Abstract
Introduction:
While wideband segmented, breath-hold late gadolinium-enhancement (LGE) cardiovascular magnetic resonance (CMR) has been shown to suppress image artifacts associated with cardiac implanted electronic devices (CIEDs), it may produce image artifacts in patients with arrhythmia and/or dyspnea. Single-shot LGE is capable of suppressing said artifacts. We sought to compare the performance of wideband single-shot free-breathing LGE against standard and wideband segmented LGEs in CIED patients.
Methods and Results:
We retrospectively identified all 54 consecutive patients (mean age: 61±15 years; 31% females) with CIED who had undergone CMR with standard segmented, wideband segmented, and/or wideband single-shot LGE sequences as part of quality assurance for determining best clinical practice at 1.5T. Two raters independently graded the conspicuity of myocardial scar or normal myocardium and the presence of device artifact level on a 5-point Likert scale (1: worst; 3: acceptable; 5: best). Summed visual score (SVS) was calculated as the sum of conspicuity and artifact scores (SVS ≥ 6 defined as diagnostically interpretable). Median conspicuity and artifact scores were significantly better for wideband single-shot LGE (F=24.2, p<0.001) and wideband segmented LGE (F=20.6, p<0.001) compared to standard segmented LGE. Among evaluated myocardial segments, 72% were deemed diagnostically interpretable –defined as SVS ≥ 6 - for standard segmented LGE, 89% were deemed diagnostically interpretable for wideband segmented LGE, and 94% segments were deemed diagnostically interpretable for wideband single-shot LGE.
Conclusions:
Wideband single-shot LGE and wideband segmented LGE produced similarly improved image quality compared to standard LGE.
Keywords: wideband LGE, image artifacts, MR safety, CIED, MRI
INTRODUCTION
Over 3 million patients in the United States are implanted with a cardiac implanted electronic device (CIED) such as implantable cardioverter-defibrillators (ICD), pacemaker, or cardiac resynchronization therapy (CRT) for increasing their lifespan and/or quality of life.1 The prevalence of CIED patients is likely to rise considerably with the aging population.1–3 Because CIED patients have underlying structural heart disease, they often require complex downstream clinical decisions, thereby warranting non-invasive imaging. As a versatile modality, cardiovascular magnetic resonance (CMR) is well-suited for a comprehensive assessment including cardiac function, structure, perfusion, hemodynamics, and viability.4–6 This makes CMR a clinically relevant tool for guiding clinical decisions regarding new or worsening cardiac symptoms, ventricular tachycardia ablation, battery replacement, or CRT upgrades. Despite the potential benefit, CMR is rarely conducted in CIED patients due to perceived safety concerns and actual image quality concerns.
While the mounting evidence has supported the safety of CMR in CIED patients with both MR-conditional and “legacy” devices7–11, severe image artifacts in as much as 33–48% of cases12,13 remain a major deterrent for CMR in CIED patients. In direct response, the UCLA group developed wideband late gadolinium enhancement (LGE)14 for suppressing image artifacts caused by CIEDs. The term wideband refers to wide frequency bandwidth of an inversion radio-frequency pulse for inverting off-resonant spins. Wideband segmented LGE has been tested in several clinical studies.13,15–17 Since the development of wideband LGE, the wideband concept has been extended for cardiac T1 mapping18,19 and cardiac perfusion20,21 CMR.
Given the high failure rate of standard LGE in CIED patients, our radiology department made a concerted effort to conduct a series of quality control projects for determining best clinical practice. Specifically, we developed and implemented two variants of wideband LGE:22,23 a segmented breath-hold version to achieve high spatial resolution and a single-shot, free-breathing version with lower spatial resolution. While wideband segmented LGE is capable of producing better image quality than wideband single-shot LGE in CIED patients with sinus rhythm and breath-holding capability, it may produce severe ghosting artifacts in patients with arrhythmia and/or dyspnea. Single-shot LGE is capable of suppressing said artifacts.24,25 To date, wideband single-shot LGE has not been evaluated in CIED patients. Thus, the purposes of this retrospective study were to determine whether wideband single-shot LGE is as effective as wideband segmented LGE in suppressing device-related image artifacts and test whether wideband single-shot LGE produces clinically acceptable image quality in CIED patients.
MATERIALS and METHODS
Patient Demographics
As per radiology practice, all patients provided written consent to an umbrella Institutional Review Board (IRB) approved protocol, which describes the risk involved with an MRI in patients with a CIED and that MRI in patients with a non-MR-conditional device is considered off-label. Given the low yield (~33% failure rate) by a clinical standard MR protocol in patients with a CIED,12 our radiology department conducted a quality assurance project by performing standard segmented LGE, wideband segmented LGE, and wideband single-shot LGE in CIED patients, in order to determine which LGE method would be adopted as standard of care. Therefore, the need for informed consent was waived. This retrospective study was approved by our local IRB and was found to comply with the Health Insurance Portability and Accountability Act (HIPAA).
We identified all fifty-four consecutive patients (mean age: 60.9 ± 15.3 years; range: 22–88 years; 31% females) with a CIED who had undergone clinical CMR with standard segmented, wideband segmented, and/or wideband single-shot LGE sequences as part of quality assurance for determining best clinical practice between February 2016 and January 2019. Electronic heath records were used to obtain information on demographics, clinical history, implantation history, device model, and interrogation records. Among 54 patients, 33 had a transvenous ICD (TV-ICD), 15 had a pacemaker, 1 had CRT-D (defibrillator), 1 had CRT-P (pacemaker), and 4 had a subcutaneous ICD (S-ICD). Out of the 54 patients who met the study criteria, 22 (42%) patients were pacer dependent. The three most common comorbidities were coronary artery disease (57%), hypertension (56%), and hyperlipidemia (54%). Nearly all patients had cardiac rhythm issues (94%), while nearly half had prior ablation (40%). 36 patients had some form of cardiomyopathy, with ischemic cardiomyopathy being the most common (24%)(Table 1). All information was stored in a HIPAA compliant REDCap (Research Electronic Data Capture) database.26,27
Table 1:
Patient demographics including age, sex, type of device, device characteristics, comorbidities, and cardiac history. TV: transvenous; S: subcutaneous.
| Age (years) | 60.9 ± 15.4 | |
| Male | 38 (70%) | |
| Female | 16 (30%) | |
| CIED | TV-ICD S-ICD | 33 (61%) 4 (7%) |
| Pacemaker | 15 (28%) | |
| CRT | 2 (4%) | |
| Pacer Dependent | 22 (41%) | |
| Manufacturer | Medtronic | 26 (48%) |
| Boston Scientific | 17 (32%) | |
| St. Jude Medical | 9 (17%) | |
| Biotronik | 2 (4%) | |
| Left Chest | 48 (89%) | |
| Right Chest | 2 (4%) | |
| Below Left Axilla | 4 (7%) | |
| Lead Location | Right Ventricle | 14 (28%) |
| Right Atrium, Right Ventricle | 24 (48%) | |
| Right Atrium, Right Ventricle, Left Ventricle | 12 (24%) | |
| Retained Lead | 2 (4%) | |
| Comorbidities | Coronary Artery Disease | 31 (57%) |
| Hypertension | 30 (56%) | |
| Hyperlipidemia | 29 (54%) | |
| Congestive Heart Failure | 27 (50%) | |
| Renal disease | 19 (35%) | |
| Obstructive Sleep Apnea | 19 (35%) | |
| Diabetes | 16 (30%) | |
| Chronic Obstructive Pulmonary Disease | 7 (13%) | |
| Cerebrovascular disease | 6 (11%) | |
| Cardiac History | Cardiac Arrhythmia | 51 (94%) |
| Prior ablation | 21 (39%) | |
| Ischemic Cardiomyopathy | 13 (24%) | |
| Non-ischemic Cardiomyopathy | 7 (13%) | |
| Dilated Cardiomyopathy | 5 (9%) | |
| Hypertrophic Cardiomyopathy | 4 (7%) | |
| Restrictive Cardiomyopathy | 1 (2%) | |
| Primary Prevention | 19 (51%) | |
| Secondary Prevention | 18 (49%) | |
MRI
Patients were scanned on a whole-body 1.5 Tesla MRI system (Magnetom Avanto, Siemens Healthcare, Erlangen, Germany) equipped with a gradient system capable of achieving a maximum gradient strength of 45 mT/m and maximum slew rate of 200 T/m/s. Body coil was used for radio-frequency excitation. Both body matrix and spine coil arrays (12–15 elements in total) were used for signal reception.
Because technologists had used their discretion to choose from standard segmented LGE, wideband segmented LGE, and/or wideband single-shot LGE, the specific LGE pulse sequences used (e.g. in some instances, not all three sequences were applied) and their order (standard LGE always went first, whereas two variants of wideband LGE order was mixed) varied across patients. The timing of LGE was approximately 10–30 minutes following administration of 0.1 to 0.2 mmol/kg of gadobutrol (Gadavist, Bayer HealthCare Pharmaceuticals, Wayne, New Jersey), where the dosage was based on estimated glomerular filtration rate. Because a standard inversion time (TI) scout pulse sequence using balanced steady state free precession produces severe image artifacts, our technologists repeatedly performed wideband single-shot LGE with variable TI to manually identify the TI that best nulls the normal myocardium signal (range: 190–450 msec). All pulse sequences used gradient echo readouts. Relevant pulse sequence parameters are summarized in Table S1 in supplementary materials.
Clinical Safety Procedure for MRI
CMR was conducted using a previously recommended guideline,7 which included: (1) device interrogation and programming before the MRI by the electrophysiology team; (2) monitoring the patient throughout the MRI by a nurse staff; (3) maintaining specific absorption rate (SAR) < 2.0 W/kg;28 (4) post-MRI device interrogation by the electrophysiology team to document any changes in device settings; (5) reprogramming device settings to the initial settings. Adverse events were defined as new arrhythmias, electrical reset, battery depletion, or any mode changes of the device as documented in the medical records. To determine whether CMR altered CIED operation, we retrieved device sensing (right atrium, right ventricle), impedance (right atrium, right ventricle, left ventricle), threshold (right atrium, right ventricle, left ventricle), and battery level information. These measurements were taken prior to CMR, immediately following CMR, and at a follow-up clinical appointment. To further evaluate CMR safety, we retrieved whole-body SAR for each LGE pulse sequence by reading the SAR value embedded in the DICOM header metadata using a custom code written in MATLAB (version R2017b; The MathWorks, Natick, MA, USA).
Visual Analysis of Image Quality
Images were visually evaluated by two clinical raters: one radiology attending (RJA) with 10 years of experience in CMR and one radiology fellow (AMS) with 4 years of experience in CMR. For each study, three short-axis images representing the basal, mid-ventricular, and apical planes of the left ventricle, and one 2-chamber plane image, when available, were chosen for visual scoring. As summarized in Table 2, the number of available planes by sequence varied, because of inconsistency in technologists performing three LGE sequences. The raters visually scored 16 myocardial segments in short-axis and 7 segments in long-axis using the American Heart Association model.29 Raters graded for conspicuity of scar and/or normal myocardium using a 5-point Likert scale (1 = nondiagnostic, 2 = poor, 3 = acceptable, 4 = good, 5 = excellent) and device-induced artifact on the heart using a 5-point Likert scale (1 = nondiagnostic, 2 = severe, 3 = moderate, 4 = mild, 5 = minimal). For 3% images with incorrect TI (i.e. normal myocardium appear grayish) caused by technologist error, the two raters were instructed to account for this in conspicuity scoring. The two raters were given training images to calibrate their scores in consensus prior to independent grading. Each rater independently graded the images blinded to pulse sequence, patient clinical history, and each other’s scores. Summed visual score (SVS) was calculated as the sum of conspicuity and artifact scores (range = 2–10). Diagnostically interpretable segment was defined as having SVS ≥ 6.
Table 2:
Summary of distribution of slices (basal, mid-ventricular, apical, and 2-chamber) across pulse sequences.
| Slice | Standard Segmented | Wideband Segmented | Wideband Single-Shot |
|---|---|---|---|
| Basal | 21 | 40 | 26 |
| Mid-Ventricular | 23 | 40 | 27 |
| Apical | 21 | 39 | 27 |
| 2-Chamber | 24 | 42 | 2 |
Quantitative Analysis of Image Quality
To supplement visual scores, we quantified coefficient of variation (CV) – defined as standard deviation divided by mean - in myocardial signal of whole left ventricle as a measure of hyper-intense image artifact. A second year medical school student (SMS) carefully drew the endocardial and epicardial contours while making sure to avoid partial volume averaging. We also quantified the blur metric30 on a 0 to 1 continuous scale, where 0 is defined as sharp and 1 is defined as blurred, as a measure of spatial resolution. For the blur metric, a medical fellow (AP) with two years of research experience cropped each image such that only the heart is included.
CXR Evaluation
To establish the relationship between visual scores and proximity of CIED to the heart, chest X-ray (CXR), when available, was used to measure lateral and vertical distances between centroids of the generator and left ventricle, as shown in Figure 1. The lateral and vertical distances were used to calculate the Euclidean distance, D = ((Lateral Distance)2 + (Vertical Distance)2)1/2.
Figure 1:
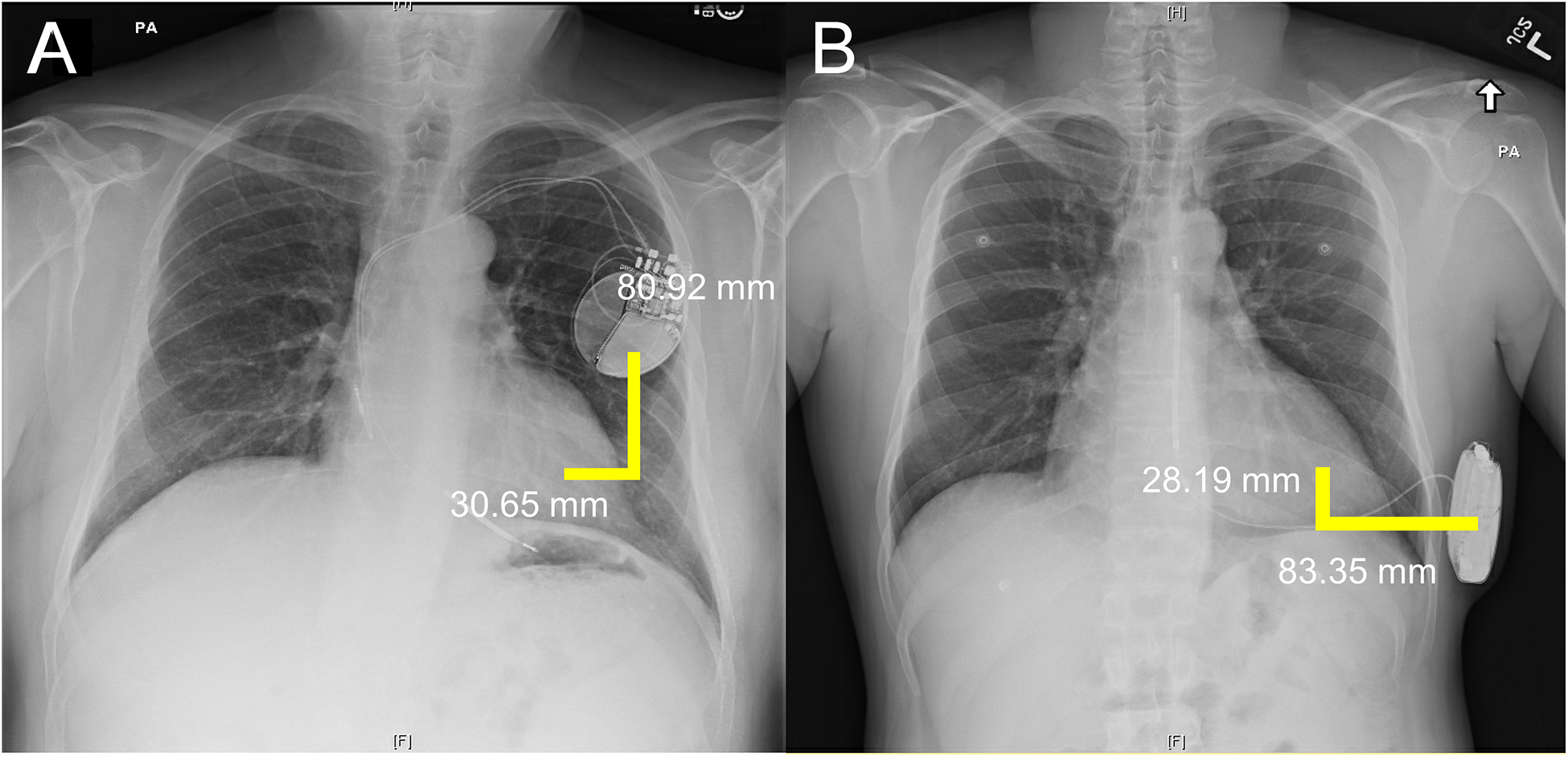
CXR measurements from the centroid of the device to the centroid of the left ventricle in a patient with a TV-ICD (Panel A) and an S-ICD (Panel B). CXR: Chest X-ray; TV: transvenous; S: subcutaneous.
Statistical Analysis
Variables were tested for normality using a Shapiro–Wilk test. Continuous data were reported as mean ± standard deviation; ordinal data were reported as median and interquartile range (IQR); 25th percentile, 75th percentile. Continuous variables with two normally distributed groups were compared using a two-tailed paired t-test. Continuous and ordinal variables with three groups were compared using ANOVA and Kruskal-Wallis, respectively, with Bonferroni correction for comparing each pair. Inter-rater reliability was calculated using weighted Cohen’s kappa coefficient.31 The association between continuous and ordinal variables was calculated using Spearman’s correlation analysis. All statistical analyses were conducted in SPSS (Version 23.0. Armonk, NY: IBM Corp). A p-value less than 0.05 was considered statistically significant for all tests.
RESULTS
Image Quality
A total of 322 images were evaluated: 89 images (28%; 21 basal, 23 mid-ventricular, 21 apical, and 24 2-chamber planes) for standard segmented LGE; 161 images (49%; 40 basal, 40 mid-ventricular, 39 apical, and 42 2-chamber planes) for wideband segmented LGE; 82 images (25%; 27 basal, 26 mid-ventricular, 26 apical, and 2 2-chamber planes) for wideband single-shot LGE. Figure 2 presents representative images obtained from three different patients with TV-ICDs using the three LGE pulse sequences. As shown, both wideband segmented LGE and wideband single-shot LGE consistently produced better image quality than standard segmented LGE.
Figure 2:
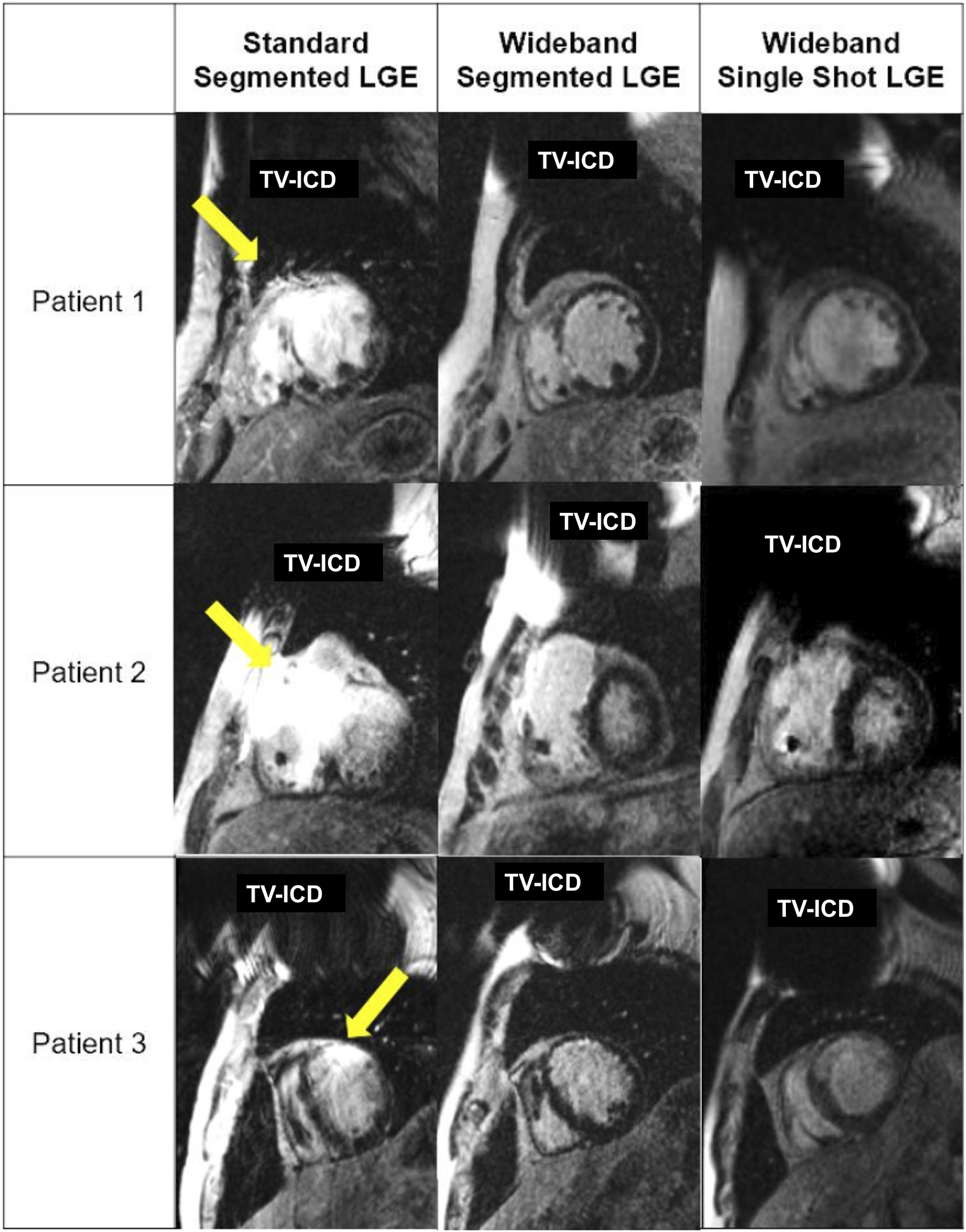
Representative images of three different patients with TV-ICDs using three LGE pulse sequences. Yellow arrows point to image artifacts caused by the device. Both wideband LGE pulse sequences consistently produced better image quality than standard LGE sequence. For patient 1 average rater conspicuity and artifact scores were 3.3 and 3.2 for standard segmented LGE, 4.7 and 4.8 for wideband segmented LGE, and 4.4 and 4.4 for wideband single-shot LGE. For patient 2 average rater conspicuity and artifact scores were 1.7 and 1.7 for standard segmented LGE, 4.8 and 4.8 for wideband segmented LGE, and 3.9 and 4.4 for wideband single-shot LGE. For patient 3 average rater conspicuity and artifact scores were 2.9 and 3.0 for standard segmented LGE, 4.9 and 4.8 for wideband segmented LGE, and 4.5 and 4.5 for wideband single-shot LGE. For patient 3, the TI for wideband single-shot LGE was sub-optimally chosen by a clinical technologist. TI: inversion time.
According to weighted Cohen’s kappa coefficient, inter-rater reliability was moderate for both conspicuity (κ=0.589; 95% CI, 0.561 to 0.617) and artifact level (κ=0.513; 95% CI, 0.491 to 0.535) scores for wideband single-shot LGE; moderate for both conspicuity (κ=0.593; 95% CI, 0.574 to 0.612) and artifact level (κ=0.505; 95% CI, 0.485 to 0.525) scores for wideband segmented LGE; moderate for both conspicuity (κ=0.567; 95% CI, 0.543 to 0.591) and artifact level (κ=0.491; 95% CI, 0.470 to 0.512) scores for standard single-shot LGE.
The remaining visual analysis results represent average reader scores. According to the Kruskal-Wallis test, conspicuity was significantly improved with wideband segmented LGE (median: 4.5, IQR [4, 4.8], χ2 = 27.774, p<0.001) and wideband single-shot LGE (median: 4.4, IQR [4,5], χ2 = 16.311, p<0.001) compared to standard segmented LGE (median: 3.3, IQR [2.8, 4.1]), but not significantly different between wideband segmented LGE and wideband single-shot LGE. Artifact level was significantly improved with wideband segmented LGE (median: 4.5, IQR [4.2, 4.8], χ2 = 609, p<0.001) and wideband single-shot LGE (median: 4.5, IQR [4.2, 5], χ2 = 597, p<0.001) when compared to standard segmented LGE (median: 3.3, IQR [2.8, 4.3]), but not significantly different between the two wideband LGEs. SVS was significantly improved with wideband segmented LGE (median: 9, IQR [8.2, 9.6], χ2 = 20.516, p<0.001) and wideband single-shot LGE (median: 8.9, IQR [8.1, 10], χ2 = 17.556, p<0.001) when compared to standard segmented LGE (median: 6.6, IQR [5.5, 8.5]), but not significantly different between the two wideband LGEs. There was a greater proportion of non-diagnostic scores (conspicuity < 3; artifact < 3, SVS < 6) for standard segmented LGE than the two wideband LGEs (Figure 3). When comparing visual scores across device types (pacemakers [n=15] vs. TV-ICDs [n=33]), as shown in Table 3, conspicuity, artifact level, and SVS scores were significantly better for pacemakers than TV-ICDs in standard LGE (p<0.001), whereas they were not significantly different for the two wideband LGEs.
Figure 3:
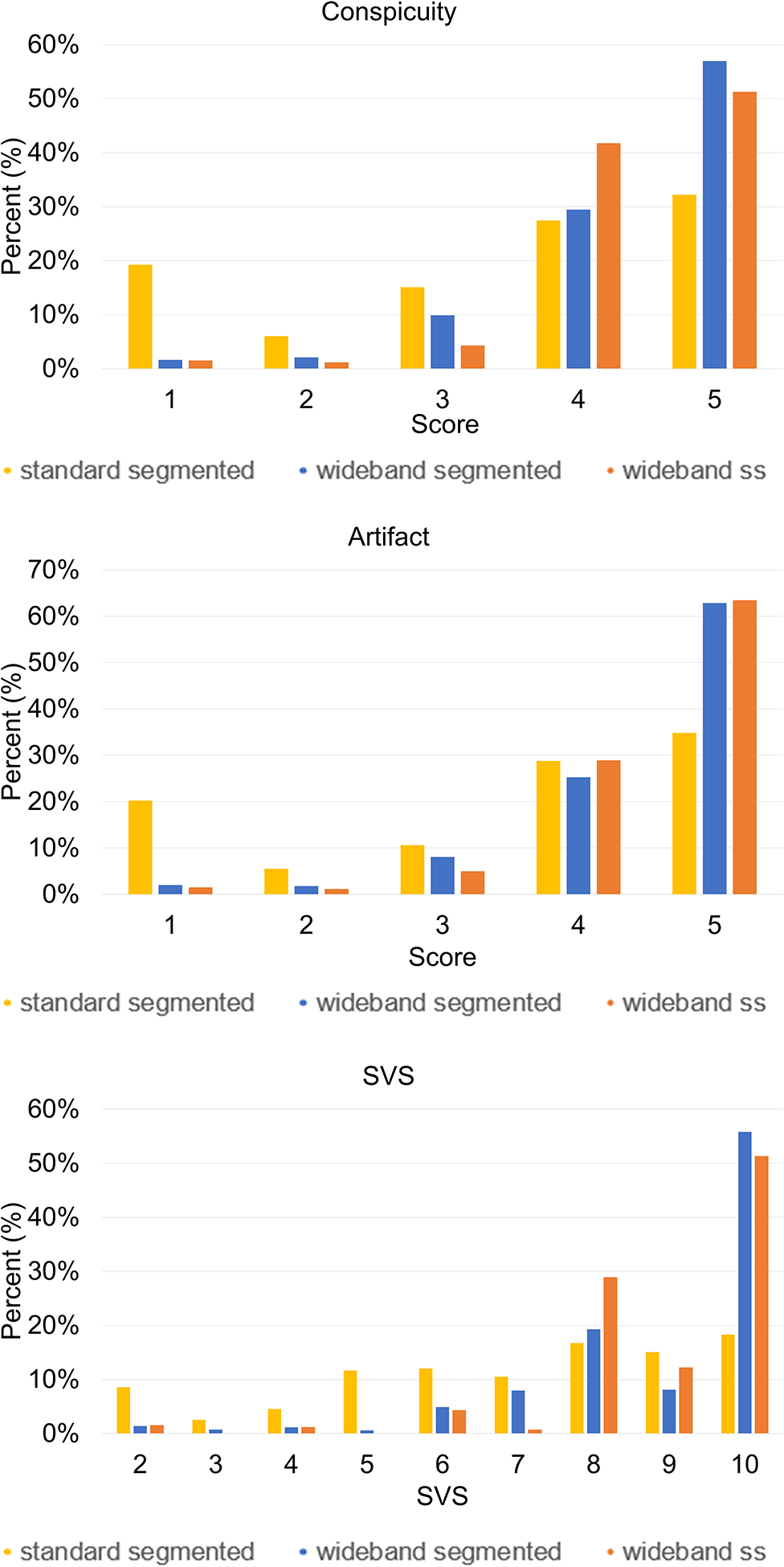
Distribution of conspicuity, artifact and SVS for three LGE pulse sequences. Standard segmented LGE produced considerably more non-diagnostic images than both wideband LGEs. SVS: summed visual score.
Table 3:
Comparison of visual scores between TV-ICDs and pacemakers across three pulse sequences. SVS: summed visual score.
| Pacemaker | TV-ICD | p-value | ||
|---|---|---|---|---|
| Standard Segmented | Conspicuity | 4.5 [4.2, 4.6] | 3.1 [2.5, 3.6] | <0.001 |
| Artifact | 4.4 [4.3, 4.6] | 3.1 [2.7, 3.5] | <0.001 | |
| SVS | 8.7 [8.6, 9.2] | 6.2 [5.2, 7.1] | <0.001 | |
| Wideband Segmented | Conspicuity | 4.4 [4.0, 4.8] | 4.6 [4.2, 4.8] | 0.977 |
| Artifact | 4.5 [4.2, 4.8] | 4.6 [4.2, 4.8] | 0.960 | |
| SVS | 8.9 [8.1, 9.5] | 9.3 [8.4, 9.6] | 0.993 | |
| Wideband Single-Shot | Conspicuity | 4.5 [4.0, 5.0] | 4.4 [3.9, 5.0] | 0.424 |
| Artifact | 4.6 [4.2, 5.0] | 4.4 [4.2, 5.0] | 0.622 | |
| SVS | 9.0 [8.3, 10.0] | 8.8 [8.1, 10.0] | 0.505 |
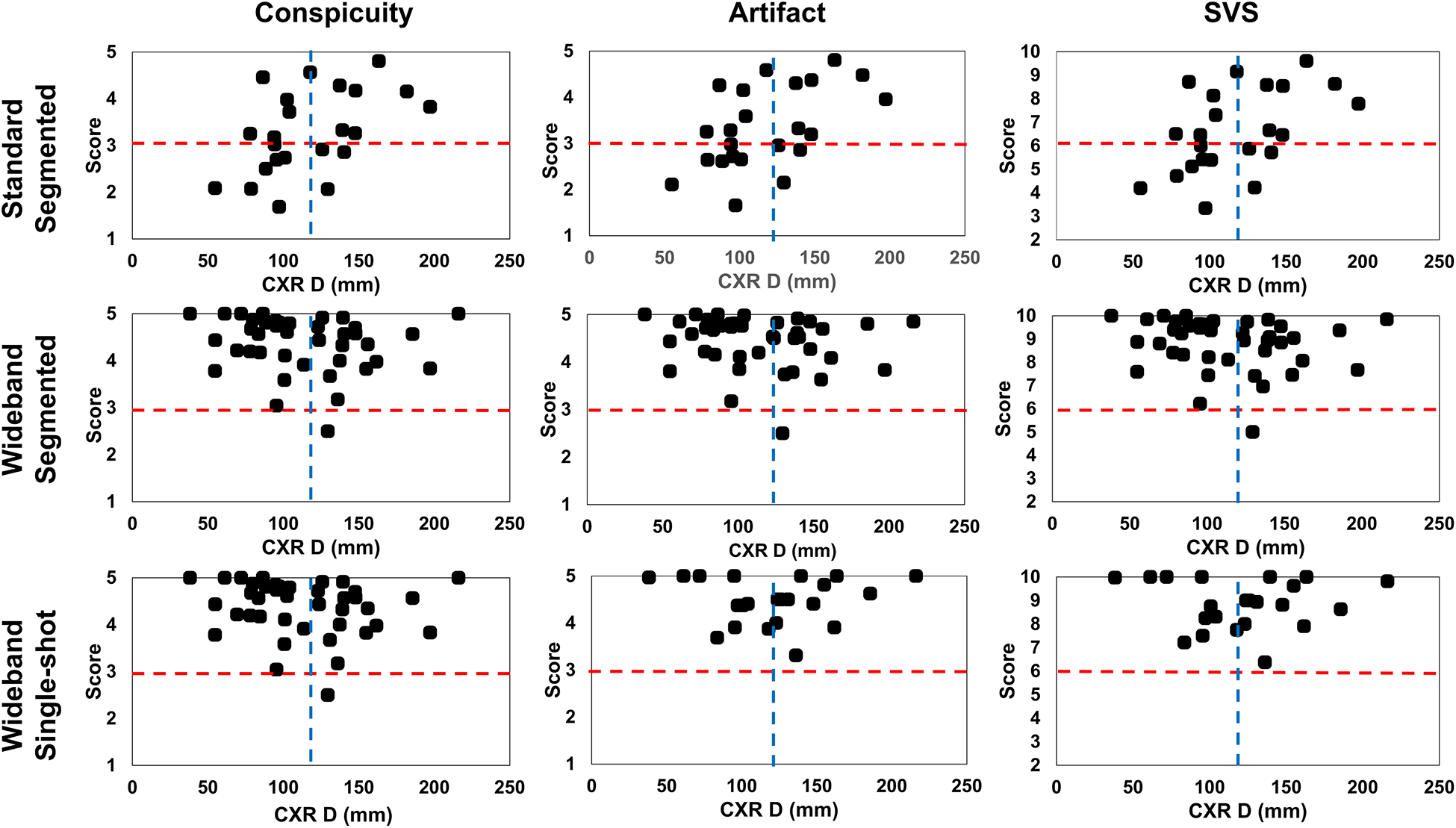
Mean CXR D was 115 ± 40 mm. According to the Spearman’s correlation analysis on average score and CXR distance, conspicuity (rho = 0.476, p = 0.022), artifact level (rho = 0.520, p = 0.011), and SVS (rho = 0.490, p = 0.018) were associated with CXR distance for standard segmented LGE. In contrast, wideband segmented (conspicuity: rho = −0.231, p = 0.151; artifact: rho = −0.205, p = 0.206; SVS: rho = −0.227, p =0.158) and wideband single-shot (conspicuity: rho = −0.024, p = 0.916; artifact: rho = 0.063, p = 0.779; SVS: rho = −0.030, p = 0.896) LGEs were not associated with CXR distance (Figure 4). The percentage of patients with diagnostically acceptable images was significantly improved with devices more than 100 mm away from the heart (96% vs 74%). The greatest variance in percentage of diagnostically acceptable images came from standard segmented LGE which improved from 50% with devices closer than 100 mm to 78% with devices farther than 100 mm.
Figure 4:
Scatter plots of average rater conspicuity (left column), artifact (middle column), and SVS (right column) versus CXR D. There were statistically significant trends for standard LGE (p <0.05), but none for both wideband LGEs. Red horizontal dashed lines indicate diagnostically acceptable cut-points: 3 for conspicuity and artifact; 6 for SVS. Blue vertical dashed lines indicate mean CXR D (115 mm): distance between centroids of left ventricle and device measured in chest X-ray; SVS: summed visual score.
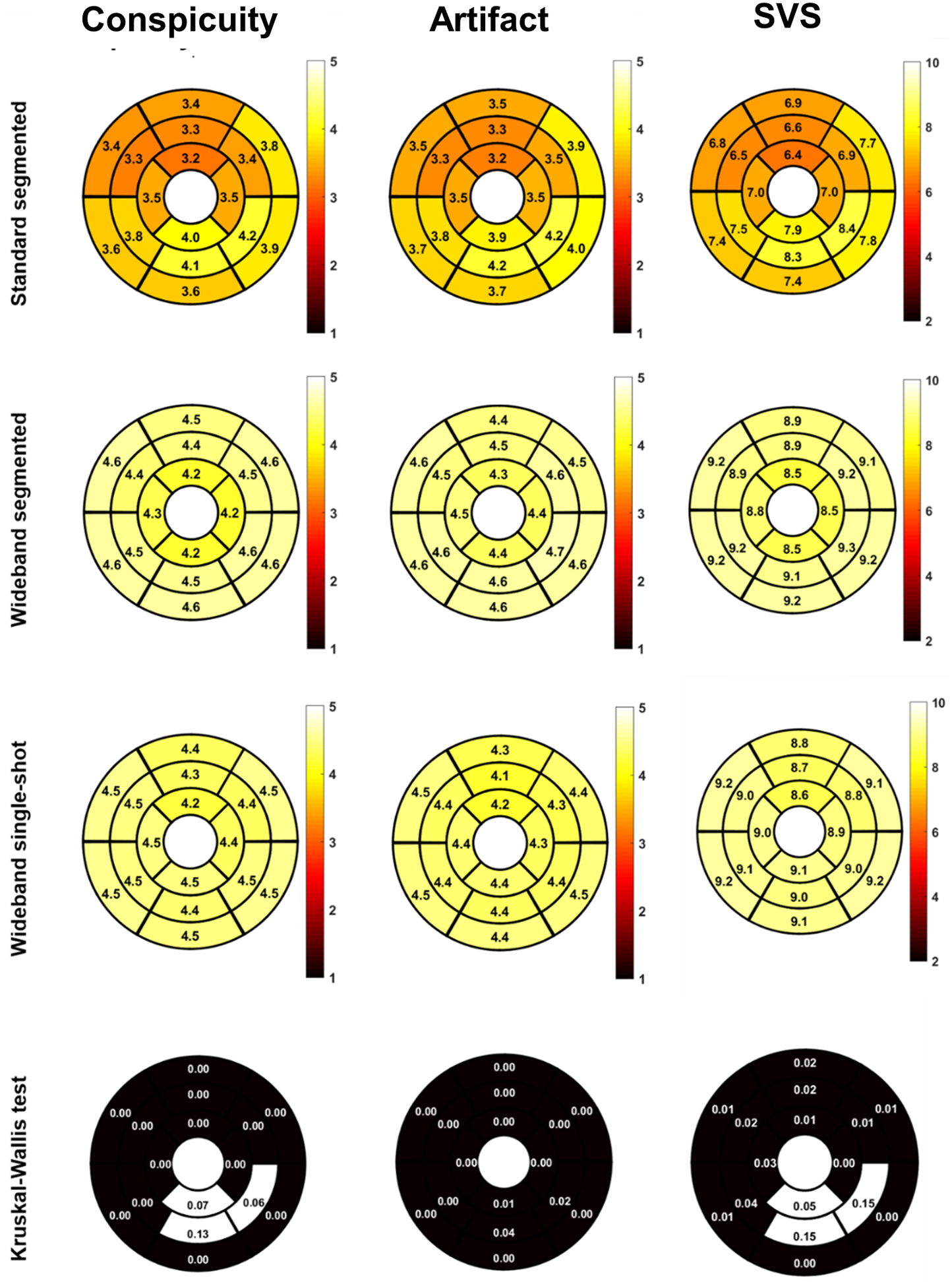
According to the Kruskal-Wallis test on segmental scores, conspicuity was significantly different between the three LGE scans for all but three segments (p<0.05), artifact level was significantly different between the three LGE scans for all segments (p<0.05), and SVS was significantly different between the three LGE scans for all but three segments (p<0.05)(Figure 5). There were greater regional variations in conspicuity, artifact level, and SVS scores for standard segmented LGE than the two wideband LGEs. Among evaluated myocardial segments, 365 out of 505 (72%) segments were deemed diagnostically interpretable (i.e. SVS ≥ 6) for standard segmented LGE, 834 out of 933 (89%) segments were deemed diagnostically interpretable for wideband segmented LGE, and 405 out of 432 (94%) segments were deemed diagnostically interpretable for wideband single-shot LGE.
Figure 5:
Segmental conspicuity, artifact, and SVS in 16 myocardial segments across pulse sequences using a Kruskal-Wallis test. As shown, segmental scores varied more for standard LGE than wideband segmented LGE and wideband single-shot LGE. These plots exclude data from four patients with S-ICD, which is implanted in a different location and transvenous devices. SVS: summed visual score; S: subcutaneous.
As shown in Figure 6, CV in myocardial signal was significantly (p<0.001) higher for standard segmented LGE (median: 0.47, IQR [0.38, 0.63]) than wideband segmented LGE (median: 0.34, IQR [0.30, 0.42]) and wideband single-shot LGE (median: 0.31, IQR [0.27, 0.37]). The blur metric was significantly (p < 0.001) higher for standard segmented LGE (median: 0.27, IQR [0.23, 0.28]) than wideband segmented LGE (median: 0.23, IQR [0.21, 0.29]) and wideband single-shot LGE (median: 0.26, IQR [0.24, 0.28]).
Figure 6:
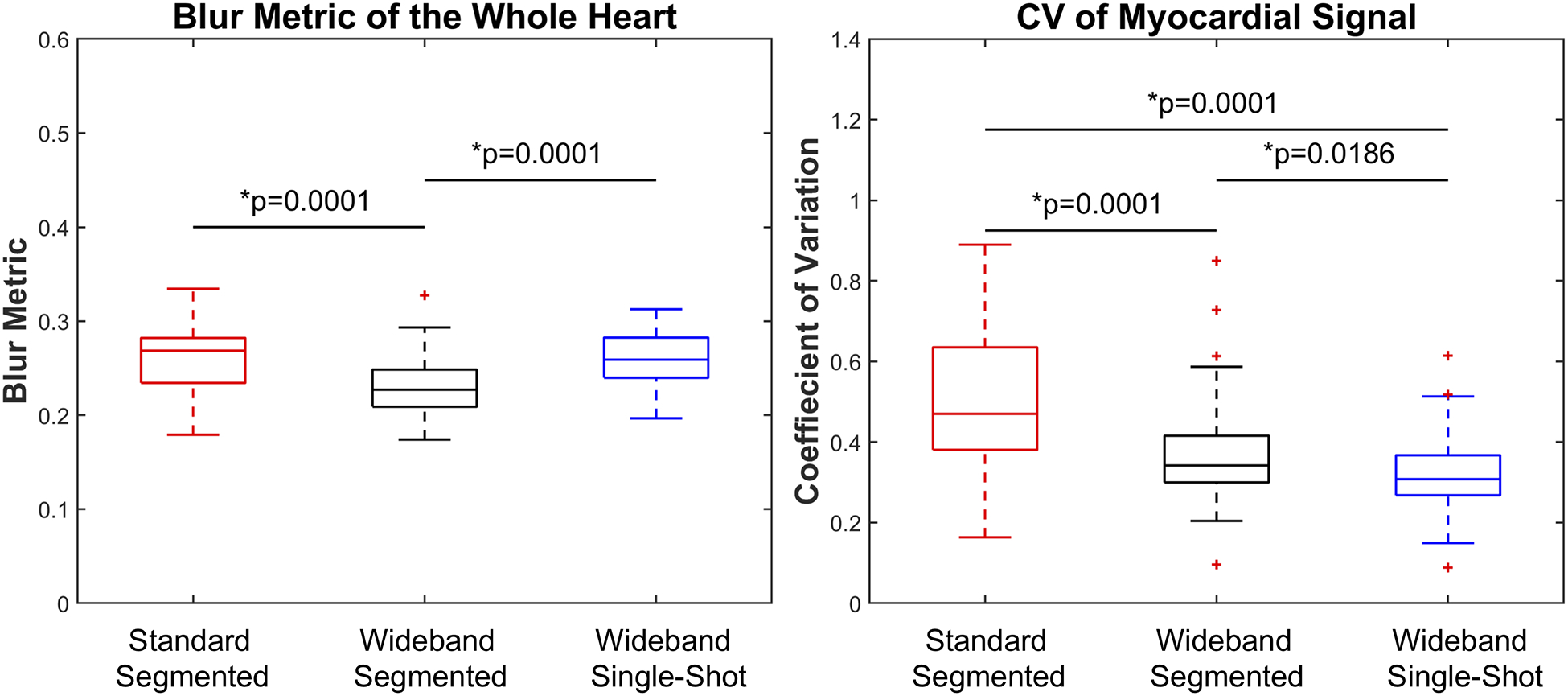
Box plots of blur metric as a measure of spatial resolution (left) and CV in myocardial signal as a measure of hyper-intense image artifact (right). There was a statistically significant difference between all groups (p < 0.001).
None of the patients experienced an adverse event during CMR. Average whole-body SAR was significantly (p < 0.001) lower for standard segmented LGE (0.058 ± 0.021 W/kg) than wideband segmented LGE (0.156 ± 0.040 W/kg) and wideband single-shot LGE (0.153 ± 0.040 W/kg). However, all values were at least one order of magnitude lower than the 2.0 W/kg Food and Drug Administration limit.28 When comparing device parameters before and after MRI, there was no significant difference in any of the device parameters (sensing [right atrium, right ventricle], impedance [right atrium, right ventricle, left ventricle], threshold [right atrium, right ventricle, left ventricle]) (Table 4). Battery level was not significantly (average change <1%) changed before and after MRI.
Table 4:
Mean and standard deviation of sensing, impedance, and threshold before and after MRI, as well as relative difference and p-values.
| Before MRI | After MRI | Relative Difference (%) | p-value | |
|---|---|---|---|---|
| RA | 3.29 ± 2.12 | 3.20 ± 1.92 | −2.6 ± 24.5 | 0.054 |
| RV | 11.83 ± 4.70 | 11.72 ± 5.42 | −0.9 ± 20.2 | 0.649 |
| RA | 543 ± 166 | 505 ± 137 | −7.1 ± 14 | 0.167 |
| RV | 514 ± 138 | 532 ± 158 | 3.4 ± 15.8 | 0.494 |
| LV | 670 ± 197 | 593 ± 181 | −11.5 ± 7.9 | 0.730 |
| RA | 0.75 ± 0.37 | 0.76 ± 0.34 | 0.7 ± 35.1 | 0.739 |
| RV | 0.81 ± 0.29 | 0.90 ± 0.28 | 10.8 ± 30.6 | 0.306 |
DISCUSSION
This retrospective study demonstrates that our wideband single-shot LGE and wideband segmented LGE similarly produce improved image quality compared with standard segmented LGE. For standard LGE, visual scores were associated with CXR distance and differed between device types, whereas the corresponding scores were consistently high for both wideband LGEs and not associated with CXR distance. Both quantitative metrics (CV, blur metric) of image quality supported visual scores.
While prior studies have evaluated the image quality and safety of various wideband LGE techniques, this study uniquely included both wideband segmented and wideband single-shot LGE sequences, evaluated image quality with respect to device type and implantation location, and included battery level changes. Prior studies reported improved image quality with wideband segmented LGE.13,15,16 Do et al.16 studied over 100 CIED patients and showed that wideband segmented LGE produces up to 87% diagnostically interpretable images. Similarly, Singh et al.13 studied 49 ICD patients and reported that wideband segmented LGE produces better image quality than standard segmented LGE. Stevens et al.15 studied wideband LGE in 18 ICD patients and found that the 15 (83%) patients with clinically acceptable wideband LGE correlated with electro-anatomical mapping. Our study differs from these prior studies because it including wideband single-shot LGE. This is important since many CIED patients have a high burden of arrhythmias and/or dyspnea, and segmented acquisitions would produce ghosting artifacts due to arrhythmias and/or dyspnea. Our study also evaluates the influence of the device type and location on image quality. Image artifacts were relatively smaller for pacemakers than ICDs for standard LGE. This finding is consistent with prior studies using standard (non-wideband) CMR.32–34 Our study further evaluated the association (or lack thereof for wideband) of image quality and CXR distance. Our study additionally includes quantitative metrics of image quality, which was not included in these prior studies. Several prior wideband LGE CMR studies reported no adverse events and non-significant changes in device parameters before and after MRI.13,16 In addition to standard device parameters, our study also included battery level before and after MRI. This is an important information to examine since battery replacement procedure is invasive, costly, and associated with risk.
This study has several elements that warrant further discussion. First, wideband single-shot LGE produced clinically acceptable image quality in 94% of myocardial segments, thereby drastically reducing the failure rate. Second, for CIED patients with a high burden of arrhythmia and/or dyspnea, wideband single-shot LGE with moderate spatial resolution might serve as a robust alternative to wideband segmented LGE with high spatial resolution, because the latter is sensitive to arrhythmia and breath-holding difficulties. Third, our results show that standard LGE is sensitive to device type and proximity, which is consistent with findings reported by a prior study.32 In contrast, wideband single-shot LGE consistent produced high image quality, regardless of device type and proximity. Fourth, our study included only four patients with S-ICDs. The degree of image quality improvement with wideband LGEs compared with standard LGE might be even greater for patients with S-ICDs, which are approximately twice as large as TV-ICDs and implanted closer to the heart.35,36 Fifth, because wideband LGE sequences produce approximately 90% of diagnostically interpretable myocardial segments, they provide a means to evaluate scar distribution in homogenous cohorts of CIED patients.
The study had several limitations. First, as a retrospective study, the pulse sequence order was not randomized, and not all patients underwent all three pulse sequences. An unknown number of technologists used their own discretion to apply a mixture of three LGE pulse sequences and adjust pulse sequence parameters and slice coverage. These inconsistencies and biases may have contributed to differences in image quality. Second, the TI was not set properly in some of the patients, which led to non-zero myocardial signal for 11 (3% of total) images. To account for this, the raters were instructed to account for TI failures during interpretation. Third, CXR was measured using an in-plane Euclidean distance which does not account for the true distance between the centroids of the generator and the left ventricle (i.e. missing through-plane distance). Fourth, arrhythmia burden during CMR was not recorded. As such, it was not possible to correlate visual scores with arrhythmia burden. Fifth, we did not quantify scar volume, because that is beyond the scope of this initial quality control study. Sixth, we did not investigate whether wideband single-shot LGE with lower spatial resolution would be able to resolve small infarcts as well as wideband segmented LGE with higher spatial resolution.
In conclusion, this retrospective study shows that wideband single-shot LGE produces clinically acceptable image quality in 94% of myocardial segments in CIED patients and that it may be an alternative for CIED patients with a high burden of arrhythmia and/or dyspnea.
Supplementary Material
Supplementary Table S1: Relevant pulse sequence parameters for three LGE pulse sequences.
Acknowledgements
The authors thank funding support from the National Institutes of Health (R01HL116895, R01HL138578, R21EB024315, R21AG055954, R01HL151079) and American Heart Association (19IPLOI34760317).
Footnotes
None of the authors have relationships with industry related to this study
References
- 1.Greenspon AJ, Patel JD, Lau E, et al. Trends in permanent pacemaker implantation in the United States from 1993 to 2009: increasing complexity of patients and procedures. J Am Coll Cardiol. 2012;60(16):1540–1545. [DOI] [PubMed] [Google Scholar]
- 2.Mond HG, Proclemer A. The 11th world survey of cardiac pacing and implantable cardioverter-defibrillators: calendar year 2009--a World Society of Arrhythmia’s project. Pacing Clin Electrophysiol. 2011;34(8):1013–1027. [DOI] [PubMed] [Google Scholar]
- 3.Lim WY, Prabhu S, Schilling RJ. Implantable Cardiac Electronic Devices in the Elderly Population. Arrhythm Electrophysiol Rev. 2019;8(2):143–146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Andreu D, Penela D, Acosta J, et al. Cardiac magnetic resonance-aided scar dechanneling: Influence on acute and long-term outcomes. Heart Rhythm. 2017;14(8):1121–1128. [DOI] [PubMed] [Google Scholar]
- 5.Berruezo A, Fernandez-Armenta J, Andreu D, et al. Scar dechanneling: new method for scar-related left ventricular tachycardia substrate ablation. Circ Arrhythm Electrophysiol. 2015;8(2):326–336. [DOI] [PubMed] [Google Scholar]
- 6.Piers SR, Tao Q, de Riva Silva M, et al. CMR-based identification of critical isthmus sites of ischemic and nonischemic ventricular tachycardia. JACC Cardiovasc Imaging. 2014;7(8):774–784. [DOI] [PubMed] [Google Scholar]
- 7.Nazarian S, Hansford R, Roguin A, et al. A prospective evaluation of a protocol for magnetic resonance imaging of patients with implanted cardiac devices. Annals of internal medicine. 2011;155(7):415–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nazarian S, Roguin A, Zviman MM, et al. Clinical utility and safety of a protocol for noncardiac and cardiac magnetic resonance imaging of patients with permanent pacemakers and implantable-cardioverter defibrillators at 1.5 tesla. Circulation. 2006;114(12):1277–1284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sommer T, Naehle CP, Yang A, et al. Strategy for safe performance of extrathoracic magnetic resonance imaging at 1.5 tesla in the presence of cardiac pacemakers in non-pacemaker-dependent patients: a prospective study with 115 examinations. Circulation. 2006;114(12):1285–1292. [DOI] [PubMed] [Google Scholar]
- 10.Russo RJ, Costa HS, Silva PD, et al. Assessing the Risks Associated with MRI in Patients with a Pacemaker or Defibrillator. N Engl J Med. 2017;376(8):755–764. [DOI] [PubMed] [Google Scholar]
- 11.Muthalaly RG, et al. MRI in Patients with Cardiac Implantable Electronic Devices. Radiology. 2018;289(2). [DOI] [PubMed] [Google Scholar]
- 12.Dandamudi S, Collins JD, Carr JC, et al. The Safety of Cardiac and Thoracic Magnetic Resonance Imaging in Patients with Cardiac Implantable Electronic Devices. Acad Radiol. 2016;23(12):1498–1505. [DOI] [PubMed] [Google Scholar]
- 13.Singh A, Kawaji K, Goyal N, et al. Feasibility of Cardiac Magnetic Resonance Wideband Protocol in Patients With Implantable Cardioverter Defibrillators and Its Utility for Defining Scar. Am J Cardiol. 2019;123(8):1329–1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rashid S, Rapacchi S, Vaseghi M, et al. Improved late gadolinium enhancement MR imaging for patients with implanted cardiac devices. Radiology. 2014;270(1):269–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stevens SM, Tung R, Rashid S, et al. Device artifact reduction for magnetic resonance imaging of patients with implantable cardioverter-defibrillators and ventricular tachycardia: late gadolinium enhancement correlation with electroanatomic mapping. Heart Rhythm. 2014;11(2):289–298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Do DH, Eyvazian V, Bayoneta AJ, et al. Cardiac magnetic resonance imaging using wideband sequences in patients with nonconditional cardiac implanted electronic devices. Heart Rhythm. 2018;15(2):218–225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hilbert S, Weber A, Nehrke K, et al. Artefact-free late gadolinium enhancement imaging in patients with implanted cardiac devices using a modified broadband sequence: current strategies and results from a real-world patient cohort. Europace : European pacing, arrhythmias, and cardiac electrophysiology : journal of the working groups on cardiac pacing, arrhythmias, and cardiac cellular electrophysiology of the European Society of Cardiology. 2018;20(5):801–807. [DOI] [PubMed] [Google Scholar]
- 18.Shao J, Rashid S, Renella P, Nguyen KL, Hu P. Myocardial T1 mapping for patients with implanted cardiac devices using wideband inversion recovery spoiled gradient echo readout. Magnetic resonance in medicine : official journal of the Society of Magnetic Resonance in Medicine / Society of Magnetic Resonance in Medicine. 2017;77(4):1495–1504. [DOI] [PubMed] [Google Scholar]
- 19.Hong K, Jeong EK, Wall TS, Drakos SG, Kim D. Wideband arrhythmia-Insensitive-rapid (AIR) pulse sequence for cardiac T1 mapping without image artifacts induced by an implantable-cardioverter-defibrillator. Magn Reson Med. 2015;74(2):336–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hong K, Collins JD, Knight BP, Carr JC, Lee DC, Kim D. Wideband myocardial perfusion pulse sequence for imaging patients with a cardiac implantable electronic device. Magn Reson Med. 2019;81(2):1219–1228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hong K, Collins JD, Freed BH, et al. Accelerated Wideband Myocardial Perfusion Pulse Sequence with Compressed Sensing Reconstruction for Myocardial Blood Flow Quantification in Patients with a Cardiac Implantable Electronic Device. Radiol Cardiothorac Imaging. 2020;2(2):e190114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ranjan R, McGann CJ, Jeong EK, et al. Wideband late gadolinium enhanced magnetic resonance imaging for imaging myocardial scar without image artefacts induced by implantable cardioverter-defibrillator: a feasibility study at 3 T. Europace. 2015;17(3):483–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rahsepar AA, Collins JD, Knight BP, Hong K, Carr JC, Kim D. Wideband LGE MRI permits unobstructed viewing of myocardial scarring in a patient with an MR-conditional subcutaneous implantable cardioverter-defibrillator. Clinical imaging. 2018;50:294–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Viallon M, Jacquier A, Rotaru C, et al. Head-to-head comparison of eight late gadolinium-enhanced cardiac MR (LGE CMR) sequences at 1.5 tesla: from bench to bedside. Journal of magnetic resonance imaging : JMRI. 2011;34(6):1374–1387. [DOI] [PubMed] [Google Scholar]
- 25.Muehlberg F, Arnhold K, Fritschi S, et al. Comparison of fast multi-slice and standard segmented techniques for detection of late gadolinium enhancement in ischemic and non-ischemic cardiomyopathy - a prospective clinical cardiovascular magnetic resonance trial. Journal of cardiovascular magnetic resonance : official journal of the Society for Cardiovascular Magnetic Resonance. 2018;20(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)—A metadata-driven methodology and workflow process for providing translational research informatics support. Journal of Biomedical Informatics. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Harris PA, Taylor R, Minor BL, et al. The REDCap consortium: Building an international community of software platform partners. Journal of Biomedical Informatics. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Indik JH, Gimbel JR, Abe H, et al. 2017 HRS expert consensus statement on magnetic resonance imaging and radiation exposure in patients with cardiovascular implantable electronic devices. Heart Rhythm. 2017;14(7):e97–e153. [DOI] [PubMed] [Google Scholar]
- 29.Cerqueira MD, Weissman NJ, Dilsizian V, et al. Standardized myocardial segmentation and nomenclature for tomographic imaging of the heart. A statement for healthcare professionals from the Cardiac Imaging Committee of the Council on Clinical Cardiology of the American Heart Association. Circulation. 2002;105(4):539–542. [DOI] [PubMed] [Google Scholar]
- 30.Crete F, Dolmiere T, Ladret P, Nicolas M. The blur effect: perception and estimation with a new no-reference perceptual blur metric. Vol 6492: SPIE; 2007.
- 31.Cohen J Weighted kappa: nominal scale agreement with provision for scaled disagreement or partial credit. Psycol Bull. 1968;70(4):213–220. [DOI] [PubMed] [Google Scholar]
- 32.Hilbert S, Jahnke C, Loebe S, et al. Cardiovascular magnetic resonance imaging in patients with cardiac implantable electronic devices: a device-dependent imaging strategy for improved image quality. Eur Heart J Cardiovasc Imaging. 2018;19(9):1051–1061. [DOI] [PubMed] [Google Scholar]
- 33.Sasaki T, Hansford R, Zviman MM, et al. Quantitative assessment of artifacts on cardiac magnetic resonance imaging of patients with pacemakers and implantable cardioverter-defibrillators. Circ Cardiovasc Imaging. 2011;4(6):662–670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesubi O, Ahmad G, Jeudy J, et al. Impact of ICD artifact burden on late gadolinium enhancement cardiac MR imaging in patients undergoing ventricular tachycardia ablation. Pacing Clin Electrophysiol. 2014;37(10):1274–1283. [DOI] [PubMed] [Google Scholar]
- 35.Westerman SB, El-Chami M. The subcutaneous implantable cardioverter defibrillator--review of the recent data. J Geriatr Cardiol. 2018;15(3):222–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Holtstiege V, Meier C, Bietenbeck M, et al. Clinical experience regarding safety and diagnostic value of cardiovascular magnetic resonance in patients with a subcutaneous implanted cardioverter/defibrillator (S-ICD) at 1.5 T. J Cardiovasc Magn Reson. 2020;22(1):35. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Relevant pulse sequence parameters for three LGE pulse sequences.


