Abstract
Objective:
To evaluate total and out-of-pocket costs for poly(ADP-ribose) polymerase (PARP) inhibitors and differences based on insurance characteristics.
Methods:
We identified ovarian cancer patients who were prescribed niraparib, olaparib, or rucaparib from the MarketScan (2014–2017) and Surveillance, Epidemiology, and End Results (SEER)-Medicare (2014–2016) databases. Drug costs were estimated for a 30-day supply. Descriptive statistics and Wilcoxon rank sum tests were performed.
Results:
590 commercially insured beneficiaries from MarketScan and 213 SEER-Medicare beneficiaries were prescribed PARP inhibitors for a median 112 days. For commercially insured beneficiaries, median total cost was $13,342 (IQR $12,022-$14,256). Median out-of-pocket cost was $44 (IQR $0-$120) and PARP inhibitors accounted for a median 90.8% of patients’ total out-of-pocket drug spending. High-deductible health plan was not associated with higher out-of-pocket costs (N=570; median $0 vs. $45, P=0.87). For SEER-Medicare beneficiaries, median total cost was $12,798 (IQR $11,704-$13,180). Median out-of-pocket cost was $370 (IQR $2-$1,234) and PARP inhibitors accounted for a median 99.0% of patients’ total out-of-pocket drug spending. Out-of-pocket costs were lower for dual-eligible patients with supplemental Medicaid prescription coverage (N=209; median $1 vs. $911, P<0.001).
Conclusions:
Although insurers are responsible for a large proportion of PARP inhibitor costs, out-of-pocket costs for PARP inhibitors account for a majority of patients’ drug spending. SEER-Medicare beneficiaries had higher out-of-pocket costs than patients with commercial insurance, which was offset for those with supplemental Medicaid prescription coverage.
Keywords: Cost of illness, Female genital neoplasms, Health expenditures, Health insurance
1. Introduction
Cancer care costs are rising with an estimated 34% increase from $183 to $246 billion between 2015 to 2030 based on population changes in the United States alone [1]. In addition, cancer treatment has intensified as more cancer therapies become available and cancer patients live longer [2]. The majority of new cancer drugs have annual total costs over $100,000 at the time of Food and Drug Administration (FDA) approval [2–3]; moreover, the trajectory of certain drug costs after launch is an 18% average increase over a mean follow-up period of 8 years [4]. For ovarian cancer, poly(ADP-ribose) polymerase (PARP) inhibitors are a paradigm-shifting class of drugs with expanding indications for use, including as maintenance therapy after adjuvant treatment or for the treatment of platinum sensitive recurrence [5]. Currently, there are three FDA-approved PARP inhibitors for ovarian cancer - niraparib, olaparib, and rucaparib - with monthly total drug costs ranging from $13–15,000 [6–10].
From the patient perspective, PARP inhibitors have unique cost sharing considerations. As oral cancer therapies, PARP inhibitors are covered by pharmaceutical drug benefits often with specialty drug tiers in which a beneficiary’s cost sharing is greater for higher tiers of prescription drugs [11]. Among Medicare Part D beneficiaries, patients are at risk for incurring significant out-of-pocket drug spending as an absolute limit on out-of-pocket spending does not exist [11]. Higher patient out-of-pocket costs for other oral cancer therapies have been associated with higher rates of prescription abandonment, delayed initiation, and non-adherence [12–14]. While the clinical benefit of PARP inhibitors is significant, published cost-effectiveness studies have demonstrated that incremental cost-effectiveness ratios per progression free life year saved often fall above currently accepted willingness to pay thresholds using current drug costs, particularly in populations that are not selected for germline or somatic BRCA mutations [6–10]. In this complex landscape, our objective was to use prescription drug coverage databases to determine the burden of total costs shared between patients and insurers for PARP inhibitors and to evaluate differences based on insurance characteristics using MarketScan commercial claims data and Surveillance, Epidemiology, and End Results (SEER)-Medicare Part D claims data.
2. Materials and Methods
2.1. Patient population
This study was categorized as exempt by the Columbia University Institutional Review Board. We utilized two databases starting in 2014, the first year a PARP inhibitor was approved by the FDA for ovarian cancer, to the most recent year of available data for each database. The IBM MarketScan Commercial Claims and Encounters and Medicare Supplemental and Coordination of Benefits database (Ann Arbor, Michigan) were used to identify patients with ovarian cancer [15]. The MarketScan data includes patients enrolled in commercial insurance plans in the United States, including Medicare-eligible retirees with employer-sponsored Medicare Supplemental plans. The database captures prescription drug use, inpatient claims, and outpatient claims and allows for longitudinal study of patient data and enrollment. All data is de-identified. Patients were eligible for inclusion in the MarketScan study cohort if they had an ovarian cancer diagnosis between 2008–2017 and at least two cancer claims that were 30 days apart. The group was further limited to patients who had at least one outpatient pharmaceutical claim for one of the commercially available PARP inhibitors from 2014–2017.
To capture elderly Medicare beneficiaries, we used the SEER-Medicare database. SEER is a group of population-based tumor registries that capture approximately 28% of the U.S. population [16]. SEER contains detailed information on tumor characteristics, sociodemographic features, and follow-up. Linkage with Medicare billing records allows for the capture of all inpatient, outpatient, and physician billed services among Medicare beneficiaries. We limited our cohort to women with Medicare Part A and B. Women enrolled in a health maintenance organization were excluded. To capture prescription drug use, the cohort was further limited to women with Medicare Part D coverage. We used similar selection criteria and included ovarian cancer patients diagnosed from 1979–2015 with at least one pharmaceutical claim for one of the commercially available PARP inhibitors from 2014–2016.
2.2. Covariates
MarketScan and SEER-Medicare Part D both capture detailed data on all costs for prescription drugs. The primary objective of this study was to analyze the overall cost of PARP inhibitors per 30-day supply including all costs liable to insurance and out-of-pocket costs incurred by patients. Our secondary objective was to evaluate differences in cost based on various insurance characteristics, such as high-deductible health plan for commercial beneficiaries in MarketScan and dual Medicaid-eligibility for prescription coverage or prescription events in the catastrophic phase for SEER-Medicare Part D beneficiaries. For 2019, the Internal Revenue Service defined a high-deductible health plan as a plan with a deductible of at least $1,350 for an individual or $2,700 for a family with a total annual out-of-pocket maximum of $6,750 for an individual or $13,500 for a family. Patients with dual Medicaid-eligibility were those who had supplemental Medicaid prescription insurance. For Medicare Part D, there are four coverage phases (i.e., initial deductible, initial coverage phase, coverage gap/donut hole, and catastrophic phase) which determine the proportion of patient cost sharing for prescription drugs and resets annually at the start of the calendar year. Because of the differing phases of prescription coverage for Medicare Part D, an individual’s monthly out-of-pocket cost can vary widely from month to month. After patients exit the deductible and initial coverage phases of their Medicare Part D benefits, patients enter into a coverage gap/donut hole where the percentage of patient cost sharing is higher. In 2020, the coverage gap/donut holes closes, but patients are still responsible for 25% of the cost of drugs in this phase. Once patients exit the coverage gap/donut hole, they enter the catastrophic phase. In 2019, Medicare beneficiaries enter the catastrophic phase once they spend more than $5,100 per year in true out-of-pocket Medicare Part D drug costs. In this phase, patients pay the greater amount of either 5% or $8.50 for brand name medications. There is no absolute maximum out of pocket drug spending for Medicare Part D.
2.3. Statistical analysis.
We calculated descriptive statistics for prescribing patterns and patient characteristics. PARP inhibitor costs were calculated as the costs for a 30-day supply by taking the sum of all the costs from the first to the last PARP inhibitor prescriptions and dividing by the total days of supply then multiplying by 30 to account for prescriptions dispensed for different durations. Costs are reported as medians and interquartile ranges. The total drug costs were calculated as the sum of all drug costs that occurred from the first to the last PARP inhibitor prescription. The out-of-pocket costs were calculated similarly. The number of other drugs was calculated as the total number of National Drug Codes other than PARP inhibitors in the same period. All costs were inflation adjusted to 2017 dollars. We compared costs based on insurance characteristics using Wilcoxon rank sum tests within each data set. Wilcoxon rank sum test is a nonparametric alternative to the two-sample t-test based on Wilcoxon scores in the linear rank statistic and tests for location differences. To evaluate trends in PARP inhibitor out-of-pocket costs for the first, second, and third 30-day supply of PARP inhibitor within each data set, we evaluated patients who had PARP inhibitor prescribed for ≥ 56 days. To minimize the variability in days of supply when determining the first, second, and third prescriptions and calculating the cost per 30-day supply for each prescription, we further limited the analysis to the patients who had the corresponding prescriptions (i.e., first, second, and third) with days of supply ranging from 28 to 30 days. All statistical tests were two-sided. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
3. Results
There were 590 patients in the MarketScan cohort. Commercial insurance beneficiaries from MarketScan had PARP inhibitor prescriptions for a median 112 days (IQR 60–210) (Table 1). The majority of patients (61.2%) were first treated with PARP inhibitors in 2017. The first filled PARP inhibitor prescriptions were for olaparib (56.6%), niraparib (31.4%), and rucaparib (12.0%) and remained similar for the second fill. Among the MarketScan cohort, the median age was 58 years (range 53–63) and all geographic regions were represented (Table 1).
Table 1:
Prescribing patterns and patient characteristics among ovarian cancer patients prescribed PARP inhibitors from MarketScan and SEER-Medicare Part D claims data
| MarketScan (n=590) n (%), median (IQR) | |
|---|---|
| Year of first PARP inhibitor | |
| 2014–2015 | 130 (22.0) |
| 2016 | 99 (16.8) |
| 2017 | 361 (61.2) |
| PARP inhibitor type – first fill | |
| Olaparib | 334 (56.6) |
| Niraparib | 185 (31.4) |
| Rucaparib | 71 (12.0) |
| PARP inhibitor type – second fill a | |
| Olaparib | 293 (60.3) |
| Niraparib | 130 (26.8) |
| Rucaparib | 63 (13.0) |
| Duration of PARP inhibitor use (days) | 112 (60–210) |
| Age (y) | 58 (53–63) |
| Region | |
| Northeast | 137 (23.2) |
| North central | 124 (21.0) |
| South | 221 (37.5) |
| West/unknown | 108 (18.3) |
|
SEER-Medicare Part D (n=213) n (%), median (IQR) | |
| Year of first PARP inhibitor | |
| 2015 | 120 (56.3) |
| 2016 | 93 (43.7) |
| PARP inhibitor type | |
| Olaparib | 213 (100.0) |
| Duration of PARP inhibitor use (days) | 112 (56–328) |
| Age (y) | 66 (58–72) |
| Race/ethnicity | |
| White | 164 (77.0) |
| Black | 13 (6.1) |
| Hispanic | 13 (6.1) |
| Other/unknown | 23 (10.8) |
| Marital status | |
| Married | 109 (51.2) |
| Unmarried/unknown | 104 (48.8) |
| Region | |
| Eastern | 31 (14.6) |
| Midwest | 66 (31.0) |
| West | 116 (54.5) |
| Location | |
| Metropolitan | 193 (90.6) |
| Urban/rural | 20 (9.4) |
| Year of diagnosis | |
| 1994–2006 | 35 (16.5) |
| 2007 | 14 (6.6) |
| 2008 | 13 (6.1) |
| 2009 | 19 (8.0) |
| 2010 | 19 (8.0) |
| 2011 | 32 (15.0) |
| 2012 | 29 (13.6) |
| 2013 | 29 (13.6) |
| 2014–2015 | 23 (10.8) |
| Stage | |
| I/II/unknown | 28 (13.2) |
| III | 109 (51.2) |
| IV | 76 (35.7) |
| Histology | |
| Serous | 156 (73.2) |
| Other epithelial | 38 (17.8) |
| Other | 19 (8.9) |
| Grade | |
| Moderate | 18 (8.5) |
| Poor | 153 (71.8) |
| Unknown | 42 (19.7) |
Patients without refill were not included.
There were 213 patients in the SEER-Medicare Part D cohort. SEER-Medicare beneficiaries also had PARP inhibitor prescriptions for a median 112 days (IQR 56–238). For this cohort, 56.3% of patients first used a PARP inhibitor in 2015 and 43.7% in 2016. All SEER-Medicare PARP inhibitor prescriptions were for olaparib during this time frame. The median age was 66 years (range 58–72) and the majority (77.0%) were white. Half (50.7%) of the patients were diagnosed between 2011–2014 (Table 1).
For commercially insured beneficiaries in MarketScan, the median total cost for a 30-day supply of PARP inhibitor was $13,342 (IQR $12,022-$14,256) and insurance was liable for a median of $12,952 (IQR $10,533-$13,912) (Figure 1). Patients paid a median of $44 (IQR $0-$125) out-of-pocket. This cohort had a median monthly copayment for PARP inhibitor of $20 (IQR $0-$80) and a median monthly coinsurance for PARP inhibitor of $0 (IQR $0–0). The median monthly out-of-pocket cost for PARP inhibitor contributing to a patient’s deductible was $0 (IQR $0-$0) with a maximum of $5,355. There were 124 (21.0%) patients who were dual-eligible for Medicare supplemental insurance plans, although information was unavailable to determine if these individuals had Part D. Coordination of benefits, which typically represented supplemental insurance that could include Medicare, covered a median of $0 (IQR $0-$0) for PARP inhibitor with a maximum of $20,523. A median of 99.8% (IQR 98.6–100.0%) of total drug cost and a median of 90.8% (63.4–100.0%) of total patient out-of-pocket cost for all drug spending was due to PARP inhibitor costs during the months of PARP inhibitor use. Among 476 commercial beneficiaries in Marketscan who had PARP inhibitor prescriptions for ≥ 56 days, the days of supply ranged from 3 to 168 days per prescription. The median out-of-pocket cost was $45 (IQR $0–109) for first (N=398), $29 (IQR $0–83) for second (N=372), and $25 (IQR $0–88) for the third 30-day supply of PARP inhibitor (N=291).
Figure 1:
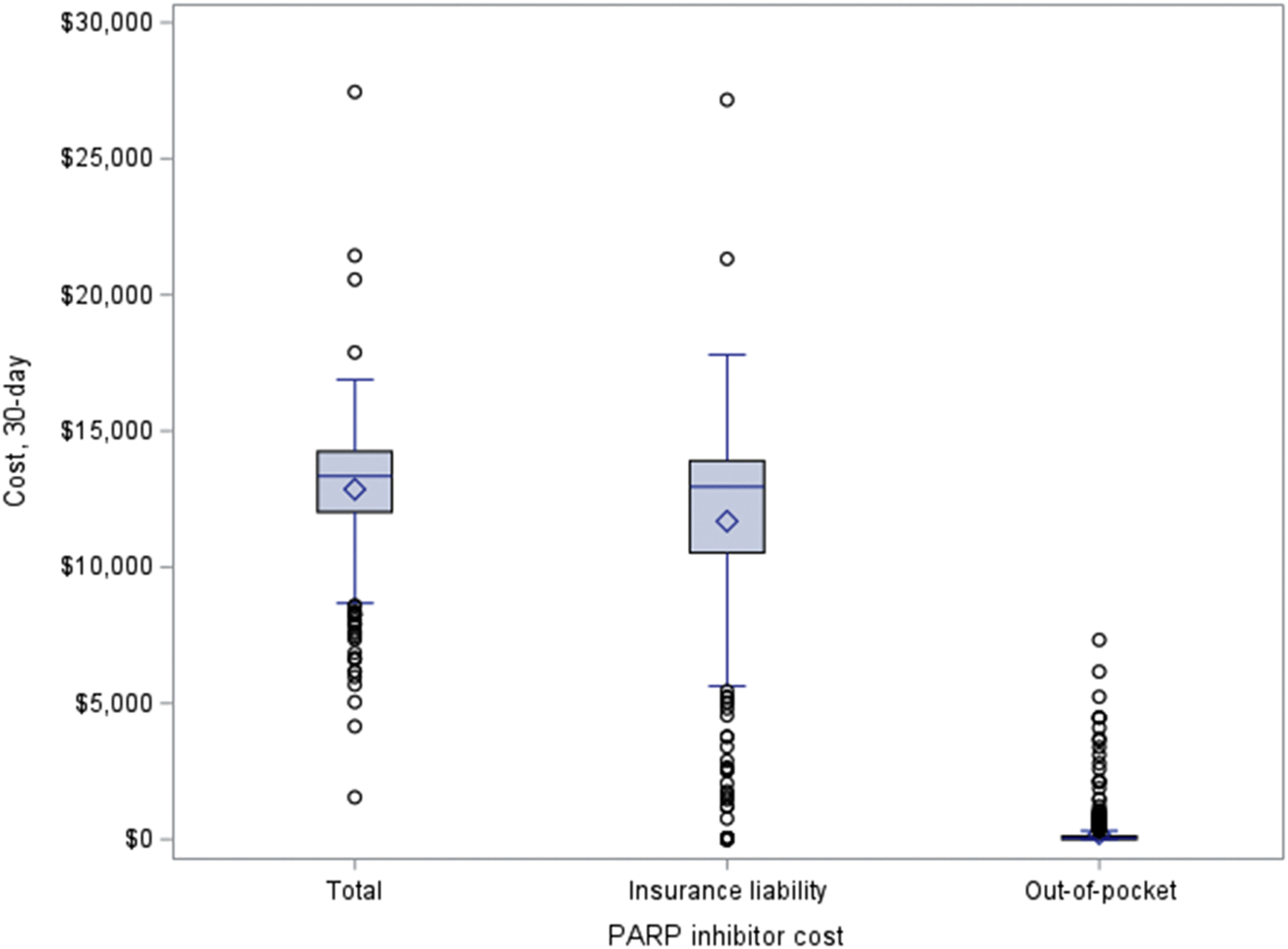
Cost sharing for 30-day supply of PARP inhibitors among ovarian cancer patients with commercial insurance from MarketScan
For SEER-Medicare beneficiaries, the median total cost of a 30-day supply of PARP inhibitor was $12,798 (IQR $11,704-$13,180) and patients paid a median out-of-pocket cost of $370 (IQR $2-$1,234) (Figure 2). A median of 100.0% (IQR 98.0–100.0%) of total drug cost and a median of 99.0% (IQR 96.0–100.0%) of total patient out-of-pocket spending on drugs were attributable to PARP inhibitor during the months of PARP inhibitor use. Among 165 SEER-Medicare patients who had PARP inhibitor prescriptions for ≥ 56 days, their days of supply ranged from 7 to 56 days per prescription. The median out-of-pocket cost was $40 (IQR $4–2,994) for first (N=90), $20 (IQR $0–645) for second (N=85), and $21 (IQR $0–655) for third (N=69) 30-day supply of PARP inhibitor. We evaluated the proportion of patients in the coverage gap/donut hole and catastrophic phase at the first and second prescription. For the first PARP inhibitor prescription, 165 (77.5%) patients were in the coverage gap compared to 75 (41.9%) who were in the coverage gap for the second prescription. While there were less than 10% of patients in the catastrophic phase at the time of the first prescription, 72 of 179 (40.2%) with a second PARP inhibitor prescription had reached the catastrophic phase by a patient’s second prescription.
Figure 2: Cost sharing for 30-day supply of PARP inhibitors among ovarian cancer patients with Medicare Part D from SEER-Medicarea.
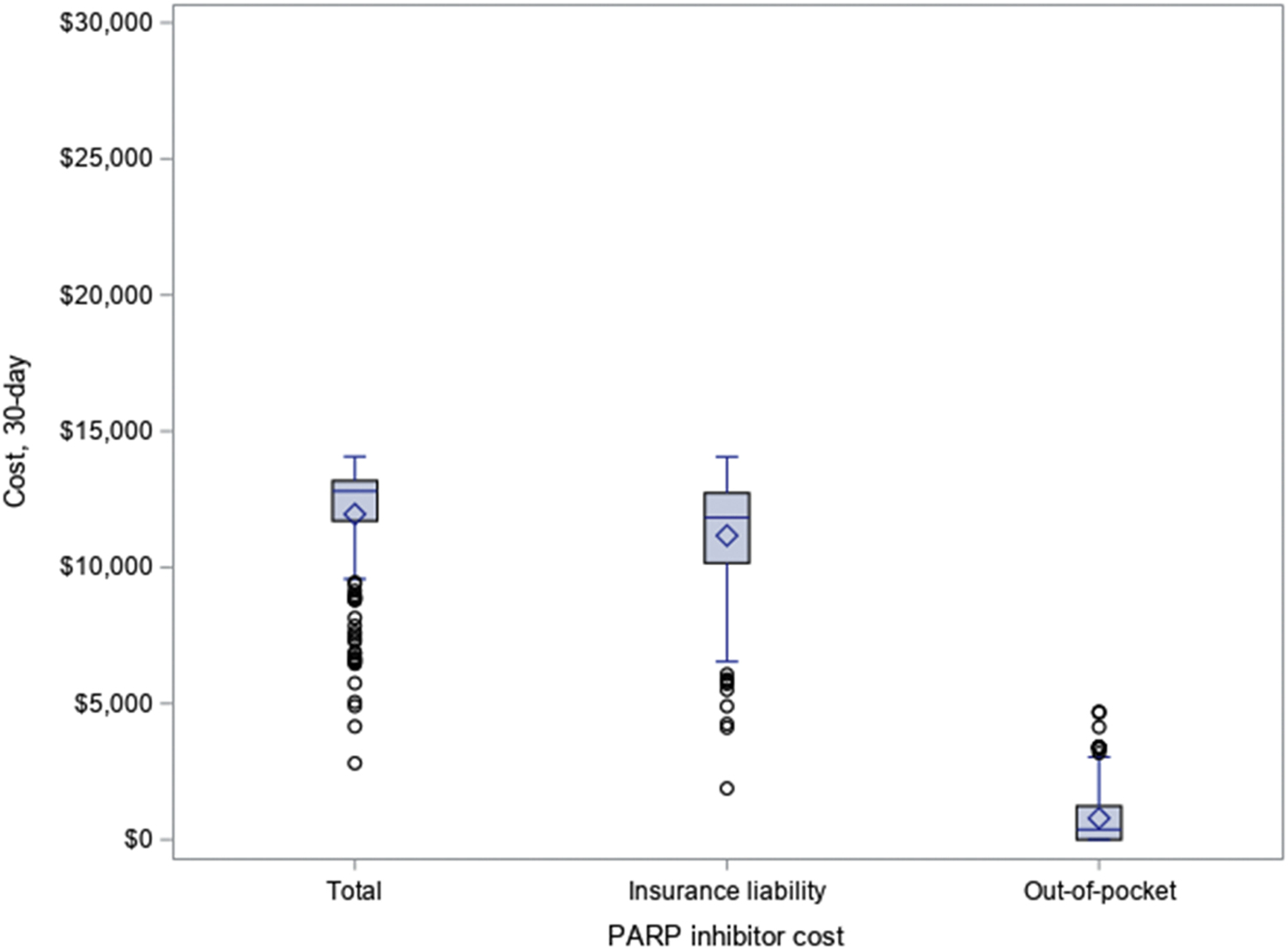
a For SEER-Medicare data, insurance liability is derived from gross drug cost (total) – patient paid amount (out-of-pocket).
For commercially insured beneficiaries in MarketScan, we compared PARP inhibitor costs among patients with and without a high-deductible health plan for the duration of PARP inhibitor use (Table 2). There were 35 patients with a high deductible prescription drug plan. The median total drug cost was $13,875 (IQR $13,307-$14,839) for those with a high-deductible health plan compared to $13,315 (IQR $11,948-$14,237) for those without (P=0.01). The median patient out-of-pocket cost with a high-deductible health plan was $0 (IQR $0-$588) compared to $45 (IQR $0-$121) for those without a high-deductible health plan (P=0.87).
Table 2.
Differences in total and patient out-of-pocket costs for 30-day supply of PARP inhibitor among ovarian cancer patients based on insurance characteristics
| n (%) | Total cost (median) | Total cost (IQR) | p-value | Patient out-of-pocket cost (median) | Patient out-of-pocket cost (IQR) | p-value | |
|---|---|---|---|---|---|---|---|
| Marketscan cohort (n=570) a | |||||||
| High deductible health plan | 35 (6.1) | $13,875 | $13,307-$14,839 | 0.01 | $0 | $0-$588 | 0.87 |
| No high deductible health plan | 535 (93.9) | $13,315 | $11,948-14,237 | $45 | $0-$121 | ||
| SEER-Medicare Part D cohort (n=209) b | |||||||
| Dual Medicaid-eligibility prescription coveragec | 50 (23.9) | $12,663 | $12,450-$13,227 | 0.57 | $1 | $0-$2 | <0.01 |
| No dual Medicaid-eligibility prescription coverage | 159 (76.1) | $12,860 | $11,470-$13,157 | $911 | $44-$1,513 | ||
| SEER-Medicare Part D cohort (n=159) c | |||||||
| Any PARP inhibitor prescription in catastrophic phase | 99 (62.3) | $12,812 | $11,209-$13,101 | 0.25 | $1,021 | $637-$1,438 | 0.19 |
| No PARP inhibitor prescription in catastrophic phase | 60 (37.7) | $12,862 | $12,104-$13,376 | $84 | $23-$2,733 | ||
IQR=interquartile range
Patients with high-deductible plan in some months during use of PARP inhibitor and patients with missing insurance type were excluded.
Patients with Medicaid supplemental in some months during use of PARP inhibitor were excluded.
Only one patient was a Low-Income Subsidy Medicare beneficiary.
Patients with any Medicaid supplemental insurance were excluded.
Next, we evaluated PARP inhibitor costs among patients who were and were not dual-eligible with supplemental Medicaid insurance for SEER-Medicare Part D beneficiaries (Table 2). We found that 50 patients (23.9%) had dual Medicaid-eligibility prescription coverage during all months of PARP inhibitor use. The median total drug cost for these patients was $12,663 (IQR $12,450-$13,227) compared to $12,860 (IQR $11,470-$13,157) for those with no dual Medicaid-eligibility during the months of PARP inhibitor use (P=0.57). However, patients with dual Medicaid-eligibility prescription coverage had a lower out-of-pocket cost (median $1, IQR $0-$2) compared to those without (median $911, IQR $44-$1,513) during the months of PARP inhibitor use (P<0.001).
Lastly, we evaluated PARP inhibitor costs for patients with any PARP inhibitor prescriptions in the catastrophic phase compared to those with no PARP inhibitor prescriptions in the catastrophic phase (Table 2). Out of 1,668 total prescriptions for the 213 SEER-Medicare beneficiaries, there were 1,116 (66.9%) prescriptions which occurred in the catastrophic phase. We excluded patients with any supplemental Medicaid prescription coverage for this portion of the analysis. Among the 159 SEER-Medicare beneficiaries with no Medicaid prescription coverage, we found that 99 patients (62.3%) had at least some PARP inhibitor prescriptions in the catastrophic phase with a median patient out-of-pocket cost of $1,021 (IQR $637-$1,438) compared to 60 patients (37.7%) who had no PARP inhibitor prescriptions in the catastrophic phase with a median patient out-of-pocket cost of $84 (IQR $23-$2,733) (P=0.19). Despite having no PARP inhibitor prescriptions in the catastrophic phase, a subset of 23 patients who did not have supplemental prescription coverage (i.e., through Programs of All-Inclusive Care for the Elderly or employer-sponsored coverage) experienced the highest median patient out-of-pocket cost of $3,031 (IQR $1,820-$3,390).
4. Discussion
We found that the median total cost for PARP inhibitors of $13–14,000 per month is consistent with the cost of other oral cancer therapies. In 2014, the total monthly cost for recently approved oral cancer drugs typically exceeded $10,000 [17]. Among ovarian cancer patients, we found that insurers bear a high proportion of PARP inhibitor drug costs. Regardless of payer type, total PARP inhibitor cost accounted for not only the majority of total drug expenditures, but also the majority of patient out-of-pocket drug spending throughout the time patients were prescribed a PARP inhibitor. Out-of-pocket costs were generally higher for patients with Medicare Part D compared to patients with commercial insurance; however, dual eligibility with supplemental Medicaid insurance played a significant role in reducing patient out-of-pocket cost burden.
While insurance is liable for a high percentage of PARP inhibitor costs, these drugs usually accounted for over 90% of patients’ out-of-pocket drug spending. In our cohort, the median monthly out-of-pocket cost was $370 for SEER-Medicare Part D beneficiaries compared to $44 for commercial insurance beneficiaries from MarketScan. This is similar to findings by Streeter et al. where patients with Medicare had higher cost sharing for oral cancer therapies than those with commercial insurance [18]. This could have important implications on clinical outcomes as prior studies have found that increased cost sharing has a negative impact on adherence to oral cancer therapies. A study of Medicare Part D beneficiaries found that for each $10 increase in monthly out-of-pocket spending for oral cancer therapies with higher out-of-pocket costs (median costs $497–774), there was an increased likelihood of discontinuation or delay ranging from 13–20% [19]. A separate study of mixed payer statuses found that patients with higher out-of-pocket costs had higher rates of prescription claim reversal, abandonment, or delayed initiation, particularly when comparing the highest out-of-pocket cost categories of greater than $2,000 to the lowest out-of-pocket cost categories of less than $10 [12].
Insurance characteristics vary based on insurance type and can have an impact on patient out-of-pocket drug costs. We found that for commercial insurance beneficiaries in MarketScan, high-deductible health plans were not associated with higher out-of-pocket costs for PARP inhibitor. This is likely explained by the minimal contribution of PARP inhibitors to patients’ deductibles ($0 for the 25th to 75th percentile) in the commercially insured population. For Medicare Part D beneficiaries, there are two unique characteristics to consider: dual eligibility for supplemental Medicaid insurance and prescription drug coverage phase. Importantly, the presence of supplemental Medicaid coverage offset patients’ out-of-pocket spending from a median of $911 for SEER-Medicare Part D beneficiaries without supplemental Medicaid prescription coverage compared to a median of $1 for those who were dual-eligible for Medicaid supplemental prescription coverage. In other cancer settings where patients are prescribed oral cancer therapies, Medicare Part D beneficiaries without cost sharing subsidies, such as supplemental Medicaid coverage, were less likely to initiate oral tyrosine kinase inhibitors for chronic myeloid leukemia than those with these subsidies [14]. We found that over 75% of SEER-Medicare patients were in the coverage gap, which has the highest proportion of patient cost sharing, at the time of first PARP inhibitor prescription. By the second PARP inhibitor prescription, approximately 40% were in the catastrophic phase, which has lower cost sharing but no absolute out-of-pocket maximum for patient drug spending.
A strength of our study is that we utilized real world data from two different populations that encompass diverse payer statuses, including patients covered by commercial insurance and Medicare Part D. We recognize several limitations. Detailed information about patients’ treatment history are not available which limits our ability to evaluate appropriateness of PARP inhibitor prescriptions, reasons for discontinuation, or the impact of cost on adherence or other clinical outcomes. Patients who are uninsured, who have insurance through national or state-run Marketplaces, or who receive free PARP inhibitor from a manufacturer-sponsored program were not represented in our study. Moreover, we are unable to measure the relative contribution of financial assistance programs or other payer-negotiated rebates and discounts as well as the impact of state oral oncology drug parity laws [20–22]. By estimating 30-day supply over the entire duration of a patient’s PARP inhibitor use, our study does not fully demonstrate the variability in month to month costs that patients may experience. To address this, we evaluated the out-of-pocket cost per 30-day supply based on the first, second, and third PARP inhibitor prescriptions among a subset of defined patients. We found SEER-Medicare patients generally had higher out-of-pocket costs for the first 30-day supply compared to subsequent prescriptions; whereas, there was less difference for commercial beneficiaries in Marketscan. We acknowledge there may be selection bias due to variations in the supply amount for individual prescriptions and patients’ insurance characteristics could differ between those with longer or shorter duration of PARP inhibitor prescriptions. While surprising that the patient out-of-pocket cost was higher for patients with a high deductible health plan, this was not statistically significant. Our analysis was limited by the inability to determine how much of the deductible or out of pocket maximum had been met at the time of PARP inhibitor prescription as well as the wide variation in benefits among commercial plans represented in MarketScan. Due to the complexities of the coverage phases for patients with Medicare Part D, which vary for individuals depending on the time of year and spending on other prescription drugs, we were unable to fully measure the impact of coverage phase on patient cost sharing. With a median duration of olaparib use of 19.4 months in SOLO2 among platinum sensitive patients with recurrent ovarian cancer, our study may not reflect cost sharing trends for longer durations of use as the median duration of use in our study was 112 days (or 3.7 months) [23]. PARP inhibitors were first FDA-approved in December 2014 with expanding indications for ovarian cancer as well as other cancer types. Our study was limited by smaller sample size due to the currently available data, but we anticipate usage will only continue to increase in future years and that our data can provide early insight into PARP inhibitor costs. Based on timing of FDA approval, only olaparib was represented in the SEER-Medicare data set. Although we inflation adjusted to 2017 dollars, only earlier years were available from the SEER-Medicare (2014–2016) databased compared to Marketscan (2014–2017), which may underestimate costs for SEER-Medicare given trends have shown that the cost of novel drugs often continue to increase above inflation after approval [4]. This would further widen the described difference in our study.
The relatively high out-of-pocket costs, particularly for Medicare beneficiaries, may limit use of PARP inhibitors for patients who cannot afford the drugs. Several types of patient assistance programs, typically sponsored by drug manufacturers or charitable organizations, exist [20]. Medicare Part D beneficiaries, however, are barred by law from accessing manufacturer-sponsored copay assistance programs, which may exacerbate financial barriers in this population. At a single specialty pharmacy, over one third of patients received financial assistance for oral targeted cancer therapies [24]. Identification of financial assistance programs for patients with high out-of-pocket costs has therefore become an essential responsibility for oncology pharmacists [25]. Although assistance programs can help reduce patients’ costs, there are concerns that their use may contribute to inequities if assistance is not available to all patients and may perpetuate overall drug prices by shielding patients and providers from high costs that might otherwise prompt discussions regarding potentially low-value care or efforts to advocate for lower drug costs [20]. Toward this end, national societies including the American Society of Clinical Oncology have released position statements outlining strategies to address drug affordability. These strategies include high value drug development, innovative drug pricing models, policy reform encouraging generic and biosimilar development, and drug cost transparency [26–27]. As PARP inhibitors have become a key component of ovarian cancer care, it is imperative that oncology practices are equipped to address financial barriers and oncology providers advocate for policies to ensure affordable access to this important class of drugs.
Highlights.
Among insured ovarian cancer patients, payers are responsible for the majority of total PARP inhibitor costs
While on PARP inhibitor, these drugs account for over 90% of patients’ total out of pocket drug spending
SEER-Medicare Part D beneficiaries had higher PARP inhibitor out of pocket costs than commercially insured in MarketScan
Medicare beneficiaries had lower out of pocket costs for PARP inhibitor if dual-eligible for Medicaid supplemental coverage
Acknowledgements:
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, CMS; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Funding disclosure:
MIL was supported by the National Institute of Child and Human Development Women’s Reproductive Health Research Career Development K-12 Grant (grant number 5K12HD001258) for this work. The funding source had no role in the study design, data collection, data analysis, data interpretation, manuscript writing, or decision to submit the manuscript for publications.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: WKH has the following disclosures, including consultant for LICOR and Altum and Data Safety Monitoring Board for Inovio. JDW has the following disclosures, including consultant for Clovis oncology and research funding from Merck. The other authors have no disclosures.
References
- 1.Mariotto AB, Enewold L, Zhao J, Zeruto CA, Yabroff KR. Medical care costs associated with cancer survivorship in the United States. Cancer Epidemiol Biomarkers Prev 2020;29(7):1304–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yabroff KR, Gansler T, Wender RC, Cullen KJ, Brawley OW. Minimizing the burden of cancer in the United States: Goals for a high-performing health care system. CA Cancer J Clin 2019;69(3):166–183. [DOI] [PubMed] [Google Scholar]
- 3.Memorial Sloan Kettering Cancer Center. Price and value of cancer drugs. https://www.mskcc.org/research-programs/health-policy-outcomes/cost-drugs Accessed December 5, 2020.
- 4.Gordon N, Stemmer SM, Greenberg D, Goldstein DA. Trajectories of Injectable Cancer Drug Costs After Launch in the United States. J Clin Oncol 2018;36(4):319–325. [DOI] [PubMed] [Google Scholar]
- 5.Washington C, Gunderson CC, Moore KN. Update in the use and evaluation of poly (ADP-ribose) polymerase inhibitors in epithelial ovarian cancer: current and pending clinical research. Curr Opin Obstet Gynecol 2019;31(1):4–11. [DOI] [PubMed] [Google Scholar]
- 6.Dottino JA, Moss HA, Lu KH, Secord AA, Havrilesky LJ. U.S. Food and Drug Administration-approved Poly(ADP-ribose) polymerase inhibitor maintenance therapy for recurrent ovarian cancer: a cost-effectiveness analysis. Obstet Gynecol 2019;133(4):795–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhong L, Tran AT, Tomasino T, Nugent E, Smith JA. Cost-effectiveness of niraparib and Olaparib as maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer. J Manag Care Spec Pharm 2018;24(12):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guy H, Walder L, Fisher M. Cost-effectiveness of niraparib versus routine surveillance, olaparib and rucaparib for the maintenance treatment of patients with ovarian cancer in the United States. Pharmacoeconomics 2019;37(3):391–405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barrington DA, Tubbs C, Smith HJ, Straughn JM Jr, Senter L, Cohn DE. Niraparib maintenance in frontline management of ovarian cancer: a cost effectiveness analysis. Int J Gynecol Cancer 2020. [DOI] [PubMed] [Google Scholar]
- 10.Smith HJ, Walters Haygood CL, Arend RC, Leath CA 3rd, Straughn JM Jr. PARP inhibitor maintenance therapy for patients with platinum-sensitive recurrent ovarian cancer: a cost-effectiveness analysis. Gynecol Oncol 2015;139(1):59–62. [DOI] [PubMed] [Google Scholar]
- 11.Juliette Cubanski WK, and Tricia Neuman. The out-of-pocket cost burden for specialty drugs in Medicare Part D in 2019. http://files.kff.org/attachment/Issue-Brief-the-Out-of-Pocket-Cost-Burden-for-Specialty-Drugs-in-Medicare-Part-D-in-2019 Accessed December 5, 2020.
- 12.Doshi JA, Li P, Huo H, Pettit AR, Armstrong KA. Association of Patient Out-of-Pocket Costs With Prescription Abandonment and Delay in Fills of Novel Oral Anticancer Agents. J Clin Oncol 2018;36(5):476–482. [DOI] [PubMed] [Google Scholar]
- 13.Farias AJ, Du XL. Association Between Out-Of-Pocket Costs, Race/Ethnicity, and Adjuvant Endocrine Therapy Adherence Among Medicare Patients With Breast Cancer. J Clin Oncol 2017;35(1):86–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winn AN, Keating NL, Dusetzina SB. Factors Associated With Tyrosine Kinase Inhibitor Initiation and Adherence Among Medicare Beneficiaries With Chronic Myeloid Leukemia. J Clin Oncol 2016;34(36):4323–4328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.IBM Marketscan Research. Marketscan Research Data. https://marketscan.truvenhealth.com/marketscanportal/ Accessed October 2, 2019.
- 16.National Cancer Institute Division of Cancer Control & Population Sciences. Surveillance E, and End Results (SEER) Medicare. https://healthcaredelivery.cancer.gov/seermedicare/ Accessed October 2, 2019.
- 17.Dusetzina SB. Drug pricing trends for orally administered anticancer medications reimbursement by commercial health plans, 2000–2014. JAMA Oncol 2016;2(7):960–1. [DOI] [PubMed] [Google Scholar]
- 18.Streeter SB, Schwartzberg L, Husain N, Johnsrud M. Patient and plan characteristics affecting abandonment of oral oncolytic prescriptions. J Oncol Pract 2011;7(3 Suppl):46s–51s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaisaeng N, Harpe SE, Carroll NV. Out-of-pocket costs and oral cancer medication discontinuation in the elderly. J Manag Care Spec Pharm 2014;20(7):669–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zafar SY, Peppercorn JM. Patient financial assistance programs: a path to affordability or a barrier to accessible cancer care? J Clin Oncol 2017;35(19):2113–2116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hernandez I, San-Juan-Rodriguez A, Good CB, Gellad WF. Changes in list prices, net prices, and discounts for branded drugs in the US, 2007–2018. JAMA 2020;323(9):854–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dusetzina SB, Huskamp HA, Winn AN, et al. Out-of-pocket and health care spending changes for patients using orally administered anticancer therapy after adoption of state parity laws. JAMA Oncol 2018;4(6):e173598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pujade-Lauraine E, Ledermann JA, Selle F, et al. Olaparib tablets as maintenance therapy in patients with platinum-sensitive, relapsed ovarian cancer and a BRCA1/2 mutation (SOLO2/ENGOT-Ov21): a double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Oncol 2017;18(9):1274–1284. doi: 10.1016/S1470-2045(17)30469-2 [DOI] [PubMed] [Google Scholar]
- 24.Olszewski AJ, Zullo AR, Nering CR, Huynh JP. Use of Charity Financial Assistance for Novel Oral Anticancer Agents. J Oncol Pract 2018;14(4):e221–e228. doi: 10.1200/JOP.2017.027896 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mackler E, Segal EM, Muluneh B, Jeffers K, Carmichael J. 2018 Hematology/Oncology Pharmacist Association Best Practices for the Management of Oral Oncolytic Therapy: Pharmacy Practice Standard. J Oncol Pract 2019;15(4):e346–e355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Society of Clinical Oncology. American Society of Clinical Oncology Position Statement on Addressing the Affordability of Cancer Drugs. J Oncol Pract 2018;14(3):187–192. [DOI] [PubMed] [Google Scholar]
- 27.Kantarjian H, Steensma D, Rius Sanjuan J, Elshaug A, Light D. High cancer drug prices in the United States: reasons and proposed solutions. J Oncol Pract 2014;10(4):e208–11. [DOI] [PubMed] [Google Scholar]


