Abstract
Scope:
Persons with metabolic syndrome (MetS) absorb less vitamin E than healthy controls. We hypothesized that absorption of fat-soluble vitamins (FSV) A and D2 would also decrease with MetS status and that trends would be reflected in lipidomic responses between groups.
Methods and Results
Following soymilk consumption (501 IU vitamin A, 119 IU vitamin D2), the triglyceride-rich lipoprotein fractions (TRL) from MetS and healthy persons (n = 10 age- and gender-matched subjects/group) were assessed using LC-MS/MS. Absorption was calculated using area under the time-concentration curves (AUC) from samples collected at 0, 3, and 6 h post-ingestion. MetS persons had ~6.4-fold higher median vitamin A AUC (retinyl palmitate) vs. healthy controls (P = 0.07). Vitamin D2 AUC was unaffected by MetS status (P = 0.48). Untargeted LC-MS lipidomics revealed 6 phospholipids and 1 cholesterol ester with concentrations correlating (r = 0.53–0.68; P < 0.001) with vitamin A concentration.
Conclusions:
The vitamin A-phospholipid association suggests increased hydrolysis by PLB, PLRP2, and/or PLA2IB may be involved in the trend in higher vitamin A bioavailability in MetS persons. Previously observed differences in circulating levels of these vitamins are likely not due to absorption. Alternate strategies should be investigated to improve FSV status in MetS.
Keywords: fat-soluble vitamins, liquid chromatography-mass spectrometry, metabolomics, phospholipids, postprandial absorption
Introduction
Metabolic syndrome (MetS) is a collection of risk factors involving central adiposity, dyslipidemia, and insulin resistance that results in a >2-fold increased risk of cardiovascular disease (CVD) [1] and a ~3-fold increased risk of type 2 diabetes mellitus (T2DM),[2] relative to healthy controls. MetS diagnosis is based on meeting ≥3 of 5 criteria: increased waist circumference, high blood triglycerides, low high-density lipoprotein cholesterol (HDL-C), high blood pressure, and high fasting blood glucose.[3]
Vitamins A, D, E, and K are fat-soluble vitamins (FSVs) essential for growth and development. Vitamins A and D help to regulate adiposity through binding to the nuclear receptors retinoic acid receptor/retinoid X receptor (RAR/RXR) and vitamin D receptor (VDR), respectively,[4,5] and thus could mediate the development of MetS. However, outcomes of observational studies are equivocal. Indeed, some reports indicate that circulating concentrations of 25(OH)D, a biomarker of vitamin D status, is inversely correlated with overt MetS or symptoms of MetS,[6,7] whereas others observed no relationship when parathyroid hormone was also considered.[8,9] Prospective vitamin D supplementation and lifestyle counseling to increase sun exposure have resulted in an inverse association between serum vitamin D concentration and prevalence of MetS at one year of follow-up.[10,11] Likewise, a randomized placebo-controlled trial in postmenopausal women observed decreased systolic blood pressure, triglycerides, and glucose, and increased HDL in subjects following 9 months of 25(OH)D supplementation.[12]
Less is known about the relationship between circulating vitamin A and MetS. A cross-sectional study in adolescents (n=79) found that body fat percentage and waist-to-hip ratio were positively correlated with serum retinol (i.e. vitamin A status marker).[13] Serum triglycerides and fasting insulin were also positively correlated with the ratio of retinol binding protein 4 (RBP4) to serum retinol in the same cohort.[14] Similar results were observed in a study of Mexican-American children, with serum retinol positively correlated with BMI, truncal fat mass, and total body fat mass.[13] In contrast, obesity, a contributing factor associated with MetS, has been associated with lower fasting serum retinol.[15]
Various studies have also linked MetS and low status of vitamins A & D to poor health outcomes. In a prospective cohort study, MetS persons (n = 1801) with sufficient serum total 25(OH)D (i.e. ≥ 75 nmol/L) had lower all-cause and CVD mortality than MetS subjects with vitamin D-deficient status.[16] In middle-aged adults, circulating retinoic acid (RA), the most potent form of vitamin A, was inversely correlated with non-alcoholic fatty liver disease (NAFLD) and its progression into non-alcoholic steatohepatitis (NASH)—a disease afflicting ~70% of those with MetS syndrome.[17,18] Thus, in the absence of controlled trials, it is unclear whether reduced FSV status is a contributor to, or a consequence of, cardiometabolic disorders.
Numerous factors may affect FSV status in MetS, including decreased dietary intakes,[19] sequestration in adipose tissue,[20] chronic inflammation,[21,22] impaired gut-to-liver trafficking,[23] and impaired liver function.[24] Reduced FSV absorption is also a potential contributor to low FSV status in MetS. Findings of a pharmacokinetics study indicated that the bioavailability of orally ingested deuterium-labeled α-tocopherol (vitamin E) was lower in MetS persons compared with age- and gender-matched healthy adults.[25] Because vitamins D and E share the same apical transporters of the small intestinal enterocyte for uptake,[26,27] it is plausible they would also be impacted. Similarly, although the transporter for vitamin A has never been conclusively identified,[28] SR-B1, stimulated by retinoic acid 6 (STRA6), and retinol binding protein-receptor 2 (RBPR2), are thought to be involved.[28,29] In this secondary study of the aforementioned vitamin E investigation, we hypothesized that absorption of vitamin A and vitamin D2 would be reduced in the same MetS subjects in which vitamin E absorption was found to be reduced,[25] in association with altered postprandial lipidomic responses.
Experimental Section
Chemicals
HPLC-grade methyl tert-butyl ether (MTBE), and ammonium acetate, and Optima-grade acetonitrile, isopropanol, water, methanol, and formic acid were purchased from Fisher Scientific (Hampton, NH, USA). Authentic standards of retinyl palmitate and ergocalciferol were purchased from Sigma Aldrich (St. Louis, MO, USA). (6, 19, 19-D3, 97%)-ergocalciferol was purchased from Cambridge Isotopes (Andover, MA, USA), and (12, 13, 20-13C3)-retinyl palmitate was purchased from Buchem BV (Apeldoorn, Netherlands). Authentic standards of sphingomyelin (SM) 18:1/16:0, phosphatidylcholine (PC) 18:1/16:0, PC 16:0/20:4, PC 18:2/18:2, and PC 18:1/18:1 were purchased from Cayman Chemical (Ann Arbor, MI, USA). PC 16:0/20:3 and PC 18:0/18:2 were purchased from Avanti Polar Lipids (Alabaster, AL, USA). Cholesterol was purchased from Sigma Aldrich (St. Louis, MO, USA) (see Supporting Information Table S1 for product numbers and full chemical names).
Clinical Trial
Full details of the original randomized, double-blind crossover clinical trial have been published previously.[25] This trial is registered at www.clinicaltrials.gov as NCT01787591. Only one treatment (soymilk meal) from the crossover design was investigated in the present study. Briefly, participants were age- and gender-matched healthy adults and MetS adults (5M and 5F per group; 24–40 years of age). MetS persons met at least 3 of the following diagnostic criteria of MetS;[3] waist circumference ≥ 102 cm (men) and ≥ 88 cm (women), fasting triglyceride ≥ 1.7 mmol/L, fasting glucose ≥ 5.6 mmol/L, resting systolic (≥ 130 mmHg) and diastolic (≥ 85 mmHg) blood pressure, and HDL-C <1.0 mmol/L (men) and <1.3 mmol/L (women).
Participants arrived at the study center in the fasted state, baseline blood samples were collected, and they then consumed a soymilk beverage (240 mL) and a hexa-deuterium (d6)-RRR-α-tocopherol capsule (nutrient composition in Supporting Information Table S2). Blood samples were collected 3, 6, 9, and 12 hours postprandially, and a lunch meal was provided after the 6 h blood draw and dinner after 12 h (nutrient composition in Supporting Information Table S3). Plasma was isolated via centrifugation before snap freezing in liquid N2 and storage at −80° C. The goal of the primary study was to evaluate vitamin E pharmacokinetics, so all meals were controlled for α-tocopherol and vitamin C content, but not vitamins A and D. Because subjects consumed a second, larger dose of vitamin A with lunch and snacks (after the 6-hour blood draw), this secondary analysis focuses on blood sampled from 0–6 hours. Although the amount of fat in the meal was low (3.5 g), fat levels as low as 2 g have elicited a retinyl ester response in the chylomicron fraction of plasma,[30] and increasing fat content in skim vs. whole milk has not been shown to influence relative serum ergocalciferol concentrations.[31]
Dosage Information
Subjects consumed 119 IU of vitamin D2 (i.e. ergocalciferol), and 501 IU of vitamin A (i.e. retinyl palmitate) as part of a soymilk beverage (240 mL) after an overnight fast. These amounts reflect a typical soymilk beverage and therefore represent a physiologically relevant dose.
Isolation of Triglyceride-Rich Lipoprotein Fraction
The TRL fraction was isolated from plasma following the method previously described.[32]
Apolipoprotein B-48 Quantitation
Aliquots of isolated TRL were analyzed in duplicate using a Shibiyagi human ApoB-48 ELISA kit [33] according to the manufacturer’s instructions.
Vitamin A and D Extraction of TRL Fraction
Vitamins A (retinyl palmitate) and D2 were extracted from TRL fractions according to a previously published method.[34]
Extraction of the TRL Fraction for Untargeted Lipidomics
Lipids were extracted from aliquots of TRL using a modified Bligh and Dyer method.[35] TRL fractions (60 μL) were placed in a glass vial and combined with methanol (190 μL) and vortexed for 20 sec. Dichloromethane (380 μL) was added, the mixture vortexed another 20 sec, then water (120 μL) added before a final 10 sec vortex. Mixtures were centrifuged at 8000 x g (Microfuge 22R, Beckman Coulter, Brea, CA) for 10 min at 10° C. The organic layer (340 μL) was removed and dried under argon. Samples were reconstituted in injection solvent (65:35 acetonitrile/isopropanol, 240 μL) and further diluted 100x.
Targeted Vitamin A & D analysis via HPLC-MS/MS
Dried TRL extracts were re-dissolved in MTBE (50μL) containing a mix of stable isotope internal standards (0.01 nmol 13C3-retinyl palmitate, 0.15 nmol D3-ergocalciferol) and were sonicated in a water bath. Methanol (100μL) was added, and samples were re-sonicated and centrifuged to remove remaining particulate, with the supernatant analyzed. The extraction efficiency has been previously validated to be ≥95%.[34] Thus, internal standards were added upon reconstitution, to control for matrix effects, and to avoid deuterium exchange between the ergocalciferol standard and methanol used in the extraction.[34]
HPLC-MS/MS analyses were performed as described with minor modification to also assess ergocalciferol.[34] Samples were analyzed on a Thermo Ultimate 3000 HPLC (Thermo Fisher Scientific, Waltham, MA, USA) interfaced with a Quantiva TSQ triple-quadrupole mass spectrometer (Thermo Fisher Scientifc, Waltham, MA, USA) using an atmospheric pressure chemical ionization (APCI) probe operated in positive ion mode, with source parameters as follows: sheath gas = 45 arbitrary units (AU), auxillary gas = 10 AU, sweep gas = 12 AU, ion transfer tube temperature = 350° C, vaporizer temperature = 450° C, positive ion discharge current = 5 μA, dwell time = 50 msec. Precursor-product ion pairs were selected, and collision energies optimized, for each native compound and the corresponding stable isotope (Supporting Information Table S4). Internal standards were used for quantitation. Representative chromatograms can be found in Supporting Information Figure S1.
Area under the time-concentration curve (AUCs) was calculated using the trapezoidal method. The AUCs from 0–6 hours were determined to be more relevant than AUCs across 0–12 hours, because subjects consumed a vitamin A-rich lunch following the 6 h blood draw. See Supporting Information Table S3 for meal components, and Supporting Information Figure S2 and Supporting Information Table S6 for 0–12 h AUCs).
Untargeted lipidomics analysis via.
UHPLC-MS
Lipid separation followed a previously described UHPLC method.[36] Sample extracts (10 μL) were injected onto an Acquity HSS T3 column (2.1 mm x 100 mm, 1.8 μm particle size; Waters, Milford, MA, USA) at 55°C in a 1290 UHPLC (Agilent, Santa Clara, CA, USA) with solvent A = 40:60 acetonitrile/water, and solvent B = 10:90 acetonitrile/isopropanol, both containing 10 mM ammonium acetate. Solvent flow = 0.4 mL/min, with a gradient as follows: increasing linearly from 40% to 100% B over 10 min, held at 100% B for 4 min, rapidly returned to initial conditions and held for 6 min. The UHPLC was interfaced with a 6545 quadrupole-time-of-flight mass spectrometer (Agilent, Santa Clara, CA, USA) operated with electrospray ionization (ESI) operated in positive ion mode. The MS scanned over 50–1700 m/z with an acquisition rate of 8119 transients per spectrum and 1 spectrum per second with source parameters as follows: voltage capillary = +3000 V, nozzle voltage = 35 V, gas temperature = 300° C, gas flow = 8 L/min, nebulizer = 30 psi, sheath gas temperature = 350° C, sheath gas flow = 10 L/min. Lipids with significant associations with vitamins A and D were initially identified using MS/MS fragmentation and the LIPID MAPS Structure Database.[37] Authentic standards of putative structures were purchased, and identities were confirmed using retention time, MS, and MS/MS spectra of authentic standards relative to sample.
Statistical Analysis
R studio was used to perform analyses,[38] with P ≤ 0.05 considered statistically significant. AUC differences between MetS and control group were compared using the Mann-Whitney U Test because the data was not normally distributed, as determined by the Shapiro-Wilk test.
Following lipidomic analyses, lipids differentially distributed between the two groups were identified using a mixed effect model, with log-transformed lipid intensities as the dependent variable; MetS status, time, and MetS status x time interaction as the fixed factors; and subjects as random factor (Supporting Information Table S5). The correlations between these lipids and log-transformed concentrations of ApoB-48-normalized and non-normalized vitamins A and D2 were determined by calculating the Pearson correlation coefficient (P < 0.05). Fold change in lipids between health groups were determined by calculating the 0–6 h AUC of lipid intensities, and performing a Wilcoxon Rank-Sum comparison.
Results
Vitamin A and D Analysis
At baseline, the TRL concentrations of vitamin A, vitamin D2, ApoB-48, and vitamin A normalized to ApoB-48 were not different regardless of health status (Supporting Information Figure S2). Only baseline concentrations of vitamin D2 normalized to ApoB-48 were higher in MetS subjects vs. healthy controls.
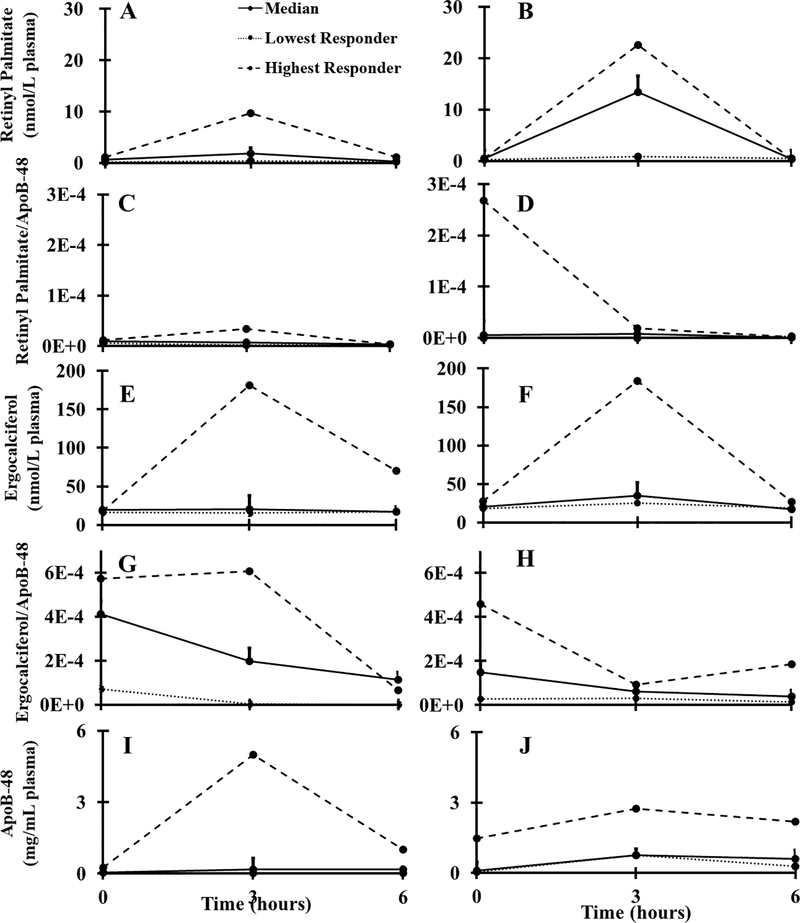
The median 0–6 h AUCs for vitamins A and D2 are shown in Figure 1 (individual AUC comparisons in Supporting Information Figure S3). Newly absorbed vitamin A was 6.4-fold greater in MetS subjects vs. controls (P = 0.07). In contrast, vitamin D2 absorption was not different between MetS subjects and control subjects whether considering 0–6 h (P = 0.48) or 0–12 h (P = 0.37, Supporting Information Figure S2). MetS subjects had 4.5-fold higher 0–6 h ApoB-48 AUC than the healthy controls (P = 0.07) (Figure 1I, J; Table 1).
Figure 1.
Postprandial curves from 0–6 h representing average response (n=10 per group), highest-, and lowest-responders for (a) vitamin A healthy, (b) vitamin A MetS, (c) vitamin A normalized to ApoB-48 healthy, (d) vitamin A normalized to ApoB-48 MetS, (e) vitamin D2 healthy, (f) vitamin D2 MetS, (g) vitamin D2 normalized to ApoB-48 healthy, (h) vitamin D2 normalized to ApoB-48 MetS, (i) ApoB-48 healthy, (j) ApoB-48 MetS. MetS, metabolic syndrome; ApoB-48, apolipoproteinB-48.
Table 1.
Median vitamin A, D2 (nmol/L plasma*hr), and ApoB-48 (ng/mL plasma) 6-hour AUCs ± SEM of MetS (n = 10) vs. healthy controls (n = 10).
| Vitamin | MetS | Healthy | P-value |
|---|---|---|---|
| Retinyl palmitate (vitamin A) | 27.1 ± 9.01 | 4.25 ± 3.10 | 0.07 |
| Ergocalciferol (vitamin D2) | 127 ± 46.0 | 2.37 ± 55.8 | 0.48 |
| ApoB-48 | 36.2 x 105 ± 1.53 x 106 | 7.91 x 105 ± 1.18 x 106 | 0.07 |
| Retinyl palmitate/ApoB-48 | 3.34 x 10−5 ± 4.36 x 10−5 | 6.79 x 10−5 ± 1.48 x 10−5 | 0.54 |
| Ergocalciferol/ApoB-48 | 3.36 x 10−4 ± 1.33 x 10−4 | 1.36 x 10−3 ± 2.29 x 10−4 | 0.02 |
When normalized to ApoB-48 levels in the same sample, the 0–6 h AUCs of vitamin A in MetS and healthy control subjects were not significantly different (P = 0.54). In contrast, vitamin D2 AUCs normalized to ApoB-48 were lower in MetS subjects than healthy controls (P = 0.02), (Figure 1E, F; Table 1).
Untargeted Lipidomic Analysis
Following data deconvolution, feature grouping and retention time alignment, 4687 lipids metabolites were detected. Peaks were manually inspected, and those present in process blanks, in ≤ 80% of the pooled quality control (QC) samples, and with relative standard deviations >30% in the pooled QC, were removed. Of the remaining 370 lipids, we found the changes over 6 h were different between health groups in seven lipid species, as determined by mixed effect modeling. Of these, the identities of five were confirmed against authentic standards (SM 18:1/16:0, PC 16:0/18:1, PC 16:0/20:4, PC 16:0/20:3, and PC 18:0/18:2), and the remaining two were identified with Level 2 and 3 confidence (as a cholesterol ester and SM 18:2/24:1, Supporting Information Table S5).[39] A majority of these lipids (6 out of 7) are classified as choline phospholipids. All seven lipids were significantly positively correlated with vitamin A TRL concentrations (both with and without normalization to ApoB-48), and vitamin D2 TRL concentrations (Table 2).
Table 2.
Pearson’s correlation coefficients of newly absorbed vitamin A and vitamin D2 AUC with select lipid AUCs over 0–6 h.
| Lipid | Neutral mass (Da) | r Vit. A | P-value | r Vit. A/ApoB-48 | P-value | r Vit. D2 | P-value | r Vit. D2 /ApoB-48 | P-value | Identification Levela |
|---|---|---|---|---|---|---|---|---|---|---|
| CE | 368.3425 | 0.54 | 1.3E-4 | 0.31 | 0.05 | 0.64 | 2.0E-6 | −0.14 | 0.37 | level 3 |
| SM 18:1/16:0 | 702.5698 | 0.58 | 3.2E-5 | 0.34 | 0.03 | 0.63 | 3.2E-6 | −0.15 | 0.34 | level 1 |
| PC 16:0/18:1 | 759.5333 | 0.62 | 5.4E-6 | 0.34 | 0.03 | 0.65 | 1.5E-6 | −0.16 | 0.32 | level 1 |
| PC 16:0/20:4 | 781.5549 | 0.58 | 3.2E-5 | 0.35 | 0.02 | 0.64 | 2.5E-6 | −0.18 | 0.25 | level 1 |
| PC 16:0/20:3 | 783.5218 | 0.61 | 8.5E-6 | 0.34 | 0.03 | 0.67 | 5.7E-7 | −0.18 | 0.24 | level 1 |
| PC 18:0/18:2 | 785.5916 | 0.60 | 1.3E-5 | 0.34 | 0.03 | 0.68 | 2.4E-6 | −0.19 | 0.22 | level 1 |
| SM 18:2/24:1 | 810.5951 | 0.53 | 2.0E-4 | 0.34 | 0.03 | 0.60 | 1.8E-6 | −0.21 | 0.19 | level 2 |
Identification level is based on criteria outlined in [39]. Lipids were selected via significant group x time interactions in a mixed-effect model applied to all detected lipids.
Discussion
Findings from this pilot study indicate that absorption of vitamin A trended higher in MetS subjects than in healthy controls. In contrast, absorption of vitamin D2 was unaffected by health status. Vitamin D2 normalized to ApoB-48 was significantly lower in MetS subjects; however, vitamin A normalized to ApoB-48 was not statistically different, indicating nutrient-specific effects for MetS on FSV chylomicron packaging and release into the blood stream. Lipidomics analyses revealed that the TRL concentrations of both vitamins were associated with PCs and SMs, indicative of intestinal-level absorptive processes, including micellarization and enterocyte trafficking.
MetS subjects had a trend toward a higher post-prandial response to a physiologically relevant dose of pre-formed vitamin A, as compared to healthy controls. Similarly, a previous study found that obese subjects (BMI 43.66 ± 2.81 kg/m2) had ~2x the AUC of chylomicron retinyl palmitate compared to controls 24 hours following a fat load test; however, the values were not statistically significant.[40] Postprandial retinyl palmitate levels were also significantly higher in men with higher visceral adiposity (a component of MetS) after a fat load test containing 60,000 IU vitamin A as compared to men with low visceral adiposity.[41]
These vitamin A results are in contrast to the absorption of isotopically labeled vitamin E in the same subjects from the same meal, where MetS subjects absorbed 11% less than the healthy controls. The Tmax of newly absorbed vitamin E in the chylomicron fraction occurred at 9 hours, in contrast to the 3 hour Tmax observed for vitamins A and D2 and ApoB-48, suggesting that enterocyte sequestration limited vitamin E release in all subjects, but with a stronger influence on MetS subjects[25]—a phenomenon not observed in the present study. It should also be noted that the dose of vitamin E was 100x higher than that of vitamin A and 5000x higher than vitamin D2 (in mg). Caco-2 experiments have previously demonstrated antagonistic effects of vitamin E on vitamin D absorption,[42] and vice versa,[27] but this interaction has not yet been studied in humans. Additionally, the approach used to determine the differences in bioavailability of vitamin E included assessing the percent of labeled vitamin E normalized to the unlabeled concentration in the TRL fraction,[25] as a labeled dose of this vitamin (but not others) was fed. Each of these factors may have contributed to the differences observed.
When newly absorbed retinyl palmitate was normalized to ApoB-48, it did not produce significant differences in AUC, indicating that the same number of retinyl ester particles were transported on each chylomicron, regardless of health status. Thus, we infer that MetS subjects absorbed more preformed vitamin A than healthy controls, suggesting that decreased levels of circulating vitamin A observed in MetS subjects from previous work[13] are not due to decreased absorption. Other factors that may reduce circulating vitamin A include decreased release of retinol bound to retinol binding protein (RBP) from the liver,[14,43] potentially due to decreased binding efficiency, and/or impaired liver function due to NAFLD and NASH in MetS.[13]
Lipidomic analysis revealed a number of PCs and SMs that were positively correlated with retinyl palmitate concentrations (Table 2). PCs are cleaved by phospholipases, particularly brush-border phospholipase B (PLB) and pancreatic lipase-related protein 2 (PLRP2).[44] Retinyl esters are also cleaved by PLB [45] and, to a smaller extent, PLRP2 [46] in the intestinal lumen (see Figure 2). Thus, because the magnitude of the fold changes were >1.5 for each PC (Supporting Information Table S7), it is possible that phospholipid and retinyl ester cleavage in the gastrointestinal lumen is increased in MetS subjects, potentially via increased activity of PLB and/or PLRP2. This would be expected to increase PC and retinol absorption in MetS, followed by re-esterification for packaging into the chylomicron. This hypothesis is further supported by in vitro and in vivo rodent work reporting increased transcription of pancreatic PLRP2 in response to increased leptin,[47,48] which is associated with MetS.[49] Likewise, a rat model of pancreatitis—a potential late-stage consequence of MetS—also reported increased activity of brush border PLB in the ileum in response to decreased pancreatic lipases in the proximal intestine.[50] There is no data to-date regarding expression of intestinal PLB or PLRP2 in MetS versus healthy humans.
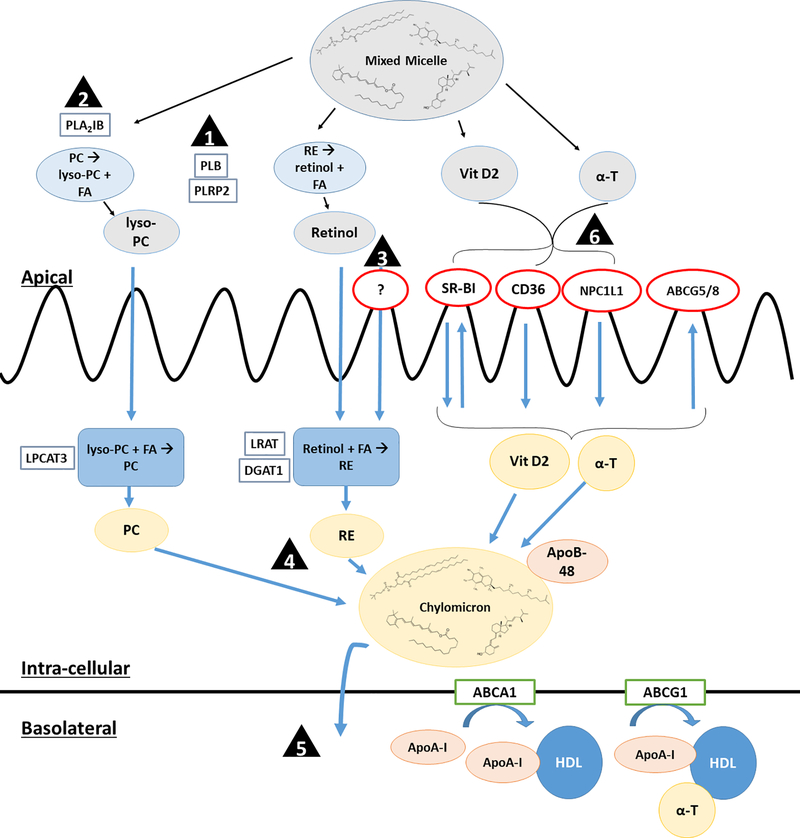
Figure 2.
Schematic of potential points of differences in FSV absorption between MetS and control subjects: (1) Increased PLB and PLRP2 expression and/or activity in MetS may increase retinyl ester hydrolysis and uptake. (2) Increased PLA2IB activity, leading to increased luminal lyso-PC, may increase chylomicron secretion and contribute to increased retinyl ester absorption in MetS subjects. (3) Unidentified retinol-specific apical transport protein may lead to increased retinol uptake in MetS. (4) Vitamin A absorption may be linked to the increased lipemic response observed in MetS, either due to increased chylomicron production and/or decreased clearance. (5) Increased SM in chylomicrons of MetS persons may delay chylomicron clearance, contributing to elevated vitamin A measured in this fraction. (6) Vitamins D and E compete for transporters in in vitro models; however, clinical studies are warranted to investigate the effects of this antagonism in vivo. Abbreviations: ABCA1 = ATP-binding cassette transporter sub-family A member 1, ABCG1 = ATP-binding cassette transporter sub-family G member 1, ABCG5/8 = ATP-binding cassette transporter sub-family G members 5/8, ApoB-48 = apolipoproteinB-48, ApoA-I = Apolipoprotein A-I, α-T = α-tocopherol, CD36 = cluster of differentiation 36, DGAT1 = diglyceride acyltransferase 1, HDL = high density lipoprotein, LPCAT3 = lysophosphatidylcholine acyltransferase 3, LRAT = lecithin retinol acyltransferase, lyso-PC = lysophosphatidylcholine, NPC1L1 = Niemann-Pick C1-Like 1, PC = phosphatidylcholine, PLA2IB = phospholipase A2 group IB, PLB = brush border phospholipase B, PLRP2 = pancreatic lipase-related protein 2, RE = retinyl ester, SM = sphingomyelin, SR-BI = scavenger receptor class B type 1, Vit D2 = ergocalciferol.
Increased concentration or activity of phospholipolytic enzymes that act earlier in the gastrointestinal tract, such as pancreatic phospholipase A2 IB (PLA2IB),[44] could also indirectly influence MetS retinyl ester absorption via increased chylomicron synthesis and secretion. Caco-2 cells incubated with micelles containing lyso-PC (the product of PLA2IB cleavage) synthesized and secreted ~1.5x more ApoB-48 than cells incubated with lyso-PC-free micelles.[51] Further supporting this theory is the trend of increased ApoB-48 concentrations in our MetS subjects relative to healthy controls, an observation also reported by others.[52–54] Higher concentrations of TRL retinyl palmitate could also be due to delayed chylomicron clearance[55] and/or increased chylomicron production[53] in MetS subjects. Indeed, the positive correlation of two SM species with retinyl palmitate concentrations further supports delayed chylomicron clearance, as increased chylomicron SM has been associated with decreased plasma lipid clearance. Rats fed lipid emulsions with increasing proportions of SM (0–100%) had the fractional plasma clearance rate of radiolabeled cholesterol ester and triglyceride to decrease ~94%,[56] potentially due to SM inhibition of lipoprotein lipase (LPL).[57] Further study of the influence of PC and SM digestion on chylomicron production and clearance in MetS is warranted in the context of vitamin A absorption.
While early kinetic studies of uptake by Hollander et al. suggest the existence of an apical transport protein for preformed vitamin A,[58] no specific transporter has been identified to date. Due to the divergent behavior between MetS and healthy subjects for vitamin A but not vitamin D2, results further support separate absorption transporters, consistent with previous findings indicating shared transport mechanisms for vitamins D, E, and K, but not A.[27]
The AUC of newly absorbed vitamin D2 was not significantly different between MetS subjects and healthy controls. Of the previous studies conducted with relevance to MetS, a significant inverse correlation was observed between peak serum vitamin D2 concentration (i.e. Cmax) and BMI in obese and healthy subjects consuming a supraphysiological dose of vitamin D2 (50,000 IU).[20] However, it should be noted that this dose was 420x higher than the dose reported herein, and measurements were taken in serum rather than the TRL fraction.
Strengths of the study included the use of physiologically relevant doses of vitamins A and D2 in age- and gender-matched MetS and healthy subjects. Limitations due to the design of the parent study precluded analyses beyond hour 6 for retinyl esters, and limited blood draws between hours 0 and 6. Previous studies of retinyl ester absorption confirm that the hour 3 time point adequately reflects the Tmax of vitamin absorption.[30,59] Likewise, the kinetics of vitamin D2 absorption at hours 3 and 6 align with the results of Compston et al., Thompson et al., and Gleize et al., where the average Tmax of chylomicron vitamin D was observed 2–3 hours following dosing, with steady decreases from hours 4–8.[60–62] Desmarchelier et al. observed a slightly later average TRL-fraction Tmax, although significant interindividual variability was reported.[63] Because the lunch meal fed a small dose of vitamin D3 (i.e. as opposed to vitamin D2 fed with the test meal), we also considered the 0–12 hour AUCs to assess differences in vitamin D2 between MetS and healthy subjects (see Supporting Information Figure S2), but no significant difference was observed. For direct comparison with vitamin A, we left the 0–6 hour AUCs for both nutrients. This time frame also eliminates the 12 hour TRL increase observed for both nutrients, previously shown to be due retinyl ester re-release on VLDL captured in the TRL,[64] a phenomenon that also likely occurs for vitamin D2.[60,61] Limitations in plasma volume also precluded the analysis of vitamin A and D status in these subjects. Future studies should consider an 8–12 hour time-course following a lipid-rich vitamin A breakfast and vitamin-free lunch, to better follow preformed vitamin A kinetics in MetS subjects vs. healthy controls.
In conclusion, 6-hour postprandial absorption of vitamin A trended toward an increase in MetS versus healthy subjects. This increase may be due to increased activity of PLB, PLRP2, and/or PLA2IB in MetS subjects relative to healthy controls, a hypothesis that warrants testing in future studies. The postprandial vitamin D2 response was not different between MetS and healthy controls. These results are in contrast to decreased vitamin E bioavailability in the same MetS subjects as compared to controls, which may be due to differences in vitamin E sequestration within the enterocyte, in relative doses, and/or the method of bioavailability assessment. Together, these data suggest that in studies of MetS where reduced circulating levels of vitamins A and D were observed, their decrease is more likely due to reduced intakes, and/or sequestration in adipose or other tissues. Further cell and animal studies of digestion and postprandial absorption are warranted to pinpoint the mechanistic differences between vitamin handling in MetS versus healthy populations.
Supplementary Material
Acknowledgements
Thank you to Dr. Matt Bernier for assistance with LC-MS instrumentation, and Mariona Vendrell-Pacheco for assistance in extracting the samples.
Funding Sources
This research was supported by a Foods for Health Discovery Themes Initiative SEEDS grant, the National Dairy Council, and the Ohio Agricultural Research and Development Center at OSU. The sample analyses were supported by NIH Award Number Grant P30 CA016058, OSU, and OSUCCC. Statistical analyses were supported by NIH grant UL1TR002733.
Abbreviations:
- ABCA1
ATP binding cassette transporter subfamily A member 1
- ABCG1
ATP binding cassette transporter subfamily G member 1
- ABCG5/8
ATP binding cassette transporter subfamily G member 5/8
- APCI
atmospheric pressure chemical ionization
- ApoB-48
Apolipoprotein B-48
- AUC
area under the curve, body mass index (BMI)
- CD36
cluster of differentiation 36
- (CVD)
cardiovascular disease
- ELISA
enzyme-linked immunosorbent assay
- FSV
fat-soluble vitamin
- HDL-C
high density lipoprotein cholesterol
- HPLC
high performance liquid chromatography
- LC-MS
liquid chromatography-mass spectrometry
- LDL-C
low density lipoprotein cholesterol
- LPL
lipoprotein lipase
- LXR
liver X receptor
- lyso-PC
lysophosphatidylcholine
- MetS
metabolic syndrome
- MTBE
methyl tert-butyl ether
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- NPC1L1
Niemann-Pick C1-Like 1
- PC
phosphatidylcholine
- PLA2IB
phospholipase A2 group IB
- PLB
phospholipase B
- PLRP2
pancreatic lipase-related protein 2
- QC
quality control
- RA
retinoic acid
- RAR
retinoic acid receptor
- RBP
retinol binding protein
- RBPR2
retinoid binding protein receptor 2
- RXR
retinoid X receptor
- SM
sphingomyelin
- SNP
single nucleotide polymorphism
- SR-B1
scavenger receptor - class B type 1
- STRA6
stimulated by retinoic acid 6
- TG
triglyceride
- TRL
triglyceride-rich lipoprotein fraction
- T2DM
type 2 diabetes mellitus
- UHPLC
ultra-high performance liquid chromatography
- VDR
vitamin D receptor
Footnotes
Conflict of Interest
The authors have no conflicts of interest to declare.
Data Availability Statement:
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
- [1].Mottillo S, Filion KB, Genest J, Joseph L, Pilote L, Poirier P, Rinfret S, Schiffrin EL, Eisenberg MJ, J. Am. Coll. Cardiol. 2010, 56, 1113–1132. [DOI] [PubMed] [Google Scholar]
- [2].Ford ES, Diabetes Care 2005, 28, 1769–1778. [DOI] [PubMed] [Google Scholar]
- [3].Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, Fruchart J-C, James PT, Loria CM, Smith SC, Circulation 2009, 120, 1640–1645. [DOI] [PubMed] [Google Scholar]
- [4].Bonet ML, Ribot J, Palou A, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2012, 1821, 177–189. [DOI] [PubMed] [Google Scholar]
- [5].Blumberg JM, Tzameli I, Astapova I, Lam FS, Flier JS, Hollenberg AN, J. Biol. Chem. 2006, 281, 11205–11213. [DOI] [PubMed] [Google Scholar]
- [6].McGill AT, Stewart JM, Lithander FE, Strik CM, Poppitt SD, Nutr. J. 2008, 7, 3–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Gagnon C, Lu ZX, Magliano DJ, Dunstan DW, Shaw JE, Zimmet PZ, Sikaris K, Ebeling PR, Daly RM, J. Clin. Endocrinol. Metab. 2012, 97, 1953–1961. [DOI] [PubMed] [Google Scholar]
- [8].Reis JP, Von Mühlen D, Kritz-Silverstein D, Wingard DL, Barrett-Connor E, Diabetes Care 2007, 30, 1549–1555. [DOI] [PubMed] [Google Scholar]
- [9].Rueda S, Fernández-Fernández C, Romero F, Martínez De Osaba MJ, Vidal J, Obes. Surg. 2008, 18, 151–154. [DOI] [PubMed] [Google Scholar]
- [10].Pham TM, Ekwaru JP, Setayeshgar S, Veugelers PJ, Nutrients 2015, 7, 7271–7284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Al-Daghri NM, Alkharfy M, Khalid, Al-Saleh Y, Al-Attas OS, Alokail MS, Al-Othman A, Moharram O, El-Kholie E, Sabico S, Kumar S, Chrousos GP, Metab. Clin. Exp. 2012, 61, 661–666. [DOI] [PubMed] [Google Scholar]
- [12].Ferreira PP, Cangussu L, Bueloni-Dias FN, Orsatti CL, Schmitt EB, Nahas-Neto J, Nahas EAP, Climacteric 2019, DOI 10/1080/13697137.2019.1611761. [DOI] [PubMed] [Google Scholar]
- [13].Gunanti IR, Marks GC, Al-Mamun A, Long KZ, Nutr J. 2014, 144, 489–495. [DOI] [PubMed] [Google Scholar]
- [14].Aeberli I, Biebinger R, Lehmann R, L’Allemand D, Spinas GA, Zimmermann MB, J. Clin. Endocrinol. Metab. 2007, 92, 4359–4365. [DOI] [PubMed] [Google Scholar]
- [15].Thomas-Valdés S, das Gracas M Tostes V, Anunciacao PC, da Silva BP, Pinheiro Sant’Ana HM, Crit. Rev. Food Sci. Nutr. 2017, 57, 3332–3343. [DOI] [PubMed] [Google Scholar]
- [16].Thomas GN, O-Hartaigh B, Bosch JA, Pilz S, Loerbroks A, Kleber M, Fischer JE, Grammer TB, Bohm BO, Marz W, Diabetes Care 2012, 35, 1158–1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Chen SH, He F, Zhou HL, Wu HR, Xia C, Li YM, J. Dig. Dis. 2011, 12, 125–130. [DOI] [PubMed] [Google Scholar]
- [18].Liu Y, Chen H, Wang J, Zhou W, Sun R, Xia M, Am. J. Clin. Nutr. 2015, 102, 130–137. [DOI] [PubMed] [Google Scholar]
- [19].Lissner L, Lindroos AK, Sjostrom L, Eur. J. Clin. Nutr. 1998, 52, 316–322. [DOI] [PubMed] [Google Scholar]
- [20].Wortsman J, Matsuoka LY, Chen TC, Lu Z, Holick MF, Am. J. Clin. Nutr. 2000, 72, 690–693. [DOI] [PubMed] [Google Scholar]
- [21].Stephensen CB, Gildengorin G, Am. J. Clin. Nutr. 2000, 72, 1170–1178. [DOI] [PubMed] [Google Scholar]
- [22].Bellia A, Garcovich C, D’Adamo M, Lombardo M, Tesauro M, Donadel G, Gentileschi P, Lauro D, Federici M, Lauro R, Sbraccia P, Intern. Emerg. Med. 2011, 8, 33–40. [DOI] [PubMed] [Google Scholar]
- [23].Traber MG, Buettner GR, Bruno RS, Redox Biol. 2019, 21, 101091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Villaca Chaves G, Pereira SE, Saboya CJ, Ramalho A, Obes. Surg. 2008, 18, 378–385. [DOI] [PubMed] [Google Scholar]
- [25].Mah E, Sapper TN, Chitchumroonchokchai C, Failla ML, Schill KE, Clinton SK, Bobe G, Traber MG, Bruno RS, Am. J. Clin. Nutr. 2015, 102, 1070–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Reboul E, Borel P, Prog. Lipid Res. 2011, 50, 388–402. [DOI] [PubMed] [Google Scholar]
- [27].Goncalves A, Roi S, Nowicki M, Dhaussy A, Huertas A, Amiot MJ, Reboul E, Food Chem. 2015, 172, 155–160. [DOI] [PubMed] [Google Scholar]
- [28].Reboul E, Nutrients 2013, 5, 3563–3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Isken A, Golczak M, Oberhauser V, Hunzelmann S, Driever W, Imanishi Y, Placzewski K, von Lintig J, Cell Metab. 2008, 7, 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Borel P, Dubois C, Mekki N, Grolier P, Partier A, Alexandre-Gouabau MC, Lairon D, Azais-Braesco V, Eur. J. Clin. Nutr. 1997, 51, 717–722. [DOI] [PubMed] [Google Scholar]
- [31].Tangpricha V, Koutkia P, Rieke SM, Chen TC, Perez AA, Holick MF, Am. J. Clin. Nutr. 2003, 77, 1478–1483. [DOI] [PubMed] [Google Scholar]
- [32].Borel P, Desmarchelier C, Nowicki M, Bott R, Morange S, Lesavre N, Am. J. Clin. Nutr. 2014, 100, 168–175. [DOI] [PubMed] [Google Scholar]
- [33].Kinoshita M, Kojima M, Matsushima T, Teramoto T, Clin. Chim. Acta 2005, 351, 115–120. [DOI] [PubMed] [Google Scholar]
- [34].Kopec RE, Schweiggert RM, Riedl KM, Carle R, Schwartz SJ, Rapid Commun. Mass Spectrom. 2013, 27, 1393–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Hu C, van Dommelen J, van der Heijden R, Spijksma G, Reijmers TH, Wang M, Slee E, Lu X, Xu G, van der Greef J, Hankemeier T, J. Proteome Res. 2008, 7, 4982–4991. [DOI] [PubMed] [Google Scholar]
- [36].Castro-Perez JM, Kamphorst J, DeGroot J, Lafeber F, Goshawk J, Yu K, Shockcor JP, Vreeken RJ, Hankemeier T, J. Proteome 2010, 9, 2377–2389. [DOI] [PubMed] [Google Scholar]
- [37].Sud M, Fahy E, Cotter D, Brown A, Dennis E, Glass C, Murphy R, Raetz C, Russell D, Subramaniam S, Nucleic Acids Res. 2006, 35, D527–D532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].RStudio Team, RStudio Inc; 2015. http://www.rstudio.com/. [Google Scholar]
- [39].Sumner LW, Amberg A, Barrett D, Beale MH, Beger R, Daykin CA, Fan TWM, Fiehn O, Goodacre R, Griffin JL, Hankemeier T, Hardy N, Harnly J, Higashi R, Kopka J, Lane AN, Lindon JC, Marriott P, Nicholls AW, Reily MD, Thaden JJ, Viant MR, Metabolomics 2007, 3, 211–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Lewis GF, Omeara NM, Soltys PA, Blackman JD, Iverius PH, Druetzler AF, Getz GS, Polonsky KS, J. Clin. Endocrinol. Metab. 1990, 71, 1041–1050. [DOI] [PubMed] [Google Scholar]
- [41].Couillard C, Bergeron N, Prud’Homme D, Bergeron J, Tremblay A, Bouchard C, Mauriège P, Després JP, Diabetes 1998, 47, 953–960. [DOI] [PubMed] [Google Scholar]
- [42].Reboul E, Goncalves A, Comera C, Bott R, Nowicki M, Landrier JF, Jourdheuil-Rahmani D, Dufour C, Collet X, Borel P, Mol. Nutr. Food Res. 2011, 55, 691–702. [DOI] [PubMed] [Google Scholar]
- [43].Mills JP, Furr HC, Tanumihardjo SA, Exp. Biol. Med. 2008, 233, 1255–1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Nilsson Å, Duan R-D, Am J Physiol Gastrointest Liver Physiol 2019, 316, 425–445. [DOI] [PubMed] [Google Scholar]
- [45].Rigtrup KM, Kakkad B, Ong DE, Biochemistry 1994, 33, 2661–2666. [DOI] [PubMed] [Google Scholar]
- [46].Reboul E, Berton A, Moussa M, Kreuzer C, Crenon I, Borel P, Biochim. Biophys. Acta 2006, 1761, 4–10. [DOI] [PubMed] [Google Scholar]
- [47].Birk RZ, Rubio-Aliaga I, Boekschoten MV, Danino H, Müller M, Daniel H, Br. J. Nutr. 2014, 112, 154–161. [DOI] [PubMed] [Google Scholar]
- [48].Elinson N, Amichay D, Birk RZ, Br. J. Nutr. 2006, 96, 691–696. [PubMed] [Google Scholar]
- [49].Correia MLG, Rahmouni K, Diabetes, Obes. Metab. 2006, 8, 603–610. [DOI] [PubMed] [Google Scholar]
- [50].Tchoua U, Ito M, Okamoto M, Tojo H, Biochim. Biophys. Acta 2000, 1487, 255–267. [DOI] [PubMed] [Google Scholar]
- [51].Nakano T, Inoue I, Katayama S, Seo M, Takahashi S, Hokari S, Shinozaki R, Hatayama K, Komoda T, J. Clin. Biochem. Nutr. 2009, 45, 227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Mekki N, Christofilis A, Charbonnier M, Atlan-Gepner C, Defoort C, Juhel C, Borel P, Portugal H, Pauli AM, Vialettes B, Lairon D, J. Clin. Endocrinol. Metab. 1999, 84, 184–191. [DOI] [PubMed] [Google Scholar]
- [53].Shojaee-Moradie F, Ma Y, Lou S, Hovorka R, Umpleby AM, Diabetes 2013, 62, 4063–4069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [54].Vine DF, Glimm DR, Proctor SD, Atheroscler. Suppl. 2008, 9, 69–76. [DOI] [PubMed] [Google Scholar]
- [55].Vine DF, Takechi R, Russell JC, Proctor SD, Atherosclerosis 2007, 190, 282–290. [DOI] [PubMed] [Google Scholar]
- [56].Redgrave TG, Rakic V, Mortimer BC, Mamo JCL, Biochim. Biophys. Acta (BBA)/Lipids Lipid Metab. 1992, 1126, 65–72. [DOI] [PubMed] [Google Scholar]
- [57].Saito H, Arimoto I, Tanaka M, Sasaki T, Tanimoto T, Okada S, Handa T, Biochim. Biophys. Acta - Mol. Cell Biol. Lipids 2000, 1486, 312–320. [DOI] [PubMed] [Google Scholar]
- [58].Hollander D, Muralidhara KS, Am. J. Physiol. Metab. 1977, 232, E471–E477. [DOI] [PubMed] [Google Scholar]
- [59].Sauvant P, Mekki N, Charbonnier M, Portugal H, Lairon D, Borel P, Metabolism. 2003, 52, 514–519. [DOI] [PubMed] [Google Scholar]
- [60].Compston JE, Merrett AL, Hammett FG, Magill P, Clin. Sci. 1981, 60, 241–243. [DOI] [PubMed] [Google Scholar]
- [61].Thompson GR, Lewis B, Booth CC, J. Clin. Invest. 1966, 45, 94–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Gleize B, Hiolle M, Meunier N, Pereira B, Richard R, Savary-Auzeloux I, Buffiere C, Peyron M-A, Halimi C, Caris-Veyrat C, Nau F, Reboul E, Mol. Nutr. Food Res. 2020, In Press, DOI 10.1002/mnfr.202000228.This. [DOI] [PubMed] [Google Scholar]
- [63].Desmarchelier C, Borel P, Goncalves A, Kopec RE, Nowicki M, Morange S, Lesavre N, Portugal H, Reboul E, J. Nutr. 2016, 146, 2421–2428. [DOI] [PubMed] [Google Scholar]
- [64].Burri BJ, Clifford AJ, Arch. Biochem. Biophys. 2004, 430, 110–119. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.