Abstract
Background:
There continues to be a need for HIV prevention options that women can initiate and use autonomously. The dapivirine vaginal ring (VR) has been shown to have a favorable safety profile and reduce the risk of HIV-1 acquisition. We report on women’s experiences with VR adherence during the MTN-025/HIV Open-label Prevention Extension (HOPE) study and responses to Residual Drug Level (RDL) results.
Setting:
Ten women at each of the six HOPE research sites in Lilongwe, Malawi; Durban (2 sites) and Johannesburg, South Africa; Kampala, Uganda; and Chitungwiza, Zimbabwe were randomly selected (n=60).
Methods:
Following confirmation of eligibility criteria, in-depth interviews were conducted where available RDL results were presented.
Results:
Many women with low RDL release measurements deflected blame onto other factors (the ring, the drug and faulty testing machines) and distrust of the testing method. The disclosure of RDL results enabled some users to discuss their challenges experienced (fear of partner objections, perceived side effects and removals during menses). Consistent users reported important motivators (support from others, protection from HIV and enhanced sexual experiences from the VR).
Conclusion:
The VR provided a sense of security for some women however adherence was still callenging for others regardless of it being a female controlled, long acting HIV prevention technology. Adherence measurements may not be sustainable in the real world implementation of the VR, though they can be seen as a benefit as they provide a better understanding of actual product use and provide women with a platform to discuss their experiences.
Keywords: women, adherence, dapivirine vaginal ring, qualitative research, sub-Saharan Africa, residual drug levels
INTRODUCTION
Women in sub-Saharan Africa are disproportionately at risk for human immunodeficiency virus (HIV) compared to men in the same region and women in other parts of the world [1]. Although condoms are effective in preventing HIV transmission during sexual intercourse, many women find it difficult to negotiate their use with male partners due to many behavioural, social and structural factors [2]. When taken as indicated, oral pre-exposure prophylaxis (PrEP) is a safe and effective HIV prevention method for both men and women, however the uptake and consistent use of the daily dosing regimen has been challenging for women in clinical trials [3, 4] and demonstration projects [5].
Studies have shown that adherence to HIV prevention technologies has been challenging for women. The FEM-PREP team attributed poor adherence to low HIV risk perception and difficulty taking daily oral pills [4]. In VOICE-C [6], a qualitative sub-study following the VOICE trial of vaginal gel and oral tablets for HIV prevention, women reported that unknown efficacy, distrust of researchers, dangers of research participation and an association of antiretroviral’s with illness led to poor adherence. In VOICE-D [7], another qualitative sub-study of VOICE, poor adherence was reported due to a variety of reasons such as mistrust of the research, community rumours, burdens of a daily regimen, unpleasant experience using products, unknown efficacy, busy lifestyles, unsupportive partners, side effects and non-use during menses. Consequently, there continues to be a need for HIV prevention options that women can initiate, use autonomously, and which mitigate daily adherence challenges.
Two recently completed Phase 3 trials, the Microbicide Trials Network (MTN)-020/A Study to Prevent Infection with a Ring for Extended Use (ASPIRE) and International Partnership for Microbicides (IPM)-027 (The Ring Study) showed the dapivirine vaginal ring (VR) (Figure 1) to be well tolerated and reduce the risk of HIV-1 infection by 27% and 31% respectively when used as indicated [8, 9]. The dapivirine VR, an investigational new drug, is an off-white flexible ring containing 25 mg of dapivirine and when inserted, provides sustained release of dapivirine for a minimum of 1 month [8, 9].
Figure 1:
Dapivirine vaginal ring
The dapivirine VR was subsequently tested for safety and adherence in multi-site, open label extension, phase III studies – MTN-025/HIV Open-label Prevention Extension (HOPE) and IPM-032/ Dapivirine Ring Access and Monitoring (DREAM) [10, 11]. Used VRs were collected and tested for residual drug levels (RDL) (i.e. the amount of dapivirine that remained in the VR, which provided an estimation of the amount of drug that was released). Overall, 90% of used VRs in HOPE and 95% of used VRs in DREAM indicated at least some use – not necessarily consistent use [10, 11]. The European Medicine Agency recently adopted a positive scientific opinion about the ring for use among women aged 18 and older to reduce the risk of HIV-1 infection. This is an important step towards regulatory approvals in African countries and will be the first female controlled, long-acting HIV prevention technology available.
As part of understanding socio-contextual and trial specific issues that impacted VR adherence, the MTN-032 Assessment of ASPIRE and HOPE Adherence (AHA) exploratory sub-study was implemented. This is one of the first studies to report on women’s experiences of dapivirine VR adherence and their responses to RDL results in the context of an open label extension trial. Findings from this analysis can be used to inform strategies for women using the vaginal ring post-licensure.
METHODS
The MTN 025 (HOPE) study evaluated the safety of and adherence to the dapivirine VR [10]. Eligible HIV-uninfected ASPIRE participants were offered the active dapivirine VR that was replaced monthly over 12 months. Study follow-up visits occurred monthly for the first three months and quarterly thereafter to allow for a more real-world type of setting such as oral PrEP. Women could decline the VR and still enroll into the study and accept the VR at any point if they changed their mind. During the HOPE study, HIV prevention options counseling sessions occurred at enrollment, follow-up months 1, 2, 3, 6, and 9, and at the Product Use End Visit. Sessions were conducted by certified counsellors and were designed to be a collaborative open conversation between counsellor and participant. Counseling sessions centered around choice to use the VR or other HIV prevention strategies, RDL result provision, accurate reporting of VR use and the participants experience with her HIV preventative method of choice. Counseling also included messages that RDL testing might not be 100% accurate due to the variability in the tests and emphasized the importance of the participant’s reported experience.
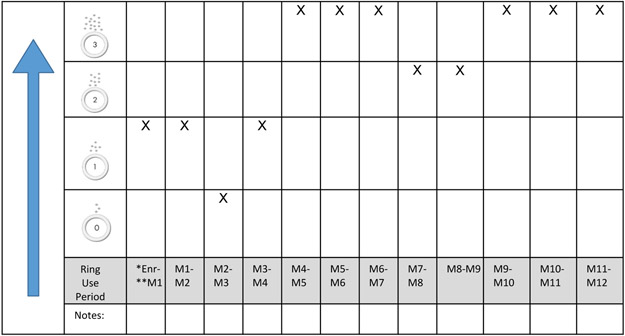
The data presented here constitute women’s ring use perceptions and experiences, captured through in-depth interviews (IDIs) conducted during the AHA study. AHA occurred 0-9 months after women exited from HOPE, and included provision of all their available RDL results, categorized from 0 (no drug release) to 3 (high rate of drug release) using the Residual Drug Feedback Over Time Tool (Figure 2).
Figure 2: Example of the tool used to capture residual drug level results.
*Enrolment
**Month
Women who received study product and provided permission to be contacted for future studies in HOPE were eligible to participate in AHA. Women were contacted in sequential order from a randomized list generated by the HOPE Data Management Centre. Ten women at each of six HOPE research sites in Lilongwe, Malawi; Durban (2 sites) and Johannesburg, South Africa; Kampala, Uganda; and Chitungwiza, Zimbabwe were randomly selected to participate in IDIs. Recruitment was stratified in a 1:3:1 ratio (low: middle: high release) according to the dapivirine RDL from the Month 1 VR to ensure a diversity of adherence perspectives.
Following confirmation of eligibility criteria, informed consent and demographic and behavioral questionnaires were administered by trained study staff. IDIs were then conducted in the language of the women’s choice (English or local language) using a semi–structured interview guide. Women were reminded that the interview was about their experience during HOPE, it was confidential and questions/concerns could be raised at any time. Thereafter, they were shown their available RDL results using the same tool that was presented to them during HOPE (Figure 2). A discussion was then facilitated on how they felt about the results, including if they believed the RDLs matched their actual use and views on the RDL testing method, barriers (RDL results of 0/1) and motivators ( RDL results of 2/3) to ring use.
IDIs were audio-recorded, transcribed and translated into English (if conducted in a local language) with quality control checks. Transcripts were then uploaded to Dedoose (Version 8.1.8), a qualitative software programme, for coding. Analysts, including the lead author, used a codebook developed iteratively and descriptively coded for key themes and topics. Intercoder consistency was confirmed at a level above a mean kappa score of 0.70 for 10% of transcripts across five coders and code application queries were discussed and resolved with the coding team. Data assigned with the parent code “adherence” and related sub-codes were stratified by average RDL results and compiled into summary memos.
For the purpose of this analysis only (not presented to the women), average RDL results were calculated by summing the total monthly RDL scores and dividing by the number of months of use. Participants were then categorized as follows: low rate of release = an average rating of between 0 to 1, middle rate of release = an average rating of > 1 to < 2, high rate of release = an average rating of between 2 to 3. Quantitative data was tabulated using Stata 15.0 (Statacorp, College Station, TX).
The AHA study protocol was approved by the Institutional Review Boards at RTI International, and at each study site and regulated by the U.S. National Institutes of Health and the Microbicide Trials Network.
RESULTS
Study Sample
Eighty-five women were screened and sixty of these women were enrolled into AHA. Two women were not included in the analysis due to inappropriate enrolment, resulting in an analytic sample of n=58. Thirteen women refused screening/enrolment citing reasons of unavailability (n=10) and disinterest (n=3).
Detailed characteristics of the study sample are presented by average RDL results in Table 1. Women averaged 32 years of age (range 23-48), with less than half (44.8%) married, and 84.5% reported having the same partner since exiting HOPE. More than half (57.1%) reported that their primary partners were HIV-negative, although many (41.1%) did not know their partner’s HIV status, or whether their partners had other sex partners (66.1%). All women, except for one, (98.2%) said that they would use a VR in future, with 74.5% being worried about acquiring HIV in the next 12 months.
Table 1:
Women’s characteristics presented by average RDL release results
| Average of Month 1 - Month 12 RDL results | ||||
|---|---|---|---|---|
| All sites (n=58)* |
Low = 0-1 (n=12) |
Middle = >1-<2 (n=24) |
High = 2-3 (n=22) |
|
| Site | ||||
| Johannesburg South Africa | 9 (15.5%) | 1 (8.3%) | 3 (12.5%) | 5 (22.7%) |
| Durban (1) South Africa | 10 (17.2%) | 3 (25.0%) | 6 (25.0%) | 1 (4.5%) |
| Durban (2) South Africa | 10 (17.2%) | 4 (33.3%) | 1 (4.2%) | 5 (22.7%) |
| Kampala Uganda | 10 (17.2%) | 3 (25.0%) | 4 (16.7%) | 3 (13.6%) |
| Chitungwiza Zimbabwe | 10 (17.2%) | 1 (8.3%) | 4 (16.7%) | 5 (22.7%) |
| Lilongwe Malawi | 9 (15.5%) | 0 (0.0%) | 6 (25.0%) | 3 (13.6%) |
| Age - mean (median, min-max) | 31.8 (30.0, 23.0-48.0) | 28.6 (27.5, 23.0-37.0) | 31.8 (31.5, 23.0-45.0) | 33.5 (30.5, 25.0-48.0) |
| Highest level of education | ||||
| Primary school, not complete | 8 (13.8%) | 2 (16.7%) | 2 (8.3%) | 4 (18.2%) |
| Primary school complete | 19 (32.8%) | 4 (33.3%) | 9 (37.5%) | 6 (27.3%) |
| Secondary school complete | 24 (41.4%) | 3 (25.0%) | 11 (45.8%) | 10 (45.5%) |
| College/university complete | 7 (12.1%) | 3 (25.0%) | 2 (8.3%) | 2 (9.1%) |
| Earn income | ||||
| Formal employment | 19 (32.8%) | 5 (41.7%) | 9 (37.5%) | 5 (22.7%) |
| Self employed | 16 (27.6%) | 4 (33.3%) | 5 (20.8%) | 7 (31.8%) |
| Other (eg. social welfare) | 8 (13.8%) | 2 (16.7%) | 4 (16.7%) | 2 (9.1%) |
| Relationship status | ||||
| Currently married | 26 (44.8%) | 4 (33.3%) | 11 (45.8%) | 11 (50.0%) |
| Has a primary sex partner | 56 (96.6%) | 12 (100.0%) | 23 (95.8%) | 21 (95.5%) |
| Same partner from HOPE | 49 (84.5%) | 10 (83.3%) | 21 (87.5%) | 18 (81.8%) |
| Primary partner has other sex partners* | ||||
| Yes | 11 (19.6%) | 2 (16.7%) | 7 (30.4%) | 2 (9.5%) |
| Unknown | 37 (66.1%) | 7 (58.3%) | 15 (65.2%) | 15 (71.4%) |
| Primary partners HIV status* | ||||
| HIV positive | 1 (1.8%) | 1 (8.3%) | 0 (0.0%) | 0 (0.0%) |
| HIV negative | 32 (57.1%) | 6 (50.0%) | 14 (60.9%) | 12 (57.1%) |
| Unknown | 23 (41.1%) | 5 (41.7%) | 9 (39.1%) | 9 (42.9%) |
| Worried about getting HIV in the next 12 months* | ||||
| Not worried at all | 14 (25.5%) | 2 (20.0%) | 6 (25.0%) | 6 (28.6%) |
| A little/somewhat worried | 21 (38.2%) | 4 (40.0%) | 11 (45.8%) | 6 (28.6%) |
| Very/Extremely worried | 20 (36.4%) | 4 (40.0%) | 7 (29.2%) | 9 (42.9%) |
| Would use a vaginal ring in future* | ||||
| Yes | 54 (98.2%) | 10 (100.0%) | 24 (100.0%) | 20 (95.2%) |
| Unknown | 1 (1.8%) | 0 (0.0%) | 0 (0.0%) | 1 (4.8%) |
Responses missing in some categories
The average RDL results indicated that 20.6% (n=12) of women were in the low rate of release category, 41.3% (n=24) were in the middle rate of release category and 37.9% (n=22) were in the high rate of release category. Approximately two-thirds of the women, (n=37; 63.7%), had all twelve months of RDL results to review. Those with less than twelve months of RDL exited early from the study, ring use was discontinued by study staff (e.g., seroconversion, pregnancy) or had chosen not to use the VR.
In agreement with RDL results
There was generally more agreement about how well the RDL results matched behavior among women in the high RDL category compared to the low/middle RDL categories. Further, those who felt that their RDLs matched VR use (n=19/58; 32.8%) predominantly trusted the RDL testing method (n=18/19; 94.7%) (Table 2).
Table 2:
Trusting/not trusting the method used to test the VR vs residual drug level results matching/not matching how the woman used the VR
| Trust/does not trust the method used to test the VR |
Residual drug level (RDL) match/do not match how the woman says she used the VR |
||
|---|---|---|---|
| RDL matched ring use | RDL did not match ring use |
Total | |
| N (%) | N (%) | N (%) | |
| Trusts the method | 18 (94.7) | 15 (38.5) | 33 (56.9) |
| Does not trust the method | 1 (5.3) | 24 (61.5) | 25 (43.1) |
| Total (%) | 19 (32.8) | 39 (67.2) | 58 (100.00) |
Women in all three RDL categories reported that support from partners, other women and study staff (counselling and engagement activities) helped them to sustain adherence to VR use. Women who faced adherence challenges mentioned that even if they had low RDL results, study staff were still encouraging during the counselling sessions by reassuring women to continue using the VR consistently.
“The staff asked us ‘please use the ring at all times’, ‘it helps’ and ‘the good thing was that you had a choice, it was up to you to choose to use the ring or not’…… It did encourage me…..” (Durban (1), South Africa, RDL Category: Low)
Protection from HIV was a key motivator for VR use among the women in all three RDL categories, across all sites. Women in the middle and high categories who felt that they were at high risk for HIV due to distrust of their male partners were motivated to use the VR, knowing that it reduced HIV risk and wanting to remain HIV negative.
“I wanted to be protected because sometimes you cannot trust your partner since you never know what he does when he goes out. That was why I was motivated to use the ring consistently.” (Lilongwe, Malawi, RDL Category: Middle)
A woman from Durban, South Africa, explained that women were more motivated to use the VR consistently in HOPE as compared to ASPIRE. This behavior was attributed both to taking the study more seriously because the VR was now shown to be well-tolerated and reduce HIV risk, and being able to have the opportunity to use the VR since their peers from ASPIRE could not join HOPE due to seroconversion post-ASPIRE.
“ Everybody was willing to use it now …… maybe everyone’s eyes started to open and they started to take this whole thing seriously…… the seriousness of the matter and the fact that they know people who were in ASPIRE who were not able to come back to HOPE because along the way they found they had become HIV positive” (Durban (1), South Africa, RDL Category: Middle)
Another motivating factor reported from the middle and high RDL categories was that the VR increased sexual pleasure for male partners. Some women reported that their male partners felt that the VR made their vaginas “tighter” while other women mentioned that male partners just enjoyed sex more when they knew that the VR was inserted.
“At the beginning he (partner) said, “Ah, it appears the ring causes sex to be more enjoyable, because it’s all different now.” …… He actually encouraged me saying, “Insert your ring.” So, I kept the ring inserted.” (Chitungwiza, Zimbabwe, RDL Category: High)
Many women with inconsistent RDL results openly discussed challenges to using the VR. The most common reported reason across all sites and RDL categories (more common in the low RDL category) were actual or perceived objections to the VR by male partners. Women described how they removed the VR before seeing their partners or when their partners told them to remove it even though they knew they should not remove it, to avoid any arguments or violence within the relationship.
“…… when you are married to somebody there are instances where you conform to his wishes even though you might have your own desires…… So, the moment he said, “Remove the ring,” I would remove it. Even though I had my own thoughts and views, there were times he would override that.” (Chitungwiza, Zimbabwe, RDL Category: Low)
Some women in the high RDL category told their partners that they had removed the VR when it was still in place, and these partners believed this because they did not feel it during sex.
Negative experiences interpreted as side effects from VR use were mentioned across all RDL categories resulting in VR removals. Reported symptoms included headaches, dizziness, increased vaginal wetness, widening of the vagina, abdominal and pelvic pain, vaginal discharge, odour, or itching. Some of these women accepted that the VR was not causing these perceived “side effects” after counselling from study staff or self-realization when the “side effect” continued after removing the VR. Some women in all RDL categories reported removing the VR for the duration of menses or for a short time during menses to wash and re-insert it as they believed it was unhygienic not to wash the menstrual blood from the VR.
Disagreement with RDL results
Most women (n=39; 67.2%), predominantly in the low and middle RDL categories, felt that their RDLs did not match their actual VR use (Table 2). Of the 39 women, 24 (61.5%) indicated that they distrusted the RDL testing method (Table 2). Women in the low and middle categories sometimes explicitly referenced the reason that the RDL results may not be 100% accurate.
Some women who reported that the RDLs were inaccurate did not describe periods of non-use. Instead, they provided several different explanations for this discrepancy between RDLs and perceived adherence to the VR. These included external factors (e.g. condoms or traditional medications affecting the release of dapivirine); biomedical factors (e.g. the body being resistant to drug uptake during periods of stress, VR contact with semen and sex itself prevented drug release, washing the VR removed some of the drug, or different blood types affecting the release of dapivirine); and technical factors (e.g. delays in inserting a new VR monthly and not inserting the VR correctly contributed to the amount of dapivirine being released or the VR having the incorrect amount of drug prior to insertion). A small number of women believed that the RDL testing machines were defective.
These are examples of women that described how their blood type and stress played a role in low RDL results:
“Some blood types quickly absorb some things. This implies that when I insert the ring and the drug coming from the ring is compatible with my blood, the drug quickly saturates my body” (Chitungwiza, Zimbabwe, RDL Category: Middle).
“I kept the ring inside inserted, …… during June when I was writing exams I was stressed, maybe my stress level or my hormonal levels affected the ring drug use in my body, so maybe that affected it.….. ” (Johannesburg, South Africa, RDL Category: Middle).
Some of their narratives suggested they felt confused about the low RDL results because they believed they had used the VR consistently and as instructed by the research team.
“……but when I would receive the results that were not true, I would be hurt, because I wouldn’t understand how? …… because I used to use the ring all the time, which meant it was always in my blood. That is where I would get confused” (Durban (1), South Africa, RDL Category: Middle)
DISCUSSION
The data in this analysis are suggestive of three key findings about women’s use of the dapivirine VR during an open-label extension trial. First, many with low release measurements reported good adherence, and cited other factors as rationale for low RDLs, including the testing method. Second, the disclosure of RDL results enabled some users to discuss their challenges experienced with VR use. Third, consistent users reported important motivators to VR use.
Many women with low RDL results voiced their distrust of the testing method, a finding similar to VOICE-D where women attributed non-detection of the drug to problems with the pharmacokinetic testing [7]. It should be recognized that RDL testing on returned VRs provided an estimate of VR use and was not 100% accurate due to the variability in the tests. It was therefore possible for a woman to receive results that were not reflective of her actual use. Counseling flipcharts contained a message that results “may not be 100% accurate”, which may have led women towards distrust of the testing method – irrespective of their actual use. Future studies with PK testing should carefully consider how these messages might be received,interpreted and adjusted.
Some women with actual low use may have been uncomfortable admitting to nonadherence, and therefore cited the VR, the drug, defective testing machines and distrust of the testing method as reasons for the low RDLs. These factors may have been named to avoid conflict or judgement by study staff and to present themselves in a way that they thought would be viewed favorably by study staff. While there were some admissions of non-use in AHA, most likely facilitated by the RDL results disclosure, women still sought to provide socially desirable explanations.
These findings are again similar to those from the VOICE sub-studies, where women reported themselves to be perfect adherers with contradicting pharmacokinetic results. Women cited multiple reasons for misreporting adherence, including human nature, self-presentation to study staff, fear of repercussions (study termination and experience of HIV-related stigma) and avoiding inconvenient additional counseling [12, 13].
During phase one of the AHA study, post-ASPIRE, women’s reported reasons for nonadherence were similar to this post-HOPE cohort, including removals during menses [14, 15], perceived side effects from VR use and fear of or actual partner opposition to the VR [16].
The consistency of the results across studies strengthens the reliability of our findings and demonstrates that African women in these settings have had adherence challenges during research trials irrespective of the type of study product, mode of administration or its efficacy. Many of these challenges are rooted in structural factors related to inequitable gender norms, HIV-related stigma, risk perception and research suspicion – all of which require broader intervention approaches than biomedical technologies. In addition, it seems that in some cases, women feel their level of adherence is adequate and acceptable given their life circumstances [16]. Some barriers, such as removals due to menses and perceived side effects can be pre-empted in future marketing of the VR through health information and counselling.
Women felt conflicted regarding removing the VR for fear of their partner feeling it during sex versus keeping the VR inserted to have some protection against HIV which alludes to the gender power inequalities still experienced in relationships and the need for a discreet HIV prevention technology. In this study we measured men’s experiences with the VR in FGDs which will be presented elsewhere [17]. Counselling and guidelines for VR rollout should speak to the level of protection offered with intermittent use. An important future research question will be to determine the level of protection offered if the VR is removed for sex.
The three main reasons women offered for their consistent VR adherence during HOPE was support from others (partners, fellow women/friends and study staff), confidence in the VR providing protection from HIV and the VR enhancing sexual experiences, which is consistent with previous qualitative research findings among women who participated in ASPIRE [18]. Using the VR seemed to give women a sense of security, when they were unsure of their partners’ fidelity and risk related behavior which reiterates the need for women to have access to a female controlled HIV prevention technology. This was confirmed in a qualitative study amongst women in ASPIRE where nine product formulations were presented to gauge preference - women preferred the long-acting products (rings, implants, injections) for its protection, ease of use, discreetness and less frequent dosing [19]. It may be useful to incorporate this into future marketing communications of the VR.
There are limitations to this research that should be taken into consideration. The data collected is only from a sub-set of women from HOPE, randomly selected based on their Month 1 adherence (low, middle and high release) using a 1:3:1 ratio and therefore may not fully reflect the ring-use experiences of all women participating in HOPE. Further, the interviews were conducted 0 – 9 months after women exited the HOPE study, so recall bias must be considered when interpreting the data.
Even though women chose to join the HOPE study and had the opportunity to decline the VR, adherence was still a challenge for some women regardless of it being a female controlled, long acting HIV prevention technology. This suggests that women may have joined for other reasons such as health benefits provided during participation; alternatively, they may have joined with an intention to use the VR but then faced challenges that led to non or intermittent adherence. Women need a diverse set of discreet options that can be incorporated in their lives depending on their immediate or long-term needs, situation, and lifestyle. Adherence measurements may not be sustainable in the real world implementation of the VR, however they can be seen as a benefit as they provide a better understanding of actual product use and provide women with a platform to discuss their experiences. In addition, understanding a woman’s reported use beyond RDL results provides insight into how the VR is understood to work and is incorporated into women’s lives. As in the trial setting, person-centered adherence counseling to identify and address misunderstandings and challenges will facilitate successful real-world implementation of the VR.
ACKNOWLEDGEMENTS
We thank all the women that participated in this study, the study staff that implemented this study and the RTI International team for their guidance and expertise with data analysis. We also acknowledge the MTN leadership, MTN 032 management team and FHI360 for protocol development and implementation.
Source of Funding: The MTN-032/Assessment of ASPIRE and HOPE Adherence (AHA) study was designed and implemented by the Microbicide Trials Network (MTN). The MTN is funded by the National Institute of Allergy and Infectious Diseases (UM1AI068633, UM1AI068615, UM1AI106707), with co-funding from the Eunice Kennedy Shriver National Institute of Child Health and Human Development and the National Institute of Mental Health, all components of the United States (US) National Institutes of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The vaginal rings used in this study were supplied by the International Partnership for Microbicides (IPM).
Footnotes
Conflicts of Interest: There are no conflicts of interest.
Meetings at which parts of the data were presented:
Data was presented at IAS 2019, Abstract number WEPEC494, July 2019, Mexico City, Mexico
REFRENCES
- 1.UNAIDS, Joint United Nations Programme on HIV/AIDS. UNAIDS Data 2019. Available at: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data_en.pdf [PubMed]
- 2.Mash R, Mash B, de Villiers P ‘Why don’t you just use a condom?’: Understanding the motivational tensions in the minds of South African women. Afr J Prm Health Care & Fam Med. 2010;2(1), Art. #79, 4 pages. DOI: 10.4102/phcfm.v2i1.79 [DOI] [Google Scholar]
- 3.Marrazzo JM, Ramjee G, Richardson BA, et al. Tenofovir-based preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2015; 372:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Van Damme L, Corneli A, Ahmed K, et al. Preexposure prophylaxis for HIV infection among African women. N Engl J Med. 2012;367(5):411–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Celum CL, Delany-Moretlwe S, Baeten JM, et al. HIV pre-exposure prophylaxis for adolescent girls and young women in Africa: from efficacy trials to delivery. JIAS. 2019, 22(Suppl 4):e25298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Van der Straten A, Stadler J, Montgomery E, et al. Women’s experiences with oral and vaginal pre-exposure prophylaxis: the VOICE-C Qualitative Study in Johannesburg, South Africa. PLoS One. 2014; 9:e89118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van der Straten A, Montgomery ET, Musara P, et al. Disclosure of pharmacokinetic drug results to understand nonadherence. AIDS. 2015; 29:2161–2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nel A, van Niekerk N, Kapiga S, et al. Safety and efficacy of a dapivirine vaginal ring for HIV prevention in women. N Engl J Med. 2016; 375:2133–2143. [DOI] [PubMed] [Google Scholar]
- 9.Baeten JM, Palanee-Phillips T, Brown ER, et al. Use of a vaginal ring containing dapivirine for HIV-1 prevention in women. N Engl J Med. 2016; 375:2121–2132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baeten JM. High adherence and sustained impact on HIV-1 incidence: Final results of an open-label extension trial of the dapivirine vaginal ring [Abstract] Presented at IAS 2019; 23 July 2019; Mexico City; Mexico [Google Scholar]
- 11.Nel A Safety, adherence and HIV-1 seroconversion in DREAM–an open-label dapivirine vaginal ring trial [Abstract]. Presented at: 9th SAAIDS Conference; 13 June 2019; Durban; South Africa [Google Scholar]
- 12.Stadler J, Scorgie F, van der Straten A, et al. Adherence and the lie in a HIV prevention clinical trial. Med Anthropol. 2016. Nov-Dec; 35(6): 503–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Montgomery ET, Mensch B, Musara P, et al. Misreporting of product adherence in the MTN- 003/VOICE trial for HIV prevention in Africa: participants’ explanations for dishonesty. AIDS Behav. 2017; 21:481–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duby Z, Katz AWK, Musara P, et al. “The state of mind tells me it’s dirty”: menstrual shame amongst women using a vaginal ring in Sub Saharan Africa. Women & Health. 60:1, 72–86, DOI: 10.1080/03630242.2019.1607803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Duby Z, Katz AWK, Browne EN, et al. Hygiene, Blood Flow, and Vaginal Overload: Why Women Removed a HIV Prevention Vaginal Ring During Menstruation in Malawi, South Africa, Uganda and Zimbabwe. AIDS Behav. 2020. February;24(2):617–628. Doi: 10.1007/s10461-019-02514-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Montgomey ET, Stadler J, Naidoo S, et al. Reasons for nonadherence to the dapivirine vaginal ring: narrative explanations of objective drug-level results. AIDS. 2018. July 17;32(11):1517–1525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Montgomery ET, Katz AWK, Duby Z, et al. Men’s sexual experiences with the dapivirine vaginal ring in Malawi, South Africa, Uganda and Zimbabwe. Presented at: Microbicide Trials Network Annual Meeting; 11 February 2020; Washington DC; USA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Montgomery ET, van der Straten A, Chitukuta M, et al. Acceptability and use of a dapivirine vaginal ring in a phase III trial. AIDS. 2017; 31:1159–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.van der Straten A, Shapley-Quinn MK, Reddy K, et al. Favoring “Peace of Mind”: A Qualitative Study of African Women’s HIV Prevention Product Formulation Preferences from the MTN-020/ASPIRE Trial. AIDS Patient Care STDS. 2017;31(7):305–314. doi: 10.1089/apc.2017.0075 [DOI] [Google Scholar]