Abstract
Background and Purpose:
The optimal endovascular stroke therapy (EVT) care delivery structure is unknown, as the treatment is highly time-sensitive but also less efficacious in lower volume centers. Here, we present our experience in creating an integrated stroke system (ISS) to expand EVT availability throughout our region while maintaining hospital and physician quality standards.
Methods:
We identified all consecutive patients with LVO AIS treated with EVT from Jan 2014 – Feb 2019 in our health care system. In Oct 2017 we implemented the ISS, in which 3 additional hospitals (4 total) became EVT performing hospitals (EPHs) and physicians were rotated between all centers. The cohort was divided by time into pre-ISS and post-ISS, and the primary outcome was time from stroke onset to EPH arrival. Secondary outcomes included hospital and procedural quality metrics. We performed an external validation using data from the SouthEast Texas Regional Advisory Council (SETRAC).
Results:
Among 513 patients with LVO AIS treated with EVT, 58% were treated pre-ISS and 43% post-ISS. Over the study period, EVT procedural volume increased overall but remained relatively low at the three new EPHs (<70 EVT/year). After ISS, the proportion of patients that underwent inter-hospital transfer decreased (46% vs. 37%, p<0.05). In adjusted quantile regression, ISS implementation resulted in a reduction of time from stroke onset to EPH arrival by 40 minutes (p<0.01) and onset to groin puncture by 29 min (p<0.05). Rates of post-procedural hemorrhage, TICI 2b/3 and 90d mRS were comparable at the higher and lower volume EPHs. The improvement in onset to arrival time was not reflective of overall improvement in secular trends in regional pre-hospital care.
Conclusions:
In our system, increasing EVT availability decreased time from stroke onset to EPH arrival. The ISS provides a framework to maintain quality in lower volume hospitals.
Keywords: stroke systems of care, pre-hospital care, endovascular treatment
Graphical Abstract
Introduction
Multiple randomized clinical trials have demonstrated dramatic improvements in clinical outcome for patients with large vessel occlusion (LVO) acute ischemic stroke (AIS) treated with endovascular stroke therapy (EVT) compared to medical management alone.1–5 In these studies, one of the key determinants of clinical outcome was the time from symptom onset to endovascular treatment.6 As a result, minimizing delays in treatment by restructuring stroke systems of care around hospital and physician EVT capability has gained considerable importance. The optimal structure for the distribution of EVT resources in any given region, however, remains unknown.
On the one hand, expanding EVT availability broadly, such that nearly all hospitals that receive AIS patients would be able to perform EVT could reduce treatment delays by eliminating inter-hospital transfer.7 Counterbalancing this benefit, however, is the finding that EVT outcomes are also tied to physician and hospital treatment volumes.8,9 Similar to findings from many other procedural outcome studies, greater annual treatments per site or physician are associated with improved outcomes. Thus, EVT availability may have to be weighed against diminishing local treatment volumes.
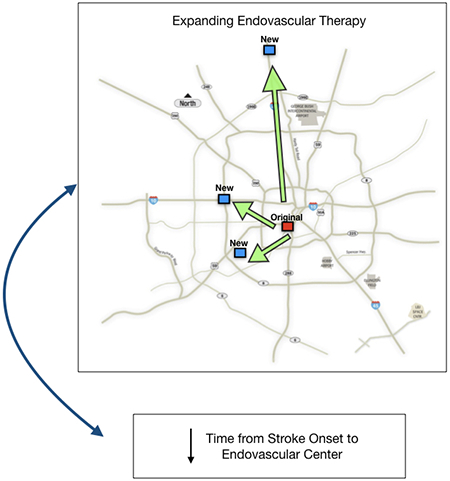
In this study, we describe our experience in creating an integrated stroke system (ISS), which sought to address the issue of access to EVT by substantially increasing the number of hospitals in our region that performed the procedure but maintained physician-level procedural competence by rotating practitioners across higher- and lower-volume sites and standardizing care protocols across all the sites. Three additional EVT-performing stroke centers were created, distributed across the city to match local population density. We hypothesized that implementation of the ISS and expansion of EVT availability in our region would reduce delays from stroke onset to treating-hospital arrival, while maintaining quality standards including reperfusion and complication rates.
Methods
Study Population
The data that support the findings of this study are available from the corresponding author on reasonable request. Our study population consisted of a subset of the PRIME study (Practical Implementation of Mechanical Thrombectomy). PRIME is a prospective observational cohort study examining all patients diagnosed with AIS or transient ischemic attack at 11 stroke centers within the same health system across the Greater Houston area. For this analysis, we identified a subset of this population that consisted of all consecutive patients with AIS and LVO who underwent EVT at any of our four EVT-performing hospitals (EPHs) between January 2014 and February 2019. Note that from January 2014 – September 2017 data were captured from a single center, and from October 2017 – February 2019 data were captured from 4 hospitals as explained below. Patients were excluded if they did not receive EVT for LVO AIS. During the study period, clinical trials were reported that supported the treatment of AIS LVO patients with EVT up to 24 hours from symptom onset.10,11 However, in order to maintain consistency of the cohort across the study period, only patients with AIS LVO who presented within six hours of symptom onset were included. A summary of the cohort can be found in the Supplemental Figure I.
In October 2017, we implemented the ISS model, described below. Patients treated from January 2014 – September 2017 comprised the pre-ISS cohort, and those treated from October 2017 – February 2019 comprised the post-ISS cohort. The EPH which existed prior to implementation of the ISS is defined as the “original EPH,” and the three additional ones that were added after implementation of ISS are defined as “new EPHs.”
Note that pre-hospital routing guidelines in the region that includes all four EPH are recommended by a single regional advisory council (which includes multiple EMS agencies) called the SouthEast Texas Regional Advisory Council (SETRAC). Because efforts were made before, during and after the study period to expedite the pre-hospital care of patients with AIS, we performed an external validation of our results using data from SETRAC. See further discussion below.
This study was reviewed and approved by the Committee for the Protection of Human Subjects at the University of Texas Health Science Center at Houston and waive of consent and HIPAA authorization was granted (IRB ID: HSC-MS-19-0367).
Study Design
Study data were abstracted from electronic medical chart review, de-identified, and entered using REDCap.12,13 NIHSS at the time of arrival was included for analysis. LVO was defined as an occlusion of the intracranial internal carotid artery (ICA), A1 or A2 segments of the anterior cerebral artery, M1 or M2 segments of the middle cerebral artery, P1 or P2 segments of the posterior cerebral artery, intracranial vertebral artery or basilar artery as determined by CT angiogram, MR angiogram or digital subtraction angiography.
The primary endpoint was time from symptom onset to EPH arrival, which was examined in pre-ISS vs. post-ISS cohorts and adjusted for age and NIHSS (see below). Note that for this analysis, only patients with witnessed stroke onset or known onset time were included, for accuracy of measurement. Secondary endpoints included onset-to-reperfusion time, and onset-to-groin puncture times, which were also evaluated in the subset of patients with known onset time. Additional endpoints included the rates of inter-hospital transfer, good functional outcome at 90 days (mRS 0-2), length of stay, and rates of substantial reperfusion (TICI 2b/3). These additional endpoints were measured in the entire cohort. Safety endpoints included post-procedural hemorrhage rates defined using ECASS criteria.
Integrated Stroke System
In October 2017, we implemented the ISS model, in which three additional hospitals simultaneously became EPHs, and clinical practices across all four hospitals were standardized. The purpose of this new system was to extend high-quality EVT care throughout the Houston area and closer to the community (S. Figure II). Under this model, a single, multidisciplinary physician group was created, including all NeuroInterventionalists, Neurologists, Telemedicine specialists, NeuroCritical Care specialists, and Neurosurgeons across the 4 EPHs. These physicians rotated across these 4 campuses (at least 2 campuses per physician), to both ensure consistency in care, as well as competency and exposure to the full range of cerebrovascular disease. All NeuroInterventional physicians were certified by the Committee for Advanced Subspecialty Training in NeuroEndovascular Surgery.14 NeuroIntensive care staff, procedural technologists, and nursing staffs were substantially expanded at each of the new EPHs. In addition, unified nursing protocols, order sets, and centralized Quality Improvement data collection were implemented to maintain uniformity of care at these settings. Of note, prior to October 2017, the hospitals that were to become EVT-performing centers had performed Neuroendovascular procedures including diagnostic cerebral angiography and interventional procedures including carotid stenting and cerebral aneurysm treatments, in low volumes. All four hospitals had dedicated neuro-angiography biplane units with dedicated neuro-angiography technologists and nurses.
Description of the four hospitals in the ISS
In October 2017, pre-hospital Emergency Medical Service agencies were notified that the three new EPHs were “seeking comprehensive stroke center status,” which allowed them to receive severe stroke patients directly. The three new EPHs attained certification during the study period in July 2018 and maintained this certification throughout the study period.
The Acute Stroke evaluation pathway at each center was standardized as much as possible and consisted of an alert from the Emergency Room to the on-call Neurologist, who then evaluated the patient emergently either in-person or through telemedicine, followed by brain imaging with decision for thrombolysis. In parallel, the on-call Neurologist would then determine whether to call the on-call NeuroInterventionalist to evaluate the patient for thrombectomy, which would be performed on-site. Inter-hospital transfer was attempted to be minimized, through training with pre-hospital emergency medical service crews; when it was required, each EPH acted as a regional hub, and patients were transferred to the closest center.
Comparison against regional outcomes
In order to assess for region-wide improvements in pre-hospital stroke care as a potential confounder to our primary outcome, we analyzed data from SETRAC, capturing data from all stroke centers in the two counties in which the four EPHs were located (Harris and Montgomery counties). SETRAC collects data on all AIS patients from each hospital in the region and performs its own adjudication of quality outcomes.15 This data collection mechanism is independent of the PRIME dataset used to conduct the primary analysis. This agency also publishes recommended routing protocols for AIS for all pre-hospital emergency medical services in its region. During the study period, there were no significant changes to the pre-hospital routing guidelines, which recommended transport of patients with suspected LVO stroke to the nearest comprehensive stroke center provided it did not result in a greater than 15-minute delay compared to the nearest primary stroke center. Four different LVO assessment tools were allowed to be used to identify LVO, including Rapid Arterial Occlusion Evaluation (RACE) score ≥5, Los Angeles Motor Scale (LAMS) score ≥4, Cincinnati Prehospital Stroke Severity Scale (CSTAT) score ≥ 2 or Vision, Aphasia, Neglect (VAN) Weakness +.16–19
For the purposes of this study, data were collected from April 2014 – February 2019. Time from onset to hospital arrival for all AIS patients across the two counties over the study period was assessed, as well as the relative proportion of these patients treated at one of the four study EPHs.
Statistical Analysis
Patient characteristics and outcomes were compared before and after the ISS implementation. The effect of ISS implementation on time from onset to treating hospital arrival was determined by quantile regression (as the outcome variable is likely non-normal) and adjusted for patient age and NIHSS. Quantile regression adjusted for age and NIHSS was also used to determine changes in time from onset to groin puncture, onset to reperfusion, hospital arrival to groin puncture, and hospital arrival to reperfusion. Fisher’s exact and Mann-Whitney U tests were used to compare categorical and continuous variables, respectively. Good functional outcome was defined as a modified Rankin Scale (mRS) score 0-2 at 90-day follow-up. Statistical analyses were performed with Stata 14 (StataCorp) LLC, College Station, TX) and Prism 7 (GraphPad, La Jolla, CA) software. All p-values were 2-sided and considered statistically significant at P < 0.05. Data are presented as median [IQR] unless otherwise indicated.
Results
Among 513 patients with LVO AIS and undergoing EVT between January 2014 and February 2019, median age was 66 years [56-76 years], 44% were female, 41% were White, and 17% were Hispanic. Across the entire cohort, 74% had hypertension, 41% had hyperlipidemia, and 27% had atrial fibrillation. 58% arrived directly to the ER, and the remaining presented as a transfer from an outside hospital. 295 (58%) patients underwent EVT prior to implementation of the ISS and 218 (42%) after. Patients treated with EVT during the pre-ISS period were younger (median age, 64 vs 69, pre-ISS vs post-ISS, p<0.001) and were more often treated with IV tPA (80% vs 65%, pre-ISS vs post-ISS, p<0.0001). Demographics and clinical characteristics of patients are detailed in Table 1.
Table 1.
Baseline characteristics of EVT patients before and after implementation of the ISS.
| Pre-ISS N=295 |
Post-ISS N=218 |
p-value | ||
|---|---|---|---|---|
| Age, median [IQR] | 64 [53-75] | 69 [59-79] | <0.001 | |
| Female, % | 40% | 49% | 0.06 | |
| Past Medical History | ||||
| Prior Stroke | 16% | 10% | 0.06 | |
| Atrial Fibrillation | 26% | 29% | 0.54 | |
| Coronary Artery Disease/Myocardial Disease | 16% | 16% | 1.00 | |
| Diabetes Mellitus | 28% | 33% | 0.32 | |
| Hyperlipidemia | 43% | 39% | 0.40 | |
| Hypertension | 76% | 70% | 0.12 | |
| Smoking | 14% | 18% | 0.25 | |
| Transferred, % | 46% | 37% | <0.05 | |
| NIH Stroke Scale, median [IQR] | 18 [13-22] | 17 [12-22] | 0.18 | |
| Pre-Stroke Modified Rankin Scale Score 0-2, % | 93% | 90% | 0.26 | |
| NCHCT ASPECTS, median [IQR] | 9 [7-10] | 8 [7-9] | <0.05 | |
| Occlusion location | 0.72 | |||
| ICA | 19% | 17% | ||
| M1 | 52% | 56% | ||
| M2 | 17% | 18% | ||
| ACA | 1% | 1% | ||
| Intracranial Vertebral | 1% | 1% | ||
| Basilar | 8% | 6% | ||
| PCA | 2% | 1% | ||
| CTP Performed, % | 64% | 53% | <0.05 | |
| CTP RAPID core volume, median [IQR] | 7 [0-31] | 7 [0-26] | 0.39 | |
| CTP RAPID penumbral volume, median [IQR] | 117 [82-164] | 118 [81-147] | 0.71 | |
| Receipt of IV t-PA, % | 80% | 65% | <0.0001 | |
The proportion of EVT patients who were transferred (as opposed to direct presentation to ER) was lower after ISS implementation (46% vs 37%, pre-ISS vs post-ISS, p<0.05) and decreased over the course of the study (45% vs 32%, 2014 vs 2019, p=0.05) (Figure 1a). EVT treatments increased over the study period at the individual hospital-level and across all 4 EPHs (Figure 1b). Annual EVT volume at the new EPHs remained lower relative to the original EPH. On review of the transferred patients, all transferred occurred for evaluation for EVT.
Figure 1. Interhospital transfer and procedural volumes.
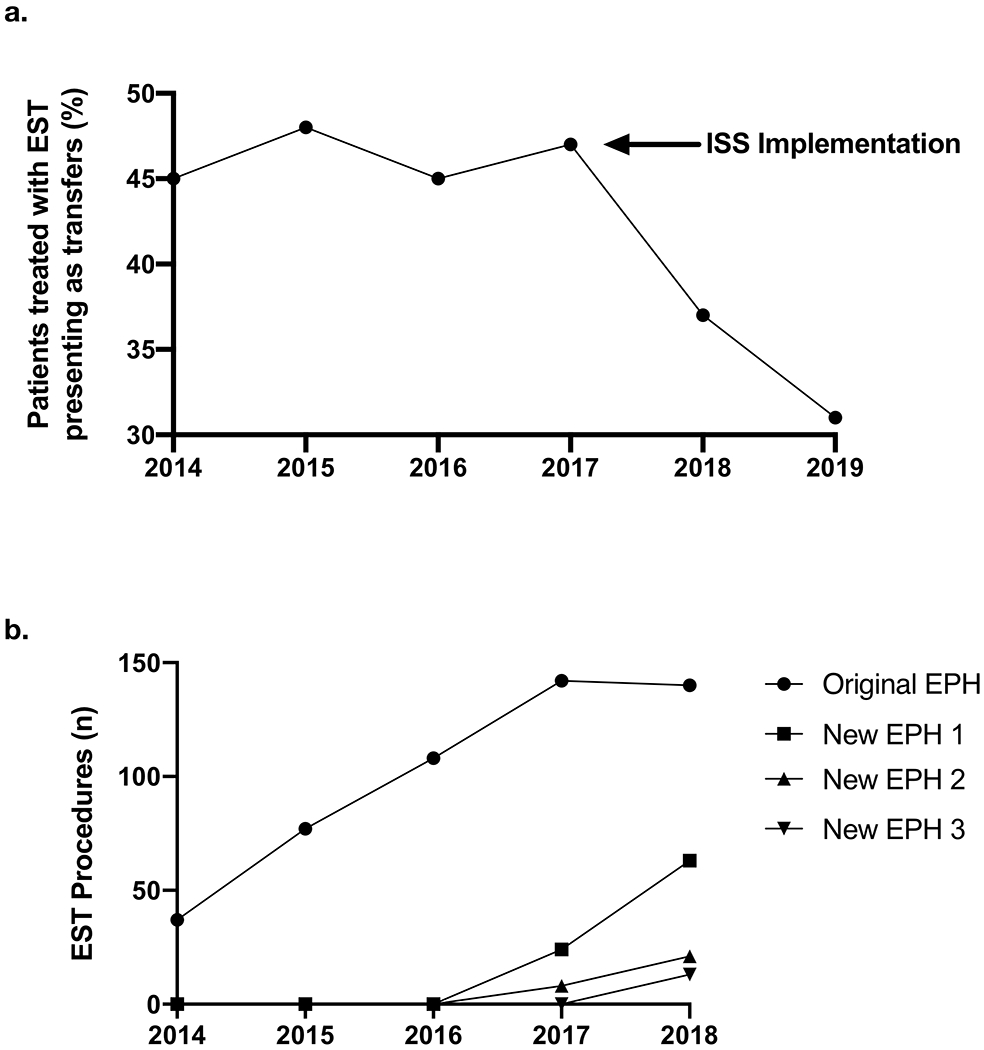
(a) Percentage of patients treated with EVT presenting as transfers over time, and (b) number of EVT procedures performed per EPH over time.
Among patients with known stroke onset time (n=352), we observed a decline in time from stroke onset to EPH arrival following implementation of ISS (Figure 2A). Unadjusted analysis demonstrated shorter times from hospital arrival to recanalization (153 vs. 129 minutes, pre-ISS vs. post-ISS, p<0.001) and from stroke onset to reperfusion (229 vs. 202 minutes, pre-ISS to post-ISS, p<0.05) after ISS implementation (Table 2).
Figure 2. Stroke onset to EPH arrival times.
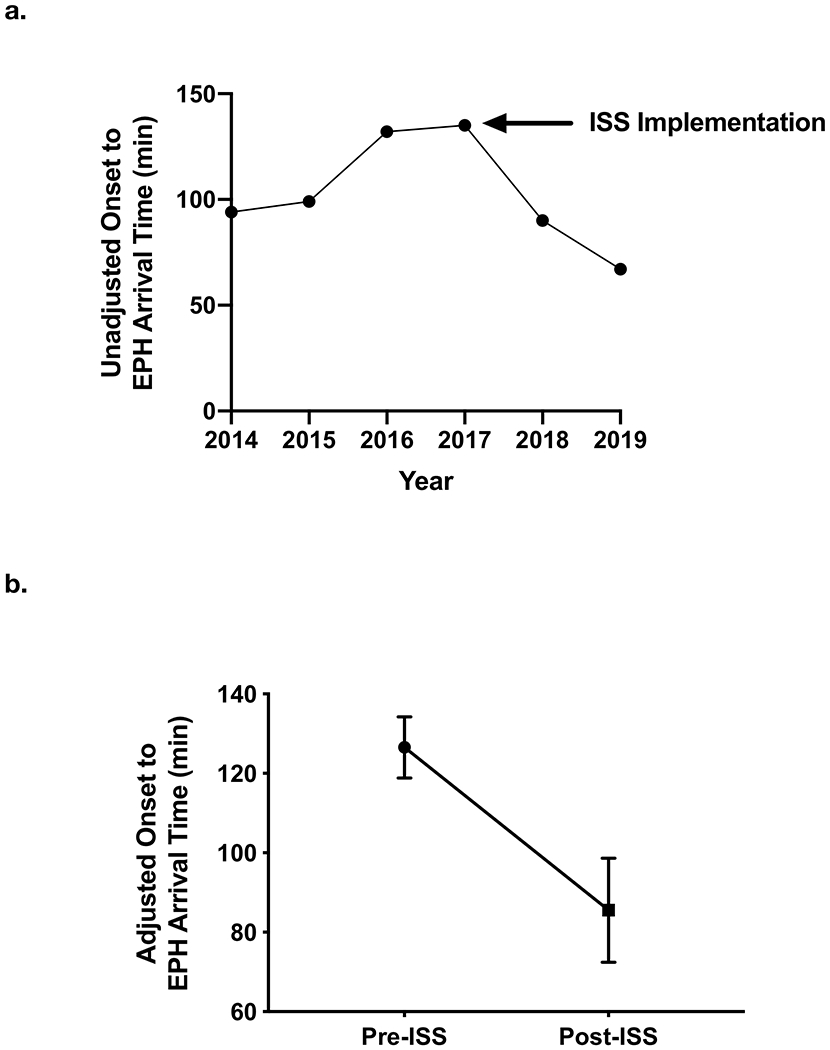
(a) Unadjusted times from stroke onset to EPH arrival and (b) adjusted onset to arrival time differences between pre-ISS and post-ISS cohorts (p<0.01, quantile regression).
Table 2.
Procedural and outcome metrics before and after implementation of the ISS
| Pre-ISS N=295 |
Post-ISS N=218 |
p-value | |
|---|---|---|---|
| Time from Door to Groin Puncture, mins, median [IQR] | 111 [85-132] | 89 [63-115] | <0.0001 |
| Time from Door to Recanalization, mins, median [IQR] | 153 [125-186] | 129 [99-166] | <0.0001 |
| Time from Onset to Hospital Arrival, mins, median [IQR] | 125 [70-180] | 89 [50-220] | 0.11 |
| Time from Onset to Groin Puncture, mins, median [IQR] | 229 [182-295] | 202 [149-307] | <0.05 |
| Time from Onset to Recanalization, mins, median [IQR] | 273 [225-339] | 251 [190-354] | 0.08 |
| Successful reperfusion (mTICI 2b/3), % | 87% | 90% | 0.33 |
| PH-2 ICH | 7% | 7% | 1.0 |
| Length of Stay, days, median [IQR] | 6 [4-10] | 5 [3-9] | 0.07 |
| 90d mRS 0-2, % (N=462) | 34% | 37% | 0.49 |
In adjusted quantile regression, implementation of ISS and expansion of EVT capability resulted in a reduction of time from stroke onset to hospital arrival (Figure 2B) by 40 minutes [95% CI 16-65, p<0.01]. Similarly, the time from onset to groin puncture was reduced by 29 minutes [95% CI 1-58, p<0.05]. The time from hospital arrival to groin puncture was reduced by 25 minutes [95% CI 17-33, p<0.001]. Further, the time from hospital arrival to reperfusion was reduced by 24 minutes [95% CI 13-36, p<0.001]. The time from stroke onset to reperfusion was decreased by 29 minutes [95% CI 6-65, p=0.15] but did not achieve statistical significance.
Across the entire cohort, clinical outcomes and procedural outcomes including rates of good 90-day functional outcome, successful reperfusion and post-procedure hemorrhage remained comparable after ISS implementation (Table 2). Individually, quality metrics and outcomes were maintained at each of the lower volume new EPHs (S. Table I). Patient demographics were largely unchanged post-ISS in the EPHs (S. Table II). A summary of endovascular devices used pre- and post-ISS is provided in S. Table III.
After implementation of the ISS, 87 (40%) EVT procedures were performed at new EPHs and 131 (60%) at the original EPH. In-hospital quality metrics including door-to-groin puncture times and door-to-recanalization times were comparable across the new EPHs and original EPH. Procedural metrics and clinical outcomes were also comparable as shown in Table 3. TICI 2b/3 rates were greater at the new EPHs. The increase in EVT procedures performed at the new EPHs occurred as a result of an increase in the overall number of patients evaluated for AIS, without a decrement in the EVT volume at the original EPH (S. Figure III A and B).
Table 3.
Procedural and outcome metrics between the original EPH and new EPHs after implementation of the ISS
| Original EPH N=131 |
New EPHs N=87 |
p-value | |
|---|---|---|---|
| Time from Door to Groin Puncture, mins, median [IQR] | 86 [67-105] | 91 [53-140] | 0.43 |
| Time from Door to Recanalization, mins, median [IQR] | 129 [105-160] | 126 [88-172] | 0.73 |
| Time from Onset to Hospital Arrival, mins, median [IQR] | 84 [45-243] | 89.5 [54-186] | 0.96 |
| Time from Onset to Groin Puncture, mins, median [IQR] | 191 [140-303] | 232 [167-329] | 0.13 |
| Time from Onset to Recanalization, mins, median [IQR] | 246 [183-379] | 275 [192-350] | 0.75 |
| Successful reperfusion (mTICI 2b/3), % | 86% | 97% | <0.05 |
| PH-2 ICH | 9% | 4% | 0.66 |
| Length of Stay, days, median [IQR] | 5 [3-8] | 5 [3-10] | 0.47 |
| 90d mRS 0-2, % (N=195) | 39% | 35% | 0.65 |
Using data from the SETRAC, over the course of the study period, approximately 25% of the AIS patients treated in the two counties containing the 4 study hospitals were treated at these centers (Figure 3a), a proportion that did not change significantly over time. Approximately 50% of the EVT procedures in the region were performed at one of the study’s EPHs (Figure 3b). Across the region, there was a decrease in the rate of presentation to stroke centers within 3.5 hours of onset after stroke (Figure 3c) (16% vs 12%, 2014 vs 2019; p<0.05) over the study period.
Figure 3. SETRAC regional data.
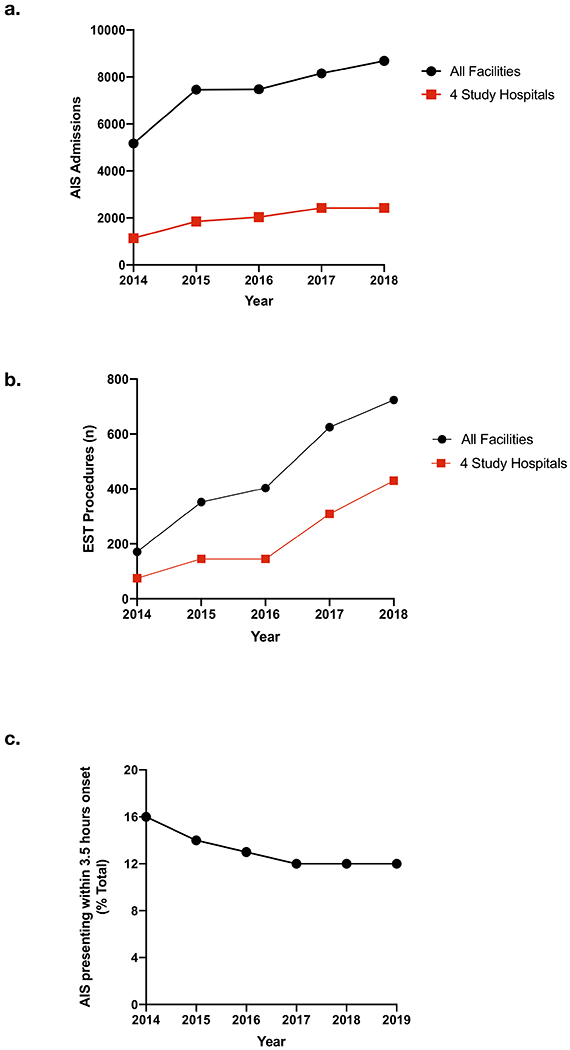
Total AIS admissions (a) and EVT procedures (b) for all facilities within the two-county region as well as the 4 study hospitals. (c) Percentage of all AIS presenting to a stroke center in the two-county region within 3.5 hours from onset over time.
Discussion
In this observational cohort study of patients who underwent EVT for LVO AIS in the early time window, we identified a significant decrease in inter-hospital transfer following the creation of 3 additional EPHs scattered throughout the region. In age- and stroke severity- adjusted comparisons, delays between stroke onset and EVT-performing hospital arrival decreased by 40 minutes, and the delay between hospital arrival and reperfusion also fell. Clinical outcomes were preserved at the lower volume new EPHs, and procedural complications did not increase despite the simultaneous establishment of 3 lower volume EVT-performing centers. Taken together, these findings suggest that an ISS, in which physician coverage and expertise is shared across new and established hospitals, can expand the availability of EVT while maintaining competency and procedural outcomes.
Despite initiating EVT programs simultaneously at three lower volume hospitals, we did not observe an increase in procedural complications. Rates of post-procedural hemorrhage were consistent with prior studies.20,21 Of note, IV tPA usage decreased in the post-ISS cohort. The reason for this change is not clear and may have been related to the slightly increased ischemic core size in the post-ISS cohort. On the other hand, 90-day disability outcomes were preserved post-ISS despite this cohort being older, harboring poorer ASPECTS, and receiving less IV tPA.
Multiple prior studies have observed a decrease in good clinical outcomes in centers with lower EVT volumes.8,9 In our study, the three new EPHs had relatively low annual EVT volumes but clinical outcomes did not differ when compared against the higher volume original EPH. In addition, we did not observe a decrement in care quality outcomes including door-to-groin puncture times and ICH rates. These findings are likely due to the standardization of care across all the campuses, including rotation of physicians, which helped ensure consistency and maintained procedural competency.
With the establishment of three additional EPHs throughout the city, we found that the rate of inter-hospital transfer dropped significantly. Transfers have been shown in several studies from well-performing stroke systems to be associated with not only significant delays in treatment, but also quantifiable deterioration in likelihood of good clinical outcomes.22,23 In our study, this decrease in transfer rates may have been the principal driver behind the reduction in onset to EVT-performing hospital arrival times. While transfer rates decreased over the course of the study, they remained >30%. This number should ideally continue to fall, substantially. Further expansion of EVT capabilities, to areas from which these patients were transferred, in addition to improved pre-hospital protocols to triage likely LVO patients to EVT-performing centers even if they are at a greater distance than primary centers continue to be needed. In addition, more direct pre-hospital evaluation through mobile stroke units has been shown to significantly reduce treatment times.24
The optimal distribution of EVT resources ultimately is likely also driven in large part by local geography. Our setting, a large urban area with multiple hospitals capable of performing at the EPH level, is not representative of every locale. However, our finding that an ISS in which protocols, best practices, and physicians are shared across larger and smaller volume hospitals, can be extended to larger areas, as it does not require immediate proximity to accomplish.
Our study has limitations. We limited our analysis to early time window patients to maintain consistency across the cohort. As such, it is unclear how our findings would affect patients presenting in the late time window, who may have different physiology than patients in the early time window. In addition, our primary outcome focused on patients with known onset of stroke, in order to more accurately measure differences in onset-to-arrival times. Doing so may have skewed the cohort towards more severe stroke; on the other hand, our analysis was adjusted for age and stroke severity. Further, over the course of the study period, in-hospital quality improvement efforts were ongoing, which may have contributed to the decline in in-hospital time metrics such as door-to-groin puncture time. However, these efforts would not have had any effect on our primary outcome, which was a pre-hospital measure. Then, because of the structure of care in our region, we cannot claim that population-level stroke care was improved by the ISS intervention. Also, to address the issue of regional secular trends, we obtained data from SETRAC, the advisory council that partners with all the pre-hospital emergency medical services agencies in the region. In these data, we found that the stroke onset to hospital presentation times, over the course of the study period, did not improve but in fact slightly worsened. As a result, we felt that our primary result, which was a reduction in time from stroke onset to hospital presentation time, was not the result of a secular trend. Finally, ideally EMS contact to arrival and/or treatment would be a key metric to study. These data, however, are not recorded by SETRAC nor available through the PRIME or institutional databases.
Conclusions
In this study of patients with AIS LVO treated with EVT, we found that increasing the number of hospitals performing EVT was associated with a decrease in onset-to-hospital arrival times. By sharing best practices and physician expertise across all four hospitals in an ISS, clinical outcomes and quality metrics were maintained in the lower volume hospitals. These findings support expansion of EVT-capabilities to hospitals closer to patient populations and provide a framework to maintain care quality.
Supplementary Material
Acknowledgments
Sources of Funding
This study was funded in part by the American Academy of Neurology Interventional Neurology Career Development award (PI: Dr Sheth). Drs. Blackburn and Kim report funding from the National Institutes of Health (NIH).
Conflict(s)-of-Interest/Disclosure(s)
Drs. Sheth reports consultancy fees from Penumbra and Cerenovus outside of the submitted work. Dr. Chen reports grants from Stryker and the NIH outside the submitted work. Dr. McCullough reports grants from NIH and grants from the American Heart Association outside the submitted work. Drs. Lopez-Rivera, Savitz, Czap, Alderazi, Spiegel, Blackburn, Dannenbaum, Wu, McCullough, Cochran, Jones, Kim, Day and Ms. Abdelkhaleq report no relevant disclosures.
Non-standard Abbreviations
- LVO
Large Vessel Occlusion
- EVT
Endovascular Stroke Therapy
- AIS
Acute Ischemic Stroke
- ISS
Integrated Stroke System
- EPH
EVT-performing Hospital
- SETRAC
SouthEast Texas Regional Advisory Council
References
- 1.Berkhemer OA, Fransen PSS, Beumer D, van den Berg LA, Lingsma HF, Yoo AJ, Schonewille WJ, Vos JA, Nederkoorn PJ, Wermer MJH, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N. Engl. J. Med. 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Saver JL, Goyal M, Bonafe A, Diener H-C, Levy EI, Pereira VM, Albers GW, Cognard C, Cohen DJ, Hacke W, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N. Engl. J. Med. 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 3.Jovin TG, Chamorro A, Cobo E, de Miquel MA, Molina CA, Rovira A, San Román L, Serena J, Abilleira S, Ribo M, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N. Engl. J. Med. 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 4.Campbell BCV, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, Yassi N, Yan B, Dowling RJ, Parsons MW, Oxley TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N. Engl. J. Med. 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 5.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, Roy D, Jovin TG, Willinsky RA, Sapkota BL, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N. Engl. J. Med. 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, van der Lugt A, Menon BK, Majoie CBLM, Dippel DW, Campbell BC, Nogueira RG, Demchuk AM, Tomasello A, et al. Time to Treatment With Endovascular Thrombectomy and Outcomes From Ischemic Stroke: A Meta-analysis. JAMA. 2016;316:1279–1288. [DOI] [PubMed] [Google Scholar]
- 7.Grotta JC, Lyden P, Brott T. Rethinking Training and Distribution of Vascular Neurology Interventionists in the Era of Thrombectomy. Stroke; a journal of cerebral circulation. 2017;48:2313–2317. [DOI] [PubMed] [Google Scholar]
- 8.Saber H, Navi BB, Grotta JC, Kamel H, Bambhroliya A, Vahidy FS, Chen PR, Blackburn S, Savitz SI, McCullough L, et al. Real-World Treatment Trends in Endovascular Stroke Therapy. Stroke; a journal of cerebral circulation. 2019;50:683–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim BM, Baek J-H, Heo J- H, Kim DJ, Nam HS, Kim YD. Effect of Cumulative Case Volume on Procedural and Clinical Outcomes in Endovascular Thrombectomy. Stroke; a journal of cerebral circulation. 2019;50:1178–1183. [DOI] [PubMed] [Google Scholar]
- 10.Nogueira RG, Jadhav AP, Haussen DC, Bonafe A, Budzik RF, Bhuva P, Yavagal DR, Ribo M, Cognard C, Hanel RA, et al. Thrombectomy 6 to 24 Hours after Stroke with a Mismatch between Deficit and Infarct. N. Engl. J. Med. 2018;378:11–21. [DOI] [PubMed] [Google Scholar]
- 11.Albers GW, Marks MP, Kemp S, Christensen S, Tsai JP, Ortega-Gutierrez S, McTaggart RA, Torbey MT, Kim-Tenser M, Leslie-Mazwi T, et al. Thrombectomy for Stroke at 6 to 16 Hours with Selection by Perfusion Imaging. N. Engl. J. Med. 2018;378:708–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Minor BL, Elliott V, Fernandez M, O’Neal L, McLeod L, Delacqua G, Delacqua F, Kirby J, et al. The REDCap consortium: Building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Day AL, Siddiqui AH, Meyers PM, Jovin TG, Derdeyn CP, Hoh BL, Riina H, Linfante I, Zaidat O, Turk A, et al. Training Standards in Neuroendovascular Surgery: Program Accreditation and Practitioner Certification. Stroke; a journal of cerebral circulation. 2017;48:2318–2325. [DOI] [PubMed] [Google Scholar]
- 15.Damani RH, Anand S, Asgarisabet P, Bissell C, Savitz S, Suarez JI. Regional Intervention of Stroke Care to Increase Thrombolytic Therapy for Acute Ischemic Stroke. Stroke; a journal of cerebral circulation. 2018;49:2008–2010. [DOI] [PubMed] [Google Scholar]
- 16.Perez de la Ossa N, Carrera D, Gorchs M, Querol M, Millán M, Gomis M, Dorado L, López-Cancio E, Hernandez-Pérez M, Chicharro V, et al. Design and validation of a prehospital stroke scale to predict large arterial occlusion: the rapid arterial occlusion evaluation scale. Stroke; a journal of cerebral circulation. 2014;45:87–91. [DOI] [PubMed] [Google Scholar]
- 17.Llanes JN, Kidwell CS, Starkman S, Leary MC, Eckstein M, Saver JL. The Los Angeles Motor Scale (LAMS): a new measure to characterize stroke severity in the field. Prehosp Emerg Care. 2004;8:46–50. [DOI] [PubMed] [Google Scholar]
- 18.McMullan JT, Katz B, Broderick J, Schmit P, Sucharew H, Adeoye O. Prospective Prehospital Evaluation of the Cincinnati Stroke Triage Assessment Tool. Prehosp Emerg Care. 2017;21:481–488. [DOI] [PubMed] [Google Scholar]
- 19.Teleb MS, Ver Hage A, Carter J, Jayaraman MV, McTaggart RA. Stroke vision, aphasia, neglect (VAN) assessment-a novel emergent large vessel occlusion screening tool: pilot study and comparison with current clinical severity indices. Journal of NeuroInterventional Surgery. 2017;9:122–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mueller-Kronast NH, Zaidat OO, Froehler MT, Jahan R, Aziz-Sultan MA, Klucznik RP, Saver JL, Hellinger FR, Yavagal DR, Yao TL, et al. Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke: Primary Results of the STRATIS Registry. Stroke; a journal of cerebral circulation. 2017;48:2760–2768. [DOI] [PubMed] [Google Scholar]
- 21.Campbell BCV, Hill MD, Rubiera M, Menon BK, Demchuk A, Donnan GA, Roy D, Thornton J, Dorado L, Bonafe A, et al. Safety and Efficacy of Solitaire Stent Thrombectomy: Individual Patient Data Meta-Analysis of Randomized Trials. Stroke; a journal of cerebral circulation. 2016;47:798–806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Froehler MT, Saver JL, Zaidat OO, Jahan R, Aziz-Sultan MA, Klucznik RP, Haussen DC, Hellinger FR, Yavagal DR, Yao TL, et al. Interhospital Transfer Before Thrombectomy Is Associated With Delayed Treatment and Worse Outcome in the STRATIS Registry (Systematic Evaluation of Patients Treated With Neurothrombectomy Devices for Acute Ischemic Stroke). Circulation. 2017;136:2311–2321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mokin M, Gupta R, Guerrero WR, Rose DZ, Burgin WS, Sivakanthan S. ASPECTS decay during inter-facility transfer in patients with large vessel occlusion strokes. Journal of NeuroInterventional Surgery. 2017;9:442–444. [DOI] [PubMed] [Google Scholar]
- 24.Czap AL, Grotta JC, Parker SA, Yamal J- M, Bowry R, Sheth SA, Rajan SS, Hwang H, Singh N, Bratina P, et al. Emergency Department Door-to-Puncture Time Since 2014. Stroke; a journal of cerebral circulation. 2019;50:1774–1780. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


