Abstract
Background:
Red blood cells (RBCs) derived from patients who receive testosterone replacement therapy (TRT) may be considered eligible for component production and transfusion. The aim of this study was to identify testosterone-dependent changes in RBC metabolism and to evaluate its impact on susceptibility to hemolysis during cold storage.
Study Design and Methods:
We characterized stored RBCs from two cohorts of TRT patients who were matched with control donors (no TRT) based upon sex, age, and ethnicity. We further evaluated the impact of testosterone deficiency (orchiectomy) on RBC metabolism in FVB/NJ mice. RBC metabolites were quantified by ultra-high-pressure liquid chromatography-mass spectrometry. RBC storage stability was determined in RBC units from TRT and controls by quantifying storage, osmotic, and oxidative hemolysis.
Results:
Orchiectomy in mice was associated with significant (P < 0.05) changes in RBC metabolism as compared with intact males including increased levels of acyl-carnitines, long-chain fatty acids (eg, docosapentaenoic acids), arginine, and dopamine. Stored RBCs from TRT patients exhibited higher levels of pentose phosphate pathway metabolites, glutathione, and oxidized purines (eg, hypoxanthine), suggestive of increased activation of antioxidant pathways in this group. Further analyses indicated significant changes in free fatty acids and acyl-carnitines in response to testosterone therapies. With regard to hemolysis, TRT was associated with enhanced susceptibility to osmotic hemolysis. Correlation analyses identified acyl-carnitines as significant modifiers of RBC predisposition to osmotic and oxidative hemolysis.
Conclusions:
These observations provide new insights into testosterone-mediated changes in RBC metabolome and biology that may impact the storage capacity and posttransfusion efficacy of RBCs from TRT donors.
1 ∣. INTRODUCTION
Recent studies that characterized genetic and biologic modifiers of hemolysis have reported a sex dichotomy in the capacity of red blood cells (RBCs) to withstand certain stress conditions including cold storage of RBC concentrates routinely used in transfusion practice.1-5 In these studies, male RBCs exhibited enhanced susceptibility to multiple measurements of hemolysis induced by cold storage, osmotic shock, or oxidative stress.3,4 Similar sex differences were observed in patients with sickle cell disease (SCD), of whom male patients had increased peripheral blood biomarkers of hemolysis (eg, lactate dehydrogenase, reticulocyte count, bilirubin) as compared with female patients.6 Investigations of the molecular mechanisms that contribute to sex differences in RBC biology, rheological properties, and pathology have identified a role for sex hormones as modifiers of hemolysis.4,7-10
Gonadectomy studies in mice revealed that orchiectomy, but not ovariectomy, significantly reduced RBC hemolytic responses to osmotic or oxidative stress and enhanced RBC storage and post-transfusion recovery as compared to intact males.4 Furthermore, testosterone repletion in orchiectomy mice restored the sex differences in RBC susceptibility to hemolysis.4 As erythrocytes lack DNA and because in vitro treatments of RBCs with testosterone had no apparent impact on hemolysis,4 this study suggested that testosterone action takes place early in male erythropoiesis via classical (genomic) pathways that resulted in the production of RBCs that were more susceptible to functional decline under the tested stress conditions. In female blood donors, sex hormone therapy and menstrual status were associated with changes in RBC predisposition to spontaneous (cold storage) and stress-induced hemolysis. Evaluation of the interaction between sex hormones and RBCs suggested that progesterone, but not 17β-estradiol or testosterone, protected against spontaneous (cold storage) or calcium-induced hemolysis via inhibition of calcium influx into RBCs in a rapid (non-classical) action that was partially attributed to regulation of membrane transient receptor potential channels (TRPCs).10 Taken together, these studies provided new evidence to direct or indirect sex hormone regulation of RBC characteristics and function that may contribute to sex differences in susceptibility to hemolysis.
The prevalence of testosterone replacement therapy (TRT) has significantly increased in recent years, for which it is estimated that 4%11 of cis and transgender men, and in some cases women, receive exogenous testosterone. Late-onset hypogonadism and female-to-male gender reassignment treatments are the major reasons for TRT; however, there is a growing concern regarding testosterone overuse among younger men.11,12 In fact, a 4-fold increase in TRT has been observed in younger men (ages 18-45) since 2003.13 Although the long-term benefits and risks associated with TRT have not been established and continue to be debated,14 a few retrospective studies have associated TRT with increased risk of cardiovascular events.15 Erythrocytosis and polycythemia secondary to TRT are common side effects of testosterone administration to patients,16,17 who often present at blood centers for therapeutic phlebotomy. In 2018, the US Food and Drug Administration allowed RBC components derived from therapeutic phlebotomies by blood donors who receive TRT to be used for allogeneic transfusion.18 Despite the growing number of unique donations by these donors, little is known about potential consequences of TRT and erythrocytosis on RBC biology, metabolic profile, susceptibility to hemolysis, as well as possible impact on RBC storage capacity and transfusion efficacy.
Advancements in omics technologies have facilitated the investigation of sex and sex hormone interactions with RBCs. These technologies have led to the discovery that RBCs exhibit a complex proteome, comprising ~3000 proteins19-21 with complex structural and enzymatic functions. Some of these enzymes have been demonstrated to be present and active in erythrocytes, paving the way for a novel understanding of the complexity of the RBC metabolome. Examples include several cytosolic isoforms of Krebs cycle enzymes, which may play a role in the homeostasis of reducing equivalents,22 purine oxidation and salvage reactions,23 carnitine-dependent24 and -independent lipid recycling25 and sphingolipid metabolism,26 arginine metabolism and the potential generation of nitric oxide through nitric oxide synthase27 and arginase,28 and one-carbon and sulfur metabolism involved in recycling oxidized proteins.29,30 In addition, RBCs express at least 77 small molecule transporters,31 which makes the analysis of RBC metabolism a window into systems-wide or organ-specific metabolic dysfunction achievable by peripheral blood sampling.32 As such, investigating RBC metabolism is not only relevant per se, but also provides clues into systems metabolism.
In the present study, we used metabolomics analyses as a tool to identify RBC-specific metabolic pathways that are modulated by testosterone therapies. Another goal was to evaluate whether the identified testosterone-mediated changes in the RBC metabolome would impact RBC function and susceptibility to hemolysis in response to selected stress conditions including cold storage. We demonstrate that RBCs from TRT donors exhibited lower ATP, enhanced glycolysis end products (lactate), and changes associated with purine (ITP), glutathione (5-oxoproline), and carnitine metabolism. These observations correlate with an altered RBC propensity to hemolyze under various stress conditions, a feature that may have consequences on the quality and transfusion efficacy of RBC products donated by TRT patients.
2 ∣. MATERIALS AND METHODS
2.1 ∣. Murine RBC metabolomic studies
All experimental procedures were approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Pittsburgh. FVB/NJ mice were obtained from The Jackson Laboratory (Bar Harbor, ME, USA). Orchiectomy was performed on 4-week-old FVB/NJ pups, which were housed with age-matched intact males or females for 12-15 months, after which all animals were sacrificed. RBCs were collected by centrifugation (1500 × g, 10 minutes, 4°C), which was followed by three washes (same conditions) with phosphate-buffered saline (PBS) to remove plasma and buffy coat. Aliquots of washed packed RBCs were snap frozen in liquid nitrogen and stored at −80°C for metabolomic studies.
2.2 ∣. TRT blood donor cohorts
The impact of testosterone therapies on RBC metabolome and predisposition to hemolysis was determined in two cohorts of TRT patients. The first cohort included five patients who presented at Vitalant’s blood bank (Pittsburgh) for the purpose of therapeutic phlebotomy due to testosterone therapy. These patients were matched with three control (no TRT) subjects based upon sex, race, and age. Each donor donated a whole blood unit from which a non-leukocyte-reduced RBC unit was produced in additive solution 1 (AS-1). All units were stored (1-6°C) for 39-42 days. For validation purposes, a second cohort of blood donors who received testosterone therapy (two males and one female) was recruited at Vitalant Denver. All subjects of this cohort were eligible allogeneic blood donors of whom one male responded yes to the blood disorders question (polycythemia secondary to testosterone). Control subjects were matched as described for the first cohort. We acquired leukocyte reduced (LR) packed RBC units from each donor, which were processed in the same manner described for the first cohort. We chose LR-RBC units in this validation cohort to further eliminate possible contamination by platelets or white blood cells. These units were stored for 42 days in additive solution-3 (AS-3). Overall, the storage conditions between the two cohorts were comparable (blood volume, bag plasticizer) with the exception of the additive solution and leukoreduction. Donor demographics and hemoglobin levels are summarized in Table S1.
2.3 ∣. Ultra-high-pressure liquid chromatography-mass spectrometry (MS) metabolomics
For metabolomic studies, stored RBCs were centrifuged (1500 × g, 10 minutes, 4°C) and the top layer was carefully removed and discarded to reduce possible contamination by residual platelets and white blood cells. Aliquots of washed packed RBCs were snap frozen in liquid nitrogen and stored at −80°C. A volume of 50 μL of frozen RBC aliquots was extracted 1:10 in ice cold extraction solution (methanol: acetonitrile: water 5:3:2 v/v).29 Samples were vortexed and insoluble material pelleted, as described before.33 Analyses were performed using a Vanquish UHPLC coupled online to a Q Exactive mass spectrometer (Thermo Fisher, Bremen, Germany). Samples were analyzed using a 3-minute isocratic condition34 or a 5-, 9-, and 17-minute gradient, as described.35,36
2.4 ∣. Evaluation of hemolysis in stored RBCs from TRT subjects
Following storage under routine blood bank conditions (1-6°C) for 42 days, aliquots (5-7 mL) of stored RBCs from blood donors of both cohorts were collected weekly into conical tubes, from which two aliquots (1 mL) were processed for the hemolysis assays. One aliquot was used for the quantification of spontaneous storage hemolysis that was determined by: . HCT is the sample hematocrit. Hbsupernatant refers to the levels of free hemoglobin obtained after centrifugation (1500×g, 10 minutes, 18°C) measured in the supernatant, whereas Hbtotal refers to the total amount of sample hemoglobin before centrifugation. In the entire study, hemoglobin concentrations (micromolar) were determined by the Drabkin’s method.37 The other aliquot was used for the osmotic and oxidative hemolysis assays. Both stress tests were proven to be highly reproducible and successful in capturing genetic (eg, glucose-6-phosphate dehydrogenase deficiency38) and biologic (eg, female sex hormones10) determinants of hemolysis in 13 403 blood donors from NHLBI’s Recipient Epidemiology Donor Evaluation Study (REDS)-III RBC-Omics study.1-3 For the evaluation of stress-induced hemolysis, stored RBCs were washed (1500×g, 10 minutes, 18°C) three times with PBS to remove plasma and additive solution, and immediately subjected to osmotic or oxidative stress assays. RBC susceptibility to osmotic hemolysis was determined by a modified pink test,39 in which washed RBCs (1.6% ±0.2%) were incubated under static conditions (4 hours at 22°C) in pink test buffer. After incubation, all samples were centrifuged (1500×g, 10 minutes, 18°C) and percent osmotic hemolysis was determined by: . Hbosmotic corresponds to supernatant cell-free hemoglobin of pink test-treated RBCs and Hbtotal refers to the total amount of hemoglobin of each sample.
RBC susceptibility to oxidative hemolysis was evaluated by incubating RBCs in the presence of 2,2′-azobis-2-methyl-propanimidamide, dihydrochloride (AAPH, 150mmoL). Thermal (37°C) decomposition of AAPH generates peroxyl radicals and, consequently, lipid peroxidation-mediated hemolysis.40
Using AAPH, we have identified genetic variants in the G6PD gene suggesting that glucose-6-phosphate dehydrogenase is activated in response to this oxidant.38 Percent AAPH-induced oxidative hemolysis was determined by: . HbAAPH corresponds to supernatant cell-free hemoglobin of AAPH-treated RBCs, Hbcontrol corresponds to supernatant cell-free hemoglobin of untreated RBCs, and Hbtotal refers to the total amount of hemoglobin of each sample.
2.5 ∣. Statistical analysis
RBC metabolomics data: Graphs and statistical analyses (either t test or repeated measures ANOVA) were prepared with GraphPad Prism 5.0 (GraphPad Software, Inc, La Jolla, CA), GENE E (Broad Institute, Cambridge, MA, USA), and MetaboAnalyst 4.0.41
Multivariate linear model for hemolysis in TRT and control donors: A multivariate linear regression analysis was performed to test the effect of testosterone intake for all three hemolysis measures at each tested time point. Donation site (cohort) was included as a covariate in the model. Due to small sample sizes, the validation cohort was analyzed with a simple One-way ANOVA (P < 0.05). Analyses were performed using R software version 3.4.4 (R Core Team, 2018).42
3 ∣. RESULTS
3.1 ∣. Sex and orchiectomy are significant modifiers of RBC metabolism in mice
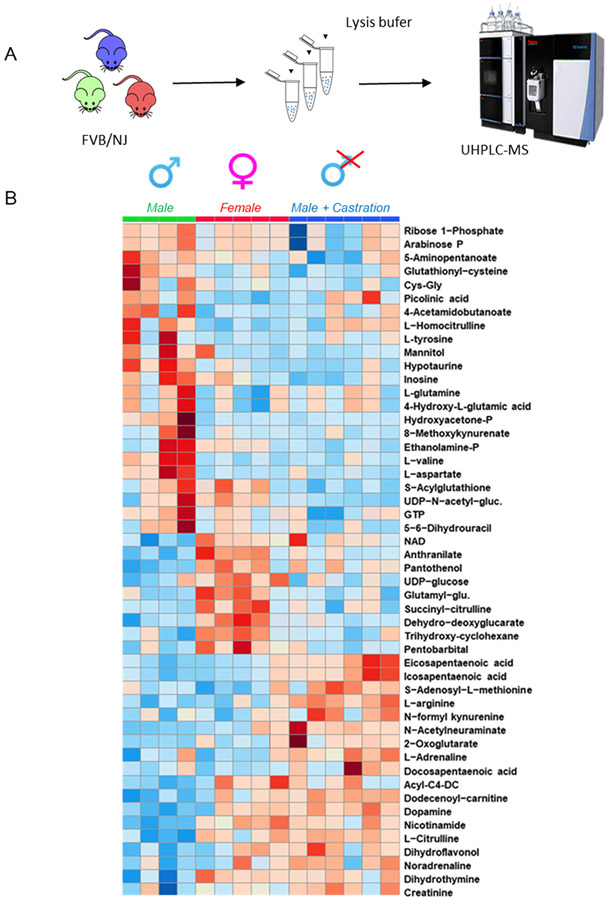
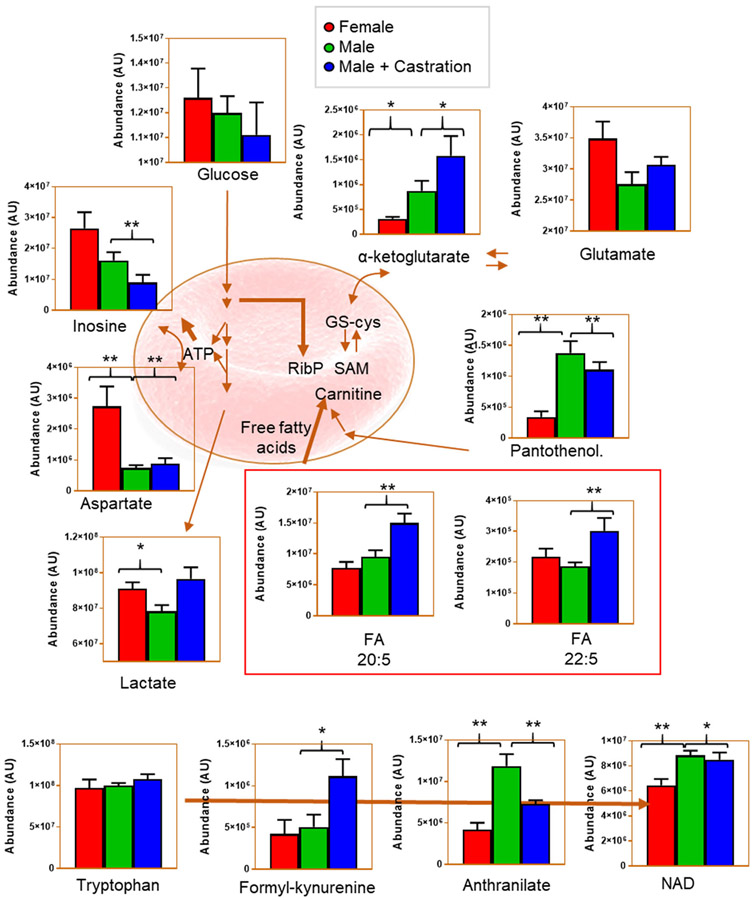
Our first set of experiments was designed to determine the impact of sex and testosterone deficiency on murine RBC metabolism. Metabolomics analyses were performed on RBCs from intact males (n = 4), females (n = 5), and orchiectomy males (n = 6; Figure 1A). The latter group was included to determine the impact of testosterone deficiency on RBC metabolism. We hypothesized that female RBCs are metabolically distinct from males, and that such differences can be reverted by decreasing the circulating levels of testosterone via castration. All results are reported in tabulated form in Table S2 (datasheet 1) and the top 50 metabolites sorted by ANOVA analysis are graphed in the heat map in Figure 1B. Male RBCs were characterized by higher levels of substrate and intermediates of glycolysis (eg, glucose and glyceraldehyde 3-phosphate). However, orchiectomy was associated with significant changes in RBC metabolism, in which certain metabolites exhibited levels similar to those observed in female RBCs, (eg, lactate, anthranilate). Conversely, certain metabolite levels were not impacted by orchiectomy and testosterone deficiency as was evidenced by the similarity to that of intact male RBCs (eg, aspartate and NAD; Figure 2). In addition, orchiectomy was characterized by increased RBC levels of several free fatty acids including eicosa- and docosapentaenoic acids, α-ketoglutarate, and short chain acyl-carnitines (acyl-C4-DC and acyl-C12:1). Orchiectomy was also associated with changes in metabolite levels from the tryptophan pathway including formyl-kynurenine and anthranilate; Figure 2 and Figure S1).
FIGURE 1.
Metabolomics analysis of RBCs from intact males, females, and orchiectomized (testosterone deficient) FVB/NJ mice. A, An overview of the experimental design. B, An overview of the top 50 significant metabolites by ANOVA, graphed in the form of a heat map with hierarchical clustering
FIGURE 2.
Pathway analysis of RBCs from intact males, females, and orchiectomized FVB/NJ mice. Bar plots (mean ± SEM) highlight the most impacted metabolites and related pathways from metabolomics analysis. Significant (*, P < 0.05, **, P < 0.01) metabolite changes were determined by two-tailed t test
3.2 ∣. Testosterone replacement therapy significantly modulated key RBC metabolic pathways during cold storage
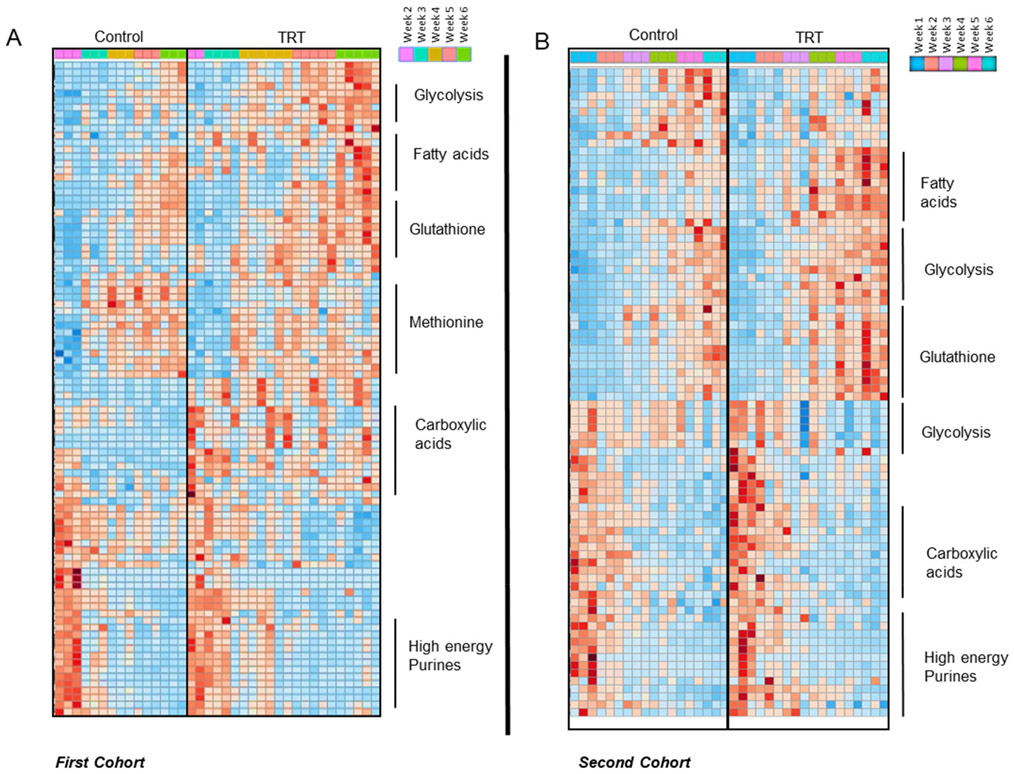
In the light of the results in mice, we set to further explore the impact of testosterone on RBC metabolism in humans. Specifically, in mice we had observed sex-specific metabolic signatures that were in part reversed by orchiectomy. Therefore, to validate and expand on these findings in human RBCs, we performed metabolomics analyses on RBCs from healthy control donors and from donors undergoing testosterone replacement therapy. The hypothesis to be tested here is that, as opposed to what was observed in mice upon castration, an intervention aimed at increasing circulating levels of exogenous testosterone would result in metabolic changes in directions (increases or decreases) consistent with those observed when comparing male vs female mouse RBCs. The first batch of RBC units was donated by five TRT male subjects who underwent therapeutic phlebotomy and three age, race and sex-matched controls (cohort 1; Vitalant Pittsburgh). Results are reported extensively in Table S2 (datasheet 2). Hierarchical clustering analyses of the significant metabolic changes by ANOVA is illustrated in the heatmap (Figure 3A). Specifically, changes were noted with respect to glycolysis, glutathione homeostasis and methionine metabolism, as well as fatty acid and acyl-carnitine metabolism, carboxylic acids and high energy purines (Figure 3A). Notably, RBCs from donor undergoing TRT did not exhibit significant changes in the levels of glycolytic metabolites; however, TRT resulted in higher levels of PPP metabolites (eg, ribose phosphate) and glutathione (Figure S2) suggestive of an opposite effect on this pathway of TRT in human RBCs in comparison to orchiectomy in mice. These changes are consistent with alterations of RBC antioxidant metabolism as a function of testosterone levels. In support of this hypothesis, we observed increases in the levels of oxidized purines (eg, hypoxanthine), tryptophan, and its oxidation products (kynurenines). Most notably, significant increases in the levels of free fatty acids and decreases in the levels of carnitine were detected in TRT RBCs. On the other hand, no significant changes were observed in the levels of carboxylic acids, while increased glutaminolysis was accompanied by increases in the levels of intracellular amino acids in TRT RBCs (eg, arginine and ornithine, but not citrulline; proline, alanine, leucine, and choline).
FIGURE 3.
Metabolomics analyses on stored human RBCs from two cohorts of patients who received testosterone replacement therapy (TRT) and their matched controls (no TRT). A, Top significant metabolites by ANOVA and related pathways are highlighted in the heat map for cohort 1, which consisted of five TRT patients who presented for therapeutic phlebotomy and three sex, age, and race/ethnicity-matched controls. B, Top significant metabolites by ANOVA and related pathways are highlighted in the heat map for cohort 2, which consisted of six eligible allogeneic blood donors (n = 3 TRT and n = 3 matched controls)
To validate these findings, a second independent cohort of blood donors was enrolled at Vitalant Denver. This cohort consisted of eligible TRT allogenic blood donors and matched controls (n = 3/group) who provided LR-RBC units. Results are extensively reported in Table S2 (datasheet 3) and hierarchical clustering analyses of the significant metabolic changes by ANOVA is illustrated in the heatmap Figure 3B. Key metabolites from cohort 2 were graphed as line plots in Figure S3. Specifically, we confirmed the observations related to increases in the PPP and glutathione metabolism, increased purine oxidation (IMP), arginine metabolites (guanidinoacetate and creatinine, but not citrulline, suggestive of deregulation of nitric oxide synthase activity), and free fatty acids including arachidonic, eicosapentaenoic, docosapentaenoic, and docosahexaenoic acid (Figure S3).
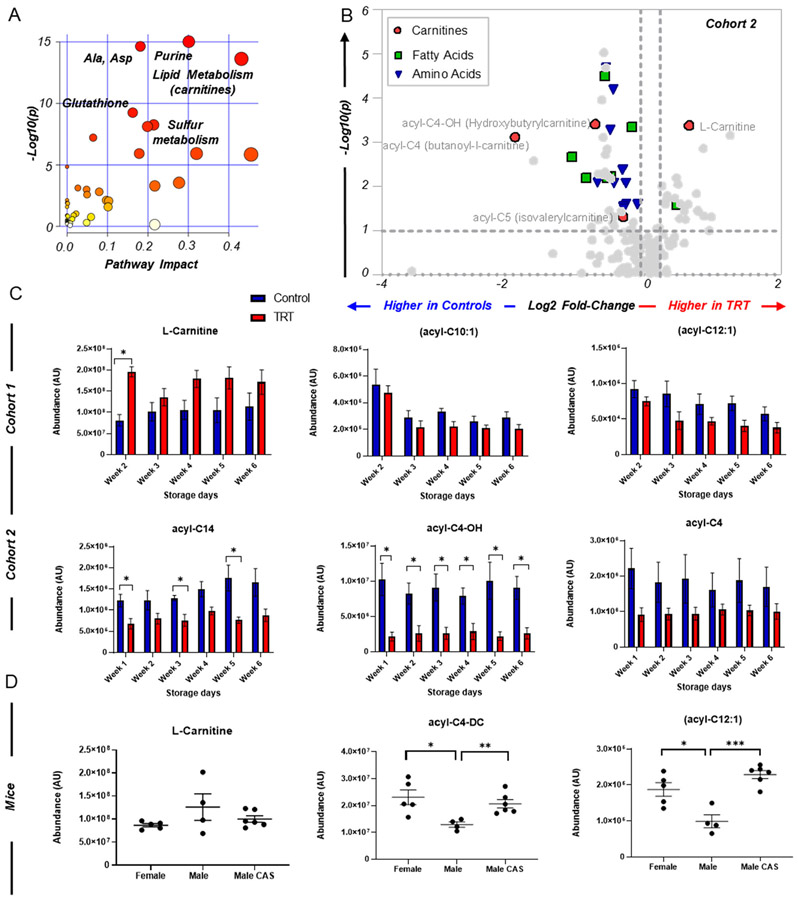
A pathway analysis of the combined significant changes as a function of TRT indicated significant changes in the RBC levels of free fatty acids and acyl-carnitines (Figure 4A), a result that was particularly evident in the second cohort of donor volunteers as highlighted by the volcano plot in Figure 4B, but in general, the directional pattern was consistent across both cohorts (Figure 4C). These altered acyl-carnitine levels were also evident within the mouse cohort (Figure 4D), indicative of significant decreases in intact male mice verses their castrated counterparts. A list of common metabolites found in murine RBCs and in stored (3-4 days) RBCs from the second human cohort is summarized in Table S3.
FIGURE 4.
Testosterone-dependent changes in fatty-acyl carnitine metabolism in red blood cells from two independent cohorts of human subjects who received testosterone replacement therapy and in mice. A, Gene Set Enrichment Analysis (GSEA) of the metabolic pathways significantly impacted by TRT in two independent cohorts of subjects undergoing TRT. B, The volcano plot highlights some of the metabolic changes in these pathways in the second cohort. C, The bar plots in the top and bottom row are used to graph changes in some representative acyl-carnitines from the first (top) and second (bottom) cohort in control (blue) and TRT (red) subjects as a function of storage duration (x axis). All significant changes (*, P < 0.05) were analyzed using a two-tailed t test. In D, the scatter plot illustrates graph changes significant by ANOVA, representative of acyl-carnitines in intact male, female, and castrated male FVB/NJ mice
3.3 ∣. Testosterone replacement therapy modulates susceptibility to hemolysis in cold stored RBCs
We evaluated the impact of TRT on RBC susceptibility to spontaneous (cold storage) and stress-induced hemolysis in the same blood donor cohorts as described in Materials and Methods. The major differences between the cohorts were the type of blood donation (ie, therapeutic in cohort 1 vs allogeneic in cohort 2) and pre-storage leukocyte reduction of whole blood that was performed on RBCs units from cohort 2 only. Despite these differences, TRT in both cohorts was associated with increased susceptibility to osmotic hemolysis, and with a trend toward increased storage hemolysis and decreased oxidative hemolysis (Figure 5). The overall hemolysis values in the first cohort were higher than that of the second cohort. Specifically, osmotic hemolysis measured at week 6 of storage was 54.4 ± 8.1% and 31.8 ± 3.5% (TRT and controls, respectively) in cohort 1 vs 37.6 ± 6.1% and 29.0 ± 8.3% (TRT and controls, respectively) in cohort 2. Similarly, average TRT donor end of storage hemolysis (week 6) measured 1.37 ± 0.64% in cohort 1 vs 0.28 ± 0.14 in cohort 2. A multivariate linear regression analysis adjusted to testosterone intake and donation site (cohort) revealed significant (P < 0.05) differences between TRT and matched controls with regards to increased osmotic hemolysis in TRT (Table S4).
FIGURE 5.
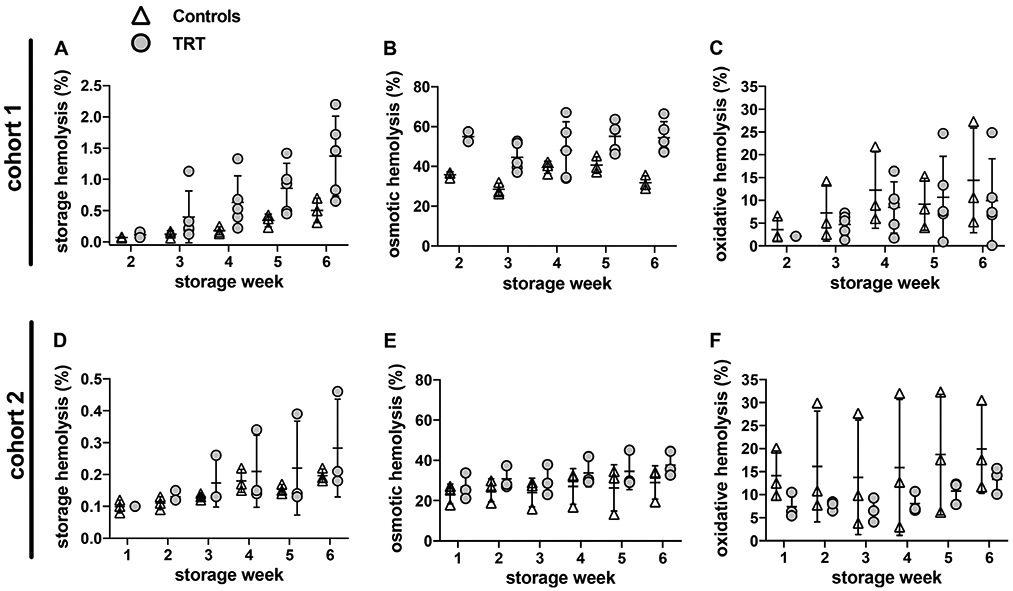
Testosterone replacement therapy modulates RBC susceptibility to hemolysis in cold storage. RBC concentrates from testosterone-treated donors (TRT) and matched controls (no TRT) were stored (1-6°C) for six weeks and tested for storage or stress-induced hemolysis as described in Materials and Methods. Cohort 1: therapeutic donations by five TRT patients and three age, sex, and race-matched controls (Vitalant Pittsburgh). Cohort 2: allogenic donations by three TRT donors and three age, sex, blood type, and race-matched controls (Vitalant Denver). A and D: percent spontaneous storage hemolysis. B and E: percent osmotic hemolysis. C and F: percent AAPH-induced oxidative hemolysis. Significant (P < 0.05) differences between TRT and matched controls were observed in osmotic hemolysis as summarized in Table S4
3.4 ∣. Metabolic correlates to storage hemolysis, oxidative hemolysis, and osmotic hemolysis
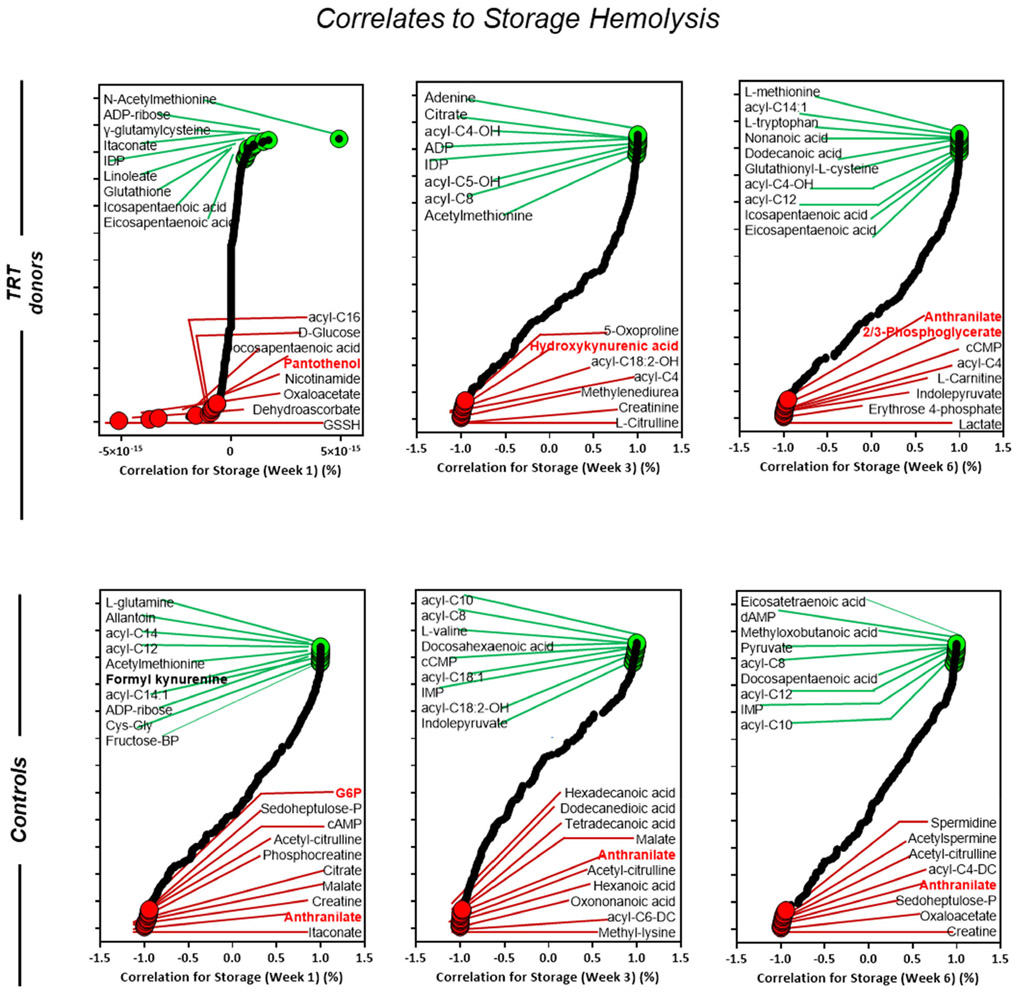
Evaluations of metabolites that are correlated with hemolysis and modified by testosterone treatments (Figures 6, 7, and 8) revealed positive correlation between high-energy phosphate compounds (eg, IDP, ADP-ribose and ADP) and storage hemolysis during the first three weeks of storage in TRT and control subjects. Notably, TRT donors exhibit a consistent positive correlation of free fatty acids from week one to week six, while control donors exhibited a consistent positive correlation to acyl-carnitines from week one to week six. Interestingly, metabolites from the glycolytic pathway and tryptophan metabolism (G6P, 2,3-Phosphoglycerate; anthranilate, hydroxy-kynurenic acid) were found to negatively correlated with storage hemolysis in both TRT and control groups at various stages of storage; Figure 6.
FIGURE 6.
Metabolic correlates of storage hemolysis in blood donors (cohort 2) who received testosterone replacement therapy (TRT) verses their matched controls (no TRT). Correlation curves represent storage weeks 1, 3, and 6. The dot plot highlighted positive (green) or negative (red) metabolic correlates to storage hemolysis. All highlighted metabolites were selected within the top 15 negative and positive correlates
FIGURE 7.
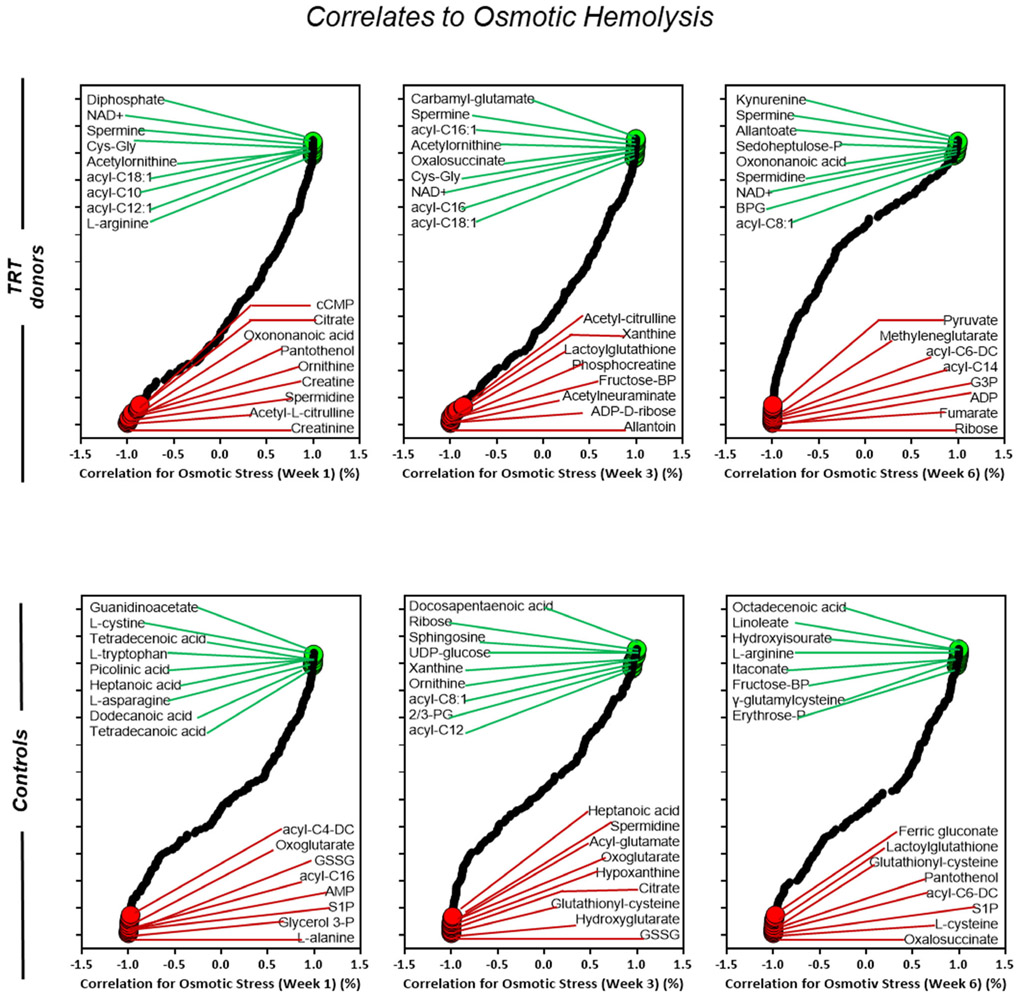
Metabolic correlates of osmotic hemolysis in blood donors (cohort 2) who received testosterone replacement therapy (TRT) verses their matched controls (no TRT). Correlation curves represent storage weeks 1, 3, and 6. The dot plot highlighted positive (green) or negative (red) metabolic correlates to osmotic hemolysis. All highlighted metabolites were selected within the top 15 negative and positive correlates
FIGURE 8.
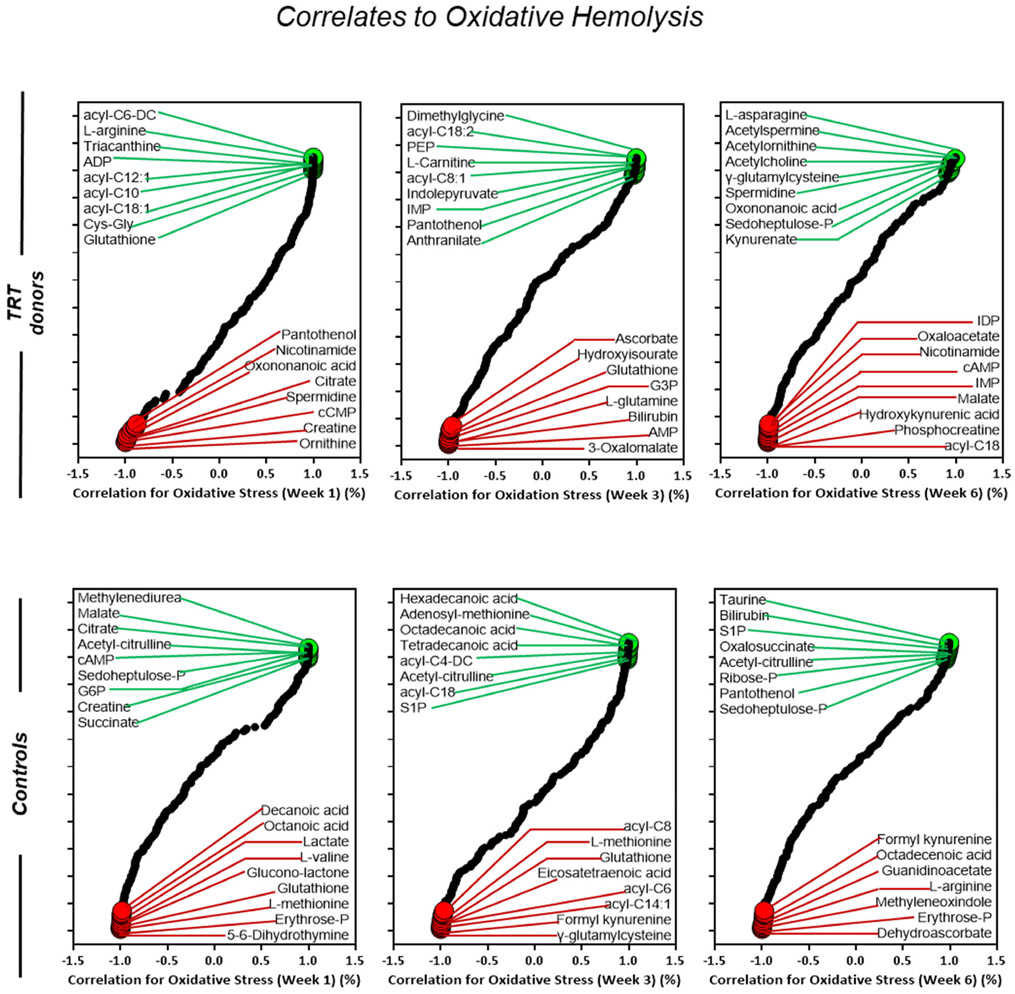
Metabolic correlates of osmotic hemolysis in blood donors (cohort 2) who received testosterone replacement therapy (TRT) verses their matched controls (no TRT). Correlation curves represent storage weeks 1, 3, and 6. The dot plot highlighted positive (green) or negative (red) metabolic correlates to oxidative hemolysis. All highlighted metabolites were selected within the top 15 negative and positive correlates
Acyl-carnitines were positively corelated with osmotic hemolysis in TRT donors from week one to week three, with a progressive reduced association through week six (Figure 7). Conversely, metabolites of the arginine (ornithine, acetyl-citrulline) and glycolytic (fructose-BP, G3P) pathway were negatively correlated with osmotic hemolysis in TRT (Figure 7). Similar to results seen in TRT samples under osmotic stress, acyl-carnitines show a positive correlation to oxidative hemolysis in week one followed by a progressive decline in week three, and no association found by week six (Figure 8). Metabolites of the arginine (creatine, ornithine, phosphocreatine) and glycolytic (G3P, AMP, cAMP) pathways were negatively associated with oxidative hemolysis in TRT donors throughout week six (Figure 8).
4 ∣. DISCUSSION
This study provided new evidence for sex hormone modulation of RBC metabolism and susceptibility to hemolysis during routine cold storage of RBC units or under applied stress (osmotic or oxidative). We have identified several metabolites and RBC metabolic pathways that are associated with testosterone therapy in humans or with testosterone deficiency in mice. We further characterized the associations between the identified metabolites and hemolysis and provided new insights into the mechanisms by which testosterone contributes to sex differences in RBC biology. Additionally, we demonstrated that TRT is associated with enhanced RBC susceptibility to osmotic hemolysis. These findings are in agreement with our previous observations in mice4 and emphasize the need to further characterize the storage capacity and posttransfusion efficacy of RBCs from TRT donors.
In support of our previous observations of testosterone-dependent sex differences in RBC biology and hemolysis,4 we demonstrated that orchiectomy in mice was associated with significant changes in RBC metabolic profile as compared with RBCs from intact males. Some of the changes in metabolite levels resembled RBCs from females (eg, nicotinamide, L-aspartate, L-valine, L-citrulline, dopamine, acyl-carnitines, purine catabolites, NAD), whereas other changes (eg, L-arginine, formyl-kynurenine) were unique to orchiectomy RBCs. However, previous studies have shown alterations of arginine metabolism in RBCs as a function of sex, with arginine metabolism being upregulated in females,38,43 and citrulline/arginine ratios being sufficient to discriminate sub-populations in subjects undergoing gender reassignment treatments.44 Furthermore, the levels of certain metabolites in female RBCs were significantly different than that of male (intact or orchiectomy) RBCs. These observations suggest that in females, other mechanisms including female sex hormones10 regulate RBC metabolism.
Interestingly, common patterns emerged when comparing RBCs from mice and humans. While some related to metabolites in the arginine pathway (eg, creatinine), others suggested a role for testosterone signaling in the regulation of sulfur metabolites involved in antioxidant responses, either directly (eg, glutathionyl-cysteine) or indirectly (eg, S-Adenoylmethionine or SAM). Of note, SAM is the main methyl group donor in the mature erythrocyte, which is relevant in the light of the role of protein methylation to repair isoaspartyl damage to structural (eg, band 3, ankyrin) and functional proteins (eg, hemoglobin, glycolytic enzymes) following storage-induced oxidant stress.29
Our evaluation of stored RBCs from therapeutic whole blood donations (cohort 1) revealed that TRT was accompanied by metabolic changes that may have contributed to the observed alterations in storage capacity and predisposition to hemolysis. Among those changes are alterations of total glutathione levels and recycling by means of reducing equivalents generated via the pentose phosphate pathway consistent with an altered redox homeostasis in TRT. Though caveats related to species-specific metabolic peculiarities have to be taken into account, PPP and glutathione metabolites decreased in orchiectomized mice, while they increased in human subjects undergoing testosterone replacement therapy. Of note, the rate-limiting enzyme of the pentose phosphate pathway, glucose 6-phosphate dehydrogenase (G6PD) is coded by a gene on chromosome X. Mutations to G6PD are common in humans, affecting ~400 million people worldwide, making it the most common enzymopathy in humans (~13% of the African American donor population in some metropolitan areas).45 Decreased activation of this pathway in G6PD deficient subjects have been noted to predispose the stored RBC to hemolysis following oxidative stress, while being protective with respect to RBC morphology.46 These observations are in agreement with what we observed in orchiectomized mice. Similarly, alterations of carboxylate metabolism opposite to those observed here in RBCs from TRT subjects have been associated with G6PD deficiency (eg, increase in fumarate upon TRT and decrease in G6PD deficient subjects).47 On the other hand, increases in S-adenosyl-methionine in orchiectomized mice and decreases in methionine metabolites in stored RBCs from subjects undergoing TRT suggest that testosterone levels may impact protein oxidant damage-repair pathways29 in an opposite direction to what observed in G6PD deficient subjects.47
To date, little is known about the impact of polycythemia and erythrocytosis on RBC function and viability. One study suggested that RBCs from blood donors with polycythemia vera or secondary polycythemia (unspecified reasons) have increased susceptibility to osmotic fragility.48 As polycythemia and erythrocytosis are common side effects of TRT,17 our observations of enhanced osmotic hemolysis in stored RBCs from therapeutic donations (cohort 1) support findings from this study. Of note, we were unable to verify if all therapeutic donations were prescribed for erythrocytosis secondary to testosterone, however, average hemoglobin levels in TRT donors (cohort 1) suggested that at least some of these patients had this condition. Another limitation is the lack of information regarding plasma testosterone levels that could be directly linked to our observations. The differences in the severity of hemolysis between the two tested TRT cohorts may stem from the reason for donation (ie, clinical condition in cohort 1 vs routine allogeneic donations in cohort 2). Other factors include differences in RBC component manufacturing procedures, such as leukocyte filtration in RBC units from allogeneic TRT donors that remove buffy coat and possibly fragile RBCs or differences in the additive solutions (additive solution-1 in cohort 1 vs additive solution-3 in cohort 2), the latter a significant contributor to the RBC metabolic storage lesion to an extent comparable to storage duration.49
Correlation analyses between top metabolites and hemolysis identified acyl-carnitines as significant modifiers of RBC susceptibility to osmotic and oxidative stress. It is worthwhile to note that orchiectomy promoted increases in acyl-carnitines and TRT was accompanied by decreases in acyl-carnitine levels by storage expiration. On the other hand, fatty acid levels (especially long chain fatty acids) increased both in orchiectomized mice and TRT patients. Dietary regimens or species-specific peculiarities may contribute to explain this discrepancy. For example, the mouse strain investigated in this study, FVB/NJ, is characterized by high basal levels of activity for the ferroreductase STEAP3 whose activity is critical during erythroid maturation50 but is absent in mature human RBCs.51 The levels and activity of STEAP3 have been shown to impact the levels of fatty acid and oxylipid in stored RBCs from FVB mice and negatively contribute to RBC storage capacity and post-transfusion recoveries.52
On the other hand, carnitine supplements have been indeed proposed two decades ago to counteract lipid remodeling that occurs in RBCs stored under blood bank conditions.53 Interestingly, acyl-carnitine levels in both cohorts were associated with predisposition to osmotic and oxidative hemolysis at the end of storage; on the other hand they show negative correlations early on during storage, when they may exert a protective role against oxidant stress and membrane lipid remodeling prior to exhaustion of the RBC capacity to cope with the storage lesion. For example, low levels of certain acyl-carnitines (eg, Acyl-C4 and Acyl-C6) observed in TRT RBCs were correlated with increased osmotic hemolysis and decreased oxidative hemolysis. We recently conducted retrospective analyses of hemolysis in 96 TRT blood donors from the National Heart, Lung and Blood Institute Red Blood Cell-Omics study54 and in agreement with the current study, TRT was associated with increased osmotic hemolysis and significant decrease in oxidative hemolysis. The observation that TRT is associated with reduced RBC acyl-carnitine concentrations may explain the changes in RBC predisposition to these markers of hemolysis.
In conclusion, we have demonstrated that modulation of testosterone signaling via castration in mice or hormone therapy in humans is associated with changes in RBC metabolism and susceptibility to hemolysis under selected stress conditions. These observations contribute to understanding the impact of sex and sex hormone therapies on RBC biology and susceptibility to hemolysis. The reported changes in RBC acyl-carnitine, arginine, and SAM metabolism warrant further evaluation to determine the quality and storage stability of RBCs from TRT donors, particularly in those with erythrocytosis secondary to testosterone.
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by the National Heart, Lung and Blood Institute (NHLBI) grant # R01 HL134653 to TK, and # R01HL146442, # R01HL149714, #R01HL148151 and # R21HL150032 to ADA; by the National Institute of General and Medical Sciences (NIGMS)grant # RM1GM131968 (ADA); by a Shared Instrument grant by the National Institute of Health grant # S10OD021641, and by funds from the Boettcher Webb-Waring Investigator Award (ADA).
Funding information
National Heart, Lung, and Blood Institute, Grant/Award Numbers: R01 HL134653, R01HL146442, R01HL148151; National Institute of General Medical Sciences, Grant/Award Number: RM1GM131968; National Institutes of Health, Grant/Award Number: S10OD021641
Footnotes
CONFLICT OF INTEREST
Though unrelated to the contents of this manuscript, the authors declare that AD is a founder of Omix Technologies Inc and Altis Biosciences LLC. AD is a consultant for Hemanext Inc. SLS is also a consultant for Tioma, Inc. All other authors disclose no conflicts of interest relevant to this study.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Lanteri MC, Kanias T, Keating S, et al. Intradonor reproducibility and changes in hemolytic variables during red blood cell storage: Results of recall phase of the REDS-III RBC-Omics study. Transfusion. 2019;59:79–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kanias T, Stone M, Page GP, et al. Frequent blood donations alter susceptibility of red blood cells to storage- and stress-induced hemolysis. Transfusion. 2019;59:67–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kanias T, Lanteri MC, Page GP, et al. Ethnicity, sex, and age are determinants of red blood cell storage and stress hemolysis: Results of the REDS-III RBC-Omics study. Blood Adv. 2017;1:1132–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kanias T, Sinchar D, Osei-Hwedieh D, et al. Testosterone-dependent sex differences in red blood cell hemolysis in storage, stress, and disease. Transfusion. 2016;56:2571–2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan A, Chen D, Yi QL, Kanias T, Gladwin MT, Acker JP. Assessing the influence of component processing and donor characteristics on quality of red cell concentrates using quality control data. Vox Sang. 2016;111:8–15. [DOI] [PubMed] [Google Scholar]
- 6.Raslan R, Shah BN, Zhang X, et al. Hemolysis and hemolysis-related complications in females vs. males with sickle cell disease. Am J Hematol. 2018;93:E376–E380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Raval JS, Waters JH, Seltsam A, et al. Menopausal status affects the susceptibility of stored RBCs to mechanical stress. Vox Sang. 2011;100:418–421. [DOI] [PubMed] [Google Scholar]
- 8.Kameneva MV, Watach MJ, Borovetz HS. Rheologic dissimilarities in female and male blood: Potential link to development of cardiovascular diseases. Adv Exp Med Biol. 2003;530:689–696. [DOI] [PubMed] [Google Scholar]
- 9.Kameneva MV, Watach MJ, Borovetz HS. Gender difference in rheologic properties of blood and risk of cardiovascular diseases. Clin Hemorheol Microcirc. 1999;21:357–363. [PubMed] [Google Scholar]
- 10.Fang F, Hazegh K, Sinchar D, et al. Sex hormone intake in female blood donors: Impact on haemolysis during cold storage and regulation of erythrocyte calcium influx by progesterone. Blood Transfus. 2019;17:263–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Morgan DJ, Dhruva SS, Wright SM, Korenstein D. 2016 update on medical overuse: A systematic review. JAMA Intern Med. 2016;176:1687–1692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Handelsman DJ. Testosterone: use, misuse and abuse. Med J Aust. 2006;185:436–439. [DOI] [PubMed] [Google Scholar]
- 13.Rao PK, Boulet SL, Mehta A, et al. Trends in testosterone replacement therapy use from 2003 to 2013 among reproductive-age men in the United States. J Urol. 2017;197:1121–1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gagliano-Juca T, Basaria S. Testosterone replacement therapy and cardiovascular risk. Nat Rev Cardiol. 2019;16:555–574. [DOI] [PubMed] [Google Scholar]
- 15.Elsherbiny A, Tricomi M, Bhatt D, Dandapantula HK. State-of-the-art: A review of cardiovascular effects of testosterone replacement therapy in adult males. Curr Cardiol Rep. 2017;19:35. [DOI] [PubMed] [Google Scholar]
- 16.Ohlander SJ, Varghese B, Pastuszak AW. Erythrocytosis following testosterone therapy. Sex Med Rev. 2018;6:77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones SD Jr, Dukovac T, Sangkum P, Yafi FA, Hellstrom WJ. Erythrocytosis and polycythemia secondary to testosterone replacement therapy in the aging male. Sex Med Rev. 2015;3:101–112. [DOI] [PubMed] [Google Scholar]
- 18.FDA. Exceptions and Alternative Procedures Approved Under 21 CFR 640.120. FDA, 2018. Available from: https://www.fda.gov. Cited on October 25, 2019. [Google Scholar]
- 19.Wilson MC, Trakarnsanga K, Heesom KJ, et al. Comparison of the proteome of adult and cord erythroid cells, and changes in the proteome following reticulocyte maturation. Mol Cell Proteomics. 2016;15:1938–1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gautier EF, Leduc M, Cochet S, et al. Absolute sproteome quantification of highly purified populations of circulating reticulocytes and mature erythrocytes. Blood Adv. 2018;2:2646–2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.D’Alessandro A, Dzieciatkowska M, Nemkov T, Hansen KC. Red blood cell proteomics update: Is there more to discover? Blood Transfus. 2017;15:182–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nemkov T, Sun K, Reisz JA, et al. Metabolism of citrate and other carboxylic acids in erythrocytes as a function of oxygen saturation and refrigerated storage. Front Med (Lausanne). 2017;4:175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nemkov T, Sun K, Reisz JA, et al. Hypoxia modulates the purine salvage pathway and decreases red blood cell and supernatant levels of hypoxanthine during refrigerated storage. Haematologica. 2018;103:361–372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nikolaos S, George A, Telemachos T, Maria S, Yannis M, Konstantinos M. Effect of L-carnitine supplementation on red blood cells deformability in hemodialysis patients. Ren Fail. 2000;22:73–80. [DOI] [PubMed] [Google Scholar]
- 25.Wu H, Bogdanov M, Zhang Y, et al. Hypoxia-mediated impaired erythrocyte Lands' cycle is pathogenic for sickle cell disease. Sci Rep. 2016;6:29637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sun K, Zhang Y, D’Alessandro A, et al. Sphingosine-1-phosphate promotes erythrocyte glycolysis and oxygen release for adaptation to high-altitude hypoxia. Nat Commun. 2016;7:12086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kleinbongard P, Schulz R, Rassaf T, et al. Red blood cells express a functional endothelial nitric oxide synthase. Blood. 2006;107:2943–2951. [DOI] [PubMed] [Google Scholar]
- 28.D’Alessandro A, Reisz JA, Zhang Y, et al. Effects of aged stored autologous red blood cells on human plasma metabolome. Blood Adv. 2019;3:884–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reisz JA, Nemkov T, Dzieciatkowska M, et al. Methylation of protein aspartates and deamidated asparagines as a function of blood bank storage and oxidative stress in human red blood cells. Transfusion. 2018;58:2978–2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barber JR, Clarke S. Membrane protein carboxyl methylation increases with human erythrocyte age. Evidence for an increase in the number of methylatable sites. J Biol Chem. 1983;258:1189–1196. [PubMed] [Google Scholar]
- 31.Nemkov T, Reisz JA, Xia Y, Zimring JC, D’Alessandro A. Red blood cells as an organ? How deep omics characterization of the most abundant cell in the human body highlights other systemic metabolic functions beyond oxygen transport. Expert Rev Proteomics. 2018;15:855–864. [DOI] [PubMed] [Google Scholar]
- 32.D’Alessandro A, Giardina B, Gevi F, Timperio AM, Zolla L. Clinical metabolomics: The next stage of clinical biochemistry. Blood Transfus. 2012;10(suppl 2):s19–s24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemkov T, Hansen KC, Dumont LJ, D’Alessandro A. Metabolomics in transfusion medicine. Transfusion. 2016;56:980–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nemkov T, Hansen KC, D’Alessandro A. A three-minute method for high-throughput quantitative metabolomics and quantitative tracing experiments of central carbon and nitrogen pathways. Rapid Commun Mass Spectrom. 2017;31:663–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.D’Alessandro A, Nemkov T, Yoshida T, Bordbar A, Palsson BO, Hansen KC. Citrate metabolism in red blood cells stored in additive solution-3. Transfusion. 2017;57:325–336. [DOI] [PubMed] [Google Scholar]
- 36.Fu X, Felcyn JR, Odem-Davis K, Zimring JC. Bioactive lipids accumulate in stored red blood cells despite leukoreduction: A targeted metabolomics study. Transfusion. 2016;56:2560–2570. [DOI] [PubMed] [Google Scholar]
- 37.Zwart A, van Assendelft OW, Bull BS, England JM, Lewis SM, Zijlstra WG. Recommendations for reference method for haemoglobinometry in human blood (ICSH standard 1995) and specifications for international haemiglobinocyanide standard (4th edition). J Clin Pathol. 1996;49:271–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.D’Alessandro A, Fu X, Kanias T, et al. Donor sex, age and ethnicity impact stored red blood cell antioxidant metabolism through mechanisms in part explained by glucose 6-phosphate dehydrogenase levels and activity. Haematologica. 2020. 10.3324/haematol.2020.246603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Judkiewicz L, Bugala I, Bartosz G. 'Pink test' and osmotic fragility test for the diagnosis of hereditary spherocytosis: Another view. Eur J Haematol. 1989;42:217. [DOI] [PubMed] [Google Scholar]
- 40.Takebayashi J, Kaji H, Ichiyama K, et al. Inhibition of free radical-induced erythrocyte hemolysis by 2-O-substituted ascorbic acid derivatives. Free Radic Biol Med. 2007;43:1156–1164. [DOI] [PubMed] [Google Scholar]
- 41.Chong J, Soufan O, Li C, et al. MetaboAnalyst 4.0: Towards more transparent and integrative metabolomics analysis. Nucleic Acids Res. 2018;46:W486–W494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.R: A lanuguage and environment for statistical computing [monograph on the internet]. Vienna, Austria: R foundation for Statistical Computing; 2018. Available from: https://www.R-project.org. [Google Scholar]
- 43.Contreras-Zentella ML, Sanchez-Sevilla L, Suarez-Cuenca JA, et al. The role of oxidant stress and gender in the erythrocyte arginine metabolism and ammonia management in patients with type 2 diabetes. PLoS One. 2019;14:e0219481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Auer MK, Cecil A, Roepke Y, et al. 12-months metabolic changes among gender dysphoric individuals under cross-sex hormone treatment: A targeted metabolomics study. Sci Rep. 2016;6:37005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Karafin MS, Francis RO. Impact of G6PD status on red cell storage and transfusion outcomes. Blood Transfus. 2019;17:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tzounakas VL, Kriebardis AG, Georgatzakou HT, et al. Glucose 6-phosphate dehydrogenase deficient subjects may be better “storers” than donors of red blood cells. Free Radic Biol Med. 2016;96:152–165. [DOI] [PubMed] [Google Scholar]
- 47.Reisz JA, Tzounakas VL, Nemkov T, et al. Metabolic linkage and correlations to storage capacity in erythrocytes from glucose 6-phosphate dehydrogenase-deficient donors. Front Med (Lausanne). 2017;4:248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ben-David A, Gavendo S. Osmotic fragility changes of ACD blood units from polycythemic donors. Transfusion. 1973;13:320–323. [DOI] [PubMed] [Google Scholar]
- 49.D’Alessandro A, Culp-Hill R, Reisz JA, et al. Heterogeneity of blood processing and storage additives in different centers impacts stored red blood cell metabolism as much as storage time: Lessons from REDS-III-Omics. Transfusion. 2019;59:89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Blanc L, Papoin J, Debnath G, et al. Abnormal erythroid maturation leads to microcytic anemia in the TSAP6/Steap3 null mouse model. Am J Hematol. 2015;90:235–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu D, Yi S, Zhang X, et al. Human STEAP3 mutations with no phenotypic red cell changes. Blood. 2016;127:1067–1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Howie HL, Hay AM, de Wolski K, et al. Differences in Steap3 expression are a mechanism of genetic variation of RBC storage and oxidative damage in mice. Blood Adv. 2019;3:2272–2285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Arduini A, Holme S, Sweeney JD, Dottori S, Sciarroni AF, Calvani M. Addition of L-carnitine to additive solution-suspended red cells stored at 4 degrees C reduces in vitro hemolysis and improves in vivo viability. Transfusion. 1997;37:166–174. [DOI] [PubMed] [Google Scholar]
- 54.Fang F, Page G, Alexander KL, et al. Abstract Presentations from the AABB Annual Meeting San Antonio, TX, October 19–22, 2019. Transfusion. 2019;59:87A. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.