Abstract
Introduction:
In March 2016, the Centers for Disease Control and prevention issued opioid prescribing guidelines for chronic non-cancer pain. In response, in April 2016 the North Carolina Medical Board launched the Safe Opioid Prescribing Initiative, an investigative program intended to limit overprescribing of opioids. This study focuses on the association of the Safe Opioid Prescribing Initiative with immediate and sustained changes in opioid prescribing among all opioid patients, and opioid discontinuation and tapering among high-dose (>90 milligrams of morphine equivalents), long-term (>90 days) opioid therapy patients.
Methods:
Controlled and single interrupted time series analysis of opioid prescribing outcomes before and after the implementation of Safe Opioid Prescribing Initiative was conducted, using de-identified data from the North Carolina Controlled Substances Reporting System from January 2010 through March 2017. Analysis was conducted in 2019–2020.
Results:
In an average study month, 513,717 patients, including 47,842 high-dose, long-term opioid therapy patients, received 660,912 opioid prescriptions at 1.3 prescriptions per patient. There was a 0.52% absolute decline (95% CI= −0.87, −0.19) in patients receiving opioid prescriptions in the month after Safe Opioid Prescribing Initiative implementation. Abrupt discontinuation, rapid tapering, and gradual tapering of opioids among high-dose, long-term opioid therapy patients increased by 1% (95% CI= −0.22, 2.23), 2.2% (95% CI=0.91, 3.47), and 1.3% (95% CI=0.96, 1.57), respectively, in the month following Safe Opioid Prescribing Initiative implementation.
Conclusions:
Although Safe Opioid Prescribing Initiative implementation was associated with an immediate decline in overall opioid prescribing, it was also associated with an unintended immediate increase in discontinuations and rapid tapering among high-dose, long-term opioid therapy patients. Better policy communication and prescriber education regarding opioid tapering best practices may help mitigate unintended consequences of statewide policies.
INTRODUCTION
In 2017 in the U.S., 47,600 opioid overdose deaths occurred, at an average of 130 per day.1 Although illicit opioids including fentanyl, carfentanil, and heroin are implicated in the majority of opioid-related deaths,1 prescription opioid overdose deaths claimed more American lives in 2017 than in any single year on record, contributing to >17,000 deaths per year.1 High doses and longer durations of opioid prescriptions are associated with overdose deaths2–5 and the inappropriate use of prescription opioids is a gateway to diversion and the use of heroin and illicit fentanyl.6–8 The Centers for Disease Control and Prevention (CDC) estimates that >700 people seek care in emergency departments every day for opioid-related harms.9 In 2017, an estimated 1.7 million Americans adults had a substance use disorder that involved prescription pain relievers10 and 9.9 million Americans used prescription opioids for non-medical purposes.10 As of 2013, the total economic burden of opioid use disorders and overdoses related to prescription opioids was estimated to be $78.5 billion.11
Though this epidemic is multifactorial, most policy-based attention has focused on curtailing opioid overprescribing.12 To limit opioid overprescribing and address prescription opioid overdoses, CDC introduced opioid prescribing guidelines for chronic non-cancer pain in March 2016.13 The following month, April 2016, the North Carolina Medical Board (NCMB) announced that it had adopted the guidelines and launched the Safe Opioid Prescribing Initiative (SOPI), an investigative program intended to address overprescribing by “identifying prescribers who manage large number of patients at high doses of opioids, as well as prescribers who have had two or more patient deaths due to opioid poisoning.”14 The NCMB sent letters and e-mails to all NC providers detailing the SOPI launch and its intention to investigate providers who met the criteria. By December 2016, 62 NC prescribers had been investigated under SOPI and 54% of those had been disciplined.14
There is concern that programs such as SOPI, which involve scrutiny and enforcement by state medical boards, may lead to inappropriate tapering or discontinuation of opioid pain medications for patients with legitimate pain control needs. CDC guidance suggests that opioid reduction of ≤10% per week may be ideal. However, many physician groups, as well as CDC, have expressed concern that some patients may be tapered and even discontinued at a much higher rate.15,16 Patients who receive high-dose, long-term opioid therapy (HD-LTOT), defined as receiving ≥90 milligrams of morphine equivalents (MMEs) of opioids for ≥90 consecutive days, may be particularly vulnerable.17 HD-LTOT patients, if abruptly tapered or discontinued, may suffer withdrawal and seek opioids elsewhere, increasing their potential for illicit drug use and overdose.
In this study, the authors examined the association of SOPI with immediate and sustained changes in: (1) overall opioid prescribing in NC and (2) tapering and discontinuation of prescription opioids among HD-LTOT patients.
METHODS
A controlled interrupted time series study was conducted to examine changes in temporal trends of opioid prescribing overall and discontinuation and tapering among HD-LTOT patients in NC, following the NCMB’s adoption of CDC opioid prescribing guidelines and launching of SOPI in April 2016. De-identified data from January 2010 through March 2017 were obtained from the NC Controlled Substances Reporting System. This database contains all outpatient prescriptions of Schedule II–V substances dispensed by NC pharmacies, except for those managed by the Veterans Administration and located on military bases.
Study Population
Any individual who filled ≥1 opioid pain medication prescription, excluding tramadol, buprenorphine, and methadone, was included; reasons for these exclusions are detailed in the Appendix. Individuals who received a daily dosage of ≥90 MMEs for ≥90 consecutive days were considered HD-LTOT patients. Ninety or more consecutive days of opioids meets the LTOT defintion,18–21 and the CDC considers 90 MMEs per day a high dose.13 MME for all opioids were calculated using CDC conversion ratios.22 Overlapping prescriptions with <7 days of overlap were staggered to follow one another; ≥7 days of overlap was considered true overlap and doses were summed for each overlapping day.
Measures
Outcomes were: (1) proportion of NC population (all ages) filling an opioid prescription or opioid prescribing rate, (2) proportion of all opioid patients who received HD-LTOT, (3) average opioid prescriptions per opioid patient, (4) proportion of HD-LTOT patients who were discontinued, (5) proportion of HD-LTOT patients who were tapered rapidly, and (6) proportion of HD-LTOT patients who were tapered gradually. Monthly outcome estimates were calculated. The first 3 months of the data series were truncated owing to the 90-day HD-LTOT eligibility requirement; hence, analyses included data from April 2010 to March 2017.
The HD-LTOT patients were considered discontinued if they were not dispensed any opioid pain medication for ≥30 consecutive days. HD-LTOT patients were considered tapered if the total MMEs they received from all prescribers decreased during a 14-day period. Then, the percentage change in opioid MMEs from day 1 to 14 among tapered patients was calculated. Those tapered by ≥19% during this 14-day period (equivalent to CDC-defined 10% per week)15 were classified as gradually tapered. Those tapered by >19% over the 2 weeks were considered rapidly tapered. This was a dynamic cohort where HD-LTOT patients who were discontinued or tapered <90 MMEs were censored from the HD-LTOT population, but could re-enter if they again met the HD-LTOT criteria.
The exposure was the launch of NCMB SOPI in April 2016. SOPI was launched simultaneously with the NCMB’s adoption of the CDC opioid prescribing guidelines for chronic non-cancer pain, which were released in March 2016. Thus, the authors compared trends in outcomes prior to and including April 2016, relative to May 2016 and thereafter.
Two control groups were included to address potential co-intervention/contamination of SOPI’s effects by the CDC guidelines. First, the guidelines addressed co-prescription of benzodiazepines and opioids, but SOPI’s investigative criteria were based solely on opioids. Hence, using benzodiazepine prescribing trends as a control would allow to remove the effects of the CDC guidelines, such that a change in opioid prescribing beyond what can be observed in benzodiazepine prescribing (as the effects of CDC’s guidelines) can be attributed to SOPI Secondly, prior studies have used prescribing trends in other medications as a control.23,24 Pertinent to opioids, other scheduled substances (e.g., stimulants) can be used, which like benzodiazepines are also prescribed for mental health disorders and sometimes co-prescribed with opioids.
Patients who receive opioids from <1 prescriber may be more likely to be rapidly tapered or discontinued. Therefore, analyses were stratified by receiving opioid medications from a single prescriber versus multiple prescribers. Any patient receiving simultaneous opioid prescriptions from <1 prescriber was considered a multiple prescriber patient for the entirety of the study.
Statistical Analysis
Autoregressive integrated moving average models were used for controlled interrupted time series analyses24 to examine the association of SOPI with the trends of opioid prescribing rate and average opioid prescriptions per patient. In addition, single series interrupted time series analysis was used for all outcomes.25 Model details are explained in the Appendix. The models included a first- and second-order autoregressive component to the model (p=1,2). Adding an autoregressive moving average component (q=1) reduced model fit and was excluded. Prior studies have noted a seasonal trend in opioid prescribing, where rates decline in November and December before increasing again in January.26 This effect was modeled using an indicator variable for November and December. Presentation of results focuses on effect sizes and precision (95% CIs) rather than statistical significance.
As the discontinuation and tapering definitions respectively required 30-day and 14-day follow ups with no opioids, very few discontinuations and tapering were observed in the first and last months of the time series. Therefore, May 2010 and March 2017 were excluded from the discontinuation and tapering analyses.
Sensitivity analyses were conducted to: (1) examine SOPI’s potential anticipatory (starting in March or April 2016) or lagged (starting in June 2016) effects by modifying the exposure definition; (2) examine discontinuations, rapid tapering, and gradual tapering among those who receive moderate-dose (≥50 MMEs) LTOT (MD-LTOT); (3) compare the effect of SOPI during the intervention period with the prior year’s HD-LTOT population as controls using a difference-in-differences framework; and (4) examine the HD-LTOT rate, discontinuation, and tapering among those receiving methadone, as methadone is usually prescribed at very high doses for <1% of chronic pain management cases (Appendix). This study was approved by the IRB at the University of North Carolina at Chapel Hill.
RESULTS
In NC, from April 2010 to March 2017, there were 57,404,706 dispensed prescriptions of opioid pain medications excluding tramadol, buprenorphine, and methadone. During this period, a total of 205,255 (2%) people received HD-LTOT (Appendix Table 1). Although daily MME trends remained constant among opioid patients from 2010 to 2017, the days’ supply increased over time (Appendix Table 1).
During an average calendar month between April 2010 and March 2017, an estimated 513,717 patients received about 660,912 opioid prescriptions, at a rate of 1.3 prescriptions per patient, and 9.3% of opioid patients received HD-LTOT (Appendix Table 2). Though single prescriber patients formed almost half of all opioid patients, only 1.8% of single prescriber opioid patients received HD-LTOT compared with 16.6% in the multiple prescriber patient group (Appendix Table 2). Single prescriber HD-LTOT patients were more often discontinued compared with the multiple prescriber HD-LTOT patients (Appendix Table 2).
The opioid prescribing rate remained stable at approximately 5%–6% population per month before SOPI, but then declined in the month immediately following the SOPI rollout by 0.52% (95% CI= −0.86, −0.19) relative to benzodiazepines and by 0.54% (95% CI= −0.87, −0.20) relative to stimulants, and remained stable thereafter (Table 1; Figures 1A and 1B). After SOPI implementation, the trend of receiving opioids from a single prescriber increased, whereas the rates of multiple prescriber opioid patients declined (Table 1; Figure 1C); both trends were stable pre-SOPI.
Table 1.
Association of Safe Opioid Prescribing Initiative (SOPI) With Opioid Prescribing Outcomes Among All Opioid Patients in NC: 2010–2017
| Control series | Immediate absolute change (β6) after SOPI (95% CI) relative to control | Sustained trend changes (β7) after SOPI (95% CI) relative to control |
|---|---|---|
| Opioid patients/100 NC population | ||
| Benzodiazepines | −0.5248 (−0.8584, −0.1911) | 0.0380 (−0.0084, 0.0844) |
| Stimulants | −0.5374 (−0.8722, −0.2026) | 0.0462 (−0.0444, 0.0486) |
| Number of opioid prescriptions per patient | ||
| Benzodiazepines | 0.0020 (−0.0249, 0.0289) | −0.0016 (−0.0021, 0.0053) |
| Stimulants | −0.0086 (−0.0341, 0.0168) | −0.0014 (−0.0049, 0.0022) |
SOPI, Safe Opioid Prescribing Initiative; NC, North Carolina.
Figure 1A, 1B, 1C.
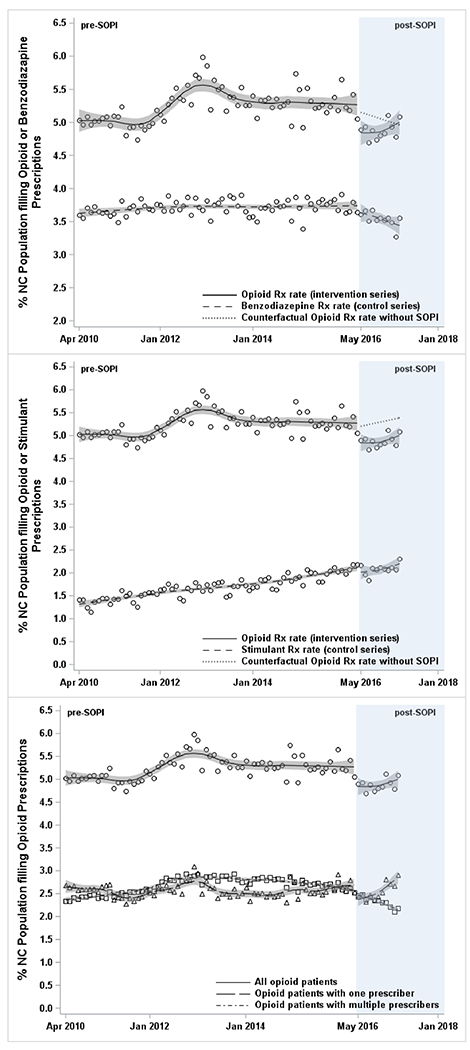
Association of NCMB SOPI with opioid prescribing rate in North Carolina using controlled and single interrupted time series analyses: 2010–2017.
HD-LTOT, high dose long term opioid therapy; Rx, prescription; SOPI, Safe Opioid Prescribing Initiative; NCMB, North Carolina Medical Board.
The proportion of all opioid patients who received HD-LTOT increased from 7% to 10% during the pre-SOPI period (Figure 2A). This trend reversed post-SOPI when the proportion of HD-LTOT started declining, falling to 8% by 2017 (Table 2; Figure 2A). This trend change was confined to multiple prescriber HD-LTOT patients. The average number of opioid prescriptions per patient declined continuously from 2010 to 2017 without any evident policy effects (Table 2; Appendix Figure 1).
Figure 2A, 2B, 2C, 2D.
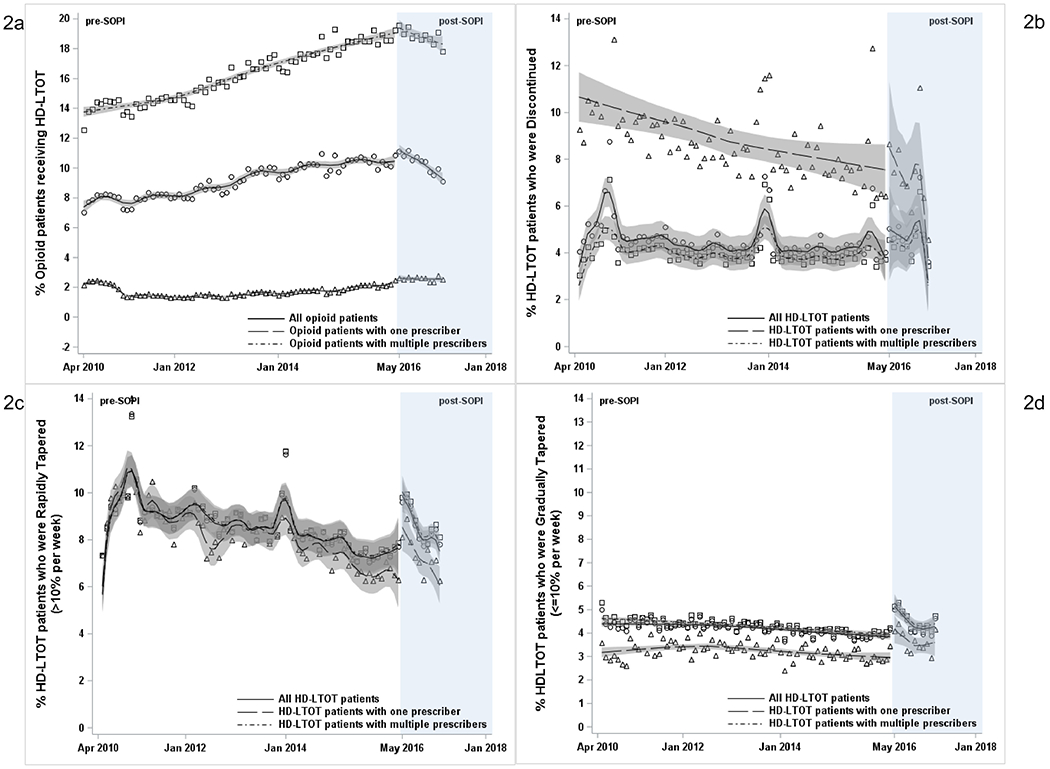
Association of NCMB SOPI with receiving HD-LTOT and being discontinued and tapered off opioids among HD-LTOT patients in North Carolina: 2010–2017.
HD-LTOT, high dose long term opioid therapy; SOPI, Safe Opioid Prescribing Initiative; NCMB, North Carolina Medical Board.
Table 2.
Association of Safe Opioid Prescribing Initiative (SOPI) With Opioid Prescribing Outcomes Among All Opioid Patients and High Dose Long Term Opioid Therapy Patients in North Carolina: 2010-2017
| Overall/Stratified | Pre-SOPI monthly trend (95% CI) | Immediate absolute change after SOPI (95% CI) | Difference between postand pre-SOPI monthly trends (95% CI) |
|---|---|---|---|
| All opioid patients | |||
| Opioid patients/100 NC population | |||
| Overall | 0.0041 (−0.0006, 0.0089) | −0.4186 (−0.7780, −0.0592) | −0.0056 (−0.0591, 0.0480) |
| Single prescriber | −0.0001 (−0.0028, 0.0026) | −0.2797 (−0.5227, −0.0366) | 0.0462 (0.0113, 0.0811) |
| Multiple prescriber | 0.0045 (−0.0046, 0.0136) | −0.0556 (−0.1994, 0.0883) | −0.0395 (−0.0801, 0.0011) |
| HD-LTOT rate/ 100 opioid patients | |||
| Overall | 0.0478 (0.0404, 0.0552) | 0.6293 (−0.0617, 1.3202) | −0.2406 (−0.3386, −0.1427) |
| Single prescriber | 0.0004 (−0.0238, 0.0247) | 0.1749 (−0.1197, 0.4695) | −0.0012 (−0.1003, 0.0979) |
| Multiple prescriber | 0.0813 (0.0740, 0.0886) | 0.4033 (−0.3508, 1.1574) | −0.2004 (−0.3059, −0.0950) |
| Opioid prescriptions per patient | |||
| Overall | −0.0009 (−0.0011, −0.0006) | −0.0011 (−0.0268, 0.0246) | −0.0015 (−0.0050, 0.0021) |
| Single prescriber | −0.0004 (−0.0005, −0.0003) | 0.0031 (−0.0091, 0.0152) | 0.0006 (−0.0012, 0.0023) |
| Multiple prescriber | −0.0016 (−0.0018, −0.0015) | −0.0035 (−0.0261, 0.0191) | 0.0011 (−0.0021, 0.0043) |
| HD-LTOT patients | |||
| Discontinuation rate/100 HD-LTOT patients | |||
| Overall | −0.0123 (−0.0219, −0.0027) | 1.0030 (−0.2214, 2.2274) | −0.0370 (−0.2266, 0.1525) |
| Single prescriber | −0.0520 (−0.0769, −0.0271) | 1.9406 (−1.0273, 4.9086) | −0.2652 (−0.7245, 0.1940) |
| Multiple prescriber | −0.0052 (−0.0124, 0.0020) | 0.6910 (−0.2788, 1.6608) | −0.0120 (−0.1627, 0.1387) |
| Rapid tapering rate/ 100 HD-LTOT patients | |||
| Overall | −0.0316 (−0.0424, −0.0208) | 2.1922 (0.9106, 3.4738) | −0.1765 (−0.3743, 0.0213) |
| Single prescriber | −0.0456 (−0.0571, −0.0342) | 1.9040 (0.5221, 3.2858) | −0.1726 (−0.3846, 0.0393) |
| Multiple prescriber | −0.0299 (−0.0408, −0.0189) | 2.2290 (0.9275, 3.5304) | −0.1692 (−0.3702, 0.0318) |
| Gradual tapering rate/ 100 HD-LTOT patients | |||
| Overall | −0.0094 (−0.0119, −0.0069) | 1.2630 (0.9599, 1.5660) | −0.1240 (−0.1704, −0.0777) |
| Single prescriber | −0.0057 (−0.0106, −0.0008) | 1.1685 (0.5970, 1.7399) | −0.1080 (−0.1949, −0.0210) |
| Multiple prescriber | −0.0105 (−0.0127, −0.0082) | 1.3171 (1.0240, 1.6103) | −0.1230 (−0.1679, −0.0781) |
HD-LTOT, high dose long term opioid therapy; SOPI, Safe Opioid Prescribing Initiative; NC, North Carolina.
Prior to SOPI, the rate of opioid discontinuation among HD-LTOT patients ranged from approximately 3% to 7% per month (Figure 2B), with a minimal decline of 0.01% per month during this period (Table 2). By contrast, during the month post-SOPI, the discontinuation rate increased by 1% (95% CI= −0.22, 2.23) for all HD-LTOT patients (Table 2; Figure 2B). Single prescriber HD-LTOT patients, who had a more pronounced decline in discontinuation pre-SOPI (−0.05% per month), experienced 1.9% increase in discontinuations in the month following SOPI implementation (Table 2). Multiple prescriber HD-LTOT patients, who had stable pre-SOPI discontinuation trend, experienced a modest 0.7% increase in discontinuation the month after SOPI.
Rapid tapering among HD-LTOT patients declined from approximately 10% per month in 2010 to <8% per month in 2016 (Figure 2C). Post-SOPI, there was an immediate increase of 2.2% (95% CI=0.91, 3.47) in rapid tapering (Table 2) and returned to close to the pre-SOPI levels within approximately 11 months. There was no effect measure modification by single or multiple prescribers (Figure 2C).
Gradual tapering among HD-LTOT patients pre-SOPI was stable at approximately 4% per month (Figure 2D; Table 2). Gradual tapering increased by 1.3% (95% CI=0.96, 1.57) in the month after SOPI and then declined but remained above pre-SOPI levels in 2017 (Table 2). Overall, the combined rates of discontinuation and rapid tapering were much higher than the rate of gradual tapering before and after SOPI (Figure 2B–D).
Sensitivity analyses ascertained the robustness of the results (see Appendix). Anticipatory or lagged effects of the policy did not change results substantively (Appendix Table 3). Although the overall rates of discontinuations, rapid tapering, and gradual tapering among MD-LTOT patients were slightly lower than those of HD-LTOT patients, their association with SOPI was very similar to that among the HD-LTOT patients (Appendix Table 4). Difference-in-differences analyses showed that compared to prior years, the introduction of SOPI in 2016 resulted in 4.3% (95% CI=3.5, 5.2) additional discontinuations, 3.2% (95% CI=2.8, 3.5) additional rapid tapering, and 0.46% (95% CI=0.42, 0.50) additional gradual tapering (Appendix Tables 5 and 6). Finally, stratified methadone analysis revealed that in an average month 65%-75% of methadone patients were HD-LTOT (median daily MMEs=120) compared with 8%–12% of HD-LTOT patients among those receiving other opioids. SOPI’s impact on discontinuations and tapering among methadone HD-LTOT patients is similar to that seen in the main analyses (Appendix Figure 2A–F
DISCUSSION
This is the first study to examine a state medical board policy initiative to address opioid overprescribing on discontinuation and tapering of opioids among HD-LTOT patients. There was a 0.52% reduction in opioid prescribing rate in NC the month after SOPI implementation that remained unchanged over the rest of the study period. There was also a decline in the proportion of patients receiving HD-LTOT post-SOPI. Immediately after the NCMB’s SOPI implementation, abrupt discontinuation, rapid tapering, and gradual tapering of opioids among HD-LTOT patients increased by 1%, 2.2%, and 1.3%, respectively.
The increase in gradual tapering, the decline in HD-LTOT, and the decline in patients receiving opioids overall are likely to be viewed as a success of SOPI. However, the simultaneous increase in discontinuations and rapid tapering may cause concern as they could lead to uncontrolled pain and induce patients to seek unregulated market for opioids.27 Although the differences in discontinuations post-SOPI were statistically non-significant, the 3%–7% abrupt discontinuations among HD-LTOT and the additional 1% increase post-SOPI may be concerning.28,29 The 0.52% decline in opioid prescribing rate (~20,000 fewer patients across the state per month) occurred in a single month following SOPI implementation, which potentially suggests a sudden reduction or even stoppage in opioid prescribing by NC prescribers.
During the past 2 decades, the pendulum has swung from a focus on pain management using opioids to regulations designed to reduce overprescribing of opioids.30 Now, there is concern that patients with chronic pain may be denied needed pain management.31–34 If changes in recommendations and policies are not well communicated, it may lead to a chilling effect, causing prescribers to decline opioid pain relievers to new patients or rapidly taper or discontinue opioids to patients already receiving them.33,34 A recent study showed that discontinuing individuals at ≥120 MMEs of LTOT was associated with increased emergency department visits.35 Owing to growing concerns,36 CDC has advised against misapplications of the guidelines that result in abrupt tapering or discontinuation.16,37,38 Rapid tapering and discontinuation results from this study support the recent consensus statement from the American Academy of Pain Medicine.15 Another study examining the impact of CDC guidelines suggested that opioid prescribing declined nationally after the CDC guidelines were released.39 However, this study did not examine rapid tapering or discontinuation.
The impact of SOPI varied by the number of prescribers a patient received opioids from. Discontinuation was higher among those receiving HD-LTOT from a single prescriber compared with multiple prescribers, whereas rapid and gradual tapering were higher among multiple prescriber HD-LTOT patients than those with a single prescriber. Multiple prescriber HD-LTOT patients may continue to receive some opioids even if 1 prescriber discontinues prescribing, hence appearing to be tapered even when 1 of their multiple prescriptions is discontinued. However, single prescriber HD-LTOT patients will only appear as discontinued when their sole prescriber discontinues opioid prescribing.
During the study period, HD-LTOT patients represented 2% of opioid patients in NC; that is, >200,000 people. The authors found that the proportion of opioid patients who received HD-LTOT increased pre-SOPI but declined post-SOPI, especially among multiple prescriber opioid patients. Simultaneously, the opioid prescription rate trend was stable (neither increasing nor decreasing) before and after SOPI, but there was an immediate decrease in opioid prescribing rate the month after SOPI implementation. Further, there was no change in the average opioid prescriptions per patient, which were already decreasing pre-SOPI and kept decreasing post-SOPI. This suggests that the HD-LTOT patient group was one of the most affected by SOPI. This is also the group of patients that is potentially most vulnerable to unintended consequences of rapid tapering and discontinuation, and it is concerning that the combined proportion of rapidly tapering and discontinuation was much higher than gradual tapering both pre- and post-SOPI. There is a need for better communication to providers about appropriate tapering strategies and harms of abrupt discontinuation.
Limitations
This study has limitations that could not be addressed. First, given the limited nature of the data, patients who died, migrated out of the state, or were incarcerated could not be identified. These individuals may erroneously appear as discontinued. However, mortality, incarcerations, and outmigrations over the study period are much smaller than the number of individuals included in this study, and any resulting overestimation of discontinuation will be expected to affect pre- and post-SOPI trends similarly, thereby not affecting the interpretation of the results. Second, the exact reasons that may have caused each specific case of discontinuation, rapid tapering, or gradual tapering are unknown. However, regardless of the exact reasons, there is an immediate change (or a disruption) in the trend of these events after SOPI implementation. Lastly, differential effects of SOPI by age, gender, race, and payer type could not be addressed due to lack of demographic information in the data available to the authors. The strengths of the study include use of a comprehensive statewide database with high-quality and detailed information opioid prescribing information, well-measured exposure and outcomes, and controlled interrupted time series design limiting potential for selection effects.
CONCLUSIONS
This study presents some of the first large-scale documented evidence of a state-level policy designed to combat opioid overprescribing on opioid tapering and discontinuation among patients receiving HD-LTOT. SOPI implementation was associated with an immediate decline in overall opioid prescribing and HD-LTOT. However, it was also associated with an immediate increase in discontinuations and rapid tapering in HD-LTOT, suggesting a need to emphasize communication with, and education of, prescribers regarding best practices in tapering of opioids.
Supplementary Material
ACKNOWLEDGMENTS
This work was supported by an award R49 CE002479 (Principal Investigator [PI]: Marshall) for an Injury Control Research Center from the National Center for Injury Prevention and Control, Centers for Disease Control and Prevention. SIR, CLR, BWP, NF and the analytic work were also supported through R21 DA046048 (PI: Pence). BLD’s time was funded through T32 AI007001 (PI: Adimora). The research and conclusions presented in this paper are that of the authors and do not reflect the views or policy of the funding agencies.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
No financial disclosures were reported by the authors of this paper.
REFERENCES
- 1.Scholl L, Seth P, Kariisa M, Wilson N, Baldwin G. Drug and opioid-involved overdose deaths—United States, 2013–2017. MMWR Morb Mortal Wkly Rep. 2018;67(5152): 1419–1427. 10.15585/mmwr.mm675152e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dunn KM, Saunders KW, Rutter CM, et al. Opioid prescriptions for chronic pain and overdose: a cohort study. Ann Intern Med. 2010; 152(2):85–92. 10.7326/0003-4819-152-2-201001190-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller M, Barber CW, Leatherman S, et al. Prescription opioid duration of action and the risk of unintentional overdose among patients receiving opioid therapy. JAMA Intern Med. 2015; 175(4):608–615. 10.1001/jamainternmed.2014.8071. [DOI] [PubMed] [Google Scholar]
- 4.Bohnert AS, Valenstein M, Bair MJ, et al. Association between opioid prescribing patterns and opioid overdose-related deaths. JAMA. 2011;305(13):1315–1321. 10.1001/jama.2011.370. [DOI] [PubMed] [Google Scholar]
- 5.Dasgupta N, Funk MF, Proescholdbell S, et al. Cohort study of the impact of high-dose opioid analgesics on overdose mortality. Pain Med. 2016;17(l):85–98. 10.1111/pme.12907. [DOI] [PubMed] [Google Scholar]
- 6.Jones CM, Logan J, Gladden M, Bohm MK. Vital signs: demographic and substance use trends among heroin users — United States, 2002–2013. MMWR Morb Mortal Wkly Rep. 2015;64(26):719–725. [PMC free article] [PubMed] [Google Scholar]
- 7.Substance Abuse and Mental Health Services Administration, Results from the 2013 National Survey on Drug Use and Health: Summary of National Findings, NSDUH Series H-48, HHS Publication No. (SMA) 14-4863. Rockville, MD: Substance Abuse and Mental Health Services Administration, 2014. [Google Scholar]
- 8.Centers for Disease Control and Prevention. Annual Surveillance Report of Drug-Related Risks and Outcomes — United States, 2017. Surveillance Special Report 1. Centers for Disease Control and Prevention, HHS; https://www.cdc.gov/drugoverdose/pdf/pubs/2017-cdc-drug-surveillance-report.pdf. Published August 31, 2017 Accessed December 12, 2017. [Google Scholar]
- 9.Lovegrove MC, Dowell D, Geller AI, et al. US emergency department visits for acute harms from prescription opioid use, 2016–2017. Am J Public Health. 2019;109(5):784–791. 10.2105/ajph.2019.305007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Substance Abuse and Mental Health Services Administration. Key Substance Use and Mental Health Indicators in the United States: Results from the 2018 National Survey on Drug Use and Health, https://www.samhsa.gov/data/sites/default/files/cbhsq-reports/NSDUHNationalFindingsReport2018/NSDUHNationalFindingsReport2018.pdf Accessed March 1, 2020.
- 11.Florence CS, Zhou C, Luo F, Xu L. The economic burden of prescription opioid overdose, abuse and dependence in the United States, 2013. Med Care. 2016; 54(10): 901–906. 10.1097/mlr.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dasgupta N, Beletsky L, Ciccarone D. Opioid crisis: no easy fix to its social and economic determinants. Am J Public Health. 2018; 108(2): 182–186. 10.2105/ajph.2017.304187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dowell D, Haegerich TM, Chou R. CDC guideline for prescribing opioids for chronic pain—United States, 2016. JAMA. 2016;315(15): 1624–1645. 10.1001/jama.2016.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North Carolina Medical Board. Annual Report. Raleigh, NC: North Carolina Medical Board; https://www.ncmedboard.org/images/uploads/disciplinary_reports/2016_Annual_Report1.pdf. Published 2016. Accessed January 1, 2019. [Google Scholar]
- 15.Kroenke K, Alford DP, Argoff C, et al. Challenges with implementing the Centers for Disease Control and Prevention opioid guideline: a consensus panel report. Pain Med. 2019;20(4):724–735. 10.1093/pm/pny307. [DOI] [PubMed] [Google Scholar]
- 16.Centers for Disease Control and Prevention. CDC Advises Against Misapplication of the Guideline for Prescribing Opioids for Chronic Pain. https://www.cdc.gov/media/releases/2019/s0424-advises-misapplication-guidelineprescribing-opioids.html. Published 2019. Accessed September 19, 2019.
- 17.Sullivan MD, Bauer AM, Fulton-Kehoe D, et al. Trends in opioid dosing among Washington State Medicaid patients before and after opioid dosing guideline implementation. J Pain. 2016;17(5):561–568. 10.1016/j.jpain.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 18.Chou R, Turner JA, Devine EB, et al. The effectiveness and risks of long-term opioid therapy for chronic pain: a systematic review for a National Institutes of Health Pathways to Prevention Workshop. Ann Intern Med. 2015;162(4):276–286. 10.7326/m14-2559. [DOI] [PubMed] [Google Scholar]
- 19.Tennant F, Hermann L. Intractable or chronic pain: there is a difference. West J Med. 2000;173(5):306 10.1136/ewjm.173.5.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Merskey H, Bogduk N. Classification of chronic pain. Descriptions of chronic pain syndromes and definitions of pain terms. Prepared by the International Association for the Study of Pain, Subcommittee on Taxonomy. Pain Suppl. 1986;3:S1–S226. [PubMed] [Google Scholar]
- 21.Baumblatt JAG, Wiedeman C, Dunn JR, et al. High-risk use by patients prescribed opioids for pain and its role in overdose deaths. JAMA Intern Med. 2014;174(5):796–801. 10.1001/jamainternmed.2013.12711. [DOI] [PubMed] [Google Scholar]
- 22.Centers for Disease Control and Prevention. Data Resources: Analyzing Prescription Data and Morphine Milligram Equivalents (MME). https://www.cdc.gov/drugoverdose/resources/data.html. Accessed October 21, 2020.
- 23.Walley AY, Xuan Z, Hackman HH, et al. Opioid overdose rates and implementation of overdose education and nasal naloxone distribution in Massachusetts: interrupted time series analysis. BMJ. 2013;346:f174 10.1136/bmj.f174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lopez Bernal J, Cummins S, Gasparrini A. The use of controls in interrupted time series studies of public health interventions. Int J Epidemiol. 2018;47(6):2082–2093. 10.1093/ije/dyy135. [DOI] [PubMed] [Google Scholar]
- 25.Ranapurwala SI, Carnahan R, Brown G, Hinmann J, Casteel C. Impact of Iowa’s prescription monitoring program on opioid pain reliever prescribing patterns: an interrupted time series study 2003–2014. Pain Med. 2019;20(2):290–300. 10.1093/pm/pny029. [DOI] [PubMed] [Google Scholar]
- 26.Goedel WC, Marshall BDL, Spangler KR, et al. Increased risk of opioid overdose death following cold weather: a case-crossover study. Epidemiology. 2019;30(5):637–641. 10.1097/ede.0000000000001041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Compton WM, Jones CM, Baldwin GT. Relationship between nonmedical prescription opioid use and heroin use. N Engl J Med. 2016; 374(2): 154–163. 10.1056/nejmra1508490. [DOI] [PubMed] [Google Scholar]
- 28.Amrhein V, Greenland S, McShane B. Scientists rise up against statistical significance. Nature. 2019;567(7748):305–307. 10.1038/d41586-019-00857-9. [DOI] [PubMed] [Google Scholar]
- 29.Wasserstein RL, Lazar NA. The ASA’s statement on p-values: context, process, and purpose. J Am Stat Assoc. 2016;70(2): 129–133. 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 30.Garcia AM. State laws regulating prescribing of controlled substances: balancing the public health problems of chronic pain and prescription painkiller abuse and overdose. J Law Med Ethics. 2013;41(suppl 1):42–45. 10.1111/jlme.12037. [DOI] [PubMed] [Google Scholar]
- 31.Fishman SM, Papazian JS, Gonzalez S, Riches PS, Gilson A. Regulating opioid prescribing through prescription monitoring programs: balancing drug diversion and treatment of pain. Pain Med. 2004;5(3):309–324. 10.1111/j.1526-4637.2004.04049.x. [DOI] [PubMed] [Google Scholar]
- 32.Goldenbaum DM, Christopher M, Gallagher RM, et al. Physicians charged with opioid analgesic-prescribing offenses. Pain Med. 2008;9(6):737–747. 10.1111/j.1526-4637.2008.00482.x. [DOI] [PubMed] [Google Scholar]
- 33.Hoffmann DE, Tarzian AJ. Achieving the right balance in oversight of physician opioid prescribing for pain: the role of state medical boards. J Law Med Ethics. 2003;31(1):21–40. 10.1111/j.1748-720x.2003.tb00057.x. [DOI] [PubMed] [Google Scholar]
- 34.von Gunten CF. The pendulum swings for opioid prescribing. J Palliat Med. 2016;19(4):348 10.1089/jpm.2016.0079. [DOI] [PubMed] [Google Scholar]
- 35.Mark TL, Parish W. Opioid medication discontinuation and risk of adverse opioid-related health care events. J Subst Abuse Treat. 2019;103:58–63. 10.1016/j.jsat.2019.05.001. [DOI] [PubMed] [Google Scholar]
- 36.Chou R, Ballantyne J, Lembke A. Rethinking opioid dose tapering, prescription opioid dependence, and indications for buprenorphine. Ann Intern Med. 2019;171(6):427–429. 10.7326/m19-1488. [DOI] [PubMed] [Google Scholar]
- 37.Dowell D, Haegerich T, Chou R. No shortcuts to safer opioid prescribing. N Engl J Med. 2019;380(24):2285–2287. 10.1056/nejmp1904190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dowell D, Compton WM, Giroir BP. Patient-centered reduction or discontinuation of long-term opioid analgesics: the HHS Guide for Clinicians. JAMA. 2019;322(19):1855–1856. 10.1001/jama.2019.16409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bohnert ASB, Guy GP Jr., Losby JL. Opioid prescribing in the United States before and after the Centers for Disease Control and Prevention’s 2016 opioid guideline. Ann Intern Med. 2018; 169(6):367–375. 10.7326/m18-1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


