Abstract
One-quarter to one-third of ischemic strokes have no established mechanism after standard diagnostic evaluation and are classified as embolic stroke of undetermined source (ESUS). Failure of randomized trials to demonstrate a benefit of direct oral anticoagulants over aspirin for the treatment of ESUS as a single homogeneous entity has led to renewed interest by stroke experts to divide ESUS into subgroups. Emerging data suggest that active cancer, which is present in 5–10% of patients with ESUS, is a distinct and important subgroup of ESUS with unique clinical characteristics, underlying pathophysiologies, and treatment and prognostic considerations. Further, the prevalence of cancer-related ESUS is expected to increase as patients with cancer, even those with distant metastases, survive longer due to improvements in cancer treatments. In this topical review, we examine the epidemiological link between ESUS and cancer, the clinical features and potential mechanistic underpinnings of ESUS with cancer (with a focus on novel biomarkers and their relationship to recurrent stroke and other thromboembolic events), and the potential treatment strategies for cancer-related ESUS. We include a critical appraisal of existing data and ongoing or planned clinical trials of different antithrombotic approaches. As cancer-related ESUS is a dynamic disease with variable course, we recommend close collaboration between neurologists and oncologists to develop individualized management plans.
Keywords: embolism, stroke, cancer, malignancy, neoplasm, review
Introduction
Stroke is a leading cause of death and disability worldwide and its incidence is increasing, particularly in younger age groups.1, 2 Most strokes are ischemic and up to one-third have no established mechanism and are considered cryptogenic.3 In 2014, Hart et al proposed a new clinical construct, whereby a non-lacunar ischemic stroke that remained cryptogenic after standard diagnostic evaluation was classified as embolic stroke of undetermined source (ESUS).4 This pragmatic construct was partly derived to serve as the basis for randomized trials comparing novel oral anticoagulant therapy to traditional antiplatelet therapy for secondary stroke prevention. The underlying premise was that most ESUS is due to thromboembolic mechanisms originating from various cardiac and atherothrombotic sources and therefore patients with ESUS may preferentially benefit from anticoagulant therapy. However, two large randomized trials, NAVIGATE ESUS and RE-SPECT ESUS, failed to demonstrate the superiority of direct oral anticoagulants versus aspirin in patients with ESUS.5, 6 These neutral results renewed stroke experts’ interest in dividing ESUS into individual subgroups, particularly for those with unique diagnostic and treatment considerations. One important subgroup is patients with active cancer. This topical review aims to critically examine data on the epidemiological relationship, pathophysiological underpinnings, clinical features, and therapeutic considerations for ESUS with cancer, while emphasizing the unique characteristics that differentiate this ESUS subgroup from others.
Epidemiological Link between Cancer and ESUS
In addition to left atrial cardiopathy, nonstenosing large artery atherosclerosis, and patent foramen ovale (PFO), cancer is an important and common subgroup of ESUS.7, 8 Claims-based data from the National Inpatient Sample suggest that about 10% of patients with ischemic stroke of all etiologies have known cancer.9 The estimated proportion of ESUS patients with cancer similarly approaches 10%, although it may be as high as 20% in some Asian populations.8, 10 However, the attributable fraction of ischemic stroke due to cancer is likely less than this because some of the association may be coincidental. The coprevalence for cancer and ischemic stroke is expected to increase further as patients with cancer survive longer. Cancer registry data have demonstrated falling mortality rates for several cancer types, including for lung, breast, and prostate cancers, the three most common types in the United States.11 For example, the two-year survival among men diagnosed with nonsmall cell lung cancer, which is often widespread at presentation, improved from 26% in 2001 to 35% in 2014.12 These improved mortality rates reflect improved cancer treatments, particularly targeted and immunological therapies, and reduced smoking.
Active cancer is an established risk factor for ischemic stroke. Multiple large studies, including prospective ones, have demonstrated an increased risk of stroke and other arterial thromboembolic events in patients with incident cancer versus matched controls.13–15 This risk is particularly high in the first 6 months after cancer diagnosis and in patients with distant metastases. Further, stroke risk varies by cancer type, and is highest with cancers most linked to venous thromboembolism risk, particularly lung and pancreatic cancer. Approximately half the ischemic strokes in patients with cancer are classified as ESUS, a higher proportion than is typical in those without cancer.3, 16–18
It is also increasingly recognized that stroke, particularly ESUS, can be the initial presentation of cancer.19, 20 In a large study using American cancer registry data linked to Medicare claims, the risk of ischemic stroke was increased 59% in the year before cancer diagnosis.21 The increased risk began 5 months before cancer was diagnosed and peaked in the month prior. Because it generally takes years for cancer to develop, these patients’ cancers were presumably present at the time of their stroke, and perhaps contributed to its development.22 Further, recent studies have reported that among patients with ESUS, 2–10% are diagnosed with cancer in the year after their stroke.23–25 Possible clues to the presence of occult cancer in ESUS include history of smoking, unexplained weight loss, infarcts in all vascular distributions (i.e., “three-territory sign”), elevated D-dimer, increased C-reactive protein, anemia, and hypoalbuminemia.10, 23, 25–27 However, studies reporting on this topic were generally small, single center, and retrospective, and therefore the true rate and indicators of occult cancer in patients with ESUS remain uncertain. It is also uncertain whether patients with ESUS should be screened for occult cancer, how they should be screened, and what the clinical utility of earlier detection might be. These uncertainties are exemplified by the cautionary tale of cancer screening for unprovoked venous thromboembolism. Early reports had suggested that approximately 10% of patients with unprovoked venous thromboembolism had occult cancer, and this led some physicians to empirically screen these patients with whole body imaging.28 However, in a large prospective randomized trial, SOME, only 3.9% of patients with an unprovoked venous thromboembolism were diagnosed with cancer in the following year, and the diagnostic yield of comprehensive screening with CT imaging was not significantly higher than limited screening with basic blood tests, chest radiography, and age- and sex-appropriate screening for breast, cervical, and prostate cancers.29 Therefore, we believe that prospective multicenter studies with systematic follow-up and outcomes adjudication are needed to determine the utility of cancer screening in ESUS before it can be considered standard practice.
Pathophysiology of ESUS with Cancer
Accumulating evidence indicates that cancer-associated ESUS may be a distinct subgroup of stroke.30 We demonstrated in a small multicenter prospective study that patients with cancer and stroke have a distinctive molecular signature in their peripheral blood gene expression as compared to cancer-only and stroke-only controls.31 Pathways specific to the cancer-stroke group primarily involved inflammation, hypoxia response, transcriptional regulation, cortical circuit plasticity, and cancer formation/progression.
While possibly a distinct stroke subgroup, the potential underlying mechanisms of cancer-associated ESUS are broad and various heterogenous pathophysiologies require consideration. These include mechanisms that develop from cancer-mediated hypercoagulability, an entity that increases the risk for not just venous thromboembolism, but also arterial events.15 The pathobiology that leads to this acquired hypercoagulable state is complex, varies by cancer site and histology, and involves multiple interconnected factors (Figure 1).
Figure 1.
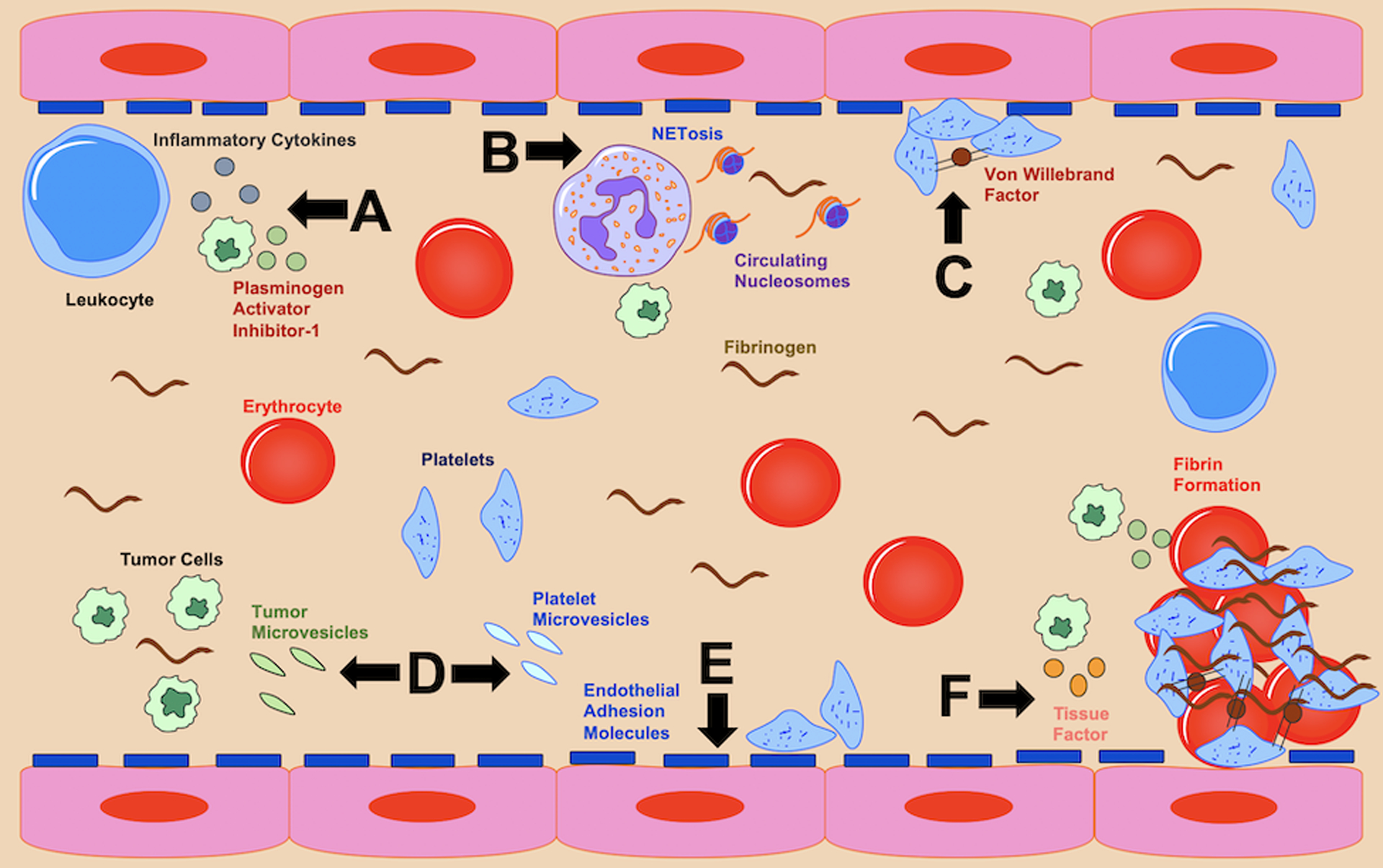
Biological factors that may promote thromboembolic events in patients with cancer, including embolic stroke of undetermined source. Black lettering and arrows are used to illustrate the different hematological pathophysiologies depicted in this imagined blood vessel. They include (A) tumor cell release of inflammatory cytokines and fibrinolysis inhibitors, (B) heightened neutrophil extracellular trap formation, (C) increased platelet aggregation, (D) circulating tumor and platelet extracellular vesicles, (E) excessive endothelial adhesiveness, and (F) increased coagulation factors.
First, pro-coagulation factors, including tissue factor, are increased, both by cancer cells and the body’s inflammatory response. This is supported clinically in patients with cancer and stroke by the observation that approximately 10% will have venous thromboembolism and most will have an elevated plasma D-dimer (a nonspecific marker of hypercoagulability).18, 32 Second, hematogenous extracellular vesicles derived from cancer cells and platelets are increased, and these vesicles can trigger the coagulation cascade. In the OASIS-Cancer prospective cohort study from Korea, patients with cancer and ESUS had higher blood levels of extracellular vesicles than patients with cancer and conventional stroke mechanisms, and higher levels than stroke-only and cancer-only controls.33 In this analysis, cancer cell-derived extracellular vesicle levels correlated with plasma D-dimer levels, and these vesicles promoted coagulation independent of tissue factor pathways. Additionally, when restricted to patients with lung cancer, extracellular vesicle levels were associated with adenocarcinoma histology, the histological type most linked to hypercoagulability, and coagulation assays in these patients demonstrated shorter clotting times.34 Cancer cell-derived extracellular vesicles contain microRNAs that have various functions;35 the OASIS-Cancer study is exploring whether circulating non-coding RNA is an important biomarker for cancer-mediated coagulopathy. Third, neutrophil extracellular trap formation (NETosis), which is part of the innate immune response and promotes platelet and coagulation factor activation, is upregulated. In a small study from Sweden, patients with cancer-associated ischemic stroke demonstrated markedly elevated NETosis levels, which were associated with thrombin-antithrombin and P-selectin levels, traditional markers of coagulation and platelet activity, respectively.36 In the OASIS-Cancer study, circulating plasma DNA and nucleosome levels, purported markers of NETosis, were higher in cancer-ESUS patients than in controls, and were associated with higher D-dimer levels.37 Fourth, platelet activity is abnormal and aggregation is increased. In a histopathological study of intracranial thrombi endovascularly retrieved from patients with large vessel occlusive strokes, 16 patients with active cancer had higher platelet and lower erythrocyte fractions—so called “white clots”—than equal numbers of patients with inactive cancer and no cancer.38 Among the active cancer group, there were seven patients with ESUS and four patients with echocardiography-confirmed nonbacterial thrombotic endocarditis and these patients had similar thrombus compositions and markedly increased platelet fractions indicating that platelet dysfunction plays a prominent role in cancer-related ESUS and hypercoagulability. Fifth, endothelial wall integrity and adhesiveness is altered. Cancer is associated with increased soluble thrombomodulin, which is believed to reduce thrombomodulin at the endothelial surface, where it normally acts as an anticoagulant.39 Additionally, cancer can increase von Willebrand factor, and this protein promotes platelet-endothelium adhesion.39 In our prospective cohort study, MOST-Cancer (ClinicalTrials.gov identifier NCT02604667), patients with cancer and stroke had increased levels of several endothelial markers (thrombomodulin, soluble intercellular adhesion molecule-1, vascular cell adhesion molecule-1) compared to matched stroke-only and cancer-only controls (B. Navi, unpublished data, 2020).
Beyond these five pathways, other factors contributing to cancer-mediated hypercoagulability may include tumor expression of fibrinolysis inhibitors and inflammatory cytokines (i.e., “thromboinflammation”), activation of the intrinsic pathway, and effects of cancer treatments such as L-asparaginase. While not completely understood, these hemostatic alterations seen with cancer are believed to promote tumor cell survival, growth, and dissemination.40
How these various hypercoagulable pathways could lead to ESUS in patients with cancer is unclear (Table 1). One possibility with considerable supporting data is nonbacterial thrombotic endocarditis, which is caused by the formation of sterile platelet-fibrin vegetations on cardiac valves. A prospective study from Korea reported transcranial Doppler (TCD) evidence for cerebral microemboli in 58% of patients with cancer and ESUS, and microemboli were associated with high D-dimer levels and adenocarcinoma histology.41 These microemboli were often bilateral suggesting a central embolic source. In a large autopsy series from the 1970s-1980s conducted at Memorial Sloan Kettering, a quaternary-care cancer center in New York, nonbacterial thrombotic endocarditis was the leading identified mechanism in cancer patients with symptomatic ischemic stroke, and this mechanism was often undetected during life.42 Considering these data and the observation that approximately 30%−70% of all ESUS with cancer involves multiple vascular distributions,17, 18, 43, 44 it is logical to hypothesize that many ESUS with cancer are due to nonbacterial thrombotic endocarditis that is missed on standard transthoracic echocardiography (TTE) whereas transesophageal echocardiography (TEE) appears superior in detecting cardiac vegetations.45
Table 1.
Possible Underlying Mechanisms and Treatment Approaches in ESUS with Cancer
| Mechanisms* | Associated Characteristics | Possible Treatment Approaches |
|---|---|---|
| Hypercoagulable | ||
| Cerebral Intravascular Coagulation | Elevated D-dimer, diffuse systemic and cerebral infarcts, disseminated cancer, sepsis | Direct oral or parenteral heparin-based anticoagulation, cytoreductive chemotherapy |
| Nonbacterial thrombotic endocarditis | Elevated D-dimer, diffuse systemic and cerebral infarcts, TCD microemboli, disseminated cancer, adenocarcinoma histology | Direct oral or parenteral heparin-based anticoagulation, cytoreductive chemotherapy |
| Paradoxical embolization | Elevated D-dimer, indwelling venous catheter, venous thromboembolism history, immobility | Direct oral or parenteral heparin-based anticoagulation, consider removing offending venous catheter, consider PFO closure |
| Non-Hypercoagulable | ||
| Aortic atheroma | Smoking, vascular risk factors, thoracic radiation | Dual antiplatelets, intensive statin therapy, vascular risk factor control |
| Atrial disease | Vascular risk factors, dilated left atrium, atrial ectopy, increased BNP | Anticoagulant vs. antiplatelet, cardiac rhythm and rate control |
| Cardiomyopathy | Anthracycline and trastuzumab chemotherapy, thoracic radiation, heart disease, cardiac symptoms | Avoid offending chemotherapy, anticoagulant vs. antiplatelet, afterload reduction |
| Infective endocarditis | Indwelling venous catheter, sepsis, recent invasive procedures | Intravenous antibiotics, avoid antithrombotics |
| Nonstenosing large artery atherosclerosis | Smoking, vascular risk factors, thoracic/head/neck radiation | Antiplatelet(s), intensive statin therapy, vascular risk factor control, consider endarterectomy/stent |
| Tumor embolism | Centrally-located lung tumor, thoracic surgery, subsequent metastasis at stroke site | Cytoreductive chemotherapy, surgical resection, anticoagulant vs. antiplatelet |
| Vasculitis | Immunotherapy, fungal or varicella infection, intravascular lymphoma | Treat underlying cause or trigger, antiplatelet |
Abbreviations: ESUS, embolic stroke of undetermined source; TCD, transcranial Doppler; B-type natriuretic peptide; PFO, patent foramen ovale.
Categorized by association with hypercoagulability and listed in alphabetic order.
Cancer-mediated hypercoagulability could cause ESUS through cerebral intravascular coagulation, which is essentially in situ thrombus formation within the cerebral vasculature. While this may occur in some ESUS patients, in the authors’ experience, this pathophysiology typically only manifests when there is profound coagulopathy meeting diagnostic criteria for disseminated intravascular coagulation with thrombocytopenia and hypofibrinogenemia, and therefore by definition is not ESUS. Further, cerebral intravascular coagulation would not be expected to produce microemboli on TCD.
Another mechanism tied to hypercoagulability that could cause ESUS with cancer is paradoxical embolization. Up to 20% of patients with cancer develop a venous thromboembolism during their lifetime and approximately 25% of the population harbor a PFO.46, 47
There are also many mechanisms unrelated to hypercoagulability that can lead to ESUS in patients with cancer. Atherosclerosis shares causal pathways with cancer, including obesity, glucose intolerance, and smoking.48 Further, radiation therapy, a cornerstone of many cancer treatments, accelerates atherosclerosis, and often causes progressive irreversible vascular injury.49 Therefore, nonstenosing large artery atherosclerosis of the head and neck may be an important cause of ESUS with cancer. Aortic arch atheroma may be another underappreciated cause of cancer-related ESUS, as thoracic radiation is performed routinely in several common cancers, including breast and lymphoma. While radiation therapy generally takes years to produce hemodynamic luminal stenosis, it can produce arterial injury and accelerate and destabilize atherosclerotic plaques within months, especially when combined with cancers’ proinflammatory effects.50 As patients with cancer live longer, aortic and other large artery atherosclerosis will likely represent an even higher attributable fraction of ESUS with cancer, particularly among survivors of childhood and young adult cancers.49, 51 The cardiac effects of various cancer treatments can also lead to ESUS. For example, anthracycline chemotherapy can produce acute and chronic cardiomyopathy, which can cause ESUS.52 Radiation therapy to the thorax can injure the coronary arteries, cardiac valves, myocardium, and pericardium, and these effects can lead to ESUS.53 Immunotherapy, which is increasingly used in modern cancer regimens, has been associated with myocarditis and vasculitis.54
Tumor embolism, while rare, is probably another underappreciated mechanism of ESUS in patients with cancer.20 This pathophysiology typically occurs in patients with centrally-located primary or metastatic lung cancers that invade the pulmonary veins or cardiac chambers and embolize to the brain. Tumor emboli can also occur in invasive head and neck cancers. A clue to this mechanism is that if the patient survives long enough, they often form a metastasis at the site of their prior stroke.
Cancer surgery can also cause ESUS. It can do so through tumor emboli, direct arterial injury, and secondary cardiac arrhythmias. Patients with cancer are often immunosuppressed and have indwelling venous catheters and therefore infective endocarditis should be considered in cancer-related ESUS. Finally, accumulating data suggest that cancer is a risk factor for atrial fibrillation.55 Besides shared risk factors, increased inflammation may link cancer to left atrial disease.
Clinical Characteristics of Cancer-Associated ESUS
Patients with active cancer and ESUS have several identifiable characteristics. First, although conflicting data exist, most studies suggest that apart from smoking, patients with cancer-associated ESUS have fewer traditional stroke risk factors than those without cancer.44 Second, their strokes tend to be more severe, although their stroke severity scales can be confounded by preexisting disability from cancer.56 Third, cancer-related ESUS most commonly occurs with disseminated solid tumor adenocarcinomas; however, all cancer types, solid or hematological, stage 1 through stage 4, are associated with an increased risk of ischemic stroke.15–17 Fourth, most will have increased D-dimer and inflammatory markers, although this profile is typical of cancer in general and with other stroke mechanisms (e.g., cardioembolic).57, 58 Fifth, anywhere from 30%−70% demonstrate embolic-appearing infarcts in bilateral anterior and posterior circulations.17, 18, 43, 44 Sixth, they face high rates of recurrent stroke, recurrent thromboembolism, early neurological deterioration, and mortality.43, 56
In a retrospective cohort study of 263 patients with active solid or hematological cancer and acute ischemic stroke at Memorial Sloan Kettering from 2005–2010, 132 had cryptogenic mechanisms, and among these patients the 1-year cumulative rate of recurrent stroke and other major thromboembolic events was 48%.43 This high rate of recurrent events has been validated in other settings.56, 59, 60 The estimated 1-year rate of recurrent stroke in patients with cancer and ESUS ranges from 14–29%, which is approximately three-fold higher than in ESUS patients without cancer.43, 56, 59, 60 Patients with ESUS and cancer also face worse long-term functional outcomes and survival than patients with ESUS and no cancer.16 However, this should not engender a nihilistic attitude towards these patients. The past decade has seen an explosion in new and more effective cancer treatments, which has prolonged the survival and quality of life of many cancer patients, including those previously deemed terminal.11, 12 There are also, in the authors’ experience, many patients with ESUS and cancer, including those with historically poor prognosis (i.e., metastatic pancreatic cancer), who survive long periods with good quality of life, and therefore it is imperative to avoid a self-fulfilling prophecy that these patients will invariably succumb. For these reasons, the care of patients with cancer and ESUS needs to be personalized and performed in close collaboration with oncologists in order to meet the needs of an individual patient.
The diagnostic evaluation of patients with cancer-related ESUS should conform to standard stroke guidelines with a few additional considerations.61 For laboratory analysis, we typically also evaluate plasma D-dimer, which may be a useful diagnostic and prognostic marker in these patients.57 Reductions in D-dimer levels after starting antithrombotic therapy are associated with a lower risk of recurrent stroke.62 If aggressive care is sought and the patient’s clinical condition allows it, we generally pursue TEE after TTE in patients whose mechanism remains cryptogenic, as TEE has a higher diagnostic yield than TTE for identifying cardioembolic mechanisms, including nonbacterial thrombotic endocarditis and aortic atheroma.45 TCD microemboli detection should be considered as the presence of bilateral emboli indicates a central embolic source and the study can help gauge antithrombotic treatment response.41 If a right-to-left shunt is identified, we typically perform bilateral lower extremity ultrasounds, upper extremity ultrasound if a central venous catheter is present, and computed tomogram of the chest to evaluate for venous thromboembolism. A pelvic magnetic resonance venogram may also be useful in these patients.63 Identification of venous thrombosis with resultant paradoxical embolization would dictate treatment with long-term anticoagulation.
Treatment Considerations for Cancer-Associated ESUS
Large industry-sponsored trials failed to demonstrate the superiority of direct oral anticoagulant therapy for the treatment of ESUS as a single homogeneous entity.5, 6 This likely occurred because ESUS reflects a heterogeneous group of underlying mechanisms, some of which may be more effectively treated with antiplatelet or other therapies. Although anticoagulant therapy can reduce the risk of recurrent thromboembolism, any reductions in this risk should be weighed against the increased risk of bleeding. Therefore, there is an urgent need for trials focused on biologically distinct, high risk subgroups, which may preferentially benefit from anticoagulant therapy. One such subgroup is active cancer.
There are strong theoretical considerations for anticoagulating patients with cancer and ESUS and this is often empirically performed in practice, although data supporting this strategy are limited. Among 29 patients with cancer-related stroke with serial D-dimer measurements during their stroke hospitalization, anticoagulant use was associated with reduction in D-dimer, a surrogate for recurrent stroke risk.41 In the prospective OASIS-Cancer study, patients with cancer and stroke whose D-dimer decreased with anticoagulation had improved 1-year survival.62 In the pilot trial of Enoxaparin versus Aspirin in Cancer Patients with Ischemic Stroke (TEACH), we conducted the only multicenter randomized clinical trial comparing anticoagulant therapy to antiplatelet therapy in patients with active cancer and acute ischemic stroke (n=20, 75% ESUS).64 In TEACH, the objective was to evaluate feasibility and the study anticoagulant was enoxaparin, an injectable subcutaneous low-molecular weight heparin; however, direct oral anticoagulants are increasingly being used in cancer patients with thrombosis, including those with ESUS.65 Further, the leading reason for enrollment failure in TEACH was patient aversion to receiving injections, and 40% of patients randomized to enoxaparin crossed-over to aspirin because of discomfort with injections, underscoring the likely preference for oral anticoagulants in future trials in this population. Fortunately, several randomized trials have demonstrated that oral factor Xa inhibitors are comparable in terms of safety and efficacy to subcutaneous low-molecular weight heparins for preventing recurrent venous thromboembolism or major bleeding in cancer patients, making them an attractive option for cancer-related ESUS.66, 67 Accordingly, the American Society of Clinical Oncology guidelines now support the use of factor Xa inhibiting direct oral anticoagulants for the treatment of cancer-associated venous thromboembolism. However, venous thromboembolic disease is distinct from ESUS, and this guideline warns that there are limited data about the risks and benefits of anticoagulation beyond 6 months in cancer patients.68
Alternatively, some data support the use of antiplatelet therapy in patients with cancer and ESUS. Among 172 patients with active cancer and acute ischemic stroke at Memorial Sloan Kettering, based on physician judgment, 102 patients received an antiplatelet and 90 received an anticoagulant at discharge (some received both).43 Although there may have been confounding by indication, there were no differences in the odds of recurrent stroke, recurrent thromboembolism, or death between treatment groups. In the NAVIGATE ESUS randomized trial comparing rivaroxaban to aspirin in patients with ESUS, 543 (7.5%) had cancer—although whether these cancers were active was not reported.69 While patients with cancer had higher rates of recurrent stroke than patients without cancer, the risk of recurrent stroke between treatment groups was not significantly different, and if anything, aspirin may have performed better at secondary prevention. Further, the risk of major bleeding was more than doubled in the rivaroxaban-randomized patients. Lastly, as indicated previously, endovascularly-retrieved intracranial thrombi from patients with cancer-related ESUS are platelet-rich, indicating that antiplatelet therapy may benefit this population.38
Given the current knowledge as reviewed, we believe there is equipoise regarding the optimal antithrombotic strategy (anticoagulant vs. antiplatelet therapy) in patients with cancer and ESUS, and that clinical trials are needed. Many longstanding indications for anticoagulation in stroke based on theoretical considerations, such as cervicocephalic artery dissection and aortic atheroma, have not been supported by randomized trials, and any reduction in stroke risk was offset by increased risk of bleeding.70, 71 This is particularly germane to cancer patients who already face up to a 20% annual risk of major bleeding, a risk that may be even higher in cancer patients with stroke and other brain pathology.72, 73 In turn, major bleeding in patients with cancer is associated with an increased risk of death, and is highest with older age, medical comorbidities, gastrointestinal or genitourinary cancers, and metastatic disease, factors which are common in cancer-related ESUS.74 A 2020 survey of 77 NIH StrokeNet study sites supported this approach as 88% reported that the majority of their stroke attendings believe there is equipoise to randomize patients with ESUS and cancer to apixaban versus aspirin (B. Navi, unpublished data, 2020).
We are in the process of planning a multicenter, double-blind, randomized trial, entitled TEACH2, to determine if anticoagulant therapy with apixaban is safe and effective compared to antiplatelet therapy with aspirin in patients with active cancer and ESUS. In this trial, we will employ a patient-centric design, whereby the composite primary outcome includes all major thromboembolic events that impact quality of life measures and cancer treatment decisions in patients with cancer. Venous thromboembolic disease, in particular, will be included as part of the composite outcome because it is the second most common cause of death in patients with cancer and often hinders their functional status.75
To the authors’ knowledge, the only active randomized trial evaluating different antithrombotic approaches in patients with cancer and ESUS is the ENCHASE trial (ClinicalTrials.gov ID NCT03570281). This pilot trial in Korea is comparing the direct oral anticoagulant, edoxaban, to the injectable low-molecular weight heparin, enoxaparin, in 40 patients with cancer-associated ESUS. Additionally, the ongoing MOST-Cancer and OASIS-Cancer prospective cohort studies are evaluating biomarkers of hypercoagulability and antithrombotic treatment effects in patients cancer-related ESUS, albeit in non-randomized designs.
Another antithrombotic strategy that might benefit patients with cancer-related ESUS is a combined anticoagulant-antiplatelet approach that has demonstrated merit in other high-risk populations such as patients with atherosclerotic vascular disease.76 As platelets and the coagulation cascade are both activated in patients with cancer and ESUS, such a dual antithrombotic approach could, in theory, more comprehensively address the mechanistic underpinnings of cancer-related ESUS.38 However, such an approach would probably increase the risk of major bleeding, which is already elevated in these patients, and therefore more data are needed before this approach can be recommended.
While antithrombotics are vital to secondary prevention in patients with cancer and ESUS, it is as important, if not more so, to target the underlying cancer through directed cancer treatments, particularly cytoreductive and targeted chemotherapy. In many cancer patients, thromboembolic events, including ESUS, are driven by cancer-mediated hypercoagulability, which is directly linked to the activity and extent of the underlying tumor. Therefore, reducing cancer activity is of paramount importance, and sometimes may be the only strategy that can halt further thromboembolic events.19 However, two practical issues make this situation challenging to navigate. First, a “catch 22” situation often occurs whereby oncologists are hesitant to prescribe chemotherapy after stroke because they worry that the patient is too disabled to tolerate side effects and that the treatment could trigger another stroke. In these situations, we recommend a discussion between treating neurologists and oncologists to review the patient’s goals of care, functional status, and global risks and benefits. If the patient prefers aggressive care and has a reasonable functional status (i.e., able to ambulate and/or care for themselves), and there are potentially effective cancer treatments available, then we would generally advocate for directed cancer treatment as soon as possible in order to reduce cancer activity, thereby reducing cancer-mediated hypercoagulability and subsequent recurrent stroke risk. Second, there may be no potentially effective cancer treatments left for the patient. In this increasingly uncommon situation, the focus should be antithrombotic therapy and long-term goals of care.
Besides antithrombotic medicines and treating the underlying cancer, we believe that the management of cancer-associated ESUS should incorporate other targeted treatments. Emerging data suggest that statins may reduce the risk of stroke in cancer patients treated with thoracic, head, or neck radiation.77 Further, among patients at risk for radiation vasculopathy, vascular risk factors, such as hypertension, should be closely monitored and controlled. Additionally, patients with cancer and ESUS who have a PFO but no diagnosed venous thromboembolism should be considered for PFO closure. When making this decision, clinicians should factor in the patient’s age, life expectancy, and vascular risk factors, as well as the PFO’s structural characteristics.
Conclusion
ESUS and cancer are epidemiologically and mechanistically linked diseases that will likely increase in coprevalence as survival from cancer improves. Accumulating data suggest that cancer-related ESUS may be a distinct subgroup of ischemic stroke with specific clinical characteristics. However, this subgroup is comprised of many possible underlying mechanisms, not all of which would be expected to preferentially benefit from anticoagulation. Therefore, the optimal antithrombotic treatment strategy for cancer-related ESUS remains uncertain, especially because of the high risk of bleeding in these patients. Consequently, there is an urgent need for randomized clinical trials in this population. Several prospective studies are ongoing or planned to enhance our understanding of the unique pathophysiologic and therapeutic considerations in cancer-related ESUS. Because cancer is a heterogeneous and dynamic disease with unique medical and psychological risks, it is paramount that neurologists and oncologists work closely together and utilize a patient-centric and comprehensive approach to manage these patients successfully.
Funding
NIH grants K23NS091395, R01HL144541, P20GM135007, and P30CA008748.
Disclosures
Babak Navi serves as a DSMB member for the PCORI-funded TRAVERSE trial and has received personal fees for medicolegal consulting on stroke. Scott Kasner has received grant funding from the NIH, Bristol Meyers Squibb, Bayer, Daiichi Sankyo, Genentech, WL Gore, and Medtronic; personal consulting fees from Bristol Meyers Squibb, Abbott, AbbVie; and royalties from UpToDate and Elsevier. Mitchell Elkind receives study drug in kind from the BMS-Pfizer Alliance for Eliquis® and ancillary research funding but no personal compensation for an NIH-funded trial of stroke prevention in patients with ESUS, grant support from Roche outside the submitted work, and royalties from UpToDate for a chapter on cryptogenic stroke. Lisa DeAngelis has received personal funds for serving on the scientific advisory board for Sapience Therapeutics. The other authors have no disclosures.
Non-standard Abbreviations and Acronyms
- ESUS
embolic stroke of undetermined source
- NAVIGATE ESUS
New Approach Rivaroxaban Inhibition of Factor Xa in a Global Trial versus Aspirin to Prevent Embolism in Embolic Stroke of Undetermined Source trial
- RE-SPECT ESUS
Randomized, Double-Blind, Evaluation in Secondary Stroke Prevention Comparing the Efficacy and Safety of the Oral Thrombin Inhibitor Dabigatran Etexilate versus Acetylsalicylic Acid in Patients with Embolic Stroke of Undetermined Source trial
- PFO
patent foramen ovale
- SOME
Screening for Occult Malignancy in Patients with Idiopathic Venous Thromboembolism trial
- OASIS-Cancer
Optimal Anticoagulation Strategy in Stroke Related to Cancer Study
- NETosis
neutrophil extracellular trap formation
- MOST-Cancer
Mechanisms of Ischemic Stroke in Cancer Patients study
- TCD
transcranial Doppler
- TTE
transthoracic echocardiography
- TEE
transesophageal echocardiography
- TEACH
Trial of Enoxaparin versus Aspirin in Patients with Cancer and Stroke
- TEACH2
Trial of Apixaban versus Aspirin in Cancer Patients with Cryptogenic Ischemic Stroke
- ENCHASE
Edoxaban for the Treatment of Coagulopathy in Patients with Active Cancer and Acute Ischemic Stroke: a Pilot Study
References
- 1.Boot E, Ekker MS, Putaala J, Kittner S, De Leeuw FE, Tuladhar AM. Ischaemic stroke in young adults: A global perspective. J Neurol Neurosurg Psychiatry. 2020;91:411–417 [DOI] [PubMed] [Google Scholar]
- 2.Feigin VL, Norrving B, Mensah GA. Global burden of stroke. Circ Res. 2017;120:439–448 [DOI] [PubMed] [Google Scholar]
- 3.Hart RG, Catanese L, Perera KS, Ntaios G, Connolly SJ. Embolic stroke of undetermined source: A systematic review and clinical update. Stroke. 2017;48:867–872 [DOI] [PubMed] [Google Scholar]
- 4.Hart RG, Diener HC, Coutts SB, Easton JD, Granger CB, O’Donnell MJ, Sacco RL, Connolly SJ, Cryptogenic Stroke EIWG. Embolic strokes of undetermined source: The case for a new clinical construct. Lancet Neurol. 2014;13:429–438 [DOI] [PubMed] [Google Scholar]
- 5.Hart RG, Connolly SJ, Mundl H. Rivaroxaban for stroke prevention after embolic stroke of undetermined source. N Engl J Med. 2018;379:987. [DOI] [PubMed] [Google Scholar]
- 6.Diener HC, Sacco RL, Easton JD, Granger CB, Bernstein RA, Uchiyama S, Kreuzer J, Cronin L, Cotton D, Grauer C, et al. Dabigatran for prevention of stroke after embolic stroke of undetermined source. N Engl J Med. 2019;380:1906–1917 [DOI] [PubMed] [Google Scholar]
- 7.Kamel H, Merkler AE, Iadecola C, Gupta A, Navi BB. Tailoring the approach to embolic stroke of undetermined source: A review. JAMA Neurol. 2019;76:855–861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ntaios G, Perlepe K, Lambrou D, Sirimarco G, Strambo D, Eskandari A, Karagkiozi E, Vemmou A, Koroboki E, Manios E, et al. Prevalence and overlap of potential embolic sources in patients with embolic stroke of undetermined source. J Am Heart Assoc. 2019;8:e012858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanossian N, Djabiras C, Mack WJ, Ovbiagele B. Trends in cancer diagnoses among inpatients hospitalized with stroke. J Stroke Cerebrovasc Dis. 2013;22:1146–1150 [DOI] [PubMed] [Google Scholar]
- 10.Kim SJ, Park JH, Lee MJ, Park YG, Ahn MJ, Bang OY. Clues to occult cancer in patients with ischemic stroke. PLoS One. 2012;7:e44959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Welch HG, Kramer BS, Black WC. Epidemiologic signatures in cancer. N Engl J Med. 2019;381:1378–1386 [DOI] [PubMed] [Google Scholar]
- 12.Howlader N, Forjaz G, Mooradian MJ, Meza R, Kong CY, Cronin KA, Mariotto AB, Lowy DR, Feuer EJ. The effect of advances in lung-cancer treatment on population mortality. N Engl J Med. 2020;383:640–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Navi BB, Howard G, Howard VJ, Zhao H, Judd SE, Elkind MSV, Iadecola C, DeAngelis LM, Kamel H, Okin PM, et al. New diagnosis of cancer and the risk of subsequent cerebrovascular events. Neurology. 2018;90:e2025–e2033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Navi BB, Reiner AS, Kamel H, Iadecola C, Elkind MS, Panageas KS, DeAngelis LM. Association between incident cancer and subsequent stroke. Ann Neurol. 2015;77:291–300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Elkind MSV, Panageas KS, DeAngelis LM. Risk of arterial thromboembolism in patients with cancer. J Am Coll Cardiol. 2017;70:926–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Cryptogenic subtype predicts reduced survival among cancer patients with ischemic stroke. Stroke. 2014;45:2292–2297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim SG, Hong JM, Kim HY, Lee J, Chung PW, Park KY, Kim GM, Lee KH, Chung CS, Bang OY. Ischemic stroke in cancer patients with and without conventional mechanisms: A multicenter study in Korea. Stroke. 2010;41:798–801 [DOI] [PubMed] [Google Scholar]
- 18.Schwarzbach CJ, Schaefer A, Ebert A, Held V, Bolognese M, Kablau M, Hennerici MG, Fatar M. Stroke and cancer: The importance of cancer-associated hypercoagulation as a possible stroke etiology. Stroke. 2012;43:3029–3034 [DOI] [PubMed] [Google Scholar]
- 19.Navi BB, DeAngelis LM, Segal AZ. Multifocal strokes as the presentation of occult lung cancer. J Neurooncol. 2007;85:307–309 [DOI] [PubMed] [Google Scholar]
- 20.Navi BB, Kawaguchi K, Hriljac I, Lavi E, DeAngelis LM, Jamieson DG. Multifocal stroke from tumor emboli. Arch Neurol. 2009;66:1174–1175 [DOI] [PubMed] [Google Scholar]
- 21.Navi BB, Reiner AS, Kamel H, Iadecola C, Okin PM, Tagawa ST, Panageas KS, DeAngelis LM. Arterial thromboembolic events preceding the diagnosis of cancer in older persons. Blood. 2019;133:781–789 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Marchetti M, Falanga A. Hemostatic biomarkers in occult cancer and cancer risk prediction. Thromb Res. 2020;191 Suppl 1:S37–S42 [DOI] [PubMed] [Google Scholar]
- 23.Selvik HA, Bjerkreim AT, Thomassen L, Waje-Andreassen U, Naess H, Kvistad CE. When to screen ischaemic stroke patients for cancer. Cerebrovasc Dis. 2018;45:42–47 [DOI] [PubMed] [Google Scholar]
- 24.Gon Y, Sakaguchi M, Takasugi J, Kawano T, Kanki H, Watanabe A, Oyama N, Terasaki Y, Sasaki T, Mochizuki H. Plasma d-dimer levels and ischaemic lesions in multiple vascular regions can predict occult cancer in patients with cryptogenic stroke. Eur J Neurol. 2017;24:503–508 [DOI] [PubMed] [Google Scholar]
- 25.Cocho D, Gendre J, Boltes A, Espinosa J, Ricciardi AC, Pons J, Jimenez M, Otermin P. Predictors of occult cancer in acute ischemic stroke patients. J Stroke Cerebrovasc Dis. 2015;24:1324–1328 [DOI] [PubMed] [Google Scholar]
- 26.Kassubek R, Bullinger L, Kassubek J, Dreyhaupt J, Ludolph AC, Althaus K, Lewerenz J. Identifying ischemic stroke associated with cancer: A multiple model derived from a case-control analysis. J Neurol. 2017;264:781–791 [DOI] [PubMed] [Google Scholar]
- 27.Uemura J, Kimura K, Sibazaki K, Inoue T, Iguchi Y, Yamashita S. Acute stroke patients have occult malignancy more often than expected. Eur Neurol. 2010;64:140–144 [DOI] [PubMed] [Google Scholar]
- 28.Carrier M, Le Gal G, Wells PS, Fergusson D, Ramsay T, Rodger MA. Systematic review: The trousseau syndrome revisited: Should we screen extensively for cancer in patients with venous thromboembolism? Ann Intern Med. 2008;149:323–333 [DOI] [PubMed] [Google Scholar]
- 29.Carrier M, Lazo-Langner A, Shivakumar S, Tagalakis V, Zarychanski R, Solymoss S, Routhier N, Douketis J, Danovitch K, Lee AY, et al. Screening for occult cancer in unprovoked venous thromboembolism. N Engl J Med. 2015;373:697–704 [DOI] [PubMed] [Google Scholar]
- 30.Bang OY, Chung JW, Lee MJ, Seo WK, Kim GM, Ahn MJ, Investigators OA-CS. Cancer-related stroke: An emerging subtype of ischemic stroke with unique pathomechanisms. J Stroke. 2020;22:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Navi BB, Mathias R, Sherman CP, Wolfe J, Kamel H, Tagawa ST, Saxena A, Ocean AJ, Iadecola C, DeAngelis LM, et al. Cancer-related ischemic stroke has a distinct blood mrna expression profile. Stroke. 2019;50:3259–3264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stefan O, Vera N, Otto B, Heinz L, Wolfgang G. Stroke in cancer patients: A risk factor analysis. J Neurooncol. 2009;94:221–226 [DOI] [PubMed] [Google Scholar]
- 33.Bang OY, Chung JW, Lee MJ, Kim SJ, Cho YH, Kim GM, Chung CS, Lee KH, Ahn MJ, Moon GJ. Cancer cell-derived extracellular vesicles are associated with coagulopathy causing ischemic stroke via tissue factor-independent way: The oasis-cancer study. PLoS One. 2016;11:e0159170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chung JW, Cho YH, Ahn MJ, Lee MJ, Kim GM, Chung CS, Bang OY. Association of cancer cell type and extracellular vesicles with coagulopathy in patients with lung cancer and stroke. Stroke. 2018;49:1282–1285 [DOI] [PubMed] [Google Scholar]
- 35.Hayes J, Peruzzi PP, Lawler S. MicroRNAs in cancer: Biomarkers, functions and therapy. Trends Mol Med. 2014;20:460–469 [DOI] [PubMed] [Google Scholar]
- 36.Thalin C, Demers M, Blomgren B, Wong SL, von Arbin M, von Heijne A, Laska AC, Wallen H, Wagner DD, Aspberg S. Netosis promotes cancer-associated arterial microthrombosis presenting as ischemic stroke with troponin elevation. Thromb Res. 2016;139:56–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bang OY, Chung JW, Cho YH, Oh MJ, Seo WK, Kim GM, Ahn MJ. Circulating DNAs, a marker of neutrophil extracellular traposis and cancer-related stroke: The oasis-cancer study. Stroke. 2019;50:2944–2947 [DOI] [PubMed] [Google Scholar]
- 38.Park H, Kim J, Ha J, Hwang IG, Song TJ, Yoo J, Ahn SH, Kim K, Kim BM, Kim DJ, et al. Histological features of intracranial thrombi in stroke patients with cancer. Ann Neurol. 2019;86:143–149 [DOI] [PubMed] [Google Scholar]
- 39.Blann AD, Dunmore S. Arterial and venous thrombosis in cancer patients. Cardiol Res Pract. 2011;2011:394740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Falanga A, Marchetti M, Vignoli A. Coagulation and cancer: Biological and clinical aspects. J Thromb Haemost. 2013;11:223–233 [DOI] [PubMed] [Google Scholar]
- 41.Seok JM, Kim SG, Kim JW, Chung CS, Kim GM, Lee KH, Bang OY. Coagulopathy and embolic signal in cancer patients with ischemic stroke. Ann Neurol. 2010;68:213–219 [DOI] [PubMed] [Google Scholar]
- 42.Graus F, Rogers LR, Posner JB. Cerebrovascular complications in patients with cancer. Medicine 1985;64:16–35 [DOI] [PubMed] [Google Scholar]
- 43.Navi BB, Singer S, Merkler AE, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Recurrent thromboembolic events after ischemic stroke in patients with cancer. Neurology. 2014;83:26–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gon Y, Okazaki S, Terasaki Y, Sasaki T, Yoshimine T, Sakaguchi M, Mochizuki H. Characteristics of cryptogenic stroke in cancer patients. Ann Clin Transl Neurol. 2016;3:280–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Merkler AE, Navi BB, Singer S, Cheng NT, Stone JB, Kamel H, Iadecola C, Elkind MS, DeAngelis LM. Diagnostic yield of echocardiography in cancer patients with ischemic stroke. J Neurooncol. 2015;123:115–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Blom JW, Doggen CJ, Osanto S, Rosendaal FR. Malignancies, prothrombotic mutations, and the risk of venous thrombosis. JAMA. 2005;293:715–722 [DOI] [PubMed] [Google Scholar]
- 47.Iguchi Y, Kimura K, Kobayashi K, Ueno Y, Inoue T. Ischaemic stroke with malignancy may often be caused by paradoxical embolism. J Neurol Neurosurg Psychiatry. 2006;77:1336–1339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016;133:1104–1114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campen CJ, Kranick SM, Kasner SE, Kessler SK, Zimmerman RA, Lustig R, Phillips PC, Storm PB, Smith SE, Ichord R, et al. Cranial irradiation increases risk of stroke in pediatric brain tumor survivors. Stroke. 2012;43:3035–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu J, Cao Y. Radiation-induced carotid artery stenosis: A comprehensive review of the literature. Interv Neurol. 2014;2:183–192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bright CJ, Hawkins MM, Guha J, Henson KE, Winter DL, Kelly JS, Feltbower RG, Hall M, Cutter DJ, Edgar AB, et al. Risk of cerebrovascular events in 178 962 five-year survivors of cancer diagnosed at 15 to 39 years of age: The TYACSS (teenage and young adult cancer survivor study). Circulation. 2017;135:1194–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chang HM, Moudgil R, Scarabelli T, Okwuosa TM, Yeh ETH. Cardiovascular complications of cancer therapy: Best practices in diagnosis, prevention, and management: Part 1. J Am Coll Cardiol. 2017;70:2536–2551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Mulrooney DA, Yeazel MW, Kawashima T, Mertens AC, Mitby P, Stovall M, Donaldson SS, Green DM, Sklar CA, Robison LL, et al. Cardiac outcomes in a cohort of adult survivors of childhood and adolescent cancer: Retrospective analysis of the childhood cancer survivor study cohort. BMJ. 2009;339:b4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ramos-Casals M, Brahmer JR, Callahan MK, Flores-Chavez A, Keegan N, Khamashta MA, Lambotte O, Mariette X, Prat A, Suarez-Almazor ME. Immune-related adverse events of checkpoint inhibitors. Nat Rev Dis Primers. 2020;6:38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vinter N, Christesen AMS, Fenger-Gron M, Tjonneland A, Frost L. Atrial fibrillation and risk of cancer: A danish population-based cohort study. J Am Heart Assoc. 2018;7:e009543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kiyuna F, Sato N, Matsuo R, Kamouchi M, Hata J, Wakisaka Y, Kuroda J, Ago T, Kitazono T, Fukuoka Stroke Registry I. Association of embolic sources with cause-specific functional outcomes among adults with cryptogenic stroke. JAMA Netw Open. 2018;1:e182953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ohara T, Farhoudi M, Bang OY, Koga M, Demchuk AM. The emerging value of serum d-dimer measurement in the work-up and management of ischemic stroke. Int J Stroke. 2020;15:122–131 [DOI] [PubMed] [Google Scholar]
- 58.Álvarez-Pérez FJ, Verde I, Usón-Martín M, Figuerola-Roig A, Ballabriga-Planas J, Espino-Ibañez A. Frequency and mechanism of ischemic stroke associated with malignancy: A retrospective series. Eur Neurol. 2012;68:209–213 [DOI] [PubMed] [Google Scholar]
- 59.Kim JM, Jung KH, Park KH, Lee ST, Chu K, Roh JK. Clinical manifestation of cancer related stroke: Retrospective case-control study. J Neurooncol. 2013;111:295–301 [DOI] [PubMed] [Google Scholar]
- 60.Shin YW, Lee ST, Jung KH, Kim DY, Park CK, Kim TM, Choi SH, Chu K, Lee SK. Predictors of survival for patients with cancer after cryptogenic stroke. J Neurooncol. 2016;128:277–284 [DOI] [PubMed] [Google Scholar]
- 61.Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, Biller J, Brown M, Demaerschalk BM, Hoh B, et al. 2018 guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the american heart association/american stroke association. Stroke. 2018;49:e46–e110 [DOI] [PubMed] [Google Scholar]
- 62.Lee MJ, Chung JW, Ahn MJ, Kim S, Seok JM, Jang HM, Kim GM, Chung CS, Lee KH, Bang OY. Hypercoagulability and mortality of patients with stroke and active cancer: The oasis-cancer study. J Stroke. 2017;19:77–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cramer SC, Rordorf G, Maki JH, Kramer LA, Grotta JC, Burgin WS, Hinchey JA, Benesch C, Furie KL, Lutsep HL, et al. Increased pelvic vein thrombi in cryptogenic stroke: Results of the paradoxical emboli from large veins in ischemic stroke (PELVIS) study. Stroke. 2004;35:46–50 [DOI] [PubMed] [Google Scholar]
- 64.Navi BB, Marshall RS, Bobrow D, Singer S, Stone JB, DeSancho MT, DeAngelis LM. Enoxaparin vs aspirin in patients with cancer and ischemic stroke: The TEACH pilot randomized clinical trial. JAMA Neurol. 2018;75:379–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nam KW, Kim CK, Kim TJ, An SJ, Oh K, Ko SB, Yoon BW. Treatment of cryptogenic stroke with active cancer with a new oral anticoagulant. J Stroke Cerebrovasc Dis. 2017;26:2976–2980 [DOI] [PubMed] [Google Scholar]
- 66.Raskob GE, van Es N, Verhamme P, Carrier M, Di Nisio M, Garcia D, Grosso MA, Kakkar AK, Kovacs MJ, Mercuri MF, et al. Edoxaban for the treatment of cancer-associated venous thromboembolism. N Engl J Med. 2018;378:615–624 [DOI] [PubMed] [Google Scholar]
- 67.Agnelli G, Becattini C, Meyer G, Munoz A, Huisman MV, Connors JM, Cohen A, Bauersachs R, Brenner B, Torbicki A, et al. Apixaban for the treatment of venous thromboembolism associated with cancer. N Engl J Med. 2020;382:1599–1607 [DOI] [PubMed] [Google Scholar]
- 68.Key NS, Khorana AA, Kuderer NM, Bohlke K, Lee AYY, Arcelus JI, Wong SL, Balaban EP, Flowers CR, Francis CW, et al. Venous thromboembolism prophylaxis and treatment in patients with cancer: ASCO clinical practice guideline update. J Clin Oncol. 2019:JCO1901461 [DOI] [PubMed] [Google Scholar]
- 69.Martinez-Majander N, Ntaios G, Liu YY, Ylikotila P, Joensuu H, Saarinen J, Perera KS, Marti-Fabregas J, Chamorro A, Rudilosso S, et al. Rivaroxaban versus aspirin for secondary prevention of ischaemic stroke in patients with cancer: A subgroup analysis of the NAVIGATE ESUS randomized trial. Eur J Neurol. 2020;27:841–848 [DOI] [PubMed] [Google Scholar]
- 70.Chimowitz MI, Lynn MJ, Howlett-Smith H, Stern BJ, Hertzberg VS, Frankel MR, Levine SR, Chaturvedi S, Kasner SE, Benesch CG, et al. Comparison of warfarin and aspirin for symptomatic intracranial arterial stenosis. N Engl J Med. 2005;352:1305–1316 [DOI] [PubMed] [Google Scholar]
- 71.Investigators CT, Markus HS, Hayter E, Levi C, Feldman A, Venables G, Norris J. Antiplatelet treatment compared with anticoagulation treatment for cervical artery dissection (CADISS): A randomised trial. Lancet Neurol. 2015;14:361–367 [DOI] [PubMed] [Google Scholar]
- 72.Kamphuisen PW, Beyer-Westendorf J. Bleeding complications during anticoagulant treatment in patients with cancer. Thromb Res. 2014;133 Suppl 2:S49–55 [DOI] [PubMed] [Google Scholar]
- 73.Mantia C, Uhlmann EJ, Puligandla M, Weber GM, Neuberg D, Zwicker JI. Predicting the higher rate of intracranial hemorrhage in glioma patients receiving therapeutic enoxaparin. Blood. 2017;129:3379–3385. [DOI] [PubMed] [Google Scholar]
- 74.Lee AYY. When can we stop anticoagulation in patients with cancer-associated thrombosis? Blood. 2017;130:2484–2490. [DOI] [PubMed] [Google Scholar]
- 75.Timp JF, Braekkan SK, Versteeg HH, Cannegieter SC. Epidemiology of cancer-associated venous thrombosis. Blood. 2013;122:1712–1723 [DOI] [PubMed] [Google Scholar]
- 76.Eikelboom JW, Connolly SJ, Bosch J, Dagenais GR, Hart RG, Shestakovska O, Diaz R, Alings M, Lonn EM, Anand SS, et al. Rivaroxaban with or without aspirin in stable cardiovascular disease. N Engl J Med. 2017;377:1319–1330 [DOI] [PubMed] [Google Scholar]
- 77.Boulet J, Peña J, Hulten EA, Neilan TG, Dragomir A, Freeman C, Lambert C, Hijal T, Nadeau L, Brophy JM, et al. Statin use and risk of vascular events among cancer patients after radiotherapy to the thorax, head, and neck. J Am Heart Assoc. 2019;8:e005996. [DOI] [PMC free article] [PubMed] [Google Scholar]


