Abstract
Objective:
To determine the incidence and progression of ankle osteoarthritis (OA) and associated risk factors in a community-based cohort of African Americans and whites. Methods: Data were from 541 participants who had standardized lateral and mortise radiography of the ankles in weight bearing at baseline (2013–2015) and follow-up (2017–2018). Incident radiographic ankle OA (rAOA) was defined as a Kellgren-Lawrence grade (KLG) ≥1 at follow-up among ankles with baseline KLG<1; progressive rAOA was a ≥1 KLG increase at follow-up among ankles with KLG≥1 at baseline. Symptoms were assessed using self-reported pain, aching, and stiffness (PAS) on most days and the Foot and Ankle Outcome Score (FAOS) symptoms subscale. Ankle-level logistic regression models were used to assess associations of ankle outcomes with covariates (age, sex, race, body mass index [BMI], smoking, number of symptomatic joints, comorbidities, prior ankle injury, and knee or foot OA).
Results:
Among ankles without rAOA at baseline, 28% developed incident rAOA, 37% had worsening FAOS symptoms, and 7% had worsening PAS. Incident rAOA and worsening ankle symptoms were associated with higher BMI and symptoms in other joints. Among ankles with baseline rAOA, 4% had progressive rAOA, 35% had worsening of FAOS symptoms, and 9% had worsening PAS. rAOA progression was associated with ankle injury and concomitant knee or foot OA; worsening of symptoms was associated with higher BMI and other symptomatic joints.
Conclusions:
Not all ankle OA is post-traumatic. Smoking prevention/cessation, a healthy weight, and injury prevention may be methods for reducing the incidence and progression of rAOA.
Keywords: Ankle, osteoarthritis, epidemiology, radiography, symptoms
Introduction
Osteoarthritis (OA) is a debilitating chronic disease which is associated with pain, dysfunction and is a leading cause of disability (1). Ankle OA in particular has been estimated to affect approximately 1% of the population (2), although a systematic review in 2018 highlights the lack of high quality epidemiology to support this figure (3). The mental and physical disability associated with end-stage ankle disease is at least as severe as that associated with end-stage hip arthrosis and hence there is a high burden of disease (4). However, in comparison to the relatively large number of studies focused on hip and knee OA, research related to ankle OA is quite limited. Additionally, most studies of ankle OA are of clinical populations with end stage ankle disease that is mainly post traumatic and not reflective of the burden in the general population (2, 5). For example, a study of 79 patients presenting to University of Iowa Hospitals and Clinics' Orthopedic department with a primary diagnosis of ankle OA (2001 to 2003) found that 79% had a history of trauma (6). Another study of 639 patients who presented to a tertiary care center between 1991 and 2004 with evidence of moderate to severe ankle arthritis found that only 46 (7%) of these patients had primary ankle OA (7).
In part due to the perception that the majority of ankle OA is post-traumatic, there has been minimal investigation into other possible contributors to ankle OA, and there is a lack of community-based epidemiological studies. Our prior work reported on the cross-sectional prevalence of radiographic ankle OA (rAOA) in the Johnston County OA Project (JoCo OA) (8). In the current analysis, we include longitudinal data to allow assessment of the incidence and progression of ankle rAOA and pertinent risk factors over a mean of 3.5 years in this large community-based cohort.
Participants and Methods
The JoCo OA is a prospective, longitudinal cohort study in African American and white men and women aged at least 45 years, who were residents of 1 of 6 Johnston County townships for at least 1 year and capable of completing the study protocol, which has been previously described (9). The JoCo OA has been continuously approved by the Institutional Review Board of the University of North Carolina at Chapel Hill (92–0583). For these analyses, all participants in the third (T3, baseline, 2013–2015, (8)) to the fourth (T4, follow-up, 2017-18) time point of the JoCo OA were included as these time points included ankle radiography (added to the study protocol at T3). All participants provided demographic and anthropometric information, completed standardized questionnaires and underwent examinations and radiographs with trained personnel.
Standardized lateral and mortise views of the ankle were obtained in weight-bearing at the two most recent data collections of the JoCo OA (2013–2018) as previously described (10), utilizing positioning guides and quality control; the tibiotalar joints were graded by an expert musculoskeletal radiologist (JBR) with excellent inter-rater reliability for ankle Kellgren-Lawrence grades (KLG; k = 0.91) (10). The KLG scale was modified for this purpose, such that grade 0 indicated no radiographic findings of OA, grade 1 indicated “minute osteophytes of doubtful clinical significance,” grade 2 included definite osteophytes and mild joint space narrowing (JSN), grade 3 reflected the presence of definite osteophytes and moderate JSN, and grade 4 was the combination of definite osteophytes and severe JSN (10). Incident radiographic ankle OA (rAOA, specifically tibiotalar OA) was defined for these analyses as a KLG ≥ 1 at follow-up among ankles with baseline KLG<1, based on our prior work (8) showing similar patterns but much higher prevalence of KLG ≥ 1 compared with KLG ≥ 2 disease. Progression of rAOA was defined as an increase of ≥1 KLG at follow-up among ankles with KLG ≥ 1 at baseline.
We also assessed the presence of pain, aching, and stiffness (PAS (11)) in the ankles, as well as the Foot and Ankle Outcome Score (FAOS) symptoms subscale (12). Participants self-reported PAS in the ankles on most days of any month in the past 12 months ranging from 0 (none) to 10 (as bad as you can imagine). The FAOS symptoms subscale includes seven items regarding symptoms and joint stiffness of the foot or ankle during the last week. Items are self-scored ranging from 0 (never/none) to 4 (always/extreme) and total scores are calculated as and range from 100 (no problems) to 0 (extreme problems). Worsening from baseine to follow-up was defined as any increase in the PAS scale or any decrease in the FAOS symptoms subscale. The four other FAOS subscales were also assessed: pain, activity of daily living, sports and recreation, and quality of life.
Person-level covariates that were evaluated included baseline age, sex (male or female), race (African American or white), baseline body mass index (BMI, measured in clinic), weight gain (defined as a 5% or greater increase in BMI from baseline to follow-up), smoking history (current, past, never), current alcohol use, history of falls in the past 12 months, a count of other joints with PAS in the past 12 months (including shoulders, elbows, wrists, hands, hips, knees, and feet, range 0-14) and the Charlson comorbidity index (a score of 2 or higher vs less than 2, (13)). Ankle-level or side-level covariates included baseline history of ankle injury (defined as an affirmative response to: “have you ever injured your right/left ankle badly enough that it limited your ability to walk for at least 2 days?”) and knee or foot OA. Weight-bearing radiographs of the knees (posteroanterior in fixed flexion (14)) and feet (anteroposterior and lateral (15)) were read by the same radiologist although without information about other sites, and OA was defined as a KLG ≥ 2 at the knee, or a score of 2 or more for osteophytes or joint space narrowing in at least one of 5 foot joints per the La Trobe radiographic atlas (16).
Statistical analysis
Ankle-level logistic regression models using generalized estimating equations with an exchangeable working correlation structure were used to examine associations of rAOA incidence or progression and covariates of interest. Models were stratified by baseline rAOA status so that estimated adjusted odds ratios and 95% confidence intervals (aOR [95% CI]) are separate for incidence or progression. Missing covariate information for at least one measure accounted for less than 5% (1.7%) and was assumed to be missing at random. Multiple imputation using logistic regression was used to impute missing binary covariates (knee or foot OA, ankle injury, history of falls, and Charlson comorbidity index) and linear regression was used to impute missing continuous covariates (count of other joints with PAS). These methods use fully conditional specification. Models were averaged over 10 imputed datasets. A sensitivity analysis was conducted excluding individuals with diabetes at baseline who may (or may not) have concomitant neuropathy.
Results
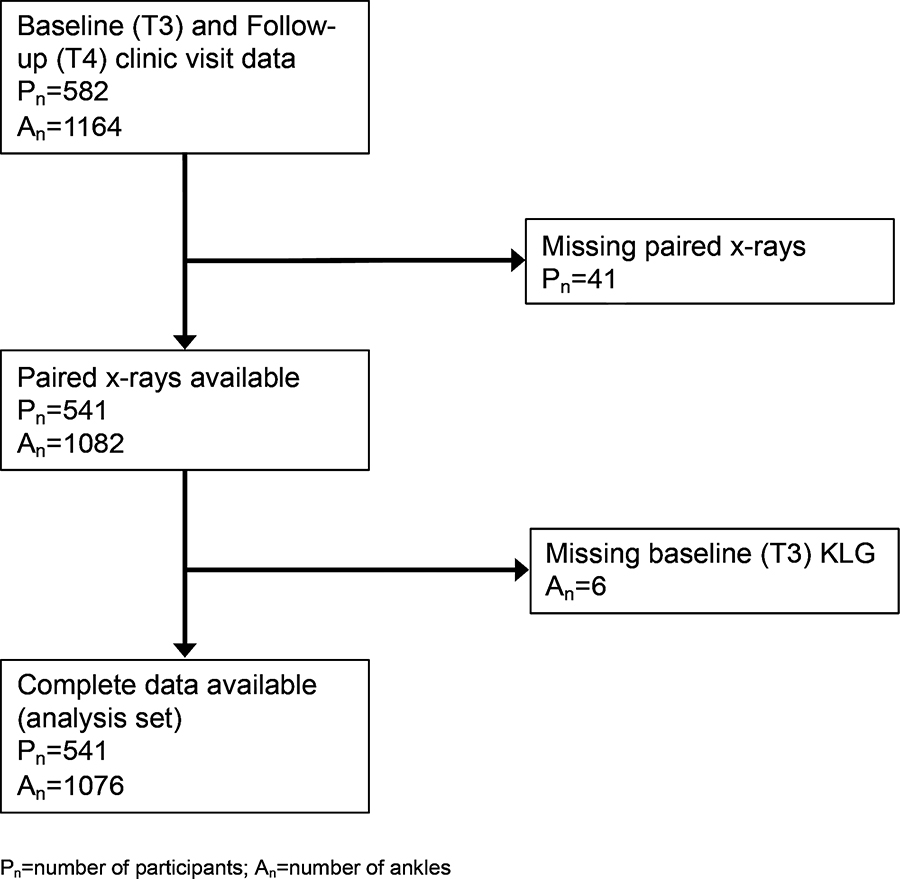
A total of 541 participants (1076 ankles) with data from baseline (T3) to follow-up (T4, mean ± standard deviation [SD] 3.5 ± 0.7 years, range 2–5 years between time points) were included in this analysis (Figure 1). There were 128 participants that had no evidence of OA (KLG=0) in both ankles and 413 participants that had KLG ≥ 1 in at least one ankle at baseline, with the majority having bilateral ankle OA (255 participants) as opposed to unilateral ankle OA (158 participants). The mean ± standard deviation (SD) age of the sample was 69 ± 7 years, the mean BMI was 31.2 ± 6 kg/m2, 71% of the participants were women and 35% were African American. Forty-three (7.9%) participants were current smokers at baseline, and 134 (24.8%) had a Charlson comorbidity index score ≥ 2 (Table 1).
Figure 1.
Flowchart of participants and ankles included in the analysis
Table 1.
Descriptive statistics of person-level and ankle/side-level variables.
| Person-level variables | |||
| Baseline (T3) values of Predictors and Covariates | Overall (n=541) | No rAOA KLG=0; (n=128) | Baseline rAOA KLG ≥1; (n=413) |
| mean (SD) or % or median (Q1-Q3) | |||
| Age, mean (SD) years | 69.4 (6.9) | 69.3 (7.1) | 69.5 (6.9) |
| Male, % | 29.4 | 18.8 | 32.7 |
| African American, % | 35.1 | 41.4 | 33.2 |
| BMI, mean (SD) kg/m2 | 32.1 (6.4) | 28.9 (5.8) | 31.9(6.4) |
| ≥5% weight gain, % | 18.7 | 20.3 | 18.2 |
| Charlson Index score ≥ 2, % | 24.8 | 27.1 | |
| Non-smoker, % | 47.0 | 53.1 | 45.0 |
| Past smoker, % | 45.1 | 34.4 | 48.4 |
| Current Smoker, % | 7.9 | 12.5 | 6.5 |
| Current alcohol drinker, % | 14.8 | 17.2 | 27.1 |
| Falls in the past 12 months | 26.2 | 22.7 | 27.4 |
| Count of other joints wiith PAS | 2 (0–4) | 1 (0–3) | 2 (0–5) |
| Ankle-level variables | |||
| Baseline (T3) values of Predictors and Covariates | Overall n=1076 | no rAOA KLG=0; (n=408) | Baseline rAOA KLG ≥1; (n=668) |
| History of ankle injury, % | 6.2 | 3.4 | 7.9 |
| Either foot OA or knee OA, % | 49.8 | 42.2 | 54.5 |
OA (osteoarthritis), BMI (body mass index), KLG (Kellgren-Lawrence grade), T3 (baseline time period (2013–2015), SD (standard deviation), Q1 (first quartile), Q3 (third quartile)
Unadjusted/descriptive results
Incident rAOA, defined as development of KLG ≥ 1 in an ankle without evidence of baseline rAOA, was present in 115/408 (28.2%) of ankles. However, incident rAOA with symptoms present at follow-up was observed in only 14/408 (3.4%) of ankles. Ankle PAS was self-reported for 7.4% of ankles without rAOA at baseline, with 6.9% of these 408 ankles having an increase (worsening) of PAS from baseline to follow-up. The median (interquartile range) for the FAOS Symptoms subscale among ankles without rAOA was 96.4 (85.7–100) with 61% having some symptoms at baseline (score<100) and 36.5% having a decrease (worsening) in the FAOS Symptoms subscale from baseline to follow-up (Table 2).
Table 2:
Ankle/Side-level Characteristics for outcomes
| Outcomes | All ankles | Ankles without baseline rAOA KLG=0; (n=408) | Ankles with baseline rAOA KLG ≥1; (n=668) |
|---|---|---|---|
| Radiographic | % | ||
| Incidence (KLG≥1) of OA or progression (increase) of KLG | 13.2% | 28.2% | 4.0% |
| Incidence (KLG≥1) of OA or progression (increase) of KLG with pain/aching/stiffness symptoms present at follow-up | 2.0% | 3.4% | 1.2% |
| Symptomatic | |||
| PAS any worsening (increase) | 8.5% | 6.9% | 9.4% |
| FAOS Symptoms subscale worsening (decrease) | 35.8% | 36.5% | 35.3% |
OA (osteoarthritis), rAOA (radiographic ankle OA), BMI (body mass index), KLG (Kellgren-Lawrence grade) JSN (joint space narrowing); baseline time period (T3: 2013–2015); follow-up time (T4: 2017–2018)
Progression of rAOA (defined by an increase in KLG) was noted in 27/668 (4%) of ankles with KLG ≥1 (those with rAOA) at baseline. However, progression of rAOA with symptoms present at follow-up was observed in only 8 (1.2%) of these ankles. PAS was reported for 16.3% of ankles with rAOA at baseline, with 9.4% of these 668 ankles having an increase (worsening) of PAS from baseline to follow-up. The median (interquartile range) for the FAOS Symptoms subscale among ankles with rAOA was 92.9 (82.1–100) with 68% having some symptoms (score<100) at baseline and 35.3% having a decrease (worsening) in the FAOS Symptoms subscale from baseline to follow-up (Table 2).
Adjusted analyses
In adjusted analyses (Table 3), among ankles without rAOA at baseline, the odds of developing incident rAOA increased by almost 50% with more symptomatic joints and more than doubled due to current smoking. Women, compared with men, had 50% lower odds of incident rAOA, while a non-significant, but strong, association was also noted for higher BMI.
Table 3.
Adjusted odds ratios with 95% confidence intervals, aOR (95% CI), for risk factors of interest and incident and progressive rAOA outcomes
| Risk factors | Incident rAOA | Progressive rAOA |
|---|---|---|
| In ankles without ankle OA (KLG=0) at baseline (n=408) | In ankles with ankle OA (KLG≥1) at baseline (n=668) | |
| aOR (95% CI) | ||
| Age: 5 years older | 1.10 (0.92, 1.32) | 0.92 (0.64, 1.33) |
| Women vs Men | 0.49 (0.28, 0.83) | 3.08 (0.90, 10.6) |
| Black vs White | 0.99 (0.61, 1.62) | 0.32 (0.11, 0.91) |
| BMI: 5 kg/m2 higher | 1.23 (0.97, 1.54) | 1.25 (0.91, 1.71) |
| 5% or more weight gain vs no 5% weight gain | 0.88 (0.48, 1.61) | 0.79 (0.21, 2.97) |
| Charlson comorbidity index, ≥2 vs <2 | 0.93 (0.54, 1.60) | 1.18 (0.47, 2.97) |
| Past smoker vs never smoker | 0.90 (0.55, 1.48) | 1.43 (0.57, 3.61) |
| Current smoker vs never smoker | 2.21 (1.05, 4.65) | 0.99 (0.16, 5.97) |
| Alcohol use vs no alcohol use | 1.07 (0.57, 2.02) | 2.83 (0.82, 9.76) |
| History of falls vs no falls | 0.70 (0.40, 1.24) | 0.84 (0.32, 2.20) |
| Other joints with symptoms: 2 additional joints with symptoms | 1.46 (1.06, 2.02) | 1.96 (0.94, 4.08) |
| Ankle injury vs no ankle injury | 0.94 (0.25, 3.49) | 3.41 (1.13, 10.3) |
| Either foot or knee OA vs neither | 1.24 (0.76, 2.04) | 2.91 (1.13, 7.49) |
OA (osteoarthritis), rAOA (radiographic ankle OA), BMI (body mass index), KLG (Kellgren-Lawrence grade), aOR (adjusted odds ratio), CI (confidence interval); Bold indicates statistically significant result
Among ankles with rAOA at baseline, previous ankle injury more than tripled the odds of progression of rAOA, and presence of concomitant foot or knee rOA also tripled the odds of progressive rAOA. African Americans, compared with whites, had over 60% lower odds of progression of rAOA (Table 3).
As shown in Table 4, in ankles without rAOA at baseline, the odds of reporting worsening FAOS symptoms increased by 40–60% with older age, higher BMI, and additional symptomatic joints. Only reporting other additional joints with symptoms was associated with worsening ankle PAS.
Table 4.
Adjusted odds ratios with 95% confidence intervals, aOR (95% CI), for risk factors of interest and worsening of symptoms by FAOS Symptoms subscale or foot PAS
| Risk factors | ||||
|---|---|---|---|---|
| No rAOA at baseline (n=408) | With rAOA at baseline (n=668) | |||
| Worsening FAOS | Worsening PAS | Worsening FAOS | Worsening PAS | |
| aOR (95% CI) | aOR (95% CI) | |||
| Age: 5 years older | 1.41 (1.12, 1.77) | 1.10 (0.80, 1.52) | 1.13 (0.97, 1.33) | 0.86 (0.64, 1.17) |
| Women vs Men | 1.53 (0.71, 3.29) | 0.94 (0.25, 3.49) | 1.54 (0.92, 2.58) | 2.25 (1.01, 5.03) |
| Black vs White | 1.41 (0.76, 2.61) | 1.73 (0.54, 5.54) | 0.95 (0.58, 1.56) | 0.82 (0.38, 1.77) |
| BMI: 5 kg/m2 higher | 1.65 (1.22, 2.24) | 0.90 (0.60, 1.36) | 1.36 (1.13, 1.64) | 1.27 (0.98, 1.63) |
| 5% or more weight gain vs no 5% weight gain | 1.16 (0.60, 2.27) | 2.03 (0.67, 6.11) | 1.33 (0.78, 2.28) | 1.03 (0.44, 2.43) |
| Charlson comorbidity index, ≥2 vs <2 | 0.60 (0.29, 1.25) | 1.34 (0.46, 3.94) | 0.97 (0.59, 1.60) | 0.45 (0.19, 1.05) |
| Past smoker vs never smoker | 1.05 (0.55, 2.01) | 0.93 (0.32, 2.73) | 1.44 (0.89, 2.33) | 2.20 (1.11, 4.34) |
| Current smoker vs never smoker | 2.25 (0.76, 6.65) | 0.26 (0.04, 1.66) | 3.56 (1.29, 9.82) | 1.27 (0.29, 5.60) |
| Alcohol use vs no alcohol use | 1.49 (0.63, 3.51) | 1.84 (0.55, 6.13) | 0.92 (0.47, 1.78) | 1.19 (0.42, 3.34) |
| History of falls vs no falls | 0.79 (0.39, 1.63) | 1.92 (0.62, 5.93) | 1.22 (0.73, 2.02) | 0.62 (0.31, 1.25) |
| Other joints with symptoms: 2 additional joints with symptoms | 1.49 (1.20, 1.86) | 1.59 (1.23, 2.04) | 1.27 (1.08, 1.50) | 1.45 (1.23, 1.71) |
| Ankle injury vs no ankle injury | 0.65 (0.29, 1.45) | 1.27 (0.68, 2.38) | 1.2 0 (0.56, 2.61) | 1.91 (0.81, 4.50) |
| Either foot or knee OA vs neither | 1.14 (0.72, 1.80) | n/a | 1.06 (0.78, 1.45) | 1.06 (0.63, 1.78) |
FAOS (foot and ankle outcome score), PAS (pain, aching, and stiffness score), OA (osteoarthritis), rAOA (radiographic ankle OA), BMI (body mass index), KLG (Kellgren-Lawrence grade), aOR (adjusted odds ratio), CI (confidence interval); Bold indicates statistically significant result; n/a, model did not converge
Among ankles with rAOA at baseline, currently versus never smoking was associated with over 3 times the odds of worsening FAOS symptoms ( Table 4). Additionally, higher BMI and additional symptomatic joints were associated with 30–40% higher odds of worsening FAOS symptoms. Women (compared with men) and past smokers (compared with never smokers) had 40–50% higher odds of worsening FAOS symptoms, although not statistically significant. However, past smoking and being female more than doubled the odds of reporting worsening PAS. Additionally, higher BMI and additional symptomatic joints were independently associated with worsening PAS at follow-up in ankles with rAOA (Table 4). Other FAOS subscales (data not shown) showed similar patterns, particularly for higher BMI or additional symptomatic joints, both of which increased the odds of worsening for all scales by 15–48%. Results considering individual progression of osteophytes or joint space narrowing (each graded 0-3) were similar to those for KLG (data not shown). Results were consistent, although the strata within some models did not converge, when individuals with diabetes at baseline were excluded (n without baseline rAOA=313; n with baseline rAOA=451, data not shown).
Discussion
In the current analysis, we found that among the ankles without rAOA at baseline, more than 1 in 4 (28%) developed incident rAOA, with symptomatic worsening noted by 37% according to the FAOS symptoms subscale and 7% by the PAS scale. The development of incident rAOA was associated with male sex, higher BMI, current smoking and the presence of other joint symptoms. Progression of rAOA over 3–4 years of follow-up was relatively infrequent, occurring in 4% of ankles with baseline rAOA, and associated with prior ankle injury and concomitant foot or knee OA. Among ankles with baseline rAOA, symptomatic worsening at follow-up was noted in about a third by the FAOS symptoms subscale and 10% by PAS, associated with smoking, higher BMI, and additional symptomatic joints. Our prior cross-sectional analysis at baseline (T3) also identified associations between higher age and BMI, prior injury, and joint symptoms and prevalent rAOA (8). This is somewhat in contrast to another cross-sectional study of rAOA among individuals with foot and ankle pain that identified female sex, age 50–64, and manual occupations as risk factors for symptomatic rAOA (3).
We found that smoking was associated with increased risk of incident rAOA and of worsening symptoms. In general, an inverse relationship between smoking and knee rOA has been reported (17), although this is dependent on OA site, definition, and population studied (18). Smoking is associated with more musculoskeletal pain and painful OA (19). However, some studies have found a negative effect of smoking on cartilage, such that being a current or former smoker was associated with increased annual loss of medial knee cartilage volume, with a dose–response relationship between 'pack-years' smoked and increased cartilage volume loss (20). Smoking also produces a chronic inflammatory state (21), and has been associated with elevated levels of C-reactive protein (a marker of inflammation) (22). There is a complex relationship between smoking, BMI, and OA that may explain some of the inconsistencies in association (23).
Elevated BMI was independently associated with increased odds of developing rAOA and symptomatic worsening. There is a clear association between elevated BMI and OA, particularly in weight-bearing joints, although more prominent in the knee (24) than in the hip (25). Our previous cross-sectional study also demonstrated that obese participants had twice the odds of ankle OA compared with nonobese individuals (8). This was consistent with the study done by Frey et al, where the odds of having ankle or foot OA were increased by 50% in overweight/obese versus normal weight individuals (though this association was not statistically significant) (26).
Another significant association was noted with the presence of symptoms in other joints. We have previously noted stronger associations between multiple joint symptoms, rather than radiographic OA at multiple sites, and functional outcomes (27), and many groups have commented on the high frequency of multiple joint pain in patients with OA (28, 29), which may represent multiple joint OA (30), a centralized pain process, shared risk factors, or other potential mechanisms. Interestingly, we did not find statistically significant associations between incident rAOA and prevalent radiographically defined knee or foot OA despite their biomechanical interconnection (31).
Although not associated with incident rAOA, prior ankle injury was a strong risk factor for progression of rAOA, increasing the odds more than 3 times compared to no injury. In this older cohort, this may indicate that those with prior injury already had rAOA by the mean age of 70 (and therefore could not develop new disease), although continued worsening was still influenced by prior injury. Similarly, Kraus et al, identified rAOA in 15% of individuals with symptomatic knee OA although less than 5% of these individuals reported any prior ankle injury or surgery (32). This also argues that, as we found in our initial cross-sectional association study (8), not all ankle rAOA is post-traumatic, a belief that is based on studies of individuals with end-stage ankle OA (2, 5) rather than more general community-based epidemiology studies such as this one which were previously lacking in the literature.
Our study had a few limitations. The mean age of the patients was 69 and the majority of our cohort was female, so results may not be generalizable to populations with substantially different age or sex distributions. To make the rAOA definition most useful, we included ankles with a KLG of 1 or more, as a KLG of 2 or more was infrequent in prior work from our group and others. As this is a large, longitudinal epidemiologic study, we did not collect exhaustive data specific to the ankle, so other etiologies/contributors for the observed associations (e.g., neuropathic conditions, tendinopathy) cannot be excluded. Similarly, information on ankle injuries was limited to the available questionnaire data (i.e., “have you ever injured your right/left ankle badly enough that it limited your ability to walk for at least 2 days?), and we did not have more specific data regarding acute injuries, instability, alignment, or specific surgical procedures. Ankle radiographs were not obtained until the third follow-up of the parent cohort, and not all of these participants returned for the fourth follow-up to allow longitudinal assessments; however, our sample size of more than 500 community-based participants makes this one of the largest studies of ankle OA available. Other strengths of this analysis include that it is in a large and well-characterized, community-based cohort including African American and white men and women, and utilized standardized, weight-bearing ankle radiographs read with high reliability by a single musculoskeletal radiologist with decades of experience. We assessed ankle symptoms in two ways, using self-reported pain, aching, and stiffness as well as a validated symptoms score (FAOS symptoms scale), and considered a variety of potentially relevant risk factors.
Conclusion
About a quarter of ankles with KLG=0 at baseline developed KLG ≥ 1 over approximately 3 years of follow up in this cohort of older community-living adults, although most of these were asymptomatic. An increase in KLG was seen in a small minority of ankles, and few of these had symptoms. However, an increase in symptoms over time by PAS was reported in 7% of ankles without baseline rAOA and 9% of those with rAOA at baseline; worsening of FAOS symptoms was reported for over a third of ankles (regardless of rAOA status). Key modifiable risk factors for developing or worsening radiographic OA and/or symptoms included smoking (current or former versus never), prior ankle injury, number of additional symptomatic joints, and higher BMI.
Supplementary Material
Acknowledgments:
We wish to thank the participants and staff of the Johnston County Osteoarthritis Project without whom this work would not be possible.
Funding for this work was provided in part by:
Association of Schools of Public Health/Centers for Disease Control and Prevention (CDC) S043, S1734, S3486; CDC U01 DP003206 and U01 DP006266; National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases P60AR30701, P60AR049465, P60AR064166, and P30AR072580. We also appreciate the support of the UNC Thurston Arthritis Research Center. The funding sources had no role in study design, data collection, writing the report, or the decision to submit the article for publication.
Abbreviations:
- OA
osteoarthritis
- rAOA
radiographic ankle OA
- KLG
Kellgren-Lawrence grade
- PAS
pain, aching, and stiffness
- FAOS
foot and ankle outcome score
- BMI
body mass index
- JoCo OA
Johnston County OA Project
- JSN
joint space narrowing
- aOR
adjusted odds ratio
- CI
confidence interval
- SD
standard deviation
Footnotes
Declaration of interest:
The authors have no competing interests in relation to this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Buckwalter JA, Martin JA. Osteoarthritis. Adv Drug Deliv Rev. 2006;58(2):150–67. doi: 10.1016/j.addr.2006.01.006. [DOI] [PubMed] [Google Scholar]
- 2.Barg A, Pagenstert GI, Hugle T, Gloyer M, Wiewiorski M, Henninger HB, et al. Ankle osteoarthritis: etiology, diagnostics, and classification. Foot Ankle Clin. 2013;18(3):411–26. doi: 10.1016/j.fcl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Murray C, Marshall M, Rathod T, Bowen CJ, Menz HB, Roddy E. Population prevalence and distribution of ankle pain and symptomatic radiographic ankle osteoarthritis in community dwelling older adults: A systematic review and cross-sectional study. PLoS One. 2018;13(4):e0193662. doi: 10.1371/journal.pone.0193662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Glazebrook M, Daniels T, Younger A, Foote CJ, Penner M, Wing K, et al. Comparison of health-related quality of life between patients with end-stage ankle and hip arthrosis. J Bone Joint Surg Am. 2008;90(3):499–505. doi: 10.2106/JBJS.F.01299. [DOI] [PubMed] [Google Scholar]
- 5.Agel J, Coetzee JC, Sangeorzan BJ, Roberts MM, Hansen ST, Jr. Functional limitations of patients with end-stage ankle arthrosis. Foot Ankle Int. 2005;26(7):537–9. [DOI] [PubMed] [Google Scholar]
- 6.Brown TD, Johnston RC, Saltzman CL, Marsh JL, Buckwalter JA. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20(10):739–44. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 7.Saltzman CL, Salamon ML, Blanchard GM, Huff T, Hayes A, Buckwalter JA, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44–6. [PMC free article] [PubMed] [Google Scholar]
- 8.Lateef S, Golightly YM, Renner JB, Jordan JM, Nelson AE. A Cross-sectional Analysis of Radiographic Ankle Osteoarthritis Frequency and Associated Factors: The Johnston County Osteoarthritis Project. J Rheumatol. 2017;44(4):499–504. doi: 10.3899/jrheum.161076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34(1):172–80. [PubMed] [Google Scholar]
- 10.Kraus VB, Kilfoil TM, Hash TW, 2nd, McDaniel G, Renner JB, Carrino JA et al. Atlas of radiographic features of osteoarthritis of the ankle and hindfoot. Osteoarthritis Cartilage. 2015;23(12):2059–85. doi: 10.1016/j.joca.2015.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Neogi T. The epidemiology and impact of pain in osteoarthritis. Osteoarthritis Cartilage. 2013;21(9):1145–53. doi: 10.1016/j.joca.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Golightly YM, Devellis RF, Nelson AE, Hannan MT, Lohmander LS, Renner JB, et al. Psychometric properties of the foot and ankle outcome score in a community-based study of adults with and without osteoarthritis. Arthritis Care Res (Hoboken). 2014;66(3):395–403. doi: 10.1002/acr.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 14.Nelson AE, Renner JB, Shi XA, Shreffler JH, Schwartz TA, Jordan JM. Cross-sectional comparison of extended anteroposterior and posteroanterior fixed flexion positioning to assess radiographic osteoarthritis at the knee: the Johnston County Osteoarthritis Project. Arthritis Care Res (Hoboken). 2010;62(9):1342–5. doi: 10.1002/acr.20210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Golightly YM, Hannan MT, Nelson AE, Hillstrom HJ, Cleveland RJ, Kraus VB, et al. Relationship of Joint Hypermobility with Ankle and Foot Radiographic Osteoarthritis and Symptoms in a Community-Based Cohort. Arthritis Care Res (Hoboken). 2019;71(4):538–44. doi: 10.1002/acr.23686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Menz HB, Munteanu SE, Landorf KB, Zammit GV, Cicuttini FM. Radiographic classification of osteoarthritis in commonly affected joints of the foot. Osteoarthritis Cartilage. 2007;15(11):1333–8. doi: 10.1016/j.joca.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 17.Kong L, Wang L, Meng F, Cao J, Shen Y. Association between smoking and risk of knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2017;25(6):809–16.. doi: 10.1016/j.joca.2016.12.020. [DOI] [PubMed] [Google Scholar]
- 18.Hui M, Doherty M, Zhang W. Does smoking protect against osteoarthritis? Meta-analysis of observational studies. Ann Rheum Dis. 2011;70(7):1231–7. doi: 10.1136/ard.2010.142323. [DOI] [PubMed] [Google Scholar]
- 19.Smuck M, Schneider BJ, Ehsanian R, Martin E, Kao MJ. Smoking Is Associated with Pain in All Body Regions, with Greatest Influence on Spinal Pain. Pain Med. 2019. doi: 10.1093/pm/pnz224. [DOI] [PubMed] [Google Scholar]
- 20.Davies-Tuck ML, Wluka AE, Forbes A, Wang Y, English DR, Giles GG, et al. Smoking is associated with increased cartilage loss and persistence of bone marrow lesions over 2 years in community-based individuals. Rheumatology (Oxford) 2009;48(10):1227–31. doi: 10.1093/rheumatology/kep211. [DOI] [PubMed] [Google Scholar]
- 21.Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89(9):1117–9. doi: 10.1016/s0002-9149(02)02284-1. [DOI] [PubMed] [Google Scholar]
- 22.Tracy RP, Psaty BM, Macy E, Bovill EG, Cushman M, Cornell ES, et al. Lifetime smoking exposure affects the association of C-reactive protein with cardiovascular disease risk factors and subclinical disease in healthy elderly subjects. Arterioscler Thromb Vasc Biol. 1997;17(10):2167–76. doi: 10.1161/01.atv.17.10.2167. [DOI] [PubMed] [Google Scholar]
- 23.Felson DT, Zhang Y. Smoking and osteoarthritis: a review of the evidence and its implications. Osteoarthritis Cartilage. 2015;23(3):331–3. doi: 10.1016/j.joca.2014.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jiang L, Tian W, Wang Y, Rong J, Bao C, Liu Y, et al. Body mass index and susceptibility to knee osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2012;79(3):291–7. doi: 10.1016/j.jbspin.2011.05.015. [DOI] [PubMed] [Google Scholar]
- 25.Jiang L, Rong J, Wang Y, Hu F, Bao C, Li X, et al. The relationship between body mass index and hip osteoarthritis: a systematic review and meta-analysis. Joint Bone Spine. 2011;78(2):150–5. doi: 10.1016/j.jbspin.2010.04.011. [DOI] [PubMed] [Google Scholar]
- 26.Frey C, Zamora J. The effects of obesity on orthopaedic foot and ankle pathology. Foot Ankle Int. 2007;28(9):996–9. doi: 10.3113/FAI.2007.0996. [DOI] [PubMed] [Google Scholar]
- 27.Nelson AE, Elstad E, DeVellis RF, Schwartz TA, Golightly YM, Renner JB, et al. Composite measures of multi-joint symptoms, but not of radiographic osteoarthritis, are associated with functional outcomes: the Johnston County Osteoarthritis Project. Disabil Rehabil. 2014;36(4):300–6. doi: 10.3109/09638288.2013.790490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Raja R, Dube B, Hensor EM, Hogg SF, Conaghan PG, Kingsbury SR. The clinical characteristics of older people with chronic multiple-site joint pains and their utilisation of therapeutic interventions: data from a prospective cohort study. BMC Musculoskelet Disord. 2016;17:194. doi: 10.1186/s12891-016-1049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Felson DT, Niu J, Quinn EK, Neogi T, Lewis C, Lewis CE, et al. Multiple Nonspecific Sites of Joint Pain Outside the Knees Develop in Persons With Knee Pain. Arthritis Rheumatol. 2017;69(2):335–42. doi: 10.1002/art.39848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gullo TR, Golightly YM, Cleveland RJ, Renner JB, Callahan LF, Jordan JM, et al. Defining multiple joint osteoarthritis, its frequency and impact in a community-based cohort. Semin Arthritis Rheum. 2019;48(6):950–7. doi: 10.1016/j.semarthrit.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ro DH, Lee J, Lee J, Park JY, Han HS, Lee MC. Effects of Knee Osteoarthritis on Hip and Ankle Gait Mechanics. Adv Orthop. 2019;2019:9757369. doi: 10.1155/2019/9757369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kraus VB, Worrell TW, Renner JB, Coleman RE, Pieper CF. Highprevalence of contralateral ankle abnormalities in association with knee osteoarthritis and malalignment. Osteoarthritis Cartilage. 2013;21(11):1693–9. doi: 10.1016/j.joca.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.