Abstract
OBJECTIVE
Expression of ZFP423, a key pro-adipogenic transcription factor in adipocyte precursor cells, is regulated by interaction of the pro-adipogenic Early B-cell Factor-1 (EBF1) and anti-adipogenic ZFP521. The ubiquitin ligase Seven-in-absentia homolog 2 (SIAH2) targets ZFP521 for degradation. Here, we asked if SIAH2 is expressed in adipocyte precursor cells and if SIAH2 interacts with ZFP521 and EBF1 to regulate ZFP521 protein levels during adipogenesis.
METHODS
Siah2 expression in precursor cells was assessed in primary cells and tissues from wild-type and Siah2 null mice fed a control or high fat diet. Primary cells, 3T3-L1 preadipocytes and HEK293T cells were used to analyze Siah2, Ebf1, and Zfp521 expression and SIAH2-mediated changes in ZFP521 and EBF1 protein levels.
RESULTS
Siah2 is expressed in PDGFRα+ and SCA1+ adipocyte precursor cells. SIAH2 depletion reduces Ebf1 gene expression and increases EBF1 protein levels in early, but not late adipogenesis. In early adipogenesis, SIAH2 forms a protein complex with EBF1 and ZFP521 to enhance SIAH2-mediated ubiquitylation and degradation of ZFP521 while increasing EBF1 protein levels.
CONCLUSION
Siah2 is expressed in PDGFRα+ adipocyte precursor cells and is linked to precursor cell commitment to adipogenesis by interacting with EBF1 and ZFP521 proteins to target the anti-adipogenic ZFP521 for degradation.
Keywords: Adipogenesis, adipocyte precursor, obesity, ubiquitin, SIAH2
Introduction
Adipose tissue expansion with obesity occurs through two mechanisms: adipocytes enlargement (hypertrophy) of existing adipocytes or recruitment of precursor cells to form new adipocytes (hyperplasia). During hypertrophic obesity, insulin resistance occurs as the lipid storage capacity of adipocytes is exceeded (1), but hyperplastic expansion is associated with improved insulin tolerance in individuals with obesity (2). The relationship between adipocyte hyperplasia and insulin sensitivity points out the importance of recruiting adipocyte precursor cells to undergo adipogenesis in maintaining healthy adipose tissue expansion (3). Forming new adipocytes from precursor cells depends on the coordinated activity of multiple transcriptional regulators, but the mechanisms underlying transcriptional regulation of precursor cell commitment to adipogenesis are not fully understood.
Adipocyte precursor cells can be identified by cell surface expression of the platelet-derived growth factor receptor α (PDGFRα) and stem cell antigen-1 (SCA1) (4–8). At the onset of diet-induced adipose tissue expansion, the PDGFRα+ cell population increases, correlating with the appearance of new adipocytes (4). Commitment of recruited PDGFRα+ precursor cells to adipogenesis is initiated by factors such as bone morphogenetic protein 4 (BMP4) (9–11), leading to downstream activation (12) of zinc-finger protein 423 (ZFP423), a key transcription factor in committing precursor cells to forming white adipocytes (13, 14). ZFP423 promotes expression of the peroxisome proliferator-activated receptor gamma (PPARγ) to stimulate conversion of the preadipocytes to mature adipocytes (13). Other factors regulating commitment to adipogenesis have also been identified. The Zinc finger protein 521 (ZFP521) promotes osteocyte development (10, 15, 16) and antagonizes ZFP423 pro-adipogenic activity by binding to the early B-cell Factor-1 (EBF1), a pro-adipogenic transcription factor that activates Zfp423 expression and activity (15–18). As adipogenesis progresses, EBF1 represses Zfp521 transcriptional expression in a negative feedback loop (16). This suggests regulation of ZFP521 and EBF1 protein levels contributes to preadipocyte commitment to adipogenesis. However, the potential mechanisms controlling ZFP521 or EBF1 protein levels during adipogenesis are unknown.
The mammalian Seven-in-absentia homolog 2 (SIAH2), a RING-type ubiquitin ligase, is expressed in adipose tissue stromal vascular fraction cells and adipocytes (19). Our earlier studies showed SIAH2 promotes adipogenesis (19, 20) and Zfp423 mRNA expression in adipose tissue primary cells through a process that involves BMP4 (20). SIAH2 also regulates zinc-finger protein 521 (ZFP521) protein levels during adipogenesis by a mechanism that is likely proteasome-dependent (20). These data support SIAH2 involvement in regulating early steps in commitment of adipocyte precursor cells to adipocyte differentiation.
In this study, we asked if SIAH2 is expressed in adipocyte precursor cells and if SIAH2 interacts with ZFP521 and EBF1 to regulate ZFP521 protein levels. Herein, we show that Siah2 is expressed in PDGFRα+ and SCA1+ cells in visceral and subcutaneous adipose tissue and that Siah2 depletion affects Ebf1 mRNA and EBF1 protein levels during adipogenesis in adipose tissue stromal cells. Experiments in HEK293 cells and 3T3-L1 preadipocytes indicate SIAH2 forms a complex with EBF1 and ZFP521 that enhances SIAH2-mediated ubiquitylation and degradation of ZFP521 and increases EBF1 levels. These results support a mechanism in which SIAH2 expression promotes adipocyte precursor cell commitment to adipogenesis by reducing ZFP521 levels via an interaction with EBF1.
Methods
Mice
Wild-type C57BL/6J male mice were purchased from Jackson Laboratory. Siah−/− (Siah2KO) C57BL/6J male mice were generated and maintained (21) as described. All mice were multi-housed on a 12 hours light/dark cycle at 24oC. At 5 weeks of age, wild-type mice were normalized by body weights and randomly assigned to a 10% fat diet (Research Diets, D12450J, LFD) or a 60% fat diet (Research Diets, D12492, HFD) for 2 months (N=8/group). Body weight was measured weekly and body composition was determined biweekly by NMR. At the end of 2 months, the mice were euthanized between 0700 – 0900 hr. Epididymal (eWAT) and inguinal (iWAT) white adipose tissue was collected for histology and stromal vascular fraction cell (SVF) isolation. A separate cohort of 4–5 week old wild-type and Siah2KO male mice (N=8–10/group) were euthanized between 0900–1100 hr and inguinal adipose tissue was collected for adipogenesis experiments. A third cohort of 10 week-old wild-type C57BL/6J male mice fed on a 10% low-fat diet (#380056) or 60% high-fat diet (#380050) for four-weeks were purchased from Jackson Laboratory (N=3 mice/group). These mice were euthanized between 0800–1000 hr. Inguinal and epididymal adipose tissue was collected to analyze obesity-induced regulation of Siah2, Ebf1, Zfp423, and Zfp521 gene expression. All animal studies were approved by Pennington Biomedical Research Center Institutional Animal Care and Use (Protocol # 974).
Tissue histology and imaging
Tissues were fixed with 10% neutral buffered formalin for 24 hours at 25oC, embedded in paraffin, and sectioned into 5μm slices. Fluorescent in situ hybridization (ISH) was performed using RNAscope (ACD) according to manufacturer’s recommendation. Sections were hybridized using a Siah2 probe (ACD) for 2 hours at 40oC. After hybridization, the sections were sequentially incubated with AMP1, AMP2, AMP3, and then C1-HRP to amplify the probe signal. Signal was detected using TSA Plus Fluorescence Kit (PerkinElmer).
Immunohistochemistry (IHC) immediately followed ISH to establish dual ISH-IHC. Primary antibodies (Table S1) were applied overnight at 4oC. Secondary antibodies were applied for 1 hour at room temperature. Sections were counterstained with DAPI in Prolong Gold Antifade Mountant (ThermoFisher). Slides were analyzed by wide field Leica DMI6000 (Leica Microsystems) fluorescence microscopy. Images were processed by LAS X software (Leica Microsystems) and ImageJ (NIH).
Magnetic cell separation
Inguinal and epididymal adipose tissue SVF was isolated (N=8/group) and single cell suspensions were established as described (22). We were unsuccessful in using available SIAH2 antibodies to immunopurify SIAH2 with specificity from permeabilized cells for flow cytometry. Thus, we analyzed Siah2 expression in the selected primary cell types using magnetic bead-based cell separation. A portion of SVF from both fat depots was collected and RNA extracted for use as a total “SVF” gene expression comparison. The remaining cells were separated into three equal volume fractions for use in cell type selection. Biotinylated antibodies (Table S1) were added to each fraction of the eWAT or iWAT SVF. The antibody-bound cell samples were mixed with Dynabead biotin binder (Invitrogen) and captured magnetically according to manufacturer’s recommendation. The bound cells were collected in TriReagent for RNA extraction. Cell number was determined using a hemocytometer and trypan blue staining. The number of cells bound onto the column was defined as the difference between input cell number and unbound cell number with the assumption of maximal column binding efficiencies. Column binding efficiency and specificity for the selected marker gene expression were analyzed by qRT-PCR (Fig. S1).
Cell culture
Stromal vascular fraction cells from pooled tissue harvested from 8–10 mice/group of wild-type and Siah2KO male mice were isolated, cultured, and induced for adipocyte differentiation as described (20). The 3T3-L1 preadipocytes were maintained and induced to undergo adipogenesis as described (20). HEK293T cells were maintained in DMEM, high glucose containing 10% fetal bovine serum and 100U penicillin/100μg streptomycin.
Immunoblotting
Whole cell extracts were prepared in RIPA buffer (50mM Tris-Cl, pH 8.0 with 150 mM NaCl, 1% NP-40, 0.5% sodium deoxycholate, 0.1% SDS, 1 μM phenylmethylsulfonyl fluoride, 1 μM pepstatin, 50 trypsin inhibitory milliunits of aprotinin, 10 μM leupeptin, and freshly prepared 10 mM N-ethyl maleimide (N-EM)) or when carrying out immunoprecipitations, a nondenaturing buffer (50mM Tris-Cl, pH 7.4 with 150 mM NaCl, 1mM EDTA, 1% Triton X-100, 0.5% Igepal, protease inhibitors and 10 mM N-EM). Protein concentrations were determined by BCA assay (Thermo Fisher Scientific).
Protein lysates from the whole cell extracts or immunoprecipitations were separated by SDS-PAGE and transferred to nitrocellulose membrane (Bio-Rad). Membranes were incubated with antibodies and the results visualized as described in Table S1.
Transient transfection
HEK293T cells were transfected at 50–60% confluence with cDNA (total 2 μg/well) encoding wild-type Flag-SIAH2 (pcDNA3.1 Flag-Siah2wt) (19), a dominant negative RING mutant SIAH2 (pcDNA3.1 Flag-Siah2H99A-C102A, referred to as Flag-SIAH2dnm) (23), pcDNA3.1 Ebf1, or hemagglutinin-tagged ZFP521 (pcDNA3.1 HA-Zfp521) (20) as indicated using Polyfect transfection reagent (Qiagen) according to manufacturer’s protocol. Cells were transfected with pcDNA3.1 alone as a negative control or to balance the total amount of DNA. pcDNA3.1-Ebf1 was generated by PCR amplification of pTRE3G-Ebf1. The amplicon was cloned into pcDNA3.1 (+) at HindIII/Xbal. All cDNAs were confirmed by dideoxy sequencing. Transfection efficiency was monitored using pCMV-Gfp as an internal transfection control. Forty-eight hours post transfection, the cells were harvested after pretreatment with DMSO as a vehicle control or MG132 (10 μM) for four hours.
Immunoprecipitation
HEK293T cells or 3T3-L1 preadipocytes were treated with MG132 (10 μM) for 4 hours with PR619 (25 μM) added for the final 30 minutes to inhibit deubiquitinase activity prior to harvesting the cells and lysate preparation. Total lysate was precleared with 50 μL of protein A/B plus agarose (Pierce) for 1 hour at 4oC. In the transiently transfected HEK293T cells, antibodies against the HA or Flag epitope tags were applied to the precleared supernatant at 1 μg/200 μg lysate for 1 hour at 4o C. Protein A/B plus agarose was then added and incubated at 4oC overnight. The 3T3-L1 preadipocyte whole cell lysates were incubated with the anti-SIAH2 antibody at 1 μg/250 μg lysate. Nonspecific rabbit anti-IgG was included as an isotype control at 1 μg/250 μg lysate.
Protein stability assay
HEK293T cells were transfected with HA-Zfp521 alone, HA-Zfp521 and FLAG-Siah2, or HA-Zfp521 with FLAG-Siah2 and Ebf1 as outlined in Transient transfection. After forty-eight hours, cells were treated with 100 μg/mL cycloheximide −/+ MG132 (10 μM) and harvested at the indicated time points. pCMV-gfp was included as an internal control. Western blot images were quantified using ImageJ. Protein decay rates were determined using first order kinetics and plotted as intensity over time.
Gene Expression
Total RNA was extracted and prepared for real-time PCR as previously described (20). Gene expression were analyzed with Taqman or SYBR green using Applied Biosystems 7900HT system. Results were normalized to UbB mRNA levels and analyzed by the 2-μμCT method with stromal vascular fraction values or Siah2KO values in Fig 3 or wild-type values in Fig 4 used as the calibrator. Primers used are included in Table S1.
Figure 3:
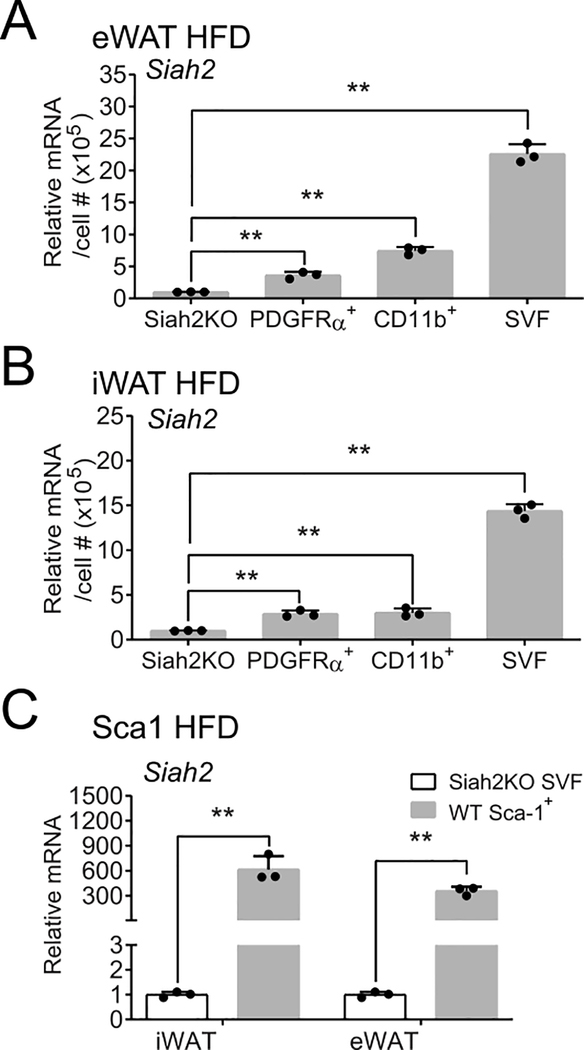
Siah2 is expressed in adipocyte precursor cells and macrophages. Adipocyte precursor (PDGFRα+) cells and macrophages (CD11b+) were positively selected from SVF obtained from 60% high fat diet (HFD) fed male mice via magnetic bead cell sorting. Siah2 mRNA levels were assayed by qRT-PCR and compared against total stromal vascular fraction (SVF) cells. Siah2 expression in SVF from SIAH2 deficient male mice (Siah2KO) was used to establish minimal siah2 expression. Siah2 relative expression was assayed in (A) eWAT or (B) iWAT. (C) Siah2 relative expression was assayed in iWAT or eWAT of SCA1+ cells. Figures depict mean ± SD; **, p < 0.0001; *, p < 0.001; #, p < 0.01. Each data point represents a technical replicate from pooled samples (N=8 mice/group) from a single cohort of HFD-fed mice. The experiment was conducted after extensive optimization of column conditions using adipose tissue from prior mouse cohorts.
Figure 4:
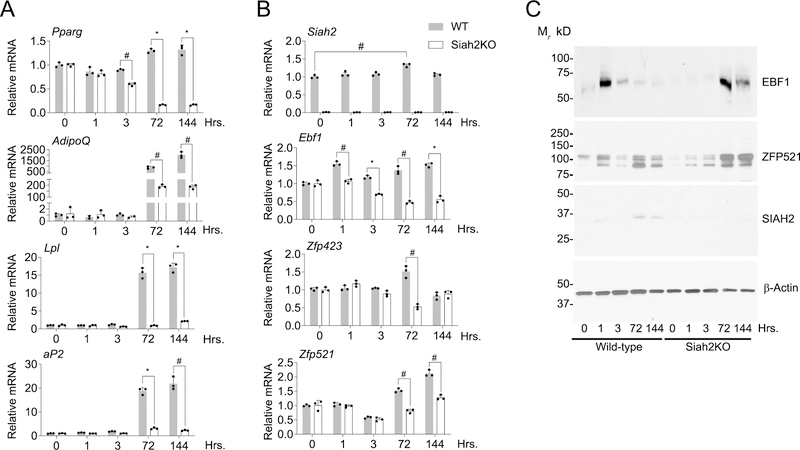
SIAH2 affects Ebf1 and Zfp521 mRNA and protein expression during adipogenesis of adipose tissue primary stromal cells. (A) Pparg, Adiponectin (AdipoQ), Lpl, aP2 and (B) Siah2, Ebf1, Zfp423 and Zfp521 expression between wild-type and Siah2 knockout (Siah2KO) by qRT-PCR at the indicated time points. (C) Western blot analysis of EBF1, ZFP521 and SIAH2 expression in wild-type and Siah2KO at early time points post induction of adipogenesis. β-actin is included as a loading control. Bar graph plotted as mean ± SD; *, p < 0.0001; #, p < 0.001. Each data point in the bar graph represents technical replicates from a pooled sample (N=8–10 mice). The experiment was performed twice independently.
Statistical analysis
Statistical significant was determined using unpaired two-tailed t-test with GraphPad Prism 8 software and reported as the mean −/+ standard deviation.
Results
Siah2 is expressed in adipocyte precursor cells
Our previous data indicated SIAH2 is involved with regulating commitment of precursor cells to undergo adipogenesis (13). To determine if Siah2 expression is regulated by a high fat diet challenge that stimulates adipose tissue expansion, we analyzed Siah2 gene expression along with Ebf1, Zfp423 and Zfp521 expression in the visceral and subcutaneous fat depots of male mice fed a high fat diet (Fig. S2). At four weeks after the onset of a high fat diet, Siah2, Ebf1 and Zfp521 expression was reduced in the stromal cells of visceral and subcutaneous fat compared to age-matched male mice fed a low fat diet (Fig. S2A, B). Zfp423 expression was reduced in the stromal cell population in visceral fat, but not subcutaneous fat, and was consistently expressed at higher levels in the adipocyte fraction compared to the stromal fraction (Fig.S2 C, D). While the distribution of Siah2 expression between the stromal and adipocyte fractions was unchanged by the diet, Ebf1 and Zfp521 expression shifted toward the adipocyte fraction with the high fat diet in the subcutaneous fat (Fig. S2C, D). This corresponds to adipose tissue expansion in which adipogenesis in visceral fat is stimulated after 4 weeks on a high fat diet while subcutaneous fat expands via adipocyte hypertrophy for up to 12 weeks on a high fat diet (24). Thus, Siah2 expression is regulated by high fat diet-induced obesity along with pro-adipogenic factors such as Ebf1 and Zfp423 that are expressed in adipocyte precursor cells in the stromal cell population. We hypothesized that Siah2 is also expressed in adipocyte precursor cells.
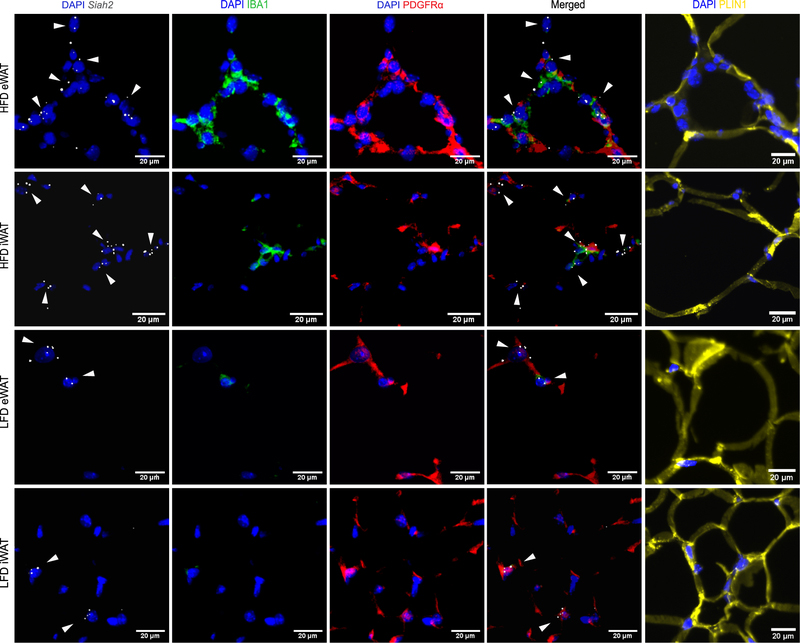
To test this prediction, Siah2 mRNA was probed using dual ISH-IHC alongside PDGFRα, protein in visceral (eWAT) and subcutaneous (iWAT) adipose tissue obtained from male mice fed a low fat or high fat diet (Fig. 1). Because PDGFRα+ precursor cells co-localize with macrophage that form the “crown-like structure” surrounding dying adipocytes (5) and SIAH2 regulates innate immune responses (25), we also probed for macrophage (IBA1). We observed that Siah2 is expressed in close proximity to crown-like-structures, where PDGFRα+ cells and macrophage overlap. However, Siah2 remained localized with PDGFRα+ cells although the crown-like-structures were not readily observed in iWAT or the LFD eWAT, indicating Siah2 localization with PDGFRα+ cells occurs in the absence of macrophage.
Figure 1:
Siah2 co-localizes with PDGFRα+ cells and macrophages (IBA1). Representative images of paraffin embedded inguinal (iWAT) and epididymal (eWAT) adipose tissue sections from male mice on a 60% high fat diet (HFD) or 10% low fat diet (LFD) stained for nuclei (DAPI, blue), adipocyte precursor cells (PDGFRα, red), and macrophage (IBA1,green). Siah2 mRNA (gray) was localized by in situ hybridization using ACD RNAscope technology. The tissue was separately stained for perilipin 1 (PLIN1, yellow) and imaged at the same area to provide details on the structure of surrounding mature adipocytes. Signal brightness of everything except Siah2 was dimmed in the merged image to enhance Siah2 visualization. The arrows are added to emphasize Siah2 location. Data represent observations from N=8/group.
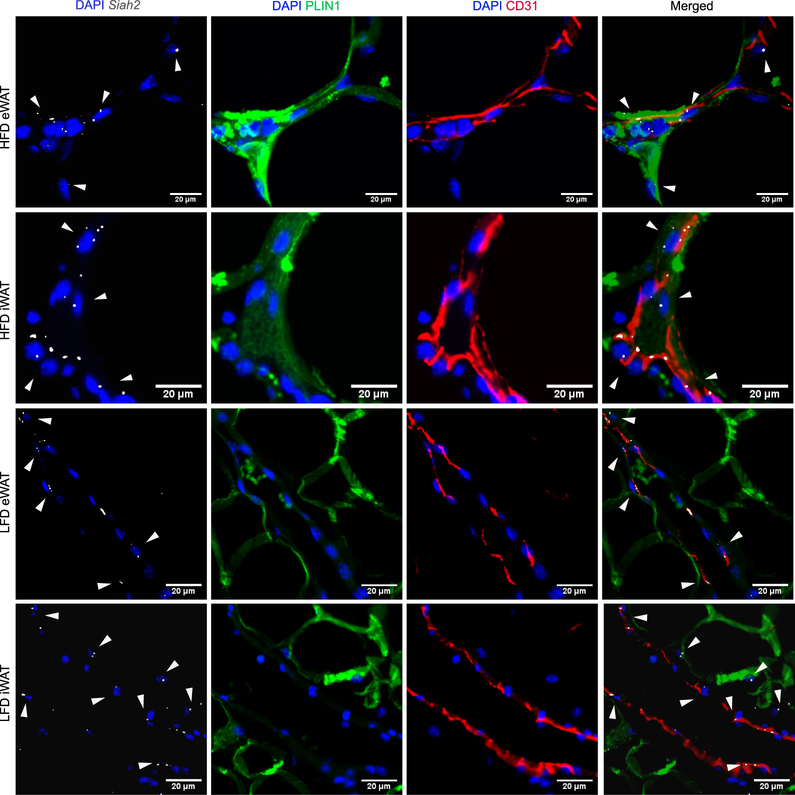
Tang et al. (26) identified adipose tissue vasculature as a niche for adipocyte precursor cells. Therefore, we also used Platelet Endothelial Cell Adhesion Molecule (PECAM-1, CD31) as a marker of vascular endothelial cells to determine if Siah2 mRNA is localized near the adipose tissue vasculature. Although we cannot determine whether the Siah2+ cells are also vasculature endothelial (CD31+) cells, Siah2 is present at the adipose tissue vascular endothelium and this was more apparent in adipose tissue obtained from HFD-fed mice (Fig. 2).
Figure 2:
Siah2 localizes near the adipose tissue vasculature. Representative images of paraffin embedded inguinal (iWAT) and epididymal (eWAT) adipose tissue sections from male mice fed a 60% high fat diet (HFD) or 10% low fat diet (LFD) were stained for nuclei (DAPI, blue), Siah2 mRNA (gray), and PLIN1 (green). CD31 (red), a marker of endothelial cells, was used to visualize the vasculature endothelial cells. Signal brightness of everything except Siah2 was dimmed in the merged image to enhance Siah2 visualization. The arrows are added to emphasize Siah2 localization. Data represent observations from N=8/group.
To confirm that Siah2 is expressed in precursor cells and/or macrophage, PDGFRα+ and macrophage (CD11b+) cells were isolated from adipose tissue stromal vascular fraction using magnetic immunoaffinity purification (Fig. 3, Fig. S1). Siah2 mRNA relative expression was normalized to cell number and compared with Siah2KO SVF as a limit-of-detection threshold. Siah2 was expressed at levels significantly higher than the limit-of-detection (Siah2KO) in both PDGFRα+ and CD11b+ fractions in each depot. Compared to total SVF in each depot, Siah2 expression in PDGFRα+ cells accounted for approximately 15% and 18% of Siah2 expression in eWAT (Fig. 3A) and iWAT (Fig. 3B), respectively. Siah2 in the CD11b+ cells constituted 32% and 17% of Siah2 expression in eWAT (Fig. 3A) and iWAT SVF (Fig. 3B), respectively. This shows approximately half of Siah2 expression in visceral adipose tissue SVF originates from PDGFRα+ and CD11b+ cells but only about a third of Siah2 is expressed in PDGFRα+ and CD11b+ cells in subcutaneous adipose tissue. As a second marker of precursor cells, SCA1 was also positively selected with magnetic immunoaffinity purification and analyzed for Siah2 expression (Fig. 3C). Although we were unable to normalize to total cell number due to an insufficient number of cells in the unbound fraction to ensure an accurate cell count, Siah2 was detected at significantly higher level in SCA1+ fraction compared to the Siah2KO cells, indicating SCA1+ cell population also expresses Siah2.
SIAH2 deletion affects Ebf1 and Zfp521 gene and protein expression
Kang et al. (16) found ZFP521 interacts with EBF1 to regulate Zfp423 gene expression in 3T3-L1 preadipocytes. Using primary cells, we asked if Siah2 also regulates EBF1 expression during adipogenesis. Impaired adipogenesis of the Siah2KO cells was reflected in reduced expression of Pparg and markers of mature adipocytes (AdipoQ, Lpl, aP2) (Fig. 4A). Siah2 deficiency is associated with reduced Ebf1 mRNA throughout induction, but Zfp521 was not reduced until 72 hours and later. Zfp423 was reduced only at 72 hours after induction in the absence of Siah2 (Fig. 4B). In the wild-type cells, EBF1 was maximally expressed at 0–1 hours post induction, corresponding to the initial SIAH2 expression, but declined as SIAH2 expression increased. ZFP521 increased at 1 hour, but declined by three hours before increasing at 72–144 hours post induction.
In contrast, EBF1 was not expressed in the early phase of induction in the Siah2KO cells but was upregulated at later induction as Ebf1 mRNA levels declined (Fig. 4C). Although Zfp521 mRNA levels were lower at later time points during adipogenesis with Siah2 deficiency compared to wild-type, ZFP521 levels increase relative to wild-type, except at the 0–1 hour time points (Fig. 4C).
SIAH2, ZFP521 and EBF1 form a complex that regulates ZFP521 degradation
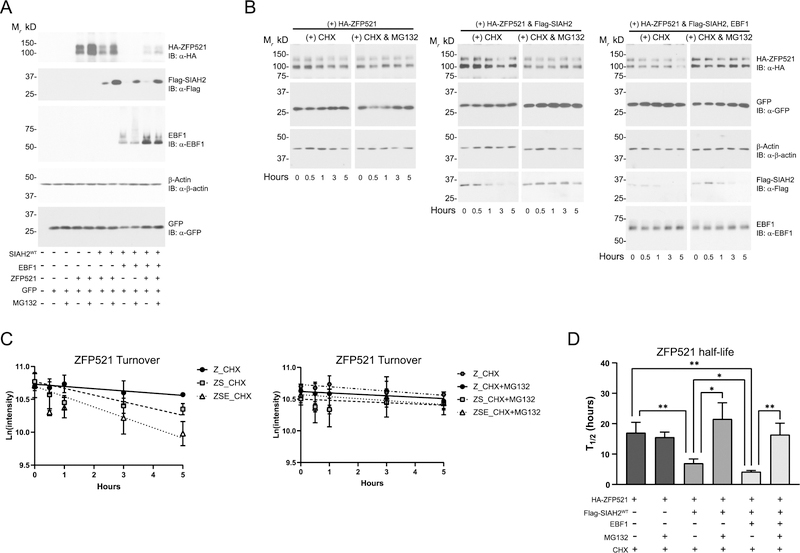
Because EBF1 and ZFP521 interact to regulate adipogenesis, we carried out a series of experiments in HEK293 cells using epitope-tagged SIAH2 and ZFP521and native EBF1 to ask if SIAH2-mediated regulation of ZFP521 protein levels is affected by EBF1. SIAH2-mediated proteasome-dependent degradation of ZFP521 was enhanced by EBF1 while the presence of ZFP521 increased EBF1 protein levels (Fig. 5A). Interestingly, EBF1 appears to destabilize SIAH2 in a manner that is partially regulated by the proteasome and is independent of EBF1 levels. To confirm that SIAH2 affects ZPF521 turnover rate, we assayed ZFP521 protein expression over 5 hours in the presence of cycloheximide (Fig. 5B). SIAH2 protein significantly reduced ZFP521 half-life and EBF1 further increased SIAH2-mediated turnover of ZFP521 (Fig. 5B–D). Proteasome inhibition with MG132 mitigated the effect of SIAH2 and EBF1 on ZFP521. Together, the data suggests a complex co-regulatory interaction between the three factors that modulates ZFP521 protein levels by a mechanism that involves proteasome-dependent degradation of ZFP521.
Figure 5:
SIAH2 controls ZFP521 protein level. HEK293T cells were transfected with equal amount of cDNA encoding wild-type Flag-SIAH2 (Flag-SIAH2wt), HA-ZFP521, or EBF1. pcDNA 3.1 was used to balance out the total cDNA amount transfected. (A) Forty-eight hours after transfection, the cells were treated with DMSO as a vehicle control or 10 μM MG132 (MG132) for four hours. (B) Forty-eight hours after transfection, the cells were treated with 100μg/mL Cyclohexamide (CHX) with DMSO or CHX with 10 μM of MG132 (CHX+MG132) at the indicated time points. (C) Using HA-ZFP521 band intensity as determined by ImageJ, the natural logarithm of the band intensity was plotted to quantify the reaction rate coefficient of first-order kinetics for Zfp521 turnover. (D) Plot comparing ZFP521 half-life calculated using the reaction rate coefficient from (C). β-actin is included as a loading control. pCMV-GFP was used as an internal control for transfection efficiency. IB, immunoblot; Z, ZFP521; ZS, ZFP521 + SIAH2; ZSE, ZFP521 + SIAH2 + EBF1; *, p < 0.05; **, p < 0.001. All graphs were plotted as mean ± SD. All experiments were performed at least 3 times.
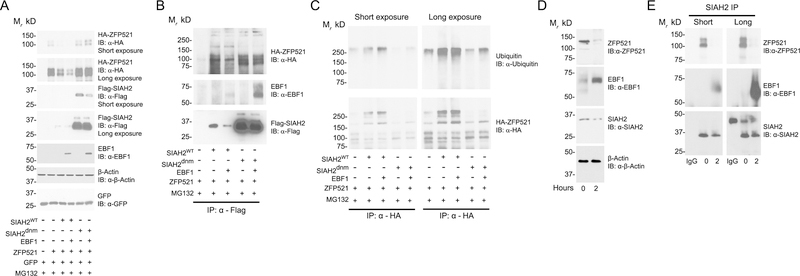
Since the RING-type ubiquitin ligases directly interact with the targeted substrate to facilitate transfer of ubiquitin from an E2 conjugating enzyme to the substrate (27), we asked if SIAH2 interacts with ZFP521 and EBF1 to regulate ZFP521 levels. In these experiments, we included a SIAH2 dominant negative RING mutant (SIAH2dnm) to determine if SIAH2 ligase activity impacts the potential protein interaction of SIAH2, EBF1, and ZFP521. The mutations at H99A-C102A in SIAH2dnm diminish SIAH2 ubiquitin-ligase activity by reducing E2 conjugating enzyme binding to SIAH2 (23, 28). ZFP521 levels were not decreased in the presence of SIAH2dnm protein regardless of EBF1 expression, indicating SIAH2 regulation of ZFP521 depends on SIAH2-ligase activity (Fig. 6A). Moreover, addition of EBF1 reduced SIAH2 protein levels independent of SIAH2 ligase activity or proteasome activity.
Figure 6:
SIAH2 forms a complex with ZFP521 and EBF1. (A – C) HEK293T cells were transfected with equal amount of cDNA encoding wild-type Flag-SIAH2 (Flag-SIAH2wt), dominant negative mutant Flag-SIAH2 (Flag-SIAH2dnm), HA-ZFP521 or EBF1. pcDNA 3.1 was used to balance out the total cDNA amount transfected. Flag-SIAH2dnm represents a H99A-C102A (RING mutant) form of SIAH2 that abolished SIAH2 ligase activity. After 48 hours transfection, cells were treated with 10 μM MG132 for 3.5 hours and then treated with 25 μM PR619 for an additional 30 minutes. (A) Cells were collected and analyzed by western blotting, representing 10% of input samples used in (B) and (C). Cell lysates were affinity purified using (B) anti-FLAG M2 antibody or (C) anti-hemagglutinin antibody. pCMV-GFP was used as an internal control for transfection efficiency. (D-E) 3T3-L1 preadipocytes were induced for adipocyte differentiation at the indicated time points. (D) Cells were collected and analyzed by western blotting, representing 10% of input samples used in (E). (E) Cell lysates were affinity purified using a rabbit anti-SIAH2 or a nonspecific rabbit anti-IgG antibody as an isotype control. β-actin is included as a loading control. IB, immunoblot; IP, immunopurified. All experiments were performed at least twice.
Immunoprecipitation of FLAG-SIAH2 showed SIAH2 co-purified with both ZFP521 and EBF1, but SIAH2dnm showed a stronger detection of ZFP521 and EBF1 interaction versus SIAH2wt (Fig. 6B), corresponding to the level of ZFP521 and EBF1 expression. Interestingly, the protein interaction of ZFP521 with SIAH2wt appeared to be stronger in the absence of EBF1, consistent with enhanced ZFP521 degradation in the presence of EBF1 (Fig. 5). Notably, ZFP521-related higher molecular weight bands copurifying with SIAH2wt protein were increased in intensity compared to SIAH2dnm protein. To determine if the banding pattern indicated SIAH2-mediated ubiquitylation of ZFP521, we immunopurified HA-ZFP521 in the absence or presence of SIAH2 and EBF1 (Fig. 6C).
The higher molecular weight forms of ZFP521 were associated with ubiquitin and the banding pattern was consistent with SIAH2-mediated ubiquitylation of ZFP521 that was reduced in the ligase-deficient SIAH2 (Fig. 6C). The short and long exposures showed SIAH2-mediated ubiquitylation of ZFP521 was increased by EBF1, indicating EBF1 promoted SIAH2-mediated ubiquitin-dependent degradation of ZFP521.
Jimenez et al. (18) and Kang et al. (16) showed Zfp521 expression decreased while Ebf1 expression increased during adipogenic induction of 3T3-L1 preadipocytes. To ascertain whether a SIAH2:ZFP521:EBF1 complex forms during adipocyte differentiation, SIAH2 was immunoprecipitated from 3T3-L1 preadipocytes at pre-induction and 2 hours post-induction to capture SIAH2 interactions during adipogenesis. EBF1 expression increased while ZFP521 expression decreased with adipogenesis (Fig. 6D). Consistent with the HEK293T-based results, increased protein expression of EBF1 in the 3T3-L1 preadipocytes correlated with a downregulation of SIAH2 protein during adipogenic induction. SIAH2 immunoprecipitation showed interaction of SIAH2 with both ZFP521 and EBF1 protein during adipogenesis (Fig. 6E). Only minimal ZFP521 protein was observed to interact with SIAH2 protein (long exposure) after 2 hours of adipogenic induction, while SIAH2 interaction with EBF1 increased alongside adipocyte differentiation. This corresponds to the reported dynamic between ZFP521 and EBF1 protein level with adipogenesis, supporting SIAH2-mediated ZFP521 degradation that is enhanced by interaction with EBF1.
Discussion
We previously identified SIAH2 as a factor promoting Zfp423 expression in a BMP4-regulated mechanism that correlates with SIAH2-mediated downregulation of ZFP521 protein (19, 20), a corepressor of Zfp423 expression (10, 16). Here, we show that Siah2 is expressed in PDGFRα+ and SCA1+ cells, both markers of adipocyte precursor cells. Moreover, Siah2 localizes with PDGFRα+ cells and adipose tissue vasculature, placing Siah2 at the site of new adipocyte formation. This is particularly evident in the high fat-fed adipose tissue where PDGFRα+ cells are found near the crown-like structure macrophage that efferocytose the dysfunctional adipocytes, forming a proposed adipogenic niche where macrophage signal activation of PDGFRα+ precursor cells to form new adipocytes (4, 5). Siah2 is also expressed in CD11b+ cells and colocalizes with macrophage at the crown-like structures. Thus, Siah2 is expressed in both cell types that influence the ability of adipose tissue to form new adipocytes (4, 26, 29, 30). Even so, Siah2 does not localize with every PDGFRα+ cell or macrophage surrounding the dying adipocyte, raising the possibility that Siah2 expression in PDGFRα+ cells or macrophage is differentially regulated. Conversely, Siah2 expression in CD11b+ and PDGFRα+ cells does not account for total Siah2 mRNA expression in adipose tissue SVF, signifying Siah2 expression is not limited to macrophages and adipocyte precursor cells in adipose tissue.
Although the current data cannot differentiate between adipocyte progenitor cells and preadipocytes as the adipocyte precursor cell type expressing Siah2, the results support a role for SIAH2 in promoting adipogenesis by influencing the conversion of preadipocytes to mature adipocytes (Fig. 7B). EBF1 promotes white adipocyte formation (18) and under cold exposure, stimulates brown fat formation along with EBF2 (31). Importantly, the substantial upregulation of EBF1 upon wild-type induction coincides with initial expression of SIAH2 and is absent when SIAH2 is depleted. Upregulation of EBF1 early in adipogenesis appears to be critical as increased expression of EBF1 in the absence of SIAH2 at the later time points did not correspond to upregulation of Zfp423 expression or adipogenesis. This inverse correlation between EBF1 and SIAH2 protein levels suggests that SIAH2 participates in a feedback loop to regulate EBF1 expression during adipogenesis. Moreover, dynamic regulation of ZFP521 levels present in early wild-type primary cells induction is absence with Siah2 deficiency, suggesting ZFP521 expression is involved in SIAH2-mediated changes in EBF1 expression.
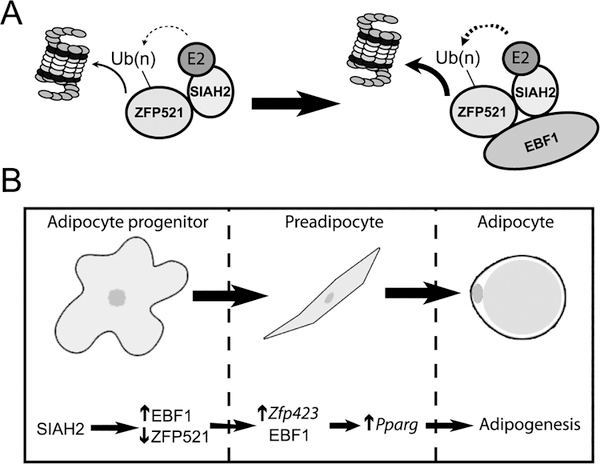
Figure 7:
Schematic of the proposed regulatory dynamic between SIAH2, ZFP521, and EBF1 in promoting adipogenesis. (A) A molecular model where SIAH2-mediated degradation of ZFP521 (A) is enhanced by interaction of EBF1 with SIAH2 and ZFP521 to accelerate ZFP521 degradation. (B) Proposed sequence of SIAH2, EBF1 and ZFP521 protein interaction to promote Zfp423 and Pparg gene expression during conversion of precursor cells to mature adipocytes.
In support of this idea, SIAH2 complexed with both EBF1 and ZFP521 in a manner indicating Siah2 acts to shift ZFP521 and EBF1 levels to favor adipogenesis (Fig. 7A). Interaction of ZFP521 with EBF1 downregulates ZFP521 by increasing SIAH2-mediated ubiquitin-dependent proteasomal degradation of ZFP521 while EBF1 protein levels were increased by the interaction. This observation is consistent with SIAH2 and EBF1 acting together to promote adipogenesis by forming a protein complex favoring destruction of ZFP521 protein as SIAH2 protein levels increase during the early phase of adipogenesis. These data agree with a role for SIAH2 in promoting adipocyte hyperplasia as depletion of SIAH2 is associated with hypertrophied adipocytes in vivo (32), and impaired adipogenesis of Siah2KO stromal vascular cells that is overridden by BMP-4-dependent regulation of Zfp423 and Zfp521 expression (40). A potential role for BMP-4 in directly affecting pro-adipogenic SIAH2 activity was not addressed in the earlier study. The current results suggest further studies are warranted to determine if BMP-4 promotes adipogenesis by controlling the interaction of SIAH2 with the ZFP521:EBF1 complex.
Proteasome-independent regulation of SIAH2 protein levels by EBF1 adds an additional layer of complexity. EBF1 is a member of the EBF family of transcription factors that bind the consensus palindromic motif 5’-TCCCNNGGGA-3’ (33) and Treiber et al. (33) showed that EBF1 protein binding acts to repress transcription. Although not tested in the current study, this represents a possible mechanism by which EBF1 decreases Siah2 mRNA expression via recognition of a binding site within the Siah2 coding region (590–603bp) of Siah2 cDNA that closely matches the EBF1 consensus motif, 5’-ACCCTTCAGGGA-3’.
This level of complex regulatory interaction is well-described for the multi-subunit RING-type ubiquitin ligases involved in cell cycle control where ligase-substrate interactions are regulated at the level of ligase transcription or ligase degradation (27). While RING-type ligases that act as homodimers are not typically described as acting in multi-subunit complexes, SIAH2 has been identified as a subunit of the nuclear receptor co-repressor complex (N-CoR1) that includes N-CoR1 and the histone deacetylase HDAC-3 (34–36). Down-regulation of N-CoR1 expression enhances adipogenesis (37) and SIAH2 functions as a subunit of the complex that regulates the stability of N-CoR1 and HDAC3 (35, 36, 38, 39). There is evidence that N-CoR1 and HDAC3 also interact with ZFP521 to control bone formation (40, 41) in conjunction with EBF1 (15). Together with evidence presented in the current study, this raises the intriguing possibility that SIAH2 acts to regulate EBF1 and ZFP521 levels (and is regulated by EBF1) as a subunit of the nuclear receptor co-repressor complex (N-CoR) to promote adipogenesis.
Supplementary Material
Study Importance Questions.
What is already known about this subject?
The ubiquitin ligase Siah2 promotes adipogenesis.
Protein-protein interaction between the Early B-cell Factor 1 (EBF1) and Zinc-Finger Protein 521 (ZFP521) contributes to commitment of precursor cells to the adipogenic pathway.
What are the new findings in your manuscript?
Siah2 is expressed in PDGFRα+ and SCA1+ adipocyte precursor cells.
EBF1 interacts with SIAH2 to increase proteasome-mediated degradation of ZFP521 protein while increasing EBF1 protein levels. A SIAH2/EBF1/ZFP521 protein complex occurs during the early steps of adipogenesis.
How might your results change the direction of research or the focus of clinical practice?
This study emphasizes the role of an enzyme of the ubiquitin-proteasome system in determining commitment of adipocyte precursor cells to adipogenesis. Future studies focused on regulation of protein stability in adipocyte precursor cells may offer new insight into how adipose tissue expansion is regulated in obesity.
Acknowledgments
FUNDING: This work is supported by the National Institutes of Health (NIDDK, 1R01DK099625, ZEF), COBRE (NIH8 P30GM118430-01, Cell Biology and Bioimaging Core; Pilot and Feasibility Studies) and NORC (NIH P30 DK072476, Genomics Core) Center grants. The work was also supported by the John S. McIlhenny Endowed Post-doctoral Fellowship (TD).
Footnotes
DISCLOSURE: The authors declared no conflict of interest.
References
- 1.Rutkowski JM, Stern JH, Scherer PE, The cell biology of fat expansion. J Cell Biol 208, 501–512 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U, Insulin resistance and impaired adipogenesis. Trends in Endocrinology & Metabolism 26, 193–200 (2015). [DOI] [PubMed] [Google Scholar]
- 3.Vishvanath L, Gupta RK, Contribution of adipogenesis to healthy adipose tissue expansion in obesity. The Journal of Clinical Investigation 129, 4022–4031 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lee YH, Petkova AP, Mottillo EP, Granneman JG, In vivo identification of bipotential adipocyte progenitors recruited by beta3-adrenoceptor activation and high-fat feeding. Cell Metab 15, 480–491 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee YH, Petkova AP, Granneman JG, Identification of an adipogenic niche for adipose tissue remodeling and restoration. Cell Metab 18, 355–367 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berry R, Jeffery E, Matthew S. Rodeheffer, Weighing in on Adipocyte Precursors. Cell Metabolism 19, 8–20 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee YH, Granneman JG, Seeking the source of adipocytes in adult white adipose tissues. Adipocyte 1, 230–236 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodeheffer MS, Birsoy K, Friedman JM, Identification of white adipocyte progenitor cells in vivo. Cell 135, (2008). [DOI] [PubMed] [Google Scholar]
- 9.Huang H et al. , BMP signaling pathway is required for commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proceedings of the National Academy of Sciences of the United States of America 106, 12670–12675 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Addison WN et al. , Direct Transcriptional Repression of Zfp423 by Zfp521 Mediates a Bone Morphogenic Protein-Dependent Osteoblast versus Adipocyte Lineage Commitment Switch. Molecular and Cellular Biology 34, 3076–3085 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bowers RR, Lane MD, A role for bone morphogenetic protein-4 in adipocyte development. Cell Cycle 6, 385–389 (2007). [DOI] [PubMed] [Google Scholar]
- 12.Hammarstedt A et al. , WISP2 regulates preadipocyte commitment and PPARγ activation by BMP4. Proceedings of the National Academy of Sciences of the United States of America 110, 2563–2568 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gupta RK et al. , Zfp423 expression identifies committed preadipocytes and localizes to adipose endothelial and perivascular cells. Cell Metab 15, 230–239 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shao M et al. , Zfp423 Maintains White Adipocyte Identity through Suppression of the Beige Cell Thermogenic Gene Program. Cell Metab 23, 1167–1184 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kiviranta R et al. , Coordinated transcriptional regulation of bone homeostasis by Ebf1 and Zfp521 in both mesenchymal and hematopoietic lineages. J Exp Med 210, 969–985 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang S et al. , Regulation of early adipose commitment by Zfp521. PLoS Biol 10, e1001433 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Griffin MJ et al. , Early B-cell factor-1 (EBF1) is a key regulator of metabolic and inflammatory signaling pathways in mature adipocytes. The Journal of biological chemistry 288, 35925–35939 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jimenez MA, Akerblad P, Sigvardsson M, Rosen ED, Critical role for Ebf1 and Ebf2 in the adipogenic transcriptional cascade. Mol Cell Biol 27, 743–757 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kilroy G, Kirk-Ballard H, Carter LE, Floyd ZE, The ubiquitin ligase Siah2 regulates PPARgamma activity in adipocytes. Endocrinology 153, 1206–1218 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kilroy G, Burk DH, Floyd ZE, Siah2 Protein Mediates Early Events in Commitment to an Adipogenic Pathway. The Journal of biological chemistry 291, 27289–27297 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kilroy G et al. , The ubiquitin ligase Siah2 regulates obesity-induced adipose tissue inflammation. Obesity (Silver Spring) 23, 2223–2232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Grant R, Youm Y-H, Ravussin A, Dixit VD, Quantification of Adipose Tissue Leukocytosis in Obesity. Methods in molecular biology (Clifton, N.J.) 1040, 195–209 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Habelhah H et al. , Stress-induced decrease in TRAF2 stability is mediated by Siah2. EMBO J 21, 5756–5765 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang QA, Tao C, Gupta RK, Scherer PE, Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med 19, 1338–1344 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polekhina G et al. , Siah ubiquitin ligase is structurally related to TRAF and modulates TNF-alpha signaling. Nat Struct Biol 9, 68–75 (2002). [DOI] [PubMed] [Google Scholar]
- 26.Tang W et al. , White fat progenitor cells reside in the adipose vasculature. Science (New York, N.Y.) 322, 583–586 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Metzger MB, Pruneda JN, Klevit RE, Weissman AM, RING-type E3 ligases: master manipulators of E2 ubiquitin-conjugating enzymes and ubiquitination. Biochim Biophys Acta 1843, 47–60 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hu G, Fearon ER, Siah-1 N-terminal RING domain is required for proteolysis function, and C-terminal sequences regulate oligomerization and binding to target proteins. Mol Cell Biol 19, 724–732 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Strissel KJ et al. , Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes 56, 2910–2918 (2007). [DOI] [PubMed] [Google Scholar]
- 30.Debels H et al. , Macrophages play a key role in angiogenesis and adipogenesis in a mouse tissue engineering model. Tissue Eng Part A 19, 2615–2625 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Angueira AR et al. , Early B Cell Factor Activity Controls Developmental and Adaptive Thermogenic Gene Programming in Adipocytes . Cell Rep 30, 2869–2878 e2864 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kilroy G et al. , The ubiquitin ligase Siah2 regulates obesity-induced adipose tissue inflammation. Obesity (Silver Spring, Md.) 23, 2223–2232 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Treiber T et al. , Early B cell factor 1 regulates B cell gene networks by activation, repression, and transcription- independent poising of chromatin. Immunity 32, 714–725 (2010). [DOI] [PubMed] [Google Scholar]
- 34.Wen YD et al. , The histone deacetylase-3 complex contains nuclear receptor corepressors. Proceedings of the National Academy of Sciences of the United States of America 97, 7202–7207 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zhang J, Guenther MG, Carthew RW, Lazar MA, Proteasomal regulation of nuclear receptor corepressor-mediated repression. Genes & development 12, 1775–1780 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Perissi V, Aggarwal A, Glass CK, Rose DW, Rosenfeld MG, A corepressor/coactivator exchange complex required for transcriptional activation by nuclear receptors and other regulated transcription factors. Cell 116, 511–526 (2004). [DOI] [PubMed] [Google Scholar]
- 37.Yu C et al. , The nuclear receptor corepressors NCoR and SMRT decrease peroxisome proliferator-activated receptor gamma transcriptional activity and repress 3T3-L1 adipogenesis. The Journal of biological chemistry 280, 13600–13605 (2005). [DOI] [PubMed] [Google Scholar]
- 38.Zhao HL, Ueki N, Hayman MJ, The Ski protein negatively regulates Siah2-mediated HDAC3 degradation. Biochemical and biophysical research communications 399, 623–628 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhang Y et al. , PIWIL2 suppresses Siah2-mediated degradation of HDAC3 and facilitates CK2α-mediated HDAC3 phosphorylation. Cell Death & Disease 9, 423 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hesse E et al. , Zfp521 controls bone mass by HDAC3-dependent attenuation of Runx2 activity. The Journal of Cell Biology 191, 1271 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang J, Kalkum M, Chait BT, Roeder RG, The N-CoR-HDAC3 nuclear receptor corepressor complex inhibits the JNK pathway through the integral subunit GPS2. Molecular cell 9, 611–623 (2002). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.