Abstract
Background and Purpose:
There is increasing recognition of the importance of cortical microinfarcts to overall brain health, cognition, and Alzheimer’s dementia. Cerebral small vessel pathologies are associated with microinfarcts, and frequently coexist with Alzheimer’s disease (AD); however, the extent to which β-amyloid and tau pathology modulates microvascular pathogenesis is not fully understood. Study objective was to examine the relationship of small vessel pathologies, arteriolosclerosis and cerebral amyloid angiopathy (CAA), with cortical microinfarcts in persons with differing levels of β-amyloid or tau tangle burden.
Methods:
Participants were 1,489 autopsied older persons (mean age-at-death=89 years; 67% women) from one of three ongoing clinical-pathologic cohort studies of aging. Neuropathological evaluation identified cortical β-amyloid and tau tangle burden using immunohistochemistry in 8 brain regions, provided semi-quantitative grading of cerebral vessel pathologies, and identified the presence of cortical microinfarcts. Logistic regression models, adjusted for demographics and atherosclerosis, and examined whether β-amyloid or tau tangle burden modified relations between small vessel pathologies and cortical microinfarcts.
Results:
Cortical microinfarcts were present in 17% of older persons, moderate-to-severe CAA pathology in 36%, and arteriolosclerosis in 34%. In logistic regression models, we found interactions with β-amyloid and tau tangles, reflecting that the association between arteriolosclerosis and cortical microinfarcts was stronger in the context of greater β-amyloid (estimate = 0.15; SE = 0.07; p = 0.02) and tau tangle burden (estimate = 0.13; SE = 0.06; p = 0.02). Interactions also emerged for CAA, suggesting that the association between CAA and cortical microinfarcts is more robust in the presence of higher β-amyloid (estimate = 0.27; SE = 0.07; p<0.001) and tangle burden (estimate = 0.16; SE = 0.06; p = 0.005).
Conclusions:
These findings suggest that in the presence of elevated β-amyloid or tangle pathology, small vessel pathologies are associated with greater microvascular tissue injury; highlighting a potential link between neurodegenerative and vascular mechanisms.
Keywords: Microvascular Pathology, Alzheimer’s disease, Small vessel disease
INTRODUCTION
In older individuals, pathological changes affecting small vessels in the brain are important risk factors for ischemic tissue injury. Common subtypes of small vessel pathologies include arteriolosclerosis which is characterized by hyaline thickening of arterioles, and cerebral amyloid angiopathy (CAA) where accumulation of β-amyloid deposits occurs within the vessel wall. We and others have shown both arteriolosclerosis and CAA are associated with increased likelihood for microinfarct tissue injury 1–5. Data from clinical pathological studies indicate that a ‘pure’ vascular burden is relatively uncommon, and that the majority of older individuals exhibit mixed pathologies at autopsy; with mixed AD and vascular being the most common 6,7. Further, there is increasing recognition that cortical microinfarcts are common in the aging brain, especially in the context of Alzheimer’s dementia, and are associated with worse cognitive performances 8–12.
While it is evident that there are numerous pathogenic changes in the aging brain, most commonly there is accumulation of β-amyloid and tau peptides associated with AD pathology, as well as vascular changes associated small vessel pathologies and ischemic tissue injuries 13,14. However, the relationship between AD pathology and vascular changes remains complex, and it remains unclear whether β-amyloid and tau burden can influence or promote an ischemic environment. Mechanistically, studies using both human tissue and animal models have reported amyloid- and tau-dependent neurotoxicity may impact endothelial biology, altering the integrity of the microvasculature in the brain, thus impacting cerebral perfusion 15–19, an etiology associated with cortical microinfarcts 20,21. Clarifying the relationship between AD pathology and vascular tissue injury will be important in understanding small vessel disease in the context of mixed pathologies.
In the present study we examined whether β-amyloid and tau burden influences cortical microinfarct pathogenesis specifically in persons with severe small vessel disease. Data came from 1,489 participants enrolled in one of 3 longitudinal clinical pathologic cohort studies of aging. A series of systematic logistic regression models were employed to examine whether β-amyloid or tau tangle modified the relationship between cerebral vessel pathologies (arteriolosclerosis and CAA) and cortical microinfarcts.
METHODS
Participants
Participants enrolled in one of three ongoing cohort studies of aging; the Religious Orders Study (ROS), the Rush Memory and Aging Project (MAP), and the Minority Aging Research Study (MARS) 22. ROS started in 1994 and recruits older Catholic priests, nuns, and brothers from across the United States, MAP started in 1997 and recruits older adults from across the Chicagoland area, and MARS started in 2004 and enrolls older African-Americans. Eligibility in all 3 studies requires enrollment without known dementia, and agreement to undergo annual clinical evaluation and interview. Participants in both ROS and MAP consent to brain donation at the time of death; for MARS, brain donation is optional. Follow-up across all studies exceeds 85%, with autopsy rates exceeding 95% for ROS, 80% for MAP, and 55% for MARS. Each study was approved by a Rush University Medical Center institutional review board. All participants signed an informed consent and Anatomical Gift Act for brain donation. Of note, all 3 studies share uniform structured neuropathologic evaluations. At the time of the analyses, 4,168 older persons were recruited and had completed baseline evaluations, of whom 1,893 died, and 1,545 were autopsied. Neuropathologic workup for diagnostic purposes was completed on 1,522; and 1,489 had complete data necessary for the current study. Age was calculated from birth date and date of death. Education was based on self-reported years of regular schooling. Sex was self-reported. Demographic and neuropathologic characteristics across the three cohorts are included (Supplemental Materials I). For dementia diagnosis, clinical data was reviewed by a neurologist with expertise in aging and dementia, blind to all neuropathologic data, as previously described 22.
Neuropathology
The average post-mortem interval was 9.25 hours (SD=8.51), and brain removal and processing followed a standard protocol, as previously described 23,24. Briefly, one hemisphere was cut into 1-cm coronal slabs and fixed in 4% paraformaldehyde for 48–72 hours. Tissue from 11 brain regions including cortical, subcortical, brainstem, and midbrain was taken for diagnostic purposes. In addition, 8 cortical brain regions were taken for β-amyloid and tau tangle image analysis. To exclude potential sources of bias, all neuropathologic data was collected blind to demographic or clinical information.
Cortical β-amyloid load and tau tangle density
Tissue blocks from 8 brain regions; entorhinal, midfrontal (Brodmann [BA] 46/9), inferior temporal (BA 20), inferior parietal (BA 39/40), calcarine (BA 17), anterior cingulate (BA 24), and superior frontal (BA 6/8) cortices, and CA1/subiculum subfield of the hippocampus, were cut into 20μm sections. During the course of the study, immunohistochemistry was performed using three monoclonal antibodies against Aβ, 4G8 (1:9000; Covance Labs, Madison, WI), 6F/3D (1:50; Dako North America Inc., Carpinteria, CA), and 10D5 (1:600; Elan Pharmaceuticals, San Francisco, CA). For β-amyloid, cortical regions of interest were manually outlined (excluding meninges) and analyzed at 20x using an Olympus BX51 microscope attached to an MBF CX9000 camera coupled to a stage driver. The systematic random sampling (SRS) grid within the Stereo Investigator software was applied to the regions of interest. Images at each sampling grid were taken, and using Image J software, a percentage of amyloid positivity was calculated using a custom positive-pixel algorithm. For analyses, percentage of amyloid positivity was averaged across, as previously described 25. For assessment of neuritic plaque burden, manual counts were obtained by experienced neuropathologists (Supplemental Materials II). For assessment of PHF-tau tangle pathology, an antibody specific to phosphorylated tau, AT8 (1:2000, Thermoscientific) was used. Manual annotation of regions of interest were outlined by experienced raters trained to recognize neurofibrillary tangles. Using the StereoInvestigator software, the SRS and optical fractionator probe were applied to regions to obtain an estimate quantitative measure for the total number of neurofibrillary tangle cell counts within a defined area, which was then calculated as a density measure per square millimeter for analyses. Other tau pathologies (glial tau and tau neurites) were not included. During the duration of the study, multiple raters have collected neurofibrillary tangle data, in which standard protocols were followed (Supplemental Materials III). For analyses, a composite measures for β-amyloid burden and PHF-tau tangle density were only computed if 4 or more regions were non-missing, and obtained by averaging the mean percentage area per region, across all regions 25. Square root transformation was also applied to tau-tangle density and cortical β-amyloid load.
Cerebrovascular pathology
Microscopic Infarcts
Microscopic infarcts were not visible to the naked eye and identified by microscopy in tissue blocks, including cortical, subcortical, midbrain, and cerebellum regions, that were paraffin-embedded, and stained with hematoxylin & eosin. Location and age were also documented 24. For this study, we considered any ischemic lesion detected on histological examination, that was not identified on gross examination, to be a microscopic infarct. Typically, chronic microinfarct lesions would present with cystic cavitation with a few remaining macrophages surrounded by fibrillary gliosis. In addition, morphology of chronic microinfarcts could also include a ‘scar’ like lesion with very little or no cavitation with possible invagination of the pial surface. Age of all microscopic infarcts were evaluated and defined by experienced neuropathologists. For analyses, only chronic infarcts were considered, and all infarct variables dichotomous, and categorized into absent vs. present. Variables were further categorized into cortical or subcortical locations.
Cerebral Amyloid Angiopathy (CAA)
Meningeal and parenchymal vessels from 4 neocortical regions (midfrontal, midtemporal, inferior parietal, and calcarine cortices) were evaluated on sections immunostained with three monoclonal antibodies against Aβ, 4G8 (1:9000; Covance Labs, Madison, WI), 6F/3D (1:50; Dako North America Inc., Carpinteria, CA), and 10D5 (1:600; Elan Pharmaceuticals, San Francisco, CA). CAA scores were scored 0–4 based on amyloid deposition, where 0 = no deposition, 1 = scant amyloid deposition, 2 = circumferential deposition in up to 10 vessels, 3 = circumferential deposition in up to 75% of vessels, and 4 = circumferential deposition in over 75% of vessels. A score for each region was created using the maximum of the meningeal and parenchymal score. A continuous summary score was created by averaging scores across regions 26.
Arteriolosclerosis
Small vessels in the basal ganglia were evaluated on hematoxylin and eosin stained sections. For analyses, grading used a semi-quantitative 4-level rating system (0=none, 1=mild, 2=moderate, and 3=severe) based on the histological changes of the small arterioles, including intimal deterioration, smooth muscle degeneration, and hyaline concentric thickening with narrowing of the vascular lumen 27. In a subset of participants, we assessed arteriolosclerosis pathology in both the subcortical frontal and parietal white matter using the same semi-quantitative grading scheme and examined the correlation between our basal ganglia arteriolosclerosis measures with other regional arteriosclerosis measures (Supplemental Materials IV). We show that our measure of arteriolosclerosis in the basal ganglia is moderately representative of arteriosclerosis measures in other brain regions closely linked to frontal and parietal lobes.
Atherosclerosis
Large vessel atherosclerosis was evaluated at the circle of Willis at the base of the brain, and included evaluation of the vertebral, basilar, posterior, middle, and anterior cerebral arteries, and their proximal branches. Visual examination included the number of atherosclerotic plaque, extent of involvement in each vessel, and the degree of vessel occlusion. For analyses, a semi-quantitative 4-level grading system (0–3) was used; where 0 (none) = no significant atherosclerosis observed, sparse number of plaques (1–2) without vessel occlusion; 1 (mild) = small number of plaques (3–7) in up to several arteries without occlusion, 2 (moderate) = moderate number of plaques (8–11), involvement of 50% - 75% of vessel length, or up to 75% vessel occlusion, and 3 (severe) = frequent number of plaques (>11), involvement of >75% of vessel, or >75% vessel occlusion 27.
Statistical Analyses
First, we used a single logistic regression model without an interaction term to examine main effects of arteriolosclerosis and CAA with cortical microinfarcts, adjusted for demographics, atherosclerosis, β-amyloid, and tau tangles burden. Next, we included an interaction term in models to examine association of arteriolosclerosis with cortical microinfarcts in persons with and without β-amyloid. We fitted a logistic regression model with cortical microinfarcts as the outcome, terms for beta-amyloid and arteriolosclerosis, as well as an interaction term between arteriolosclerosis and β-amyloid, and terms to adjust for demographics, tau burden, atherosclerosis and CAA. Next, we repeated analyses, but included an interaction term between CAA and β-amyloid, adjusting for demographics, tau burden, atherosclerosis, and arteriolosclerosis. To examine differences in the association of arteriolosclerosis with cortical microinfarcts in persons with and without tau tangle burden, we repeated similar analyses as previously stated, but replaced the interaction term for β-amyloid and arteriolosclerosis with tau tangle burden and arteriolosclerosis. Models that included an interaction term between arteriolosclerosis and PHF-tau tangles were adjusted for demographics, β-amyloid, atherosclerosis and CAA. Models that included an interaction term between CAA and tau tangles were adjusted for demographics, β-amyloid, atherosclerosis and arteriolosclerosis. All analyses were conducted with SAS/STAT software version 9.4 (SAS Institute Inc, Cary, NC). Statistical significance was determined at nominal α level 0.05.
Data Availability
Raw data are available by request through the Rush Alzheimer’s Disease Center (RADC) Research Resource Sharing Hub https://www.radc.rush.edu/
RESULTS
Participants
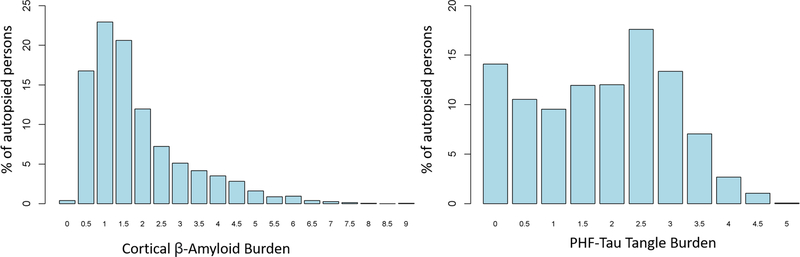
1,489 participants were included. Characteristics of participants are presented in Table 1. Mean age-at-death was 89 years, with 67% women. Cortical microinfarcts were present in 17% of older persons. Those with cortical microinfarcts were older at death, more likely to be over 90 years, and more likely to have microinfarcts in subcortical regions (basal ganglia or thalamus nuclei). Moderate-to-severe small vessel pathologies were present in about 40% of older persons with cortical microinfarcts, and 35% of persons without cortical microinfarcts. Individuals with cortical microinfarcts mixed with moderate-severe arteriolosclerosis pathology were more likely to be older (90.7 years) compared to those without either pathology (88.8 years) (Z=3.1; p=0.001), with 64.7% having dementia at last evaluation. Similarly, those with cortical microinfarcts mixed with moderate-severe CAA were more likely to be older (90.9 years vs. 88.8 years) (Z=3.0; P=0.001) with 60.0% having dementia at last evaluation. Only 12% (n=179) of participants had both moderate-to-severe arteriolosclerosis and CAA pathology. The distribution of β-amyloid load was weakly bimodal, with values ranging from 0 to 5 units; the 95th percentile was 3.5 units. By contrast, the distribution of tau tangle burden was right skewed, with the 95th percentile being 4.5 units, and the largest value being 9 units. (Figure 1).
Table 1.
Characteristics of participants
| All (n=1,489) | With cortical microinfarcts (n=256) | Without cortical microinfarcts (n=1,233) | p-Value | |
|---|---|---|---|---|
| Demographics, Mean (SD) or N (%) | ||||
| Age-at-death | 88.9 (6.79) | 89.9 (6.95) | 88.7 (6.74) | 0.004 |
| 90+ | 788 (53%) | 142 (55.5%) | 547 (44.4%) | <0.001 |
| Sex, Women | 1004 (67%) | 168 (65.6%) | 834 (67.6%) | 0.64 |
| Education | 16.2 (3.63) | 18.8 (3.67) | 16.3 (3.63) | 0.08 |
| Neuropathologic, Mean (SD) or N (%) | ||||
| Pathologic diagnosis of AD | 958 (64.3%) | 166 (64.8%) | 791 (64.2%) | 0.72 |
| Cortical β-Amyloid burden | 1.59 (1.13) | 1.72 (1.11) | 1.56 (1.14) | 0.04 |
| PHF-tau Tangle burden | 1.61 (1.34) | 1.68 (1.39) | 1.60 (1.14) | 0.26 |
| Moderate-to-severe CAA | 517 (35.5%)* | 106 (42.2%) | 412 (34.2%) | 0.02 |
| Moderate-to-severe arteriolosclerosis | 504 (34.1%)* | 100 (39.5%) | 404 (33.1%) | 0.05 |
| Moderate-to-severe atherosclerosis | 504 (34.1%)* | 86 (34.0%) | 418 (34.0%) | 0.99 |
| Subcortical Microinfarcts | 218 (14.6%) | 66 (25.8%) | 152 (12.3%) | <0.001 |
Data missing for n=32 for CAA; n=12 for arteriolosclerosis; n=6 for atherosclerosis. Abbreviations: SD – standard deviation, N- number, AD – Alzheimer’s disease, PHF – paired helical filament, CAA – cerebral amyloid angiopathy
Figure 1. Distribution of β-amyloid and tau tangle burden.
The histogram reveals a bimodal pattern in the distribution for cortical β-amyloid burden and a right-skewed distribution for PHF-tau tangle burden.
Small Vessel Pathologies and Microinfarct Burden
First, we examined the association between arteriolosclerosis and CAA pathology with cortical and subcortical microinfarcts, and fitted logistic regression models without an interaction term, adjusting for age, sex, education, atherosclerosis, β-amyloid, and tau tangle burden, with regional microinfarcts as the outcome. Persons with arteriolosclerosis pathology had 17% higher odds of having cortical microinfarcts (OR=1.17; 95% CI: 1.01–1.38; p=0.04) and 37% higher odds of having subcortical microinfarcts (OR=1.37; 95% CI: 1.16 – 1.62; p<0.01). Those with CAA had 16% higher odds of having cortical microinfarcts, but did not reach the threshold for statistical significance (OR=1.16; 95% CI: 0.99 – 1.35; p=0.06), and were not associated with subcortical microinfarcts (OR=0.98; 95% CI: 0.83 – 1.16; p=0.80). β-amyloid and tau tangle pathology were not related to cortical or subcortical microinfarcts (both p’s>0.10).
Small Vessel Pathologies, β-Amyloid, and Microinfarct Burden
To examine whether β-amyloid burden modified the association between small vessel pathologies (arteriolosclerosis and CAA) and cortical microinfarcts, logistic regression models included an interaction term between β-amyloid and the presence of arteriolosclerosis (Table 2). β-amyloid burden modified the association between arteriolosclerosis and cortical microinfarcts. With each additional 1 unit of β-amyloid, arteriolosclerosis pathology was associated with 0.15-unit higher log-odds for cortical microinfarcts, suggesting the association of arteriolosclerosis with cortical microinfarcts occurs only in the presence of higher β-amyloid levels. Table 3 illustrates this interaction for persons with differing levels of β-amyloid. For example, a person with β-amyloid at the 75th percentile would have about 1.5 times the likelihood of microinfarcts compared to someone at the lowest quartile (Table 3).
Table 2.
Interaction of cerebral vessel pathology with β-amyloid/ PHF-tau tangle burden in relation to probability of microinfarct burden.
| Model Set | Predictors | Interaction | Cortical microinfarcts | Subcortical microinfarcts |
|---|---|---|---|---|
| 1 | Arteriolosclerosis | −0.10 (0.14,0.48) | 0.35 (0.14,0.01) | |
| β-amyloid | −0.12 (0.11,0.26) | 0.09 (0.12,0.48) | ||
| Arteriolosclerosis x β-amyloid | 0.15 (0.07,0.02) | 0.02 (0.07,0.73) | ||
| 2 | CAA | −0.29 (0.14,0.05) | −0.05 (0.15,0.76) | |
| β-amyloid | −0.24 (0.11,0.03) | 0.04 (0.11,0.73) | ||
| CAA x β-amyloid | 0.27 (0.07, <0.001) | 0.02 (0.08,0.84) | ||
| 3 | Arteriolosclerosis | −0.06 (0.12,0.64) | 0.11 (0.13,0.39) | |
| PHF-tau | −0.22 (0.10,0.03) | −0.34 (0.13,0.01) | ||
| Arteriolosclerosis x PHF-tau | 0.13 (0.06,0.02) | 0.13 (0.07,0.04) | ||
| 4 | CAA | −0.10 (0.12,0.40) | 0.09 (0.13,0.49) | |
| PHF-tau | −0.36 (0.13,0.01) | −0.01 (0.13,0.93) | ||
| CAA x PHF-tau | 0.16 (0.06,0.005) | −0.08 (0.07,0.27) |
Log-odds ratio estimates were derived from logistic regression models in which cortical microinfarct burden was the outcome. Models included an interaction term between vessel pathologies, either CAA or arteriolosclerosis, and β-amyloid or tau tangle burden. All model sets were adjusted for age, sex, education, and atherosclerosis. In addition, model set 1 was further adjusted for CAA and tangle burden. Model set 2 for arteriolosclerosis and tangles. Model set 3 for CAA and β-amyloid. Model set 4 for arteriolosclerosis and β-amyloid. Cell value represents log-odds estimate (SE, p-value).
Table 3.
Estimated probability for the association between cerebral vessel pathology and cortical microinfarcts at differing percentiles of β-amyloid/tau tangle burden.
| Vessel Pathology | β-Amyloid Burden (percentile) | Tau Tangle Burden (percentile) | Cortical Microinfarct OR (95% CI) | |
|---|---|---|---|---|
| Arteriolosclerosis | X | 25th | 0.98 (0.79–1.22) | |
| 50th | 1.17 (1.00–1.37) | |||
| 75th | 1.32 (1.09–1.59) | |||
| CAA | X | 25th | 0.87 (0.69–1.08) | |
| 50th | 1.18 (1.00–1.38) | |||
| 75th | 1.46 (1.20–1.78) | |||
| Arteriolosclerosis | X | 25th | 1.03 (0.85–1.25) | |
| 50th | 1.10 (0.93–1.30) | |||
| 75th | 1.23 (1.05–1.46) | |||
| CAA | X | 25th | 1.01 (0.84–1.21) | |
| 50th | 1.10 (0.93–1.29) | |||
| 75th | 1.28 (1.08–1.51) |
Odds ratio (OR) estimates were derived from logistic regression models in which cortical microinfarct burden was the outcome. Models included an interaction term between small vessel pathologies, either CAA or arteriolosclerosis, with β-amyloid or tau tangle burden.
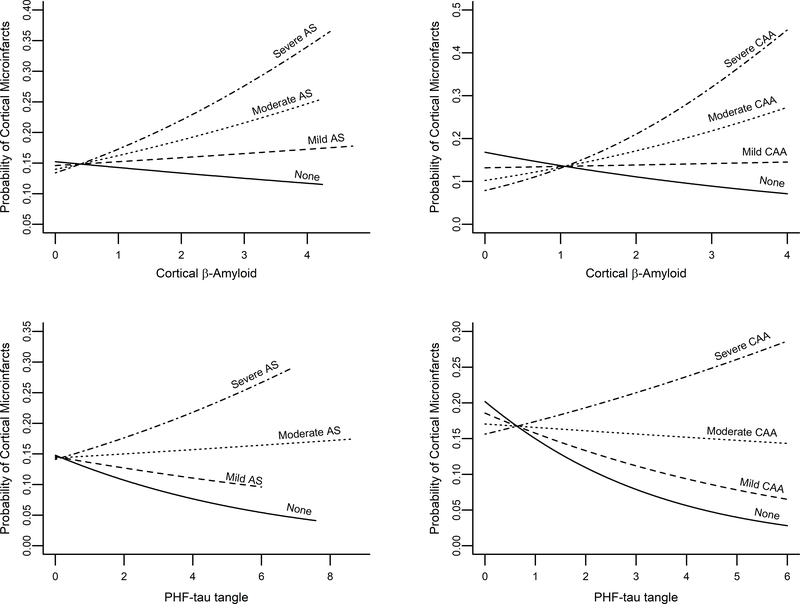
Next, we included an interaction term between β-amyloid and CAA (Table 2). The interaction term was positive (0.27 units) and significant (p<0.001), suggesting that the association between CAA and cortical microinfarcts is stronger with more severe β-amyloid. The estimated probability for cortical microinfarcts was about 2-times higher in those with β-amyloid burden at the 75th percentile compared to the 25th percentile (Table 3). Figure 2A and 2B displays the estimated probability of cortical microinfarct tissue injury versus level of β-amyloid burden by each level of arteriolosclerosis and CAA pathology.
Figure 2. Probability of cortical microinfarct tissue injury versus level of β-amyloid and PHF-tau tangle burden by level of arteriolosclerosis and CAA pathology.
Figures show the estimated probability of cortical microinfarcts for representative women with an average age-at-death at 89 years, 16 years of education, and no atherosclerosis. Abbreviations: AS – Arteriolosclerosis; CAA – Cerebral Amyloid Angiopathy; PHF – Paired-Helical Filament
In contrast, β-amyloid burden did not modify the association between either arteriolosclerosis or CAA with subcortical microinfarcts (Table 2). In sensitivity analyses, we also ran similar logistic regression models and included an interaction term between neuritic plaques and either arteriolosclerosis or CAA on microinfarct burden (Supplemental Materials V). We find a significant interaction remained between CAA and neuritic plaque burden on cortical microinfarcts (estimate = 0.21; SE = 0.09; p = 0.02), while the significance between arteriolosclerosis and neuritic plaque burden on cortical microinfarcts became attenuated (estimate = 0.12; SE = 0.09; p = 0.15), an effect likely due to power since neuritic plaque data is collected on 4 brain regions vs. using β-amyloid data which is collected on 8 brain regions and is collected with greater sampling methods, thus getting a more granular measure of amyloid burden.
Small Vessel Pathologies, Tau Tangle Burden, and Microinfarct Burden
Next, we examined whether arteriolosclerosis or CAA increased likelihood of cortical microinfarcts in the presence of tau tangle burden. In the same approach, we included an interaction term between tau tangle burden and arteriolosclerosis in logistic regression models (Table 2). With every 1 additional unit of tau tangle load, arteriolosclerosis pathology was associated with 0.13-unit higher log-odds for cortical microinfarcts. Given the level of arteriolosclerosis pathology, those with tau tangle burden at the 75th percentile were associated with a higher odds ratio than those at the 25th percentile (Table 3).
Including an interaction term for tau tangle pathology also demonstrated modification between CAA pathology and cortical microinfarcts (Table 2). Odds ratio for the effect of CAA on cortical microinfarcts was higher in the presence of tangle burden at the 75th percentile (OR=1.28; 95% CI: 1.08–1.51) versus tangle burden at the 50th (OR=1.10) or 25th percentile (OR=1.01) (Table 3. The estimated probability of cortical microinfarct tissue injury versus level of tau tangle burden by level of arteriolosclerosis and CAA pathology is displayed in Figure 2C and 2D. Unexpectedly, in models with the interaction term, we found that in the absence of arteriolosclerosis or CAA pathology, there was an inverse association between tau tangle pathology and cortical microinfarcts (Table 2). In sensitivity analyses, we found this association was driven by those individuals within the highest tau tangle percentile with few microinfarcts (Supplemental Materials VI).
Finally, including an interaction term for tau tangle pathology modified the association between arteriolosclerosis and subcortical microinfarcts, but not between CAA and subcortical microinfarcts. With every 1 additional unit of tau tangle load, arteriolosclerosis pathology was associated with 0.13-unit higher log-odds for subcortical microinfarcts (Table 2).
DISCUSSION
In the present study, we explored a potential interaction between AD pathology and small vessel disease on microvascular tissue injury. We examined neuropathologic data from 1,489 community-based older persons to examine whether elevated levels of β-amyloid deposits or tau tangle pathology modified the association between arteriolosclerosis or cerebral amyloid angiopathy and presumably downstream microinfarct tissue injury. We found that the association between arteriolosclerosis and CAA with cortical microinfarcts became more robust in the context of greater β-amyloid and tau tangle burden. While only the association between arteriolosclerosis and subcortical microinfarcts was greater with elevated tau tangle burden.
There is very limited data regarding direct or indirect involvement of amyloid and tau deposits on microinfarct pathogenesis. While multiple studies have shown that individuals with both AD and vessel brain pathologies do worse clinically, and having both pathologies is additive symptomatically 27–29, there is limited information regarding the mechanistic relationship of these pathologies in the context of ischemic tissue injury. To our knowledge, this is the first study to report accumulation of β-amyloid and tau tangle pathology modifies the likelihood of ischemic tissue injuries using data from human-based studies. There is increasing awareness of the contribution of microinfarct burden, especially cortical microinfarcts, to overall brain health, cognition and dementia 11,30–32. Microinfarcts in pathology are identified microscopically, and are most commonly considered to be a result of an ischemic-hypoxic environment in the brain. We and others have previously reported small vessel pathologies are associated with microinfarct tissue injury 1,2. Our current study extends these findings to provide evidence that higher levels of amyloid and tau may work to exacerbate the effect of vessel pathologies on ischemic tissue lesions, specifically cortical ischemic lesions, thus lowering an individual’s threshold for developing vascular-related lesions. In the case of both arteriolosclerosis and CAA pathology, we found the higher burden of amyloid and tangles you have, the more likely you are to have microinfarcts in the cortex. While only those with arteriosclerosis and higher burden of tau tangles were more likely to have a greater burden of microinfarcts in subcortical regions. Furthermore, our data suggests that a higher number of cortical microinfarcts is largely driven in those individuals with at least moderate severity in vessel disease, and with β-amyloid/tau levels above the 50th percentile. Within and surrounding a cortical microinfarct lesion, there are extensive pathogenic changes, including microvasculature integrity, inflammation, and axonal damage, which likely contribute to the pathogenesis of microinfarcts 14,33. Our arteriolosclerosis measure used in the current study documents SVD changes in the basal ganglia; however, we also demonstrate that half of the brains with basal ganglia pathology also have more widespread SVD changes in areas more closely connected to cortical lobes. It is plausible that in these individuals there is a more widespread insufficiency of blood flow contributing to microinfarct lesions throughout cortical and subcortical brain regions. By examining interactions between cerebral vessel pathologies and presence of AD proteins on cortical microinfarct burden, our study provides new mechanistic insight into additional factors that contribute to microinfarct pathogenesis. Overall, these findings have important implications on SVD pathogenesis by unravelling complex relationships between AD pathogenesis, cerebral vessel pathologies, and ischemic brain tissue injury
The microvasculature is a complex network of arterioles, capillaries, and venules, and is central to maintaining adequate brain perfusion, as well as supplying the metabolic demands for optimal neuronal activity 34. One postulated hypothesis regarding microinfarct etiology is that they are the result of local changes within the brain’s environment 13. Pathological stresses, for example accumulation of Aβ and tau peptides in AD, can drastically change the brain’s microenvironment, disrupt the neurovascular link, leading to multiple downstream effects. Prior studies have suggested that both Aβ and tau peptides may promote vasoconstriction via structural and non-structural processes, thus leading to poor perfusion and reduced brain tissue oxygenation 16,18,35. One proposed mechanism suggests that early Braak tau pathology is related to degradation of arterial smooth muscle cells and elastin, which over time can result in increasing levels of microvessel wall stress 16. In addition, microvessels within a cluster of dystrophic neurites comprising of a ‘neuritic’ amyloid plaque have been reported to be 20–50% narrower than microvessels outside the plaque environment 36. Furthermore, in brains of patients with AD, cortical microinfarcts tend to located close to vessels with CAA, as well as preferentially located in the arterial border zones, highlighting that cerebral hypoperfusion is an important pathophysiological mechanism that may contribute to the etiology of cortical microinfarcts 37. The current study is cross-sectional, such that the order of the events cannot be determined. However, it is plausible that a greater burden of β-amyloid or tau protein aggregates may activate a cascade of pathological processes that reduces the integrity of the vasculature, promoting hypoperfusion, thus leading to an increased susceptibility to ischemic tissue injury. Alternatively, mechanisms related to protein aggregation or clearance could be disrupted leading to higher levels of AD pathological changes, especially in those individuals with ischemic brain injury. In line with this, recent studies have shown that ischemic stroke may impact tau phosphorylation via upregulation of microtubule kinase, a protein critical to hyperphosphorylation of the tau protein 38. Future studies targeted to understanding how vascular and AD pathologies interact in vivo using neuroimaging will be important going forward. In addition, future work to identify molecular signatures that drive vascular damage and neurodegeneration, will be imperative in understanding complex relationships between vascular changes, AD pathology, and neurodegeneration.
Overall, findings from this study advances our knowledge regarding microvascular disease in the context of comorbid AD pathology. A major strength of this study is that findings are based on a large sample of autopsied individuals, which allows us to quantify associations more accurately, with comprehensive neuropathologic evaluation, including β-amyloid and PHF-tau tangle burden from 8 brain regions, and multiple vascular pathogenic markers. In addition, by examining continuous measures of amyloid and tangles pathology rather than a dichotomous measure of pathologic AD diagnosis allows us to examine relations across the spectrum of amyloid and tangle burden in more detail. We combined data from three different cohorts to increase the number of cases for statistical power. However, we do recognize there are statistical differences across the cohorts with age-at-death and education, but no differences with sex. For neuropathologic findings, there was a significant difference across cohorts for amyloid burden and atherosclerosis pathology. All other pathologic findings were comparable across ROS, MAP, MARS cohorts. Limitations are also noted. As previously noted, the study is cross-sectional, and cannot infer cause or consequence. Although multiple vascular pathogenic markers were used in the current study, we did not systematically investigate capillary CAA or microhemorrhages with AD pathology on vasculopathic change. We used standard and commonly used antibodies for amyloid immunohistochemistry; however, the use of multiple amyloid antibodies during the course of the study was not taken into account, as well as, we did not dissociate our amyloid findings by different amyloid species, for example by β-amyloid-42 vs. −40. In addition, we used neurofibrillary tangles as a measure for tau burden; however, glial- and neuritic-tau are common pathological findings in the aging brain, therefore future studies using a total tau burden would provide more accuracy. Finally, our study sample was highly educated and mostly white non-Latino and therefore may limit generalizability.
Supplementary Material
Acknowledgements:
We thank participants from the Rush Memory and Aging Project, Religious Orders Study, and Minority Aging Research Study. We also thank investigators and staff at Rush Alzheimer’s Disease Center (RADC).
Funding: The study is funded by the National Institute on Aging (NIA) Grants (R01AG017917, P30AG010161, RF1AG022018, and R01AG015819
Non-standard Abbreviations and Acronyms
- Aβ
Amyloid-beta
- AD
Alzheimer’s Disease
- BA
Brodmann
- CAA
Cerebral amyloid angiopathy
- MAP
Memory and Aging Project
- MARS
Minority Aging Research Study
- ROS
Religious Orders Study
- SVD
Small vessel disease
Footnotes
Disclosures and Competing Interests: JAS disclosure: Eli Lilly (AVID radiophamaceuticals). All other authors report no disclosures or competing interests.
Supplemental Materials I - VI
(I) Cohort Characteristics
(II) Assessment for neuritic plaque pathology
(III) Inter-rater protocol for PHF-tau neurofibrillary tangle burden
(IV) Assessment for subcortical frontal and parietal white matter arteriolosclerosis
(V) Interaction of cerebral vessel pathology with neuritic plaque burden in relation to probability of microinfarct burden
(VI) Sensitivity Analyses
References
- (1).Arvanitakis Z, Capuano AW, Leurgans SE, Buchman AS, Bennett DA, Schneider JA. The Relationship of Cerebral Vessel Pathology to Brain Microinfarcts. Brain Pathol. 2017; 27: 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (2).Kovari E, Herrmann FR, Gold G, Hof PR, Charidimou A. Association of cortical microinfarcts and cerebral small vessel pathology in the ageing brain. Neuropathol Appl Neurobiol. 2016; 505–513. [DOI] [PubMed] [Google Scholar]
- (3).Longstreth WT Jr, Sonnen JA, Koepsell TD, Kukull WA, Larson EB, Montine TJ. Associations between microinfarcts and other macroscopic vascular findings on neuropathologic examination in 2 databases. Alzheimer Dis Assoc Disord. 2009; 23: 291–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Haglund M, Passant U, Sjobeck M, Ghebremedhin E, Englund E. Cerebral amyloid angiopathy and cortical microinfarcts as putative substrates of vascular dementia. Int J Geriatr Psychiatry. 2006; 21: 681–687. [DOI] [PubMed] [Google Scholar]
- (5).van den Brink H, Zwiers A, Switzer AR, Charlton A, McCreary CR, Goodyear BG, Frayne R, Biessels GJ, Smith EE. Cortical Microinfarcts on 3T Magnetic Resonance Imaging in Cerebral Amyloid Angiopathy. Stroke. 2018; 49: 1899–1905. [DOI] [PubMed] [Google Scholar]
- (6).Kapasi A, DeCarli C, Schneider JA. Impact of multiple pathologies on the threshold for clinically overt dementia. Acta Neuropathol. 2017; 134: 171–186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (7).Jellinger KA. Pathology and pathogenesis of vascular cognitive impairment-a critical update. Front Aging Neurosci. 2013; 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (8).White L Brain lesions at autopsy in older Japanese-American men as related to cognitive impairment and dementia in the final years of life: a summary report from the Honolulu-Asia aging study. J Alzheimers Dis. 2009; 18: 713–725. [DOI] [PubMed] [Google Scholar]
- (9).Kovari E, Gold G, Herrmann FR, Canuto A, Hof PR, Michel JP, Bouras C, Giannakopoulos P. Cortical microinfarcts and demyelination significantly affect cognition in brain aging. Stroke. 2004; 35: 410–414. [DOI] [PubMed] [Google Scholar]
- (10).van Veluw SJ, Jolink WM, Hendrikse J, Geerlings MI, Luijten PR, Biessels GJ, Klijn CJM. Cortical microinfarcts on 7 T MRI in patients with spontaneous intracerebral hemorrhage. J Cereb Blood Flow Metab. 2015; 35: 1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (11).Arvanitakis Z, Leurgans SE, Barnes LL, Bennett DA, Schneider JA. Microinfarct pathology, dementia, and cognitive systems. Stroke. 2011; 42: 722–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (12).Kapasi A, Leurgans SE, James BD, Boyle PA, Arvanitakis Z, Nag S, Bennett DA, Buchman AS, Schneider JA. Watershed microinfarct pathology and cognition in older persons. Neurobiol Aging. 2018; 70: 10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Ter Telgte A, Scherlek AA, Reijmer YD, van der Kouwe AJ, van Harten T, Duering M, Bacskai BJ, de Leeuw FE, Frosch MP, Greenberg SM, et al. Histopathology of diffusion-weighted imaging-positive lesions in cerebral amyloid angiopathy. Acta Neuropathol. 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (14).Yilmazer-Hanke D, Mayer T, Muller HP, Neugebauer H, Abaei A, Scheuerle A, Weis J, Forsberg KME, Althaus K, Meier J, et al. Histological correlates of postmortem ultra-high-resolution single-section MRI in cortical cerebral microinfarcts. Acta Neuropathol Commun. 2020; 8: 33–020-00900–1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (15).You Y, Perkins A, Cisternas P, Munoz B, Taylor X, You Y, Garringer HJ, Oblak AL, Atwood BK, Vidal R, et al. Tau as a mediator of neurotoxicity associated to cerebral amyloid angiopathy. Acta Neuropathol Commun. 2019; 7:26–019-0680-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (16).Merlini M, Wanner D, Nitsch RM. Tau pathology-dependent remodelling of cerebral arteries precedes Alzheimer’s disease-related microvascular cerebral amyloid angiopathy. Acta Neuropathol. 2016; 131: 737–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Bennett RE, Robbins AB, Hu M, Cao X, Betensky RA, Clark T, Das S, Hyman BT. Tau induces blood vessel abnormalities and angiogenesis-related gene expression in P301L transgenic mice and human Alzheimer’s disease. Proc Natl Acad Sci U S A. 2018; 115: E1289–E1298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Niwa K, Kazama K, Younkin L, Younkin SG, Carlson GA, Iadecola C. Cerebrovascular autoregulation is profoundly impaired in mice overexpressing amyloid precursor protein. Am J Physiol Heart Circ Physiol. 2002; 283: H315–23. [DOI] [PubMed] [Google Scholar]
- (19).Miners JS, Palmer JC, Tayler H, Palmer LE, Ashby E, Kehoe PG, Love S. Abeta degradation or cerebral perfusion? Divergent effects of multifunctional enzymes. Front Aging Neurosci. 2014; 6:238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (20).Ferro DA, Mutsaerts HJ, Hilal S, Kuijf HJ, Petersen ET, Petr J, van Veluw SJ, Venketasubramanian N, Yeow TB, Biessels GJ, et al. Cortical microinfarcts in memory clinic patients are associated with reduced cerebral perfusion. J Cereb Blood Flow Metab. 2019: 271678X19877403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Suter OC, Sunthorn T, Kraftsik R, Straubel J, Darekar P, Khalili K, Miklossy J. Cerebral hypoperfusion generates cortical watershed microinfarcts in Alzheimer disease. Stroke. 2002; 33: 1986–1992. [DOI] [PubMed] [Google Scholar]
- (22).Bennett DA, Buchman AS, Boyle PA, Barnes LL, Wilson RS, Schneider JA. Religious Orders Study and Rush Memory and Aging Project. J Alzheimers Dis. 2018; 64: S161–S189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Bennett DA, Wilson RS, Schneider JA, Evans DA, Aggarwal NT, Arnold SE, Cochran EJ, Bienias KJL. Apolipoprotein E epsilon4 allele, AD pathology, and the clinical expression of Alzheimer’s disease. Neurology. 2003; 60: 246–252. [DOI] [PubMed] [Google Scholar]
- (24).Schneider JA, Wilson RS, Cochran EJ, Bienias JL, Arnold SE, Evans DA, Bennet DA. Relation of cerebral infarctions to dementia and cognitive function in older persons. Neurology. 2003; 60: 1082–1088. [DOI] [PubMed] [Google Scholar]
- (25).Bennett DA, Schneider JA, Wilson RS, Bienias JL, Arnold SE. Neurofibrillary tangles mediate the association of amyloid load with clinical Alzheimer disease and level of cognitive function. Arch Neurol. 2004; 61: 378–384. [DOI] [PubMed] [Google Scholar]
- (26).Arvanitakis Z, Leurgans SE, Wang Z, Wilson RS, Bennett DA, Schneider JA. Cerebral amyloid angiopathy pathology and cognitive domains in older persons. Ann Neurol. 2011; 69: 320–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Arvanitakis Z, Capuano AW, Leurgans SE, Bennett DA, Schneider JA. Relation of cerebral vessel disease to Alzheimer’s disease dementia and cognitive function in elderly people: a cross-sectional study. Lancet Neurol. 2016; 15: 934–943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (28).Chui HC, Ramirez-Gomez L. Clinical and imaging features of mixed Alzheimer and vascular pathologies. Alzheimers Res Ther. 2015; 7: 21–015-0104–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Vemuri P, Lesnick TG, Przybelski SA, Knopman DS, Preboske GM, Kantarci K, Raman MR, Machulda MM, Mielke MM, Lowe VJ, et al. Vascular and amyloid pathologies are independent predictors of cognitive decline in normal elderly. Brain. 2015; 138: 761–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (30).van Veluw SJ, Shih AY, Smith EE, Chen C, Schneider JA, Wardlaw JM, Greenberg SM, Biessels GJ. Detection, risk factors, and functional consequences of cerebral microinfarcts. Lancet Neurol. 2017; 16: 730–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Summers PM, Hartmann DA, Hui ES, Nie X, Deardorff RL, McKinnon ET, Helpern JA, Jensen JH, Shih AY. Functional deficits induced by cortical microinfarcts. J Cereb Blood Flow Metab. 2017; 37: 3599–3614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Corrada MM, Sonnen JA, Kim RC, Kawas CH. Microinfarcts are common and strongly related to dementia in the oldest-old: The 90+ study. Alzheimers Dement. 2016; 12: 900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Freeze WM, Bacskai BJ, Frosch MP, Jacobs HIL, Backes WH, Greenberg SM, Van Veluw SJ. Blood-Brain Barrier Leakage and Microvascular Lesions in Cerebral Amyloid Angiopathy. Stroke. 2019; 50: 328–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Bogorad MI, DeStefano JG, Linville RM, Wong AD, Searson PC. Cerebrovascular plasticity: Processes that lead to changes in the architecture of brain microvessels. J Cereb Blood Flow Metab. 2019; 39: 1413–1432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (35).Mattsson N, Tosun D, Insel PS, Simonson A, Jack CR,Jr, Beckett LA, Donohue M, Jagust W, Schuff N, Weinder MW. Association of brain amyloid-beta with cerebral perfusion and structure in Alzheimer’s disease and mild cognitive impairment. Brain. 2014; 137: 1550–1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Hansra GK, Popov G, Banaczek PO, Vogiatzis M, Jegathees T, Goldbury CS, Culen KM. The neuritic plaque in Alzheimer’s disease: perivascular degeneration of neuronal processes. Neurobiol Aging. 2019; 82: 88–101. [DOI] [PubMed] [Google Scholar]
- (37).Okamoto Y, Yamamoto T, Kalaria RN, Senzaki H, Maki T, Hase Y, Kitamura A, Washida K, Yamada M, Ito H, et al. Cerebral hypoperfusion accelerates cerebral amyloid angiopathy and promotes cortical microinfarcts. Acta Neuropathol. 2012; 123: 381–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- (38).Hayden EY, Putman J, Nunez S, Shin WS, Oberoi M, Charreton M, Dutta S, Li Z, Komuro Y, Joy MT, et al. Ischemic axonal injury up-regulates MARK4 in cortical neurons and primes tau phosphorylation and aggregation. Acta Neuropathol Commun. 2019; 7: 135–019-0783–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw data are available by request through the Rush Alzheimer’s Disease Center (RADC) Research Resource Sharing Hub https://www.radc.rush.edu/