Abstract
In chemical exchange saturation transfer (CEST) imaging, the signal at 2.6 ppm from the water resonance in muscle has been assigned to phosphocreatine (PCr). However, this signal has limited specificity for PCr since the signal is also sensitive to exchange with protein and macromolecular protons when using some conventional quantification methods, and will vary with changes in the water longitudinal relaxation rate. Correcting for these effects while maintaining reasonable acquisition times is challenging. As an alternative approach to overcome these problems, here we evaluate chemical exchange rotation transfer (CERT) imaging of PCr in muscle at 9.4T. Specifically, the CERT metric AREXdouble,cpw at 2.6 ppm was measured in solutions containing the main muscle metabolites, in tissue homogenates with controlled PCr content, and in vivo in rat leg muscles. PCr dominates CERT metrics around 2.6 ppm (though with non-trivial confounding baseline contributions), indicating CERT is well-suited to PCr specific imaging, and has the added benefit of requiring a relatively small number of acquisitions.
Keywords: CEST, CERT, muscle, phosphocreatine, creatine
INTRODUCTION
Phosphocreatine (PCr) and creatine (Cr) are two major metabolites of the creatine kinase (CK) reaction that play vital roles in muscle energetics (1,2). PCr serves as a mobile energy reserve. During the CK reaction, these two molecules convert:
| (1) |
Measurements of both PCr and Cr are essential for evaluating the CK reaction and for assessing metabolism in muscle. While proton magnetic resonance spectroscopy (1H MRS) has been used to measure total Cr, it cannot differentiate between PCr and Cr because they resonate at almost the same frequency (3). Thus proton spectroscopy has a limited ability to measure energy metabolism. Phosphorus-31 (31P) MRS has been used to measure [PCr], but suffers from low spatial resolution and sensitivity (4).
Chemical exchange saturation transfer (CEST) is a contrast mechanism which applies long, frequency selective RF pulses to saturate protons in exchanging pools and detect subsequent changes in water signals. Due to the accumulated effects of the exchange, CEST provides an indirect measurement of solutes with significantly enhanced sensitivity compared to direct detection via conventional magnetic resonance spectroscopy (MRS). In addition, although Cr and PCr have similar resonance frequency offsets at around 3 ppm from tetramethylsilane (TMS) in MRS spectra (which measure non-exchanging carbon-bound protons), they show quite different resonance frequency offsets in CEST spectra (which indirectly measure, and hence have the resonance frequencies of, exchanging amine protons), with Cr at around 2 ppm and PCr at both 2 ppm and 2.6 ppm from the water line. Thus the peak at 2.6 ppm potentially provides a way to distinguish signal contributions from these two molecules. Previously, CEST has been used for high-sensitivity Cr imaging in muscle (5-7). More recently, CEST and CEST variants have been used for PCr imaging by us (8) and others (9).
There are multiple approaches for CEST imaging of Cr and PCr. In some previous CEST creatine imaging studies, a magnetization transfer ratio with asymmetric analysis (MTRasym) has been used to quantify CEST signals (5,6). Although this method requires only three sampling points and is very simple, the results are influenced by semi-solid magnetization transfer (MT) asymmetry effects and relayed nuclear overhauser enhancement (rNOE) effects originating from the other side of the water peak. In addition, asymmetry approaches typically require significant additional data acquisitions to correct for B0 inhomogeneities (10). An alternative multiple-pool Lorentzian fit has been used to quantify the CEST signals from Cr and PCr (7,9), but its application is limited by the long acquisition time necessary to obtain the entire Z-spectrum. In this work, we apply our previously reported CEST variant, chemical exchange rotation transfer (CERT) imaging (11-13), to quantify CEST signals from PCr around 2.6 ppm in muscle. In addition, since components of the CEST and CERT signals do not add linearly, the direct subtraction of label and reference signals employed in previous methods can only remove 0th-order effects, but not higher order effects (14). Hence, we combine CERT with an inverse analysis method based on the previously published apparent exchange-dependent relaxation (AREX) approach (15) to further increase the specificity of PCr imaging. Our goal is to examine the specificity and sensitivity of this CERT measure of PCr when using as few as three data points.
MATERIALS AND METHODS
CEST methods and metrics
The conventional CEST metric MTRasym is the difference of CEST signals acquired at the solute resonance and the opposite side of the water line:
| (2) |
where (−) represents the offset of the exchanging species and (+) is the offset on the opposite side of the water peak. S0 is the signal acquired in the non-irradiated control case.
CERT is a CEST variant that detects exchanging solutes via their selective rotation by RF pulses. Most commonly CERT signals are acquired using trains of pulses with irradiation flip angles (θ) of π and 2π, while keeping the RF frequency offset and average irradiation power (Bavg power) constant (11-13,16). Eq. (3) shows the definition of a particular CERT metric which uses a constant pulse width (cpw):
| (3) |
The duty cycle (dc) is varied to maintain constant pulse width (pw). Here dc is set to be 0.18 for 2π irradiation and 0.72 for π irradiation so that the pulse widths for the two irradiations are both 13 ms with an average irradiation power of 1 μT.
To further increase specificity, we combined the CERT and AREX approaches to define a new metric:
| (4) |
where R1obs (=1 / T1obs) is the observed water longitudinal relaxation rate. By taking the difference of inverted signals, the AREX approach corrects for non-linear contributions to the measured signal (17). Further, by scaling the metric by R1obs, AREX accounts for the effect of water relaxation rate changes on the measured signal after irradiating at the solute resonance. Finally, direct water and macromolecular saturation effects are cancelled by using the same average irradiation power and offset when applying pulse trains of π pulses or train 2π pulses.
In vitro validation
To study the ability of the CERT approach to quantify [PCr], 5 solutions with metabolites at the high end of their physiological concentrations (20 mM Adenosine triphosphate ATP, 50 mM creatine Cre, 50 mM phosphocreatine PCr, 300 mM Glycogen, and 50 mM Lactate) were prepared in 1× (phosphate buffered saline) PBS and titrated to pH of 7.0. Ex vivo tissue samples were prepared to evaluate the ability of CERT to quantify the PCr. Specifically, tissue homogenates were prepared by removing whole rat brains from freshly sacrificed rats (18,19). The intact brain tissue was weighed and washed quickly in buffer composed of 70 mM HEPES with 70mM KCl. After addition of 4 times CK buffer (w/w) (45 μl D2O + 22.5 μl HEPES + 22.5 μl KCl + 5 μl MgCl2 + 5 μl N-Acetyl-L-Cysteine (NAC)), the tissues were homogenized, and centrifuged. The supernatants were divided into two samples. Sample #1 was used as a control; PCr powder was added to sample #2 to reach 10 mM.
Animal experiments
Five healthy rats were immobilized and anesthetized with a 2%/98% isoflurane/oxygen mixture. Respiration was monitored and a constant rectal temperature of 37°C was maintained throughout the experiments using a warm-air feedback system (SA Instruments, Stony Brook, NY). All procedures were approved by the Animal Care and Usage Committee of Vanderbilt University.
MRI
All measurements on animals and samples were performed on a Varian DirectDrive™ horizontal 9.4T magnet with a 38-mm Litz RF coil (Doty Scientific Inc. Columbia, SC) at 37°C. Continuous wave (CW) and pulsed Z-spectra were acquired with RF offsets from −2000 Hz to 2000 Hz (−5 ppm to 5 ppm at 9.4 T) with an interval of 50 Hz (0.125 ppm at 9.4 T). A control scan was performed to acquire S0 by setting the RF offset to 100,000 Hz. For Z-spectra studies on solutions and tissue samples, the first points of each free induction decay (FID) were used for data analysis. For Z-spectra from in vivo rat imaging, a single-shot SE-EPI sequence was employed using a FOV of 30 × 30 mm2, a slice thickness of 2 mm, a matrix of 64 × 64, a receiver bandwidth of 250 kHz, and one acquisition. Bavg power (the square root of the mean of the square of the field amplitude) was 1 μT for all irradiations, based on our previous experience isolating the effects of slower-exchanging protons from those of intermediate and fast-exchanging protons in Cr and proteins, along with direct and MT effects (12,20). The total irradiation duration was 5s for animal experiments and 8s for sample experiments performed before acquisition with a 2s delay time before the next acquisition. CW CEST signals were acquired with a long rectangular pulse. CERT signals were acquired using a pulsed-CEST sequence with a series of Gaussian RF irradiation pulses followed by acquisition (11-13). R1obs for animal experiments was obtained using a selective inversion recovery (SIR) sequence (21). R1obs for samples was obtained using an inversion recovery fast spin-echo (FSE) sequence.
RESULTS
In order to determine the sensitivity and, more importantly, the specificity of CERT approaches to imaging PCr, we acquired data from chemical phantoms, tissue homogenates with controlled PCr content, and in vivo rat leg muscle. These samples provide a means for examining the signal specificity under increasing complexity, from 1) simple known molecular content, to 2) complex molecular content but with no large background macromolecular contributions and with controlled PCr content, and finally to 3) the full in vivo case.
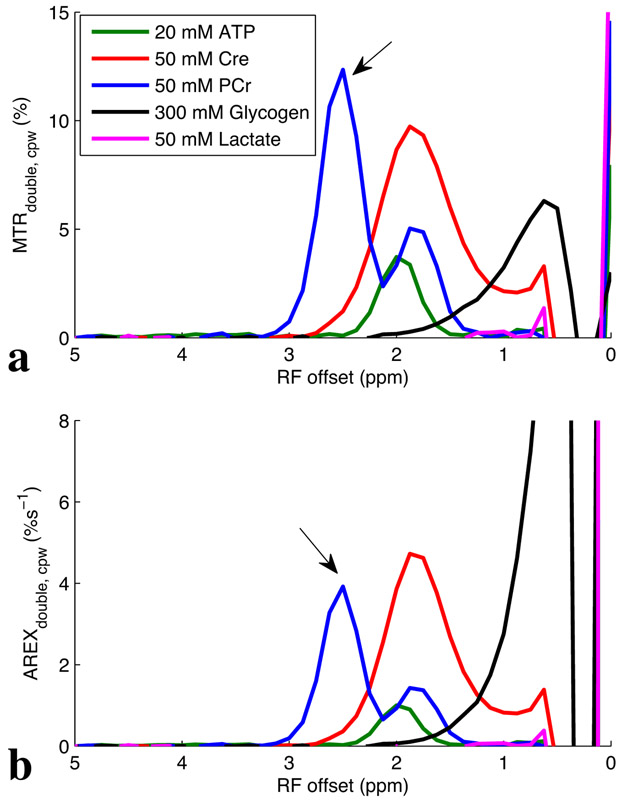
Fig. 1 shows the experimental MTRdouble,cpw and AREXdouble,cpw spectra from solutions containing the main metabolites in muscle. MTRdouble,cpw and AREXdouble,cpw at 2.6 ppm are dominated by contributions from PCr, while MTRdouble,cpw and AREXdouble,cpw at 2 ppm have sizable contributions from Cr, PCr, and ATP. The results in vivo will depend on the corresponding metabolic concentrations and may not match our phantoms, which were taken on the high end of physiological ranges.
Fig. 1:
Measured MTRdouble,cpw and AREXdouble,cpw spectra from solutions containing the main metabolites in muscle. T1obs are around 4 s for all samples.
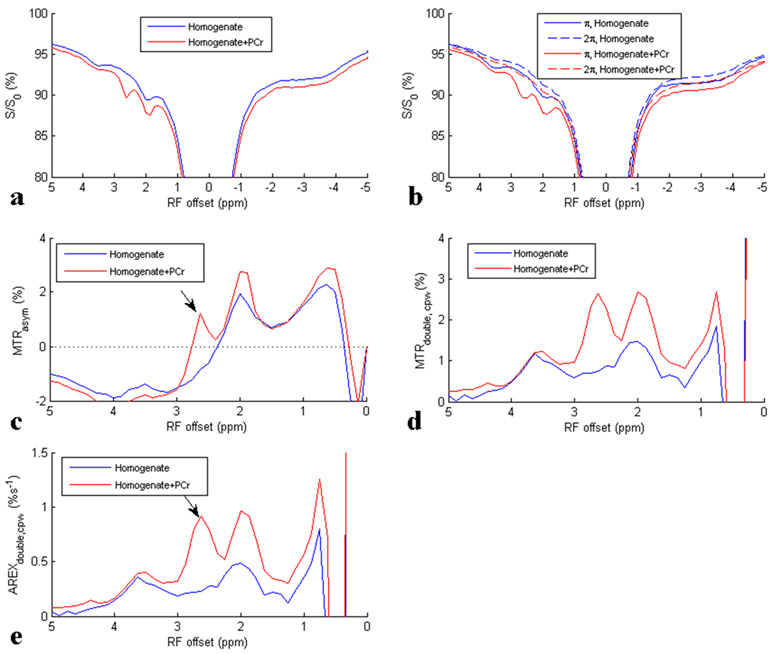
To further study the ability of CEST and CERT to detect PCr, we performed CEST experiments on tissue homogenate and tissue homogenate + PCr. Results are shown in Fig. 2. We used rat brain as the source for the homogenate, rather than muscle, to start with very low concentrations of PCr as our baseline condition (22). After adding PCr to the homogenate, the CEST effect at 2.6 ppm increased, confirming the PCr basis for the CEST peak at 2.6 ppm. Also note that although MTRasym shows a negative sloping baseline in Fig. 2c, MTRdouble,cpw in Fig. 2d and AREXdouble,cpw in Fig. 2e show reduced and flatter baseline, suggesting that CERT is effectively more specific, or at least has a simpler relationship to PCr than the conventional asymmetric analysis.
Fig. 2:
CW Z-spectra (a), CERT Z-spectra (b), MTRasym spectra using CW CEST data (c), MTRdouble, cpw spectra (d), and AREXdouble, cpw spectra (e) for sample 1 homogenate, and sample 2 homogenate + PCr. Arrows indicate the CEST signals from PCr at around 2.6ppm. T1obs are 3.7 s and 3.5 s for sample 1 and sample 2, respectively. Note that the 2π spectra in figure (b) serve as baselines with no rotation-exchange effects in the CERT approach.
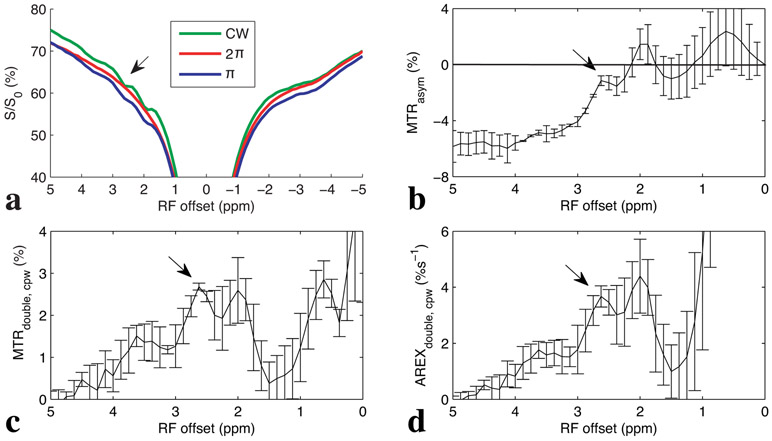
Fig. 3 shows the experimental CW and pulsed Z-spectra, along with the resulting MTRasym, MTRdouble,cpw, and AREXdouble,cpw spectra from five healthy rat legs. Note the negative MTRasym values at farther offsets from water, indicating possible confounding contributions from asymmetric MT or nuclear Overhauser enhancement (NOE) effects originating from the other side of the water peak. These effects cause a sloping negative baseline signal, making interpretation difficult. In contrast, the MTRdouble,cpw and AREXdouble,cpw metrics specifically quantify the CEST signals from PCr without large unwanted baseline distortions, though there is still the possibility of non-specific signal contributions at both 2 and 2.6 ppm. Fig. 4 shows corresponding maps of anatomy, and AREXdouble,cpw maps at 2 ppm and 2.6 ppm.
Fig. 3:
Experimental CW and CERTcpw Z-spectra (a), MTRasym spectrum (b), MTRdouble,cpw spectrum (c), and AREXdouble,cpw spectrum (d) from rat legs (N=5). Arrows indicate the CEST signals from PCr at around 2.6ppm. T1obs is 2 ± 0.07 s.
Fig. 4:
Maps of tissue anatomy (a), AREXdouble, cpw at 2 ppm (b), and AREXdouble, cpw at 2.6 ppm (c) from a representative healthy rat leg. ROI was drawn from region where shimming is good (the root mean square deviation of B0 field map was < 60 Hz). Anatomy image was from the control scan in the CEST experiment, and was cropped to match the PCr image and to avoid an aliasing artifact at the edge of the image.
DISCUSSION
MTRasym has been widely used to isolate the CEST signal from direct water saturation (DS) and semi-solid MT effects. While MTRasym has the benefit of being a simple metric for quantification, it is sensitive to confounding influences from upfield rNOE effects and/or semi-solid macromolecular asymmetry, creating a negative sloping baseline, that be seen in the current work (figure 2c). In this case, the contribution of PCr to the homogenate + PCr MTRasym values would be difficult to isolate without comparison to the values in homogenate. Such a tissue-comparison approach has been applied in the CEST literature (e.g. (23)) due to the difficulty of separating the targeted signal from the negative sloping baseline. Note, however, that the need for such comparisons and the relative contributions of confounding signals depends strongly on the pulse sequence parameters. For example, one approach to Cr imaging uses MTRasym with higher-power and shorter duration irradiations than the current work in order to use exchange-rate filtering to increase specificity (6).
The CERT results (Figs. 2d, 2e, 3c and 3d) show positive MTRdouble,cpw or AREXdouble,cpw signals at 2.6 ppm and appear to have a baseline signal flatter and closer to zero, though this assessment is somewhat arbitrary and inexact. A benefit of this diminished and simpler baseline is the viability of acquiring data only at the offset of interest, in this case 2.6 ppm, because fitting the baseline dependence on frequency is not necessary, or at least less necessary. (A separate R1obs measure is still necessary to calculate the AREX metric.) In contrast, MTRasym or raw Z-spectra analysis typically uses data acquired at many frequency offsets to correct for baseline effects. For example, a three-point method, which uses the average of two nearby signals as reference, has been previously used to quantify amides at 3.5 ppm in brain (24,25). However, this method is unlikely to accurately quantify the CEST signal at 2.6 ppm because it is close to the nearby amides at 3.5 ppm and amines at 2 ppm, and we know of no attempts to quantify PCr using such an approach. There has been a recent study that used a multiple pool Lorentizian fitting of the Z-spectrum to image PCr (9). Note that this study fit the data using 78 frequency offsets and did so at 11.7 T, the very high field facilitating fitting offset dependent models. In contrast, the AREXdouble, cpw metric uses a single offset and no signal modeling.
Proteins may also produce significant CEST signals from amides, amines, and hydroxyls (18,19,26,27). Our previous studies on bovine serum albumin (BSA) and egg white albumin (EWA) indicate that the guanidino amine of proteins may have significant contributions to the CEST signal at 2 ppm, but no significant contributions at around 2.6 ppm (13,18). In addition, although fast exchanging amines have broad peaks which may influence the CEST signals at 2.6 ppm, their CEST effects can be mostly removed in CERT due to its exchange filter effect (11,13). This suggests that proteins in muscle make negligible contributions to CERT PCr imaging. This paper thus shows that CERT may offer a viable approach to PCr imaging and a means to study the CK reaction. However, some aromatic and amide protons between 1.5 ppm and 4 ppm, which have slow exchange rates, may affect the CERT signal at 2.6 ppm. This issue of spectral selectivity is worse at clinical field strengths, where distinct solute peaks are difficult, though not impossible (28), to discern. On the plus side, CERT methods avoid contributions from the rNOE at −3.5 ppm, which is on the other side of the water peak and can thus confound CEST metrics based on asymmetry, such as MTRasym.
While we have emphasized the benefits of a small and flat baseline using the CERT approach, this baseline is not zero. Results in phantoms can give a very rough estimate of the size of this confounding factor in vivo. In Fig. 1b, the AREXdouble,cpw signal at 2.6 ppm from PCr is 4.9%s−1, suggesting 0.1% s−1 per 1mM PCr. In Fig. 2e, the subtraction of AREXdouble,cpw signal at 2.6 ppm between the homogenate and homogenate+Pcr is 0.7%s−1, suggesting 0.07%s−1 per 1mM PCr. Note that the two calibrations from a simple sample and tissue homogenate are comparable. Using 0.07%s−1 per 1mM PCr to calibrate the rat leg results at 2.6 ppm in Fig. 3d gives 52.4 mM PCr in rat legs. Typical in vivo PCr mM concentrations are in the mid-thirties, suggesting that a confounding baseline of roughly 1/3 the signal value at 2.6 ppm, which seems visually reasonable looking at Fig. 3d. A more precise estimation of the baseline, or a more specific measure, requires non-trivial quantitative tissue modelling and parameter fitting. This would likely require longer data acquisitions and is beyond the scope of this work, which is focused on the utility of a rapidly-acquired model-free metric.
There are two notes of caution in overinterpreting the homogenate results. First, we used tissue homogenates from rat brain to evaluate the CEST signal in muscle due to the difficulty making homogenates from muscle fibers. Although the tissue homogenates in muscle and brain are different, both of them have rNOE and asymmetric MT effects and can be used to evaluate the ability of CERT to remove the influence from the resulting slope in the baseline that confounds conventional asymmetric analysis. Second, any assessment of the sensitivity of CEST or CERT metrics to changes in solute concentrations is possibly confounded by similar dependencies on changes in exchange rates, which are related to variations in tissue pH and/or temperature. Quantitative fittings can avoid this co-dependency, but require assumptions about tissue models.
The AREXdouble, cpw metric can be acquired in as little as 3 points, though this does not include the required measurement of R1obs or correct for any possible B0 and B1 inhomogeneities. However, in situations where R1obs variations are negligible, R1obs normalization may not be essential. We previously showed little B0 and B1 dependence when using a similar CERT metric applied to the amide resonance at 3.5ppm (11), but it is still an open question for the current PCr study. Supporting Figure S1 show MTRasym, MTRdouble, cpw, and AREXdouble, cpw spectra from the shimmed area and the non-shimmed area. Much bigger standard deviations from the non-shimmed area than that from the shimmed area were found in the spectrum of MTRasym, but not MTRdouble, cpw, and AREXdouble, cpw. In summary, while the proposed CERT approach shows promise in imaging PCr, especially in producing a relatively flat and small confounding baseline signal, there are still several unresolved issues, including robustness (under field inhomogeneities and tissue variations), separation of concentration and exchange effects, and viability under clinical field strengths and acquisition times. The current study demonstrates PCr sensitivity in phantoms and healthy muscle, but we have not shown spatial correlation vs gold standard or independence from tissue state under pathologies. Given its low signal-to-noise, such validations using 31P will be challenging.
CONCLUSION
AREXdouble,cpw at 2.6 ppm can produce reasonably specific PCr imaging using three acquisitons, though with non-negligible baseline signal contributions and with separate measurement of R1obs.
Supplementary Material
Supporting Figure S1. MTRasym spectrum (a), MTRdouble,cpw spectrum (b), and AREXdouble,cpw spectrum (c) from shimmed area (blue) and non-shimmed area (red) on rat legs (N=5). Arrows indicate the CEST signals from PCr at around 2.6ppm. Note that the standard deviations, especially those close to water peak, are relatively bigger in the non-shimmed area than that in the shimmed area.
Acknowledgments
Grant Sponsor: NIH/NIBIB R01 EB017767, NIH/NIBIB R01 EB024525, NIH/NCI R01 CA184693, NIH/NCI R01 CA109106, and NIH/NIAMS R21 AR074261
Abbreviations used
- CEST
chemical exchange saturation transfer
- CERT
chemical exchange rotation transfer
- MT
magnetization transfer
- AREX
apparent exchange-dependent relaxation
- cpw
constant pulse width
- pw
pulse width
- dc
duty cycle
- CW
Continuous wave
- Bavg power
average irradiation power
- MTRdouble, cpw
CERT metric using a constant pulse width
- AREXdouble, cpw
CERT metric using AREX and a constant pulse width
- MTRasym
magnetization transfer ratio with asymmetric analysis
- 1H MRS
proton magnetic resonance spectroscopy
- 31P MRS
phosphorus magnetic resonance spectroscopy
- FID
Free induction decay
- SIR
Selective inversion recovery
- FSE
Fast spin-echo
- PCr
phosphocreatine
- Cr
creatine
- CK
creatine kinas
- TMS
Tetramethylsilane
- ATP
Adenosine triphosphate
REFERENCE
- 1.Chance B, Williams GR. Respiratory Enzymes in Oxidative Phosphorylation .3. The Steady State. Journal of Biological Chemistry 1955;217(1):409–427. [PubMed] [Google Scholar]
- 2.Davies RE. A Molecular Theory of Muscle Contraction - Calcium-Dependent Contractions with Hydrogen Bond Formation Plus Atp-Dependent Extensions of Part of Myosin-Actin Cross-Bridges. Nature 1963;199(489):1068-&. [DOI] [PubMed] [Google Scholar]
- 3.Bottomley PA, Lee YH, Weiss RG. Total creatine in muscle: Imaging and quantification with proton MR spectroscopy. Radiology 1997;204(2):403–410. [DOI] [PubMed] [Google Scholar]
- 4.Moon RB, Richards JH. Determination of Intracellular Ph by P-31 Magnetic-Resonance. Journal of Biological Chemistry 1973;248(20):7276–7278. [PubMed] [Google Scholar]
- 5.Haris M, Singh A, Cai KJ, Kogan F, McGarvey J, DeBrosse C, Zsido GA, Witschey WRT, Koomalsingh K, Pilla JJ, Chirinos JA, Ferrari VA, Gorman JH, Hariharan H, Gorman RC, Reddy R. A technique for in vivo mapping of myocardial creatine kinase metabolism. Nature medicine 2014;20(2):209–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kogan F, Haris M, Debrosse C, Singh A, Nanga RP, Cai KJ, Hariharan H, Reddy R. In Vivo Chemical Exchange Saturation Transfer Imaging of Creatine (CrCEST) in Skeletal Muscle at 3T. Journal Of Magnetic Resonance Imaging 2014;40(3):596–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rerich E, Zaiss M, Korzowski A, Ladd ME, Bachert P. Relaxation-compensated CEST-MRI at 7T for mapping of creatine content and pH - preliminary application in human muscle tissue in vivo. NMR in biomedicine 2015;28(11):1402–1412. [DOI] [PubMed] [Google Scholar]
- 8.Zu Z, Lin E, Louie E, Jiang X, Lankford CL, Damon BM, Does MD, Gore JC, Gochberg DF. Chemical exchange rotation transfer imaging of phosphocreatine in muscle. In: Proceedings of the 26th Annual Meeting of ISMRM, Paris, France 2018:5106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen L, Barker PB, Weiss RG, Van Zijl P, Xu JD. Creatine and phosphocreatine mapping of mouse skeletal muscle by a polynomial and Lorentzian line-shape fitting CEST method. Magn Reson Med 2018:DOI: 10.1002/mrm.27514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim M, Gillen J, Landman BA, Zhou J, van Zijl PC. Water saturation shift referencing (WASSR) for chemical exchange saturation transfer (CEST) experiments. Magnetic Resonance in Medicine 2009;61(6):1441–1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zu ZL, Janve VA, Xu JZ, Does MD, Gore JC, Gochberg DF. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magn Reson Med 2013;69(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zu ZL, Xu JZ, Li H, Chekmenev EY, Quarles CC, Does MD, Gore JC, Gochberg DF. Imaging Amide Proton Transfer and Nuclear Overhauser Enhancement Using Chemical Exchange Rotation Transfer (CERT). Magnetic Resonance In Medicine 2014;72(2):471–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zu ZL, Louie EA, Lin EC, Jiang XY, Does MD, Gore JC, Gochberg DF. Chemical exchange rotation transfer imaging of intermediate-exchanging amines at 2 ppm. Nmr in Biomedicine 2017:DOI: 10.1002/nbm.3756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zu ZL. Towards the complex dependence of MTRasym on T-1w in amide proton transfer (APT) imaging. NMR in biomedicine 2018;31(7). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zaiss M, Bachert P. Exchange-dependent relaxation in the rotating frame for slow and intermediate exchange - modeling off-resonant spin-lock and chemical exchange saturation transfer. NMR in biomedicine 2013;26(5):507–518. [DOI] [PubMed] [Google Scholar]
- 16.Zu Z, Li K, Janve VA, Does MD, Gochberg DF. Optimizing pulsed-chemical exchange saturation transfer imaging sequences. Magnetic Resonance in Medicine 2011;66(4):1100–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zaiss M, Bachert P. Chemical exchange saturation transfer (CEST) and MR Z-spectroscopy in vivo: a review of theoretical approaches and methods. Phys Med Biol 2013;58(22):R221–R269. [DOI] [PubMed] [Google Scholar]
- 18.Zhang XY, Xie JP, Wang F, Lin EC, Xu JZ, Gochberg DF, Gore JC, Zu ZL. Assignment of the molecular origins of CEST signals at 2ppm in rat brain. Magnetic Resonance In Medicine 2017;78(3):881–887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cui J, Zu ZL. Towards the molecular origin of glutamate CEST (GluCEST) imaging in rat brain. Magnetic Resonance In Medicine 2019. [DOI] [PubMed] [Google Scholar]
- 20.Zu Z, Janve VA, Xu J, Does MD, Gore JC, Gochberg DF. A new method for detecting exchanging amide protons using chemical exchange rotation transfer. Magnetic Resonance in Medicine 2013;69(3):637–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gochberg DF, Gore JC. Quantitative magnetization transfer imaging via selective inversion recovery with short repetition times. Magnetic Resonance in Medicine 2007;57(2):437–441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hetherington HP, Spencer DD, Vaughan JT, Pan JW. Quantitative P-31 spectroscopic imaging of human brain at 4 tesla: Assessment of gray and white matter differences of phosphocreatine and ATP. Magn Reson Med 2001;45(1):46–52. [DOI] [PubMed] [Google Scholar]
- 23.Zhou JY, Hong XH, Zhao XN, Gao JH, Yuan J. APT-weighted and NOE-weighted image contrasts in glioma with different RF saturation powers based on magnetization transfer ratio asymmetry analyses. Magn Reson Med 2013;70(2):320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jin T, Wang P, Zong XP, Kim SG. MR imaging of the amide-proton transfer effect and the pH-insensitive nuclear overhauser effect at 9.4 T. Magnetic Resonance in Medicine 2013;69(3):760–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu JZ, Zaiss M, Zu ZL, Li H, Xie JP, Gochberg DF, Bachert P, Gore JC. On the origins of chemical exchange saturation transfer ( CEST) contrast in tumors at 9.4 T. Nmr Biomed 2014;27(4):406–416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jin T, Kim SG. High field MR imaging of proteins and peptides based on the amine-water proton exchange effect. ISMRM 2012:2339. [Google Scholar]
- 27.Chen L, Zeng HF, Xu X, Yadav NN, Cai SH, Puts NA, Barker PB, Li T, Weiss RG, Van Zijl PC, Xu JD. Investigation of the contribution of total creatine to the CEST Z-spectrum of brain using a knockout mouse model. Nmr in Biomedicine 2017:DOI: 10.1002/nbm.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lin EC, Li H, Zu ZL, Louie EA, Lankford CL, Dortch RD, Does MD, Gore JC, Gochberg DF. Chemical exchange rotation transfer (CERT) on human brain at 3 Tesla. Magnetic Resonance In Medicine 2018;80(6):2609–2617. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Figure S1. MTRasym spectrum (a), MTRdouble,cpw spectrum (b), and AREXdouble,cpw spectrum (c) from shimmed area (blue) and non-shimmed area (red) on rat legs (N=5). Arrows indicate the CEST signals from PCr at around 2.6ppm. Note that the standard deviations, especially those close to water peak, are relatively bigger in the non-shimmed area than that in the shimmed area.