Abstract
Aims:
To assess the intervention effects of BREATHE (BRief intervention to Evaluate Asthma THErapy), a novel brief shared decision-making intervention and evaluate feasibility and acceptability of intervention procedures.
Design:
Group-randomized longitudinal pilot study.
Methods:
80 adults with uncontrolled persistent asthma participated in a trial comparing BREATHE (N=40) to a dose-matched attention control intervention (N=40). BREATHE is a one-time shared decision-making intervention delivered by clinicians during routine office visits. Ten clinicians were randomized and trained on BREATHE or the control condition. Participants were followed monthly for 3 months post-intervention. Data were collected from December 2017 – May 2019 and included surveys, lung function tests and interviews.
Results:
Participants were Black/multiracial (100%) mostly female (83%) adults (mean age 45). BREATHE clinicians delivered BREATHE to all 40 participants with fidelity based on expert review of audiorecordings. While the control group reported improvements in asthma control at 1-month and 3-month follow-up, only BREATHE participants had better asthma control at each timepoint (β=0.77; standard error (SE)[0.17]; p=<.0001; β=0.71; SE[0.16]; p=<.0001; β=0.54; SE[0.15]; p=0.0004), exceeding the minimally important difference. BREATHE participants also perceived greater shared decision-making occurred during the intervention visit (β=7.39; SE[3.51]; p=0.03) and fewer symptoms at follow-up (e.g., fewer nights woken, less shortness of breath and less severity of symptoms) than the controls. Both groups reported improved adherence and fewer erroneous medication beliefs.
Conclusion:
BREATHE is a promising brief tailored intervention that can be integrated into office visits using clinicians as interventionists. Thus, BREATHE offers a pragmatic approach to improving asthma outcomes and shared decision-making in a health disparity population.
Impact:
The study addressed the important problem of uncontrolled asthma in a high-risk vulnerable population. Compared with the dose-matched attention control condition, participants receiving the novel brief tailored shared decision-making intervention had significant improvements in asthma outcomes and greater perceived engagement in shared decision-making. Brief interventions integrated into office visits and delivered by clinicians may offer a pragmatic approach to narrowing health disparity gaps. Future studies where other team members (e.g., office nurses, social workers) are trained in shared decision-making may address important implementation science challenges as it relates to adoption, maintenance and dissemination.
Trail registration:
Keywords: nursing, minority, pragmatic, motivational interviewing, health beliefs, community–engaged research, adherence, disease management, implementation science
I. INTRODUCTION
Asthma, one of the most common chronic illnesses, affects an estimated 339 million individuals worldwide (Global Asthma Network, 2018). An estimated 15 million disability-adjusted life-years are lost due to asthma (1.8% of the total global disease burden) (Vos et al., 2012) and 420,000 asthma deaths are reported annually (Global Asthma Network, 2018). The global costs of asthma are substantial (Braman, 2006; Global Asthma Network, 2018).
In the United States (U.S.), 19 million adults (7.7%) have asthma (Centers for Disease Control [CDC], 2017). Those living below 100% of the poverty level have higher asthma prevalence than those living above (11.7% vs 7.9%) (CDC, 2017). Relative to white and Hispanic adults, Blacks have higher asthma prevalence (CDC, 2017), higher death rates (CDC, 2017) and are ~3 times more likely to have severe asthma (Barnes et al., 2007; Gamble et al., 2010). While nearly 40% of all adults have uncontrolled asthma (CDC, 2017), 92% of those with severe asthma have uncontrolled disease (Chipps et al, 2018). Inhaled corticosteroids (ICS) are a safe and effective treatment for uncontrolled asthma and if used regularly, could prevent nearly every asthma-related hospitalization and death (Global Initiatives for Asthma, 2019). Yet, ICS nonadherence is common with Black adults having lower ICS adherence than Hispanics or whites (Deshpande et al., 2014; Wu et al., 2015). A significant factor contributing to ICS nonadherence is erroneous beliefs about asthma management and ICS treatment and such beliefs are more common among Blacks, relative to whites (Le, et al., 2008). Thus, erroneous beliefs are a critical target for tailored interventions aimed at improving ICS adherence and, by extension, asthma outcomes in this population.
1.1. Background
Shared decision-making (SDM) (Satterfield et al., 2009), a patient engagement model, includes strategies that promote patient and clinician engagement to jointly consider management options. Optimal treatment decisions are informed by patient preferences, the best available scientific evidence and clinician expertise. The clinician’s role in SDM is to facilitate discussion of the risks and merits associated with options in the context of the patient’s preferences with the goal of reconciling differences. Mutually agreed treatment is characterized by higher quality decisions that align patient’s preferences with evidence-based guideline-directed care (Battersby et al., 2010; Elwyn et al., 2012; Street et al., 2009).
A directed, patient-centered strategy for eliciting behavior change, motivational interviewing (MI) can be paired with SDM to help individuals explore and resolve ambivalence about recommended health advice (Miller & Rollnick, 2012). MI is characterized by a collaborative empathetic approach that cedes decision-making to the individual. The clinician attempts to tip the “decisional balance” in favor of the desired behavior by helping draw connections between what the patient states is their goal and current behavior. SDM and MI has been postulated to work synergistically by promoting an understanding of personal motivations. Together, SDM and MI “power” brief tailored interventions in a way that more time- and labor-intensive generic interventions cannot match (Elwyn et al., 2014),
SDM interventions have been evaluated in U.S. populations with mixed results (Kew et al., 2017; Tapp et al., 2017). A study by Wilson et al. (2010) used multiple SDM sessions led by trained interventionists outside of clinical care. Those in the SDM arm had improved ICS adherence and asthma control compared with usual care but not relative to the control condition. When this intervention was adapted to six community-based practices, clinicians received intensive training (8–12 hours) on how to integrate the SDM intervention into medical visits. These efforts were supported by a health coach and an SDM toolkit (Tapp et al., 2017). In this trial, adults receiving SDM did not have improved asthma outcomes compared with a matched control.
Successfully integrating SDM into clinical visits offers several advantages for patient care, including keeping clinicians more informed regarding their patients’ health needs and offering clinicians the requisite knowledge needed to improve their practice. Since the combination of SDM and MI has been postulated to work synergistically, this trial set out to explore if a brief intervention incorporating SDM and MI integrated into a clinical visit could be effective in improving outcomes among Black adults with uncontrolled asthma. If successful, such an approach would have the potential to be both sustainable and scalable.
2. THE STUDY
2.1. Aims
The aims of this trial were:
To assess the intervention effects of BREATHE (BRief intervention to Evaluate Asthma THErapy) compared with a dose-matched attention control condition in Black adults with uncontrolled asthma in federally-qualified health centers (FQHCs) over a 3-month follow-up period; and
To evaluate clinical trial feasibility and acceptability of the intervention
2.2. Design
A group-randomized longitudinal study was conducted with 80 adults with uncontrolled persistent asthma who received BREATHE (N=40) or a dose-matched attention control intervention (N=40). Interventions were delivered by clinicians during office visits. Participants were followed monthly for 3 months post-intervention. A purposive sample of clinicians, patients and their loved ones completed interviews post-trial to ascertain satisfaction with participation and suggestions for refinement. Data were collected December 2017 – May 2019.
2.3. Participants and setting
Adults (≥18 years of age) who self-identified as Black/African American (AA)/multiracial (with one race reported as Black/AA), of any ethnicity, prescribed ICS in the prior 12 months, with both uncontrolled asthma and endorsement of erroneous beliefs about asthma management or treatment were enrolled from two FQHCs. A specialized type of primary care setting in the U.S., FQHCs receive enhanced government reimbursement because their patients are poor, uninsured, or underinsured (National Association of Community Health Centers [NACHC], 2019). Blacks are overrepresented in FQHC populations and FQHCs care for clients with higher rates of asthma (21%) than found in the general population (8–14%) (NACHC, 2019).
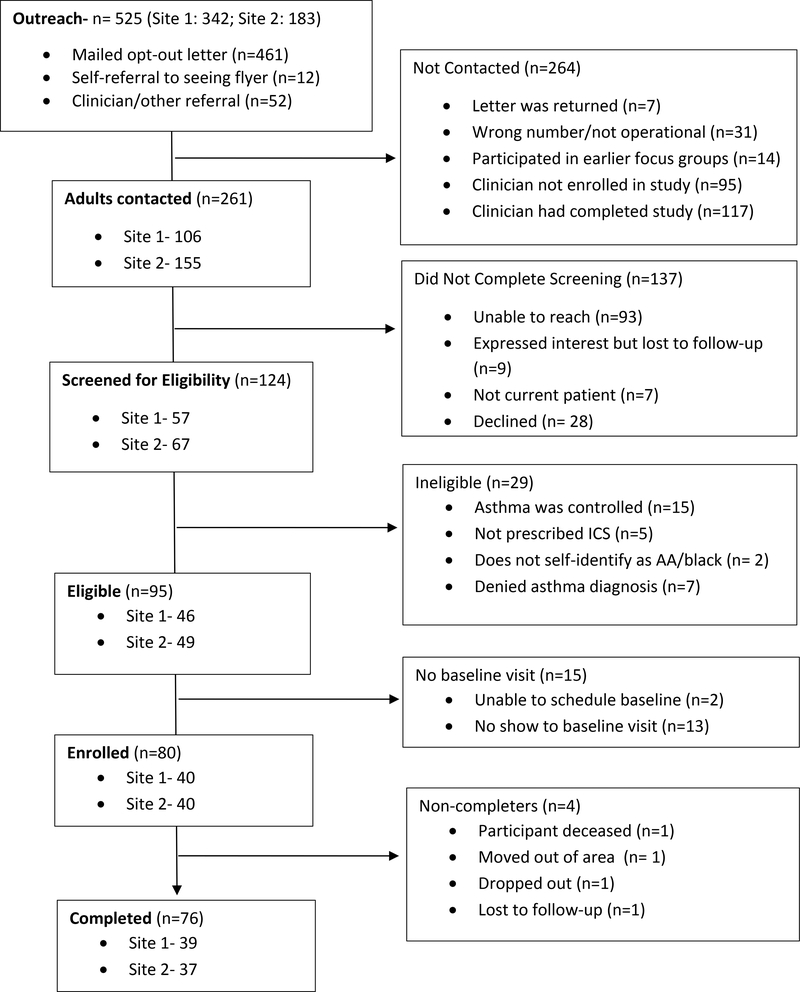
Participants were identified by self-referral, clinician referral and queries of the electronic health record (EHR). Opt-out letters were mailed to all individuals identified by EHR queries using a combination of ICD-10 (Asthma 493) codes filtered for clinician, age, race and ICS prescription. Using an institutional review board (IRB)-approved telephone consent and screening script, referrals and those who did not opt-out were contacted (Figure 1). Those who indicated interest in participating had age and race confirmed; those who self-reported they were Black adults then underwent a two-step eligibility screening. First, the Asthma Control Questionnaire (ACQ) (Juniper et al., 1999) was administered to determine if they currently had uncontrolled asthma; those who did were notified and scheduled for an office visit. They were also informed that they should seek urgent/emergent care if their status worsened before their scheduled visit.
Figure 1:
CONSORT Diagram
Written consent was obtained at the visit and a second screening was performed using the Conventional and Alternative Management for Asthma (CAM-A) questionnaire (George et al., 2014). This survey confirmed that they endorsed at least one erroneous belief about asthma management or ICS. Exclusion criteria were participation in focus groups used to inform intervention development (George et al., 2020) and comorbid disorders that would preclude longitudinal data collection. Clinicians were eligible to participate if they managed a panel of adult asthma patients.
2.4. Intervention conditions
2.4.1. BREATHE intervention
BREATHE is a one-time brief SDM intervention that uses MI to enhance ICS adherence. The intervention consists of a semi-scripted discussion based on the brief negotiation interview (D’Onofrio et al., 2012). Clinicians were trained on BREATHE in a 2-hour session comprised of didactics and role-playing. Immediately after training, clinicians were tested using a standardized patient scenario to demonstrate proficiency at delivering the intervention in four steps: 1) raising the subject of ICS non-adherence and asthma control; 2) making an explicit connection between non-adherence and uncontrolled asthma; 3) enhancing motivation to increase ICS use by exploring and resolving ambivalence towards treatment; and 4) engaging in SDM. Clinicians used a tablet and/or laminated card to prompt them through the intervention with a goal of delivering the steps in seven minutes. An infographic visualization of the patient’s ACQ score was used to focus attention on uncontrolled asthma (Arcia & George, 2020; Arcia et al., 2019). BREATHE was integrated into a single office visit. Detailed information on the intervention has been reported elsewhere (George et al., 2019).
2.4.2. Dose-matched attention control condition
Clinicians were trained using a self-guided instructional manual followed by an in-person demonstration of tablet features (~10-minutes). The dose-matched attention control condition was an unscripted discussion of healthy lifestyles (e.g., diet). The control condition was rigorous in that it was equivalent in contact time, was credible and interesting, yet exerted limited treatment effects (Safer & Hugo, 2006). Tablets were used to track time and facilitate masking.
2.4.3. Intervention fidelity
Standardized procedures for training, implementation and monitoring of intervention fidelity were used in both conditions (Bellg et al., 2004). These included training BREATHE clinicians on the manualized intervention and audiorecording all visits (active and control) to confirm accurate delivery, ensure no spillover from BREATHE to the control and to record intervention length. Within 24 hours of the intervention, audiofiles were uploaded to a secure site. Within 24 hours of uploading, audiofiles were reviewed and clinicians received feedback by email.
2.5. Procedures
2.5.1. Randomization
Prior to trial initiation, all eligible clinicians were randomized equally into the BREATHE and the dose-matched attention control condition stratified for provider type, using a computer-generated randomization list. The Principal Investigator obtained informed consent from the first eight clinicians on the randomization list: four/site; two physicians and two nurse practitioners [NPs] randomized to BREATHE; two physicians and two NPs randomized to control. Those remaining in the list were placed on “standby” if replacement was needed.
2.5.2. Contamination
Contamination was minimized by: (1) training only BREATHE clinicians to deliver the active intervention; and (2) encouraging confidentiality regarding training content.
2.5.3. Masking
Patients, data collectors and the statistician were blinded to assignment. Consent materials informed patients that the focus of the trial was on their communication with their clinician about asthma management and control. Immediately after the intervention, patients were asked to guess the condition to which their clinician had been randomized. At the end of patients’ final visit, data collectors were also asked to guess whether patients had been in the active or control arm.
2.6. Data collection
Clinical outcomes were selected based on their congruence with SDM, as well as for their established use in asthma and psychometric properties (Table 1).
Table 1.
Constructs, outcomes and measurement instruments
| Construct | Instrument | Characteristics | Minimal clinically important difference (MCID) | Miscellaneous |
|---|---|---|---|---|
| Primary Outcome | ||||
| Asthma control | Asthma Control Questionnaire (ACQ) (Juniper et al., 1999) | 6-items collect patient-reported symptoms of asthma; scores range from 0 ‘no impairment’ to 6 ‘maximum impairment in symptoms and rescue medicine use’. Scores exceeding 1.5 indicate uncontrolled asthma and have a positive predictive value of 88% in identifying uncontrolled asthma in clinical trials (Juniper et al., 2006). | Yes. MCID of 0.5 has been established (Juniper et al., 2005) | |
| Secondary Outcomes | ||||
| ICS adherence | The Medication Adherence Record Scale - Asthma (MARS-A (Cohen et al., 2009; Horne & Weinman, 2002). | 10-item survey of self-reported ICS adherence; mean score across these 10-items is computed, with scores > 4.5 indicating high adherence | No | MARS-A scores correlate with objective measures of ICS adherence as measured by electronic monitors (Menckeberg et al., 2008) and pharmacy refills (Cohen, et al., 2009). |
| Asthma quality of life | Asthma Quality of Life Questionnaire (AQLQ) (Juniper et al., 1993). | 32-item 4-domain disease-specific health-related quality of life instrument; uses a two-week recall to measure both physical and emotional impact of asthma Scores range from 7 ‘not impaired at all’ to 1 ‘severely impaired’. | Yes. A MCID of 0.5 for overall quality of life and for each of the individual domains has been established (Juniper et al., 1994). | |
| Lung function | Forced Expiratory Volume in 1s (FEV1) | Volume of air exhaled in the first second of maximum exhalation. A FEV1 < 80% may indicate airway obstruction (Graham et al., 2019). | No. The minimal clinically important difference (MCID) in FEV1 has not been rigorously established for asthma, but it is likely that changes of 100–200 mL in FEV1 are clinically important (Tepper et al, 2012) | |
| Shared decision-making | Shared Decision-making Questionnaire-9 (SDMQ-9) (Kriston et al. 2010) | 9 items measure perception of the degree to which a clinician uses SDM during medical visits; scores range from 0 ‘completely disagree’ to 5 ‘completely agree’ | Not applicable (measured once) | |
| Symptom burden | Individual items from the ACQ | 6 items: nighttime symptoms, daytime symptoms, breathlessness, wheeze, activity limitations, rescue medicine use) | No. | |
| Erroneous asthma management and negative ICS beliefs | Conventional and Alternative Management for Asthma (CAM-A) (George et al., 2014) | 17-item instrument composed of two subscales: 9 erroneous asthma management beliefs and 6 negative ICS beliefs (two items are not scored); summary score is calculated for each subscale; higher scores indicate more erroneous beliefs | No | Higher rates of erroneous asthma belief endorsement have been found to predict uncontrolled asthma (George et al., 2014). |
| Measures to characterize patient participants | ||||
| Health literacy | Newest Vital Sign (NVS) (Weiss et al., 2005) | 6-item survey that estimates likelihood of health literacy; number of correct answers reflect health literacy: 0–1 indicates high likelihood of limited literacy; 2–3 possibility of limited literacy; 4–6 almost always indicates adequate literacy | Not applicable (measured once) | |
| Pain intensity and health domains | The Patient-Reported Outcomes Measurement Information System-29 (PROMIS-29) (Health Measures, n.d.). | 29 items assess pain intensity using a single 0–10 numeric rating item and seven health domains (physical function, fatigue, pain interference, depressive symptoms, anxiety, ability to participate in social roles and activities, and sleep disturbance) using four items per domain; higher scores represents more of that concept being measure | Not applicable (measured once) | |
Primary outcome and secondary outcomes (except SDMQ-9) have established reliability and validity in adult asthma populations. Permission was obtained from all survey copyright holders.
2.7. Process assessments
Recruitment and eligibility were tracked.
2.8. Implementation assessments
Retention was followed as were data collection completion, proficiency following training, fidelity to intervention delivery, contamination and masking.
2.9. Acceptability
Patients’ satisfaction with participation was measured with the Client Satisfaction Questionnaire-8 (CSQ-8) (Larsen et al., 1979). Satisfaction with participation was also assessed by semi-structured interviews with clinicians, patients and patients’ loved ones. Interviewees were purposively selected based on the research team’s assessment of their engagement. All informants were asked to describe what they liked and disliked about participation, what revisions were needed and how the intervention had (or had not) changed their asthma management and communication. Clinicians were also asked about their satisfaction with training; BREATHE clinicians were asked if they had used or planned to use the intervention after the trial ended. The PI conducted all interviews.
2.10. Safety Monitoring Committee (SMC)
A SMC composed of a biostatistician, pulmonologist and asthma intervention expert established thresholds for adverse events (AEs), provided guidance and supervision. AEs in each arm were tracked.
2.11. Ethical considerations
The IRBs of Columbia University and the University of Pennsylvania approved this protocol.
2.12. Compensation
Clinicians received $400 for trial participation and $100 for post-trial interviews. Patients received $150 for complete data collection; patients and their loved ones received $50 for interviews.
2.12. Statistical analysis
While the primary goal was to examine feasibility, the trial was designed to test intervention effects. Descriptive data analysis, including examining patterns of missing data, proceeded with formal hypothesis testing and model building to understand the data distribution and to check for outliers. Linear mixed models were used to adjust for the heterogeneity of the sites (i.e., including in the model as a random effect) and clustering of data due to the repeated measurement of the same patient by including clinician- and patient-specific random interceptors. Analyses followed the intention-to-treat principle and considered both between and within group differences.
Hypotheses 1: Intervention effects were assessed using linear mixed models. Hypothesis testing was two-sided at level α=0.05 except for the six related asthma symptoms (α=0.05/6) using Bonferroni correction for multiple tests. The analysis was conducted using SAS© version 9.4 (2014).
2.13. Sample Size and Power
A power analysis estimated the sample size needed to detect significant differences between BREATHE and control participants on the primary outcome, ACQ score. Calculations considered four repeated observations over the three-month follow-up with repeated measures correlation of ρ = 0.8, an intra-cluster correlation among clinicians of 0.2 to account for clustering of patients from the same clinician, linear mixed models to estimate mean score differences and 10% attrition over the 3-months. Thus, for ACQ (assuming SD=0.76), this study had 91% power to detect a medium effect size with Cohen’s d=0.5, or a 0.33-point difference in ACQ.
2.14. Qualitative Analysis and Rigor
Directed content analysis was used to summarize responses (Hsieh & Shannon, 2005) guided by constructs from the Theoretical Framework of Acceptability (e.g., burden, user experience, attitudes towards the intervention and intention to participate) (Sekhon et al., 2017). Coding was performed by one team member (MG) and trustworthiness was enhanced by performing member checking (i.e., support for constructs found in earlier interviews was explored in later interviews), creating codebooks and recording coding decisions (i.e., an audit trail) (Guba, 1981).
3. RESULTS
Eighty adults (Table 2; Figure 1) and ten clinicians were enrolled (Table 3).
Table 2.
Baseline characteristics of patient participants (N=80)
| Total Sample (N=80) | BREATHE (N=40) | Dose-matched Attention Control (N=40) | |
|---|---|---|---|
| Age M (SD); range | 45.1 (13.1); 20–75 | 47.35 (12.80); 20–75 | 42.9 (13.06); 20–63 |
| Sex (Female) n (%) | 66 (82.5) | 30 (75) | 36 (90) |
| Race/Ethnicity n (%) | |||
| Black/African American | 73 (91.3) | 38 (95) | 35 (87.5) |
| More than 1 race in addition to black/African American | 7 (8.8) | 2 (5) | 5 (12.5) |
| Hispanic (Yes) n (%) | 1 (1.3) | 0 | 1 (2.5) |
| Insurance n (%) | |||
| Government (Medicaid/Medicare/SSI#) | 78 (97.5) | 39 (97.5) | 39 (97.5) |
| Commercial/other | 1 (1.3) | 1 (2.5) | 0 |
| Uninsured | 1 (1.3) | 0 | 1 (2.5) |
| Education n (%) | |||
| Some high school, no diploma | 19 (23.8) | 10 (25) | 9 (22.5) |
| High school graduate or GED | 42 (52.5) | 19 (47.5) | 23 (57.5) |
| Some college or trade school | 9 (11.3) | 5 (12.5) | 4 (10) |
| Associate or Bachelor’s degree | 10 (12.5) | 6 (15) | 4 (10) |
| Marital status n (%) | |||
| Single | 66 (82.5) | 34 (85) | 32 (80) |
| Married/living with partner | 7 (8.8) | 3 (7.5) | 4 (10) |
| Divorced/Separated/Widowed | 7 (8.8) | 3 (7.5) | 4 (10) |
| NVS† n (%) | |||
| High likelihood of limited literacy | 21 (26.25) | 14 (35) | 7 (17.5) |
| Possibility of limited literacy | 34 (42.5) | 14 (35) | 20 (50) |
| Almost always indicates adequate literacy | 25 (31.25) | 12 (30) | 13 (32.5) |
| PROMIS-29‡ M (SD); range | |||
| Physical Function | 12.64 (4.03); 4–20 | 12.43 (3.88); 4–20 | 12.85 (4.22); 4–20 |
| Anxiety | 10.53 (4.55); 4–20 | 10.13 (4.93); 4–20 | 10.93 (4.16); 4–20 |
| Depression | 9.55 (4.62); 4–20 | 9.68 (4.80); 4–20 | 9.43 (4.51); 4–20 |
| Fatigue | 11.86 (4.13); 4–20 | 12.23 (3.83); 6–20 | 11.5 (4.43); 4–20 |
| Sleep Disturbance | 13.64 (4.22); 5–20 | 13.5 (4.19); 6–20 | 13.78 (4.29); 5–20 |
| Satisfaction with Social Role Pain | 11.96 (4.48); 4–20 | 11.58 (3.84); 4–20 | 12.35 (5.06); 4–20 |
| Interference | 12.65 (5.21); 4–20 | 12.75 (4.86); 4–20 | 12.55 (5.60); 4–20 |
| Pain Intensity | 6.3 (3.02); 0–10 | 6.4 (2.94); 0–10 | 6.2 (3.14); 0–10 |
SSI=Supplemental Security Income; GED= General Education Diploma
NVS Newest Vital Sign: number of correct answers reflect health literacy; 0–1 indicates high likelihood of limited literacy, 2–3 possibility of limited literacy; 4–6 almost always indicates adequate literacy)
PROMIS-29 Patient-Reported Outcomes Measurement Information System” higher score represents more of that concept being measure.
Table 3.
Baseline characteristics of clinician participants (N=10)
| Total | Site 1 (n=4) | Site 2 (n=6) | |
|---|---|---|---|
|
N (%) or M (SD); range | |||
| Provider Type | |||
| Physician | 6 (60) | 3 | 3 |
| Nurse practitioner | 4 (40) | 1 | 3 |
| Training specialty | |||
| Family practice | 7 (70) | 3 | 4 |
| Internal medicine | 2 (20) | 1 | 1 |
| Primary care | 1 (10) | 0 | 1 |
| Age | 39.25 (5.86); 33–50 | 39.60 (7.94); 33–50 | 39.02 (4.88); 3445 |
| Sex | |||
| Female | 8 (80) | 3 | 5 |
| Male | 2 (20) | 1 | 1 |
| Race/Ethnicity | |||
| Black/African American | 5 (50) | 3 | 2 |
| White | 4 (40) | 1 | 3 |
| Biracial (White + Asian) | 1 (10) | 0 | 1 |
| Hispanic yes | 0 | 0 | 0 |
| Number of asthma | |||
| patients seen monthly | 31.7 (25.7); 15–100 | 48.3 (36.3); 15–100 | 20.7 (5.7); 15–30 |
3.1. Intervention effects
As between group tests were not significant, we report only within group differences (Table 4).
Table 4.
Linear regression of treatment effects of BREATHE on outcomes (N =80)
| BREATHE | Control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean (standard error [SE]) | β | lower limit* | upper limit* | P^ | Mean (SE) | β | lower limit* | upper limit* | P^ | |
| Primary Outcome | ||||||||||
| ACQ | ||||||||||
| Baseline | 2.94 (0.15) | 2.63 (0.15) | ||||||||
| 1-month | 2.17 (0.17) | 0.77 | 0.45 | 1.09 | <.0001 | 2.16 (0.16) | 0.47 | 0.15 | 0.79 | 0.0028 |
| 2-month | 2.22 (0.16) | 0.71 | 0.39 | 1.03 | <.0001 | 2.35 (0.16) | 0.28 | −0.04 | 0.60 | 0.077 |
| 3-month | 2.40 (0.15) | 0.54 | 0.24 | 0.84 | 0.0004 | 2.24 (0.15) | 0.39 | 0.09 | 0.69 | 0.011 |
| Secondary Outcomes | ||||||||||
| Adherence (MARS-A) | ||||||||||
| Baseline | 3.95 (0.12) | 3.88 (0.12) | ||||||||
| 1-month | 4.07 (0.13) | −0.12 | −0.34 | 0.10 | 0.3038 | 4.12 (0.12) | 0.25 | 0.03 | 0.47 | 0.0229 |
| 2-month | 4.28 (0.12) | −0.33 | −0.55 | −0.11 | 0.0027 | 4.18 (0.12) | −0.31 | −0.53 | −0.09 | 0.0054 |
| 3-month | 4.08 (0.12) | −0.13 | −0.33 | 0.07 | 0.211 | 4.03 (0.12) | −0.16 | −0.36 | 0.04 | 0.389 |
| FEV1 | ||||||||||
| Baseline | 1.92 (0.11) | 1.91 (0.11) | ||||||||
| 1-month | 2.00 (0.11) | −0.09 | −0.21 | 0.03 | 0.1452 | 1.98 (0.11) | −0.07 | −0.19 | 0.05 | 0.1977 |
| 2-month | 1.92 (0.11) | −0.0005 | −0.12 | 0.12 | 0.9928 | 1.93 (0.11) | −0.01 | −0.13 | 0.11 | 0.7897 |
| 3-month | 1.99 (0.11) | −0.07 | −0.17 | 0.03 | 0.1847 | 1.86 (0.11) | −0.67 | −0.77 | −0.57 | 0.288 |
| AQLQ | ||||||||||
| Baseline | 3.50 (0.18) | 3.65 (0.18) | ||||||||
| 1-month | 4.03 (0.20) | −0.53 | −0.88 | −0.18 | 0.003 | 4.07 (0.19) | −0.42 | −0.76 | −0.08 | 0.0139 |
| 2-month | 3.97 (0.19) | −0.47 | −0.81 | −0.13 | 0.006 | 4.18 (0.19) | −0.53 | −0.87 | −0.19 | 0.002 |
| 3-month | 4.02 (0.18) | −0.51 | −0.83 | −0.19 | 0.0019 | 4.22 (0.18) | −0.57 | −0.89 | −0.25 | 0.0006 |
| Other Outcomes | ||||||||||
| Nights Woken | ||||||||||
| Baseline | 2.80 (0.19) | 2.48 (0.19) | ||||||||
| 1-month | 1.74 (0.22) | 1.06 | 0.57 | 1.55 | 0.0001 | 2.01 (0.20) | 0.47 | 0.00 | 0.94 | 0.051 |
| 2-month | 1.78 (0.20) | 1.02 | 0.55 | 1.49 | 0.0001 | 2.10 (0.21) | 0.37 | −0.10 | 0.84 | 0.1234 |
| 3-month | 2.20 (0.19) | 0.6 | 0.15 | 1.05 | 0.009 | 2.05 (0.19) | 0.43 | −0.02 | 0.88 | 0.0657 |
| Severity of Symptoms upon Awakening | ||||||||||
| Baseline | 2.98 (0.22) | 2.18 (0.22) | ||||||||
| 1-month | 2.13 (0.25) | 0.85 | 0.28 | 1.42 | 0.0034 | 1.79 (0.23) | 0.38 | −0.15 | 0.91 | 0.1663 |
| 2-month | 2.29 (0.23) | 0.68 | 0.15 | 1.21 | 0.014 | 2.21 (0.24) | −0.03 | −0.58 | 0.52 | 0.9043 |
| 3-month | 2.47 (0.22) | 0.5 | −0.03 | 1.03 | 0.0601 | 1.95 (0.22) | 0.22 | −0.31 | 0.75 | 0.3984 |
| Activity Limitations | ||||||||||
| Baseline | 2.70 (0.29) | 2.75 (0.29) | ||||||||
| 1-month | 2.01 (0.31) | 0.69 | 0.16 | 1.22 | 0.01 | 2.30 (0.30) | 0.45 | −0.04 | 0.94 | 0.0784 |
| 2-month | 2.16 (0.30) | 0.54 | 0.03 | 1.05 | 0.0372 | 2.28 (0.30) | 0.47 | −0.04 | 0.98 | 0.0669 |
| 3-month | 2.22 (0.29) | 0.48 | −0.01 | 0.97 | 0.0541 | 1.99 (0.29) | 0.76 | 0.27 | 1.25 | 0.0022 |
| Shortness of Breath | ||||||||||
| Baseline | 3.65 (0.24) | 3.22 (0.24) | ||||||||
| 1-month | 2.54 (0.27) | 1.11 | 0.52 | 1.70 | 0.0002 | 2.83 (0.25) | 0.4 | −0.17 | 0.97 | 0.1664 |
| 2-month | 2.75 (0.25) | 0.9 | 0.33 | 1.47 | 0.002 | 2.93 (0.26) | 0.29 | −0.28 | 0.86 | 0.3113 |
| 3-month | 2.94 (0.24) | 0.71 | 0.16 | 1.26 | 0.0114 | 2.67 (0.24) | 0.56 | 0.01 | 1.11 | 0.0444 |
| Wheeze | ||||||||||
| Baseline | 3.28 (0.24) | 3.15 (0.24) | ||||||||
| 1-month | 2.78 (0.27) | 0.59 | 0.02 | 1.16 | 0.03999 | 2.21 (0.26) | 0.94 | 0.39 | 1.49 | 0.0008 |
| 2-month | 2.09 (0.26) | 1.28 | 0.73 | 1.83 | <.0001 | 2.56 (0.26) | 0.59 | 0.04 | 1.14 | 0.0351 |
| 3-month | 2.50 (0.24) | 0.87 | 0.34 | 1.40 | 0.0012 | 2.47 (0.24) | 0.68 | 0.15 | 1.21 | 0.0107 |
| Rescue Use in Last Week | ||||||||||
| Baseline | 2.10 (0.17) | 1.85 (0.17) | ||||||||
| 1-month | 1.51 (0.19) | 0.58 | 0.19 | 0.97 | 0.0043 | 1.28 (0.18) | 0.57 | 0.20 | 0.94 | 0.0034 |
| 2-month | 1.58 (0.18) | 0.52 | 0.15 | 0.89 | 0.008 | 1.41 (0.18) | 0.44 | 0.05 | 0.83 | 0.0258 |
| 3-month | 1.75 (0.17) | 0.35 | −0.02 | 0.72 | 0.0626 | 1.44 (0.17) | 0.41 | 0.04 | 0.78 | 0.028 |
| CAM-A | ||||||||||
| Erroneous management beliefs | ||||||||||
| Baseline | 4.69 (0.36) | 4.28 (0.36) | ||||||||
| 3-month | 4.63 (0.34) | 0.057 | −0.30 | 0.41 | 0.7489 | 4.51 (0.34) | −0.23 | −0.58 | 0.12 | 0.1884 |
| Erroneous ICS beliefs | ||||||||||
| Baseline | 2.69 (0.23) | 2.28 (0.23) | ||||||||
| 3-month | 1.88 (0.22) | 0.81 | 0.59 | 1.03 | <.0001 | 1.94 (0.22) | 0.34 | 0.12 | 0.56 | 0.0035 |
Definition of abbreviations: ACQ - Asthma Control Questionnaire (lower scores indicate better asthma control); FEV1 - Forced Expiratory Volume in 1 second (objective measure of lung function); AQLQ – Asthma Quality of Life Questionnaire (higher scores indicate better quality of life); CAM-A - Conventional and Alternative Management of Asthma (higher scores indicate more endorsement of erroneous beliefs).
Linear regression adjusting for heterogeneity of the and clustering of data due to the repeated measurement of the same patient.
lower and upper limits of 95% confidence interval
3.1. Asthma control
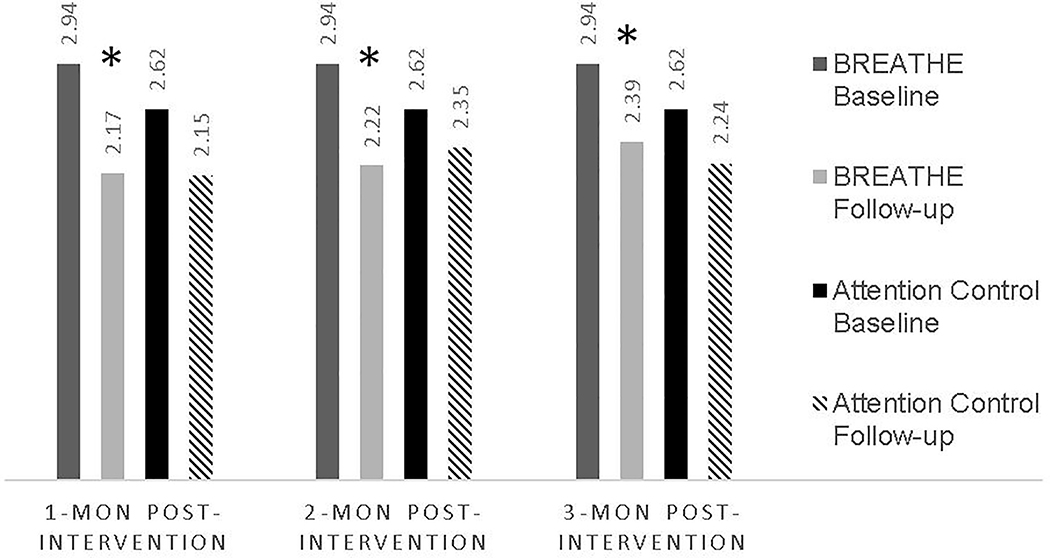
While both BREATHE and control participants had statistically significant improvements in asthma control at 1-month and 3-month follow-up (Table 4), only BREATHE participants had better asthma control at all timepoints, exceeding the minimal clinically important difference (MCID) of 0.5 at each timepoint (Fig. 2).
Figure 2. Within group differences on Asthma Control Test from baseline at each timepoint by treatment condition.
*Denotes within-group statistical and clinical significance (exceeding minimally important difference in score of 0.5)
3.2. ICS adherence, asthma quality of life and lung function
As detailed in Table 4, compared with baseline, ICS adherence was significantly better at the 2-month follow-up for the BREATHE group; ICS adherence was also significantly improved at 1-month and 2-months for the control group. Asthma quality of life scores improved for both groups at all timepoints exceeding the MCID at 1-month and 3-month post-baseline for BREATHE and at the 2-month and 3-month point for the control group. Neither group experienced improved lung functioning.
3.3. SDM, symptoms and erroneous beliefs
Relative to attention control, BREATHE participants reported statistically higher perceived SDM measured immediately after the intervention (β = 7.39; standard error (SE) [3.51]; p = 0.03). The BREATHE group also experienced significant improvements in patient-reported outcomes of fewer nights awoken and reduced shortness of breath at 1- month and 2-month post-baseline; these gains were not observed in the controls (Table 4). Only BREATHE participants reported lower severity of asthma symptoms on awakening at 1-month. Wheeze and rescue medicine use reflected a mixed pattern. While both groups reported fewer erroneous ICS beliefs, the change was larger in BREATHE compared with control; this difference was not statistically different.
3.4. Process assessment outcomes
3.4.1. Recruitment
As seen in Figure 1, there was study outreach to 525 potentially eligible adults with asthma: 461 were mailed opt-out letters; 12 responded to posted flyers and 52 were referred by their clinician or another participant. Of these, 261 were contacted and 124 screened; the remaining 137 were not screened because we were unable to reach them by phone (N=93); they declined to be screened (N= 28); they failed to follow-up after screening (N=9); or were not a current patient at the sites (N=7). Reasons for not contacting the remaining 264 included their clinician having completed trial participation (N=117); their clinician not participating in the study (N=95); having a wrong or non-operational phone number (N=31); having participated in the pre-intervention focus groups (N=14); or having the opt-out letter returned as undeliverable (N=7).
3.4.2. Eligibility
Of the 124 adults screened, 95 (77%) had ACQ scores ≥1.5 indicating uncontrolled asthma. Of these 95, the first 80 presenting for follow-up were screened for endorsement of erroneous beliefs using the CAM-A; 100% were eligible by this criterion.
3.5. Implementation assessment outcomes
3.5.1. Retention
Over 3 months 76 of 80 patient participants (95%) completed the trial. Reasons for non-completion included death (N=1), relocation outside of study area (N=1), self-withdrawal (N=1) and lost to follow-up (N=1). Two of the non-completers were in the active arm and two in the control arm.
Seventy-five percent (6/8) clinicians were retained; one clinician left employment and one relocated to a non-participating site. Of the two withdrawing, one was in the active arm and one in the control. These clinicians were replaced with the next two clinicians on the standby list.
3.5.2. Data collection completion
Over the 3 months of data collection, 289 of 320 (90%) visits were completed.
3.5.3. Proficiency after training
Five clinicians were trained either one-on-one, or in small groups, on the active BREATHE condition (See 2.4). Clinicians demonstrated 100% proficiency using a standardized patient scenario to demonstrate he/she could deliver the intervention content in seven minutes. Clinicians in the control group received one-on-one brief instruction (< 15 minutes) on tablet use.
3.5.4. Fidelity of intervention delivery
The five clinicians randomized to BREATHE delivered the intervention to all 40 BREATHE participants in ñine minutes (mean 9:11 minutes; range 4:10–15:50). This was two minutes longer than goal. Fidelity to the steps were as follows: Step 1=95.0%; Step 2=96.6%; Step 3=87.8%; Step 4=73.7%. Lower scores in Step 4 were attributable to a failure to plan follow-up. No BREATHE clinician required retraining for drift.
The five control clinicians delivered the control intervention to 92.5% of participants (37/40; a single clinician was retrained after failing to deliver the intervention three times). Clinicians led a healthy lifestyle discussion for ñine minutes (mean 8:40 minutes; range 3:40–30:18). This was two minutes longer than the goal, but consistent with the length of the BREATHE sessions. Seventy percent of the discussions focused on nutrition and exercise.
There were no statistically significant differences in total length of office visits between the active and control arms: BREATHE visits lasted an average of 16:35 minutes (range 8:10–28:35) relative to 19:06 minutes (range 4:59–35:42) for the control group (t=1.1769; confidence interval 0.418, 5.5409; p=0.09).
3.5.6. Contamination
Audiorecordings of the visits and post-trial interviews confirmed that BREATHE clinicians had not shared and control clinicians had not received, BREATHE content.
3.5.7. Masking
Forty-five percent (36/80) of patients correctly guessed their group assignment, representing no better than chance (i.e., masking achieved). Data collectors correctly guessed patient assignment in 49 of 80 cases (61%) which may indicate that masking was not fully achieved.
3.6. Acceptability
There was no significant difference between groups on the CSQ-8 (data not shown). A ceiling effect was noted with all participants rating trial satisfaction as high. As described above interviews were conducted in a purposeful sample of 17 individuals: six patients (five in the BREATHE arm and one in the control arm); five patients’ loved ones (four whose loved one was in the BREATHE arm and one whose loved one was in the control arm) and six clinicians (four randomized to BREATHE and two to the control condition).
Patients, regardless of condition, rated their interactions with study staff very positively, felt that they had learned a great deal about asthma and would recommend enrollment in a clinical trial. All six described having improved asthma communication with their clinician with two BREATHE participants describing these discussions as more “honest.” Participants made no suggestions for improvement or refinement of study components.
Loved ones felt that trial participation had improved their loved one’s ability to manage asthma. Though most supported their loved one’s decision to enroll, two expressed concerns that their loved one would be “experimented on” or “be a guinea pig.” They revealed their loved ones had disliked two study components: lung function testing and daily diaries.
Clinicians felt that being part of a clinical trial reduced the time pressures they typically felt during office visits and appreciated having time to focus only on asthma. BREATHE clinicians were extremely happy with the training content, structure and feedback and many planned to use, or were already using, the intervention with other patients (e.g., smokers, those with substance use/abuse and mental health disorders, uncontrolled diabetes). Three of the clinicians in the active arm used words like “awkward” and “scripted” to describe a feeling of artificiality when integrating the protocol into an office visit. None offered suggestions for revisions or improvements. All were surprised to learn that their patients reported low ICS adherence and endorsed erroneous beliefs. Interestingly, two BREATHE clinicians expressed suspicion that patients lied on screening surveys to be eligible for study enrollment.
3.7. AEs
There was a total of 13 asthma-related AEs. Eight occurred in the active condition: five emergency department (ED) visits for asthma (four characterized as not related and one as unlikely related), two self-initiated use of oral corticosteroids (OCS) for asthma and one urgent visit where OCS was prescribed (all OCS use characterized as unlikely related). In the control condition there were five AEs: two ED visits, one intensification of bronchodilators, one OCS use and one hospitalization for nebulization.
There was one serious adverse event (SAE) that occurred in the study: death. The SAE occurred in the active group; it did not meet the definition of an Unanticipated Problem and its relatedness to the study was characterized as “unlikely related.” The SMC held a special meeting to discuss the SAE; the higher ED rates in the active arm were discussed at the final scheduled meeting. The number of ED visits was considered small in absolute number and not unexpected or unanticipated in this population. The possibility that the intervention heightened BREATHE participants awareness of uncontrolled asthma and represented an appropriate response to loss of asthma control may explain the higher AE rates seen. The SMC recommended that the team continue to investigate whether the number of ED visits was or was not unexpectedly large for the population.
4. DISCUSSION
In this 3-month longitudinal trial of a tailored SDM intervention, statistically and clinically significant improvement in asthma control was seen in the active group at each time point. Asthma quality of life improved in both groups while lung function did not. BREATHE participants also reported significantly higher SDM scores and fewer symptoms. The reduction in erroneous ICS beliefs was greater in the BREATHE group but not statistically different than the control group. Process and implementation outcomes demonstrated that the study was feasible and acceptable.
Measures included core (e.g., ACQ; lung function) and supplemental (e.g., asthma-related quality of life, daytime and nocturnal symptoms) outcomes that have been proposed as common data elements for prospective efficacy studies of adults (Busse et al., 2012). While ACQ scores improved in both the active and control arms, only BREATHE participants had improvements at each timepoint that exceeded the MCID. This stands in contrast to the Wilson et al. (2010) trial where asthma control in the SDM group was not significantly better relative to the attention control group at one-year, although control was significantly better compared with usual care. In the Tapp study (2017) rates of exacerbations and acute health care use were not significantly different for adults at the 3-, 6- or 12-month follow-up. In a small RCT comparing asthma education with and without a decision aid (not specifically SDM), there were no differences in asthma control between groups (Gagné et al., 2017).
Wilson reported that the SDM group also had significantly higher ICS adherence at one-year relative to usual care, but not compared with attention control. In a small trial of an SDM portal for parents of children with asthma, higher controller medication possession rates were reported over 26 weeks for SDM parents compared with controls; this was associated with lower rates of asthma exacerbations (Fiks et al., 2015). Conversely, Lauffenburger and colleagues (2019) found that a brief SDM intervention for diabetes improved glucose control but not diabetes medication adherence. In our study, only BREATHE participants had improved asthma control that exceeded the MCID at each time point but ICS adherence improved only at 2-months; the control group also had improvements in ICS adherence at 1-month and 2-months. A possible explanation for improved control - but not improved adherence - may be the use of the infographic visualization of ACQ score during the intervention. While the infographic may have focused patients’ attention on asthma control the intervention may have failed to adequately make the connection between asthma control and ICS non-adherence. Thus, participants may have been prompted to take other health actions to improve asthma control, such as avoiding or reducing exposures to environmental triggers. It will be important to monitor self-management behaviors more comprehensively in future trials.
Despite its importance as a patient-centered communication strategy, implementing SDM in clinical settings is challenging. Interventions need to be brief and it is difficult to determine if patients perceive that they are engaged in SDM. If they do, it remains unknown if the intervention must be of a certain quality to effect the targeted decision (Gruffydd-Jones & Hansen, 2020; Joseph-Williams et al., 2014; Légaré & Witteman, 2013). Compared with controls, BREATHE participants reported significantly higher perceived SDM. However, participants were unable to correctly guess assignment (masking achieved), suggesting that patient-centered communication of any type, in this case healthy lifestyles discussions, might have been misconstrued by patients as SDM.
SDM interventions are purposefully scripted. As a result, some BREATHE clinicians ascribed a certain artificiality to the intervention. As SDM is a skill like any other, clinicians report an awkwardness until scripts are memorized and integrated into everyday practice, after which they make it their own (D’Onofrio, et al., 2012). Our clinicians also reported that being part of a trial afforded them more time to focus solely on asthma then a “regular” visit would allow. While it’s true that a trial provides structure for participation, this time is needed until clinicians become adept at integrating SDM into the daily flow of their practice. Like the initial sense of artificiality, the idea of needing “protected time” diminishes with ongoing coaching and accountability (D’Onofrio, et al., 2012).
Success in meeting process and implementation goals in FQHCs is notable. Retention and data collection completion was high in FQHCs which often struggle with appointment-keeping (Cruz et al., 2018). This success may reflect the long-standing relationship the research team has developed with the community and the FQHCs. Having a patient co-investigator from the community added legitimacy to the project; positive engagement with the research assistant also likely helped. Clinician retention was lower than patient retention with providers seeking better working conditions with new employers or at new sites. Clinician burnout among FQHC staff has been well-documented (Friedberg et al., 2017).
Data also confirmed the feasibility of integrating SDM into brief office visits, demonstrating potential for sustainability and scalability. These office visits were shorter (~18 minutes) than visits previously reported in similar sites (George et al., 2014) or reported nationally (Irving et al., 2017), suggesting that dissemination to primary care settings, more broadly, might be possible. Despite the brevity of the visits, asthma control improved signifying that the content and patient-centeredness of the visits may be more important than absolute length.
4.1. Limitations
This trial in not without limitations. Selection bias was likely introduced by allowing self-referrals and clinician-referrals. This may have been mitigated somewhat by outreaching to those identified by EHR queries. That said, those who enrolled in the trial were likely different than those who did not. It is also possible that social desirability could have led participants to answer questions about asthma control, symptoms, adherence, SDM and erroneous beliefs that they believed the research team wanted to hear. Having a two-step process for study eligibility and a dose-matched attention control comparator may have addressed this risk. Further, while self-reports of asthma control and adherence may result in over-reporting, we selected instruments that reduced this risk. For example, the shortened version of the ACQ has been found to be as good a measure of asthma control as those that include objective measures of lung function (Juniper et al., 2005). In addition, high scores on the Medication Adherence Record-Scale for Asthma have been found to correlate with objective measures of ICS adherence obtained via electronic monitoring (Cohen et al., 2009). Lastly, although two independently-operated FQHCs served as study sites, the trial took place in two contiguous urban areas that share similar demographic populations. Future studies should be conducted in more racial-ethnic groups across a more geographical diverse area.
5. CONCLUSION
Compared with the control condition, participants receiving the novel brief tailored SDM intervention had significant improvements in asthma outcomes and perceived greater SDM. The intervention was brief, integrated into an office visit and delivered by clinicians, offering a pragmatic approach to scaling up an intervention that shows early evidence of narrowing the health disparity gap in under resourced primary care settings. Using the patient’s own clinician as the interventionist has the added benefit of keeping them apprised of the challenges to asthma management their patient faces. This intervention can serve as a template for other patient populations who may be experiencing similar challenges. Future studies should examine how brief interventions enhance the perception of SDM and create behavioral intentions for health actions other than medication adherence. Understanding factors to adoption and implementation of SDM interventions in real-world settings that serve vulnerable populations is important, as is its applicability to other team members (e.g., office nurses, social workers) who may be well-suited to assume the role of SDM interventionist. Thus, this study answers important implementation science questions as it relates to adoption and maintenance; dissemination needs to be explored more fully in future studies. Taken together, this trial provides early evidence in support of a real-world approach to sustaining and building capacity to meet the needs of an underserved population.
Acknowledgements:
We’d like to thank Obumneke “Ify” Obi and William Schneider for their assistance with data collection.
Funding:
R21 NR016507 George (PI)
National Center for Advancing Translational Sciences, National Institutes of Health
TL1 TR001875
Footnotes
No conflict of interest has been declared by the authors.
Trial registration: Registered at clincialtrials.gov as NCT03036267 and NCT03300752
Contributor Information
Maureen GEORGE, Columbia University School of Nursing.
Jean-Marie BRUZZESE, Columbia University School of Nursing.
Marilyn (Lynn) S. SOMMERS, University of Pennsylvania School of Nursing.
Michael V. PANTALON, Department of Emergency Medicine, Yale University School of Medicine.
Haomiao JIA, Columbia University School of Nursing, Columbia University Mailman School of Public Health.
Joseph RHODES, University of Pennsylvania School of Nursing.
Allison A. NORFUL, Columbia University School of Nursing.
Annie CHUNG, Center for Health Behavior Research, Perelman School of Medicine, University of Pennsylvania.
Jesse CHITTAMS, University of Pennsylvania School of Nursing.
Danielle COLEMAN, Philadelphia, PA.
Karen GLANZ, Perelman School of Medicine and School of Nursing, University of Pennsylvania.
References
- Arcia A, & George M Reference range number line format preferred by adults for display of asthma control status. (2019). Journal of Asthma, 3, 1–7. 10.1080/02770903.2019.1590597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcia A, George M, Lor. M, Mangal S, & Bruzzese JM (2019). Design and comprehension testing of tailored asthma control infographics for adults with persistent asthma. Applied Clinical Informatics, 10(4), 643–654. 10.1055/s-0039-1693713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes KC, Grant AV, Hansel NN, Gao P, &. Dunston GM (2007). African Americans with asthma: Genetic insights. Proceedings of the American Thoracic Society, 4(1): 58–68. 10.1513/pats.200607-146JG [DOI] [PMC free article] [PubMed] [Google Scholar]
- Battersby M, Von Korff M, Schaefer J,. Davis C, Ludman E, Greene SM, Parkerton M, & Wagner E,H (2010). Twelve evidence-based principles for implementing self-management support in primary care. Joint Commission Journal of Quality and Patient Safety, 36(12), 561–570. 10.1016/S1553-7250(10)36084-3 [DOI] [PubMed] [Google Scholar]
- Bellg AJ, Borrelli B, Resnick B, Hecht J, Minicucci DS, Ory M, Ogedegbe G, Orwig D, Ernst D, Czajkowski S; Treatment Fidelity Workgroup of the NIH Behavior Change Consortium. (2004). Enhancing treatment fidelity in health behavior change studies: Best practices and recommendations from the NIH Behavior Change Consortium. Health Psychology, 23(5), 443–451. 10.1037/0278-6133.23.5.443 [DOI] [PubMed] [Google Scholar]
- Braman SS (2006). The global burden of asthma. Chest, 130(1 Suppl), 4S–12S. 10.1378/chest.130.1_suppl.4S [DOI] [PubMed] [Google Scholar]
- Busse WW, Morgan WJ, Taggart V, & Togias A (2012). Asthma uutcomes workshop: Overview. Journal of Allergy & Clinical Immunology 129(30), S1–S8. 10.1016/j.jaci.2011.12.985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centers for Disease Control. (2017). Asthma surveillance data. https://www.cdc.gov/asthma/asthmadata.htm
- Chipps BE, Haselkorn T, Paknis B, Ortiz, Bleecker ER, Kianifard F, Foreman AJ, Szefler SJ, & Zeiger RS (2018). More than a decade follow-up in patients with severe or difficult-to-treat asthma: The Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) II. Journal of Allergy and Clinical Immunology, 141, 1590–1597. 10.1016/j.jaci.2017.07.014. [DOI] [PubMed] [Google Scholar]
- Cohen JL, Mann DM, Wisnivesky JP, Home R, Leventha l H., Musumeci-Szabo TJ, Halm EA (2009). Assessing the validity of self-reported medication adherence among inner-city asthmatic adults: The Medication Adherence Report Scale for Asthma. Annals of Allergy Asthma and Immunology, 103, 325–331. 10.1016/S1081-1206(10)60532-7 [DOI] [PubMed] [Google Scholar]
- Cruz H, Gawrys J, Thompson D, Mejia J, Rosul L, & Lazar D (2018). A multipronged initiative to improve productivity and patient access in a Federally Qualified Health Center network. Journal of Ambulatory Care Management, 41(3), 225–237. 10.1097/JAC.0000000000000230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deshpande M, Chewning B, Mott D, Thorpe JM, & Young HN (2014). Asthma medication use among U.S. adults 18 and older. Research in Social Administrative Pharmacy, 10(6), e113–e123. 10.1016/j.sapharm.2014.02.006 [DOI] [PubMed] [Google Scholar]
- D’Onofrio G, Fiellin DA, Pantalon MV, Chawarski MC, Owens PH, Degutis LC, Busch SH, Bernstein SL, O’Connor PG (2012). Brief Intervention Reduces Hazardous and Harmful Drinking in Emergency Department Patients. Annals of Emergency Medicine, 60, 181–192. 10.1016/j.annemergmed.2012.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn G, Frosch D, Thomson R, Joseph-Williams N, Lloyd A, Kinnersley P, Cording E, Tomson D, Dodd C, Rollnick S, Edwards A, & Barry M (2012). Shared decision making: A model for clinical practice. Journal of General Internal Medicine, 27(10), 1361–1367. 10.1007/s11606-012-2077-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elwyn G, Dehlendorf C, Epstein RM, Marrin K, White J, Frosch DL. (2014). Shared decision making and motivational interviewing: achieving patient-centered care across the spectrum of health care problems. Annals of Family Medicine, 12(3), 270–275. 10.1370/afm.1615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiks AG, Mayne SL, Karavite DJ, Suh A, O’Hara R, Localio AR, Ross M & Grundmeier RW (2015). Parent-reported outcomes of a shared decision-making portal in asthma: a practice-based RCT. Pediatrics, 135(4), e965–973. 10.1542/peds.2014-3167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedberg MW, Reid RO, Timbie JW, Setodji C, Kofner A, Weidmer B, & Kahn K (2017). Federally Qualified Health Center clinicians and staff increasingly dissatisfied with workplace conditions. Health Affairs (Millwood), 36(8),1469–1475. 10.1377/hlthaff.2017.0205 [DOI] [PubMed] [Google Scholar]
- Gagné ME, Légaré F, Moisan J, & Boulet LP (2017). Impact of adding a decision aid to patient education in adults with asthma: A randomized clinical trial. PLOS ONE, 12(1), e0170055 10.1371/journal.pone.0170055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gamble C, Talbott E, Youk A, Holguin F, Pitt B, Silveira. L, Bleecker E, Busse W, Calhoun W, Castro M, Chung KF, Erzurum S, Israel E, & Wenzel. S (2010). Racial differences in biologic predictors of severe asthma: data from the Severe Asthma Research Program. Journal of Allergy and Clinical Immunology, 126, 1149–1156. 10.1016/j.jaci.2010.08.049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Arcia A, Chung A, Coleman D, & Bruzzese JM (2020). African Americans want a focus on shared decision-making in asthma adherence interventions. Patient, 13(1), 71–81. 10.1007/s40271-019-00382-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Pantalon MV, Sommers MS, Glanz K, Jia H, Chung A, Norful AA, Poghosyan L, Coleman D, Bruzzese J-M (2019). Shared decision-making in the BREATHE asthma intervention trial: A research protocol. Journal of Advanced Nursing, 75(4), 876–887. 10.1111/jan.13916 [DOI] [PMC free article] [PubMed] [Google Scholar]
- George M, Topaz M, Rand C, Sommers MS, Glanz K, Pantalon MV, Mao JJ, & Shea J (2014). Inhaled corticosteroid beliefs, complementary and alternative medicine and uncontrolled asthma in urban minority adults. Journal of Allergy and Clinical Immunology, 134, 1252–1259. 10.1016/j.jaci.2014.07.044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Global Asthma Network (2018). The Global Asthma Report 2018. Auckland, New Zealand: http://www.globalasthmareport.org/Global%20Asthma%20Report%202018.pdf [Google Scholar]
- Global Initiatives for Asthma [GINA]. (2019). Global strategy for asthma management and prevention. https://ginasthma.org/gina-reports/
- Graham BL, Steenbruggen I, Miller MR, Barjaktarevic IZ, Cooper BG, Hall GL, Hallstrand TS, Kaminsky DA, McCarthy K, McCormack MC, Oropez CE, Rosenfeld M, Stanojevic S, Swanney MP, & Thompson BR, (2019). Standardization of spirometry 2019 update. America Journal of Respiratory and Critical Care Medicine, 200(8) e70–e88. 10.1164/rccm.201908-1590ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruffydd-Jones K &, Hansen K (2020). Working for better asthma control: how can we improve the dialogue between patients and healthcare professionals? Adv Ther 37(1):1–9. 10.1007/s12325-019-01131-0 [DOI] [PubMed] [Google Scholar]
- Guba E (1981). Criteria for assessing the trustworthiness of naturalistic inquiries. Educational Communication and Technology Journal, 29(2), 75–91. 10.1007/BF02766777 [DOI] [Google Scholar]
- Health Measures. (n.d.). Patient reported outcomes measurement information system measures. http://www.healthmeasures.net/explore-measurement-systems/promis/measure-development-research/validation
- Hsieh H, & Shannon SE (2005). Three approaches to qualitative content analysis. Qualitative Health Research, 15; 1277–1288. 10.1177/1049732305276687 [DOI] [PubMed] [Google Scholar]
- Horne R, & Weinman J (2002). Self-regulation and self-management in asthma: exploring the role of illness perceptions and treatment beliefs in explaining non-adherence to preventer medication. Psychology & Health, 17, 17–32. 10.1080/08870440290001502 [DOI] [Google Scholar]
- Irving G, Neves AL, Dambha-Miller H, Oishi A, Tagashira H, Verho A, & Holden J (2017). International variations in primary care physician consultation time: a systematic review of 67 countries. BMJ Open, 7, e017902 10.1136/bmjopen-2017-017902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph-Williams N, Edwards A, & Elwyn G (2014). Power imbalance prevents shared decision making. British Medical Journal, 348, 3178 10.1136/bmj.g3178 [DOI] [PubMed] [Google Scholar]
- Juniper EF, Bousquet J, Abetz L, & Bateman ED (2006). Identifying ‘well-controlled’ and ‘not well-controlled’ asthma using the asthma control questionnaire. Respiratory Medicine, 100(4), 616–621. 10.1016/j.rmed.2005.08.012 [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Ferrie PJ, & Griffith LE (1993). Measuring quality of life in asthma. American Review of Respiratory Disease, 147(4), 832–838. 10.1164/ajrccm/147.4.832 [DOI] [PubMed] [Google Scholar]
- Juniper EF, Guyatt GH, Willan A, & Griffith LE (1994). Determining a minimal important change in a disease-specific quality of life questionnaire. Journal of Clinical Epidemiology, 47(1), 81–87. 10.1016/0895-4356(94)90036-1 [DOI] [PubMed] [Google Scholar]
- Juniper EF, O’Byrne PM, Guyatt GH, Ferrie PJ, & King DR (1999). Development and validation of a questionnaire to measure asthma control. European Respiratory Journal, 14(4), 902–907. 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- Juniper EF, Svensson K, Mörk AC, & Ståh l E. (2005). Measurement properties and interpretation of three shortened versions of the asthma control questionnaire. Respiratory Medicine, 99(5), 553–558. 10.1016/j.rmed.2004.10.008 [DOI] [PubMed] [Google Scholar]
- Kew KM, Malik P, Aniruddhan K, & Normansell R (2017). Shared decision-making for people with asthma. Cochrane Database of Systematic Reviews, 10, CD012330 10.1002/14651858.CD012330.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kriston L, Scholl I, Hölzel L, Simon D, Loh A, & Härter M (2010). The 9-item shared decision making questionnaire (SDM-Q-9). Development and psychometric properties in a primary care sample. Patient Education and Counseling, 80(1), 94–99. 10.1016/j.pec.2009.09.034 [DOI] [PubMed] [Google Scholar]
- Larsen DL, Attkisson CC, Hargreaves WA, & Nguyen TD (1979). Assessment of client/patient satisfaction: Development of a general scale. Evaluation and Program Planning, 2(3), 197–207. 10.1016/0149-7189(79)90094-6 [DOI] [PubMed] [Google Scholar]
- Lauffenburger JC, Ghazinouri R, Jan S, Makanji S, Ferro CA, Lewey J, Wittbrodt E, Lee J, Haff N, Fontanet CP, & Choudhry NK (2019). Impact of a novel pharmacist-delivered behavioral intervention for patients with poorly-controlled diabetes: The ENhancing outcomes through Goal Assessment and Generating Engagement in Diabetes Mellitus (ENGAGE-DM) pragmatic randomized trial. PLoS One, 14(4), e0214754 10.1371/journal.pone.0214754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le TT, Bilderback A, Bender B, Wamboldt FS, Turner CF, Rand CS, & Bartlett SJ (2008). Do asthma medication beliefs mediate the relationship between minority status and adherence to therapy? Journal of Asthma, 45(1), 33–37. 10.1080/02770900701815552 [DOI] [PubMed] [Google Scholar]
- Légaré F, & Witteman HO (2013). Shared decision making: examining key elements and barriers to adoption into routine clinical practice. Health Affairs (Millwood, 32(2), 276–284. 10.1377/hlthaff.2012.1078 [DOI] [PubMed] [Google Scholar]
- Menckeberg TT, Bouvy ML, Bracke M, Kaptein AA, Leufkens HG, Raaijmakers JA, Horne R. Beliefs about medicines predict refill adherence to inhaled corticosteroids. J Psychosom Res 2008. January;64(1):47–54. 10.1016/j.jpsychores.2007.07.016 [DOI] [PubMed] [Google Scholar]
- Miller WR, & Rollnick S. (2012). Motivational interviewing: Helping people change. The Guilford Press. [Google Scholar]
- National Association of Community Health Centers. (2019). Community health centers chartbook. http://www.nachc.org/wp-content/uploads/2019/01/Community-Health-Center-Chartbook-FINAL-1.28.19.pdf
- SAS Institute Inc. 2013. Base SAS® 9.4 Procedures Guide: Statistical Procedures, Second Edition Cary, NC: SAS Institute Inc. [Google Scholar]
- Safer DL & Hugo EM. (2006). Designing a control for a behavioral group therapy Behavioral Therapies, 37(2), 120–130. 10.1016/j.beth.2005.06.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satterfield JM., Spring B, Brownson RC, Mullen EJ, Newhouse RP, Walker BB, & Whitlock EP (2009). Toward a transdisciplinary model of evidence-based practice. The Milbank Quarterly, 87(2), 368–390. 10.1111/j.1468-0009.2009.00561.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sekhon M, Cartwright M, & Francis JJ (2017). Acceptability of healthcare interventions: an overview of reviews and development of a theoretical framework. BMC Health Services Research, 17(1), 88–100. 10.1186/s12913-017-2031-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Street RL Jr, Makoul G, Arora NK, & Epstein RM (2009). How does communication heal? pathways linking clinician-patient communication to health outcomes. Patient Education and Counseling, 74(3), 295–301. 10.1016/j.pec.2008.11.015 [DOI] [PubMed] [Google Scholar]
- Tapp H, Shade L, Mahabaleshwarkar R, Taylor YJ, Ludden T, & Dulin M,F (2017). Results from a pragmatic prospective cohort study: Shared decision making improves outcomes for children with asthma. Journal of Asthma, 54(4), 392–402. 10.1080/02770903.2016.1227333 [DOI] [PubMed] [Google Scholar]
- Tepper RS, Wise RS, Covar R, Irvin CG,, Kercsmar CM, Kraft M, Liu MC, O’Connor GT, Peters SP, Sorkness R, & Togias A (2012). Asthma outcomes: pulmonary physiology. Journal of Allergy & Clinical Immunology, 129(3 Suppl), S65–87. 10.1016/j.jaci.2011.12.986 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss BD, Mays MZ, Martz W, Castro KM, DeWalt DA, Pignone MP, Mockbee J, & Hale FA (2005). Quick assessment of literacy in primary care: The newest vital sign. Annals of Family Medicine, 3(6), 514–522. 10.1370/afm.405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SR, Strub P, Buist AS, Knowles SB, Lavori PW, Lapidus J, & Vollmer WM (2010). Shared treatment decision making improves adherence and outcomes in poorly controlled asthma. American Journal of Respiratory and Critical Care Medicine, 181(6). 566–577. 10.1164/rccm.200906-0907OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu AC, Butler MG, Li L, Fung V, Kharbanda EO, Larkin EK, Vollmer WM, Miroshnik I, Davis RL, Lieu TA, & Soumerai SB(2015). Primary adherence to controller medications for asthma is poor. Annals of the American Thoracic Society, 12(2), 161–166. 10.1513/AnnalsATS.201410-459OC [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos T, Flaxman AD, Naghavi M, Lozano R, Michaud C, Ezzati M, Shibuya K, Salomon JA, Abdalla S, Aboyans V, Abraham J, Ackerman I, Aggarwal R, Ahn SY, Ali MK, Alvarado M. anderson, anderson HR, L.M. andrews KG, … Memish ZA. (2012). Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet, 380(9859), 2163–2196. 10.1016/S0140-6736(12)61729-2 [DOI] [PMC free article] [PubMed] [Google Scholar]