Abstract
Objectives
Delirium is common in older adults, especially following hospitalization. As low vitamin D levels may be associated with increased delirium risk, we aimed to determine the prognostic value of blood vitamin D levels, extending our previous genetic analyses of this relationship.
Design
Prospective cohort analysis.
Setting
Community-based cohort study of adults from 22 cities across the United Kingdom (the UK Biobank).
Participants
Adults aged 60 years or greater by the end of follow-up in the linked hospital inpatient admissions data, up to 14 years after baseline (n=351,320).
Measurements
At baseline, serum vitamin D (25-OH-D) levels were measured. We used time-to-event models to estimate Hazard Ratios (HR) and 95% Confidence Intervals (CIs) for the association between vitamin D deficiency and incident hospital-diagnosed delirium, adjusted for age, sex, assessment month, assessment center, and ethnicity. We performed Mendelian randomization genetic analysis in European participants to further investigate vitamin D and delirium risk.
Results
3,634 (1.03%) participants had at least one incident hospital-diagnosed delirium episode. Vitamin D deficiency (<25 nmol/L) predicted a large incidence in delirium (HR 2.49: 95% CIs 2.24 to 2.76, p=3*10−68; compared to >50 nmol/L). Increased risk was not limited to the deficient group: insufficient levels (25–50 nmol/L) were also at increased risk (HR 1.38: 1.28 to 1.49, p=4*10−18). The association was independent of calcium levels, hospital-diagnosed fractures, dementia, and other relevant co-factors. In genetic analysis, participants carrying more vitamin D-increasing variants had reduced likelihood of incident delirium diagnosis (HR 0.80 per standard deviation increase in genetically instrumented vitamin D: 0.73 to 0.87; p=2*10−7).
Conclusion
Progressively lower vitamin D levels predicted increased risks of incident hospital-diagnosed delirium, and genetic evidence supports a shared causal pathway. As low vitamin D levels are simple to detect and inexpensive and safe to correct, an intervention trial to confirm these results is urgently needed.
Keywords: Delirium, vitamin D, risk factor, biomarker, genetic
Introduction
Delirium is an acute fluctuating change in cognition associated with inattention, disorganized thinking, or altered level of consciousness, and is common among hospitalized older adults1. It is potentially preventable and often under-recognized in clinical practice2, affecting 23% of acute hospital admissions in adults3, with considerable economic and societal costs4. Diagnosis rates in the community are much lower (1–2%)5. Causes of delirium are multi-factorial involving both underlying or predisposing (e.g. dementia, advanced age), and precipitating factors, often acute events (e.g. hospitalization, surgery, anesthesia, infection), with inflammation, polypharmacy, constipation, catheterization, environment, pain, and stroke also implicated6.
There is increasing interest in the role of vitamin D in delirium and dementia, with a recent meta-analysis demonstrating a correlation between low vitamin D and reduced cognition7. Nevertheless, most of the studies included were observational and this effect has not been replicated in interventional studies with supplementation of vitamin D7. A further systematic review indicated a potential link between low levels of vitamin D and development of dementia8. In our previous genetics study using Mendelian randomization methods we found evidence for a causal link between lower vitamin D levels and higher risks of incident episodes of delirium in hospital inpatient records in the United Kingdom (UK) Biobank, however serum vitamin D levels were not available at the time9. Here we build upon this work by combining serum vitamin D levels, genetic information, and 4 additional years of hospital inpatient follow-up to further investigate this relationship.
In this study we aimed to estimate the association between serum vitamin D levels and risk of incident hospital-diagnosed delirium in a large community volunteer sample. Although delirium is underdiagnosed in the hospital setting2 previous work has shown that delirium diagnoses made in the hospital setting are accurate with a high level of specificity10. We also aimed to extend our previous genetic analysis9 with the increased numbers of incident delirium cases now available in the UK Biobank (n=3,634 up from 544 in our previous work).
Methods
The UK Biobank recruited 503,325 community-based volunteers aged 40–70 between 2006 and 2010 from across the UK11. Data collected at the baseline assessment included extensive questionnaires on demographic, health, and lifestyle information. Anthropometric measures were also taken, in addition to blood samples for future biochemical and genetic analysis. Ethical approval for the UK Biobank study was obtained from the North West Multi-Centre Research Ethics Committee.
Serum vitamin D
Serum 25-hydroxyvitamin D (25[OH]D, a proxy for vitamin D levels) measurement (in nmol/L) was performed by the immunoassay analyzer DiaSorin Liaison XL, with data on 448,376 participants at baseline passing the quality control procedures applied by the UK Biobank central team12 (see UK Biobank report for sensitivity, inter-assay variability, and other information13).
We performed exploratory analysis on the following potential vitamin D covariates: age, sex, self-reported ethnicity (split into 6 groups: “White,” “Asian,” “Black”, “Other,” “Mixed,” or “Missing”), assessment month (season), and assessment center (see Supplementary Methods for details). We adjusted for these covariates in all analyses.
Participants were split into three vitamin D level groups according to the United Kingdom National Institute for Health and Care Excellence (NICE) guidelines for the management of vitamin D deficiency or insufficiency in adults14: deficient for vitamin D if serum 25[OH]D levels are less than 25 nmol/L; insufficient vitamin D if serum 25(OH)D levels are in the range of 25–50 nmol/L; vitamin D levels sufficient if serum 25(OH)D levels are above 50 nmol/L.
Delirium diagnosis
Follow-up disease ascertainment from hospital admissions records were available up to 14 years after assessment (end March 2020: data from Wales or Scotland were censored to 29 Feb 2016 and 31 October 2016, respectively). 270,299 of the 351,320 participants (76.9%) included in this analysis had at least one hospital admission after the baseline assessment. Diagnosis of delirium was ascertained using ICD-10 code F05 (see Supplementary Table S1 for all ICD-10 codes used in this analysis). Due to the rarity of delirium diagnoses before age 6015 (of the 3,634 participants with a hospital diagnosis of delirium only 44 occurred before the age of 60), and that delirium in younger groups may have different aetiology, participants were excluded if they did not reach the age of 60 by the date of censoring. Participants with a previous delirium diagnosis at baseline (n=45) were excluded. In the primary analysis no other exclusions were made: see Supplementary Methods for details on sensitivity analyses such as the effect of exclusions, e.g. bone fractures.
Analysis of vitamin D association with incident delirium
351,320 participants aged 60 plus at any time during follow-up had sufficient data for analysis of vitamin D and risk of incident delirium. STATA (v15.1) was used for analysis. Cox’s proportions hazards regression models estimated the association between vitamin D and incident delirium, with adjustment for age, sex, assessment center, assessment month, and self-reported ethnicity (see Supplementary Methods for details). Visual inspection of Kaplan-Meier plots, and application of the STATA function èstat phtest, detail` to estimate Schoenfeld residuals, were used to test for violations of the proportional-hazards assumption.
To model the non-linear effect of vitamin D (nmol/L) on rate of incident delirium diagnosis from Cox’s proportions hazards regression models we used the natural polynomial smoothing spline function in R (v4.0.2) package `psplinè (v1.0–18) and package `survival` (v3.1–12). We used default options for the smoothing parameters (modifying these did not meaningfully affect the results).
Genetic data
Genotyping and quality control were performed centrally by the UK Biobank team16. In brief, directly genotyped genetic variants (n=805,426) are available in 488,377 UK Biobank participants, from two almost identical platforms sharing >95% of variants: the Affymetrix Axiom UKB array (in 438,427 participants) and the Affymetrix UKBiLEVE array (in 49,950 participants). Genotype imputation was successful in 487,442 participants and increased the number of genetic variants to ~96 million16.
Mendelian randomization analysis
Mendelian randomization (MR) analyses are used to determine whether an association between a risk factor (e.g. vitamin D) and an outcome (e.g. delirium) may share a causal pathway. If individuals carrying more vitamin D-increasing genetic variants have greater risk of delirium, this supports the hypothesis that there is a shared causal pathway. We previously applied these methods to an earlier version of the UK Biobank data9 and here extend the analysis using the longer follow-up now available (n=3,405 delirium cases, up from 544 in our previous work). Briefly, known genetic variants associated with circulating 25[OH]D concentration were extracted from a large meta-analysis by Jiang et al.17 that was independent of the UK Biobank cohort. R (v4.0.2) packages `MendelianRandomization` (v0.4.2) and RadialMR (v0.4)18 were used. See Supplementary Methods for details.
Results
We analyzed 351,320 UK Biobank participants who reached the age of 60 before the end of the follow-up period and had complete data (end March 2020, see Methods for details, see Supplementary Figure S1 for cohort flowchart). There were 3,634 (1.0%) participants with an incident delirium diagnosis in the hospital admissions data (Table 1).
Table 1:
Summary statistics of 351,320 UK Biobank participants eligible for primary analysis
| Mean (SD) | Min, Max | |
|---|---|---|
| Age at baseline, years | 60.39 (5.76) | 47.06, 73.89 |
| Age at end of follow-up or death, years | 70.95 (5.71) | 60.00, 86.28 |
| Vitamin D at baseline, nmol/L | 49.45 (20.93) | 10, 335 |
| Time to first delirium episode, years (n=3,634) | 8.63 (2.25) | 0.11, 12.84 |
| Time to death, years (n=23,584) | 7.22 (3.07) | 0.01, 13.06 |
| N | %* | |
| Sex, females | 187,032 | 53.24 |
| Vitamin D, categories | ||
| Sufficient (>50 nmol/L) | 162,514 | 46.26 |
| Insufficient (25 to 50 nmol/L) | 145,890 | 41.53 |
| Deficient (<25 nmol/L) | 42,916 | 12.22 |
| Self-reported ethnicity^ | ||
| White | 335,517 | 95.5 |
| Asian | 6,135 | 1.8 |
| Black | 4,121 | 1.2 |
| Other | 2,486 | 0.7 |
| Mixed | 1,469 | 0.4 |
| Prefer not to answer / Do not know / Missing | 1,592 | 0.5 |
| Highest education level attained^ | ||
| None | 70,843 | 20.4 |
| CSEs/GCSEs/O-levels | 56,432 | 16.3 |
| A-levels/NVQ/HND/HNC | 59,282 | 17.1 |
| Professional qualification (e.g. nursing, teaching) | 53,392 | 15.4 |
| College or University Degree | 107,108 | 30.9 |
| Smoking status | ||
| Never | 185,203 | 53.0 |
| Former | 131,260 | 37.6 |
| Current | 33,053 | 9.5 |
| Died during follow-up | 23,584 | 6.7 |
| Delirium during follow-up | 3,634 | 1.0 |
| Any recorded hospital admission during follow-up | 270,299 | 76.9 |
| Genetically European, with vitamin D genetics | 326,558 | 93.0 |
% of total participants without missing data for that phenotype
combined groups - see Supplementary Information for more detailed sub-groups
We observed significant variation in vitamin D associated with season (highest average levels recorded in August, lowest in February: Supplementary Table S2 and Supplementary Figure S2), assessment center (highest average levels recorded in Cardiff, lowest in Glasgow: Supplementary Table S3), and self-reported ethnicity (highest average levels in participants reporting “white” ethnicity, lowest in those reporting any Asian ethnicity: Supplementary Table S4 and Supplementary Table S5).
Vitamin D deficiency is associated with increased risk of incident delirium
We estimated the effect of serum vitamin D (nmol/L) on rates of incident delirium diagnosis in Cox’s proportional hazards regression models adjusted for age, sex, assessment center, assessment month, and self-reported ethnicity, first using a smoothing spline parameter to model continuous and non-linear effects: decreasing vitamin D was significantly associated with risk of incident delirium (spline p=1.8*10−32), with risk progressively increasing below ~75nmol/L (Figure 1A).
Figure 1: Serum vitamin D and rates of incident delirium diagnosis.
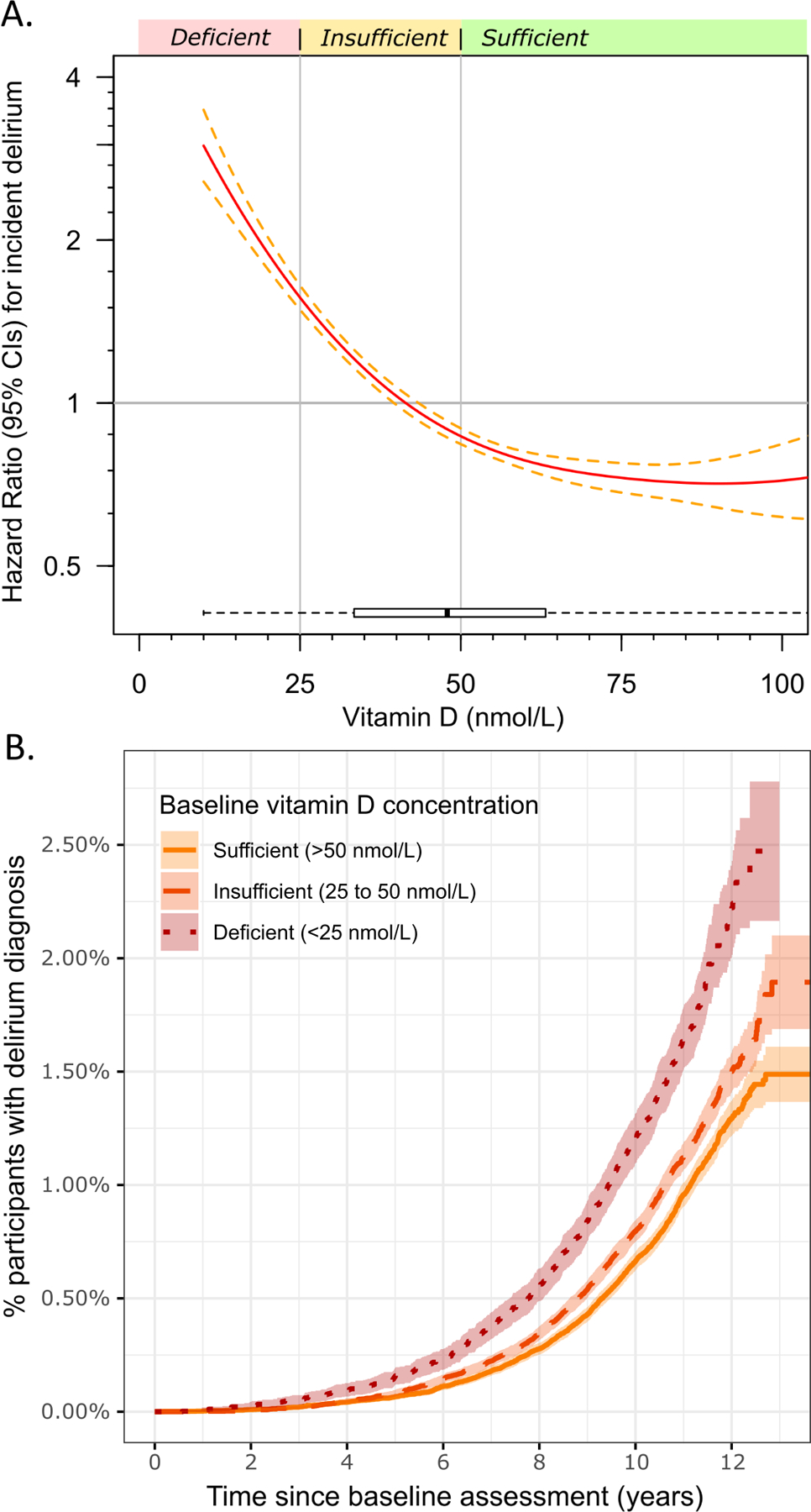
A) Analysis of serum vitamin D (nmol/L) at baseline and rates of incident delirium diagnosis using Cox’s proportional hazards regression models adjusted for age, sex, assessment center, assessment month, and self-reported ethnicity. A smoothing spline function was applied to determine the non-linear effect of vitamin D on risk of incident delirium. The x-axis is limited to 100 nmol/L for clarity, see Supplementary Figure S3 for the unrestricted plot. B) Unadjusted cumulative event plot showing the proportion of the participants with a diagnosis of delirium in the hospital in three groups, based on baseline vitamin D sufficiency. R package `survminer` (v0.4.8) used for plot B).
Participants with deficient vitamin D levels (<25 nmol/L) at the baseline assessment were at increased risk for incident delirium (Hazard Ratio 2.49: 95% Confidence Intervals 2.25 to 2.76, p=3*10−68) compared to those with sufficient levels (≥50 nmol/L) in Cox’s proportional hazards regression models adjusted for age, sex, assessment center, assessment month, and self-reported ethnicity (Figure 1B). Participants with insufficient levels were also at increased risk (HR 1.38: 1.28 to 1.49, p=4*10−18).
In sex-stratified analysis the effect of vitamin D deficiency on risk of incident delirium was similar in males compared to females (n=164,288 males, HR 2.51: 2.19 to 2.88, p=3*10−39; n=187,032 females, HR 2.50: 2.14 to 2.92, p=7*10−31), and there was no significant interaction (p>0.05).
We repeated the analysis only in the 335,517 participants (96%) who self-reported as white, as individuals of other ethnic groups are known to have lower vitamin D levels whilst retaining the same level of bioavailable vitamin D24. The association between vitamin D deficiency and incident delirium was very similar to the overall estimate (HR 2.58: 2.32 to 2.87, p=7*10−70) and there was no significant interaction (p>0.05). Due to low numbers of non-white participants (see Table 1) analysis of other ethnic groups was underpowered.
Sensitivity analyses
In sensitivity analyses using Fine & Gray competing risks regression – accounting for mortality as the competing risk (23,584 of 351,320 participants died during follow-up) – the results remained consistent (deficient sub HR 2.33: 2.10 to 2.58, p=3*10−56; insufficient sHR 1.36: 1.26 to 1.47, p=1*10−15).
The results were also consistent after multiple additional adjustments and exclusions were made (Table 2). First with adjustment for baseline smoking status and educational attainment, a proxy for socio-economic status (vitamin D deficiency HR 2.38: 2.14 to 2.64, p=2*10–59). Next excluding 30,528 participants who reported taking vitamin D supplements at baseline (HR 2.46: 2.21 to 2.74, p=2*10−59). Next, additionally excluding 45,197 participants with hospital-diagnosed bone fractures, CKD, dementia, liver disease (any), or Parkinson’s disease (HR 2.40: 2.00 to 2.89, p=6*10−21). Similar trends were seen for insufficient vitamin D levels (Table 2).
Table 2:
Vitamin D deficiency is associated with increased rates of incident delirium
| Model | Vitamin D at baseline | N | N delirium | Person-years | HR (95% CIs) | p-value |
|---|---|---|---|---|---|---|
| Model 1* | Sufficient (>50 nmol/L) | 162,514 | 1,484 | 1,728,790 | ||
| Insufficient (25–50 nmol/L) | 145,890 | 1,521 | 1,530,801 | 1.38 (1.28 to 1.49) | 3.9*10–18 | |
| Deficient (<25 nmol/L) | 42,916 | 629 | 440,778 | 2.49 (2.24 to 2.76) | 3.4*10–68 | |
| + adj. for education + smoking status~ | 160,120 | 1,439 | 1,703,633 | |||
| Insufficient (25–50 nmol/L) | 143,609 | 1,472 | 1,507,279 | 1.37 (1.27 to 1.48) | 1.3*10–16 | |
| Deficient (<25 nmol/L) | 42,012 | 618 | 431,646 | 2.38 (2.14 to 2.64) | 1.5*10–59 | |
| + excl. vitamin D supplements | 140,274 | 1,246 | 1,491,439 | |||
| Insufficient (25–50 nmol/L) | 134,247 | 1,374 | 1,408,789 | 1.40 (1.29 to 1.52) | 6.2*10–17 | |
| Deficient (<25 nmol/L) | 40,692 | 598 | 418,019 | 2.46 (2.21 to 2.74) | 2.1*10–59 | |
| + excl. co-morbidities^ | 121,070 | 448 | 1,290,397 | |||
| Insufficient (25–50 nmol/L) | 115,022 | 473 | 1,209,590 | 1.32 (1.15 to 1.51) | 4.1*10–5 | |
| Deficient (<25 nmol/L) | 33,924 | 211 | 349,874 | 2.40 (2.00 to 2.89) | 6.0*10–21 | |
| + adj. for calcium~ | 110,391 | 406 | 1,176,061 | |||
| Insufficient (25–50 nmol/L) | 105,366 | 447 | 1,107,064 | 1.37 (1.19 to 1.58) | 8.9*10–6 | |
| Deficient (<25 nmol/L) | 31,194 | 197 | 321,497 | 2.45 (2.02 to 2.97) | 2.6*10–20 | |
| + adj. Frailty Index~ | 92,593 | 327 | 987,394 | |||
| Insufficient (25–50 nmol/L) | 86,341 | 348 | 907,548 | 1.31 (1.12 to 1.54) | 6.1*10–4 | |
| Deficient (<25 nmol/L) | 24,556 | 153 | 253,166 | 2.33 (1.88 to 2.89) | 1.3*10–14 | |
Excl.=excluding diagnoses of. Adj.=models adjusted for. N=sample size included in the model. N delirium=the number of incident delirium cases included in the model. HR=Hazard Ratio. CIs=Confidence Intervals.
Cox’s proportional hazards regression models adjusted for age, sex, assessment centre, assessment month, and self-reported ethnicity.
at baseline assessment, participants with missing data excluded.
Ever diagnosed with bone fracture, CKD, dementia, liver disease, or Parkinson’s disease in the hospital admissions data to March 2020, prevalent or incident.
Vitamin D deficiency was associated with lower calcium levels (coef −0.020 mmol/L: −0.019 to −0.021, p=4*10−281), but there was no linear association between calcium levels and incident delirium diagnosis (HR per mmol/L 0.76: 0.52 to 1.10, p=0.14). In non-linear analysis calcium levels below 2.27mmol/L or greater than 2.57mmol/L were associated with increased delirium risk using a smoothing spline in Cox’s proportional hazards regression models adjusted for age, sex, assessment center, assessment month, and self-reported ethnicity (spline p=0.03, see Supplementary Figure S4). The association between vitamin D and incident delirium remained consistent after inclusion of the calcium spline term in the model (vitamin D deficiency HR 2.52: 2.24 to 2.83, p=2*10−54).
To explore the known reduced sun exposure in frail, less mobile individuals25 we performed an analysis adjusted for baseline frailty using the Frailty Index count of health deficits23 as a continuous covariate (n=232,087 with complete data); the estimate was modestly attenuated (HR 2.16: 1.89 to 2.47, p=8*10−30) but not significantly different (FI~vitamin D interaction p>0.05) (Table 2).
We performed a single analysis with adjustment for age, sex, assessment center, assessment month, self-reported ethnicity, smoking status, educational attainment, calcium levels, and Frailty Index, and excluding participants taking vitamin D supplements, with hospital-diagnosed CKD, bone fractures, dementia, liver disease, and Parkinson’s disease: the association between vitamin D deficiency and incident delirium remained consistent, albeit slightly attenuated, in Cox’s proportional hazards regression models (n=203,490 in analysis, HR 2.33: 1.88 to 2.89, p=1*10−14: Table 2). Participants with insufficient vitamin D levels were also still at increased risk (HR 1.31: 1.12 to 1.54, p=6*10−4).
Separately, we repeated the main analysis restricted to those participants hospitalized during the follow-up period (270,299 of 351,320, 76.9%) and found that the association between vitamin D deficiency and incident delirium was similar to that in all participants (HR 2.45: 2.21 to 2.72, p=8*10−66).
We also investigated whether the effect of vitamin D on delirium was dependent on the delirium diagnosis resulting from a surgical procedure (i.e. post-operative delirium only). Of 3,634 incident delirium cases, 1,473 (40.5%) were <72 hours after a recorded hospital operation. The effect of vitamin D deficiency on risk of incident delirium was consistent in an analysis restricted to only post-operative cases (HR 2.60: 2.22 to 3.06, p=2*10−31) compared to an analysis restricted to those delirium diagnoses made where no surgical procedure was recorded (n delirium=2,161: HR 2.48: 2.17 to 2.82, p=2*10−42).
Vitamin D-increasing genetic variants are associated with vitamin D levels
A genetic risk score (GRS) for the number of vitamin D-increasing variants each participant carried (weighted by the published effect on vitamin D levels by Jiang et a. 201817) were computed in the 326,558 UK Biobank participants of European ancestry that met the inclusion criteria for analysis (see Methods). The GRS was strongly associated with serum vitamin D (nmol/L) in linear regression models adjusted for age at vitamin D assessment, sex, assessment center, assessment month, and ethnicity (coefficient per SD of GRS 3.37: 95% CIs 3.30 to 3.44, p=1*10−2029). The proportion of the variation in vitamin D levels explained by the GRS was 1.2%).
Calcium serum levels (μmol/L) were increased in individuals with greater vitamin D GRS in linear regression models (coefficient per SD of GRS 1.03: 95% CIs 0.68 to 1.37, p=8*10−9). However, this association lost significance when vitamin D was included as a covariate (p=0.3), suggesting the effect of vitamin D GRS on calcium is via the effect on vitamin D.
Vitamin D-increasing genetic variants confirm reduced likelihood of incident delirium diagnosis
In MR analysis we found consistent evidence that higher circulating vitamin D reduced likelihood of incident hospital diagnosis of delirium: the primary analysis was of the MR-IVW penalized robust regression estimate (change in log(HR) for delirium per log(nmol/L) vitamin D = −0.48: 95% CIs −0.66 to −0.30, p=2×10−7) using the six vitamin D-associated variants from Jiang et al.17 (Table 3; Figure 2A). The HR for delirium per SD genetically instrumented log(vitamin D) is 0.799 (0.735 to 0.869). Sensitivity analysis using Radial IVW (Figure 2B) or excluding the large-effect SNP rs3755967 showing consistent effect size (Table 3), albeit attenuated significance. We found no evidence for horizontal pleiotropy with MR-Egger regression or Radial MR-Egger (intercept p>0.05, Table 3, Figure 2C). See Supplementary Table S6 for detailed results. In sensitivity analysis only analyzing the cases in the updated hospital admissions data since our previous publication9 (n=2,861 between Feb 2016 and March 2020) the association is consistent in effect but attenuated in significance compared to the analysis of diagnoses before Feb 2016 (n=544; see Supplementary Table S7 and Supplementary Figure S5).
Table 3:
Mendelian randomization estimates for the effect of circulating vitamin D on delirium
| Method * | Estimate^ | 95% CI | P | |
|---|---|---|---|---|
| IVW | −0.48 | −0.66 | −0.30 | 2×10−7 |
| Weighted median | −0.51 | −1.02 | 0.00 | 0.048 |
| MR-Egger | −0.41 | −0.73 | −0.10 | 0.010 |
| MR-Egger (intercept) | 0.00 | −0.03 | 0.02 | 0.755 |
| Radial IVW | −0.48 | −0.76 | −0.20 | 0.001 |
| Radial MR-Egger | −0.49 | −1.01 | 0.04 | 0.144 |
| Radial MR-Egger (intercept) | 0.01 | −0.87 | 0.90 | 0.977 |
| IVW (excluding rs3755967) | −0.41 | −1.01 | 0.18 | 0.173 |
IVW = penalized robust inverse-variance weighted regression (assumes there is no unbalanced horizontal pleiotropy); Weighted median = penalized weighted median estimate (assumes less than 50% of the weight in the analysis comes from invalid instruments); MR-Egger = penalized robust Egger regression (assumes the genetic variants’ effect is not correlated with any pleiotropic effect on the outcome); MR-Egger (intercept) = like IVW but the MR-Egger incept is not fixed, as deviation from the null is used to test for possible horizontal pleiotropy; Radial IVW = radial inverse-variance weighted regression using modified second-order weights (no significant outliers detected); Radial MR-Egger = intercept in unconstrained and assumes that pleiotropic effects are independent of the Radial weights.
ln(HR) per ln(vitamin D). See Supplementary Table S6 for full details.
Figure 2: Vitamin D-increasing genetic variants associated with reduced likelihood of incident delirium diagnosis.
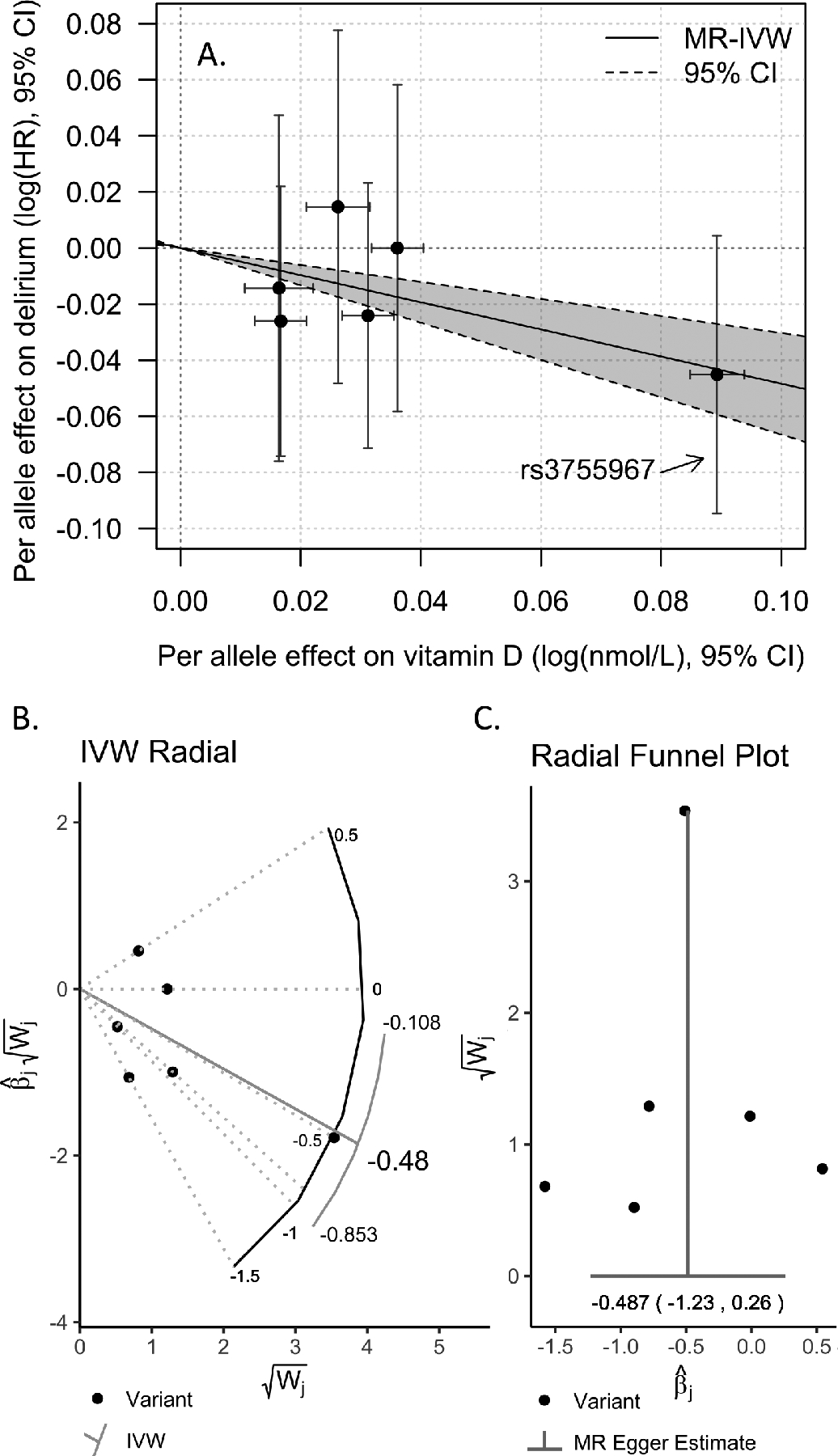
A) Six genetic variants are known to affect circulating 25(OH)D (vitamin D) levels (effect shown on the x-axis). We determined the association with risk of incident delirium for each genetic variant (shown on the y-axis). Mendelian randomization penalized robust inverse-weighted regression (MR-IVW) results (including 95% Confidence Intervals) show that genetic predisposition to higher serum vitamin D is associated with reduced likelihood of delirium diagnosis in the follow-up. B) Radial IVW plot of results using modified second-order weights (no significant outliers were detected). C) Radial MR-Egger funnel plot showing the regression intercept is not significantly different from the null (no evidence of pleiotropy).
Discussion
In this large prospective study of 351,320 community-based UK Biobank participants, aged 60 plus by the end of the 14 years follow-up, vitamin D levels predicted increased risks of incident hospital diagnosed delirium. Genetic evidence supports a shared causal pathway. The highest risk for delirium occurred in the vitamin D deficient group (<25 nmol/L), compared to those with sufficient levels (>50 nmol/L). Participants with vitamin D insufficiency (25 to 50 nmol/L) also had an increased risk of delirium at follow up, with a smaller effect size, suggesting a dose-response relationship. Genetic results with a larger sample than we previously reported (n=3,405: up from 544) continue to support a causal role overall, showing 20% reduction in delirium hazard per standard deviation of genetically instrumented vitamin D (limitations discussed below). This research has important clinical relevance and consequences; although delirium is an acute diagnosis, it is known to increase the risk of dementia26. Whether this is a causative role or an unmasking of underlying cognitive vulnerability is unclear, however prevention of delirium can potentially delay irreversible cognitive impairment27. Additionally, economic analyses in the USA have estimated that healthcare costs for patients with delirium are 2.5x greater than in patients without delirium28.
This work is consistent with the results of a previous retrospective cross sectional study revealing lower levels of vitamin D levels in patients with delirium in whom the levels were checked29. Low vitamin D levels were also more prevalent in patients with hip fractures and delirium (although the numbers included were small and prone to confounding)30. A further retrospective cohort study of 4,508 participants showed higher levels of hospital acquired delirium in those with lower vitamin D levels (checked pre-hospitalization). However, the number of delirium cases was only 4% and they concluded that a future randomized controlled trial would need to be conducted31.
The association between vitamin D and delirium is plausible considering its hypothesized neuroprotective role in preventing oxidative damage to nervous tissue and influence on neuromediator synthesis32. Vitamin D is also thought to affect the inflammatory processes within the brain that increase vulnerability to injury33. Studies in rat models have shown vitamin d has a protective effect on neurons from oxidative stress and the role of vitamin D in the growth and protection of neurones34. Vitamin D3 receptors have been found not only in brain neurons but also in spinal cord and peripheral nervous system35. In addition, vitamin D receptors (VDR) has been found in the hippocampus, an area of the brain affected by Alzheimer’s disease and other neurodegenerative conditions36. Research has also investigated the possible role of vitamin D in the reduction of β-amyloid in mouse models37. Vitamin D is also thought to impact systemic inflammation38, which during aging impacts comorbidities, frailty, and other outcomes implicated in the pathogenesis of delirium.
Previous Mendelian randomization studies with vitamin D have shown an association between vitamin D increasing alleles and Alzheimer’s disease39. However, this evidence is inconclusive or tentative in a Mendelian randomization systematic review40. Our previous paper was, to the best of our knowledge, the first report of Mendelian randomization to estimate the relationship between vitamin D and delirium9. The current paper takes the previous work forward by combining serum vitamin D levels and genetic information to further investigate this relationship. The genetic variants associated with vitamin D are known to directly influence its synthesis and metabolism17 suggesting a direct relationship between vitamin D and delirium; our Mendelian randomization analysis results are consistent with lack of pleiotropic effects (i.e. via other pathways than vitamin D). Although, the strength of the association between vitamin D genetic variants and incident delirium is attenuated when only using the “new” cases not included in our previous report9; further work is required for full confirmation. Supporting a causal role for vitamin D in mental health is a recent randomized clinical trial of vitamin D supplementation for 1 year in 200 older (≥70 years) adults who experienced a fall in the previous year; there were significant improvements in mental health (the Mental Component Summary of the Short Form Health Survey 36-item patient health survey) in the groups achieving the highest vitamin D levels at 12 months41, strongest in those who were deficient at baseline.
In this analysis we used vitamin D levels >50nmol/L to define “sufficient” levels, in line with the relevant United Kingdom National Institute for Health and Care Excellence (NICE) guidelines14. However in our non-linear analysis of vitamin D levels with risk of delirium (see Figure 1A) the lowest risk participants are those with ≥75nmol/L, a target recommended by The Endocrine Society in the USA for treating and preventing vitamin D deficiency42. Our data suggests that 50nmol/L may not be the optimum target for delirium prevention, though more data are needed.
This study has some limitations. The diagnosis of delirium for the purpose for this study was extracted from HES data and delirium is known to be underdiagnosed in hospital settings2. However, hospital diagnosis of delirium has been shown to be more precise than in the community, with a higher level of specificity10. UK Biobank is reflective of a generally younger age group, who were fit and able to attend assessment appointments and clinic visits. Although studying a younger population may limit generalizability to older adults, the mean age at end of follow up was 71 years old (range 60 to 86), meaning our ability to capture more typical delirium cases seen in older hospitalized patients is improving. Remarkably, measured vitamin D levels are highly predictive of incident delirium up to 14 years prior to diagnosis, showing it is a good biomarker. A related point to consider is that previous hypotheses suggested that the link between cognition and vitamin D deficiency may just be a marker of increased frailty associated with cognitive decline and less sun exposure25. We included adjustments for season and month of assessment, and test center as a measure to ameliorate the influence of variable sun exposure. Frail individuals are at increased risk of delirium22 yet the association between vitamin D deficiency and incident delirium was robust to adjustment for baseline frailty index (of course, frailty status can change, but this observation means deficiency was not just a marker of baseline frailty). Vitamin D deficiency can be associated with other medical conditions that could increase the incidence of delirium: we found the association to be robust to exclusion of hospital-diagnosed bone fractures, chronic kidney disease, and liver disease. Taken together our results, including genetic analysis which is not susceptible to reverse causation and other traditional confounding factors, suggest that vitamin D deficiency could be playing a more central role in delirium susceptibility.
Conclusion
In this study we have demonstrated that measured low vitamin D is associated with incident delirium, with genetic evidence supporting a causal role. Vitamin D deficiency is simple to rectify, at low cost and with minimal side effects. This study provides rationale for further interventional trials assessing the relationship between vitamin D supplementation and cognition, with a focus on delirium prevention. Our results suggest that older adults should be routinely screened for vitamin D levels during GP visits to help ensure that they are at sufficient levels in the event that they require hospitalization where risk for delirium increases considerably.
Supplementary Material
Supplementary Methods
Supplementary Results
Supplementary Table S1: ICD-10 codes for clinical conditions from the hospital inpatient data
Supplementary Table S2: vitamin D levels associated with assessment month
Supplementary Table S3: Vitamin D variation by assessment centre
Supplementary Table S4: UK Biobank ethnic group coding
Supplementary Table S5: collapsed groups and associations with vitamin D levels
Supplementary Table S6: Expanded version of Table 3.
Supplementary Table S7: Mendelian Randomization stratified by date of diagnosis
Supplementary Figure S1: Cohort flowchart
Supplementary Figure S2: Boxplot of vitamin D by assessment month
Supplementary Figure S3: Time-to-event model with smoothing spline function (vitamin D)
Supplementary Figure S4: Time-to-event model with smoothing spline function (calcium)
Supplementary Figure S5: Mendelian Randomization stratified by date of diagnosis
Acknowledgements
This study was generously supported by an award to LCP by the NIDUS delirium network (NIA R24AG054259 subaward 9581). JA was supported by an award to DM by the UK Medical Research Council (MR/M023095/1). DM and LCP are also supported by the University of Exeter Medical School and the University of Connecticut School of Medicine. JD is supported by the Alzheimer’s Society [grant: 338 (AS-JF-16b-007)]. JB is funded by an Establishing Excellence in England (E3) research award. GAK is a member of the Scientific Advisory Board for NIDUS (NIA R24AG054259) and is supported by the Travelers Chair in Geriatrics and Gerontology.
Access to UK Biobank Resource was granted under Application Number 14631. We would like to thank UK Biobank participants and coordinators for this dataset. The authors would like to acknowledge the use of the University of Exeter High-Performance Computing (HPC) facility in carrying out this work.
Sponsor’s Role
The sponsor’s had no role in the design, methods, subject recruitment, data collections, analysis or preparation of paper.
Footnotes
Conflict of Interest
The authors have no conflicts.
References
- 1.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: Development and Validation of a New Scoring System for Delirium Severity in 2 Cohorts. Ann Intern Med. 2014;160(8):526. doi: 10.7326/M13-1927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ritter SRF, Cardoso AF, Lins MMP, Zoccoli TLV, Freitas MPD, Camargos EF. Underdiagnosis of delirium in the elderly in acute care hospital settings: lessons not learned. Psychogeriatrics. 2018;18(4):268–275. doi: 10.1111/psyg.12324 [DOI] [PubMed] [Google Scholar]
- 3.Gibb K, Seeley A, Quinn T, et al. The consistent burden in published estimates of delirium occurrence in medical inpatients over four decades: a systematic review and meta-analysis study. Age Ageing. 2020;49(3):352–360. doi: 10.1093/ageing/afaa040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Leslie DL, Inouye SK. The importance of delirium: Economic and societal costs. J Am Geriatr Soc. 2011;59(SUPPL. 2):241–243. doi: 10.1111/j.1532-5415.2011.03671.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fong TG, Tulebaev SR, Inouye SK. Delirium in elderly adults: diagnosis, prevention and treatment. Nat Rev Neurol. 2009;5(4):210–220. doi: 10.1038/nrneurol.2009.24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Goodwill AM, Szoeke C. A Systematic Review and Meta-Analysis of The Effect of Low Vitamin D on Cognition. J Am Geriatr Soc. 2017;65(10):2161–2168. doi: 10.1111/jgs.15012 [DOI] [PubMed] [Google Scholar]
- 8.Sommer I, Griebler U, Kien C, et al. Vitamin D deficiency as a risk factor for dementia: a systematic review and meta-analysis. BMC Geriatr. 2017;17(1):16. doi: 10.1186/s12877-016-0405-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bowman K, Jones L, Pilling LC, et al. Vitamin D levels and risk of delirium: A mendelian randomization study in the UK Biobank. Neurology. 2019;92(12):e1387–e1394. doi: 10.1212/WNL.0000000000007136 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Leslie DL, Agostini JV. A chart-based method for identification of delirium: Validation compared with interviewer ratings using the confusion assessment method. J Am Geriatr Soc. Published online 2005. doi: 10.1111/j.1532-5415.2005.53120.x [DOI] [PubMed] [Google Scholar]
- 11.Sudlow C, Gallacher J, Allen N, et al. UK Biobank: An Open Access Resource for Identifying the Causes of a Wide Range of Complex Diseases of Middle and Old Age. PLoS Med. 2015;12(3). doi: 10.1371/journal.pmed.1001779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.UK Biobank. UK Biobank companion document for serum biomarker data. Published 2019. Accessed May 20, 2019 http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=1227
- 13.UK Biobank. UK Biobank biochemistry assay quality procedures. Published 2019. http://biobank.ctsu.ox.ac.uk/crystal/refer.cgi?id=5636
- 14.NICE - National Institute for Health and Care Excellence. Vitamin D deficiency in adults - treatment and prevention guidelines. Published 2018. Accessed April 20, 2019 https://cks.nice.org.uk/vitamin-d-deficiency-in-adults-treatment-and-prevention
- 15.Inouye SK, Westendorp RGJ, Saczynski JS. Delirium in elderly people. Lancet. 2014;383(9920):911–922. doi: 10.1016/S0140-6736(13)60688-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bycroft C, Freeman C, Petkova D, et al. The UK Biobank resource with deep phenotyping and genomic data. Nature. 2018;562(7726):203–209. doi: 10.1038/s41586-018-0579-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jiang X, O’Reilly PF, Aschard H, et al. Genome-wide association study in 79,366 European-ancestry individuals informs the genetic architecture of 25-hydroxyvitamin D levels. Nat Commun. 2018;9(1):260. doi: 10.1038/s41467-017-02662-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bowden J, Spiller W, Del Greco MF, et al. Improving the visualization, interpretation and analysis of two-sample summary data Mendelian randomization via the Radial plot and Radial regression. Int J Epidemiol. 2018;47(4):1264–1278. doi: 10.1093/ije/dyy101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jason P Fine RJG. A Proportional Hazards Model for the Subdistribution of a Competing Risk. J Am Stat Assoc. 1999;94(446):496–509. doi: 10.1080/01621459.1999.10474144 [DOI] [Google Scholar]
- 20.Ebersbach G, Ip CW, Klebe S, et al. Management of delirium in Parkinson’s disease. J Neural Transm. 2019;126(7):905–912. doi: 10.1007/s00702-019-01980-7 [DOI] [PubMed] [Google Scholar]
- 21.Critchley RJ, Khan SK, Yarnall AJ, Parker MJ, Deehan DJ. Occurrence, management and outcomes of hip fractures in patients with Parkinson’s disease: Fig. 1. Br Med Bull. 2015;115(1):135–142. doi: 10.1093/bmb/ldv029 [DOI] [PubMed] [Google Scholar]
- 22.Quinlan N, Marcantonio ER, Inouye SK, Gill TM, Kamholz B, Rudolph JL. Vulnerability: The crossroads of frailty and delirium. J Am Geriatr Soc. 2011;59(SUPPL. 2):262–268. doi: 10.1111/j.1532-5415.2011.03674.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Williams DM, Jylhävä J, Pedersen NL, Hägg S. A Frailty Index for UK Biobank Participants. Journals Gerontol Ser A. 2019;74(4):582–587. doi: 10.1093/gerona/gly094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Powe CE, Evans MK, Wenger J, et al. Vitamin D-binding protein and vitamin D status of black Americans and white Americans. N Engl J Med. 2013;369(21):1991–2000. doi: 10.1056/NEJMoa1306357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sato Y, Asoh T, Oizumi K. High prevalence of vitamin D deficiency and reduced bone mass in elderly women with Alzheimer’s disease. Bone. 1998;23(6):555–557. [DOI] [PubMed] [Google Scholar]
- 26.Witlox J, Eurelings LSM, de Jonghe JFM, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304(4):443–451. doi: 10.1001/jama.2010.1013 [DOI] [PubMed] [Google Scholar]
- 27.Fong TG, Davis D, Growdon ME, Albuquerque A, Inouye SK. The interface between delirium and dementia in elderly adults. Lancet Neurol. 2015;14(8):823–832. doi: 10.1016/S1474-4422(15)00101-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leslie DL. One-Year Health Care Costs Associated With Delirium in the Elderly Population. Arch Intern Med. 2008;168(1):27. doi: 10.1001/archinternmed.2007.4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ford J, Hategan A, Bourgeois JA, Tisi DK, Xiong GL. Hypovitaminosis D in Delirium: a Retrospective Cross-sectional Study. Can Geriatr J. 2013;16(4):186. doi: 10.5770/CGJ.16.79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Torbergsen AC, Watne LO, Frihagen F, Wyller TB, Brugaard A, Mowe M. Vitamin deficiency as a risk factor for delirium. Eur Geriatr Med. 2015;6(4):314–318. doi: 10.1016/j.eurger.2014.09.002 [DOI] [Google Scholar]
- 31.Quraishi SA, Litonjua AA, Elias KM, et al. Association between pre-hospital vitamin D status and hospital-acquired new-onset delirium. Br J Nutr. 2015;113(11):1753–1760. doi: 10.1017/S0007114515001245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wrzosek M, Łukaszkiewicz J, Wrzosek M, et al. Vitamin D and the central nervous system. Pharmacol Rep. 2013;65(2):271–278. [DOI] [PubMed] [Google Scholar]
- 33.Anjum I, Jaffery SS, Fayyaz M, Samoo Z, Anjum S. The Role of Vitamin D in Brain Health: A Mini Literature Review. Cureus. 2018;10(7):e2960. doi: 10.7759/cureus.2960 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.AlJohri R, AlOkail M, Haq SH. Neuroprotective role of vitamin D in primary neuronal cortical culture. eNeurologicalSci. 2019;14:43–48. doi: 10.1016/j.ensci.2018.12.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kalueff AV, Eremin KO, Tuohimaa P. Mechanisms of Neuroprotective Action of Vitamin D3. Biochem. 2004;69(7):738–741. doi: 10.1023/B:BIRY.0000040196.65686.2f [DOI] [PubMed] [Google Scholar]
- 36.Gezen-Ak D, Dursun E, Yilmazer S. Vitamin D inquiry in hippocampal neurons: consequences of vitamin D-VDR pathway disruption on calcium channel and the vitamin D requirement. Neurol Sci. 2013;34(8):1453–1458. doi: 10.1007/s10072-012-1268-6 [DOI] [PubMed] [Google Scholar]
- 37.Grimm M, Thiel A, Lauer A, et al. Vitamin D and Its Analogues Decrease Amyloid-β (Aβ) Formation and Increase Aβ-Degradation. Int J Mol Sci. 2017;18(12):2764. doi: 10.3390/ijms18122764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gonçalves de Carvalho CMR, Ribeiro SML. Aging, low-grade systemic inflammation and vitamin D: a mini-review. Eur J Clin Nutr. 2017;71(4):434–440. doi: 10.1038/ejcn.2016.177 [DOI] [PubMed] [Google Scholar]
- 39.Mokry LE, Ross S, Morris JA, Manousaki D, Forgetta V, Richards JB. Genetically decreased vitamin D and risk of Alzheimer disease. Neurology. 2016;87(24):2567–2574. doi: 10.1212/WNL.0000000000003430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kuźma E, Hannon E, Zhou A, et al. Which Risk Factors Causally Influence Dementia? A Systematic Review of Mendelian Randomization Studies Gustafson D, ed. J Alzheimer’s Dis. 2018;64(1):181–193. doi: 10.3233/JAD-180013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gugger A, Marzel A, Orav EJ, et al. Effect of Monthly High-Dose Vitamin D on Mental Health in Older Adults: Secondary Analysis of a RCT. J Am Geriatr Soc. 2019;67(6):1211–1217. doi: 10.1111/jgs.15808 [DOI] [PubMed] [Google Scholar]
- 42.Holick MF, Binkley NC, Bischoff-Ferrari HA, et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline. J Clin Endocrinol Metab. 2011;96(7):1911–1930. doi: 10.1210/jc.2011-0385 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods
Supplementary Results
Supplementary Table S1: ICD-10 codes for clinical conditions from the hospital inpatient data
Supplementary Table S2: vitamin D levels associated with assessment month
Supplementary Table S3: Vitamin D variation by assessment centre
Supplementary Table S4: UK Biobank ethnic group coding
Supplementary Table S5: collapsed groups and associations with vitamin D levels
Supplementary Table S6: Expanded version of Table 3.
Supplementary Table S7: Mendelian Randomization stratified by date of diagnosis
Supplementary Figure S1: Cohort flowchart
Supplementary Figure S2: Boxplot of vitamin D by assessment month
Supplementary Figure S3: Time-to-event model with smoothing spline function (vitamin D)
Supplementary Figure S4: Time-to-event model with smoothing spline function (calcium)
Supplementary Figure S5: Mendelian Randomization stratified by date of diagnosis


