Abstract
Aim:
Determine 1) frequency and risk factors for acute kidney injury (AKI) after in-hospital cardiac arrest (IHCA) in the Therapeutic Hypothermia after Pediatric Cardiac Arrest In-Hospital (THAPCA-IH) trial and associated outcomes; 2) impact of temperature management on post-IHCA AKI.
Methods:
Secondary analysis of THAPCA-IH; a randomized controlled multi-national trial at 37 children’s hospitals.
Eligibility:
Serum creatinine (Cr) within 24 hours of randomization.
Outcomes:
Prevalence of severe AKI defined by Stage 2 or 3 Kidney Disease Improving Global Outcomes Cr criteria. 12-month survival with favorable neurobehavioral outcome. Analyses stratified by entire cohort and cardiac subgroup. Risk factors and outcomes compared among cohorts with and without severe AKI.
Results:
Subject randomization:
159 to hypothermia, 154 to normothermia.
Overall, 80% (249) developed AKI (any stage), and 66% (207) developed severe AKI. Cardiac patients (204, 65%) were more likely to develop severe AKI (72% vs 56%,p=0.006). Preexisting cardiac or renal conditions, baseline lactate, vasoactive support, and systolic blood pressure were associated with severe AKI. Comparing hypothermia versus normothermia, there were no differences in severe AKI rate (63% vs 70%,p=0.23), peak Cr, time to peak Cr, or freedom from mortality or severe AKI (p=0.14). Severe AKI was associated with decreased hospital survival (48% vs 65%,p=0.006) and decreased 12-month survival with favorable neurobehavioral outcome (30% vs 53%,p<0.001).
Conclusion:
Severe post-IHCA AKI occurred frequently especially in those with preexisting cardiac or renal conditions and peri-arrest hemodynamic instability. Severe AKI was associated with decreased survival with favorable neurobehavioral outcome. Hypothermia did not decrease incidence of severe AKI post-IHCA.
Keywords: acute kidney injury, post-cardiac arrest, in-hospital, therapeutic hypothermia
Introduction:
In-hospital (IH) cardiac arrest (IHCA) occurs in ≥15,000 children/year in the United States.1, 2 IHCA with return of circulation (ROC) is associated with morbidity and mortality including multiorgan dysfunction involving the brain, heart, liver, kidneys, and other organs.3–6 Recently, the Therapeutic Hypothermia after Pediatric Cardiac Arrest (THAPCA) trials demonstrated targeted temperature management at 32–34°C versus 36.0–37.5°C did not impact 1-year survival with favorable neurobehavioral outcome after either IHCA or out of hospital (OH) cardiac arrest (OHCA).7, 8
Acute kidney injury (AKI) frequently occurs after cardiac arrest (CA). Studies in pediatric medical and cardiac intensive care unit (ICU) patients report AKI is associated with poor outcomes.9, 10 Current literature suggests therapeutic hypothermia (TH) may improve outcomes and ameliorate AKI after CA in adults.11–15 It is unknown if TH can decrease the incidence or severity of AKI after pediatric IHCA.16, 17 We recently reported a secondary analysis of AKI in the THAPCA-OH trial that found 41% of patients developed severe AKI (Stages 2–3), which was associated with decreased survival and decreased survival with favorable neurobehavioral outcome. However, TH did not impact outcomes.18
The epidemiology of AKI after pediatric IHCA is not well studied.19 To expand our understanding of AKI and outcomes in children following IHCA, we performed a secondary analysis of the THAPCA-IH trial with the following aims to determine: 1) the frequency and risk factors for AKI in comatose children after IHCA; 2) the association of severe AKI with outcomes; 3) the impact of TH on the incidence of severe AKI after IHCA and survival; and 4) if these findings differ between two distinct IHCA populations: cardiac and non-cardiac patients.
Methods:
Parent trial
The THAPCA-IH trial was a randomized controlled trial at 37 children’s hospitals in the US, Canada, and England between 9/1/2009–2/27/2015.7 All comatose children >48 hours and <18 years old who had IHCA, chest compressions for ≥2 minutes, and mechanical ventilation after ROC were eligible. Key exclusions included: Glasgow Coma Scale (GCS) motor-response subscale of 5 or 6, inability to be randomized within 6 hours after ROC, active and refractory severe bleeding, antecedent illness conferring a life expectancy <12 months, and clinical discretion to withhold aggressive treatment.
Randomization to therapeutic normothermia (TN) 36.8°C (range 36–37.5°C) versus TH 33°C (range 32–34°C) occurred in a 1:1 ratio. Target temperature was sustained for 120 hours. TH involved cooling to 33°C (range 32–34°C) for 48 hours, warming to 36.8°C (range 36–37.5°C) over 16 hours, and then maintenance at 36.8°C through 120 hours. TN involved maintenance at 36.8°C (range 36–37.5°C) for 120 hours.
See the THAPCA-IH Trial primary publication for details.7
AKI secondary analysis
Inclusion criteria
A serum creatinine (Cr) within 24 hours of randomization was required. Children ≤7 days old were excluded.
Definitions
Surrogates of renal function or injury were calculated for 3 timepoints: estimated baseline Cr, preexisting peri-CA AKI, and post-CA AKI. A baseline Cr measured prior to CA was not available in most patients (n=40 with baseline Cr value), therefore, estimated baseline Cr was determined by 2 methods. First, Cr was calculated based on height if available using the Schwartz formula whereby Cr = 0.413 x height in cm/100.20 Alternatively, if height was not available, Cr was assigned value based on age (<5 years = 0.3 mg/dL, 5–12 years = 0.5 mg/dL, ≥12 years = 0.7 mg/dL). This baseline Cr was compared to Cr obtained between CA and at most 2 hours post-targeted temperature control initiation to calculate preexisting AKI. Because Cr is a late marker of renal injury, an immediate Cr rise in the peri-arrest period is less likely and thus, Cr may reflect AKI status at the time of CA. To ascertain the presence of post-CA AKI, baseline Cr was compared to Cr obtained serially every 12 hours from initiation to termination of the intervention period (120 hours). The highest Cr value was used to categorize patients by peak post-CA AKI stage.
Preexisting and post-CA AKI were calculated via the modified Kidney Disease: Improving Global Outcomes (KDIGO) Cr-based definition.21 The urine output (UOP) based definition was not used because granular UOP data pre and peri-arrest were unavailable. KDIGO divides AKI into: Stage 1: Cr increase to 1.5–1.9 times baseline or of ≥0.3 mg/dL within 48 hours; Stage 2: Cr increase to 2–2.9 times baseline; Stage 3: Cr increase to ≥3 times baseline or to ≥4 mg/dL or renal replacement therapy (RRT) initiation. Preexisting and post-CA severe AKI were defined as KDIGO Stages 2 or 3 as detailed in the largest multinational prospective pediatric ICU AKI study.22
A cardiac patient was defined as having congenital heart disease, acquired heart disease, or preexisting arrhythmia. Post-operative cardiac status was defined as having undergone cardiac surgery during the current hospitalization.
Outcome
The primary endpoint was 12-month survival with a favorable neurobehavioral outcome. Secondary endpoints were 12-month survival and 12-month survival without change in neurobehavioral function greater than 15 points. Neurobehavioral function was assessed via the Vineland Adaptive Behavior Scales 2nd edition (VABS-II; mean score 100, standard deviation 15), which is a caregiver-report measure.23 Baseline VABS-II reflecting functioning pre-arrest was obtained within 24 hours of randomization. A favorable neurobehavioral outcome was defined two ways 1) a score of ≥70 at 12 months (only those with baseline score of ≥70 were included) and 2) a decrease of ≤15 below baseline. If no baseline VABS-II was obtained, a Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) in the normal or mild disability category met eligibility criteria.
Statistical analysis
For demographic, clinical, and outcome data, quantitative analyses describe the overall study population and univariate analyses compare cohorts with and without severe AKI. Categorical variables were expressed as total number and percentage (Fisher’s exact test), and continuous variables were expressed as median with interquartile ranges given non-parametric distribution (Wilcoxon rank-sum test). Variables with p value <0.1 in univariate analyses were included in multivariable analyses. Multivariable analyses of severe AKI used logistic regression in a stepwise, chronological manner. Variables were partitioned into 4 phases: pre-arrest, time of ROC, post-arrest peri-temperature intervention initiation, and Day 0–1 after temperature control. Variables kept by model selection at earlier time phases in the clinical course remained part of the model as additional variables were considered in each phase. To compare time from ROC to peak post-CA severe AKI between study arms, a Wilcoxon rank-sum test was used. The Kaplan-Meier approach was used to compare freedom from the composite endpoint of mortality or severe AKI between TN and TH. A subgroup analysis of cardiac patients used the aforementioned statistical techniques. A p value of <0.05 was considered statistically significant. Adjustment for multiple p values was not performed. SAS version 9.4 software package (SAS Institute, Cary, NC) was used.
Results:
Whole cohort
Demographic data
Of the 329 subjects enrolled in the parent THAPCA-IH study, 313 met Cr eligibility criteria. Sixteen subjects were excluded; 2 subjects did not have a Cr within 24 hours of ROC, and 14 subjects were ≤7 days old at time of randomization. Randomization allocated 159 subjects to TH and 154 to TN. Demographic and clinical data including CA and therapeutic characteristics are displayed by overall population and cohorts without and without severe AKI (Table 1). About half were less than 1 year old. Over 2/3 (204/313) had cardiac disease; 85% of which had congenital heart disease and 48% were postoperative cardiac surgical patients. The most common CA aetiology was cardiovascular (49%). Bradycardia was the most common initial rhythm (57%). The median duration of chest compressions was 22 minutes, and about half required extracorporeal membrane oxygenation (ECMO) initiation prior to study intervention.24
Table 1.
Characteristics of patients experiencing IHCA separated into severe and no severe AKI cohorts
| Severe AKI |
||||
|---|---|---|---|---|
| Overall (N = 313) | Yes (N = 207) | No (N = 106) | P-value | |
| Age Group at Randomization | ||||
| <1 year | 151 (48%) | 110 (53%) | 41 (39%) | 0.0171 |
| 1–4 years | 75 (24%) | 46 (22%) | 29 (27%) | 0.3301 |
| 5–12 years | 48 (15%) | 28 (14%) | 20 (19%) | 0.2461 |
| > 13 years | 39 (12%) | 23 (11%) | 16 (15%) | 0.3661 |
| Male sex | 184 (59%) | 126 (61%) | 58 (55%) | 0.3321 |
| Preexisting severe AKI (up to 2 hours after intervention initiation)2 | 150 (48%) | 150 (72%) | 0 (0%) | <.0011 |
| Preexisting conditions | ||||
| None | 30 (10%) | 20 (10%) | 10 (9%) | 1.0001 |
| Lung or airway disease | 101 (32%) | 56 (27%) | 45 (42%) | 0.0071 |
| Neurologic condition | 100 (32%) | 61 (29%) | 39 (37%) | 0.2021 |
| Gastrointestinal disorder | 97 (31%) | 63 (30%) | 34 (32%) | 0.7971 |
| Prenatal condition | 77 (25%) | 49 (24%) | 28 (26%) | 0.6781 |
| Congenital heart disease | 173 (55%) | 119 (57%) | 54 (51%) | 0.2821 |
| Cyanotic heart disease | 39 (12%) | 28 (14%) | 11 (10%) | 0.4741 |
| Two ventricles | 109 (35%) | 72 (35%) | 37 (35%) | 0.3961 |
| Post-operative cardiac surgery patient | 98 (31% | 81 (39%) | 17 (16%) | <.0011 |
| Norwood procedure | 12 (4%) | 11 (5%) | 1 (1%) | 0.6861 |
| Acquired heart disease | 51 (16%) | 37 (18%) | 14 (13%) | 0.3341 |
| Arrhythmia | 65 (21%) | 51 (25%) | 14 (13%) | 0.0191 |
| Immunocompromised condition or taking immunosuppressive medication | 42 (13%) | 28 (14%) | 14 (13%) | 1.0001 |
| Transplant | 19 (6%) | 15 (7%) | 4 (4%) | 0.3181 |
| Endocrine condition | 20 (6%) | 16 (8%) | 4 (4%) | 0.2261 |
| Renal condition | 38 (12%) | 32 (15%) | 6 (6%) | 0.0111 |
| Other | 138 (44%) | 92 (44%) | 46 (43%) | 0.9051 |
| Primary aetiology of cardiac arrest | ||||
| Cardiovascular event | 154 (49%) | 111 (54%) | 43 (41%) | 0.0321 |
| Respiratory event | 99 (32%) | 54 (26%) | 45 (42%) | 0.0051 |
| Congenital heart disease | 45 (14%) | 32 (15%) | 13 (12%) | 0.4991 |
| Neurological event | 4 (1%) | 2 (1%) | 2 (2%) | 0.6061 |
| Multiple organ system failure | 2 (1%) | 2 (1%) | 0 (0%) | 0.5511 |
| Drug overdose | 2 (1%) | 1 (0%) | 1 (1%) | 1.0001 |
| Electrolyte imbalance | 1 (0%) | 1 (0%) | 0 (0%) | 1.0001 |
| Unknown | 6 (2%) | 4 (2%) | 2 (2%) | 1.0001 |
| Initial rhythm | ||||
| Asystole | 22 (7%) | 15 (7%) | 7 (7%) | 1.0001 |
| Bradycardia | 179 (57%) | 118 (57%) | 61 (58%) | 1.0001 |
| Pulseless electrical activity (PEA) | 66 (21%) | 43 (21%) | 23 (22%) | 0.8841 |
| Ventricular fibrillation or tachycardia | 33 (11%) | 21 (10%) | 12 (11%) | 0.8461 |
| Unknown | 13 (4%) | 10 (5%) | 3 (3%) | 0.5541 |
| Arrest occurred at study hospital | 291 (93%) | 194 (94%) | 97 (92%) | 0.4891 |
| Estimated duration of chest compressions (minutes) – Median (Q1, Q3) | 22.0 (7.0, 47.0) | 33.0 (8.0, 54.0) | 12.0 (5.0, 32.0) | <.0013 |
| Total number of doses of adrenaline (epinephrine) administered – Median (Q1, Q3) | 4.0 (2.0, 8.0) | 5.0 (2.0, 9.0) | 3.0 (2.0, 6.0) | 0.0053 |
| Assigned to Hypothermia treatment | 159 (51%) | 100 (48%) | 59 (56%) | 0.2341 |
| ECMO at treatment initiation | 167 (53%) | 133 (64%) | 34 (32%) | <.0011 |
| PRBC volume Day 0–1 per day (cc/kg) – Median (Q1, Q3) | 6.6 (0.0, 16.6) | 8.1 (0.7, 20.8) | 0.0 (0.0, 8.8) | <.0013 |
| Minimum percentile of gender/height-adjusted systolic blood pressure: Median (Q1, Q3) | 1.0 (1.0, 5.0) | 1.0 (1.0, 2.0) | 2.0 (1.0, 23.0) | <.0013 |
| Aminoglycoside (Day 0–1) | 27 (9%) | 17 (8%) | 10 (9%) | 0.8321 |
| Vancomycin (Day 0–1) | 166 (53%) | 109 (53%) | 57 (54%) | 0.9051 |
| Baseline Lactate4 (mmol/L) – Median (Q1, Q3) | 6.3 (2.5, 11.9) | 8.1 (4.0, 13.8) | 3.4 (1.8, 6.9) | <.0013 |
| Peak Lactate (Day 0–1) (mmol/L) – Median (Q1, Q3) | 7.2 (3.2, 12.9) | 9.6 (4.9, 16.0) | 3.8 (2.0, 7.7) | <.0013 |
| Baseline glucose concentration (mmol/L) – Median (Q1, Q3) | 186 (116, 276) | 177 (106, 274) | 191 (125, 278) | 0.3433 |
| Baseline ALT concentration (U/L) – Median (Q1, Q3) | 49 (25, 146) | 55 (28, 161) | 36 (23, 93) | 0.0143 |
| Peak ALT (Day 0–5) concentration (U/L) – Median (Q1, Q3) | 95 (42, 300) | 134 (53, 438) | 67 (36, 136) | <.0013 |
| Milrinone (Day 0–1) | 155 (50%) | 112 (54%) | 43 (41%) | 0.0311 |
| Vasopressin (Day 0–1) | 56 (18%) | 45 (22%) | 11 (10%) | 0.0131 |
| Vasoactive Agents (Day 0–1) | ||||
| Adrenaline | 189 (60%) | 136 (66%) | 53 (50%) | 0.0101 |
| Dopamine | 110 (35%) | 84 (41%) | 26 (25%) | 0.0061 |
| Noradrenaline (Norepinephrine) | 40 (13%) | 30 (14%) | 10 (9%) | 0.2831 |
| Dobutamine | 22 (7%) | 16 (8%) | 6 (6%) | 0.6421 |
| Phenylephrine | 9 (3%) | 3 (1%) | 6 (6%) | 0.0661 |
| Number of Vasoactive Agents (Day 0–1) | 0.0181 | |||
| 0 | 85 (27%) | 49 (24%) | 36 (34%) | |
| 1 | 112 (36%) | 68 (33%) | 44 (42%) | |
| 2 | 93 (30%) | 72 (35%) | 21 (20%) | |
| 3 | 93 (30%) | 15 (7%) | 5 (5%) | |
| 4 | 3 (1%) | 3 (1%) | 0 (0%) | |
P-value is based on Fisher’s exact test.
Preexisting severe AKI status was unknown for 25 (8%) subjects.
P-value is based on the Wilcoxon rank-sum test.
First non-missing lactate value occurring = 8 hours after randomization.
AKI data
A total of 249 (80%) patients developed AKI: stage 1 13% (42/313); stage 2 16% (49/313); stage 3 50% (158/313). Overall, 207/313 (66%) had severe AKI. RRT was instituted on 70 patients (22%), and severe AKI was observed in 80% of patients requiring ECMO initiation. Preexisting severe AKI was found in 48% (150/313) of patients and represented the majority of post-CA severe AKI cases, 72% (150/207). Of the 138 (44%) without preexisting severe AKI, only 30% (42/138) subsequently developed severe AKI. Subjects with preexisting severe AKI were more likely to require dialysis (Odds Ratio [OR] 3.3, 95% Confidence Interval [1.8, 6.1], p<0.001) and still have severe AKI at the end of the 5-day study period (OR 8.6, 95% Confidence Interval [5.0, 14.6], p<0.001). The peak percent Cr increase was higher in those with preexisting severe AKI compared to those without (median 271% increase vs 59%, p<0.001).
Severe post-IHCA AKI risk factors
Multiple pre-, peri-, and post-CA patient and treatment characteristics (Table 1) had association with development of severe AKI in the overall population. After multivariable analysis, preexisting renal condition, post-operative cardiac surgery status, baseline lactate, number of vasoactive agents on Day 0–1, and minimum percentile of gender/height-adjusted systolic blood pressure were associated with severe AKI (Table 2). Blood transfusion was not associated with severe AKI.
Table 2.
Multivariable predictors of severe AKI after IHCA at four time points
| Predictors1 | Through Step 1 Model AUC = 68.2% |
Through Step 2 Model AUC = 73.9% |
Through Step 3 Model AUC = 79.4% |
Through Step 4 Model AUC = 81.5% |
||||
|---|---|---|---|---|---|---|---|---|
| Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | |
| Step 1: Pre-arrest | ||||||||
| Preexisting lung or airway disease | 0.50 (0.30, 0.85) | 0.01 | 0.52 (0.30, 0.90) | 0.02 | 0.62 (0.34, 1.11) | 0.11 | 0.63 (0.35, 1.15) | 0.14 |
| Preexisting renal condition | 4.00 (1.55, 10.32) | 0.004 | 4.58 (1.74, 12.07) | 0.002 | 6.07 (2.11, 17.48) | <.001 | 7.14 (2.31, 22.03) | <0.001 |
| Patient post-operative cardiac surgery | 3.05 (1.67, 5.58) | <.001 | 2.51 (1.35, 4.69) | 0.004 | 2.74 (1.42, 5.29) | 0.003 | 2.55 (1.30, 5.01) | 0.007 |
| Step 2: At time of ROC | ||||||||
| Estimated duration of chest compressions | 1.02 (1.01, 1.03) | <.001 | 1.01 (1.00, 1.02) | 0.051 | 1.01 (1.00, 1.03) | 0.055 | ||
| Step 3: Post-arrest, near interv ention start | ||||||||
| Baseline Lactate2 (mmol/L) | 1.16 (1.09, 1.23) | <.001 | 1.14 (1.08, 2.04) | <.001 | ||||
| Step 4: Day 0–1 | ||||||||
| Number of Vasoactive Agents (Day 0–1) | 1.48 (1.08, 2.04) | 0.02 | ||||||
| Minimum percentile of gender/height-adjusted systolic blood pressure | 0.99 (0.97, 1.00) | 0.04 | ||||||
Predictors considered for logistic regression models: age, preexisting lung or airway disease, preexisting arrhythmia, preexisting congenital heart disease, preexisting renal condition, post-operative cardiac surgery, cardiac patient, aetiology of arrest, doses of adrenaline, estimated duration of chest compressions, extracorporeal membrane oxygenation (ECMO) used, baseline lactate, packed red blood cells (PRBC) volume (cc/kg) per day (day 0 and 1), minimum percentile of gender/height-adjusted systolic blood pressure, milrinone used (day 0 or 1), vasopressin used (day 0 or 1), adrenaline used (day 0 or 1), dopamine used (day 0 or 1), phenylephrine used (day 0 or 1), number of vasoactive agents used (day 0 or 1).
First non-missing lactate value occurring ≤ 8 hours after randomization.
Severe AKI after IHCA and outcomes
Of the 313 patients, 144 (46%) died prior to hospital discharge (Table 3). Severe AKI was associated with decreased 12-month survival with favorable neurobehavioral outcome by both metrics: a VABS-II score of ≥70, (30% vs 53%; p<0.001) and decrease of ≤15 below baseline (25% vs 38%; p=0.016). Severe AKI was associated with both decreased survival to hospital discharge (48% vs 65%; p=0.006) and to 12 months (42% vs 60%; p=0.004).
Table 3.
Outcomes of patients experiencing IHCA separated into severe and no severe AKI cohorts
| Severe AKI |
||||
|---|---|---|---|---|
| Overall (N = 313) | Yes (N = 207) | No (N = 106) | P-value | |
| Peak serum creatinine: Median (Q1, Q3) | 0.8 (0.5, 1.5) | 1.2 (0.8, 2.0) | 0.4 (0.3, 0.6) | <.0011 |
| Peak BUN: Median (Q1, Q3) | 27 (17, 45) | 36 (22, 49) | 18 (12, 25) | <.0011 |
| Peak ALT (Day 0–5) (U/L): Median (Q1, Q3) | 95 (42, 300) | 134 (53, 438) | 67 (36, 136) | <.0011 |
| UOP < 0.5 cc/kg/hr in a 24 hour period (Days 1–3) | 46/279 (16%) | 43/184 (23%) | 3/95 (3%) | <.0012 |
| Average UOP (cc/kg/hr) (Days 1–3): Median (Q1, Q3) | 3.5 (1.8, 5.2) | 3.3 (1.2, 5.1) | 4.1 (2.5, 5.2) | 0.0331 |
| Lowest UOP in a 24 hour period (cc/kg/hr) (Days 1–3): Median (Q1, Q3) | 1.9 (0.8, 3.6) | 1.6 (0.6, 3.7) | 2.2 (1.3, 3.6) | 0.0051 |
| Hospital Mortality | 144/312 (46%) | 107/206 (52%) | 37/106 (35%) | 0.0062 |
| 1 year Mortality | 161/311 (52%) | 119/206 (58%) | 42/105 (40%) | 0.0042 |
| 1 year survival with VABS-II score ≥703 | 92/246 (37%) | 49/165 (30%) | 43/81 (53%) | <.0012 |
| 1 year survival with VABS-II score decreased no more than 15 points or improved | 88/301 (29%) | 49/199 (25%) | 39/102 (38%) | 0.0162 |
Denominators reflect subjects with available or eligible data.
P-value is based on the Wilcoxon rank-sum test.
Fisher’s exact test.
Among those with baseline score ≥ 70. If no baseline VABS-II was obtained, a Pediatric Overall Performance Category (POPC) and Pediatric Cerebral Performance Category (PCPC) in the normal or mild disability category met eligibility criteria.
Impact of TH on post-IHCA severe AKI and survival
Severe AKI was not different between TH and TN (63% vs 70%, p=0.23) nor was dialysis utilization (23% vs 22%, p=1.00). TH did not impact time to peak severe AKI, which occurred at median 18 hours (2.9, 63.2) after ROC (Appendix, Fig. A1, p=0.35). Peak Cr in TH was 0.8 mg/dL (0.5, 1.4) versus 0.9 mg/dL (0.5, 1.7) in TN (p=0.08). The TH cohort had a smaller percent increase between baseline and peak post-IHCA Cr compared to TN (139% [43, 294] vs 204% [77, 402], p=0.009). Freedom from the composite endpoint of mortality or severe AKI was not affected by TH (Fig. 1, p=0.14). The impact of TH on other outcomes can be seen in the primary THAPCA-IH Trial publication.7
Figure 1.
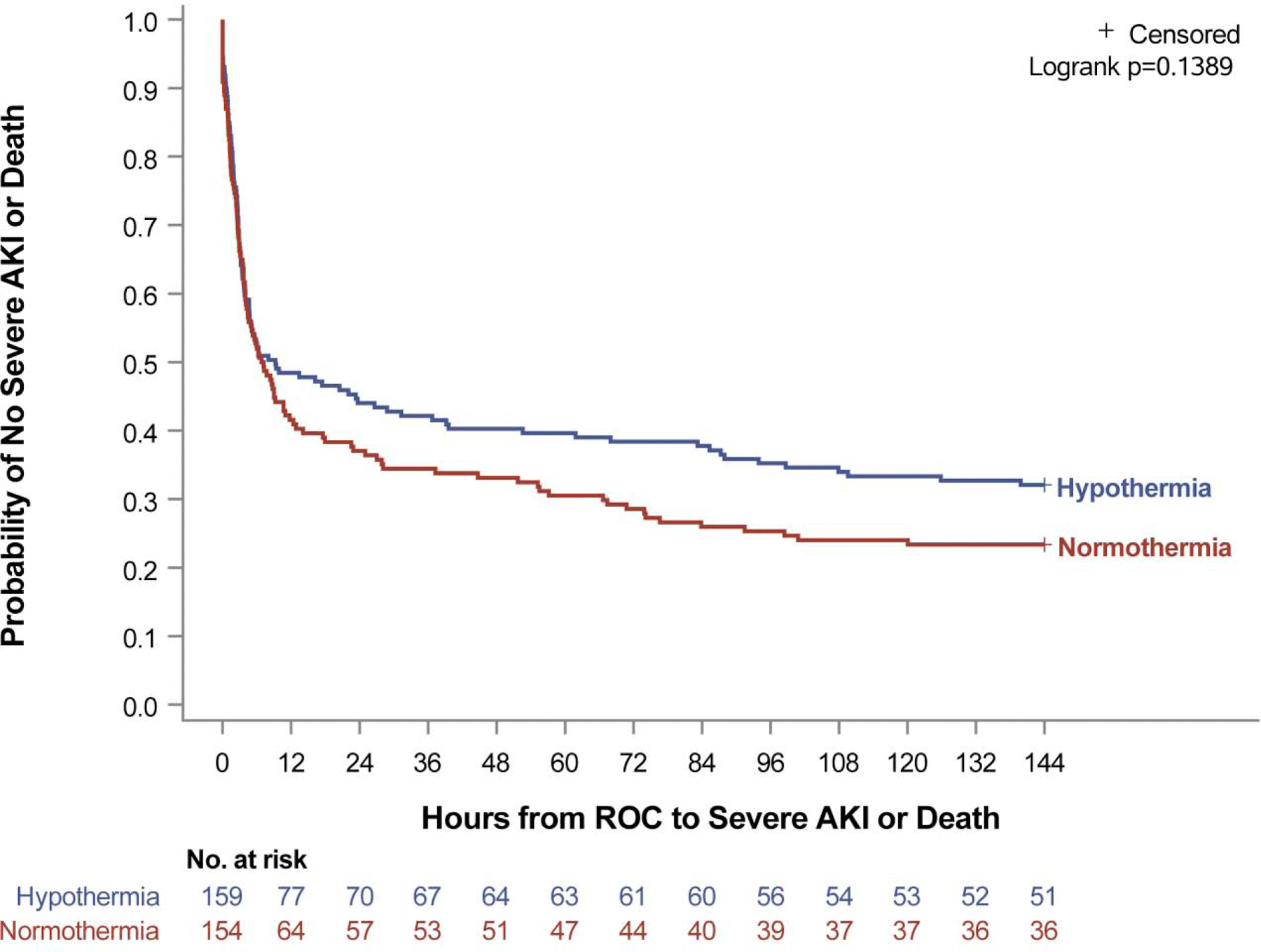
Kaplan-Meier rates of freedom from mortality or severe AKI of patients experiencing IHCA did not differ between targeted temperature control cohorts (p=0.14, log-rank test).
Cardiac subgroup analysis
Demographic data
Of the 313 overall patients, the majority (n=204) were cardiac patients, including congenital heart disease (85%), acquired heart disease, or preexisting arrhythmia. Demographic and clinical characteristics of this cardiac cohort (Table 4) include a <1 year of age predominance, cardiovascular being the most common CA aetiology, and bradycardia being the initial rhythm in 61%. CA occurred in the post-operative period in about half of cardiac subjects. Compared to non-cardiac patients, cardiac patients received longer duration of cardiopulmonary resuscitation (CPR) (30 vs 13 minutes, p<0.001), and were more likely to receive ECMO (64%, 130/204 vs 34%, 37/109).
Table 4.
Characteristics of cardiac patients experiencing IHCA separated into severe and no severe AKI cohorts
| Severe AKI |
||||
|---|---|---|---|---|
| Overall (N = 204) | Yes (N = 146) | No (N = 58) | P-value | |
| Age Group at Randomization | ||||
| <1 year | 117 (57%) | 90 (62%) | 27 (47%) | 0.0601 |
| 1–4 years | 52 (25%) | 32 (22%) | 20 (34%) | 0.0751 |
| 5–12 years | 22 (11%) | 13 (9%) | 9 (16%) | 0.2101 |
| > 13 years | 13 (6%) | 11 (8%) | 2 (3%) | 0.3571 |
| Male sex | 124 (61%) | 90 (62%) | 34 (59%) | 0.7511 |
| Preexisting severe AKI (up to 2 hours after intervention initiation)2 | 114 (56%) | 114 (78%) | 0 (0%) | <.0011 |
| Preexisting conditions | ||||
| Lung or airway disease | 56 (27%) | 36 (25%) | 20 (34%) | 0.1671 |
| Neurologic condition | 64 (31%) | 43 (29%) | 21 (36%) | 0.4041 |
| Gastrointestinal disorder | 72 (35%) | 51 (35%) | 21 (36%) | 0.8721 |
| Prenatal condition | 56 (27%) | 38 (26%) | 18 (31%) | 0.4901 |
| Congenital heart disease | 173 (85%) | 119 (82%) | 54 (93%) | 0.0501 |
| Cyanotic heart disease | 39 (19%) | 28 (19%) | 11 (19%) | 1.0001 |
| Two ventricles | 109 (53%) | 72 (49%) | 37 (64%) | 0.3961 |
| Post-operative cardiac surgery patient | 98 (48%) | 81 (55%) | 17 (29%) | 0.0011 |
| Norwood procedure | 12 (6%) | 11 (8%) | 1 (2%) | 0.6861 |
| Acquired heart disease | 51 (25%) | 37 (25%) | 14 (24%) | 1.0001 |
| Other | 164 (80%) | 119 (82%) | 45 (78%) | 0.5601 |
| Primary aetiology of cardiac arrest | ||||
| Cardiac | 158 (77%) | 117 (80%) | 41 (71%) | 0.1931 |
| ALTE/SUID | 1 (0%) | 0 (0%) | 1 (2%) | 0.2841 |
| Other Respiratory | 41 (20%) | 25 (17%) | 16 (28%) | 0.1201 |
| Other/Unknown | 4 (2%) | 4 (3%) | 0 (0%) | 0.5791 |
| Initial rhythm noted | ||||
| Asystole | 8 (4%) | 7 (5%) | 1 (2%) | 0.4451 |
| Bradycardia | 125 (61%) | 89 (61%) | 36 (62%) | 1.0001 |
| Pulseless electrical activity (PEA) | 45 (22%) | 30 (21%) | 15 (26%) | 0.4551 |
| Ventricular fibrillation or tachycardia | 20 (10%) | 15 (10%) | 5 (9%) | 0.8011 |
| Unknown | 6 (3%) | 5 (3%) | 1 (2%) | 0.6771 |
| Estimated duration of chest compressions (minutes) – Median (Q1, Q3) | 30.0 (8.0, 52.5) | 36.0 (13.0, 55.0) | 12.0 (4.0, 33.0) | <.0013 |
| Total number of doses of adrenaline administered – Median (Q1, Q3) | 4.0 (2.0, 9.0) | 5.0 (3.0, 9.0) | 3.0 (2.0, 7.0) | 0.0063 |
| Assigned to Hypothermia treatment | 107 (52%) | 72 (49%) | 35 (60%) | 0.1651 |
| ECMO at treatment initiation | 130 (64%) | 106 (73%) | 24 (41%) | <.0011 |
| PRBC volume Day 0–1 per day (cc/kg) – Median (Q1, Q3) | 7.5 (0.0, 19.8) | 9.7 (4.9, 26.4) | 0.0 (0.0, 10.0) | <.0013 |
| Minimum percentile of gender/height-adjusted systolic blood pressure: Median (Q1, Q3) | 1.0 (1.0, 4.0) | 1.0 (1.0, 2.0) | 4.0 (1.0, 26.0) | <.0013 |
| Aminoglycoside (Day 0–1) | 19 (9%) | 13 (9%) | 6 (10%) | 0.7921 |
| Vancomycin (Day 0–1) | 104 (51%) | 77 (53%) | 27 (47%) | 0.4391 |
| Baseline Lactate4 (mmol/L) – Median (Q1, Q3) | 6.6 (2.5, 12.7) | 8.8 (4.2, 14.3) | 3.3 (1.7, 7.2) | <.0013 |
| Peak Lactate (Day 0–1) (mmol/L) – Median (Q1, Q3) | 7.7 (3.6, 13.7) | 10.5 (5.1, 16.0) | 3.5 (2.0, 7.9) | <.0013 |
| Baseline glucose concentration (mmol/L) – Median (Q1, Q3) | 180 (119, 243) | 194 (115, 263) | 175 (123, 227) | 0.7863 |
| Baseline ALT concentration (U/L) – Median (Q1, Q3) | 45 (25, 156) | 52 (28, 165) | 32 (21, 89) | 0.0263 |
| Peak ALT (Day 0–5) concentration (U/L) – Median (Q1, Q3) | 83 (40, 296) | 117 (49, 500) | 41 (31, 125) | <.0013 |
| Milrinone (Day 0–1) | 128 (63%) | 94 (64%) | 34 (59%) | 0.5211 |
| Vasopressin (Day 0–1) | 35 (17%) | 28 (19%) | 7 (12%) | 0.3041 |
| Vasoactive Agents (Day 0–1) | ||||
| Adrenaline | 126 (62%) | 96 (66%) | 30 (52%) | 0.0791 |
| Dopamine | 67 (33%) | 58 (40%) | 9 (16%) | <.0011 |
| Noradrenaline | 18 (9%) | 15 (10%) | 3 (5%) | 0.2891 |
| Dobutamine | 18 (9%) | 12 (8%) | 6 (10%) | 0.5961 |
| Phenylephrine | 7 (3%) | 2 (1%) | 5 (9%) | 0.0211 |
| Number of Vasoactive Agents (Day 0–1) | 0.1371 | |||
| 0 | 55 (27%) | 35 (24%) | 20 (34%) | |
| 1 | 76 (37%) | 51 (35%) | 25 (43%) | |
| 2 | 60 (29%) | 49 (34%) | 11 (19%) | |
| 3 | 12 (6%) | 10 (7%) | 2 (3%) | |
| 4 | 1 (0%) | 1 (1%) | 0 (0%) | |
P-value is based on Fisher’s exact test.
Preexisting severe AKI status was unknown for 16 (8%) subjects.
P-value is based on the Wilcoxon rank-sum test.
First non-missing lactate value occurring = 8 hours after randomization.
AKI data
Cardiac patients had an increased rate of post-IHCA severe AKI (72%, 146/204 vs 56%, 61/109, p=0.006). The rate of preexisting severe AKI was higher in cardiac patients (56%, 114/204 vs 33%, 36/109, p≤0.001). Patients with preexisting severe AKI represented the majority of severe AKI cases in cardiac patients (78%, 114/146). Cardiac patients without preexisting severe AKI were less likely to develop severe AKI than cardiac patients with preexisting severe AKI (31%, 23/74 vs 100%, 114/114).
Severe post-IHCA AKI risk factors
Multiple pre-, peri-, and post-CA patient and treatment characteristics (Table 4) were associated with development of severe AKI in the cardiac cohort. After multivariable analysis, post-operative cardiac surgery status, baseline lactate, and minimum percentile of gender/height-adjusted systolic blood pressure remained associated with severe AKI. Congenital heart disease diagnosis was also associated with severe AKI in cardiac patients (Table 5).
Table 5.
Multivariable predictors of severe AKI after IHCA in cardiac patients at four time points
| Through Step 1 Model AUC = 68.8% |
Through Step 2 Model AUC = 75.7% |
Through Step 3 Model AUC = 80.8% |
Through Step 4 Model AUC = 83.2% |
|||||
|---|---|---|---|---|---|---|---|---|
| Predictors1 | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value | Odds Ratio (95% CI) | P-value |
| Step 1: Pre-arrest | ||||||||
| Preexisting congenital heart disease | 0.18 (0.06, 0.55) | 0.003 | 0.19 (0.06, 0.61) | 0.005 | 0.21 (0.06, 0.70) | 0.01 | 0.16 (0.04, 0.60) | 0.007 |
| Patient post-operative cardiac surgery | 4.20 (2.12, 8.32) | <.001 | 3.75 (1.84, 7.63) | <.001 | 4.05 (1.90, 8.62) | <.001 | 3.44 (1.59, 7.44) | 0.002 |
| Step 2: At time of ROC | ||||||||
| Estimated duration of chest compressions | 1.02 (1.01, 1.04) | 0.001 | 1.01 (1.00, 1.03) | 0.07 | 1.01 (0.99, 1.02) | 0.23 | ||
| Step 3: Post-arrest, near interv ention start | ||||||||
| Baseline Lactate2 (mmol/L) | 1.14 (1.06, 1.23) | <.001 | 1.15 (1.06, 1.24) | <0.001 | ||||
| Step 4: Day 0–1 | ||||||||
| Minimum percentile of gender/height-adjusted systolic blood pressure | 0.97 (0.95, 0.99) | 0.008 | ||||||
Predictors considered for logistic regression models: age, preexisting lung or airway disease, preexisting arrhythmia, preexisting congenital heart disease, preexisting renal condition, post-operative cardiac surgery, aetiology of arrest, doses of adrenaline, estimated duration of chest compressions, extracorporeal membrane oxygenation (ECMO) used, baseline lactate, packed red blood cells (PRBC) volum e (cc/kg) per day (day 0 and 1), minimum percentile of gender/height-adjusted systolic blood pressure, milrinone used (day 0 or 1), vasopressin used (day 0 or 1), adrenaline used (day 0 or 1), dopamine used (day 0 or 1), phenylephrine used (day 0 or 1).
First non-missing lactate value occurring ≤ 8 hours after randomization.
Impact of TH on post-IHCA severe AKI
Similar to the overall population, the relationship of a lower percent change from post-IHCA Cr baseline to peak Cr in TH remained in the cardiac subgroup (159% [67, 321] vs 216% [109, 412], p=0.025).
Discussion:
This study found AKI occurred in the majority of comatose survivors after pediatric IHCA in the THAPCA-IH trial, with severe AKI occurring in 2/3, and RRT required in 22%. Half of the patients already had evidence of severe AKI in the peri-arrest period (aka “preexisting” severe AKI). For subjects without preexisting severe AKI, only 30% developed severe AKI. Preexisting severe AKI, congenital heart disease, recent cardiac surgery, and surrogates of peri-arrest hypoperfusion were associated with severe post-IHCA AKI. Severe post-IHCA AKI was associated with decreased survival and decreased survival with favorable neurobehavioral outcome. TH did not decrease the severe AKI rate; however, percent peak Cr increase was lower with TH.
A notable finding was the high rate of severe AKI and dialysis after IHCA. The prevalence is the highest reported after pediatric CA,10, 14, 15, 19, 25, 26 and over 50% higher than the secondary analysis of THAPCA-OH.18 The majority of findings are driven by the high rate of preexisting severe AKI. Preexisting severe AKI was 33% for non-cardiac subjects and 56% for cardiac subjects, which is consistent with overall high rates of AKI in multicenter studies of pediatric ICU (27%, AWARE) and cardiac ICU (54%, NEPHRON)22, 27, 28 patients suggesting our cohort is representative of a high acuity subpopulation within these settings.
The epidemiology of AKI post IHCA likely represents two distinct cohorts – those with and without co-morbidity at the time of CA (including AKI). Most children who suffer IHCA are located in the ICU, and thus a CA episode in these patients often represents a second physiologic insult to already injured or at-risk kidneys.1 The post-operative cardiac cohort is particularly vulnerable as AKI is common after cardiopulmonary bypass. This may manifest as a larger Cr increase in those with preexisting severe AKI compared to those without preexisting severe AKI; a 4.6-fold greater median increase was observed in this study. Any potential TH benefit in patients with preexisting severe AKI might be relegated to mitigating AKI progression. In contrast, only 30% of patients without preexisting severe AKI developed severe post-IHCA AKI, again supporting the finding that the patient’s pre-arrest condition (including preexisting severe AKI) is partly responsible for driving the outcomes in this study. In the cohort without preexisting severe AKI, the mode of post-IHCA severe AKI is likely ischaemia reperfusion injury from the CA episode. Only 42 patients without preexisting severe AKI developed severe AKI after IHCA, limiting power for comparisons between these two cohorts as well as ability to assess the potential of TH to prevent AKI after IHCA. In the context of these limitations, similar to OHCA, a benefit of TH compared to TN for decreasing the prevalence of severe post-IHCA AKI was not seen.18
Given the high rate of preexisting severe AKI, peak Cr change from baseline after IHCA (Cr used to calculate preexisting AKI) as a surrogate of AKI progression after CA was evaluated. While there was no difference in peak Cr between TN and TH, the median peak rise was 65% lower in TH. This supports a possible benefit of TH for mitigating severity of AKI progression after IHCA. This observation may be in accordance with adult CA studies in which Cr and other markers of kidney injury were improved with TH, in addition to improved kidney graft function in transplant donors treated with TH.29, 30 This study cannot determine whether the decreased Cr rise in TH is a reflection of improved kidney function, decreased Cr production rate, or both. However, this finding warrants consideration for further investigating TH as a modality for decreasing the deleterious impact of IHCA on AKI development and progression. Such a study would need to address the limitations of the current study and have detailed pre-CA Cr values, detailed UOP, and other biomarkers in addition to Cr to more accurately detect AKI.
Many of this study’s results were driven by the high proportion of cardiac disease (65%) in the population. The AKI rate is known to be higher in this group compared to the medical ICU population, especially those post cardiac surgery – both of which were supported by this study. Another distinguishing feature of this cardiac cohort is its increased risk of post-arrest AKI; awareness of which may be valuable for those caring for the pediatric cardiac population. For both cardiac and overall cohorts, multiple, similar variables reflecting increased CA episode severity, multi-organ injury, and increased peri-CA haemodynamic insufficiency were associated with severe AKI. Of these variables, baseline lactate, number of post-CA vasoactive agents, and minimum percentile of gender/height-adjusted systolic blood pressure were predictors of severe AKI. The first two variables were also associated with severe AKI after OHCA while the latter was not included in the OHCA model.
This study suggests IHCA and subsequent severe AKI are associated with poor outcomes, which is consistent with the THAPCA-OH trial. Comatose survivors of the CPR episode had <50% chance of survival, and severe AKI was associated with increased in-hospital mortality. Severe AKI may serve as a surrogate for the magnitude of hypoxic ischaemic injury which affects long-term outcomes beyond survival to discharge. After IHCA, a patient’s likelihood of 12-month survival with favorable neurobehavioral outcome was decreased in the setting of severe AKI. This suggests the sequela of both CA and severe AKI may have longstanding neurodevelopmental effects.31–34
Notable limitations apply to this study. First, this was a secondary analysis of the THAPCA-IH trial, which did not target AKI as a primary or secondary outcome. Second, an estimate for baseline Cr was used because baseline Cr availability was limited. Baseline Cr for the majority of subjects was normal Cr for height or age. Also, the Cr-based KDIGO definition and not UOP criteria were used to calculate AKI. Third, patients with milder degrees of encephalopathy (GCS motor 5 or 6) who likely experienced a lower dose of hypoxic ischaemic injury and less resultant AKI were excluded. Fourth, children who did not survive the study period may have led to underestimation of AKI that would have developed at later time points. As detailed above, the high proportion of cardiac patients and preexisting severe AKI biased these results and applicability to other populations. Fifth, the amount of time required to achieve target temperature in the TH group may have decreased the optimal TH impact for mitigation of severe AKI, as peak AKI occurred, on average, 12 hours later. In this context, the impact of TH on renal recovery would have been an ideal endpoint, but was not feasible as the study period for Cr collection was only 5 days.
Conclusions:
Severe AKI is a common complication after pediatric IHCA. Preexisting cardiac or renal conditions and surrogates of peri-arrest hypoperfusion were associated with development of severe AKI. In this cohort of predominantly cardiac patients with a high rate of pre-existing severe AKI, TH did not decrease the prevalence of post-IHCA severe AKI. Development of severe post-IHCA AKI is associated with increased mortality and decreased survival with favorable neurobehavioral status.
Acknowledgements:
Supported by grants from the National Heart, Lung, and Blood Institute (HL094345, to Dr. Moler; and HL094339, to Dr. Dean), federal planning grants for the planning of the THAPCA trials (HD044955 and HD050531, both to Dr. Moler), cooperative agreements from the Pediatric Emergency Care Applied Research Network (U03MC00001, U03MC00003, U03MC00006, U03MC00007, and U03MC00008) and the Collaborative Pediatric Critical Care Research Network (U10HD500009, U10HD050096, U10HD049981, U10HD049945, U10HD049983, U10HD050012 and U01HD049934), and a National Emergency Medical Services for Children Data Analysis Resource Center Demonstration grant (U07MC09174). Several centers were supported by supplemental grants or cooperative agreements (UL1RR024986, UL1TR000433, U54HD087011, UL1TR000003, and P30HD040677).
Timothy T. Cornell receives funding for studies not related to this manuscript from National Institutes of Health – NHLBI (R01 HL119542). David J. Askenazi is consultant for Baxter, CHF Solutions Inc. and Medtronic. He also receives grant funding for studies not related to this project from Baxter, CHF Solutions Inc., and National Institutes of Health NIH-FDA (R01 FD005092) and the Pediatric and Infant Center for Acute Nephrology (PICAN). PICAN is part of the Department of Pediatrics at the University of Alabama at Birmingham (UAB), and is funded by Children’s of Alabama Hospital, the Department of Pediatrics, UAB School of Medicine, and UAB’s Center for Clinical and Translational Sciences (CCTS, NIH grant UL1TR001417). Kenneth E. Mah receives funding for studies not related to this manuscript from the National Science Foundation (SBIR Phase I 2028008).
Appendix:
Figure A1.
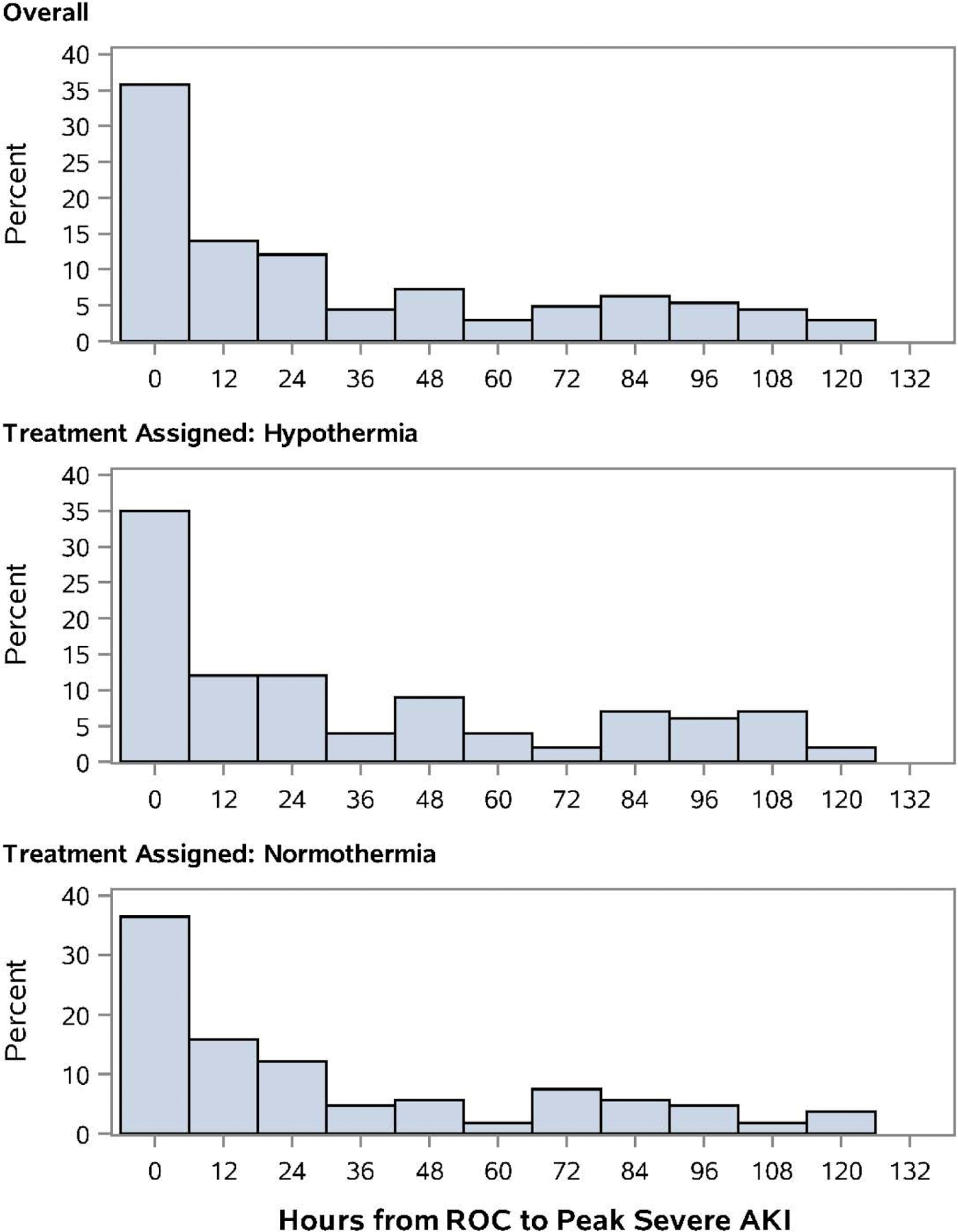
Time to peak severe AKI in patients experiencing IHCA did not differ between TH and TN cohorts (median = 18 hours [2.9, 63.2], p=0.35, Wilcoxon rank-sum test).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of interest:
The views expressed in this article are solely those of the authors and do not necessarily represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
References:
- 1.Berg RA, Nadkarni VM, Clark AE, et al. Incidence and Outcomes of Cardiopulmonary Resuscitation in PICUs. Crit Care Med 2016;44:798–808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmberg MJ, Wiberg S, Ross CE, et al. Trends in Survival After Pediatric In-Hospital Cardiac Arrest in the United States. Circulation. 2019;140:1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Meert KL, Donaldson A, Nadkarni V, et al. Multicenter cohort study of in-hospital pediatric cardiac arrest. Pediatr Crit Care Med 2009;10:544–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topjian AA, French B, Sutton RM, et al. Early Postresuscitation Hypotension Is Associated With Increased Mortality Following Pediatric Cardiac Arrest. Crit Care Med 2014;42:1518–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Neumar RW, Nolan JP, Adrie C, et al. Post-cardiac arrest syndrome: epidemiology, pathophysiology, treatment, and prognostication. A consensus statement from the International Liaison Committee on Resuscitation (American Heart Association, Australian and New Zealand Council on Resuscitation, European Resuscitation Council, Heart and Stroke Foundation of Canada, InterAmerican Heart Foundation, Resuscitation Council of Asia, and the Resuscitation Council of Southern Africa); the American Heart Association Emergency Cardiovascular Care Committee; the Council on Cardiovascular Surgery and Anesthesia; the Council on Cardiopulmonary, Perioperative, and Critical Care; the Council on Clinical Cardiology; and the Stroke Council. Circulation. 2008;118:2452–83. [DOI] [PubMed] [Google Scholar]
- 6.Topjian AA, De Caen A, Wainwright MS, et al. Pediatric Post-Cardiac Arrest Care: A Scientific Statement From the American Heart Association. Circulation. 2019;140:e194. [DOI] [PubMed] [Google Scholar]
- 7.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic Hypothermia after In-Hospital Cardiac Arrest in Children. N Engl J Med. 2017;376:318–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moler FW, Silverstein FS, Holubkov R, et al. Therapeutic hypothermia after out-of-hospital cardiac arrest in children. N Engl J Med 2015;372:1898–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mah KE, Hao S, Sutherland SM, et al. Fluid overload independent of acute kidney injury predicts poor outcomes in neonates following congenital heart surgery. Pediatr Nephrol 2018;33:511–20. [DOI] [PubMed] [Google Scholar]
- 10.Geri G, Guillemet L, Dumas F, et al. Acute kidney injury after out-of-hospital cardiac arrest: risk factors and prognosis in a large cohort. Intensive Care Med 2015;41:1273–80. [DOI] [PubMed] [Google Scholar]
- 11.Holzer M, Cerchiari E, Martens P, et al. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–56. [DOI] [PubMed] [Google Scholar]
- 12.Bernard SA, Gray TW, Buist MD, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–63. [DOI] [PubMed] [Google Scholar]
- 13.Nielsen N, Wetterslev J, Cronberg T, et al. Targeted temperature management at 33°C versus 36°C after cardiac arrest. N Engl J Med 2013;369:2197–206. [DOI] [PubMed] [Google Scholar]
- 14.Susantitaphong P, Alfayez M, Cohen-Bucay A, Balk EM, Jaber BL. Therapeutic hypothermia and prevention of acute kidney injury: a meta-analysis of randomized controlled trials. Resuscitation. 2012;83:159–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tujjar O, Mineo G, Dell’anna A, et al. Acute kidney injury after cardiac arrest. Crit Care. 2015;19:169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Doherty DR, Parshuram CS, Gaboury I, et al. Hypothermia therapy after pediatric cardiac arrest. Circulation. 2009;119:1492–500. [DOI] [PubMed] [Google Scholar]
- 17.Fink EL, Clark RSB, Kochanek PM, Bell MJ, Watson RS. A tertiary care center’s experience with therapeutic hypothermia after pediatric cardiac arrest. Pediatr Crit Care Med 2010;11:66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cornell TT, Selewski DT, Alten JA, et al. Acute kidney injury after out of hospital pediatric cardiac arrest. Resuscitation. 2018;131:63–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Neumayr TM, Gill J, Fitzgerald JC, et al. Identifying Risk for Acute Kidney Injury in Infants and Children Following Cardiac Arrest. Pediatr Crit Care Med 2017;18:E446–E54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz GJ, Munoz A, Schneider MF, et al. New Equations to Estimate GFR in Children with CKD. J AmSoc Nephrol 2009;20:629–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kidney Disease: Improving Global Outcomes (KDIGO) Acute Kidney Injury Work Group. KDIGO Clinical Practice Guideline for Acute Kidney Injury. Kidney inter, Suppl 2012;2:1–138. [Google Scholar]
- 22.Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL, Investigators A. Epidemiology of Acute Kidney Injuryin Critically Ill Children and Young Adults. N Engl J Med 2017;376:11–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sparrow SS, Balla DA, Cicchetti DV, Doll EA. Vineland-II : Vineland adaptive behavior scales. Second edition. ed: Pearson Assessments; 2006. [Google Scholar]
- 24.Meert KL, Guerguerian A-M, Barbaro R, et al. Extracorporeal Cardiopulmonary Resuscitation: One-Year Survival and Neurobehavioral Outcome Among Infants and Children With In-Hospital Cardiac Arrest. Crit Care Med 2019;47:393–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Beitland S, Nakstad ER, Staer-Jensen H, et al. Impact of acute kidney injury on patient outcome in out-of-hospital cardiac arrest: a prospective observational study. Acta Anaesthesiol Scand 2016;60:1170–81. [DOI] [PubMed] [Google Scholar]
- 26.Storm C, Krannich A, Schachtner T, et al. Impact of acute kidney injury on neurological outcome and long-term survival after cardiac arrest - A 10 year observational follow up. J Crit Care. 2018;47:254–9. [DOI] [PubMed] [Google Scholar]
- 27.Basu RK, Kaddourah A, Terrell T, et al. Assessment of Worldwide Acute Kidney Injury, Renal Angina and Epidemiology in Critically Ill Children (AWARE): A Prospective Study to Improve Diagnostic Precision. Journal Of Clinical Trials. 2015;5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gist KM, Blinder JJ, Bailly D, et al. Neonatal and Paediatric Heart and Renal Outcomes Network: design of a multi-centre retrospective cohort study. Cardiol Young. 2019;29:511–8. [DOI] [PubMed] [Google Scholar]
- 29.Hasslacher J, Barbieri F, Harler U, et al. Acute kidney injury and mild therapeutic hypothermia in patients after cardiopulmonary resuscitation - a post hoc analysis of a prospective observational trial. Critical Care. 2018;22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Niemann CU, Feiner J, Swain S, et al. Therapeutic Hypothermia in Deceased Organ Donors and Kidney-Graft Function. N Engl J Med 2015;373:405–14. [DOI] [PubMed] [Google Scholar]
- 31.Yap SC, Lee HT. Acute Kidney Injury and Extrarenal Organ Dysfunction New Concepts and Experimental Evidence. Anesthesiology. 2012;116:1139–48. [DOI] [PubMed] [Google Scholar]
- 32.Adachi N, Lei B, Deshpande G, et al. Uraemia suppresses central dopaminergic metabolism and impairs motor activity in rats. Intensive Care Med 2001;27:1655–60. [DOI] [PubMed] [Google Scholar]
- 33.Liu M, Liang Y, Chigurupati S, et al. Acute kidney injury leads to inflammation and functional changes in thebrain. J Am Soc Nephrol 2008;19:1360–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fitzgerald JC, Basu RK, Akcan-Arikan A, et al. Acute Kidney Injury in Pediatric Severe Sepsis: An Independent Risk Factor for Death and New Disability. Crit Care Med 2016;44:2241–50. [DOI] [PMC free article] [PubMed] [Google Scholar]


