) | p value | |
|---|---|---|---|
| Baseline Characteristics | |||
| Age category | 0.91 | ||
| <1 year old | 12 (44.4) | 12 (50.0) | |
| 1–13 years old | 13 (48.1) | 10 (41.7) | |
| >13 year old | 2 (7.4) | 2 (8.3) | |
| Female sex | 16 (59.3) | 12 (50.0) | 0.51 |
| Weight (kg) | 10.4 (6.5, 23.5) | 9.3 (6.1, 26.5) | 0.79 |
| Pediatric Index of Mortality 2 | 0.03 (0, 0.2) | 0.03 (0, 0.1) | 0.42 |
| Race and ethnicity | 0.96 | ||
| Non-Hispanic white | 17 (63.0) | 14 (58.3) | |
| Non-Hispanic black | 4 (14.8) | 5 (20.8) | |
| Non-Hispanic mixed | 1 (3.7) | 0 (0) | |
| Hispanic | 5 (18.5) | 5 (20.8) | |
| Presence of cancer | 1 (3.7) | 0 (0) | 1.00 |
| Presence of systemic infection | 15 (55.6) | 15 (62.5) | 0.62 |
| Presence of congenital heart disease | 2 (7.4) | 2 (8.3) | 1.00 |
| Presence of permanent immobility | 3 (11.1) | 2 (8.3) | 1.00 |
| Presence of recent surgery | 0 (0) | 2 (8.3) | 0.22 |
| Personal history of venous thromboembolism | 1 (3.7) | 0 (0) | 1.00 |
| Size of central venous catheter | 0.35 | ||
| 3 French (F) | 0 (0) | 2 (8.3) | |
| 4 F | 13 (48.1) | 11 (45.8) | |
| 5 F | 10 (37) | 10 (41.7) | |
| 7 F | 4 (14.8) | 1 (4.2) | |
| Number of lumens of central venous catheter | 0.50 | ||
| 1 | 0 (0) | 2 (8.3) | |
| 2 | 15 (55.6) | 12 (50.0) | |
| 3 | 12 (44.4) | 10 (41.7) | |
| Site of insertion of central venous catheter | 1.00 | ||
| Left femoral | 4 (14.8) | 4 (16.7) | |
| Right internal jugular | 15 (55.6) | 13 (54.2) | |
| Right femoral | 8 (29.6) | 7 (29.2) | |
| Platelet count (x 103/mm3) | 281 (191, 315) | 250 (172, 310) | 0.37 |
| Hemoglobin (g/dL) | 9.7 (9.1, 11.3) | 9.9 (8.9, 11.5) | 0.81 |
| International normalized ratio | 1.2 (1.0, 1.4) | 1.2 (1.1, 1.4) | 0.37 |
| Activated partial thromboplastin time (sec) | 33.4 (28.7, 38.5) | 32.1 (29.3, 37.0) | 0.52 |
| Co-interventions | |||
| Vasopressor | 14 (51.9) | 12 (50.0) | 0.90 |
| Non-invasive ventilation | 7 (25.9) | 6 (25.0) | 0.94 |
| Mechanical ventilation | 26 (96.3) | 21 (87.5) | 0.33 |
| Neuromuscular blockade | 21 (77.8) | 9 (37.5) | 0.004 |
| Intravenous unfractionated heparin | 18 (66.7) | 18 (75.0) | 0.51 |
| Subcutaneous unfractionated heparin | 0 (0) | 0 (0) | – |
| Low molecular weight heparin | 0 (0) | 0 (0) | – |
| Aspirin | 0 (0) | 0 (0) | – |
| Vitamin K antagonist | 0 (0) | 0 (0) | – |
| Mechanical thromboprophylaxis | 2 (7.4) | 2 (8.3) | 1.00 |
| Platelet transfusion | 1 (3.7) | 1 (4.2) | 1.00 |
| Red blood cell transfusion | 5 (18.5) | 4 (16.7) | 1.00 |
| Plasma transfusion | 1 (3.7) | 1 (4.2) | 1.00 |
| Parenteral nutrition | 4 (14.8) | 5 (20.8) | 0.72 |
Data is presented as count (percentage) or median (inter-quartile range). – not estimable.
Efficacy of Early Prophylaxis on CADVT
All children randomized to enoxaparin received the drug (median: 6 doses; IQR: 3, 13 doses). Of 272 scheduled doses, 8 (2.9%) were not given. First dose was administered at a median of 21.1 hours (IQR: 14.7, 23.5 hours) after insertion of CVC. Of 21 children with at least 1 anti-Xa level, target was achieved in 12 (57.1%) children at a median of 70.4 hours (IQR: 47.4, 93.2 hours) after insertion of CVC (Supplemental Table 1). The remaining children completed the study before achieving target (Supplemental Table 2). Anti-Xa level was within target in 6 (28.6%) children when first measured.
Ultrasonography was performed in 47 (92.2%) children. Of 4 who did not have ultrasonography, 3 had impending death and 1 was withdrawn after enrollment when the CRETE Trial was terminated. A total of 7 of 23 (30.4%) children with ultrasonography in the enoxaparin arm and 13 of 24 (54.2%) children in the usual care arm had CADVT (p=0.10; Table 2). The posterior risks of CADVT were 0.34 (95% Crl: 0.18, 0.54) in the enoxaparin arm and 0.24 (95% CrI: 0.17, 0.32) in the usual care arm. Posterior risk ratio of CADVT with enoxaparin was 0.55 (95% CrI: 0.24, 1.11; Figure 2). All CADVT in the enoxaparin arm were nonocclusive. In the usual care arm, 11 were nonocclusive and 2 were occlusive (p=0.15). Clinically relevant CADVT developed in 1 (3.7%) of 27 children in the enoxaparin arm and 7 (29.2%) of 24 children in the usual care arm (p=0.02). Of children who did not have ultrasonography, none had signs of CADVT. Other outcome measures were not statistically different between trial arms (Table 2).
Table 2.
Comparison of efficacy and safety outcomes between enoxaparin and usual care arms.
| Enoxaparin Arm (n=27) | Usual Care Arm (n=24) | p value | |
|---|---|---|---|
| Efficacy Outcome Measures | |||
| Central venous catheter-associated deep venous thrombosis1 | 7 (30.4) | 13 (54.2)2 | 0.10 |
| Other thromboembolic event | 1 (3.7) | 1 (4.2) | 1.00 |
| Pediatric ICU length of stay | 12 (6, 22) | 8 (4, 16) | 0.31 |
| Hospital length of stay | 16 (6, 35) | 16 (7, 23) | 0.58 |
| Safety Outcome Measures | |||
| Any bleed | 4 (14.8) | 2 (8.3) | 0.67 |
| Clinically relevant bleed | 1 (3.7) | 0 (0) | 1.00 |
| Heparin-induced thrombocytopenia | 0 (0) | 0 (0) | – |
| Death | 5 (18.5) | 2 (8.3) | 0.43 |
Only 23 children in the enoxaparin arm had ultrasonography. Data is presented as count (percentage). ICU – intensive care unit. – not estimable.
Figure 2.
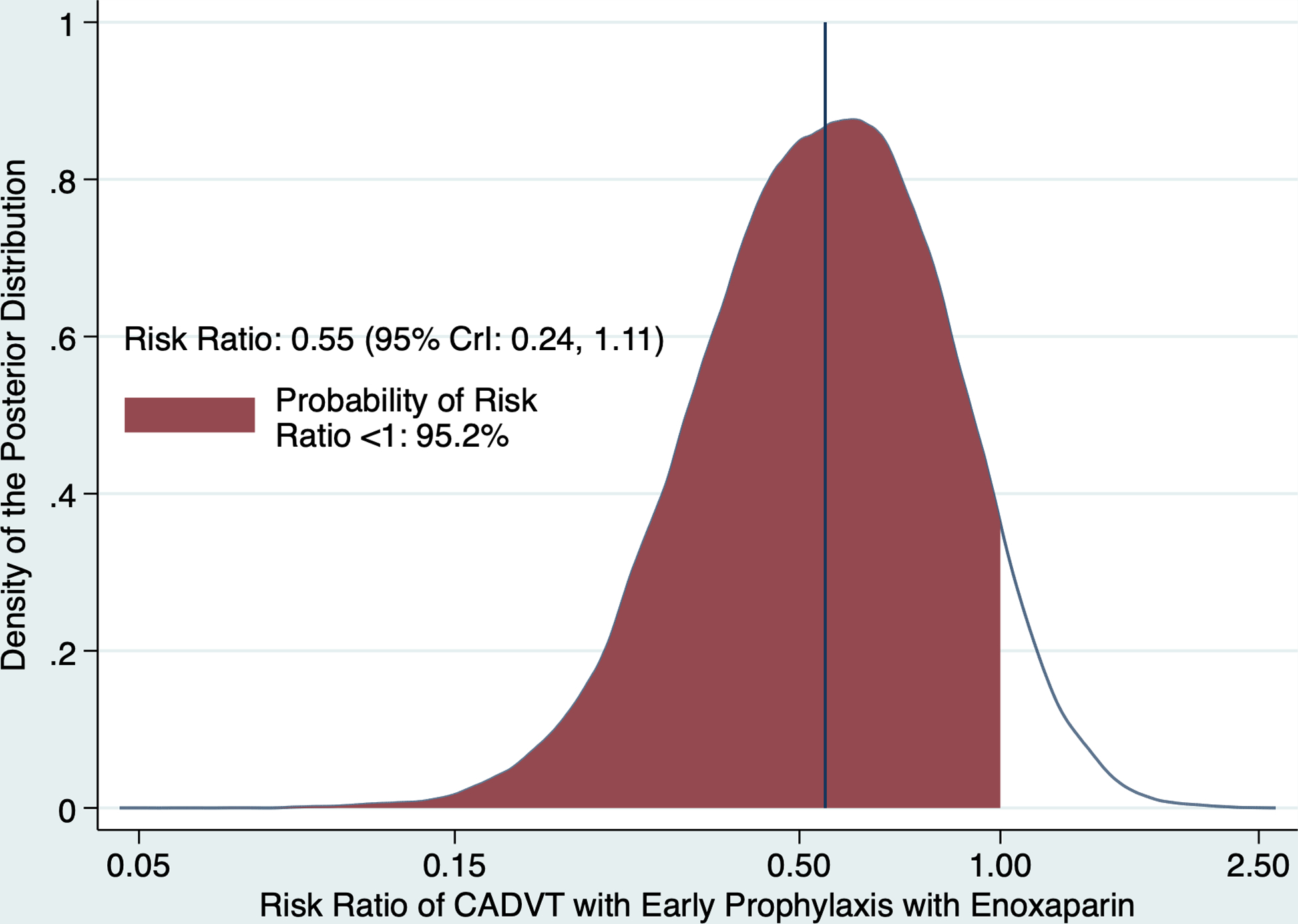
Posterior distribution of the risk ratio of central venous catheter-associated deep venous thrombosis (CADVT) with early prophylaxis with enoxaparin. The vertical line represents the posterior median of the probability distribution of the risk ratio while the shaded area represents the posterior probability that the risk ratio is <1. CrI – credible interval.
A total of 4 (14.8%) children in the enoxaparin arm bled with 1 (3.7%) clinically relevant bleed in the upper airway that required platelet transfusion (Table 2). In the usual care arm, 2 (8.3%) children bled, none of which were clinically relevant (p=1.00). A total of 5 (18.5%) children in the enoxaparin arm and 2 (8.3%) in the usual care arm died (p=0.43). The deaths were unrelated to enoxaparin.
Effect of Anti-Xa Level-Directed Prophylactic Strategy on Thrombin Generation
ETP on the day of insertion of the CVC was not statistically different between enoxaparin (median: 919.70 nM.min; IQR: 418.71, 1150.77 nM.min) and usual care (median: 1035.60 nM.min; IQR: 800.22, 1455.05 nM.min; p=0.22) arms (Figure 3). Response profiles of ETP, factor VIII activity and D-dimer level were not statistically different between trial arms (Figure 3 and Supplemental Table 3).
Figure 3.
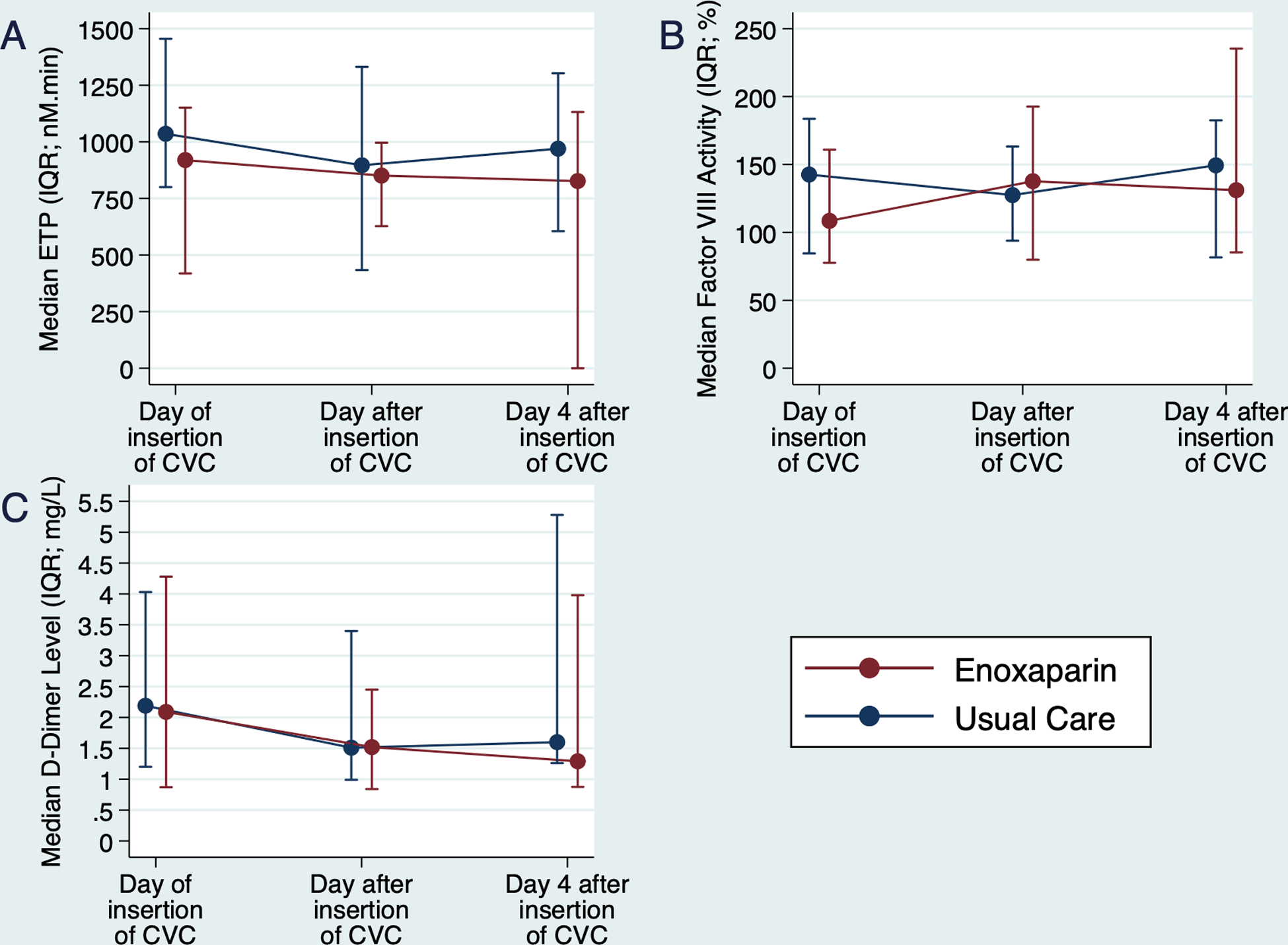
Response profiles of endogenous thrombin potential (ETP; A), factor VIII activity (B) and D-dimer level (C) across sampling days and between trial arms. None of the response profiles were statistically different between trial arms. CVC – central venous catheter; IQR – interquartile range.
DISCUSSION
The CRETE Trial was designed to obtain preliminary evidence on the efficacy of early prophylaxis against CADVT in critically ill children. It was terminated early based on misspecification in the pre-approved Bayesian analysis. Nevertheless, the risk ratio of CADVT with enoxaparin was 0.55 (95% CrI: 0.24, 1.11) in the interim analysis. The risk of clinically relevant bleeding was not statistically different with enoxaparin. We did not observe a reduction in ETP with the anti-Xa level-directed strategy. Although the CRETE Trial was terminated early for futility and the CrI of treatment effect with half the total sample size was wide and included no effect, our findings support the conduct and inform the design of a future pivotal RCT.
Bayesian inference is sensitive to choice of prior (28). We based our prior on the strong information about the risk of CADVT without prophylaxis from multiple studies (6). Unfortunately, our study population likely were sicker on enrollment with the proportion of children in the usual care arm who developed CADVT being 3-fold higher than previously reported (6, 29). In the Bayesian analysis, the strong prior risk of 0.18 with little variability shrunk the observed information from the proportion of CADVT in the usual care arm of 0.54 to the posterior risk of 0.24. The observed proportion of CADVT in the usual care arm was higher, but the posterior risk was lower, than the posterior risk of CADVT in the enoxaparin arm of 0.34. Shrinkage did not occur in the enoxaparin arm because its prior was minimally informative with large variability. Due to the misspecified prior about the risk of CADVT, the direction in estimated treatment effect was reversed. Consequently, the predictive probability met the criteria for termination. Given the pre-approved statistical approach and unusually high proportion of children with CADVT, continuing the CRETE Trial would be futile and unable to prove that the posterior risk of CADVT with enoxaparin was ≥0.06 points lower than without enoxaparin. The supportive analysis using minimally informative priors on the risk ratio of CADVT with enoxaparin resolved the misspecification and provided analyses that were consistent with the observed data and analogous frequentist analyses. We plan to use the risk ratio of CADVT from the CRETE Trial to design a future pivotal RCT. This risk ratio does not depend on the risks of CADVT and will avoid misspecification.
The potential efficacy of early prophylaxis was supported by proportionately less clinically relevant CADVT and less severe venous occlusion with enoxaparin. These findings are consistent with the natural history of CADVT in critically ill children. In these children, the risk of CADVT is <2.5% within 24 hours after insertion of an untunneled CVC (19). Starting pharmacologic prophylaxis when the risk of CADVT is low likely increased its efficacy. In children with acute leukemia, enoxaparin appeared to reduce the risk of thromboembolism when started prior to asparaginase (30). Delayed administration of reviparin in the PROTEKT Trial likely contributed to its negative findings (10). The risk of clinically relevant bleeding in the CRETE Trial was consistent with published reports (31, 32).
Thrombin generation, e.g., as measured by ETP, is the pharmacodynamic target of pharmacologic prophylaxis against CADVT. Anti-Xa level is a pharmacokinetic parameter and has moderate correlation with ETP (33, 34). We hypothesized that an anti-Xa targeted strategy of prophylaxis with enoxaparin would reduce ETP and D-dimer level, an indirect measure of thrombin generation, but not factor VIII activity, a pro-coagulant unaffected by enoxaparin (35–37). Enoxaparin did not alter the response profiles of ETP, factor VIII activity or D-dimer level, likely due to lack of statistical power, particularly because the CRETE Trial was terminated early. We achieved target anti-Xa level in only 51.4% of children and at nearly day 4 after insertion of the CVC when most CADVT would have already developed (19). A higher dose of enoxaparin that will result in lower ETP and shorter time to reach target anti-Xa level should be investigated given the high proportion of CADVT in our enoxaparin arm at 30.4% (38). Lower dose comparable to that in adults may also not be inferior to the dose we used and achieve the same efficacy against CADVT (39). Other mechanisms that promote thrombus formation, e.g., platelet, contact and complement activation, should be studied because enoxaparin may be preventing CADVT through other mechanisms (40).
The CRETE Trial has limitations. We used systematic screening with ultrasonography to define CADVT. Although ISTH recommended this definition and FDA use this to approve enoxaparin, debate is ongoing about the clinical relevance of DVT in children that has been identified by ultrasonography alone (17, 41, 42). We showed a reduction in risk of clinically relevant CADVT, but this may be biased as our design was open-label and our definition, like others’, depended on practitioners’ behavior (21, 22). Lack of ultrasonography may affect the magnitude of treatment effect, including on clinically relevant CADVT. While those who did not have ultrasonography did not have signs of CADVT, it is possible, though unlikely, that they had CADVT on ultrasonography or that CADVT would be treated given their clinical status (19). Significance of our findings on long term outcomes, such as post-thrombotic syndrome, is unclear as children were not followed after discharge (20). Risk of bleeding with enoxaparin may have been higher if those at high risk of bleeding were not excluded (32).
In conclusion, early prophylaxis with enoxaparin may safely reduce the risk of CADVT in critically ill children. Extreme caution should be exercised when attempting to use our findings to support clinical practice. The CrI of treatment effect was wide and included no effect. However, our preliminary evidence on the efficacy and safety of early prophylaxis against CADVT support the conduct of a future pivotal RCT.
Supplementary Material
Supplemental Figure. Prior distributions of the risk of central venous catheter-associated deep venous thrombosis that was used for each trial arm.
ACKNOWLEDGMENT
The authors would like to thank Dr. Henry Rinder, Ms. Gowri Ananthanarayanan, Mr. Michael Belcourt, Mr. Ralph Jacob and Ms. Parveen Bahel for all their contributions to the CRETE Trial.
Copyright form disclosure: Drs. Faustino and Shabanova’s institutions received funding from NICHD, American Heart Association, and the National Center for Advancing Translational Science. Drs. Faustino, Shabanova, Hanson, Sharathkumar, and Thomas received support for article research from the NIH. Dr. Faustino, Raffini, Kandil, Hanson, and McPartland disclosed off-label product use of enoxaparin. Dr. Raffini received funding from CSL Behring, XaTek, and Bayer. Dr. Pinto’s institution received funding from the NIH. Dr. Hanson’s institution received funding from National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development. Dr. Thomas’ institution received funding from Yale University. The remaining authors have disclosed that they do not have any potential conflicts of interest.
Footnotes
CONFLICT OF INTEREST:
FUNDING INFORMATION: EVSF and PCS received funding from the National Institutes of Health/Eunice Kennedy Shriver National Institute of Child Health and Human Development to conduct the trial (R21HD089131). EVSF received funding from the American Heart Association to conduct the trial (16RNT31180018). VS received funding through the Clinical and Translational Science Award (CTSA) Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR) and the National Center for Advancing Translational Science (NCATS), components of the National Institutes of Health (NIH), and NIH roadmap for Medical Research.
REFERENCES
- 1.Goudie A, Dynan L, Brady PW, et al. Costs of venous thromboembolism, catheter-associated urinary tract infection, and pressure ulcer. Pediatrics 2015;136(3):432–439. [DOI] [PubMed] [Google Scholar]
- 2.Boulet S, Amendah D, Grosse S, et al. Health care expenditures associated with venous thromboembolism among children. Thromb Res 2012;129(5):583–587. [DOI] [PubMed] [Google Scholar]
- 3.Raffini L, Huang YS, Witmer C, et al. Dramatic increase in venous thromboembolism in children’s hospitals in the United States from 2001 to 2007. Pediatrics 2009;124(4):1001–1008. [DOI] [PubMed] [Google Scholar]
- 4.Amos LE, Silvey M, Hall M, et al. Primary thromboprophylaxis in hospitalized children: A multi-center retrospective analysis. Thromb Res 2019;176:1–7. [DOI] [PubMed] [Google Scholar]
- 5.Mahajerin A, Branchford BR, Amankwah EK, et al. Hospital-associated venous thromboembolism in pediatrics: A systematic review and meta-analysis of risk factors and risk assessment models. Haematologica 2015;100(8):1045–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vidal E, Sharathkumar A, Glover J, et al. Central venous catheter-related thrombosis and thromboprophylaxis in children: A systematic review and meta-analysis. J Thromb Haemost 2014;12:1096–1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lyren A, Brilli R, Bird M, et al. Ohio Children’s Hospitals’ Solutions for Patient Safety: A framework for pediatric patient safety improvement. J Healthc Qual 2016;38(4):213–222. [DOI] [PubMed] [Google Scholar]
- 8.Lyren A, Brilli RJ, Zieker K, et al. Children’s Hospitals’ Solutions for Patient Safety Collaborative impact on hospital-acquired harm. Pediatrics 2017;140(3). [DOI] [PubMed] [Google Scholar]
- 9.Monagle P, Chan AK, Goldenberg NA, et al. Antithrombotic therapy in neonates and children: Antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians evidence-based clinical practice guidelines; Chest 2012;141(2 Suppl):e737S–801S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massicotte P, Julian JA, Gent M, et al. An open-label randomized controlled trial of low molecular weight heparin for the prevention of central venous line-related thrombotic complications in children: The PROTEKT trial. Thromb Res 2003;109(2–3):101–108. [DOI] [PubMed] [Google Scholar]
- 11.Massicotte MP, Sofronas M, deVeber G. Difficulties in performing clinical trials of antithrombotic therapy in neonates and children. Thromb Res 2006;118(1):153–163. [DOI] [PubMed] [Google Scholar]
- 12.Faustino EV, Spinella PC, Li S, et al. Incidence and acute complications of asymptomatic central venous catheter-related deep venous thrombosis in critically ill children. J Pediatr 2013;162(2):387–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li S, Silva CT, Brudnicki AR, et al. Diagnostic accuracy of point-of-care ultrasound for catheter-related thrombosis in children. Pediatr Radiol 2016;46(2):219–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cook D, Meade M, Guyatt G, et al. Dalteparin versus unfractionated heparin in critically ill patients. N Engl J Med 2011;364(14):1305–1314. [DOI] [PubMed] [Google Scholar]
- 15.Josephson CD, Granger S, Assmann SF, et al. Bleeding risks are higher in children versus adults given prophylactic platelet transfusions for treatment-induced hypoproliferative thrombocytopenia. Blood 2012;120(4):748–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannarino CN, Faustino EV. Clinical equipoise on prophylaxis against catheter-associated thrombosis in critically ill children. J Crit Care 2016;32:26–30. [DOI] [PubMed] [Google Scholar]
- 17.Mitchell LG, Goldenberg NA, Male C, et al. Definition of clinical efficacy and safety outcomes for clinical trials in deep venous thrombosis and pulmonary embolism in children. J Thromb Haemost 2011;9(9):1856–1858. [DOI] [PubMed] [Google Scholar]
- 18.White LJ, Fredericks R, Mannarino CN, et al. Epidemiology of bleeding in critically ill children. J Pediatr 2017;184:114–119. [DOI] [PubMed] [Google Scholar]
- 19.Beck C, Dubois J, Grignon A, et al. Incidence and risk factors of catheter-related deep vein thrombosis in a pediatric intensive care unit: A prospective study. J Pediatr 1998;133(2):237–241. [DOI] [PubMed] [Google Scholar]
- 20.Jones S, Butt W, Monagle P, et al. The natural history of asymptomatic central venous catheter-related thrombosis in critically ill children. Blood 2019;133(8):857–866. [DOI] [PubMed] [Google Scholar]
- 21.Jaffray J, Witmer C, O’Brien SH, et al. Peripherally inserted central catheters lead to a high risk of venous thromboembolism in children. Blood 2020;135(3):blood.2019002260. [DOI] [PubMed] [Google Scholar]
- 22.Jaffray J, Mahajerin A, Young G, et al. A multi-institutional registry of pediatric hospital-acquired thrombosis cases: The Children’s Hospital-Acquired Thrombosis (CHAT) project. Thromb Res 2018;161:67–72. [DOI] [PubMed] [Google Scholar]
- 23.Lemoine NP. Moving beyond noninformative priors: Why and how to choose weakly informative priors in Bayesian analyses. Oikos 2019;128(7):912–928. [Google Scholar]
- 24.Kalil AC, Sun J. Bayesian methodology for the design and interpretation of clinical trials in critical care medicine: A primer for clinicians. Crit Care Med 2014;42(10):2267–2277. [DOI] [PubMed] [Google Scholar]
- 25.Saville BR, Connor JT, Ayers GD, et al. The utility of Bayesian predictive probabilities for interim monitoring of clinical trials. Clin Trials 2014;11(4):485–493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laptook AR, Shankaran S, Tyson JE, et al. Effect of therapeutic hypothermia initiated after 6 hours of age on death or disability among newborns with hypoxic-ischemic encephalopathy: A randomized clinical trial. JAMA 2017;318(16):1550–1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fitzmaurice GM, Ravichandran C. A primer in longitudinal data analysis. Circulation 2008;118(19):2005–2010. [DOI] [PubMed] [Google Scholar]
- 28.McNeish D On using Bayesian methods to address small sample problems. Struct Equ Modeling 2016;23(5):750–773. [Google Scholar]
- 29.Marquez A, Shabanova V, Faustino EV. Prediction of catheter-associated thrombosis in critically ill children. Pediatr Crit Care Med 2016;17(11):e521–e528. [DOI] [PubMed] [Google Scholar]
- 30.Greiner J, Schrappe M, Claviez A, et al. THROMBOTECT - A randomized study comparing low molecular weight heparin, antithrombin and unfractionated heparin for thromboprophylaxis during induction therapy of acute lymphoblastic leukemia in children and adolescents. Haematologica 2019;104(4):756–765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Klaassen ILM, Sol JJ, Suijker MH, et al. Are low-molecular-weight heparins safe and effective in children? A systematic review. Blood Rev 2018;33:33–42. [DOI] [PubMed] [Google Scholar]
- 32.Bidlingmaier C, Kenet G, Kurnik K, et al. Safety and efficacy of low molecular weight heparins in children: A systematic review of the literature and meta-analysis of single-arm studies. Semin Thromb Hemost 2011;37(7):814–825. [DOI] [PubMed] [Google Scholar]
- 33.Bates SM, Weitz JI. Coagulation assays. Circulation 2005;112(4):e53–60. [DOI] [PubMed] [Google Scholar]
- 34.Chowdary P, Adamidou D, Riddell A, et al. Thrombin generation assay identifies individual variability in responses to low molecular weight heparin in pregnancy: Implications for anticoagulant monitoring. Br J Haematol 2015;168(5):719–727. [DOI] [PubMed] [Google Scholar]
- 35.Desjardins L, Bara L, Boutitie F, et al. Correlation of plasma coagulation parameters with thromboprophylaxis, patient characteristics, and outcome in the MEDENOX study. Arch Pathol Lab Med 2004;128(5):519–526. [DOI] [PubMed] [Google Scholar]
- 36.Verhamme P, Tangelder M, Verhaeghe R, et al. Single intravenous administration of TB-402 for the prophylaxis of venous thromboembolism after total knee replacement: A dose-escalating, randomized, controlled trial. J Thromb Haemost 2011;9(4):664–671. [DOI] [PubMed] [Google Scholar]
- 37.Faustino EV, Li S, Silva CT, et al. Factor VIII may predict catheter-related thrombosis in critically ill children: A preliminary study. Pediatr Crit Care Med 2015;16(6):497–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kamdar AB, Raffini LJ, Witmer CM. Children with CVC-VTE: A very high risk group for recurrent thrombosis. Blood 2017;130(Suppl 1):1098–1098. [Google Scholar]
- 39.Samama MM, Cohen AT, Darmon JY, et al. A comparison of enoxaparin with placebo for the prevention of venous thromboembolism in acutely ill medical patients. Prophylaxis in Medical Patients with Enoxaparin Study Group. N Engl J Med 1999;341(11):793–800. [DOI] [PubMed] [Google Scholar]
- 40.Jaffer IH, Fredenburgh JC, Hirsh J, et al. Medical device-induced thrombosis: What causes it and how can we prevent it? J Thromb Haemost 2015;13 Suppl 1:S72–81. [DOI] [PubMed] [Google Scholar]
- 41.Sharathkumar AA, Biss T, Kulkarni K, et al. Epidemiology and outcomes of clinically unsuspected venous thromboembolism in children: A systematic review. J Thromb Haemost 2020;18(5):1100–1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones S, Monagle P, Newall F. Do asymptomatic clots in children matter? Thromb Res 2020;189:24–34. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure. Prior distributions of the risk of central venous catheter-associated deep venous thrombosis that was used for each trial arm.


