Abstract
Background:
To identify novel myofibrillar components of the Drosophila flight muscles, we carried out a proteomic analysis of chemically demembranated flight muscle myofibrils, and characterized the knockdown phenotype of a novel gene identified in the screen, CG1674.
Results:
The CG1674 protein has some similarity to vertebrate synaptopodin 2-like, and when expressed as a FLAG-tagged fusion protein, it was localized during development to the Z-disc and cytoplasm. Knockdown of CG1674 expression affected the function of multiple muscle types, and defective flight in adults was accompanied by large actin-rich structures in the flight muscles that resembled overgrown Z-discs. Localization of CG1674 to the Z-disc depended predominantly upon presence of the Z-disc component alpha-actinin, but also depended upon other Z-disc components, including Mask, Zasp52, and Sals. We also observed re-localization of FLAG-CG1674 to the nucleus in Alpha-actinin and sals knockdown animals.
Conclusions:
These studies identify and characterize a previously unreported myofibrillar component of Drosophila muscle, that is necessary for proper myofibril assembly during development.
Keywords: Drosophila, muscle, flight muscle, Synaptopodin 2-like, CHAP, Z-disc, myofibril
Introduction.
The sarcomere, the basic unit of muscle, is a protein machine that uses chemical energy to promote force generation. Central to understanding the mechanisms of muscle contraction and the maintenance of sarcomere structure under force generation is a complete understanding of the proteins that are present in the myofibril, and their roles in myofibril assembly and function. Substantial research has contributed to our current understanding of myofibril structure: force is generated by the interaction of interdigitating thick and thin filaments, and while myosin-containing thick filaments are anchored at the M-line, actin-containing thin filaments are anchored at the Z-disc (reviewed in 1).
Despite extensive research efforts to identify myofibrillar components, the Z-disc remains somewhat enigmatic in both its structure and function. New components of the Z-disc continue to be identified, including the ZASP family of proteins in Drosophila and vertebrates (2) among numerous others (see for example 3). Moreover, recent studies have defined the Z-disc as a signaling nexus in the muscle, where both upstream and downstream signals impinge upon the Z-disc (reviewed in 4), and a number of Z-disc associated proteins are capable of shuttling from the myofibril to the nucleus (5–7). Recent studies have also shown that nascent myofibrils can be identified as ZASP-enriched foci localized to the surface of the nucleus, indicating that the Z-disc is a critical determinant of initial myofibril assembly (8). Finally, genes encoding Z-disc proteins are also human disease genes, underlining the importance of understanding this structure (9,10).
The Drosophila model has emerged as a powerful tool to understand mechanisms of muscle development and function, where traditional cell biological and biochemical approaches can be paired with genetic mutation or knockdown technology. In particular, the large thoracic flight muscles are amenable to histological, biochemical, and mechanical approaches, and are dispensable for survival of the adult fly, meaning that fundamental rules for myofibril assembly and function can be uncovered in the intact animal (reviewed in 11). As a result, several new myofibrillar components have recently been identified in Drosophila muscles and many of these localize to the Z-disc. These include Sarcomere length short (Sals; ref. 12), Ball and Mask (13), and several members of the ZASP protein family (14–16), suggesting that there may be more Z-disc components yet to be identified.
In this study, we carried out a proteomic analysis of chemically demembranated Drosophila flight muscle myofibrils to identify new structural muscle components. Alongside numerous well-characterized muscle proteins, we identified a previously uncharacterized protein encoded by the CG1674 gene. When an epitope-tagged isoform of CG1674 was expressed in the adult muscles, it localized to the Z-disc of the flight muscles, identifying it as a new component of the Z-disc. We found that knockdown of CG1674 in the developing adult muscles resulted in defective flight muscle function and altered myofibril structure. Localization of tagged CG1674 to the Z-disc was dependent upon a number of other Z-disc components, including Alpha-actinin, Sals and Mask. Interestingly, reduction in the levels of either Alpha-actinin or Sals in the adult muscles resulted in a re-localization of CG1674 to the nucleus, suggesting a role for this protein in myofibril-nucleus communication. These studies identify a new component of the flight muscle myofibril and identify a potentially new mechanism for transmitting changes in myofibril organization to the nucleus.
Results.
Identification of CG1674 as a component of the flight muscle proteome.
To identify the protein components of the adult Drosophila flight muscles, we isolated dorsal longitudinal muscles from y w adults and subjected them to chemical demembranation and homogenization to separate the insoluble myofibrils from cytosolic proteins. Proteins were then separated by SDS-PAGE and visualized using Coomassie Blue staining (Figure 1A). A gel strip corresponding to the myofibrillar fraction was cut from the gel. The intense band corresponding to Myosin heavy-chain (MHC) was removed so that signal from this protein did not overwhelm the signal from less abundant proteins. The resulting gel slice was then submitted for LC-MS/MS peptide sequencing to identify the protein components of the flight muscle myofibril. This identification was carried out in duplicate. Proteins identified in both replicates, or those represented by at least ten peptides in one run, are indicated in Figure 1B.
Figure 1. Identification of the Drosophila flight muscle myofibril proteome.
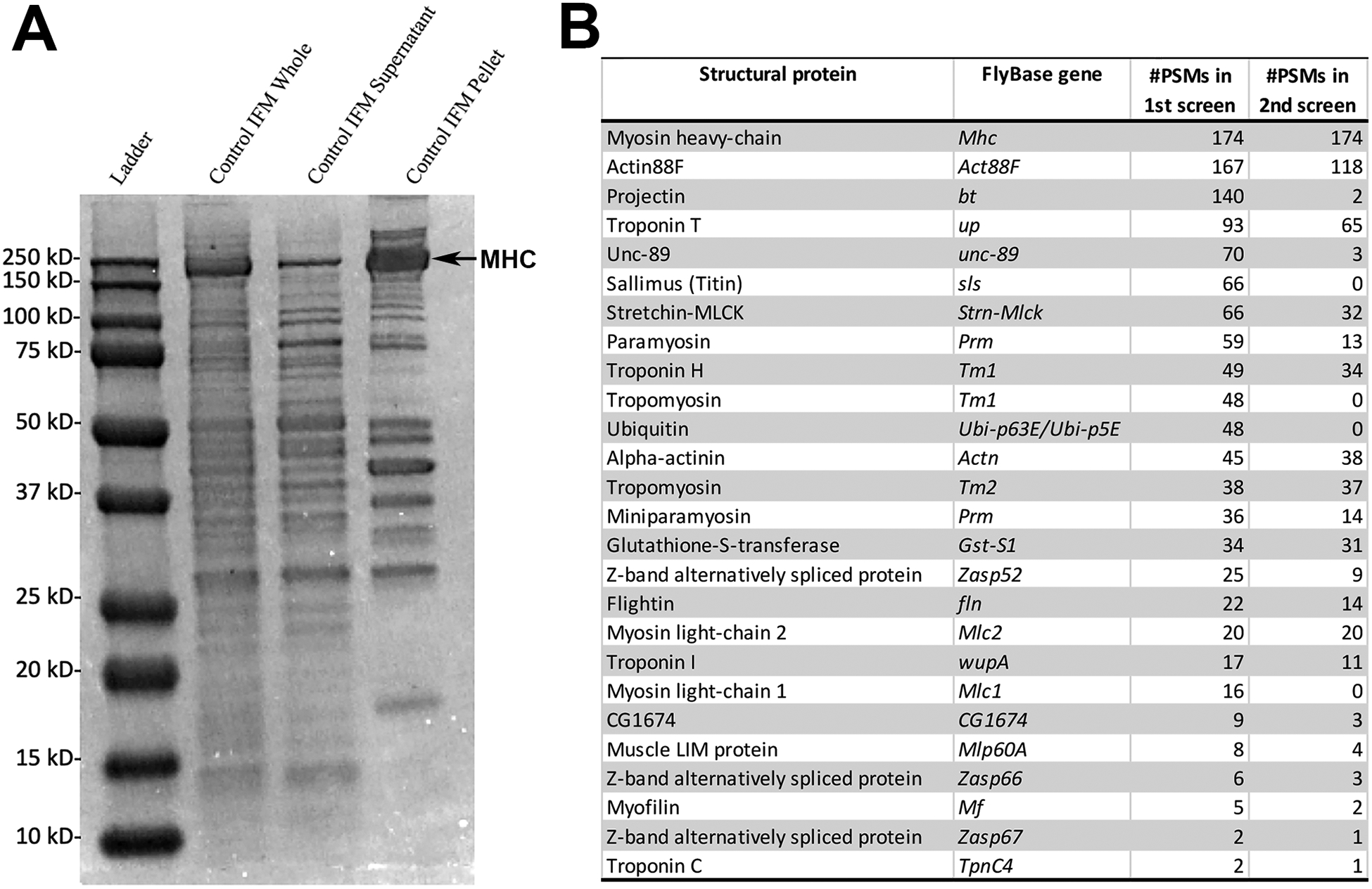
A. Flight muscles were dissected from Drosophila adults and fractionated into cytoplasmic and myofibrillar components. Shown are representative samples of whole muscles (lane 2), cytoplasmic fraction (lane 3), and myofibrillar fraction (lane 4). A gel strip corresponding to the myofibrillar fraction, with the myosin heavy-chain (MHC) band removed, was submitted for LC-MS/MS analysis. A separate dissection and fractionation was used for a duplicate analysis. B. List of identified proteins and their corresponding Drosophila gene. Number of different peptide-to-spectrum matches (PSMs) for each protein identified in first or second LC-MS/MS screens are indicated in columns 2 and 3, respectively.
As would be expected, this analysis identified a large number of known myofibrillar proteins, although interestingly there were some notable absences, or proteins that were only identified in one replicate and not the other. These included Mask and Ball, that were not detected at all; and Sallimus, Tm1 and MLC1 that were only identified in the first analysis, albeit at high levels. We interpret from these findings that our analysis has not yet been saturating to identify all myofibrillar components.
Nevertheless, alongside the known myofibrillar proteins, we identified a previously uncharacterized protein encoded by the CG1674 gene. CG1674 was represented in nine peptides in the first replicate and three peptides in the second replicate.
The CG1674 gene is located on the fourth chromosome and consists of 16 exons that show a complex pattern of splicing (Figure 2A; also see FlyBase.org (17), and ref. 18). By examining the peptides identified by the MS/MS analysis that are isoform-specific, it is possible to imply some of the isoforms of CG1674 that may exist in the flight muscles, in particular protein isoforms PK or PL must be present (encoded by transcripts RK and RL, respectively, Figure 2B). However, this analysis does not rule out the possibility that multiple isoforms are synthesized in the flight muscles.
Figure 2. The CG1674 gene.
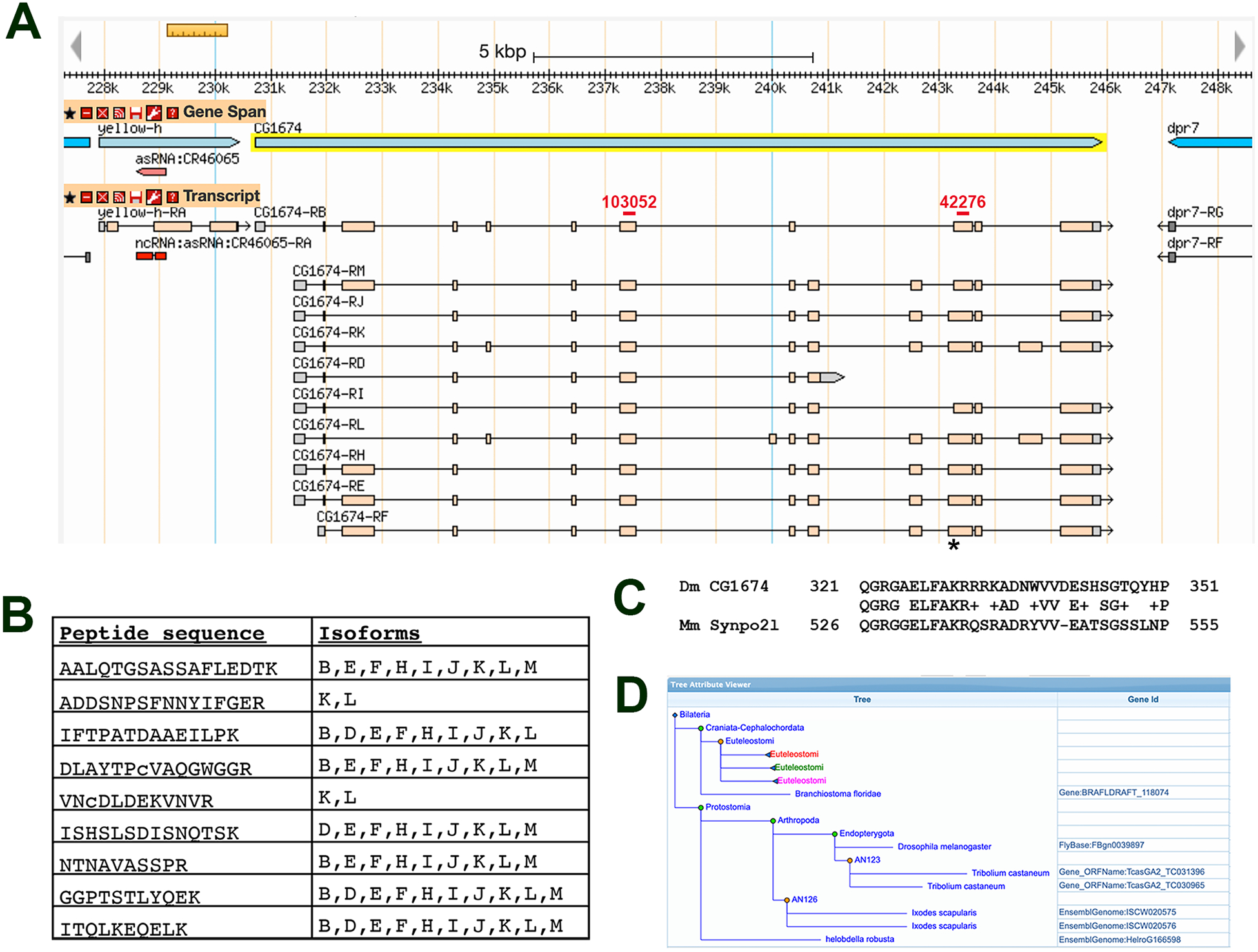
A. CG1674 is located on the fourth chromosome and spans approximately 15kb. It directs the expression of up to ten different transcripts (labeled CG1674-RB, D, E, F, H, I, J, K, L, M). The location of sequence encoding the putative nuclear localization signal is indicated with an asterisk, and the regions targeted by RNAis used in this study are indicated by red bars. GBrowse image from FlyBase.org. B. Sequences of CG1674 peptides identified in the IFM proteome, and identification of which CG1674 isoforms contain each peptide. Note that the RB transcript directs the formation of the PB isoform (indicated as B in the table), RC encodes the PC isoform (indicated as C), etc. C. Region of similarity between Drosophila CG1674-PJ and mouse Synaptopodin 2-like. The Drosophila sequence is shared among protein isoforms E, F, J, K, L and M. D. Tree from pantherdb.org describing the PTHR24217 putative related protein family. Gene names are indicated where individual species are listed. The tree is collapsed for Euteleostomes, whose genomes encode Synaptopodin proteins highlighted in red, green and pink, in order to illustrate the phylogenetic distribution of family members.
Sequence comparisons did not identify specific functional domains within CG1674 polypeptides, although a putative nuclear localization signal (NLS) was observed within CG1674 (LFAKRRRKADNW, identified using NLS-mapper, http://nls-mapper.iab.keio.ac.jp, 19). The sequence in CG1674 is encoded by sequence at the 5’ end of exon 13 (see asterisk in Figure 2A). Note that this sequence is not included in all exon 13-containing transcripts due to the presence of an alternate 3’ splice acceptor site within exon 13.
To gain further insight into the potential function of CG1674, we assessed the presence of orthologs in other species as reported at FlyBase.org. Close orthologs are observed in other Dipterans, and a similar gene to CG1674 is found in non-Dipteran insects such as Tribolium castaneum (flour beetle; 49.3% identity over 268 amino acids of the predicted protein product). In the non-insect arthropod Ixodes scapularis (deer tick), there is a similar gene showing 34% identity over 247 amino acids of the predicted product. All of these regions of similarity overlap the predicted NLS described above.
FlyBase.org curates multiple ortholog prediction tools, and while many sites do not predict orthologs outside of arthropods, Panther and Compara indicate that the mammalian protein Synaptopodin 2-like (Figure 2C; also called Synpo2l and CHAP), along with Synaptopodin and Synaptopodin 2, share some short sequence similarity with the predicted CG1674 amino acid sequence. Most of this similarity also overlaps the putative NLS (Figure 2C), although it is not clear if the corresponding region of Synpo2l corresponds to a nuclear localization signal since several basic amino acids present in CG1674 are missing in Synpo2l.
The tools at Pantherdb.org also indicate that CG1674 contains a domain that places it into a putative related protein family termed PTHR24217 (20). Other members of the family include the Arthropod proteins described above plus, more distantly, the Synaptopodin family of proteins within the Euteleostomi (“bony vertebrate”) clade (Figure 2D). Proteins within the PTHR24217 putative family are predicted to have actin binding activity, and to segregate to the Z-disc, actin cytoskeleton, and nucleus of the cells. Overall, these observations suggest that CG1674 is part of an ancient family of proteins with potential roles in muscle, but that the primary sequence of these orthologs has not been stringently conserved over evolutionary time.
CG1674 localizes to the Z-disc.
To gain greater insight into CG1674 function, we generated a CG1674 cDNA where nucleotides encoding a 3xFLAG tag were fused to the 5’ end of the coding sequence. This construct, encoding a FLAG-tagged isoform of CG1674-PJ was placed into a UAS vector, making it responsive to activation by Gal4 protein, and was stably inserted into the Drosophila genome (Figure 3A).
Figure 3. A FLAG-tagged isoform of CG1674 stably localizes to the flight muscle Z-disc.
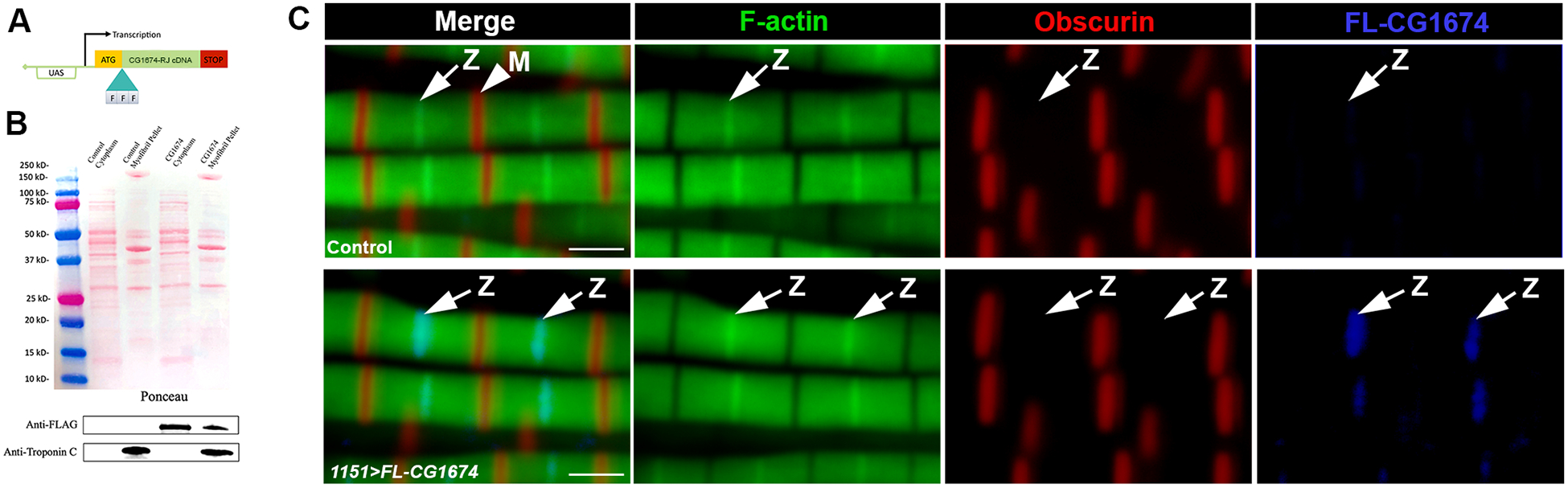
A. Diagram of the UAS-FLAG-CG1674 cDNA that was generated and inserted stably into the fly genome. B. Fractionation of Control (lanes 2 and 3) and 1151>FLAG-CG1674 (lanes 4 and 5) flight muscles into cytoplasmic (lanes 2 and 4) and myofibrillar (lanes 3 and 5) fractions. Top, Ponceau S-stained gel showing equal loading of samples. Bottom, western blots using: anti-FLAG that shows accumulation of FLAG-CG1674 in the cytoplasmic and myofibrillar fractions of transgenic samples (lanes 4 and 5); and anti-TpnC that shows correct fractionation of muscle samples. C. Staining of y w control (top) and 1151>FLAG-CG1674 (bottom) flight muscle myofibrils to determine localization of FLAG-CG1674. Phalloidin (green) labels F-actin throughout most of the sarcomere, and at higher levels at the Z-discs (arrows, Z). Obscurin (red) is localized to the M-line (arrowhead, M). In the 1151>FLAG-CG1674 muscles, the tagged protein (blue) localizes to the Z-disc. Bar, 1.5μm.
To identify the cellular compartment in which CG1674 is localized, we activated expression of this recombinant cDNA in adult muscles using the 1151-Gal4 driver. We then dissected adult muscles and fractionated the muscles into an insoluble myofibril pellet and a soluble cytoplasmic fraction. Control samples were also prepared from non-transgenic animals. Samples were separated by SDS-PAGE, transferred to nitrocellulose membrane, and transferred proteins were visualized using Ponceau S stain (Figure 3B, top). The membrane was then cut horizontally so that it could be separately probed for myofibrillar Troponin C (TpnC) and anti-FLAG.
Consistent with effective fractionation of the samples, TpnC was detected in the myofibrillar pellets of control and CG1674-FLAG samples, but not in the cytoplasmic fractions (Figure 3C, lower panel). For FLAG, this was not detected in the control samples, but was readily detected in both the cytoplasmic and myofibrillar fractions of the FLAG-CG1674 samples (Figure 3C, lower panel). We interpret from these results that CG1674 expressed in muscles can be a stable component of the myofibrils, but that some of it may exist either as a cytoplasmic form or loosely associated with the myofibrils that becomes dislodged during fractionation.
To determine if CG1674 is localized to a specific region in the myofibrils, we sectioned control and 1151>FL-CG1674 adults and stained them for accumulation of FL-CG1674, Obscurin (also called Unc-89, which is localized to the M-line; 21), and F-actin. In control animals, F-actin labels much of the sarcomere and is enriched at the location of the Z-line, whereas Obscurin is localized to specific bands corresponding to the M-line (Figure 3C, top). When FL-CG1674 was expressed, we detected strong accumulation of signal at the F-actin-rich Z-line (Figure 3C, bottom). This result demonstrated that, when associated with the myofibrils, tagged CG1674 is localized to the Z-disc. We also detected FLAG-CG1674 in areas between the myofibrils, consistent with the dual localization of the tagged protein as determined by Western blotting. The cytoplasmic localization is not visible in Figure 3 but is visible in later figures.
CG1674 is required for normal flight and muscle structure.
To determine the requirement of CG1674 for muscle function, we knocked down its expression in muscles using muscle specific Gal4 drivers and UAS-RNAi transgenes. To ensure that our findings were robust, we used two different RNAi lines targeting CG1674 transcripts (named 103052 and 42276) and three different Gal4 drivers: 1151-Gal4, that is active in all adult myoblasts and muscles (22); Mef2-Gal4, that is active in all muscles (23); and Act88F-Gal4, that is active in the flight muscles only (24).
Control y w flies were capable of strong flight, as were control 1151-Gal4/+ and UAS-CG1674 RNAi 42276/+ (Figure 4A, left three columns). By contrast, all of the CG1674 knockdowns were flightless, with most of the flies incapable of any flight and simply falling to the bottom of the testing chamber (Figure 4A). Clearly, CG1674 is necessary for normal flight muscle function. Interestingly, despite two of the Gal4 drivers being active in all adult muscles, we did not observe a strong effect of CG1674 knockdown upon viability, suggesting that CG1674 is especially critical for flight muscle performance, or that defects in the performance of other muscles can be more easily tolerated than can defects in flight muscle function.
Figure 4. CG1674 function is required for normal flight ability and function.
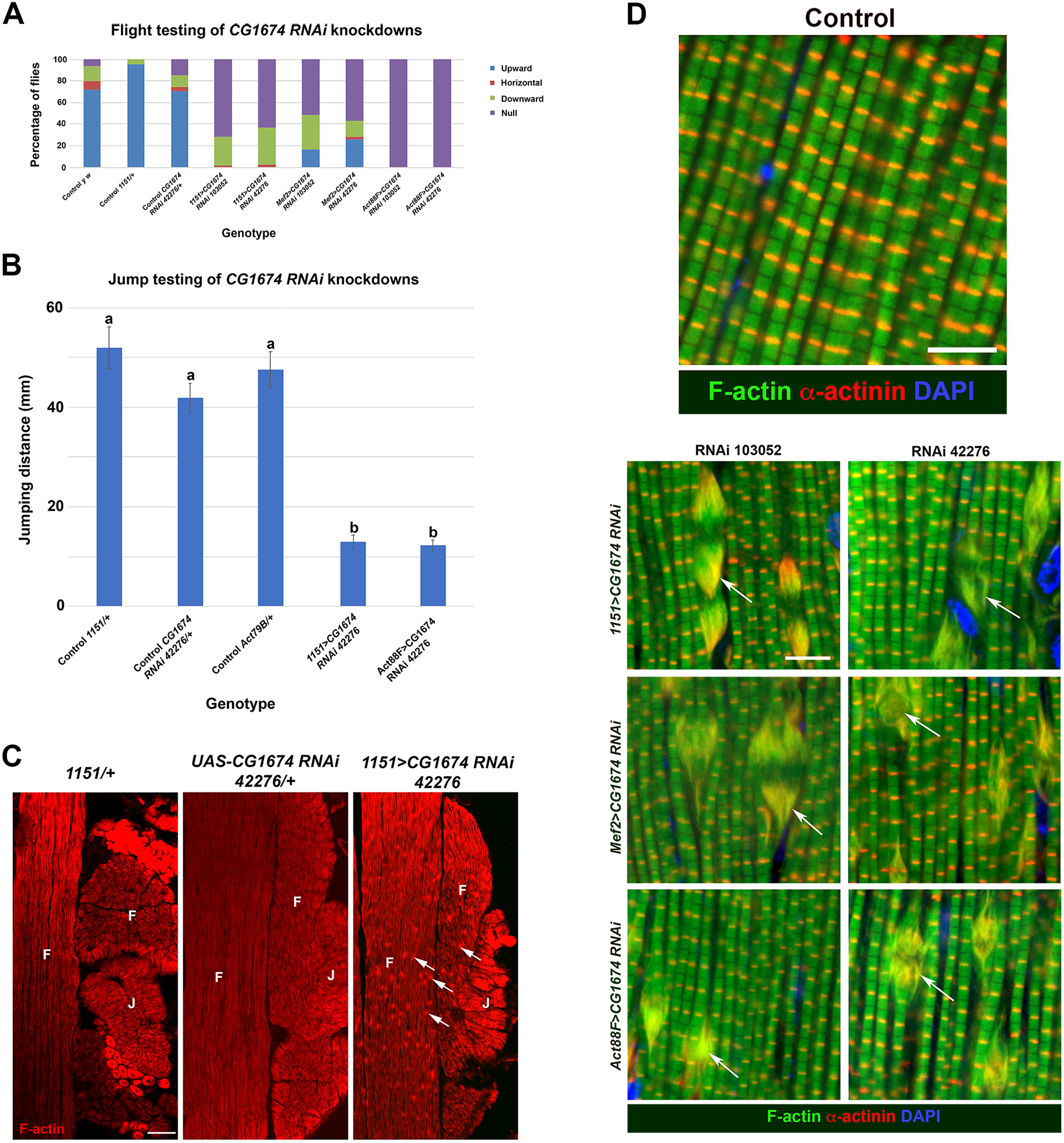
A. CG1674 expression was knocked down in flight muscles using three different Gal4 drivers, and two different UAS-CG1674 RNAi lines. Flight ability of Control and knockdown female flies is shown. Note that control flies (first three columns) predominantly fly upwards in the assay, whereas all of the knockdown lines showed strongly reduced flight, with the majority of the flies unable to fly at all (null category). B. Jumping ability of control (columns 1, 2 and 3) and CG1674 knockdown (columns 4 and 5) females. a and b refer to statistically different categories, showing that control animals (category a) did not differ from each other in jumping ability, but did differ significantly from the jumping ability of knockdown animals (category b). Jumping ability of knockdowns did not differ significantly from each other. C. Horizontal sections of y w control and CG1674 knockdown females stained for accumulation of F-actin (red) in flight (F) and jump (J) muscles. F-actin-rich deposits were only observed in the knockdown animals. Additionally, the morphology of the jump muscles was abnormal in the knockdown animals. Bar, 250μm. D. Myofibril organization in control y w flight muscles of pharate adults, stained for accumulation of F-actin (green), alpha-actinin to label the Z-discs (red), and DAPI to label nuclei (blue). Bar, 5μm. D. Phenotypes of knockdown myofibrils, stained as in B. Note the large actin-rich structures observed in all experimental conditions, that also accumulate alpha-actinin (arrows). Bar, 5μm.
To test the effect of CG1674 knockdown more stringently in non-flight muscles, we determined the jumping ability of knockdowns using one of the RNAi lines combined with two different Gal drivers, 1151-Gal4, and Act79B-Gal4 that is active specifically in the jump muscles, a distinct muscle fiber types in the thorax (24, 25). The control genotypes 1151/+, UAS-CG1674 RNAi 42276/+ and Act79B/+ all showed strong jumping ability that did not differ significantly from one another. By contrast, both knockdowns showed significantly reduced jumping ability (Figure 4B). These observations indicate that CG1674 is also critical to the function of the jump muscle.
To determine the effects upon gross muscle morphology of the knockdowns, we analyzed F-actin accumulation in horizontal sections of control and 1151>CG1674 RNAi animals at low magnification. Here, we observed that whereas there was robust F-actin staining in the muscles of all genotypes, knockdown animals show large actin-rich inclusions in the muscles that were not observed in controls (Figure 4C, arrows). We quantified the prevalence of these structures over multiple samples (n=5 for each genotype). We never observed the structures in control animals, but in the knockdowns they occurred on average 42.8 ± 2.5 times within a 300μm × 300μm area (mean plus standard error of the mean). We also found that the knockdown animals had a defective jump muscle structure where the fibers of the jump muscle were arranged in a linear fashion rather than as a rosette observed in controls (Figure 4C).
To analyze the flight muscle defects in greater detail, we studied the relationship of these F-actin rich structures to the myofibrils. Control and knockdown samples at the pharate adult stage were sectioned and stained for accumulation of F-actin and the Z-disc protein Alpha-actinin, which clearly labels the Z-disc in y w control samples (Figure 4D, top panel). In all of the knockdowns, using three different Gal4 drivers and two different RNAi lines, we consistently observed the actin-rich structures. These appeared as a fraying of the myofibril, and also showed strong accumulation of Alpha-actinin (Figure 4D, lower panels, arrows), suggesting that they might be malformed Z-discs.
The severe disruption of muscle structure provides an explanation for the loss of flight ability in the CG1674 knockdowns. Importantly, since these sections were prepared from pharate adults (prior to eclosion from the pupal case), we conclude that the defects that we observed reflect defects in myofibril assembly rather than a loss of muscle integrity following use. These findings are consistent with and extend those of Schnorrer et al (26), who showed that CG1674 is required for normal flight ability and myofibril structure.
Requirement of Z-disc proteins for CG1674 localization.
To determine the dependence of CG1674 accumulation upon other myofibrillar proteins, we next knocked down the expression of genes encoding Z-disc components, while at the same time expressing the FLAG tagged CG1674 transgene. The efficacy of knockdown was monitored by flight testing the different experimental animals. All samples were compared to y w controls, that showed normal flight ability and myofibril structure (Figure 5A).
Figure 5. Localization of FLAG-CG1674 to the Z-disc depends upon Z-disc proteins.
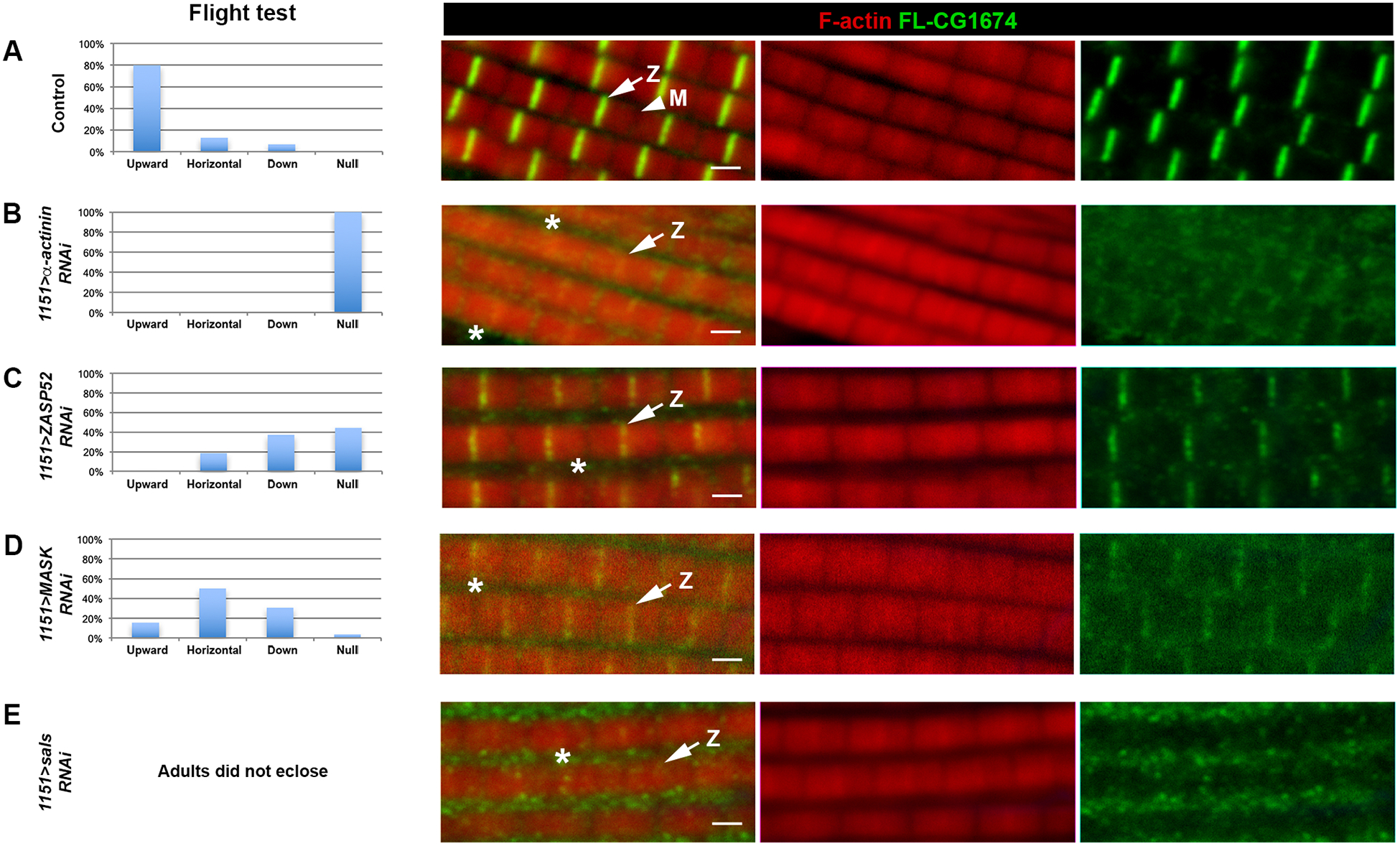
Control 1151>FLAG-CG1674 flies (A), and flies where expression of different Z-disc protein genes had been knocked down (B-E), were flight tested (left column) and stained for accumulation of F-actin (red) and FLAG-CG1674 (green). Note that whereas in control muscles FLAG-CG1674 was localized to the Z-disc, the intense FLAG-CG1674 staining was reduced or absent in the knockdown animals, and more FLAG-CG1674 accumulation was observed between the myofibrils in the cytoplasm (asterisks). Arrows denote Z-discs (Z), arrowheads denotes M-line (M). Bar, 1.5μm.
When we knocked down expression of Alpha-actinin, the adults were flightless and showed a strongly-diminished localization of FL-CG1674 at the Z-disc, and some modest accumulation at the M-line (Figure 5B, arrows denote Z-discs). In knockdowns for Zasp52, there was a diminution of FL-CG1674 at the Z-disc, and the Z-disc staining that was observed was irregular and patchy (Figure 5C). A similar result was observed for knockdown of Mask (Figure 5D), and in sals knockdowns there was also a loss of FL-CG1674 localization (Figure 5E). Taken together, these observations demonstrate a rather non-specific dependence of FL-CG1674 localization upon Z-disc components. Instead, it appears that loss of any Z-disc proteins are sufficient to induce de-localization of FL-CG1674 from the Z-disc. In several cases, we saw clear accumulation of FL-CG1674 in the cytoplasm (asterisks in Figure 5, and Figure 6A).
Figure 6. Knockdown of some Z-disc genes results in re-localization of FLAG-CG1674 to the nucleus.
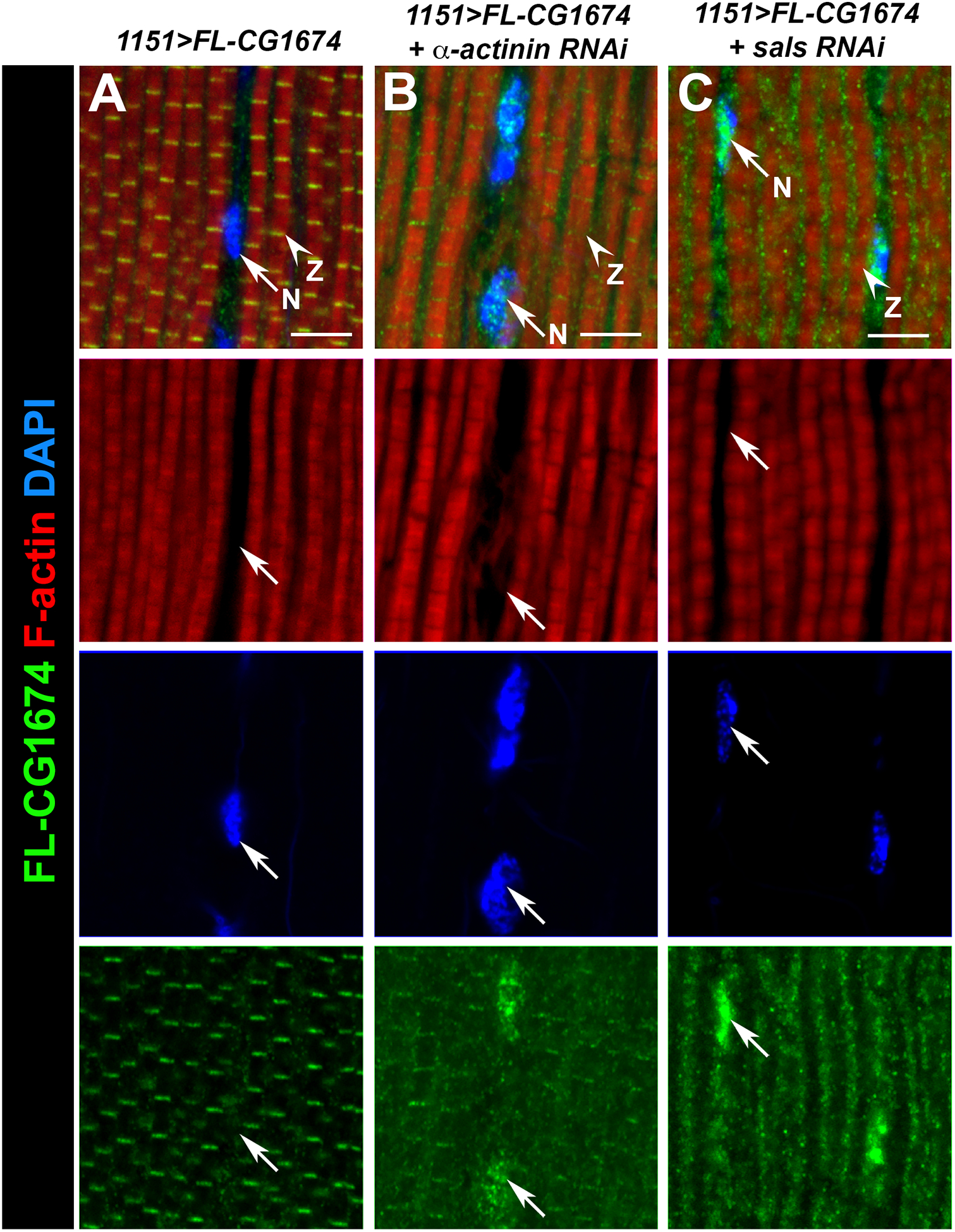
Control 1151>FLAG-CG1674 (A) and knockdown samples (B, C) were sectioned and stained for accumulation of F-actin (red), FLAG-CG1674 (green) and DAPI (blue). In controls (left column), FLAG-CG1674 was detected in the Z-discs and faintly in the cytoplasm, but not at the nucleus. In Alpha-actinin and sals knockdown samples (B and C, respectively), FLAG-CG1674 staining was reduced at the Z-disc and FLAG-CG1674 accumulation was observed associated with the nuclei. Arrows indicate nuclei (N) and arrowheads indicate Z-discs (Z). Bar, 5μm.
Interestingly, in the Alpha-actinin or sals knockdowns, a portion of the FL-CG1674 was localized to the nucleus (Figure 6B, C, arrows). However, this was not observed for knockdown of the other Z-disc protein genes, Zasp52 and Mask, suggesting that there is some specificity as to what kind of myofibrillar defects will trigger FL-CG1674 re-localization. Possible interpretations of this result are discussed below.
Discussion.
In this paper we present an initial analysis of the Drosophila flight muscle myofibril proteome, and characterize the function in muscle of one novel protein that we identified, named CG1674. Our proteomic analysis identified 26 proteins that were present either in both replicates of the LC-MS/MS assay, or that were represented by at least ten peptides in one assay. This is likely to be an underestimate of the total myofibril proteome, since we believe that this screen in not yet saturating. For example, several myofibrillar proteins were either not identified in the analysis, or detected only in one assay and not the other. This is a common challenge when analyzing proteomes of biological structures, because the large dynamic range in protein abundance can result in a failure to detect rarer polypeptides that are still essential for cell or tissue function. In particular, it is likely that components of the Z-disc and M-line will be under-represented given their very small volume within the myofibril compared to the volume filled by thick and thin filaments. Therefore, biochemical approaches to enrich for specific myofibril compartments should be effective in identifying more minor components of the myofibril. While approaches for purifying the M-line have not been described, purification of Drosophila Z-discs was achieved by Saide et al (27) therefore Z-discs purified in this manner might be a good source of material for identification of the Z-disc proteome.
Our findings are the first to identify the Drosophila flight muscle proteome. The adult Drosophila heart proteome was characterized by Cammarato et al (28), and notably CG1674 was also identified in that tissue, suggesting that CG1674 is a component common to striated muscle. Our findings also complement several studies of the muscle proteome from mammalian tissues, and while several of these approaches have concentrated upon the entire muscle proteome including cytosolic proteins (reviewed in 29, 30), other studies have characterized myofibrillar components (see for example 31). An important direction for future studies will be to apply quantitative technology to define how the muscle proteome alters during aging and disease. Moreover, novel post-translational modifications of myofibrillar components increase with aging (see for example 32) and it will be important to define how these changes might impact muscle function.
Expression of a FLAG-tagged isoform of CG1674 showed localization in control muscles to the Z-disc and cytoplasm. While this dual localization might be reflective of the wild-type location of CG1674, we note that the Gal4 expression system that we use might cause expression of CG1674 protein above normal physiological levels, and thereby saturate its normal location. Given that we observe structural defects in CG1674 knockdowns, and since several Z-disc proteins are required for localization of FLAG-CG1674, we believe that at least the myofibrillar localization of FLAG-CG1674 is physiologically relevant.
We also note that the isoform that we tagged, CG1674-PJ, may not be the flight muscle-specific isoform, since our proteomics identified some peptides that were unique to isoforms PK and PL. As a result, a mis-match between protein isoform and the muscle type in which it is expressed might provide inaccurate information regarding its localization. On the other hand, it is also highly possible that more than one CG1674 isoform is present in the flight muscles. Moreover, support for a role for CG1674 in the Z-disc comes from prior studies. Lowe et al (33) demonstrated that a protein trap allele of CG1674 resulted in localization of CG1674-YFP to the Z-disc.
Knockdown of CG1674 expression in the flight muscles produced profound changes in myofibril structure. Throughout the muscle there were myofibrils that, at a certain point along their length, show a dramatic fraying and dispersion of the myofilaments, and these dispersed and actin-rich structures also showed accumulation of the Z-disc protein alpha-actinin. These structures are reminiscent of the F-actin blobs that were observed in several muscle-specific knockdowns (26), although in those cases it was not apparent if the actin-rich structures also accumulated Z-disc components. Mutations affecting ZASP accumulation result in the overgrowth of Z-disc material (34), and several human muscle diseases are characterized by aggregates containing Z-disc components (reviewed in 35). Therefore, further analysis of the role of CG1674 in myofibril assembly may provide insight into human disease mechanisms. One possibility is that CG1674 acts to restrain filament growth at the Z-disc, and in its absence, there is overgrowth and a fractured appearance. Why this might occur at only a subset of locations within the muscle is unclear.
Some insight into the molecular function of CG1674 might be gained from knowledge of its orthologs and their functions. Blast searches revealed only a small region of similarity between CG1674 and any mammalian proteins. This similarity was with a PDZ domain protein named Synaptopodin 2-like, also called Synpo2l and CHAP. In vertebrates, Synpo2l is localized to the Z-disc of mouse cardiomyocytes where it binds to alpha-actinin, and knockdown of the expression of one ortholog in zebrafish, named Chap-1, results in defects in myofibril structure (36). Moreover, truncated isoforms of murine Synpo2l expressed in Cos cells can localize to the nucleus (36). These observations for Synpo2l are similar to our observations for CG1674, despite the apparently large differences in the protein sequences and the absence of a PDZ domain in CG1674. Indeed, phylogenetic reconstructions of gene families suggest that CG1674 arose from a progenitor protein with actin binding activity and a role in the cytoskeleton that is related to Synpo2l (see Figure 2).
The translocation of FL-CG1674 to the nucleus in two of our knockdowns was also interesting to note, however the precise localization of FL-CG1674 in these muscles remains to be fully clarified. One possible interpretation is that FL-CG1674 released from the myofibrils in these knockdowns enters the nucleus. In this model, CG1674 might function in a similar manner to Synpo2l in signaling from the myofibril to the nucleus. Alternatively, the nuclear accumulation of FL-CG1674 may represent the protein coating the outside of the nucleus. The biological significance of this is not clear, although nascent myofibrils in embryonic Drosophila muscles arise from perinuclear deposits of Z-disc components (8). In this scenario, nuclear localization of FL-CG1674 in the knockdowns may represent aberrant Z-disc formation.
Experimental procedures.
Drosophila stocks and crosses.
Drosophila were maintained using Jazz Mix food (VWR Scientific Products), in vials or bottles stored at 25°C. Control flies were y w. Crosses for RNAi experiments were carried out at 29°C to maximize effect of the Gal4 driver lines. Gal4 lines used and their sources are as follows: Act88F-Gal4 (24; our laboratory); 1151-Gal4 (22, kindly provided by Dr. L.S. Shashidara, Indian Institute of Science Education and Research, Pune, India); and Mef2-Gal4 (23, kindly provided by A. N. Johnson, Washington University in St. Louis). UAS-RNAi lines were from either the Vienna Drosophila RNAi Center (VDRC) or Bloomington Drosophila Stock Center (BDSC) as follows: CG1674, VDRC lines 103052 and 42276; Alpha-actinin, VDRC 110719; sals, VDRC 103534; Zasp52, VDRC 36563; Mask, BDSC 34571.
Flight and jump testing.
Flight testing was carried out as described by Drummond et al (37). Briefly, flies aged 1–2 days were released inside a plastic box that was illuminated from the top. Individual flies were scored for whether they flew upwards (U), horizontally (H), downwards (D), or not-at-all (N). At least 20 female flies were tested for each genotype. Jump testing was carried out as described by Cripps et al (38), where flies from which the wings had been removed were encouraged to jump from an elevated platform, and horizontal distance from the edge of the platform to the landing point was measured. At least 20 female flies were tested for each genotype. Data for jump tests were initially analyzed by One-way ANOVA to determine if significant differences occurred between genotypes. The F value was 44, indicating a probability approaching zero. Following this, Tukey post-hoc comparisons were carried out for each pairwise combination of genotypes. Genotypes with similar jumping abilities (i.e., those not differing significantly from one another) were grouped together, and dissimilar genotypes based upon p<0.01 were assigned to different groups. These analyses were carried out at https://statpages.info/anova1sm.html.
DNA methods and generation of transgenic animals.
The CG1674 cDNA clone IP15312 was obtained from the Drosophila Genomics Resource Center; this clone contains the entire coding sequence for the CG1674-PJ protein isoform. To generate the N-terminal FLAG-tagged UAS construct, CG1674 coding sequence was amplified by PCR using this plasmid as a template, and the primers:
5’- GGGGACAAGTTTGTACAAAAAAGCAGGCTTCATGGATTCTACTTTAAATATTGAG; and
5’- GGGGACCACTTTGTACAAGAAAGCTGGGTCCTAAAAATCAGAGTACGGTAGATTT.
These primers contain attB sites (underlined) and the resulting PCR product was inserted into the Gateway vector pDONR221 using Gateway cloning (Clontech, Inc.). A verified pDONR221/CG1674 clone was then recombined with the Drosophila Gateway plasmid pTFW (https://emb.carnegiescience.edu/drosophila-gateway-vector-collection) to generate a UAS-FLAG-CG1674 recombinant construct that was verified to be in-frame by DNA sequencing.
The pTFW/CG1674 plasmid was inserted into the Drosophila genome using P-element mediated germline transformation (39).
Protein sample preparation and western blotting.
Dorsal longitudinal indirect flight muscles were dissected from 1–2 day old adult Drosophila for all experiments. Chemical demembranation (“skinning”) was carried out essentially as described by Cripps and Sparrow (40). Briefly, muscles from six adult flies were dissected in PBS, and dispersed in 50μl of an homogenization solution containing Triton X-100 to disrupt membranes. The insoluble myofibrillar and soluble cytoplasmic fractions were separated by centrifugation. The cytosolic supernatant was removed, mixed with 17μl of 4X Laemmli sample buffer, and heated at 95°C for five minutes. The myofibrillar pellet was washed one more time with homogenization solution containing Triton X-100 and then two times with homogenization solution lacking the detergent. The final myofibrillar pellet was resuspended in 60μl of 1X Laemmli sample buffer, and heated as above. 5–10μl of each sample was used for electrophoresis.
The IFM proteome was identified by running a sample of w1118 skinned flight muscles on a 4–20% SDS-PAGE gel. The entire lane was excised from the gel, excluding the highly intense band corresponding to Myosin heavy-chain. The gel slice was then submitted to the Arizona Proteomics Consortium for protein digestion and peptide sequencing using liquid chromatography followed by tandem mass spectrometry (University of Arizona, AZ). This procedure was carried out in duplicate using two separate dissections of flight muscles. Proteins identified within the myofibril fraction were identified by comparing peptide sequences with the predicted Drosophila proteome, and only those proteins that were identified in both replicates, or that were present at high levels in at least one replicate, are represented in Figure 1.
Western blotting was carried out using standard procedures. Primary antibodies used were: rabbit anti-FLAG (Thermo Fisher, 1:1,000); and rat anti-Troponin C (Abcam ab51106, 1:1,000). Secondary antibodies were anti-rabbit and anti-rat, and were visualized using enhanced chemiluminescent detection (Pierce) and a ChemiDoc imager (BioRad).
Cryosectioning, immunofluorescence, and confocal microscopy.
Cryosectioning and immunofluorescence were carried out as described by Morriss et al (41). Antibodies used were: mouse anti-FLAG (Sigma, 1:1,000); rabbit anti-Obscurin (kindly provided by Dr. Belinda Bullard, University of York, UK, 1:200); and rat anti-alpha-actinin (Abcam ab50599, 1:1,000). Secondary antibodies used were Alexa-488 or Alexa-568 conjugated (Roche), and used at 1:2,000. DAPI (Sigma) was used at a 1:10,000 dilution of a 10mg/ml stock. Alexa-conjugated Phalloidin (Roche) was used as 1:500.
Acknowledgements.
This work was supported by GM061738, awarded by the NIH/NIGMS to R.M.C. M.C. was supported by a Minority Access to Research Careers training grant (T34 GM008751) and by a Postbaccalaureate Research and Education Program training grant (R25 GM075149). B.S.G. was supported by an Initiatives to Maximize Student Diversity training grant (R25 GM060201). We acknowledge technical support from the Molecular Biology Facility and the Cell Biology Facility at the Department of Biology, University of New Mexico, supported by NIH grant P20 GM103452 from the Institute Development Award (IDeA) Program of NIGMS. Mass spectrometry and proteomics data were acquired by the Arizona Proteomics Consortium supported by NIEHS grant ES06694 to the SWEHSC, NIH/NCI grant CA023074 to the UA Cancer Center and by the BIO5 Institute of the University of Arizona.
Footnotes
Conflict of interest statement.
The authors declare that they have no conflicts of interest with the contents of this article.
Literature cited.
- 1.Squire J Muscle: design, diversity, and disease. Benjamin-Cummings Publishing Company; 1996. [Google Scholar]
- 2.Sheikh F, Bang ML, Lange S, Chen J. “Z”eroing in on the role of Cypher in striated muscle function, signaling and human disease. Trends Cardiovasc Med. 17: 258–262. 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen HH, Chen WP, Yan WL, et al. NRIP is newly identified as a Z-disc protein, activating calmodulin signaling for skeletal muscle contraction and regeneration. J Cell Sci 128: 4196–4209. 2015. [DOI] [PubMed] [Google Scholar]
- 4.Frank D, Frey N. Cardiac Z-disc signaling network. J Biol Chem 286: 9897–9904. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cattaruzza M, Lattrich C, Hecker M. Focal adhesion protein zyxin is a mechanosensitive modulator of gene expression in vascular smooth muscle cells. Hypertension 43: 726–730. 2004. [DOI] [PubMed] [Google Scholar]
- 6.Frank D, Kuhn C, Katus HA and Frey N. The sarcomeric Z-disc: a nodal point in signaling and disease. J Mol Med 84: 446–468. 2006. [DOI] [PubMed] [Google Scholar]
- 7.Weins A, Schwarz K, Faul C, Barisoni L, Linke WA, Mundel P. Differentiation- and stress-dependent nuclear cytoplasmic redistribution of myopodin, a novel actin bundling protein. J Cell Biol 155: 393–404. 2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auld AL, Folker ES. Nucleus-dependent sarcomere assembly is mediated by the LINC complex. Mol Biol Cell 27: 2351–2359. 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hauser MA, Horrigan SK, Salmikangas P, et al. Myotilin is mutated in limb girdle muscular dystrophy 1A. Hum. Mol. Genet 9: 2141–2147. 2000. [DOI] [PubMed] [Google Scholar]
- 10.Selcen D, Engel AG. Mutations in ZASP define a novel form of muscular dystrophy in humans. Ann. Neurol 57: 269–276. 2005. [DOI] [PubMed] [Google Scholar]
- 11.Taylor MV. Comparison of Muscle Development in Drosophila and Vertebrates Pp 169–203 in: Muscle Development in Drosophila. Molecular Biology Intelligence Unit Springer, New York, NY: 2006. [Google Scholar]
- 12.Bai J, Hartwig JH, Perrimon N. SALS, a WH2-domain-containing protein, promotes sarcomeric actin filament elongation from pointed ends during Drosophila muscle growth. Dev Cell 13: 828–842. 2007. [DOI] [PubMed] [Google Scholar]
- 13.Katzemich A, West RJH, Fukuzawa A, et al. Binding partners of the kinase domains in Drosophila obscurin and their effect on the structure of the flight muscle. J Cell Sci 128: 3386–3397. 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chechenova MB, Bryantsev AL, Cripps RM. The Drosophila Z-disc protein Z(210) is an adult muscle isoform of Zasp52, which is required for normal myofibril organization in indirect flight muscles. J Biol Chem 288: 3718–3726. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Katzemich A, Liao KA, Czerniecki S, Schöck F. Alp/Enigma family proteins cooperate in Z-disc formation and myofibril assembly. PLoS Genet 9(3): e1003342 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.González-Morales N, Marsh TW, Katzemich A, et al. Different evolutionary trajectories of two insect specific paralogous proteins involved in stabilizing muscle myofibrils. Genetics 212: 743–755. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Thurmond J, Goodman JL, Strelets VB et al. FlyBase 2.0: the next generation. Nucleic Acids Res 47(D1) D759–D765. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oas ST, Bryantsev AL, Cripps RM. Arrest is a regulator of fiber-specific alternative splicing in the indirect flight muscles of Drosophila. Journal of Cell Biology 206: 895–908. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kosugi S, Hasebe M, Tomita M, Yanagawa H. Systematic identification of yeast cell cycle-dependent nucleocytoplasmic shuttling proteins by prediction of composite motifs. Proc. Natl. Acad. Sci. USA 106: 10171–10176. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mi H, Muruganujan A, Ebert D, Huang X, Thomas PD. PANTHER version 14: more genomes, a new PANTHER GO-slim and improvements in enrichment analysis tools. Nucl Acids Res doi: 10.1093/nar/gky1038. (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Katzemich A, Kreisköther N, Alexandrovich A, et al. The function of the M-line protein obscurin in controlling the symmetry of the sarcomere in the flight muscle of Drosophila. J Cell Sci 125: 3367−−3379. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anant S, Roy S, VijayRaghavan K. Twist and Notch negatively regulate adult muscle differentiation in Drosophila. Development 125: 1361−−1369. 1998. [DOI] [PubMed] [Google Scholar]
- 23.Ranganayakulu G, Elliott DA, Harvey RP, Olson EN. Divergent roles for NK-2 class homeobox genes in cardiogenesis in flies and mice. Development 125: 3037–3048. 1998. [DOI] [PubMed] [Google Scholar]
- 24.Bryantsev AL, Baker PW, Lovato TL, Jaramillo MS, Cripps RM. Differential requirements for Myocyte Enhancer Factor-2 during adult myogenesis in Drosophila. Dev Biol 361: 191–207. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryantsev AL, Duong S, Brunetti TM, Chechenova MB, Lovato TL, Nelson C, Shaw E, Uhl JD, Gebelein B, Cripps RM.. Extradenticle and Homothorax control adult muscle fiber identity in Drosophila. Dev Cell 23(3): 664−−673. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schnorrer F, Schönbauer C, Langer CC et al. Systematic genetic analysis of muscle morphogenesis and function in Drosophila. Nature 464: 287−−291. 2010 [DOI] [PubMed] [Google Scholar]
- 27.Saide JD, Chin-Bow S, Hogan-Sheldon J, et al. Characterization of components of Z-bands in the fibrillar flight muscle of Drosophila melanogaster. J Cell Biol 109: 2157–2167. 1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cammarato A, Ahrens CH, Alayari NN, et al. A mighty small heart: the cardiac proteome of adult Drosophila melanogaster. PLoS ONE 6: e18497 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Solaro RJ, Warren CM, Scruggs SB Why is it important to analyze the cardiac sarcomere subproteome? Expert Rev Proteomics 7: 311–314. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gonzalez-Freire M, Semba RD, Ubaida-Mohien C et al. The human skeletal muscle proteome project: a reappraisal of the current literature. J Cachexia Sarcopenia Muscle 8: 5–18. 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fraterman S, Zeiger U, Khurana TS, Wilm M, Rubinstein NA. Quantitative proteomics profiling of sarcomere associated proteins in limb and extraocular muscle allotypes. Mol Cellular Proteomics 6: 728–737. 2007. [DOI] [PubMed] [Google Scholar]
- 32.Wei L, Gregorich ZR, Lin Z et al. Novel sarcopenia-related alterations in sarcomeric protein post-translational modifications (PTMs) in skeletal muscles identified by top-down proteomics. Mol Cellular Proteomics 17: 134–145. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lowe N, Rees JS, Roote J et al. Analysis of the expression patterns, subcellular localisations and interaction partners of Drosophila proteins using a pigP protein trap library. Development 141: 3994–4005. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.González-Morales N, Xiao YS, Schilling MA, Marescal O, Liao KA, Schöck F. Myofibril diameter is set by a finely tuned mechanism or protein oligomerization in Drosophila. eLife 8:e50496 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kley RA, Olivé M, Schröder R. New aspects of myofibrillar myopathies. Current Opinion in Neurology 29: 628–634. 2016. [DOI] [PubMed] [Google Scholar]
- 36.Beqqali A, Monshouwer-Kloots J, Monteiro R et al. CHAP is a newly identified Z-disc protein essential for heart and skeletal muscle function. J Cell Sci 123: 1141–1150. 2010. [DOI] [PubMed] [Google Scholar]
- 37.Drummond DR, Hennessey ES, Sparrow JC. Characterisation of missense mutations in the Act88F gene of Drosophila melanogaster. Mol. Gen. Genet 226: 70−−80. 1991. [DOI] [PubMed] [Google Scholar]
- 38.Cripps RM, Becker KD, Mardahl M, Kronert WA, Hodges D, Bernstein, SI. Transformation of Drosophila melanogaster with the wild-type myosin heavy-chain gene: rescue of mutant phenotypes and analysis of defects caused by overexpression. J Cell Biol 126(3): 689−−699. 1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rubin GM, Spradling AC. Genetic transformation of Drosophila with transposable element vectors. Science 218: 348–353. 1992. [DOI] [PubMed] [Google Scholar]
- 40.Cripps RM, Sparrow JC. Polymorphism in a Drosophila indirect flight muscle -specific tropomyosin isozyme does not affect flight ability. Biochem. Genet 30: 159–168. 1992. [DOI] [PubMed] [Google Scholar]
- 41.Morriss GR, Bryantsev AL, Chechenova M, LaBeau EM, Lovato TL, Ryan KM and Cripps RM. Analysis of skeletal muscle development in Drosophila In: Myogenesis: methods and protocols, DiMario J, ed. Springer, New York: 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]


