Abstract
Background and objectives
Risk markers for breast cancer include earlier onset of menarche (age at menarche [AAM]) and peak height velocity (PHV). Insulin-like growth factor-1 (IGF-1) is associated with pubertal milestones, as well as cancer risk. This study examined the relationships between pubertal milestones associated with breast cancer risk and hormone changes in puberty.
Methods
This is a longitudinal study of pubertal maturation in 183 girls, recruited at ages 6–7, followed up between 2004 and 2018. Measures included age at onset of puberty, and adult height attained; PHV; AAM; adult height, and serum IGF-1, and estrone-to-androstenedione (E:A) ratio.
Results
PHV was greatest in early, and least in late maturing girls; length of the pubertal growth spurt was longest in early, and shortest in late maturing girls. Earlier AAM was related to greater PHV. IGF-1 concentrations tracked significantly during puberty; higher IGF-1 was related to earlier age of PHV, earlier AAM, greater PHV, and taller adult height. Greater E:A ratio was associated with earlier AAM.
Conclusions
Factors driving the association of earlier menarche and pubertal growth with breast cancer risk may be explained through a unifying concept relating higher IGF-1 concentrations, greater lifelong estrogen exposure, and longer pubertal growth period, with an expanded pubertal window of susceptibility.
Keywords: Puberty, IGF-1, Windows of susceptibility, Breast cancer risk
Researchers have noted that several pubertal milestones are associated with risk of breast cancer, and puberty has been suggested to be a window of susceptibility for breast cancer [1,2]. Younger age at menarche (AAM) is a well-documented risk marker for breast cancer; pooled analyses revealed the risk of pre menopausal and postmenopausal breast cancers decreased by 9% and 4%, respectively, for each year that AAM is delayed [3]. The pubertal peak height velocity (PHV, the greatest velocity during the pubertal growth spurt) [4], as well as the age at which adult height is attained [5]. are also related to the risk of breast cancer.
Insulin-like growth factor 1 (IGF-1) is associated with the pubertal growth spurt [6] and is a hormone critical for breast development [7]. IGF-1 is also associated with breast density [8–10], as well as breast cancer risk [11]; in addition, women with acromegaly have an increased risk for breast cancer [12]. The ratio of estrone-to-androstenedione (E:A) ratio has been recommended as a surrogate measure of aromatase activity and estrogen exposure [13]; a previous work has noted the relationship of breast cancer, particularly postmenopausal breast cancer, with estrogen exposure [14,15]. Given the associations of pubertal parameters to breast cancer risk, estrogen exposure, and IGF-1 concentrations to pubertal milestones and breast cancer, we examined the relationship of several pubertal parameters—PHV, age at PHV, duration of the pubertal growth spurt, ages of menarche and achievement of adult height—with several explanatory variables, including body mass index (BMI), sex hormone concentrations (estradiol, estrone, and testosterone), estrone-to-androstenedione ratio (E:A), and IGF-1 concentrations in a group of prepubertal girls followed up over the course of 14 years.
Methods
Participants in this analysis were part of the Cincinnati epidemiology project of the Breast Cancer and the Environment Research Program. Study aims and design of this longitudinal project have been described in detail [16]. Participants in Cincinnati were recruited at ages 6 and 7 years through the public and parochial schools in the Cincinnati metropolitan area and the Breast Cancer Registry of Greater Cincinnati. The study was approved by the Institutional Review Board of Cincinnati Children’s Hospital Medical Center, with written informed consent from parents/guardians, assent from participants ages 6–17, and consent when ages 18 and over.
Girls were seen every 6 months between 2004 and 2010 and every 12 months until 2018, with a visit window of 4 weeks. Two measurements of height were performed by trained staff at each visit, with a third measure obtained if the first two were more than .5 cm apart, or if height was outside the 5th and 95th percentile values for age. The average of the 2 (or closest two of 3) measures was used in data analysis. Breast maturation was assessed using the Marshall and Tanner criteria [17], incorporating breast palpation as described previously [16]. AAM was determined through parental and participant report of date of menarche or through AAM, as described previously [18]. Parents completed a detailed questionnaire in years 1 and 5 regarding family history of breast cancer.
Participants with height differences less than 2 cm over their last three annual visits were considered as having reached their final adult height. Participants were selected for these analyses if they had sex hormone concentrations measured previously, at least one serum specimen available for the IGF-1 measurement within 6 months of breast development, and had attained adult height, as defined previously. A subgroup of these participants had multiple measurements of IGF-1 from 24 months before to 18 months after breast development, and IGF-1 interclass correlation coefficients were calculated to determine tracking across the peripubertal period.
We categorized participants into three groups based on race-specific relative timing of breast development: early, on time, and late onset, using the 20th percentile and 80th percentile ages of breast stage 2 as the cut points. We examined difference in IGF-1 concentrations by race-specific age at onset of breast development, using Wilcoxon rank-sum test.
Three or more IGF-1 measures were available on 172 (of 183) participants in the window between 24 months before, and 18 months after, breast development. “Pubertal onset” serum IGF-1 measures were obtained from samples within the 6 months of the onset of breast maturation. IGF-1 concentrations were determined by an IGF-binding protein blocked enzyme immunoassay (ALPCO, Salem, NH), which measures total IGF-1 concentrations with a sensitivity of .09 ng/mL, intra-assay coefficient of variation of 5.8%, and interassay coefficient of variation of 3.9%. Sex hormone analyses were performed using high-performance liquid chromatography with tandem mass spectroscopy, as described previously [19].
Growth parameters were estimated from the Preece-Baines model 1 (PB1) [20], incorporating the recently published modification [21]. The PB1 model has been used in several longitudinal studies of adolescent growth [22–25]. SAS, version 9.3 Proc NLMixed (SAS Institute Inc) was used to fit the data to the PB1 model, and the growth parameters were calculated and stratified by race-specific early, on-time, and late-onset maturation. We also calculated annualized height velocity, determining change in height between two consecutive visits divided by the time interval in years. The maximum value of the calculated height velocity determined an individual’s PHV for correlation analyses; age at that time was used to define age at PHV. Duration of the pubertal growth spurt was calculated from the difference of age at breast stage 2 to age at which adult height was achieved.
Scatter plots and LOESS (locally weighted scatterplot smoothing) fit of pubertal and hormone variables were first examined to inspect nonlinear relationships. We performed stepwise regression analyses with pubertal parameters including age of breast onset, AAM, and PHV, as well as estradiol, estrone, and testosterone, measured at time of breast development as outcome variables; we incorporated IGF-1 concentrations and BMI, and IGF-1, BMI, and race in the stepwise regression. IGF-1 concentrations at onset of puberty, as well as IGF-1 concentrations obtained 6 months earlier, were independently examined in the regression analyses, to examine earlier as well as current hormonal milieu. We performed a separate regression analysis on the outcome AAM, incorporating E:A ratio, BMI, and race as explanatory variables.
Results
The 183 participants in these analyses included 119 non-Hispanic white and 64 black participants. Among white participants, early maturers entered puberty under 7.67 years and late maturers over 9.94 years. Among black participants, early maturers entered puberty under 7.25 years and late maturers over 9.39 years of age. There were 16 participants (8.8% overall) who had a family history of breast cancer.
A statistically significant difference in age at PHV was found between the three pubertal timing groups (Table 1): early versus on time, t = 2.87; df = 158; p = .0046; on time versus late, t = 3.35; df = 142; p < .0001. When examining pubertal variables associated with PHV, the strongest negative correlate was AAM (that is, earlier AAM was associated with greater PHV) (R =−.427, p < .0001) and PHV with age at which adult height is achieved (R = .396, p < .0001). The duration of the pubertal growth spurt was greatest in the earliest-maturing girls (Table 1) and least in the late-maturing girls, and both were different compared to duration of girls who matured on time (Kruskal-Wallis test, χ2 = 86.2, df = 2, p < .0001). Longer duration was associated with lower concentrations of estrone (Pearson R = −.539, p < .0001) and testosterone (R = −.424, p = .001), but duration was not associated with PHV (R = .123, p =.22).
Table 1.
Selected pubertal parameters, by relative timing of puberty
| Pubertal parameter | Early onset | On-time onset | Late onset | |
|---|---|---|---|---|
| Age at onset of breast development | Black participants (N = 64) | <7.25 | 7.25–9.39 | >9.39 |
| White participants (N = 119) | <7.67 | 7.67–9.94 | >9.94 | |
| Peak height velocity (PHV), cm/year | 7.18 | 6.96 | 6.60 | |
| Age of PHV, year | 10.50 | 11.09 | 11.86 | |
| Duration pubertal growth, year | 6.90 | 5.45 | 4.50 | |
IGF-1 concentrations tracked significantly within an individual across the peripubertal period (interclass correlation coefficient = .67, p < .001). Black participants had greater IGF-1 concentrations at onset of breast development, contrasted to white participants (329 ng/mL vs. 277 ng/mL, p = .0084). IGF-1 concentrations were not related to maternal history of breast cancer (p = .33). Greater IGF-1 concentrations obtained 6 months before the onset of breast development (mean IGF-1 = 280 ng/mL) were correlated with earlier age of breast development (p = .03), earlier AAM (p = .001), longer duration of puberty (p = .0027), and earlier age of PHV (p = .005) (Table 2). Greater IGF-1 was also correlated with greater final adult height (p = .023) and marginally correlated with higher PHV (p = .065). IGF-1 concentrations at onset of breast development were correlated with greater concentrations of estradiol (p = .014), estrone (p < .0001), and testosterone (p < .0001) obtained at the same time (Table 2). E:A ratio was significantly associated with AAM (R = −.166, p = .0005), but not age at breast development (R = −.09, p = .31).
Table 2.
Pearson correlation coefficients of serum IGF-1 concentrations, at time of onset of breast development (t = 0) and 6 months before breast development (t = −6), with pubertal parameters and serum sex steroid levels (at time of B2)
| Age of onset of breast development | Duration puberty | PHV (peak height velocity) | Age at PHV | Age at menarche | Age adult height attained | Final height | Estrone level at B2 | Estradiol level at B2 | Testosterone level at B2 | |
|---|---|---|---|---|---|---|---|---|---|---|
| T = 0 correlation coefficients (p-value) | −.04 (.58) | .168 (.27) | .220 (<.004) | −.178 (.020) | −.307 (.0001) | .105 (.54) | .149 (.052) | .397 (.0001) | .224 (.014) | .351 (.0001) |
| T = −6 correlation coefficients (p-value) | −.172 (.030) | .467 (.003) | .148 (.065) | −.222 (.005) | −.284 (.001) | .08 (.43) | .183 (.023) | .275 (.002) | .101 (.16) | .081 (.44) |
IGF-1 = insulin-like growth factor-1.
In the stepwise regression models, adding BMI and BMI with race into the regression model which contained IGF-1 concentrations, resulted in decreased significance of IGF-1 concentrations at 6 months before puberty with several pubertal outcomes, such as age of breast onset, duration of puberty, and age of PHV (Table 3). The relationship of IGF-1 concentrations at onset of puberty with estrone, estradiol, and testosterone concentrations were nearly identical with the addition of BMI and race into the stepwise regression. Of note, in the separate regression analysis of AAM with E:A ratio, BMI, and race, E:A ratio remained in the regression model for AAM after inclusion of BMI and race (β = −.016, p = .052).
Table 3.
Stepwise regression of outcome variables pubertal parameters and serum sex steroid levels (at time of B2), with explanatory variables IGF-1 concentrations, BMI, and race; beta estimates with standard deviations and probability estimates from analyses of IGF-1 concentrations at 6 months prior to breast development
| Est. (SD), p-value | Age at onset breast | Duration puberty | PHV | Age at PHV | Age adult ht | Final height | E2 | E1 | T |
|---|---|---|---|---|---|---|---|---|---|
| IGF-1 | −.002 (.001), .03 | .006 (.002), <.01 | .003 (.001), .07 | −.005 (.002), <.01 | .001 (.002), .63 | .03 (.01), .02 | .006 (.005), .25 | .006 (.002), <.01 | .003 (.003), .38 |
| IGF1, BMI | −.001 (.001), .35 | .004 (.002), .03 | .004 (.001), .02 | −.004 (.002), .02 | .002 (.002), .40 | .03 (.01), .02 | .006 (.006), .27 | .007 (.002), <.001 | .003 (.003), .35 |
| IGF1, BMI, | −.0004 (.001), .71 | .004 (.002), .03 | .003 (.002), .03 | −.003 (.002), .06 | .002 (.002), .35 | .02 (.01), .06 | .007 (.006), .26 | .008 (.002), <.001 | .004 (.003), .27 |
| race | |||||||||
BMI = body mass index; IGF-1 = insulin-like growth factor-1; PHV = peak height velocity; SD = standard deviation.
Discussion
This study sought to understand better the physiological links relating pubertal parameters to risk of breast cancer. This study, as others, noted earlier pubertal timing, determined by breast development or AAM, is related to greater peak height velocity, and earlier breast development is related to a longer duration of the pubertal growth spurt. IGF-1 concentrations tracked during puberty and were greater in participants with a maternal history of breast cancer, with earlier onset of breast development, and earlier AAM. In addition, the ratio of estrone to androstenedione (E:A) was associated with earlier AAM.
Age of menarche is a useful epidemiologic tool for the assessment of breast cancer risk; not only is AAM associated with breast cancer risk [3], but it also can be recalled by adolescent and adult women with a high degree of accuracy [26]. The underlying physiological basis relating menarche to risk of breast cancer may be the association of the timing of menarche with IGF-1 concentrations; sex steroid concentrations, specifically the E:A ratio; and duration of the pubertal growth spurt—an important window of exposure[27]—rather than an increased number of lifetime menstrual cycles [28,29]. Although it has been proposed that the association of earlier AAM with breast cancer may be mediated through a greater number of menstrual cycles [30], earlier studies have reported no association of AAM and age at natural menopause (ANM) [31], or earlier AAM with earlier ANM [32,33], as well as later AAM with later ANM [34]. In addition, there are two large studies noting genetic factors that are associated with both AAM and ANM [35,36].
This study and others have noted that AAM is associated with several pubertal parameters, including age of onset of puberty (defined as breast stage 2), earlier onset and greater degree of PHV, and duration of puberty [24,37–40]. Earlier AAM leads to a longer duration of puberty (as defined by onset of breast development to the end of the pubertal growth spurt) [24,41], resulting in an expanded window of susceptibility. Although earlier work had proposed the importance of the critical timing of exposure that could impact development of breast cancer, the vast majority have emphasized prenatal exposures. In a jointly sponsored workshop in 1999, scientists strove to identify “critical windows of exposures,” including several additional windows of susceptibility [27]. For example, women exposed to radiation from atomic bombs in Nagasaki and Hiroshima were most likely to develop breast cancer if exposed between 10 and 19 years of age [42]. Similarly, findings from animal model studies have identified puberty as one of the perilous “windows of susceptibility” [2,43].
As noted, estrogen exposure has been related to the risk of breast cancer [14,15], and the relationship of obesity and overweight with postmenopausal breast cancer is mediated through the peripheral conversion of adrenal androgens into estrogen by the action of aromatase. This is consistent with findings reporting greater expression of aromatase in normal breast tissue of patients with breast cancer [44] and in mammographically dense breast areas [45]. Earlier AAM is associated with greater levels of estrogen in the follicular phase in young adults [46,47] and greater lifelong estrogen exposure. These findings would suggest that endocrine and paracrine effects of greater aromatase activity would provide greater stimulation of the endometrial lining, leading to earlier menarche; greater stimulation of hormonally responsive breast tissue, leading to greater breast density; and ultimately greater risk of breast cancer.
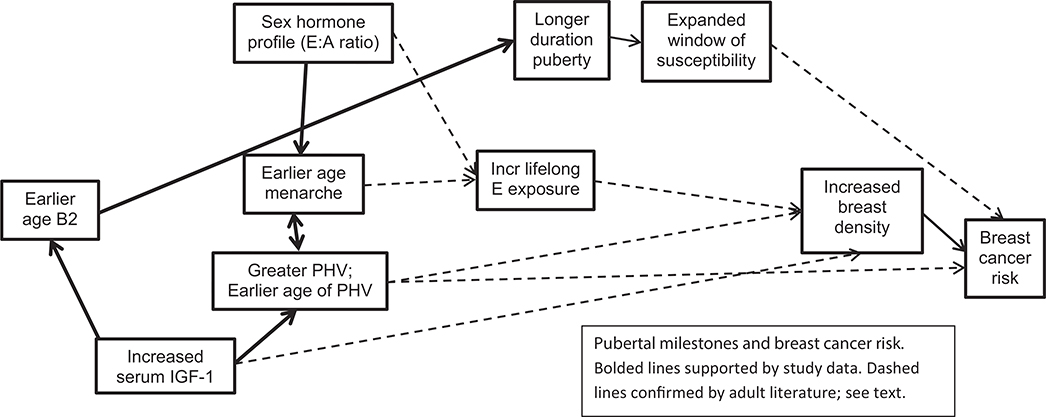
Studies have contrasted the associations between AAM, PHV, and age at which adult height is attained with breast cancer risk. PHV has been noted to be more strongly associated than AAM with risk of breast cancer [4]. Age at which adult height is reached has been reported as an independent risk factor for breast cancer [5,48] and related to more aggressive breast cancers than those associated with AAM [5]. We propose that the mechanism relating these pubertal parameters to risk of breast cancer can be explained, in part, by IGF-1 concentrations and the E:A ratio (Figure 1). We noted longitudinal associations of IGF-1 to earlier AAM, greater PHV, and earlier age of PHV; additionally, PHV was strongly related to AAM and age at which adult height is achieved. It is possible that some of our explanatory variables in the regression models were collinear with IGF-1, decreasing the statistical significance of IGF-1. Others have reported that higher IGF-1 concentrations are associated with earlier onset of puberty and menarche [49–51]. IGF-1 concentrations are consistent when resampled [52–57], and investigators have reported the significant association of age at PHV when compared to IGF-1 concentrations measured decades later [58]. IGF are mitogens that regulate cell proliferation and differentiation, as well as apoptosis [59], and a meta-analysis noted increased risk of elevated IGF concentrations with several cancers, including premenopausal breast and prostate cancers [60]. In addition, we found black girls had greater IGF-1 concentrations at onset of puberty, similar to adult women [61]. Of note, black women also have higher rates of premenopausal breast cancer [62].
Figure 1.
Pubertal milestones and breast cancer risk. Bolded lines supported by study data. Dashed lines confirmed by adult literature; see text.
IGF-1 could also explain the risk of breast cancer with two potentially inconsistent findings: both earlier maturation (associated with greater IGF-1 concentrations, and which may result in normal or shorter adult height) [24]as well as taller women [4,63] (who are also noted to have higher IGF-1 concentrations) [51,64]. IGF-1 is also associated with increased breast density in most [8,65], but not all, studies [66]. In a pooled analysis of 17 prospective studies, the odds of developing breast cancer were 1.28 when comparing those within the first and fifth quintiles of serum IGF-1 concentrations measured in adult women, and even greater odds (1.38) for breast cancers that were estrogen receptor positive [64]. In addition, the IGF-1 gene was one of the seven genes identified in a meta-analysis of genome-wide association studies that examined the relationship between breast density and breast cancer [65], although the association of IGF-1 with breast cancer may be restricted to premenopausal women [9,67].
There are several potential limitations to this study. We measured total circulating IGF-1 concentrations; previous studies have noted that there may be important differences between circulating (that is, endocrine) contrasted to tissue/cellular (paracrine) concentrations of IGF-1 in breast cancer risk and progression [11]. Measurements in tissue are not feasible in adolescent girls. In addition, we measured total IGF-1, not IGF-1 isoforms [68]. The participants in this study were recruited from the greater Cincinnati area, and not nationally representative, with few Hispanic or Asian girls, which limits the ability to generalize our findings. However, enrollment was community based, and it is highly unlikely participants were self-selected on pubertal maturation status or IGF-1 concentrations, since status on those factors would be unknown when they enrolled at 6 and 7 years of age. Our analyses, especially those stratified by race, had limited sample size, thereby limiting our power to detect differences. Finally, we do not have proximal (breast density) or clinically relevant (breast cancer) outcomes, although other studies have reported on the relationships of IGF-1 with breast density, and with breast cancer, as noted previously. Future work could address these issues, as well as explore the use of phytoestrogens, especially the flavonoids, to delay pubertal milestones [69] and in high-risk young women, to act as chemoprevention [70,71].
The current data suggest that the mechanisms underlying the association between earlier AAM with greater risk of breast cancer may be driven through higher concentrations of IGF-1, greater ratio of E:A, and an expanded window of susceptibility. We have provided a rigorous theoretical framework for the interrelationship of events during puberty, supported by evidence of the empirical data. Stronger empirical results will require larger sample sizes and directed studies in adult women.
IMPLICATIONS AND CONTRIBUTION.
Peak height velocity, IGF-1, and estrone-to-androstendione ratio were greatest in girls who matured earliest. The association of earlier maturation with breast cancer risk may be explained by higher exposures to IGF-1 and estrogen with an expanded window of susceptibility to potential carcinogens.
Acknowledgments
The authors would like to thank the research staff, study helpers, participants and their families, and gratefully acknowledge the clerical assistance of Jan Clavey. They would also like to thank the public and parochial schools in the greater Cincinnati metropolitan area, as well as the Breast Cancer Registry of Greater Cincinnati, to allow us to recruit study participants from their organizations.
Funding Sources
All phases of the study were supported by U01ES019453 (NCI and NIEHS); UL1RR026314 (USPHS) and P30ES006096 (NIEHS); and R21ES017315 (NIEHS).
Footnotes
Conflicts of interest: The authors have no conflicts of interest to disclose.
Financial Disclosure: The authors have no financial relationships relevant to this article to disclose.
References
- [1].Berkey CS, Frazier AL, Gardner JD, Colditz GA. Adolescence and breast carcinoma risk. Cancer 1999;85:2400–9. [DOI] [PubMed] [Google Scholar]
- [2].Russo J, Tay LK, Russo IH. Differentiation of the mammary gland and susceptibility to carcinogenesis. Breast Cancer Res Treat 1982;2:5–73. [DOI] [PubMed] [Google Scholar]
- [3].Clavel-Chapelon F Differential effects of reproductive factors on the risk of pre-and postmenopausal breast cancer: Results from a large cohort of French women. Br J Cancer 2002;86:723–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Ahlgren M, Melbye M, Wohlfahrt J, Sørensen TIA. Growth patterns and the risk of breast cancer in women. N Engl J Med 2004;351:1619–26. [DOI] [PubMed] [Google Scholar]
- [5].Li CI, Littman AJ, White. Relationship between age maximum height is attained, age at menarche, and age at first full-term birth and breast cancer risk. Cancer Epidemiol Biomarkers Prev 2007;16:2144–9. [DOI] [PubMed] [Google Scholar]
- [6].Juul A, Dalgaard P, Blum WF, et al. Serum levels of insulin-like growth factor (IGF)-binding protein-3 (IGFBP-3) in healthy infants, children, and adolescents: The relation to IGF-I, IGF-II, IGFBP-1, IGFBP-2, age, sex, body mass index, and pubertal maturation. J Clin Endocrinol Metab 1995;80: 2534–42. [DOI] [PubMed] [Google Scholar]
- [7].Russo J, Russo IH. Development of the human mammary gland In: Neville MC, Daniel CW, eds. The mammary gland: development, regulation and function. New York, NY: Plenum Press; 1987:67–93. [Google Scholar]
- [8].Diorio C, Pollak M, Byrne C, et al. Insulin-like growth factor-I, IGF-binding protein-3, and mammographic breast density. Cancer Epidemiol Biomarkers Prev 2005;14:1065–73. [DOI] [PubMed] [Google Scholar]
- [9].dos Santos Silva I, Johnson N, De Stavola B, et al. The insulin-like growth factor system and mammographic features in premenopausal and postmenopausal women. Cancer Epidemiol Biomarkers Prev 2006;15:449–55. [DOI] [PubMed] [Google Scholar]
- [10].Meggiorini ML, Cipolla V, Borgoni G, et al. Possible effects of insulin-like growth factor-I, IGF-binding protein-3 and IGF-1/IGFBP-3 molar ratio on mammographic density: A cross-sectional study. Eur J Gynaecol Oncol 2011;33:74–8. [PubMed] [Google Scholar]
- [11].Christopoulos PF, Msaouel P, Koutsilieris M. The role of the insulin-like growth factor-1 system in breast cancer. Mol Cancer 2015;14:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Nabarro JD. Acromegaly. Clin Endocrinol (Oxf) 1987;26:481–512. [DOI] [PubMed] [Google Scholar]
- [13].Probst-Hensch NM, Ingles SA, Diep AT, et al. Aromatase and breast cancer susceptibility. Endocr Relat Cancer 1999;6:165–73. [DOI] [PubMed] [Google Scholar]
- [14].Clemons M, Goss P. Estrogen and the risk of breast cancer. N Engl J Med 2001;344:276–85. [DOI] [PubMed] [Google Scholar]
- [15].Yaghjyan L, Colditz GA. Estrogens in the breast tissue: A systematic review. Cancer Causes Control 2011;22:529–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Biro FM, Galvez MP, Greenspan LC, et al. Pubertal assessment method and baseline characteristics in a mixed longitudinal study of girls. Pediatrics 2010;126:e583–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969;44:291–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Biro FM, Pajak A, Wolff MS, et al. Age of menarche in a longitudinal US cohort. J Pediatr Adolesc Gynecol 2018;31:339–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Biro FM, Pinney SM, Huang B, et al. Hormone changes in peripubertal girls. J Clin Endocrinol Metab 2014;99:3829–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Preece MA, Baines MJ. A new family of mathematical models describing the human growth curve. Ann Hum Biol 1978;5:1–24. [DOI] [PubMed] [Google Scholar]
- [21].Sayers A, Baines M, Tilling K. A new family of mathematical models describing the human growth curve—erratum: Direct calculation of peak height velocity, age at take-off and associated quantities. Ann Hum Biol 2013;40:298–9. [DOI] [PubMed] [Google Scholar]
- [22].Martin DD, Hauspie RC, Ranke MB. Total pubertal growth and markers of puberty onset in adolescents with GHD: Comparison between mathematical growth analysis and pubertal staging methods. Horm Res 2005;63: 95–101. [DOI] [PubMed] [Google Scholar]
- [23].Thomis M, Claessens AL, Lefevre J, et al. Adolescent growth spurts in female gymnasts. J Pediatr 2005;146:239–44. [DOI] [PubMed] [Google Scholar]
- [24].Biro FM, Huang B, Crawford PB, et al. Pubertal correlates in black and white girls. J Pediatr 2006;148:234–40. [DOI] [PubMed] [Google Scholar]
- [25].Aksglaede L, Olsen LW, Sorensen TI, Juul A. Forty years trends in timing of pubertal growth spurt in 157,000 Danish school children. PLoS One 2008; 3:e2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Koprowski C, Coates RJ, Bernstein L. Ability of young women to recall past body size and age at menarche. Obes Res 2001;9:478–85. [DOI] [PubMed] [Google Scholar]
- [27].Selevan SG, Kimmel CA, Mendola P. Identifying critical windows of exposure for children’s health. Environ Health Perspect 2000;108:451–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Wallace RB, Sherman BM, Bean JA, et al. Menstrual cycle patterns and breast cancer risk factors. Cancer Res 1978;38:4021–4. [PubMed] [Google Scholar]
- [29].Wu X, Cai H, Kallianpur A, et al. Age at menarche and natural menopause and number of reproductive years in association with mortality: Results from a median follow-up of 11.2 years among 31,955 naturally menopausal Chinese women. PLoS One 2014;9:e103673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Clavel-Chapelon F, E3N Group. Cumulative number of menstrual cycles and breast cancer risk: Results from the E3N cohort study of French women. Cancer Causes Control 2002;13:831–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Whelan EA, Sandler DP, McConnaughey DR, Weinberg CR. Menstrual and reproductive characteristics and age at natural menopause. Am J Epidemiol 1990;131:625–32. [DOI] [PubMed] [Google Scholar]
- [32].Mishra GD, Pandeya N, Dobson AJ, et al. Early menarche, nulliparity and the risk for premature and early natural menopause. Hum Reprod 2017;32: 679–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Whitcomb BW, Purdue-Smithe A, Hankinson SE, et al. Menstrual cycle characteristics in adolescence and early adulthood are associated with risk of early natural menopause. J Clin Endocrinol Metab 2018;103:3909–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Hardy R, Kuh D. Reproductive characteristics and the age at inception of the perimenopause in a British national cohort. Am J Epidemiol 1999;149: 612–20. [DOI] [PubMed] [Google Scholar]
- [35].Day FR, Ruth KS, Thompson DJ, et al. Large-scale genomic analyses link reproductive aging to hypothalamic signaling, breast cancer susceptibility and BRCA1-mediated DNA repair. Nat Genet 2015;47: 1294–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Wang G, Lv J, Qiu X, An Y. Integrating genome-wide association and eQTLs studies identifies the genes associated with age at menarche and age at natural menopause. PLoS One 2019;14:e0213953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Largo RH, Prader A. Pubertal development in Swiss girls. Helv Paediatr Acta 1983;38:229–43. [PubMed] [Google Scholar]
- [38].Taranger J, Engström I, Lichtenstein H, Svennberg-Redegren I. Somatic pubertal development. Acta Paediatr 1976;65:121–35. [PubMed] [Google Scholar]
- [39].de Ridder CM, Thijssen JH, Bruning PF, et al. Body fat mass, body fat distribution, and pubertal development: A longitudinal study of physical and hormonal sexual maturation of girls. J Clin Endocrinol Metab 1992;75: 442–6. [DOI] [PubMed] [Google Scholar]
- [40].Vizmanos B, Martí-Henneberg C, Cliville R, et al. Age of pubertal onset affects the intensity and duration of pubertal growth peak but not final height. Am J Hum Biol 2001;13:409–16. [DOI] [PubMed] [Google Scholar]
- [41].Martí-Henneberg C, Vizmanos B. The duration of puberty in girls is related to the timing of its onset. J Pediatr 1997;131:618–21. [DOI] [PubMed] [Google Scholar]
- [42].Land CE, Tokunaga M, Koyama K, et al. Incidence of female breast cancer among atomic bomb survivors, Hiroshima and Nagasaki, 1950–1990. Radiat Res 2003;160:707–17. [DOI] [PubMed] [Google Scholar]
- [43].Fenton SE. Endocrine-disrupting compounds and mammary gland development: Early exposure and later life consequences. Endocrinology 2006; 147:s18–24. [DOI] [PubMed] [Google Scholar]
- [44].Agarwal VR, Bulun SE, Leitch M, et al. Use of alternative promoters to express the aromatase cytochrome P450 (CYP19) gene in breast adipose tissues of cancer-free and breast cancer patients. J Clin Endocrinol Metab 1996;81:3843–9. [DOI] [PubMed] [Google Scholar]
- [45].Vachon CM, Sasano H, Ghosh K, et al. Aromatase immunoreactivity is increased in mammographically dense regions of the breast. Breast Cancer Res Treat 2011;125:243–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].MacMahon B, Trichopoulos D, Brown J, et al. Age at menarche, probability of ovulation and breast cancer risk. Int J Cancer 1982;29:13–6. [DOI] [PubMed] [Google Scholar]
- [47].Apter D, Reinilä M, Vihko R. Some endocrine characteristics of early menarche, a risk factor for breast cancer, are preserved into adulthood. Int J Cancer 1989;44:783–7. [DOI] [PubMed] [Google Scholar]
- [48].Bodicoat DH, Schoemaker MJ, Jones ME, et al. Timing of pubertal stages and breast cancer risk: The breakthrough generations study. Breast Cancer Res 2014;16:R18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Thankamony A, Ong KK, Ahmed ML, et al. Higher levels of IGF-I and adrenal androgens at age 8 years are associated with earlier age at menarche in girls. J Clin Endocrinol Metab 2012;97:E786–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Sørensen K, Aksglaede L, Petersen JH, et al. Serum IGF1 and insulin levels in girls with normal and precocious puberty. Eur J Endocrinol 2012;166:903–10. [DOI] [PubMed] [Google Scholar]
- [51].Cole TJ, Ahmed ML, Preece MA, et al. The relationship between insulin-like growth factor 1, sex steroids and timing of the pubertal growth spurt. Clin Endocrinol 2015;82:862–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [52].Kaaks R, Toniolo P, Akhmedkhanov A, et al. Serum C-peptide, insulin-like growth factor (IGF)-I, IGF-binding proteins, and colorectal cancer risk in women. J Natl Cancer Inst 2000;92:1592–600. [DOI] [PubMed] [Google Scholar]
- [53].Muti P, Quattrin T, Grant BJB, et al. Fasting glucose is a risk factor for breast cancer: A prospective study. Cancer Epidemiol Biomarkers Prev 2002;11: 1361–8. [PubMed] [Google Scholar]
- [54].Borofsky ND, Vogelman JH, Krajcik RA, Orentreich N. Utility of insulin-like growth factor-1 as a biomarker in epidemiologic studies. Clin Chem 2002; 48:2248–51. [PubMed] [Google Scholar]
- [55].Missmer SA, Spiegelman D, Bertone-Johnson ER, et al. Reproducibility of plasma steroid hormones, prolactin, and insulin-like growth factor levels among premenopausal women over a 2-to 3-year period. Cancer Epidemiol Biomarkers Prev 2006;15:972–8. [DOI] [PubMed] [Google Scholar]
- [56].Rollison DE, Newschaffer CJ, Tao Y, et al. Premenopausal levels of circulating insulin-like growth factor I and the risk of postmenopausal breast cancer. Int J Cancer 2006;118:1279–84. [DOI] [PubMed] [Google Scholar]
- [57].Baglietto L, English DR, Hopper JL, et al. Circulating insulin-like growth factor-I and binding protein-3 and the risk of breast cancer. Cancer Epidemiol Biomarkers Prev 2007;16:763–8. [DOI] [PubMed] [Google Scholar]
- [58].Sandhu J, Smith GD, Holly J, et al. Timing of puberty determines serum insulin-like growth factor-I in late adulthood. J Clin Endocrinol Metab 2006;91:3150–7. [DOI] [PubMed] [Google Scholar]
- [59].Yu HS, Rohan TE. Role of the insulin-like growth factor family in cancer development and progression. J Natl Cancer Inst 2000;92:1472–89. [DOI] [PubMed] [Google Scholar]
- [60].Renehan AG, Zwahlen M, Minder C, et al. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: Systematic review and meta-regression analysis. Lancet 2004;363:1346–53. [DOI] [PubMed] [Google Scholar]
- [61].Fowke JH, Matthews CE, Yu HS, et al. Racial differences in the association between body mass index and serum IGF1, IGF2, and IGFBP3. Endocr Relat Cancer 2010;17:51–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Brinton LA, Sherman ME, Carreon JD, Anderson WF. Recent trends in breast cancer among younger women in the United States. J Natl Cancer Inst 2008;100:1643–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Tretli S Height and weight in relation to breast cancer morbidity and mortality: A prospective study of 570,000 women in Norway. Int J Cancer 1989;44:23–30. [DOI] [PubMed] [Google Scholar]
- [64].Key TJ, Appleby PN, Reeves GK, Roddam AW, Endogenous Hormones and Breast Cancer Collaborative Group. Insulin-like growth factor 1 (IGF1), IGF binding protein 3 (IGFBP3), and breast cancer risk: Pooled individual data analysis of 17 prospective studies. Lancet Oncol 2010;11:530–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Lindström S, Thompson DJ, Paterson AD, et al. Genome-wide association study identifies multiple loci associated with both mammographic density and breast cancer risk. Nat Commun 2014;5:5303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Rice MS, Tworoger SS, Rosner BA, et al. Insulin-like growth factor-1, insulin-like growth factor-binding protein-3, growth hormone, and mammographic density in the Nurses’ Health Studies. Breast Cancer Res Treat 2012;136:805–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [67].Fletcher O, Gibson L, Johnson N, et al. Polymorphisms and circulating levels in the insulin-like growth factor system and risk of breast cancer: A systematic review. Cancer Epidemiol Biomarkers Prev 2005;14:2–19. [PubMed] [Google Scholar]
- [68].Philippou A, Maridaki M, Pneumaticos S, Koutsilieris M. The complexity of the IGF1 gene splicing, posttranslational modification and bioactivity. Mol Med 2014;20:202–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Mervish NA, Teitelbaum SL, Pajak A, et al. Peripubertal dietary flavonol and lignan intake and age at menarche in a longitudinal cohort of girls. Pediatr Res 2017;82:201–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Yasuda MT, Sakakibara H, Shimoi K. Estrogen- and stress-induced DNA damage in breast cancer and chemoprevention with dietary flavonoid. Genes Environ 2017;39:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].van Duursen MBM. Modulation of estrogen synthesis and metabolism by phytoestrogens in vitro and the implications for women’s health. Toxicol Res (Camb) 2017;6:772–94. [DOI] [PMC free article] [PubMed] [Google Scholar]