Abstract
RNA binding protein motif 3 (RBM3) is an RNA-binding and cold shock protein that protects myoblasts and promotes skeletal muscle hypertrophy by enhancing mRNA stability and translation. Muscle size is decreased during aging; however, it is typically delayed in models of extended lifespan such as the long-lived Ames Dwarf (df/df) mice and calorie restricted (CR) animals compared to age-match controls. In light of the protective and anabolic effects of RBM3 in muscle, we hypothesized that RBM3 expression is higher in long-lived animal models. Young and old df/df mice, and adult and old UM-HET3 CR mice were used to test this hypothesis. Gastrocnemius muscles were harvested and protein was isolated for RBM3 protein measurements. CR induced a 1.7 and 1.3-fold elevation in RBM3 protein abundance compared to adult and old male mice fed ad libitum (AL) diets, respectively; this effect was shared between males and females. Ames dwarfism induced a 4.6 and 2.7-fold elevation in RBM3 protein abundance in young and old df/df mice compared to normal control littermates, respectively. In contrast, there was an age-associated decrease in cold-inducible RNA-binding protein (CIRP), suggesting these effects are specific for RBM3. Lastly, there was an age-associated increase in RNA degradation marker decapping enzyme 2 (DCP2) in UM-HET3 mice that was mitigated by CR. These results show that muscle RBM3 expression is correlated with extended lifespan in both df/df and CR animals. Identifying how RBM3 exerts protective effects in muscle may yield new insights into healthy aging of skeletal muscle.
Keywords: Skeletal muscle, RBM3, aging, RNA-binding proteins, longevity, RNA
1. Introduction:
Lower core body temperature is associated with extended lifespan in fruit flies (22), c. elegans (36), rhesus monkeys (18), rodents (5, 15) and humans (30). Insight into the relationship between colder body temperatures and long life has been gained largely through manipulating lifespan in rodents. The long-lived Ames dwarf (df/df) mice, which lack thyroid stimulating hormone (TSH) and growth hormone (GH) display a lower core body temperature and live up to 1 year longer than normal control littermates (4). Similarly, calorie restriction (CR), which extends life span (18) is also associated with a lower body temperature in rodents as well as other species (2, 5, 15, 18, 29). However, mechanisms underlying the relationship between lower core body temperatures and extended lifespan remain to be determined.
Hibernating animals represent the most extreme case of an organism’s ability to lower their core body temperature in order lower metabolic demand (3). Indeed, hibernation in mammals results in a drastic drop in whole body metabolism along with a corresponding drop in core body temperature (3). Despite the strong physiological stresses accompanied with the hibernation process, skeletal muscle mass and the integrity of other tissues are largely retained over long and/or repeated bouts of hibernation. These facts have led to multiple lines of research investigating how hibernating animals are able to maintain muscle mass despite long periods of disuse, which consistently leads to a loss of muscle mass in humans (1). The remarkable feat of hibernating animals to maintain their muscle size despite decreased use is perhaps explained by a coordinated shift in protein metabolic processes where proteins necessary for muscle size are protected. Interestingly, it has been shown in multiple hibernators that specific RNA species accumulate and are translated preferentially (32), suggesting a post-transcriptional regulatory mechanism that bolsters homeostatic processes that confer protection against depressed energy supply/use. Indeed, despite lowered whole body metabolism, recent whole transcriptome analysis of hibernating grizzly bears has demonstrated dramatic changes in gene expression that serve to both lower skeletal muscle protein degradation and increase muscle protein synthesis in efforts to preserve muscle mass (16). These findings extend to black bears (12), artic ground squirrels (40), and the golden-mantled ground squirrel (39). While hibernating animals represent an extreme case of lowered body temperature, it is possible that there is conservation of mechanisms protecting RNA species in the lower core body temperature CR and Ames Dwarf animals, each of which associate with longevity.
RNA-binding proteins constitute a potential post-transcriptional regulatory mechanism that may protect or regulate RNA degradation in both hibernating and long-lived animals. RNA-binding proteins have roles throughout RNA metabolism including alternative splicing, nuclear export, translation and RNA decay (35). It is possible that in animals with extended lifespan RNA-binding proteins contribute to post-transcriptional regulation by stabilizing transcripts important for cellular homeostasis. Accordingly, it has been shown that RNA degradation increases with age (14) and that removal of the RNA-binding protein pumilio 2 in aged mouse skeletal muscle improves mitophagy and restores homeostasis, emphasizing the importance of RNA-binding proteins as a regulatory node in aging (6). We have previously identified RNA binding protein motif 3 (RBM3) to be robustly expressed in the skeletal muscle of young and old animals late during atrophy, suggesting a compensatory protective role in skeletal muscle (10). Interestingly, RBM3 is also the gene most prominently expressed when core body temperature is lowered (26). Activation of RBM3 and other RNA binding proteins (RBPs) such as cold inducible RNA binding protein, CIRP, confer stability to nascent mRNA to prevent degradation and aid in the maintenance of protein synthesis (41). RBM3 has been demonstrated to have the additional properties of enhancing global protein synthesis rates (9) while also promoting the biogenesis of a large percentage of microRNAs (28). Such effects, coupled with the stabilization of target mRNAs, may sculpt the proteome to augment homeostatic protective processes. It is possible that induction of RBM3 and CIRP is a convergent response to hypothermia in long-lived animal models and hibernators that serves to protect RNA homeostasis and orchestrate adaptive changes in the proteome that confer muscle mass stability (9). However, it is currently unknown whether changes in RBM3 and CIRP expression are correlated with extended lifespan. Thus, the goal of this study was to investigate the changes in skeletal muscle RBM3 and CIRP expression in two established murine models of delayed aging.
2. Materials and Methods:
2.1. Animal models
2.11. Calorie Restricted Mice
Adult and old male and female UM-HET3 mice kept on a 12-hour light/dark cycle, were used for CR studies (21) (Figure 1). Procedures and conditions for animal care were approved by the University of Michigan Committee on Use and Care of Animals and met standards for animal housing as described in the Animal Welfare Act, the Guide for Care and Use of Laboratory Animals, and the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching. UM-HET3 mice used in this study are genetically heterogeneous offspring of CB6F1 female and C3D2F1 male mice. Briefly, mice were either fed ad libitum a standard chow diet (AL) or restricted to 80% of ad libitum food consumption at 6 weeks of age and progressed to 60% at 10 weeks of age until sacrifice (21). At 12 (Adult) and 22 (Old) months of age, animals were euthanized following overnight fasting and both gastrocnemius muscle and hippocampus were immediately dissected and flash frozen in liquid nitrogen before being stored at −80°C. n ≥ 5 for all groups.
Figure 1. Experimental Design.
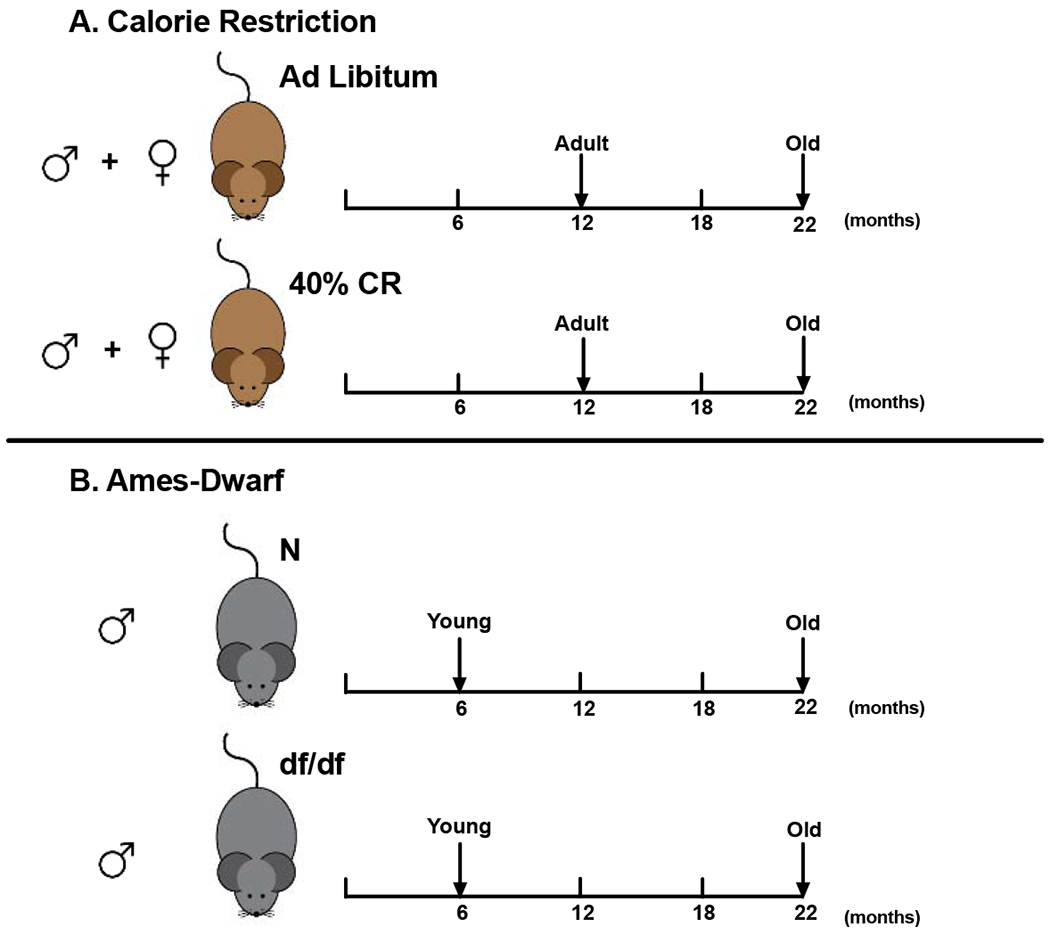
A) Male and female mice at 12 (adult) 22 (old) months of age fed ad libitum (AL) or CR B) Male 6 (young) and 22 (old) months old Ames dwarf (df/df) or normal control (N) mice were used.
2.12. Ames Dwarf Mice
All animal protocols were approved by the Laboratory Animal Care and Use Committee (LACUC) at the Southern Illinois University, School of Medicine. Young (6 months) and old (22 months) male normal controls (N) and Ames Dwarf (df/df) female mice were bred and maintained under temperature and light-controlled conditions: 22 ± 2°C, 12-hour light/12-hour dark cycle (Figure 1). Mice were euthanized following overnight fasting and gastrocnemius muscles were immediately dissected and flash frozen in liquid nitrogen before being stored at −80°C. Gastrocnemius muscles were collected at 6 (Young) and 22 (Old) months of age. n = 5 for all groups.
2.2. Western Blotting
Protein abundance was assessed by Western blotting, which was performed as described previously (24) with slight modifications. Briefly, samples were removed from −80°C storage, homogenized in radio-immunoprecipitation assay (RIPA) buffer supplemented with 10 μl ml−1 protease inhibitor cocktail (Roche, Indianapolis, IN, USA), 5 mM benzamidine, 5 mM, N-ethylmaleimide, 50 mM NaF, 25 mM B-glycerophosphate, 1 mM EDTA, and 1 mM phenylmethane sulfonyl fluoride (Boston Bioproducts, Ashland, MA, USA) and centrifuged at 5000 g to remove cell debris. Protein concentrations were determined using the Pierce BCA Protein Assay Kit (Thermo Scientific, Waltham, MA, USA). For quantification of protein abundance, 30 μg total protein was loaded and separated on 4–15% acrylamide gradient gels (Bio-Rad, Hercules, CA, USA), followed by transfer of proteins to polyvinylidene difluoride membranes with 0.22 μm pore size (Millipore, Burlington, MA, USA). Membranes were incubated in Odyssey Blocking Buffer (Li-Cor, Lincoln, NE, USA) followed by incubation with the appropriate primary antibody overnight at 4°C. The following primary antibodies were used: RNA-binding protein motif 3 (RBM3(27, 31)) (1:1,000), cold-inducible RNA-binding protein (CIRP) (Abcam, Cambridge, UK; 1:1,000), mRNA-decapping protein 2 (DCP2) (Abcam; 1:1,000) and vinculin (ProSci; 1:200). After primary antibody incubation, membranes were washed and further incubated with highly cross-absorbed infrared-labelled secondary antibodies for 1 h at room temperature (goat anti-rabbit; 1:15,000, Licor, Lincoln, NE, USA or goat anti-mouse; 1:15,000, Invitrogen, Omaha, NE, USA)). Membranes were scanned using an Odyssey infrared imaging system (Licor) to detect specific antibody binding and to perform quantification. Ponceau S staining of the membranes was used to ensure equal loading for muscle. Vinculin was used to ensure equal loading for hippocampus samples. The anti-RBM3 antibody has been extensively validated previously (27). Densitometric analyses were performed by assessors blinded to control and treatment groups.
2.3. Statistical Analysis
Two-way analysis of variance (ANOVA) was used for comparisons in both df/df and CR mice for treatment and age using GraphPad Prism (GraphPad Software, San Diego, CA, USA). A Grubb’s test was used to determine outliers where values were removed if they fell two standard deviations above/below the mean. All values reported are means ± standard error of the mean (SEM) and statistical significance was assumed at P < 0.05.
3. Results:
3.1. RBM3 is elevated in the skeletal muscle of Ames Dwarf and calorie restricted mice.
Skeletal muscle RBM3 protein abundance was 1.7 and 1.3 times higher in adult and old male UM-HET3 calorie restricted (CR) mice, respectively, compared to those fed ad libitum (AL) (p<0.0001) (Figure 2A). Similarly, female UM-HET3 mice showed 1.6 and 1.4 times higher skeletal muscle RBM3 protein abundance in adult and old CR mice, respectively, compared to age matched AL fed mice (p<0.004) (Figure 2B). There were no main effects for age in male nor female UM-HET3 mice. RBM3 protein abundance was 4.6 and 2.7 times higher in young and old df/df mice, respectively, compared to N littermate controls (p<0.0001) (Figure 3). There was no age effect on RBM3 levels in df/df mice. These data indicate that changes in skeletal muscle RBM3 protein abundance are associated with both CR and dwarfism, which are established mouse models of extended lifespan.
Figure 2. RBM3 is elevated in skeletal muscle of calorie restricted mice.
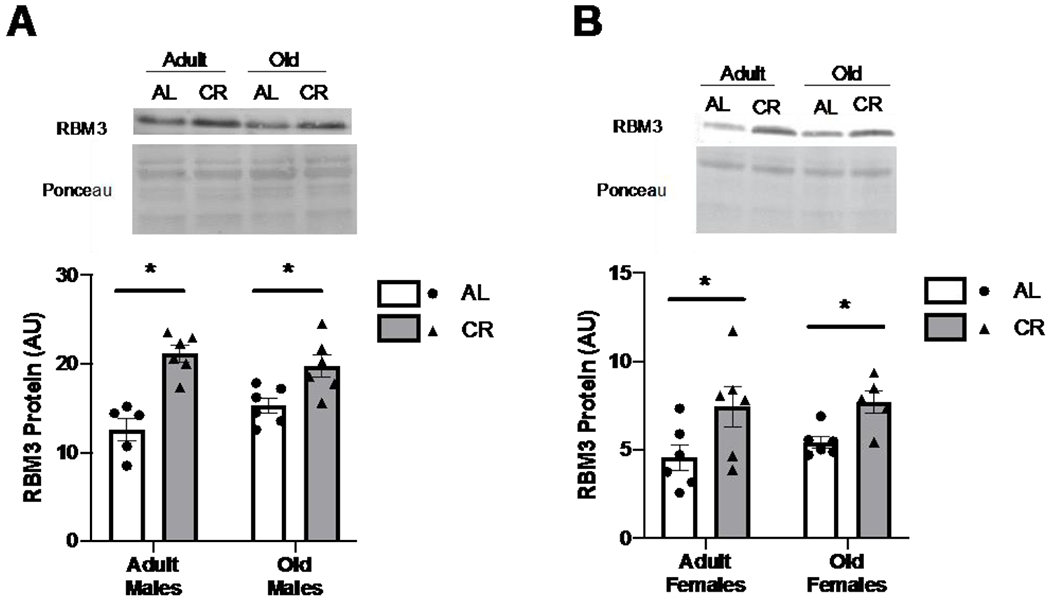
RBM3 protein abundance from muscles from A) male and B) female mice at 12 (adult) and 22 (old) months of age fed ad libitum (AL) or calorie restricted (CR). Values presented as mean ± SEM; n=6 for all groups. *p<0.05 denotes main effect for CR.
Figure 3. RBM3 is elevated in skeletal muscle of Ames-Dwarf mice.
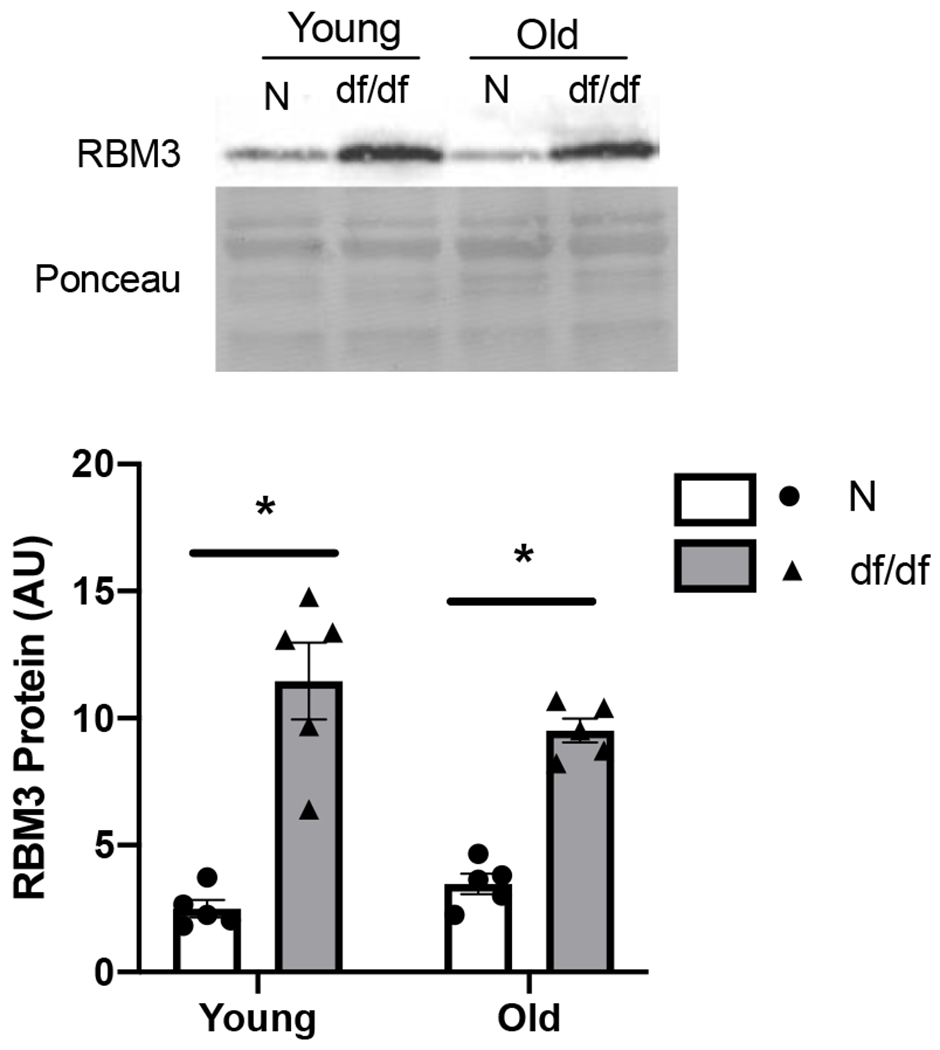
RBM3 protein abundance from male 6 (young) and 22 (old) months old Ames dwarf (df/df) or normal control (N) mice. Values presented as mean ± SEM; n=5 for all groups. *p<0.05 denotes main effect for genotype.
3.2. Age-dependent elevation of DCP2 is mitigated by CR in UM-HET3 mice.
There was a main effect for age in DCP2 protein abundance, a marker for mRNA degradation (33), in the skeletal muscle of aged compared with adult male UM-HET3 mice (p<0.001). Further, an interaction effect of DCP2 abundance in aged mice revealed that mice fed an AL diet was mitigated by CR (p<0.05) (Figure 4A). There was no effect of CR on DCP2 protein abundance in young UM-HET3 mice. Since males and females exhibited similar responses for RBM3 abundance, DCP2 was measured only in males. There was no difference in DCP2 protein abundance in young nor old df/df mice (Figure 4B).
Figure 4. DCP2 elevation in aged mice fed ad libitum is mitigated by calorie restriction only.
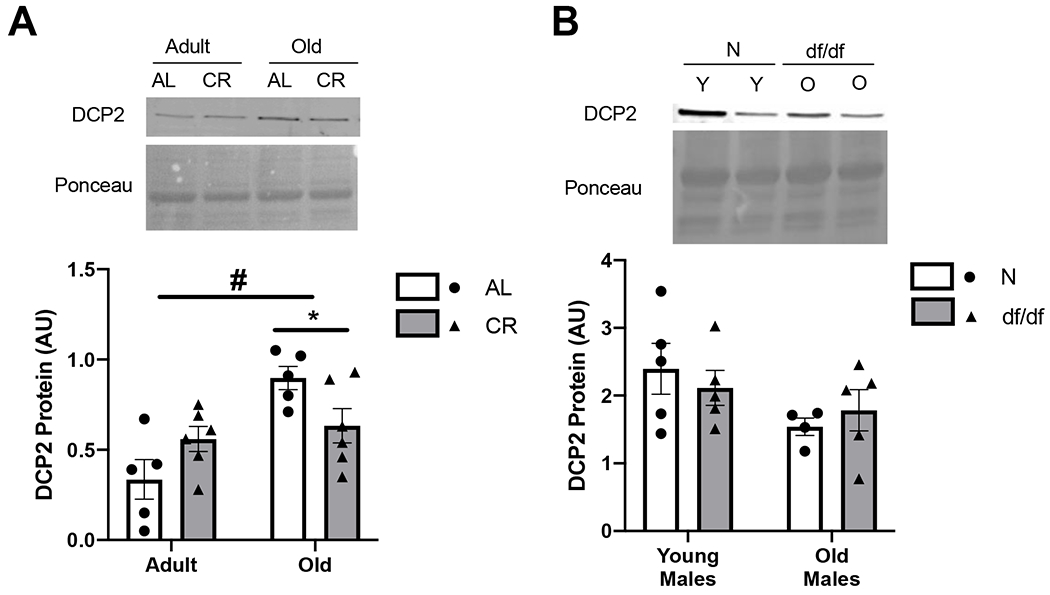
A) DCP2 protein abundance from adult and old mice fed ad libitum (AL) or calorie restricted (CR). B) DCP2 protein abundance from young and old Ames dwarf (df/df) or normal control (N) mice. Values presented as mean ± SEM; n=4-6 for CR animals. n=4-5 for df/df mice. #p<0.05 denotes main effect for age. *p<0.05 denotes difference between AL and CR.
3.3. Elevated cold shock protein in CR mice is specific for RBM3.
Both CIRP and RBM3 are upregulated by mild hypothermia (7, 25), in a temperature range seen in the longevity models studied here, and therefore we measured CIRP levels in muscle. CIRP protein abundance in skeletal muscle was lower with age in UM-HET3 AL mice compared to adult (p<0.05), but there was no difference with CR (Figure 5). RBM3 protein abundance in brain (hippocampus) of CR UM-HET3 mice was not different from AL mice, although there was a trend towards higher levels in CR animals (Supplementary Figure 1).
Figure 5. Elevated cold shock proteins in CR mice is specific to RBM3.
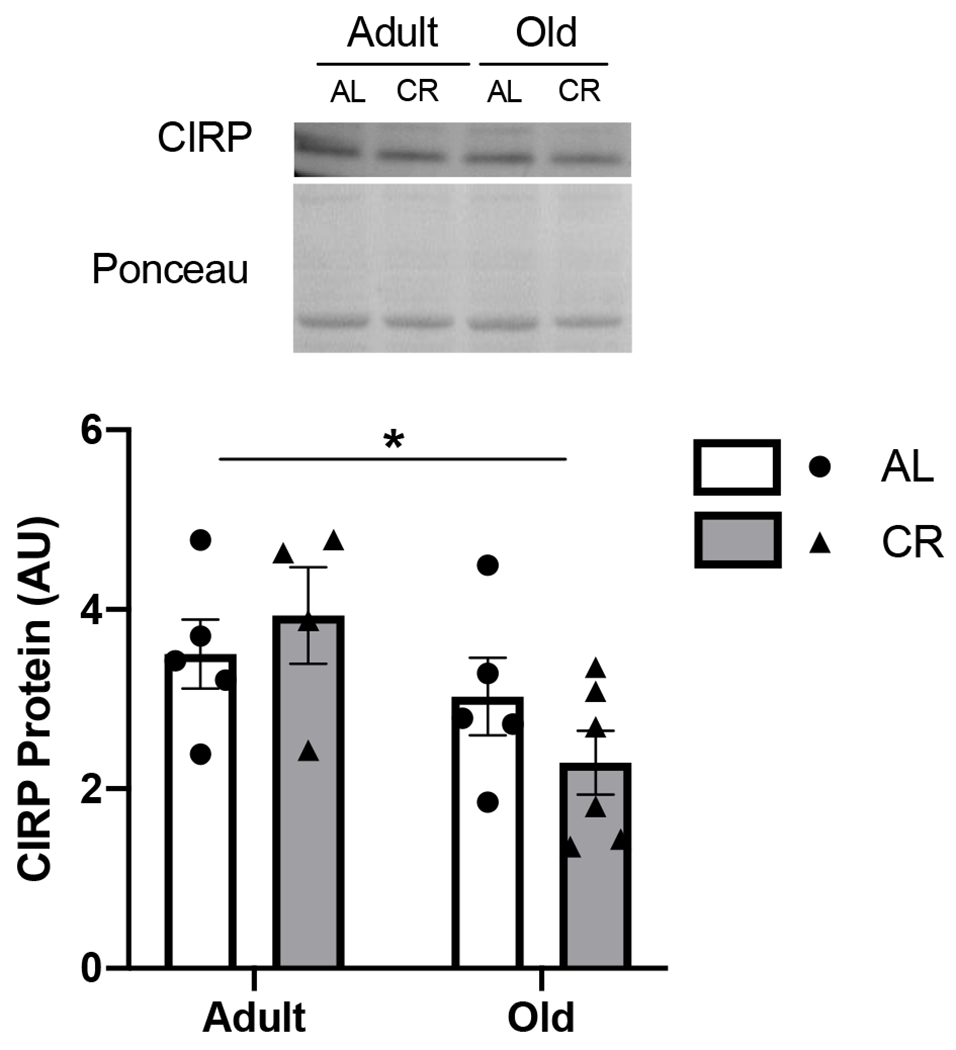
CIRP protein abundance from male mice at 12 (adult) and 22 (old) months of age fed either ad libitum (AL) or 40% calorie restricted (CR). Values presented as mean ± SEM; n=6 for all groups. *p<0.05 denotes main effect for age.
4. Discussion:
The main finding of the present study is that skeletal muscle RBM3 expression is elevated in df/df mice, and mice under CR conditions, two well-established murine models of extended lifespan. As both df/df and CR mice display long life, the results from this study suggest that RBM3 and one or more of its RNA processing functions may be associated with skeletal muscle health during extended lifespan. Moreover, as skeletal muscle health is a strong predictor of mortality in humans (20, 23), interventions that seek to maintain muscle health in old age may do so through RBM3 and its direct and/or indirect effect on muscle size regulation.
RNA-binding proteins are post-transcriptional regulators of RNA with significant influence on RNA metabolism. Indeed, it has been recently reported that ~8% of protein coding genes transcribe RNA-binding proteins involved in either the transport or processing of RNA (13). In skeletal muscle, we have previously reported that RBM3 induced muscle hypertrophy and prevented atrophy in vitro and in vivo (34). Since RBM3 is a cold shock protein which is highly elevated during hibernation – a state in which core body temperature is reduced and muscle mass is spared from the effects of metabolic stress – it became of interest to investigate its levels in muscles of animals with extended lifespan, since they also have lower body core temperatures (8). RNA-binding proteins are understudied in muscle, but a few studies point towards roles in mitochondrial biogenesis. For example, decreases in RNA-stabilizing human antigen R (HuR) and increases in destabilizing (AUF1) affect the turnover of mRNA transcripts necessary for mitochondrial biogenesis (17). Moreover, it was recently reported that RNA-binding proteins influence muscle aging via mitophagy; genetic knockdown of RNA-binding protein pumilo2 (PUM2) in c. elegans, nematodes and the muscle of elderly mice resulted in improved mitochondrial capacity and extended lifespan (6). By contrast, our results show a positive association of RNA-binding protein RBM3 in the skeletal muscle of extended lifespan, both in the df/df mice and following a CR diet, while there was an age-dependent decrease in CIRP expression in the skeletal muscle of CR animals. These results demonstrate the apparently divergent and yet specific roles that RNA-binding proteins play in RNA metabolism in aging muscle. Future studies are needed to define the mechanisms by which individual RBPs exert their actions and whether manipulation of RBPs in skeletal muscle such as RBM3 has an effect on life span.
RNA metabolism is a complex and central process in the regulation of cellular proteastasis and function, yet it is surprisingly understudied in skeletal muscle aging. Serving as the bridge between DNA and protein, the splicing, stabilization, degradation, transport and translation of messenger RNA dictates the protein pool available for numerous cellular processes. However, studies in which the proteomes and transcriptomes of cells or tissues are analyzed in parallel have highlighted the discrepancy between mRNA levels and protein abundance, suggesting highly regulated post-transcriptional mechanisms (38). In the context of aging, changes in RNA homeostasis, such as the balance between RNA degradation and stabilization, or in changes to alternative splicing (11, 19), may dramatically influence the protein pool and cellular processes, not only considering mRNA, but also of ribosomal RNA and small non-coding RNA. Moreover, previous reports have shown that skeletal muscle RNA oxidation associates with increasing age (15), perhaps contributing to altered RNA homeostasis. The present study corroborates previous findings by showing an age-related accumulation of DCP2 in mice under CR conditions. Indeed, our data show normalization of DCP2 expression in older animals fed a CR diet, suggesting the process of RNA degradation may be influenced by CR. The benefits of CR have long been thought to be mediated by metabolic changes (31). Interestingly, it has recently been shown that the metabolic changes seen under CR conditions are in part regulated at the RNA level, as Redman and colleagues show a preservation of transcripts important for metabolic reprogramming (29). In corroboration, recent reports have highlighted that other aspects of RNA metabolism play an important role in the youthful phenotype of df/df mice. Analysis of microRNA (miRNA) in serum of df/df mice identified miRNA profiles specific to advanced age df/df mice in this model (37), including those regulating tumor suppression, anti-inflammatory processes and MAPK signaling. These processes were differentially regulated in the old df/df mice compared to normal control littermates (37). Taken together, it appears that changes to RNA metabolism associates with the longevity advantages seen in both df/df mice and mice under CR conditions. Moreover, as RBM3 and other RBPs have been shown to exert their influence on RNA homeostasis, future studies would benefit from investigating the impact of RBPs in aging muscle.
5. Conclusion
In conclusion, the results from this study show that rodent models of extended lifespan demonstrate higher abundance of RBM3 protein in skeletal muscle which is specific for this cold shock RBP. The beneficial effects of RBM3 on neurons (neuroprotective (31)) and muscles (growth promoting (34)) lead us to speculate that higher levels of RBM3 in muscles from long-lived mouse models may play a role in preserved tissue homeostasis and lifespan, although exact mechanisms remain to be determined.
Supplementary Material
Acknowledgments
Funding from the Endowed Professor in Health Sciences for EDV. NIH/NIA funding for MM (R56AG061414, RO3AG059846). We would like to thank Drs. Susan Brooks and Richard Miller (University of Michigan) for the kind gift of gastrocnemius muscles from UM-HET3 mice through the Pepper Center’s Core Facility for Aged Rodents (P30-AG024824).
Funding
Funding for this study was received from NIH/NIA for MM (R56AG061414, R03AG059846, R15AG059190) and for the UM-HET mice to Dr. Richard Miller (P30-AG024824). In addition, funds were received from the Endowed Professor in Health Sciences at the University of Kentucky.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest
None.
Ethics Approval
All procedures and conditions for animal care were approved by the University of Michigan Committee on Use and Care of Animals and met standards for animal housing as described in the Animal Welfare Act, the Guide for Care and Use of Laboratory Animals, and the Guide for the Care and Use of Agricultural Animals in Agricultural Research and Teaching, along with the Laboratory Animal Care and Use Committee (LACUC) at the Southern Illinois University School of Medicine.
Availability of Data and Material
All data and information of materials will be made available upon reasonable request with the corresponding author.
References:
- 1.Baehr LM, West DW, Marcotte G, Marshall AG, De Sousa LG, Baar K, and Bodine SC. Age-related deficits in skeletal muscle recovery following disuse are associated with neuromuscular junction instability and ER stress, not impaired protein synthesis. Aging (Albany NY) 8: 127–146, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bartke A and Brown-Borg H. Life extension in the dwarf mouse. Curr Top Dev Biol 63: 189–225, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Boyer BB and Barnes BM. Molecular and Metabolic Aspects of Mammalian Hibernation: Expression of the hibernation phenotype results from the coordinated regulation of multiple physiological and molecular events during preparation for and entry into torpor. BioScience 49: 713–724, 1999. [Google Scholar]
- 4.Brown-Borg HM, Borg KE, Meliska CJ, and Bartke A. Dwarf mice and the ageing process. Nature 384: 33, 1996. [DOI] [PubMed] [Google Scholar]
- 5.Conti B, Sanchez-Alavez M, Winsky-Sommerer R, Morale MC, Lucero J, Brownell S, Fabre V, Huitron-Resendiz S, Henriksen S, Zorrilla EP, de Lecea L, and Bartfai T. Transgenic mice with a reduced core body temperature have an increased life span. Science 314: 825–828, 2006. [DOI] [PubMed] [Google Scholar]
- 6.D’Amico D, Mottis A, Potenza F, Sorrentino V, Li H, Romani M, Lemos V, Schoonjans K, Zamboni N, Knott G, Schneider BL, and Auwerx J. The RNA-Binding Protein PUM2 Impairs Mitochondrial Dynamics and Mitophagy During Aging. Mol Cell 73: 775–787.e710, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Danno S, Nishiyama H, Higashitsuji H, Yokoi H, Xue JH, Itoh K, Matsuda T, and Fujita J. Increased transcript level of RBM3, a member of the glycine-rich RNA-binding protein family, in human cells in response to cold stress. Biochem Biophys Res Commun 236: 804–807, 1997. [DOI] [PubMed] [Google Scholar]
- 8.Darcy J, McFadden S, Fang Y, Huber JA, Zhang C, Sun LY, and Bartke A. Brown Adipose Tissue Function Is Enhanced in Long-Lived, Male Ames Dwarf Mice. Endocrinology 157: 4744–4753, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dresios J, Aschrafi A, Owens GC, Vanderklish PW, Edelman GM, and Mauro VP. Cold stress-induced protein Rbm3 binds 60S ribosomal subunits, alters microRNA levels, and enhances global protein synthesis. Proc Natl Acad Sci U S A 102: 1865, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dupont-Versteegden EE, Nagarajan R, Beggs ML, Bearden ED, Simpson PM, and Peterson CA. Identification of cold-shock protein RBM3 as a possible regulator of skeletal muscle size through expression profiling. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology 295: R1263–R1273, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Faragher RGA and Ostler EL. Resveralogues: From Novel Ageing Mechanisms to New Therapies? Gerontology 66: 231–237, 2020. [DOI] [PubMed] [Google Scholar]
- 12.Fedorov VB, Goropashnaya AV, Toien O, Stewart NC, Chang C, Wang H, Yan J, Showe LC, Showe MK, and Barnes BM. Modulation of gene expression in heart and liver of hibernating black bears (Ursus americanus). BMC Genomics 12: 171, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerstberger S, Hafner M, and Tuschl T. A census of human RNA-binding proteins. Nat Rev Genet 15: 829–845, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hofer T, Marzetti E, Xu J, Seo AY, Gulec S, Knutson MD, Leeuwenburgh C, and Dupont-Versteegden EE. Increased iron content and RNA oxidative damage in skeletal muscle with aging and disuse atrophy. Exp Gerontol 43: 563–570, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunter WS, Croson WB, Bartke A, Gentry MV, and Meliska CJ. Low body temperature in long-lived Ames dwarf mice at rest and during stress. Physiol Behav 67: 433–437, 1999. [DOI] [PubMed] [Google Scholar]
- 16.Jansen HT, Trojahn S, Saxton MW, Quackenbush CR, Evans Hutzenbiler BD, Nelson OL, Cornejo OE, Robbins CT, and Kelley JL. Hibernation induces widespread transcriptional remodeling in metabolic tissues of the grizzly bear. Communications Biology 2: 336, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lai RYJ, Ljubicic V, D’Souza D, and Hood DA. Effect of chronic contractile activity on mRNA stability in skeletal muscle. Am J Physiol Cell Physiol 299: C155–C163, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lane MA, Baer DJ, Rumpler WV, Weindruch R, Ingram DK, Tilmont EM, Cutler RG, and Roth GS. Calorie restriction lowers body temperature in rhesus monkeys, consistent with a postulated anti-aging mechanism in rodents. Proc Natl Acad Sci U S A 93: 4159–4164, 1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Latorre E, Birar VC, Sheerin AN, Jeynes JCC, Hooper A, Dawe HR, Melzer D, Cox LS, Faragher RGA, Ostler EL, and Harries LW. Small molecule modulation of splicing factor expression is associated with rescue from cellular senescence. BMC Cell Biol 18: 31, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laukkanen P, Heikkinen E, and Kauppinen M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing 24: 468–473, 1995. [DOI] [PubMed] [Google Scholar]
- 21.Li W, Li X, and Miller RA. ATF4 activity: a common feature shared by many kinds of slow-aging mice. Aging Cell 13: 1012–1018, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Loeb J and Northrop JH. Is There a Temperature Coefficient for the Duration of Life? Proc Natl Acad Sci U S A 2: 456–457, 1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Metter EJ, Talbot LA, Schrager M, and Conwit R. Skeletal muscle strength as a predictor of all-cause mortality in healthy men. J Gerontol A Biol Sci Med Sci 57: B359–365, 2002. [DOI] [PubMed] [Google Scholar]
- 24.Miller BF, Hamilton KL, Majeed ZR, Abshire SM, Confides AL, Hayek AM, Hunt ER, Shipman P, Peelor FF 3rd, Butterfield TA, and Dupont-Versteegden EE. Enhanced skeletal muscle regrowth and remodelling in massaged and contralateral non-massaged hindlimb. J Physiol 596: 83–103, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nishiyama H, Itoh K, Kaneko Y, Kishishita M, Yoshida O, and Fujita J. A glycine-rich RNA-binding protein mediating cold-inducible suppression of mammalian cell growth. J Cell Biol 137: 899–908, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Phadtare S, Alsina J, and Inouye M. Cold-shock response and cold-shock proteins. Current Opinion in Microbiology 2: 175–180, 1999. [DOI] [PubMed] [Google Scholar]
- 27.Pilotte J, Cunningham BA, Edelman GM, and Vanderklish PW. Developmentally regulated expression of the cold-inducible RNA-binding motif protein 3 in euthermic rat brain. Brain Res 1258: 12–24, 2009. [DOI] [PubMed] [Google Scholar]
- 28.Pilotte J, Dupont-Versteegden EE, and Vanderklish PW. Widespread regulation of miRNA biogenesis at the Dicer step by the cold-inducible RNA-binding protein, RBM3. PLoS One 6: e28446, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Redman LM, Smith SR, Burton JH, Martin CK, Il’yasova D, and Ravussin E. Metabolic Slowing and Reduced Oxidative Damage with Sustained Caloric Restriction Support the Rate of Living and Oxidative Damage Theories of Aging. Cell Metab 27: 805–815.e804, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roth GS, Lane MA, Ingram DK, Mattison JA, Elahi D, Tobin JD, Muller D, and Metter EJ. Biomarkers of caloric restriction may predict longevity in humans. Science 297: 811, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Smart F, Aschrafi A, Atkins A, Owens GC, Pilotte J, Cunningham BA, and Vanderklish PW. Two isoforms of the cold-inducible mRNA-binding protein RBM3 localize to dendrites and promote translation. Journal of Neurochemistry 101: 1367–1379, 2007. [DOI] [PubMed] [Google Scholar]
- 32.Srere HK, Wang LC, and Martin SL. Central role for differential gene expression in mammalian hibernation. Proc Natl Acad Sci U S A 89: 7119–7123, 1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Dijk E, Cougot N, Meyer S, Babajko S, Wahle E, and Séraphin B. Human Dcp2: a catalytically active mRNA decapping enzyme located in specific cytoplasmic structures. Embo j 21: 6915–6924, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Van Pelt DW, Confides AL, Judge AR, Vanderklish PW, and Dupont-Versteegden EE. Cold shock protein RBM3 attenuates atrophy and induces hypertrophy in skeletal muscle. J Muscle Res Cell Motil 39: 35–40, 2018. [DOI] [PubMed] [Google Scholar]
- 35.Van Pelt DW, Hettinger ZR, and Vanderklish PW. RNA-binding proteins: The next step in translating skeletal muscle adaptations? J Appl Physiol (1985) 127: 654–660, 2019. [DOI] [PubMed] [Google Scholar]
- 36.Van Voorhies WA and Ward S. Genetic and environmental conditions that increase longevity in Caenorhabditis elegans decrease metabolic rate. Proc Natl Acad Sci U S A 96: 11399–11403, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Victoria B, Dhahbi JM, Nunez Lopez YO, Spinel L, Atamna H, Spindler SR, and Masternak MM. Circulating microRNA signature of genotype-by-age interactions in the long-lived Ames dwarf mouse. Aging cell 14: 1055–1066, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vogel C and Marcotte EM. Insights into the regulation of protein abundance from proteomic and transcriptomic analyses. Nat Rev Genet 13: 227–232, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Williams DR, Epperson LE, Li W, Hughes MA, Taylor R, Rogers J, Martin SL, Cossins AR, and Gracey AY. Seasonally hibernating phenotype assessed through transcript screening. Physiol Genomics 24: 13–22, 2005. [DOI] [PubMed] [Google Scholar]
- 40.Yan J, Burman A, Nichols C, Alila L, Showe LC, Showe MK, Boyer BB, Barnes BM, and Marr TG. Detection of differential gene expression in brown adipose tissue of hibernating arctic ground squirrels with mouse microarrays. Physiol Genomics 25: 346–353, 2006. [DOI] [PubMed] [Google Scholar]
- 41.Zhu X, Bührer C, and Wellmann S. Cold-inducible proteins CIRP and RBM3, a unique couple with activities far beyond the cold. Cellular and Molecular Life Sciences 73: 3839–3859, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


