Abstract
Objective:
Late-onset neutropenia (LON) is an underrecognized complication of rituximab. Our objective is to describe its incidence, risk factors, clinical features, management, and recurrence.
Methods:
We conducted a single-center retrospective cohort study of 738 adult patients with autoimmune disease treated with rituximab-induced continuous B cell depletion. The primary outcome was LON, defined as an unexplained absolute neutrophil count < 1000 cells/μL during B cell depletion. Secondary outcomes included incidental diagnosis, fever, sepsis, filgrastim use, and recurrent LON. We assessed predictors of LON using Cox regression models.
Results:
We identified 107 episodes of LON in 71 patients. The cumulative incidence at 1 year was 6.6% (95% CI, 5.0 - 8.7). The incidence rate in the first year was higher compared to thereafter (7.2 (95% CI, 5.4 - 9.6) vs. 1.5 (95% CI, 1.0 - 2.3) per 100 person-years, respectively). Systemic lupus erythematosus and combination therapy with cyclophosphamide were each independently associated with an increased risk of LON (adjusted hazards ratios 2.96, (95% CI, 1.10 - 8.01) and 1.98 (95% CI, 1.06 - 3.71), respectively). LON was not observed with minimal change disease / focal segmental glomerulosclerosis. 59.4% of episodes were asymptomatic. Fever and sepsis complicated 31.3% and 8.5% of episodes, respectively. Most patients (69%) were treated with filgrastim. Rituximab rechallenge occurred in 87% of patients, of whom 21% developed recurrent LON.
Conclusion:
LON is common and often incidental. Most cases are reversible and respond well to filgrastim. However, it can be associated with serious infections and thus warrants vigilant monitoring.
Keywords: late-onset neutropenia, rituximab, continuous B cell depletion, autoimmune disease
INTRODUCTION
B cell depletion with the anti-CD20 monoclonal antibody rituximab has emerged as an important therapeutic strategy for many antibody-mediated autoimmune diseases. A single course of rituximab has demonstrated efficacy in inducing remission in antineutrophil cytoplasmic antibody (ANCA)-associated vasculitis and rheumatoid arthritis (1, 2). However, relapses are common following cessation of rituximab, and are often preceded by B cell reconstitution (3, 4). For this reason, extended courses of rituximab are often used as maintenance therapy (5). However, the increasing use of B cell depletion has led to the emergence of treatment-related complications.
One such complication of B cell depletion is late-onset neutropenia (LON). This entity was originally recognized in lymphoma patients treated with rituximab (6–8). Initial reports in the lymphoma population portrayed LON to be rare (9), but later reports demonstrated higher incidences ranging between 5-27% (10). More recently, it was recognized to also occur in patients with autoimmune disease treated with rituximab (11–15). However, these reports remain limited in size and scope.
We conducted a single-center retrospective cohort analysis of 738 patients undergoing B cell depletion for autoimmune disease to characterize the incidence, risk factors, clinical features, management, and recurrence of LON. This study intends to advance the field because it has the greatest number of LON and recurrent LON cases published to date. Furthermore, it has the longest follow-up of continuous B cell depletion published to date.
PATIENTS AND METHODS
Study population
We retrospectively identified patients 18 years of age or older treated with rituximab for autoimmune disease between November 8, 2002 and September 30, 2018 at the Vasculitis and Glomerulonephritis Center at the Massachusetts General Hospital. This is a tertiary-care referral and treatment center that provides care for a diverse patient population in eastern Massachusetts and the surrounding region. All data were abstracted from the electronic medical record. The study was approved by the Partners HealthCare Human Research Committee. Informed consent was not required due to the retrospective nature of the study.
Treatment Regimen and Continuous B Cell Depletion
All patients received rituximab, typically starting with two 1000 mg IV doses separated by 2 - 4 weeks. Thereafter, rituximab was most commonly administered as one 1000 mg IV dose every 4 - 6 months. This dosing interval has been demonstrated to achieve continuous B cell depletion in >95% of patients (16–18). If rituximab was continued for >2 years, then attempts were made to extend the dosing interval so long as documented B cell depletion was maintained. B cell count was typically monitored every 3 months, including prior to each dose, with peripheral flow cytometry by examining the population of CD19+CD20+ lymphocytes. We defined B cell depletion as total CD19+CD20+ cell count < 5 cells/mm3. All patients had a complete blood count (CBC) with differential count checked routinely prior to each dose of rituximab, for any report of fever, as well as for other clinical indications at the discretion of the treating physician.
Prednisone and other immunosuppressive agents were initially administered with rituximab in most patients. Aside from prednisone, a two-month course of low-dose daily oral cyclophosphamide (CYC) was the most commonly used additional agent, which is part of a standard induction treatment that we have used for ANCA-associated vasculitis (AAV), membranous nephropathy (MN), and other patients (18, 19).
Outcomes
The primary outcome was the first episode of late-onset neutropenia (LON), which we defined as an unexplained ANC < 1000 cells/μL during the period of B cell depletion. To determine if patients had another identifiable etiology of neutropenia, medical records surrounding the neutropenia event were reviewed. Resolution of a LON episode was defined as an increase in the ANC ≥ 1000 cells/μL.
Secondary outcomes included incidental diagnosis, chief complaint, nadir ANC, nadir absolute lymphocyte count (ALC), fever, sepsis, source of infection, prednisone dose at the time of event, serum immunoglobulin gamma (IgG) level at the time of event, filgrastim use, therapeutic antibiotic use, and recurrent LON, defined as a second episode of LON occurring after recovery from the first. Fever was defined as a single oral temperature of ≥38.0°C (100.4°F). Sepsis was defined as the presence of systemic inflammatory response syndrome with a source of infection (20). Determination of source of infection was based on available history, exam, imaging, culture data, and treating physician’s documentation.
Covariates of LON
The following covariates were examined based on biological relevance: age, sex, underlying disease, CYC use (never, prior, concurrent), and exposure to other immunosuppressants (methotrexate (MTX), azathioprine (AZA), mycophenolic acid (MPA)) during continuous B cell depletion.
Statistical analysis
Continuous variables are presented as median (interquartile range [IQR]) or mean (standard deviation), as appropriate. Categorical variables are presented as percentages. Person-years (PY) at risk of LON were calculated for each person as time from the index date, defined as the first rituximab infusion, through the last documented date of B cell depletion prior to B cell return. If documented B cell return was not available, then we ended the at-risk period 6 months after the last infusion or on the last date of documented B cell depletion, whichever was later. We estimated the cumulative incidence of LON, using days from rituximab initiation as the time scale and present LON rates per 100 PY of continuous B cell depletion with 95% confidence intervals (CI). Poisson regression was used to generate CIs for LON incidence rates. Among patients who sustained an episode of LON, recurrence upon rechallenge was measured from the day of rituximab rechallenge to the first day of the second episode of LON. Univariate and multivariate Cox proportional hazards models were used to estimate the hazards ratio (95% CI) for LON associated with each potential predictor. The multivariable model was adjusted for age, sex, ANCA-associated vasculitis, lupus nephritis, membranous nephropathy, other disease category, no CYC exposure, concurrent CYC use, MPA use, AZA use, and MTX use. The model could not accommodate the minimal change disease / focal segmental glomerulosclerosis group due to the absence of any LON events. As such, it was excluded from the regression model. Instead, the log-rank test was used to compare it with other underlying diseases. All analyses were conducted using STATA 15 (StataCorp, College Station, TX).
RESULTS
Study population
We identified 738 adult patients treated with rituximab-induced continuous B cell depletion for autoimmune disease. The average age at initiation of therapy was 58 years (SD 17) and 53% were female. Among the 738 patients, 529 (72%) had ANCA-associated vasculitis (AAV), 73 (10%) had membranous nephropathy (MN), 59 (8%) had minimal change disease or focal segmental glomerulosclerosis (MCD / FSGS), 24 (3%) had lupus nephritis (LN), and 53 (7%) had another autoimmune disease. The underlying diseases in the other category are listed in Supplemental Table 1. Of the 529 patients with AAV, 271 (51.2%) had renal involvement, as defined by the presence of hematuria, red blood cells casts, or a rise in serum creatinine by 30%. Their median (IQR) creatinine at the start of rituximab was 1.7 mg/dL (1.11 - 2.65). All patients with MCD / FSGS had frequently relapsing, steroid-dependent, or steroid-resistant disease. Patient characteristics are presented in Table 1.
Table 1.
Study population characteristics.*
| Patients - no. | 738 |
| Age - years, mean (SD) | 58 (17) |
| Sex - no. (%) female | 392 (53) |
| Disease - no. (%) | |
| AAV | 529 (72) |
| LN | 24 (3) |
| MN | 73 (10) |
| MCD/FSGS | 59 (8) |
| Other | 53 (7) |
| Rituximab - no. of doses | 8 (5, 12) |
| Duration of B cell depletion - years | 2.5 (1.4, 4.5) |
| AAV | 2.8 (1.4, 5.1) |
| LN | 2.4 (1.8, 4.6) |
| MN | 2.2 (1.4, 2.6) |
| MCD/FSGS | 2.4 (1.5, 3.2) |
| Other | 1.4 (0.7, 3.6) |
| CYC use - no. (%) | |
| Never | 170 (23) |
| Concurrenta | 515 (70) |
| Priorb | 53 (7) |
| < 6 months of CYC | 29 (55) |
| ≥ 6 months of CYC | 20 (38) |
| Other immunosuppressant use - no. (%) | |
| Mycophenolic Acid | 59 (8) |
| Azathioprine | 119 (16) |
| Methotrexate | 33 (4) |
| Glucocorticoids | 698 (95) |
Except where indicated otherwise, values are the median (interquartile range). AAV = antineutrophil cytoplasmic autoantibody (ANCA) associated vasculitis; CYC = cyclophosphamide; LN = lupus nephritis; MN = membranous nephropathy; MCD/FSGS = minimal change disease / focal segmental glomerulosclerosis.
Concurrent use is defined as 8 weeks of daily oral cyclophosphamide beginning with the first dose of rituximab. Dosing was typically 2.5 mg/kg/day for the first week, and 1.5 mg/kg/day for 7 weeks, with adjustments made for renal function
Prior CYC is defined as a course of CYC administered prior to initiation of rituximab, as part of a separate induction of remission therapy. Missing data on 4 patients with unclear duration of CYC exposure.
Treatment Regimen and Continuous B cell depletion
All patients were treated with rituximab-induced continuous B cell depletion. Patients received a median 8 doses (IQR 5 - 12) and were in a state of continuous B cell depletion for a median of 2.5 years (IQR 1.4 - 4.5) with a total of 2214 person-years (PY).
A two-month course of low-dose daily oral CYC was utilized concurrently with initiation of rituximab in 515 (70%) patients. The remainder never received CYC (n = 170; 23%) or received it in the past (n = 53; 7%). AZA, MPA, and MTX were utilized in 119 (16%), 59 (8%), and 33 (4%) patients, respectively. Glucocorticoids were used in 698 (95%) patients.
Outcomes
Incidence of LON
During the follow-up, there were 107 episodes of LON in 71 patients. The cumulative incidence of LON at 1, 2, and 5 years of continuous B cell depletion was 6.6% (95% CI, 5.0 - 8.7), 7.9% (95% CI, 6.1 - 10.2), and 13.5% (95% CI, 10.4 - 17.4), respectively. A KM curve depicting time to LON is shown in Figure 1A. The overall incidence rate of first-time LON was 3.2 (95% CI 2.5 - 4.0) per 100 PY. The incidence rate of LON was higher in the first year following treatment initiation compared to the incidence rate thereafter (7.2 (95% CI, 5.4 - 9.6) per 100 PY vs. 1.5 (95% CI, 1.0 - 2.3) per 100 PY, respectively). Supplemental Figure 1 provides a zoomed in version of Figure 1A on the first year of treatment.
Figure 1. Cumulative incidence of LON.
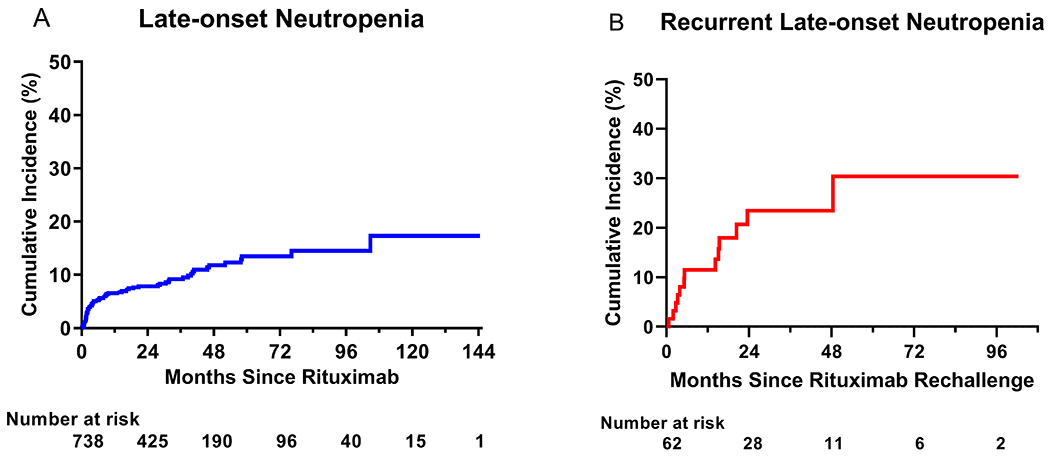
Shown are Kaplan-Meier curves for time to initial LON episode in all patients undergoing continuous B cell depletion therapy with rituximab (panel A), as well as time to second episode (recurrence) of LON in patients who were rechallenged with rituximab (panel B).
Recurrence of LON after rechallenge
Among the 71 patients who had LON, 9 patients were not rechallenged. Of these nine, 4 had second episodes of LON at 19, 54, 66, and 435 days after the first episode, respectively. All remained B cell depleted at the time of their second episode.
The remaining 62 (87%) patients were rechallenged with rituximab, of which 13 (21%) developed a second episode over a median follow-up duration of 2.4 (IQR,1.3 - 4.1) years of continuous B cell depletion after rituximab rechallenge, corresponding to a recurrent incidence rate of LON of 8.5 (95% CI, 4.9 - 14.7) per 100 PY after the date of rechallenge. The cumulative incidence of recurrent LON at 1, 2, and 5 years of continuous B cell depletion after rechallenge was 11.5% (95% CI, 5.6 - 22.6), 23.4% (95% CI, 13.8 - 38.2), and 30.4% (95% CI, 16.9 - 50.9), respectively. A KM curve for time to recurrent LON is shown in Figure 1B.
Clinical features of LON
Clinical features of LON are shown in Table 2. LON occurred at a median 4.1 months (IQR, 1.6 - 23.1) after the first infusion of rituximab. After any preceding infusion, LON occurred at a median of 2.3 months (IQR, 0.8 – 4.0). In the subset of patients who developed recurrent LON, the second episode occurred at a median of 5.3 months (IQR 3.3 – 15.4) after rituximab rechallenge. The median ANC nadir for all episodes was 490 cells/μL (IQR, 12 - 770), and the median (IQR) nadir neutrophil percentage was 16.1% (3.0 – 32.9). The median nadir ANC for symptomatic episodes was significantly lower than for asymptomatic episodes (134 cells/μL (IQR, 15.5 - 417) versus 700 cells/μL (IQR, 459 - 882), respectively, p < 0.001). The median ALC and lymphocyte percentage concurrent to the nadir ANC was 775 cells/μL (IQR, 504 – 1440) and 45.5% (IQR, 35.5 – 64.0), respectively. Lymphopenia (defined by ALC < 1000 cells/μL) occurred in 62.3% of the LON episodes. By chi-squared analysis, there was no association between occurrence of lymphopenia and infections during neutropenia (p = 0.77).
Table 2.
Clinical features and treatment of late-onset neutropenia (LON) episodes.*
| Patients with LON – no. | 71 |
| LON episodes (initial + recurrent) – no. | 107 |
| Time to initial episode – months | 4.1 (1.6 - 23.1) |
| Nadir ANC – cells/μL | 490 (12 - 770) |
| Asymptomatic episodes | 700 (459 - 882) |
| Symptomatic episodes | 128 (11 - 420) |
| ALC at time of nadir ANC – cells/μL | 775 (504 - 1440) |
| Febrile neutropenia – no. episodes (%) | 30 (31.3) |
| Sepsis syndrome – no. episodes (%) | 8 (8.4) |
| Chief complaints – no. episodes (%) | |
| Asymptomatic/Incidental | 63 (59.4) |
| Fever | 30 (28.3) |
| Gum soreness | 4 (3.8) |
| Rash | 3 (2.8) |
| Fall | 1 (0.9) |
| Photophobia | 1 (0.9) |
| Productive cough | 1 (0.9) |
| Pain at tunneled catheter site | 1 (0.9) |
| Nausea/vomiting | 1 (0.9) |
| Malaise | 1 (0.9) |
| Source of infection – no. episodes (%) | |
| No apparent infection | 63 (65.0) |
| Gingivitis | 7 (7.2) |
| SSTI | 7 (7.2) |
| URI | 6 (6.2) |
| UTI | 5 (5.2) |
| GI (gastroenteritis or colitis) | 4 (4.1) |
| Pneumonia | 2 (2.1) |
| Conjunctivitis | 1 (1.0) |
| Bacteremia | 1 (1.0) |
| Vascular access catheter tunnel infection | 1 (1.0) |
| Prednisone dose at event - mg/day | 5 (0 - 12.5) |
| Serum IgG level at eventa - mg/dL | 557 (454 - 733) |
| Treatment of LON | |
| Antibiotic useb - no. episodes (%) | 34 (34.7) |
| Filgrastim use - no. episodes (%) | 70 (69.3) |
| Filgrastim use - doses/episode | 1 (1 - 2) |
| Duration of episode - days | 4 (3 - 7) |
Except where indicated otherwise, values are the median (interquartile range). Missing data excluded from calculations. ALC = absolute lymphocyte count; ANC = absolute neutrophil count; GI = gastrointestinal; IgG = immunoglobulin gamma; SSTI = skin and soft tissue infection; URI = upper respiratory infection; UTI = urinary tract infection.
Serum reference ranges (mg/dL): IgG 614 – 1295.
Therapeutic dosing.
Sixty-three episodes of LON (59.4%) were discovered incidentally in asymptomatic patients presenting for routine follow-up. No infections were identified in asymptomatic episodes. In contrast, an infection was identified in all episodes that were symptomatic. Forty-three (40.6%) LON episodes were discovered because a patient complained of a symptom, which prompted the treating physician to check a CBC. The most common chief complaint was subjective fever (n = 30), followed by gingival soreness (n = 4). LON was complicated by sepsis in 8 (8.5%) episodes. All cases of sepsis resolved with standard of care therapy. In the entire cohort, one patient died with multiple relapsing LON. At the time of LON, patients were on a median prednisone dose of 5 mg daily (IQR, 0 - 12.5), and the median serum IgG was 557 mg/dL (IQR, 454 - 733).
Management of LON
Treatments used for LON in our cohort are shown in Table 2. All patients with LON and fever were hospitalized for management of febrile neutropenia (n = 30; 31.3%). Therapeutic antibiotics were used in 34.7% of all episodes, of which most had a fever (81.3%). Filgrastim was used in 83.6% and 69% of LON episodes with a nadir ANC < 500 and < 1000 cells/μL, respectively. Patients who were administered filgrastim had a lower median nadir ANC compared to those who were not administered filgrastim (337 cells/μL (IQR, 50 – 576) vs. 717 cells/μL (IQR, 472 - 908), respectively; p < 0.0001). In patients treated with filgrastim, a median of 1 dose (IQR 1 - 2, range 1 - 8) was administered per episode. The median duration for all episodes was 4 days (IQR, 3 - 7).
Covariates of LON
Effect of underlying disease
LON occurred more in patients with lupus nephritis (n = 6; 25%) compared to patients with AAV (n = 55; 10.4%), MN (n = 6; 8.2%), or other diseases (n = 4; 7.6%) (log-rank p = 0.03) (Figure 2). LON was not observed in any patient with MCD / FSGS. The corresponding crude incidence rates of LON for LN, AAV, MN, and other diseases were 8.9 (95% CI 4.0 - 19.7), 3.2 (95% CI 2.5 - 4.2), 4.1 (95% CI 1.8 - 9.1), and 3.5 (95% CI 1.1 - 10.9) per 100 PY, respectively. In a multivariable model, the presence of a lupus nephritis was associated with a higher odds of developing LON (adjusted HR 2.96, 95% CI [1.10 – 8.01]) (Table 3).
Figure 2. Cumulative incidence of LON, stratified by disease.

Shown is a Kaplan-Meier curve for time to LON stratified by disease. Higher rates of LON are seen in LN, while no events occurred in MCD/FSGS (log-rank p = 0.03).
AAV = ANCA-associated vasculitis; LN = lupus nephritis; MCD/FSGS = minimal change disease / focal segmental glomerulosclerosis; MN = membranous nephropathy.
Table 3.
Covariates of late-onset neutropenia (LON).*
| Odds Ratio (95% CIs) | ||
|---|---|---|
| Variable | Univariate | Multivariate |
| Age (per 1 year) | 1.01 (0.99 - 1.02) | 1.01 (0.99 - 1.03) |
| Female | 0.92 (0.58 - 1.47) | 0.85 (0.53 - 1.38) |
| Disease | ||
| AAV | 1.17 (0.66 - 2.08) | −1.0 (Ref) |
| MN | 1.01 (0.43 - 2.34) | 0.91 (0.39 - 2.15) |
| LN | 2.91 (1.26 - 6.72) | 2.96 (1.10 - 8.01) |
| MCD/FSGS | n/a | n/a |
| Other | 0.93 (0.29 - 2.95) | 0.97 (0.30 - 3.12) |
| CYC use | ||
| Never | 0.52 (0.27 - 1.01) | −1.0 (Ref) |
| Concurrent | 2.23 (1.22 - 4.08) | 1.98 (1.06 - 3.71) |
| Prior | ||
| < 6 months of CYC | 0.26 (0.04 - 1.90) | |
| ≥ 6 months of CYC | 0.88 (0.22 - 3.62) | |
| Other concurrent IS use | ||
| RTX without MPA/AZA/MTX/CYC | 0.68 (0.29 - 1.57) | −1.0 (Ref) |
| MPA | 1.44 (0.72 - 2.91) | 1.41 (0.65 - 3.08) |
| AZA | 0.37 (0.17 - 0.82) | 0.46 (0.20 - 1.04) |
| MTX | 1.07 (0.39 - 2.93) | 1.24 (0.44 - 3.54) |
Hazards ratios and 95% confidence intervals were calculated using a Cox Proportional Hazards Model. The multivariable model was adjusted for age, sex, AAV, LN, MN, other disease category, no CYC exposure, concurrent CYC use, MPA use, AZA use, and MTX use. The model could not accommodate the MCD / FSGS group due to the absence of any LON events. As such, it was excluded from the regression model. Instead, the log-rank test was used to compare it with other underlying diseases. AAV = antineutrophil cytoplasmic autoantibody (ANCA) associated vasculitis; AZA = azathioprine; CI = confidence interval; CYC = cyclophosphamide; IS = immunosuppressant; LN = lupus nephritis; MCD/FSGS = minimal change disease / focal segmental glomerulosclerosis; MN = membranous nephropathy; MPA = mycophenolic acid; MTX = methotrexate; n/a = not applicable; RTX = rituximab.
Effect of cyclophosphamide
LON occurred more in patients treated with combination CYC and rituximab for induction therapy (n = 58; 11.3%) compared to patients who were never exposed to CYC (n = 10; 5.9%), or who had CYC exposure prior to initiation of rituximab (n = 3; 5.7%) (log-rank p = 0.03). In a multivariable model, treatment with combination CYC and rituximab was associated with a higher odds of developing LON compared to those who did not receive CYC (adjusted HR 1.98, 95% CI [1.06 - 3.71]) (Table 3). In a subgroup analysis of patients who received rituximab without CYC (n = 170), the 12-month cumulative incidence of LON was 3.70% (95% CI, 1.68 – 8.07) (Supplemental Figure 2).
Effect of age, sex, and other immunosuppressants
In a multivariable model, age, sex, and exposure to MPA, AZA, or MTX concurrent with rituximab were not associated with the development of LON (Table 3).
DISCUSSION
In this retrospective cohort study of 738 patients on rituximab-induced continuous B cell depletion for autoimmune disease, we provide several key insights into the incidence, risk factors, clinical features, management, and recurrence of late-onset neutropenia. First, we describe 71 patients with LON, the largest reported to date, corresponding to a cumulative incidence of 6.6% at one year. Second, we observe the highest rate of LON to be in the first year of treatment. Third, we highlight systemic lupus erythematosus and combination therapy with cyclophosphamide each as positive independent risk factors for development of LON. Fourth, we expand on initial observations by others (11–13) on the clinical features of LON, including the often-incidental nature of discovering LON in asymptomatic patients, as well as the potential for serious infectious complications. Fifth, we describe the safety and efficacy of filgrastim treatment in this patient population. Finally, we find rituximab rechallenge does not always lead to recurrent LON.
We report a 6.6% one-year cumulative incidence of LON. Our findings are consistent with and expand on prior reports on LON, including studies using alternative rituximab dosing schedules (10–13, 21). By comparison, leukopenia was reported in 3 (3%) of 99 patients in the rituximab arm at 6 months in the RAVE trial, and neutropenia was reported in 2 (6%) of 33 patients in the rituximab arm at 12 months in the RITUXVAS trial (1, 22). Both trials used a dose of 375 mg/m2 weekly for 4 consecutive weeks for AAV. Given the long-term, relapsing nature of many antibody-mediated autoimmune diseases, the rising incidence of LON we observed with extended durations of B cell depletion is of particular concern, underscoring the need for on-going awareness of neutropenia during maintenance of remission therapy.
The mechanism of LON remains unknown. Neutrophils and their precursors do not express CD20, arguing against direct cytotoxicity. Yet, late neutropenic events have been observed with other anti-CD20 monoclonal antibodies as well as with anti-CD19 chimeric antigen receptor (CAR) T cells (23, 24). This would support it is the state of B cell depletion causing the neutropenia. It is postulated that B cell depletion leads to alterations in hematopoietic growth factors that promote lymphopoiesis and downregulate granulopoiesis. One such cytokine is B cell activating factor (BAFF). Serum BAFF levels rise during B cell depletion, with excess levels correlating with neutropenic events (21, 25). Another implicated cytokine is stromal-derived factor 1 (SDF-1), which promotes early B cell development and regulates neutrophil egress from bone marrow. Perturbations in SDF-1 levels have correlated with neutropenic events during B cell depletion (24, 26). Other hypotheses have been proposed, but additional investigation is needed to more accurately define the mechanism of LON (8, 26–30).
Our results suggest the underlying disease independently informs the risk of LON. Indeed, we found the risk of LON is three-fold higher among patients with systemic lupus erythematosus compared with AAV. The interpretation of this association may be limited by sample size and confounding, given neutropenia is an established manifestation of SLE; however, SLE-associated neutropenia is usually characterized by mild neutropenia (ANC between 1000 and 1500 cells/μL), which we excluded from our definition of LON. Furthermore, our results are consistent with prior reports describing higher rates of LON in SLE (11, 21). Distinct roles for BAFF and a proliferation-inducing ligand (APRIL) have been postulated to explain the unique immunologic phenomenon in SLE (21). In contrast to SLE, we did not observe LON in patients with the podocytopathies MCD / FSGS treated with rituximab, including those that received concurrent cyclophosphamide. It is possible that urinary loss of rituximab in the setting of heavy proteinuria alters its pharmacodynamics (31), or alternatively, a larger sample size is required to detect LON in these patients. A prior report described LON in 11 (5.2%) of 213 pediatric patients treated with rituximab for steroid-resistant nephrotic syndrome (32).
Cyclophosphamide can lead to neutropenia in a dose-dependent fashion (33). It seems plausible that the combination of rituximab and cyclophosphamide can act synergistically to cause neutropenia. Indeed, in our study, when low-dose cyclophosphamide was utilized in combination with rituximab for induction therapy, we found a two-fold higher risk of LON compared to those not exposed to cyclophosphamide. Combination therapy with cyclophosphamide and rituximab is a regimen used at several large centers (18, 34). The basis for this regimen is that the therapeutic effect of rituximab has a delayed onset of action, thus necessitating high doses of glucocorticoids in the first two months of therapy (1). The addition of a short course of cyclophosphamide allows for a more rapid prednisone taper. The steroid minimization and high efficacy of combination therapy in remission induction of AAV needs to be weighed against its higher risk of LON.
We describe how LON patients in our cohort were managed. We found most episodes were discovered incidentally in asymptomatic patients. Recombinant-granulocyte colony-stimulating factor (G-CSF), namely filgrastim, was utilized in the majority of episodes with severe neutropenia and/or fever. While there is no data in the literature to suggest G-CSF impacts morbidity or mortality in LON, data from our cohort suggests a role in shortening its duration. Indeed, the duration of an episode in our cohort (median 4 days) is shorter than prior reports in the literature, which ranged from 9 to 54 days (10, 26, 29). Importantly, no patient in our cohort had a relapse of their underlying autoimmune disease after filgrastim.
Our report provides the largest data to date on rituximab rechallenge in patients with LON. About one-fifth of patients in our cohort who sustained LON developed a second episode within 2 years of being rechallenged with rituximab, which is more than double the rate of the initial episode. This expands on a prior report that observed a second episode in 16% of re-treated patient (12).
Our experience has generated a protocolized approach to the management of LON (Figure 3). For any B cell depleted patient, we advocate checking a CBC with differential count at a minimum for any report of fever. In patients with recurrent LON, rituximab should be discontinued unless ongoing maintenance therapy is deemed essential and no reasonable therapeutic alternatives exist.
Figure 3. Protocolized management of LON.
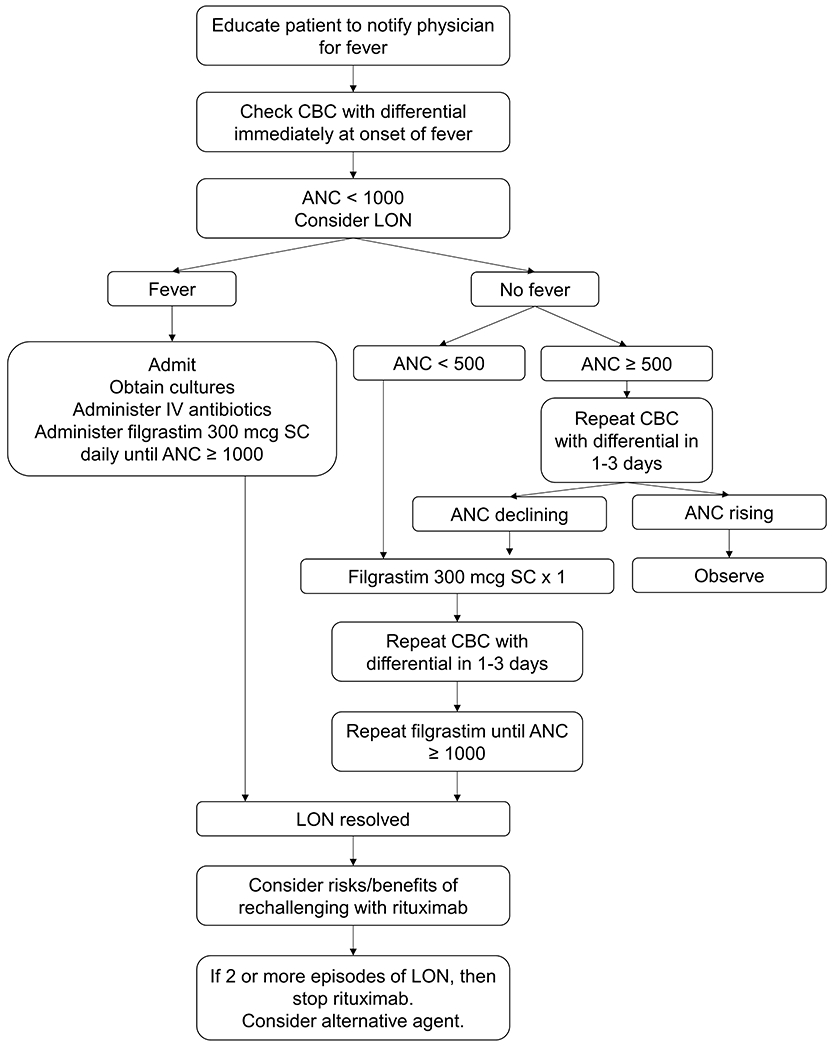
Shown is the general strategy we use at the Vasculitis and Glomerulonephritis Center at the Massachusetts General Hospital for the management of LON. For any B cell depleted patient, we advocate checking a CBC with differential count at a minimum for any report of a febrile illness. We advocate filgrastim use in patients with febrile neutropenia or an ANC < 500 cells/μL. ANC values are expressed in cells/μL.
ANC = absolute neutrophil count; CBC = complete blood count; IV = intravenous; LON = late-onset neutropenia; SC = subcutaneous.
Our study has several strengths and weaknesses. The greatest strengths are the large size of the cohort on continuous B cell depletion, long duration of follow-up, and the inclusion of patients across a broad spectrum of antibody-mediated autoimmune disease encountered in clinical practice. The main weaknesses are inherent to data collection in retrospective studies, as well as data derived from a single center. Our center is a nephrology-based practice, which explains the low number of patients with certain diseases, specifically rheumatoid arthritis, and it is possible that rates of LON are different. A large majority of patients had AAV, such that comparisons of LON based on underlying diagnosis warrant some caution pending larger studies. Lastly, CBCs were only monitored for infusions and symptoms, and it is likely that more frequent monitoring would have captured more episodes of LON.
Since the use of B cell depletion with rituximab is likely to increase, it is essential to be aware of the long-term implications of this treatment. LON is common and can be asymptomatic but has the potential to be complicated by serious infection. Further studies are required to help balance the risks of B cell depletion against the risk of disease relapse.
Supplementary Material
Supplemental Figure 1. Cumulative incidence of LON in the first year following rituximab. Shown is the Kaplan-Meier curve for time to initial LON in all patients in the first year of treatment. This is a zoomed in version of Figure 1A.
Supplemental Figure 2. Cumulative incidence of LON in patients receiving rituximab without cyclophosphamide. Shown is the Kaplan-Meier curve for time to initial LON episode in patients who received rituximab without cyclophosphamide for induction-remission therapy.
RTX = rituximab; CYC = cyclophosphamide.
Acknowledgments
Funding
Dr. Reza Zonozi’s work was supported by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) grant 5T32-DK-007540 from the NIH. Dr. Zachary Wallace was supported by funding from NIH/NIAMS [K23AR073334 and L30 AR070520].
Financial Support
There is no relevant financial support or other benefit that any of the authors have that would create a potential conflict of interest regarding this work.
REFERENCES
- 1.Stone JH, Merkel PA, Spiera R, Seo P, Langford CA, Hoffman GS, et al. Rituximab versus cyclophosphamide for ANCA-associated vasculitis. N Engl J Med. 2010;363(3):221–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Edwards JC, Szczepański L, Szechiński J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell–targeted therapy with rituximab in patients with rheumatoid arthritis. New England Journal of Medicine. 2004;350(25):2572–81. [DOI] [PubMed] [Google Scholar]
- 3.Cartin‐Ceba R, Golbin JM, Keogh KA, Peikert T, Sánchez‐Menéndez M, Ytterberg SR, et al. Rituximab for remission induction and maintenance in refractory granulomatosis with polyangiitis (Wegener's): Ten‐year experience at a single center. Arthritis & Rheumatism. 2012;64(11):3770–8. [DOI] [PubMed] [Google Scholar]
- 4.Smith RM, Jones RB, Guerry MJ, Laurino S, Catapano F, Chaudhry A, et al. Rituximab for remission maintenance in relapsing antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis & Rheumatism. 2012;64(11):3760–9. [DOI] [PubMed] [Google Scholar]
- 5.Guillevin L, Pagnoux C, Karras A, Khouatra C, Aumaître O, Cohen P, et al. Rituximab versus azathioprine for maintenance in ANCA-associated vasculitis. New England Journal of Medicine. 2014;371(19):1771–80. [DOI] [PubMed] [Google Scholar]
- 6.Maloney DG, Grillo-López AJ, White CA, Bodkin D, Schilder RJ, Neidhart JA, et al. IDEC-C2B8 (Rituximab) anti-CD20 monoclonal antibody therapy in patients with relapsed low-grade non-Hodgkin's lymphoma. Blood. 1997;90(6):2188–95. [PubMed] [Google Scholar]
- 7.McLaughlin P, Grillo-López AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. Journal of clinical oncology. 1998;16(8):2825–33. [DOI] [PubMed] [Google Scholar]
- 8.Chaiwatanatorn K, Lee N, Grigg A, Filshie R, Firkin F. Delayed‐onset neutropenia associated with rituximab therapy. British journal of haematology. 2003;121(6):913–8. [DOI] [PubMed] [Google Scholar]
- 9.Voog E Neutropenia in patients treated with rituximab. N Engl J Med. 2003;348:2691–4. [DOI] [PubMed] [Google Scholar]
- 10.Tesfa D, Palmblad J. Late-onset neutropenia following rituximab therapy: incidence, clinical features and possible mechanisms. Expert review of hematology. 2011;4(6):619–25. [DOI] [PubMed] [Google Scholar]
- 11.Tesfa D, Ajeganova S, Hägglund H, Sander B, Fadeel B, Hafström I, et al. Late‐onset neutropenia following rituximab therapy in rheumatic diseases: Association with B lymphocyte depletion and infections. Arthritis & Rheumatism. 2011;63(8):2209–14. [DOI] [PubMed] [Google Scholar]
- 12.Salmon J, Cacoub P, Combe B, Sibilia J, Pallot-Prades B, Fain O, et al. Late-onset neutropenia after treatment with rituximab for rheumatoid arthritis and other autoimmune diseases: data from the AutoImmunity and Rituximab registry. RMD open. 2015;1(1):e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monaco WE, Jones JD, Rigby WF. Rituximab associated late-onset neutropenia—a rheumatology case series and review of the literature. Clinical rheumatology. 2016;35(10):2457–62. [DOI] [PubMed] [Google Scholar]
- 14.Biotti D, Lerebours F, Bonneville F, Ciron J, Clanet M, Brassat D. Late-onset neutropenia and neurological relapse, during long-term rituximab therapy in myelin oligodendrocyte glycoprotein-antibody spectrum disorder. Multiple Sclerosis Journal. 2018;24(12):1645–7. [DOI] [PubMed] [Google Scholar]
- 15.Abdulkader R, Dharmapalaiah C, Rose G, Shand LM, Clunie GP, Watts RA. Late-onset neutropenia in patients with rheumatoid arthritis after treatment with rituximab. The Journal of rheumatology. 2014;41(5):858–61. [DOI] [PubMed] [Google Scholar]
- 16.Pendergraft WF, Cortazar FB, Wenger J, Murphy AP, Rhee EP, Laliberte KA, et al. Long-term maintenance therapy using rituximab-induced continuous B-cell depletion in patients with ANCA vasculitis. Clinical Journal of the American Society of Nephrology. 2014;9(4):736–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cortazar FB, Pendergraft III WF, Wenger J, Owens CT, Laliberte K, Niles JL. Effect of continuous B cell depletion with rituximab on pathogenic autoantibodies and total IgG levels in antineutrophil cytoplasmic antibody–associated vasculitis. Arthritis & Rheumatology. 2017;69(5):1045–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cortazar FB, Muhsin SA, Pendergraft III WF, Wallace ZS, Dunbar C, Laliberte K, et al. Combination therapy with rituximab and cyclophosphamide for remission induction in ANCA vasculitis. Kidney international reports. 2018;3(2):394–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cortazar FB, Leaf DE, Owens CT, Laliberte K, Pendergraft WF, Niles JL. Combination therapy with rituximab, low-dose cyclophosphamide, and prednisone for idiopathic membranous nephropathy: a case series. BMC nephrology. 2017;18(1):44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Physicians ACoC, Committee SoCCMCC. American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference: definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. Crit Care Med. 1992;20:864–74. [PubMed] [Google Scholar]
- 21.Parodis I, Söder F, Faustini F, Kasza Z, Samuelsson I, Zickert A, et al. Rituximab-mediated late-onset neutropenia in systemic lupus erythematosus–distinct roles of BAFF and APRIL. Lupus. 2018;27(9):1470–8. [DOI] [PubMed] [Google Scholar]
- 22.Jones RB, Cohen Tervaert JW, Hauser T, Luqmani R, Morgan MD, Peh CA, et al. Rituximab versus cyclophosphamide in ANCA-associated renal vasculitis. New England Journal of Medicine. 2010;363(3):211–20. [DOI] [PubMed] [Google Scholar]
- 23.Cohen BA. Late-onset neutropenia following ocrelizumab therapy for multiple sclerosis. Neurology. 2019;92(9):435–6. [DOI] [PubMed] [Google Scholar]
- 24.Fried S, Avigdor A, Bielorai B, Meir A, Besser MJ, Schachter J, et al. Early and late hematologic toxicity following CD19 CAR-T cells. Bone marrow transplantation. 2019;54(10):1643–50. [DOI] [PubMed] [Google Scholar]
- 25.Terrier B, Ittah M, Tourneur L, Louache F, Soumelis V, Lavie F, et al. Late-onset neutropenia following rituximab results from a hematopoietic lineage competition due to an excessive BAFF-induced B-cell recovery. Haematologica. 2007;92(2):e20–e3. [DOI] [PubMed] [Google Scholar]
- 26.Dunleavy K, Hakim F, Kim HK, Janik JE, Grant N, Nakayama T, et al. B-cell recovery following rituximab-based therapy is associated with perturbations in stromal derived factor-1 and granulocyte homeostasis. Blood. 2005;106(3):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Papadaki T, Stamatopoulos K, Stavroyianni N, Paterakis G, Phisphis M, Stefanoudaki-Sofianatou K. Evidence for T-large granular lymphocyte-mediated neutropenia in Rituximab-treated lymphoma patients: report of two cases. Leukemia research. 2002;26(6):597–600. [DOI] [PubMed] [Google Scholar]
- 28.Stamatopoulos K, Papadaki T, Pontikoglou C, Athanasiadou I, Stavroyianni N, Bux J, et al. Lymphocyte subpopulation imbalances, bone marrow hematopoiesis and histopathology in rituximab-treated lymphoma patients with late-onset neutropenia. Leukemia. 2008;22(7):1446. [DOI] [PubMed] [Google Scholar]
- 29.Tesfa D, Gelius T, Sander B, Kimby E, Fadeel B, Palmblad J, et al. Late-onset neutropenia associated with rituximab therapy: evidence for a maturation arrest at the (pro) myelocyte stage of granulopoiesis. Medical Oncology. 2008;25(4):374–9. [DOI] [PubMed] [Google Scholar]
- 30.Fukuno K, Tsurumi H, Ando N, Kanemura N, Goto H, Tanabashi S, et al. Late-onset neutropenia in patients treated with rituximab for non-Hodgkin’s lymphoma. International journal of hematology. 2006;84(3):242–7. [DOI] [PubMed] [Google Scholar]
- 31.Stahl K, Duong M, Schwarz A, Wagner A, Haller H, Schiffer M, et al. Kinetics of rituximab excretion into urine and peritoneal fluid in two patients with nephrotic syndrome. Case reports in nephrology. 2017;2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kamei K, Takahashi M, Fuyama M, Saida K, Machida H, Sato M, et al. Rituximab-associated agranulocytosis in children with refractory idiopathic nephrotic syndrome: case series and review of literature. Nephrology Dialysis Transplantation. 2014;30(1):91–6. [DOI] [PubMed] [Google Scholar]
- 33.Langford C Cyclophosphamide as induction therapy for Wegener's granulomatosis and microscopic polyangiitis. Clinical & Experimental Immunology. 2011;164:31–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McAdoo SP, Medjeral-Thomas N, Gopaluni S, Tanna A, Mansfield N, Galliford J, et al. Long-term follow-up of a combined rituximab and cyclophosphamide regimen in renal anti-neutrophil cytoplasm antibody-associated vasculitis. Nephrology Dialysis Transplantation. 2019;34(1):63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Cumulative incidence of LON in the first year following rituximab. Shown is the Kaplan-Meier curve for time to initial LON in all patients in the first year of treatment. This is a zoomed in version of Figure 1A.
Supplemental Figure 2. Cumulative incidence of LON in patients receiving rituximab without cyclophosphamide. Shown is the Kaplan-Meier curve for time to initial LON episode in patients who received rituximab without cyclophosphamide for induction-remission therapy.
RTX = rituximab; CYC = cyclophosphamide.


