To the Editor:
Primary testicular lymphoma (PTL) and testicular involvement of systemic lymphoma (secondary testicular lymphoma, STL) are uncommon1,2. Most testicular lymphomas are aggressive B-cell lymphomas with the most common histologic type being diffuse large B-cell lymphoma (DLBCL) and most common cell of origin being activated B-cell like phenotypes1. PTL is generally ascribed to cases where testis are the primary or main site of lymphoma involvement, but has also been defined more restrictively as testicular involvement without lymph node or bone marrow involvement2. Lymphomas with testicular involvement portend a high risk of extranodal relapse, including CNS relapse and evaluation often includes a diagnostic lumbar puncture while treatment is typically intensified to include CNS prophylactic therapy such as intrathecal chemotherapy or systemic methotrexate1,2. Determination of testicular involvement at diagnosis is therefore essential for appropriate evaluation and management of this aggressive disease.
F-18 fluorodeoxyglucose positron emission tomography/computed tomography (FDG-PET/CT) is a standard imaging modality to stage aggressive lymphoma. There is often visible uptake in the testes that raises concern with clinicians, but there is a lack of consensus data to determine whether the uptake is physiologic or pathologic. Physiologic testicular avidity on FDG-PET/CT has been described in a Japanese study, which reported a mean standard uptake value (SUV) of 2.44 +/− 0.45 (maximum SUV not reported) for all age groups based on FDG-PET/CT of 203 men 3 who underwent FDG-PET/CT for cancer diagnosis or cancer screening with no history of malignancy in the testis and no abnormal findings in the testis on CT images. While there are reports on normal testicular uptake on FDG-PET/CT, no clear SUV thresholds to raise suspicion for testicular involvement by lymphoma have been established. An Australian retrospective study reviewed 3,781 male patients with a diagnosis of lymphoma without a clinical suspicion of testicular involvement. 12 had abnormal testicular or scrotal FDG above that of background activity as determined visually and quantitatively by PET-accredited nuclear medicine physicians. Seven of these 12 patients were subsequently diagnosed with testicular involvement by lymphoma, of which 6 were aggressive lymphomas including DLBCL and Burkitt lymphoma with mean testicular SUVmax 10.2 (range 3.3 – 17.5) 4. This suggests a population of patients with unsuspected STL may be identified on FDG-PET/CT. However, no particular FDG cut off level was defined in that study. A testicular SUV threshold to alert for lymphoma involvement would be clinically relevant and help guide management. In this study, we compared testicular FDG avidity in patients with known testicular involvement by lymphoma with physiologic testicular FDG avidity from patients without known lymphoma.
Records of patients from Mayo Clinic diagnosed with PTL or STL from 2002–2015 enrolled in the University of Iowa/Mayo Clinic Lymphoma Specialized Programs of Research Excellence (SPORE P50 CA97274) Molecular Epidemiology Resource 5 were reviewed, yielding 10 pts with testicular lymphoma with intact testes at the time of staging FDG-PET/CT. Normal physiologic testicular avidity by FDG-PET/CT was determined from a subset analysis of our previously published normal value study of 350 male patients6 including 70 patients, 10 patients per decade ages 18 to 90 years, who received FDG-PET/CT from 2013–2018 prior to treatment for non-lymphoma indications (solitary pulmonary nodule, lung carcinoma, head & neck squamous cell carcinoma, gastrointestinal carcinomas, metastatic melanoma), without known hematologic malignancy, testicular pathology, or history of testicular infection/surgery by medical chart review. All FDG-PET/CT exams utilized a standard oncologic protocol with three-dimensional lutetium yttrium orthosilicate detectors (128×128 matrix, 70 cm field of view) acquired 60–70 minutes after intravenous injection of 370–555 MBq (10–15 mCi) 18F-FDG. PET reconstruction technique included standard three dimensional iterative reconstructions with ordered subset expectation maximization. Time-of-flight, point spread function (PSF), and Bayesian penalized likelihood iterative reconstruction data was excluded as these were not available for all patients. Acquisition time per PET bed position was 3–5 minute (BMI < 35 = 3 minutes, BMI 35–45 = 4 minutes, BMI >45 = 5 minutes). CT technique for attenuation correction and anatomic localization was non-enhanced, low dose with 16 to 128 detectors; ~90 mA, 0.5 s rotation time, ~ 45 mAs, 120 kVp, and 3.75–5.0 mm slice thickness. The patients fasted for >6 hours prior to the exam and had a blood glucose <200 mg/dL at time of exam. Images were reviewed by a board certified nuclear radiologist using a clinical picture archive and communication system. Imaging parameters collected included normal hepatic SUVmax and SUVmean for quality control along with bilateral testicular PET patterns (diffuse / focal / multifocal), SUVmax and SUVmean. Comparisons of SUVmax and SUVmean between testicular lymphoma and controls were assessed via plots and Wilcoxon rank-sum p-values.
Of the 10 pts with intact testes at the time of initial staging FDG-PET/CT, 1 patient with low-grade lymphoma was excluded. The median age in this analysis cohort was 62 years (range 35–88). The 70 control patients had a median age of 55 years (range 18–90). Among the 9 patients with aggressive lymphoma who had FDG-PET/CT scan prior to orchiectomy, there were 7 patients with diffuse large B cell lymphoma (DLBCL) and 1 patient each with Burkitt lymphoma and peripheral T-cell lymphoma, not otherwise specified. Of the 7 analyzed DLBCL patients, 6 were non-germinal center B-cell and 1 was germinal center B-cell. Testicular lymphoma was diagnosed by orchiectomy after PET scan in 3 patients and by percutaneous biopsy in 3 patients. The remaining 3 patients did not have tissue sampling of testes or scrotal contents, but had biopsy-proven lymphoma at another site (parotid gland, bone marrow, stomach, respectively) with scrotal ultrasound showing hypoechoic hyper-vascular testicular masses with overtly FDG-avid testicle highly suggestive of lymphomatous involvement (mean testicular SUVmax 17.2 among these 3 patients). With regards to timing of PET scan relative to biopsy in the percutaneously diagnosed patients, 1 with PTCL NOS and 1 with DLBCL, had testicular biopsies 1 week and 1 month, respectively, prior to FDG-PET/CT. An additional patient with DLBCL previously confirmed on pleural biopsy had right testicular biopsy 1 day prior to FDG-PET/CT, which showed increased avidity in testes bilaterally. Five patients had interim FDG-PET/CT with intact testes available for review.
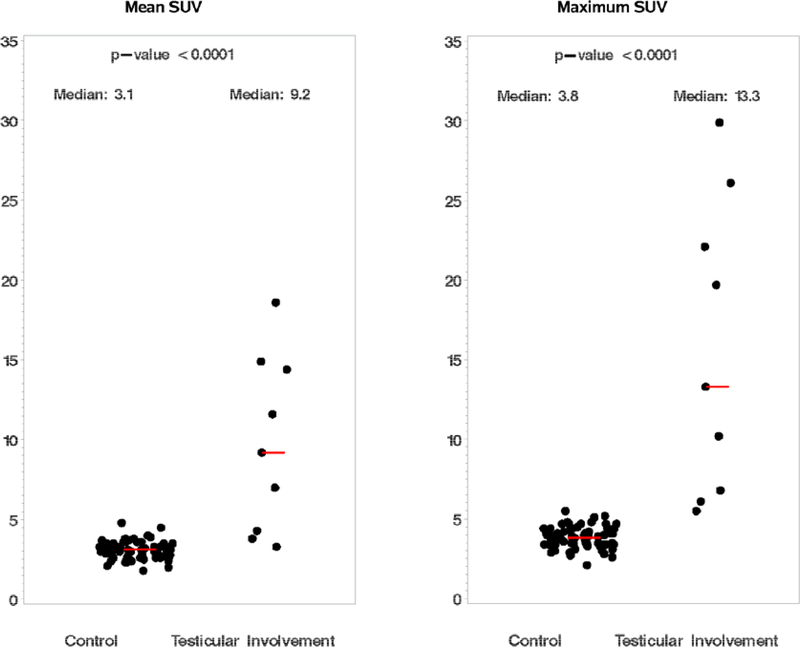
In known testicular lymphoma cases and control patients, SUVmax and SUVmean were assessed from the most FDG-avid testicle. Median SUVmax for the testicular lymphoma cases was 13.3 (range 5.5–29.9), compared to a median of 3.8 (range 2.1–5.5) for controls without lymphoma (Wilcoxon p <0.0001). Median SUVmean for testicular lymphoma was 9.2 (range 3.3–18.6), compared to a median of 3.1 (range 1.8–4.8) for controls (Wilcoxon p <0.0001) [Figure 1]. The SUVmax of previously affected testes normalized to median of 2.5 (range 1.8–4.0) by interim PET scan. Based on this dataset, an SUVmax cutoff of 5.0 has a sensitivity of 100% (95% CI 66.3–100) and a specificity of 98.6% (98% CI 92.3–99.9) for the detection of testicular involvement by an aggressive lymphoma.
Figure 1.
FDG-PET/CT results for cases of aggressive testicular lymphoma (n = 9) vs. controls without known testicular pathology (n = 70): SUVmean (left), and SUVmax (right). Each dot represents a testicular lymphoma or control case. Median values for SUVmean and SUVmax shown in red lines.
The results of this study suggest that FDG-PET/CT shows greater FDG avidity for testicular aggressive lymphomas as compared with normal physiologic testicular FDG uptake. Testicular FDG avidity greater than an SUVmax of 5.0 during initial staging of aggressive lymphoma may be suggestive of testicular involvement and warrants a clinical exam and testicular ultrasound.
In the setting of a rare disease, a limitation of this study is the small sample size as most patients with testicular involvement of lymphoma under orchiectomy prior to FDG-PET/CT. Larger pooled studies are necessary to validate these findings and further define the SUV cut-off values. Another limitation is that 3 patients had testicular biopsies within a week prior to staging PET/CT scan, which may have altered FDG uptake. However, the typical linear pattern of post-biopsy uptake was not identified in these patients, but rather the abnormal FDG uptake was either very intense, focal and round, or diffusely involving the testicle. The SUV values we report may not be comparable between different institutions and may drift with the evolution of more advanced PET detectors and reconstruction methods.
Unique strengths of this study include cases from a defined cohort study, centralized radiology review of cases and 70 controls, and the direct relevance of the findings to clinical practice. In summary, a testicle with SUVmax >5.0 on FDG-PET/CT represents a reasonable starting point to establish concern for testicular involvement by lymphoma and should prompt further work-up, including clinical exam, ultrasound of the scrotum, and possibly central nervous system evaluation as part of staging. In addition, testicles with SUVmax <5.0 in a patient with known aggressive lymphoma and no symptoms or signs of testicular involvement are less suspicious for lymphomatous involvement.
Acknowledgments:
HK and GT designed the research study. HK, AH, LKH, and JRY performed the research and clinical data extraction. GT, HK, AH, and MJM analyzed the data. HK and AH wrote the manuscript. AH, HK, LKH, TMH, GSN, CAT, PBJ, TEW, CA, MJM, JRC, JRY, and GT contributed to the interpretation of the data and revised the paper critically. All authors approved the submitted version.
Funding: This work was supported by the National Institutes of Health (National Institutes of Health Lymphoma SPORE [P50 CA97274]) and the Mayo Clinic Center for Clinical and Translational Science, KL2 Mentored Career Development Award, funded by National Center for Advancing Translational Sciences (KL2 TR002379).
Footnotes
Disclosures: None
Data availability:
Available upon request to corresponding author.
References
- 1.Cheah CY, Wirth A, Seymour JF. Primary testicular lymphoma. Blood 2014;123:486–93. [DOI] [PubMed] [Google Scholar]
- 2.Ollila TA, Olszewski AJ. Extranodal Diffuse Large B Cell Lymphoma: Molecular Features, Prognosis, and Risk of Central Nervous System Recurrence. Curr Treat Options Oncol 2018;19:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kitajima K, Nakamoto Y, Senda M, Onishi Y, Okizuka H, Sugimura K. Normal uptake of 18F-FDG in the testis: an assessment by PET/CT. Ann Nucl Med 2007;21:405–10. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu P, Lin P, Son H, Rosenfeld D, Lin M. Testicular fluorine-18 fludeoxyglucose uptake on positron emission tomography CT in patients with lymphoma: clinical significance and management impact. Br J Radiol 2014;87:20140472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerhan JR, Link BK, Habermann TM, et al. Cohort Profile: The Lymphoma Specialized Program of Research Excellence (SPORE) Molecular Epidemiology Resource (MER) Cohort Study. Int J Epidemiol 2017;46:1753–4i. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Harper LK, Simmons C, Nathan M, Thanarajasingam G, Kim H, Young J. Establishing Normal Testicular F-18 FDG PET-CT Standard Uptake Values American Journal of Roentgenology (in press). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Available upon request to corresponding author.