Abstract
Reduced nigrostriatal uptake on 123I-FP-CIT SPECT reflects dopamine dysfunction, while other imaging markers could be complementary when used together. We assessed how well 123I-FP-CIT SPECT differentiates Dementia with Lewy Bodies (DLB) from Alzheimer’s Disease Dementia (ADem) and whether multimodal imaging provides additional value. 123I-FP-CIT SPECT, MRI, FDG-PET, and PiB-PET were assessed in 35 DLB and 14 ADem participants (autopsy confirmation in 9 DLB and 4 ADem). Nigrostriatal dopamine transporter uptake was evaluated with 123I-FP-CIT SPECT using DaTQUANT software. Hippocampal volume was calculated with MRI, cingulate island sign (CIS) ratio with FDG-PET, and global cortical PiB retention with PiB-PET. The DaTQUANT z-scores of putamen showed the highest c-statistic of 0.916 in differentiating DLB from ADem among the analyzed imaging biomarkers. Adding another imaging modality to 123I-FP-CIT SPECT had c-statistics ranging from 0.968 to 0.975, and 123I-FP-CIT SPECT in combination with two other imaging modalities presented c-statistics ranging from 0.987 to 0.996. These findings suggest that multimodal imaging with 123I-FP-CIT SPECT aids in differentiating DLB and ADem, and in detecting comorbid Lewy-related and AD pathology in DLB and ADem patients.
Keywords: Dementia with Lewy bodies, 123I-FP-CIT SPECT, DaTQUANT, PiB-PET, FDG-PET, MRI
Graphical Abstract
1. Introduction
Dementia with Lewy Bodies (DLB) is the second most common cause of neurodegenerative dementia after Alzheimer’s Disease Dementia (ADem) and is characterized by several distinctive features including REM sleep behavior disorder (RBD), parkinsonism, visual hallucinations, and fluctuating cognition (McKeith et al., 2017). The key neuropathologic hallmark of DLB is deposition of neuronal intracellular deposits called Lewy bodies comprised of aggregated α-synuclein protein. Besides the presence of Lewy-related pathology, over half of cases with DLB have co-existing is known to have Alzheimer’s Disease (AD) pathology (Ferman et al., 2020; Jellinger and Attems, 2008). Likewise, Lewy-related pathology has been observed in the context of ADem (Hamilton, 2000). DLB may go underrecognized or misdiagnosed as ADem (Vann Jones and O’Brien, 2014) and patients with co-existing AD pathology may differ in phenotypic expression or may show a delay in the expression of DLB (Ferman et al., 2020). The latest 4th consensus diagnostic criteria of DLB was created to improve detection rates of DLB by incorporating recent data including multiple DLB imaging biomarkers (McKeith et al., 2017).
Reduction of dopamine transporter (DAT) uptake in basal ganglia and loss of nigrostriatal dopaminergic neurons in DLB patients were observed in postmortem series (Piggott et al., 1999). N-(3-Fluoropropyl)-2β-carbomethoxy-3β-(4-[123I]iodophenyl) nortropane (123I-FP-CIT) SPECT has been used to assess striatal DAT concentration and has demonstrated good diagnostic accuracy either by clinical (Colloby et al., 2004; McKeith et al., 2007; Walker et al., 2002) or autopsy diagnosis (Colloby et al., 2012; Maltais et al., 2020; Thomas et al., 2017; Walker et al., 2007). In the latest DLB diagnostic criteria, reduced DAT uptake in basal ganglia by SPECT or PET is regarded as one of the three “indicative biomarkers” of probable DLB (McKeith et al., 2017).
Current clinical assessment of 123I-FP-CIT SPECT often relies on qualitative visual interpretations of the images and semi-quantification analysis of binding ratios from manually selected regions of interest (ROIs) (Djang et al., 2012). Qualitative visual interpretations and selection of ROIs are susceptible to inter-rater and intra-rater variability (Augimeri et al., 2016). DaTQUANT (GE Healthcare) is one of several automated semi-quantification software programs for 123I-FP-CIT SPECT (Brogley, 2019). DaTQUANT eliminates the need for manual ROI selection and potentially diminishes inter-rater and intra-rater variability by applying predefined volume of interest (VOI). There are only a few DaTQUANT studies in DLB patients. DaTQUANT was shown to be useful in differentiating DLB from ADem clinically (Shimizu et al., 2017), and we recently reported that DaTQUANT provided excellent discriminatory power between patients with underlying Lewy Body Disease (LBD) and non-LBD pathology and was comparable to other semi-quantitative methods (Maltais et al., 2020). There was no clear preference between DaTQUANT z-scores for putamen and caudate in detecting presence of LBD pathology, and unilateral putamen or caudate lower z-scores had marginally better discrimination than average putamen or caudate z-scores. The proposed cutoff value for the putamen z-score in detecting the presence of LBD pathology was −0.82 (Maltais et al., 2020).
Amyloid PET such as 11C-Pittsburgh Compound B (PiB) PET, [18F]2-fluoro-deoxy-D-glucose (FDG) PET, and MRI each assess a different aspect of DLB and/or ADem. Hippocampal volume is relatively preserved in DLB compared to ADem (Barber et al., 2000; Burton et al., 2002; Kantarci et al., 2012b). FDG-PET in DLB shows decreased metabolism in the occipital lobe (Albin et al., 1996; Higuchi et al., 2000; Ishii et al., 1998; Minoshima et al., 2001; Mosconi et al., 2008). Along with the reduced occipital FDG activity, sparing of the posterior cingulate relative to the precuneus and cuneus, termed as cingulate island sign (CIS), has been reported as a characteristic FDG-PET imaging feature of DLB (Graff-Radford et al., 2014; Lim et al., 2009). In our former study, CIS ratio had higher discriminatory power than occipital lobe FDG uptake as a single imaging biomarker in distinguishing DLB and ADem, although the two imaging biomarkers were comparable when multimodal imaging models were considered (Kantarci et al., 2012a). Accumulation of brain β-amyloid seen on amyloid PET is common in DLB but at lower levels than in ADem (Edison et al., 2008; Foster et al., 2010; Gomperts et al., 2008) and predominantly detects the diffuse amyloid-β plaques that are abundant in DLB patients rather than neuritic plaques specific for AD (Kantarci et al., 2020; Kantarci et al., 2012b).
The usefulness of combination of 123I-FP-CIT SPECT and FDG-PET findings in the clinical workup of dementia has been reported (Garibotto et al., 2013). We previously reported the value of multimodal imaging (MRI, PiB-PET, and FDG-PET) in differentiating DLB and ADem (Kantarci et al., 2012a).
Our objective in this study is to expand our former study to include 123I-FP-CIT SPECT findings, assessed semi-quantitatively by DaTQUANT software in a multimodal imaging analyses to determine if combining other imaging modalities would provide complementary value to 123I-FP-CIT SPECT imaging.
2. Material and Methods
2.1. Participants
Participants in this study were recruited and evaluated from the Mayo Clinic Alzheimer’s Disease Research Center (ADRC) who participated in the 123I-FP-CIT SPECT study between November 2011 and November 2019. The study was approved by Mayo Clinic Institutional Review Board, and informed consent was obtained from every participant and their surrogate. Inclusion criteria included: 1. clinical diagnosis of “probable DLB” (McKeith et al., 2017) or “probable ADem” (McKhann et al., 2011), 2. an 123I-FP-CIT SPECT scan after the diagnosis of dementia was made, and 3. other imaging modalities (MRI, FDG PET, or PiB PET) within six months of 123I-FP-CIT SPECT scan. Patients with Parkinson’s disease with dementia (PDD) and those with comorbid illnesses or treatments affecting cognitive function were excluded.
Each study participant underwent annual neurological and neuropsychometric evaluation according to the Mayo Clinic ADRC protocol. Reduced DAT uptake in basal ganglia with 123I-FP-CIT SPECT was not used in diagnosing probable DLB. The presence of fluctuations was determined by a score of 3 or 4 points on the Mayo Clinic Fluctuations questionnaire (Ferman et al., 2004). Visual hallucinations were regarded as present if they were experienced recurrently with well-formed and detailed images of people, animals or objects. The presence of RBD was determined by RBD criteria from 3rd edition of the International Classification of Sleep Disorders (ICSD-3). Cardinal clinical features of parkinsonism were evaluated with motor subsection of the Fahn 1987 version of the Unified Parkinson’s Disease Rating Scale (UPDRS part III) (Fahn S, 1987), and presence of parkinsonism was defined when bradykinesia was observed concurrently with at least one of the following neurological features; rest tremor, rigidity, or postural instability (Buck et al., 2011). The 2011 National Institute on Aging and the Alzheimer’s Association (NIA-AA) criteria were used for the diagnosis of probable ADem (McKhann et al., 2011). Participants were evaluated using the Mini Mental State Examination (Folstein et al., 1975) (MMSE), Global Deterioration Scale (Reisberg et al., 1982) (GDS), and CDR® Dementia Staging Instrument sum of boxes (CDR®-SB) (Hughes et al., 1982; Morris, 1993).
2.2. 123I-FP-CIT SPECT acquisition
A 100 mg of Lugol’s solution was administered for thyroid blocking at least an hour before the slow intravenous injection of 111 to 185 MBq (3 to 5 mCi) 123I-ioflupane. SPECT imaging was performed three to six hours post 123I-ioflupane injection. Images were obtained on a gamma camera set to a photopeak of 159 keV with a ± 10% energy window mounted with GE D670/D630 SPECT systems with low energy, high resolution collimators. Over 1.5 million counts and a pixel size between 3.5 mm and 4.5 mm were needed for optimal projection data. Data were reconstructed by the ordered subset expectation maximization (OSEM) method and the planer images were pre-filtered by a Butterworth filter (power = 10, cut off = 0.6 cycles/cm). No attenuation correction was used. The 123I-FP-CIT SPECT images were co-registered with MRI images with the VINCI software (Max Planck Institute).
2.3. Semi-quantitative analysis for 123I-FP-CIT SPECT
DaTQUANT (GE Healthcare) software was used for semi-quantification of striatal uptake. DaTQUANT uses predefined semiautomatic 123I-ioflupane VOI templates for measurements of striatum-to-background (SBR) of left and right entire striatum/caudate nucleus/entire putamen/anterior putamen/posterior putamen. The software calculates the SBR by dividing the mean counts per pixel in the corresponding VOI by the mean count per pixel in the reference background region (occipital lobe) VOI, then subtracting overall by 1. Z-scores are calculated by applying age-matched normal database from the Parkinson’s Progression Markers Initiative. We chose to use the lowest unilateral putamen z-score value for analyses according to our previous report (Maltais et al., 2020).
2.4. MRI acquisition and structural MRI analysis
A 3D high resolution T1-weighted sagittal magnetization-prepared rapid acquisition gradient echo MRI scan was acquired using 3T with approximately 1 mm cubic resolution for anatomical segmentation and labeling. Hippocampal volume analysis on T1-weighted images included a correction for B0 inhomogeneities and tissue-class segmentation performed using SPM12 (Ashburner and Friston, 2005) with an in-house population-based template. Total intracranial volume (TIV) measurements were calculated from these segmentations. Hippocampal volume values are reported as a percentage of TIV (HP/TIV) and were calculated by using the ANTs SyN algorithm (Avants et al., 2008) to propagate atlas hippocampal ROI labels from the in-house template onto each subject scan. These methods were previously described and validated (Schwarz et al., 2016).
2.5. Acquisition and analysis of 11C-PiB PET and 18F-FDG PET
PET scan images were acquired using GE PET/CT scanner operating in 3-dimensional mode. A 20 minutes PiB-PET scan was performed after 40 minutes from 11C-PiB injection. An 8 minutes (4 × 2 min dynamic frames) FDG-PET scan was performed 30 minutes after injection of 18F-FDG. Each PET scan was rigidly registered to the corresponding T1-weighted MRI using SPM12, and the above MRI-based segmentations were used to evaluate the median PET signal among the voxels in each atlas region. These median values were divided by the median cerebellar crus ROI value to form the standardized uptake value ratio (SUVR). The global cortical PiB retention ratio was calculated by averaging PiB SUVR values of the bilateral prefrontal, orbitofrontal, parietal, temporal, anterior cingulate, and posterior cingulate or precuneus grey matter regions (Jack et al., 2008; Lowe et al., 2009). The CIS ratio derived from the FDG-PET images was calculated by dividing the median value in the posterior cingulate gyrus ROI by median value in the precuneus plus cuneus ROI (Lim et al., 2009).
2.6. Neuropathologic evaluation
Thirteen participants underwent autopsy. Autopsy cases were evaluated by neuropathologists blinded to clinical and imaging findings. Sampling was performed according to the 3rd Report of the DLB Consortium (McKeith et al., 2005) and Consortium to Establish a Registry for AD protocol (Mirra et al., 1991). The pathological classification of LBD was made according to the DLB consensus criteria (McKeith et al., 2017; McKeith et al., 2005). “Diffuse-neocortical or transitional-limbic LBD” was categorized as LBD in this study. The pathological classification of AD was done according to NIA-AA criteria by satisfying “intermediate or high AD neuropathologic change” which was transformed from “ABC” score (Hyman et al., 2012; Montine et al., 1982) according to combination of Braak neurofibrillary tangle (NFT) stage (Braak et al., 2006; Braak and Braak, 1991), CERAD neuritic plaque score (Mirra et al., 1991), and Thal Aβ/amyloid plaque score (Thal et al., 2002). Pathological classification of “LBD +AD” was defined when the both “diffuse-neocortical or transitional-limbic LBD” and “intermediate or high AD neuropathologic change” were observed.
2.7. Statistical Analyses
Characteristics of the subjects were summarized using means and standard deviations, or counts and percentages. ADem and DLB groups were compared using Student t-tests for continuous variables and chi-squared tests for categorical variables. Due to skewness, the PiB-PET was analyzed with a log transformation. Logistic regressions with one, two, or three modalities as predictors of ADem vs. DLB were run and summarized using odds ratios (ORs) and associated standard errors, p-values, and C-statistics. Scatter plots and Pearson correlations were used for describing associations between continuous imaging biomarkers. P-values of <0.05 were considered as statistically significant for the analyses. Statistical analyses were performed in SAS (version 9.4; SAS Institute, Inc.) and R statistical software, version 3.4.2 (RFoundation).
3. Results
Demographic and clinical features of the study participants are shown in Table 1. There were 35 probable DLB participants and 14 probable ADem participants who each had 123I-FP-CIT SPECT, FDG-PET, PiB-PET, and MRI imaging. The DLB participants included more males, had lower frequency of possessing APOEε4 allele, presented more frequent core clinical features of DLB, and had higher scores in the UPDRS part III (p<0.01). The ADem participants had more severe cognitive and/or functional impairment than the DLB participants reflected by the lower MMSE score, higher Global Deterioration Scale, and higher CDR sum of boxes (p<0.05). From the FDG-PET, PiB-PET and structural MRI analyses, the DLB participants had higher FDG uptake ratio of posterior cingulate to the sum of the precuneus and cuneus, lower global cortical PiB retention ratio, and larger hippocampal volumes (p<0.01).
Table 1.
Characteristics table by diagnostic group
| ADem n = 14 | DLB n = 34 | P-value | |
|---|---|---|---|
| Age at 123I-FP-CIT SPECT , yrs | 64.0 (9.8) | 67.3 (9.7) | 0.30 |
| Male, no. (%) | 6 (43%) | 32 (94%) | <0.001 |
| APOE4, no. (%) | 10 (71.4%) | 8 (25.0%) | 0.003 |
| Education, yrs | 14.9 (2.6) | 15.5 (3.3) | 0.54 |
| RBD, no. (%) | 0 (0%) | 29 (85%) | <0.001 |
| Visual hallucinations, no. (%) | 0 (0%) | 19 (56%) | <0.001 |
| Parkinsonism, no. (%) | 1 (7%) | 31 (91%) | <0.001 |
| Fluctuations score | 1.8 (1.3) | 2.4 (1.2) | 0.18 |
| Duration of cognitive decline, yrs | 5.6 (3.1) | 5.9 (4.9) | 0.84 |
| UPDRS part III | 4.5 (3.7) | 19.0 (11.6) | 0.019 |
| Global Deterioration Scale | 4.8 (0.7) | 3.8 (1.2) | 0.006 |
| MMSE | 16.7 (5.8) | 23.6 (4.8) | <0.001 |
| CDR®-SB | 6.7 (3.4) | 4.6 (2.6) | 0.031 |
| DaTQUANT Putamen, z-score | 0.27 (1.93) | −3.30 (1.31) | <0.001 |
| PiB-PET: global SUVr | 2.40 (0.33) | 1.65 (0.47) | <0.001 |
| MRI: HP/TIV | 0.44 (0.05) | 0.48 (0.05) | 0.009 |
| FDG-PET: CIS ratio | 0.99 (0.10) | 1.13 (0.08) | <0.001 |
Abbreviations: RBD, REM sleep behavior disorder; UPDRS, Unified Parkinson’s Disease Rating Scale motor subtest; GDS, Global Deterioration Scale; MMSE, Mini Mental State Examination; CDR®-SB, CDR® Dementia Staging Instrument sum of boxes; SUVr, standardized uptake value ratio; HP/TIV, hippocampal volume/total intracranial volume percentage; CIS, cingulate island sign
The mean (SD) listed for the continuous variables and count (%) for the categorical variables.
P-values for differences between groups come from a t-test for the continuous variables or a chi-squared test for the categorical variables.
The putamen z-scores of the 123I-FP-CIT SPECT in DLB and AD participants are shown in Fig. 1. The putamen z-scores of the DLB participants were lower than AD participants, and the distribution of putamen z-scores differentiated well between the two groups. The ADem case with the lowest z-score (Case 10) was eventually confirmed to have underlying LBD pathology along with AD neuropathologic change.
Figure 1.
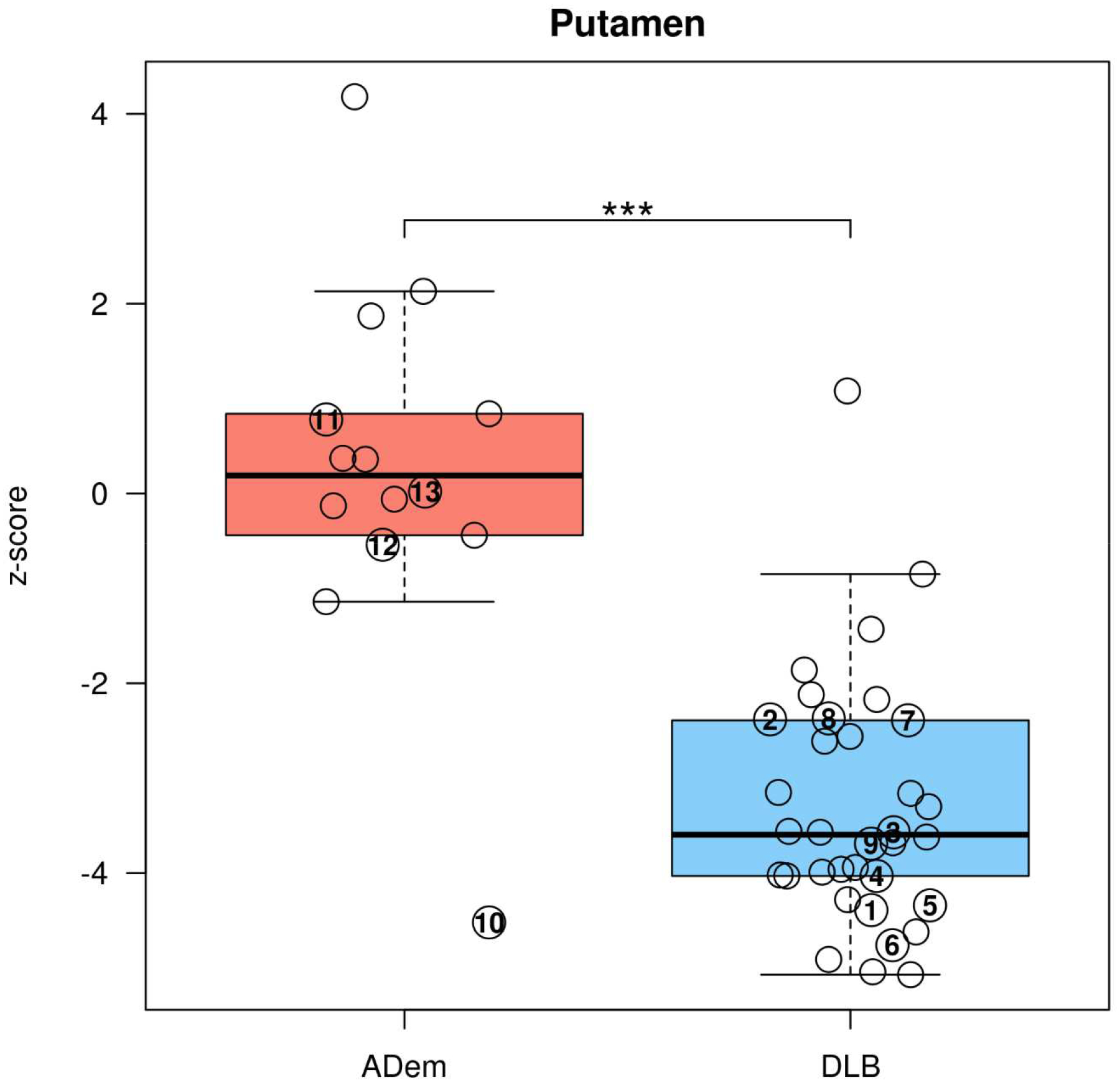
Box-and-whisker plots showing the distribution of DaTQUANT z-scores by 123I-FP-CIT SPECT for the putamen of DLB and ADem.
***p<0.001 for the difference between DLB and ADem (Student t-test).
The cases with autopsy confirmation were numbered on the plots by the same case number as Table 3. Underlying pathology for Cases 1–7 were LBD, Cases 8–11 were LBD+AD, and Cases 12–13 were AD.
The DLB cases without apparent parkinsonism which presented with 123I-FP-CIT SPECT putamen z-scores < −2.0 are shown in Sup 1. Four cases demonstrated imaging evidence of nigrostriatal dysfunction by 123I-FP-CIT SPECT when parkinsonism was not yet clinically defined (bradykinesia plus one of the followings; rest tremor/rigidity/postural instability). The UPDRS part III scores were <10 in all of these cases.
Logistic regression modeling of single or multimodal imaging markers for distinguishing DLB from ADem is shown in Table 2. Among the four imaging modalities, 123I-FP-CIT SPECT had the highest (c-statistic 0.916, p<0.001) and MRI had the lowest (c-statistic 0.739, p=0.016) discriminatory power by a single imaging modality. A logistic regression model for adding one more imaging marker showed enhanced discriminatory power from the single imaging modality model. Any two-imaging modality model that included the 123I-FP-CIT SPECT had a higher c-statistic (c-statistics 0.968–0.975) than any other two imaging models without the 123I-FP-CIT SPECT (c-statistics 0.910–0.964). The three-imaging modality marker model including 123I-FP-CIT SPECT presented very good differentiation (c-statistics 0.987–0.996).
Table 2:
Logistic regression modeling with AD as the outcome
| Single modality | OR (95% CI) | C-statistic (95%CI) | P-value |
|---|---|---|---|
| DaTQUANT: Putamen z-score | 1.13 (1.06, 1.21) | 0.916 (0.792, 1.0) | <0.001 |
| MRI: HP/TIV | 0.17 (0.04, 0.72) | 0.739 (0.575, 0.904) | 0.016 |
| FDG-PET: CIS ratio | 0.09 (0.02, 0.43) | 0.878 (0.775, 0.981) | 0.002 |
| PiB-PET: global SUVr | 2.10 (1.33, 3.31) | 0.884 (0.789, 0.980) | 0.001 |
| Two modalities | |||
| DaTQUANT + MRI | |||
| DaTQUANT | 1.16 (1.06, 1.27) | 0.968 (0.922, 1.0) | 0.001 |
| MRI | 0.03 (0.00, 0.58) | 0.02 | |
| DaTQUANT + FDG | |||
| DaTQUANT | 1.11 (1.03, 1.19) | 0.975 (0.941, 1.0) | 0.006 |
| FDG | 0.06 (0.00, 0.96) | 0.047 | |
| DaTQUANT + PiB | |||
| DaTQUANT | 1.13 (1.04, 1.24) | 0.975 (0.934, 1.0) | 0.005 |
| PiB | 1.96 (1.10, 3.50) | 0.022 | |
| MRI + FDG | |||
| MRI | 0.20 (0.03, 1.22) | 0.910 (0.818, 1.0) | 0.08 |
| FDG | 0.10 (0.02, 0.46) | 0.003 | |
| MRI + PiB | |||
| MRI | 0.05 (0.00, 0.70) | 0.931 (0.862, 0.999) | 0.026 |
| PiB | 2.60 (1.34, 5.03) | 0.005 | |
| FDG + PiB | |||
| FDG | 0.09 (0.01, 0.63) | 0.964 (0.920, 1.0) | 0.015 |
| PiB | 2.09 (1.17, 3.71) | 0.012 | |
| Three modalities | |||
| DaTQUANT + FDG + PiB | |||
| DaTQUANT | 1.14 (1.00, 1.30) | 0.992 (0.976, 1.0) | 0.048 |
| FDG | 0.05 (0.00, 1.84) | 0.10 | |
| PiB | 2.32 (0.94, 5.73 | 0.069 | |
| DaTQUANT + MRI + PiB | |||
| DaTQUANT | 1.28 (1.00, 1.63) | 0.996 (0.986, 1.0) | 0.0496 |
| MRI | 0.00 (0.00, 1.73) | 0.066 | |
| PiB | 3.43 (1.06, 11.11) | 0.0399 | |
| DaTQUANT + MRI +FDG | |||
| DaTQUANT | 1.16 (1.03, 1.30) | 0.987 (0.964, 1.0) | 0.014 |
| MRI | 0.04 (0.00, 1.08) | 0.055 | |
| FDG | 0.04 (0.00, 1.69) | 0.093 |
Odds ratio (OR) represent a 0.1 unit change. DLB is the reference group, so when the OR is greater than 1 this is more likely to occur in ADem and conversely when the OR is less than 1 then it is least likely to occur in ADem individuals. PiB was log transformed.
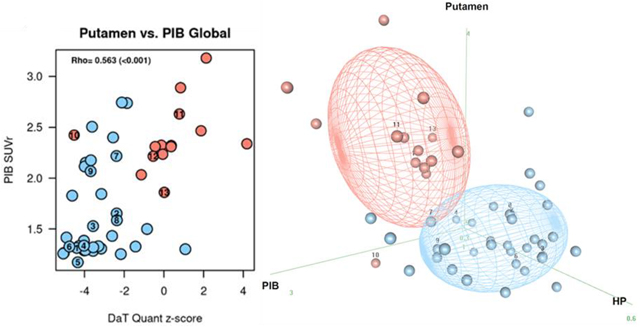
Scatter plots differentiating the DLB from ADem by using two imaging modality are shown in Figure 2. Pearson correlations between the two modalities in DLB participants are shown with scatter plots in Sup 2. There was no significant correlation between any of the two imaging biomarkers. Three dimensional plots of three imaging modality markers including 123I-FP-CIT SPECT differentiating DLB from ADem are shown in Figure 3, where 50% of the expected proportion of bivariate-observations would fall within the concentrated ellipsoid for each diagnostic group. The majority of the DLB and ADem cases were separated accordingly to their diagnosis using any of the three imaging modality models.
Figure 2. Two imaging biomarkers scatter plots differentiating DLB from ADem.
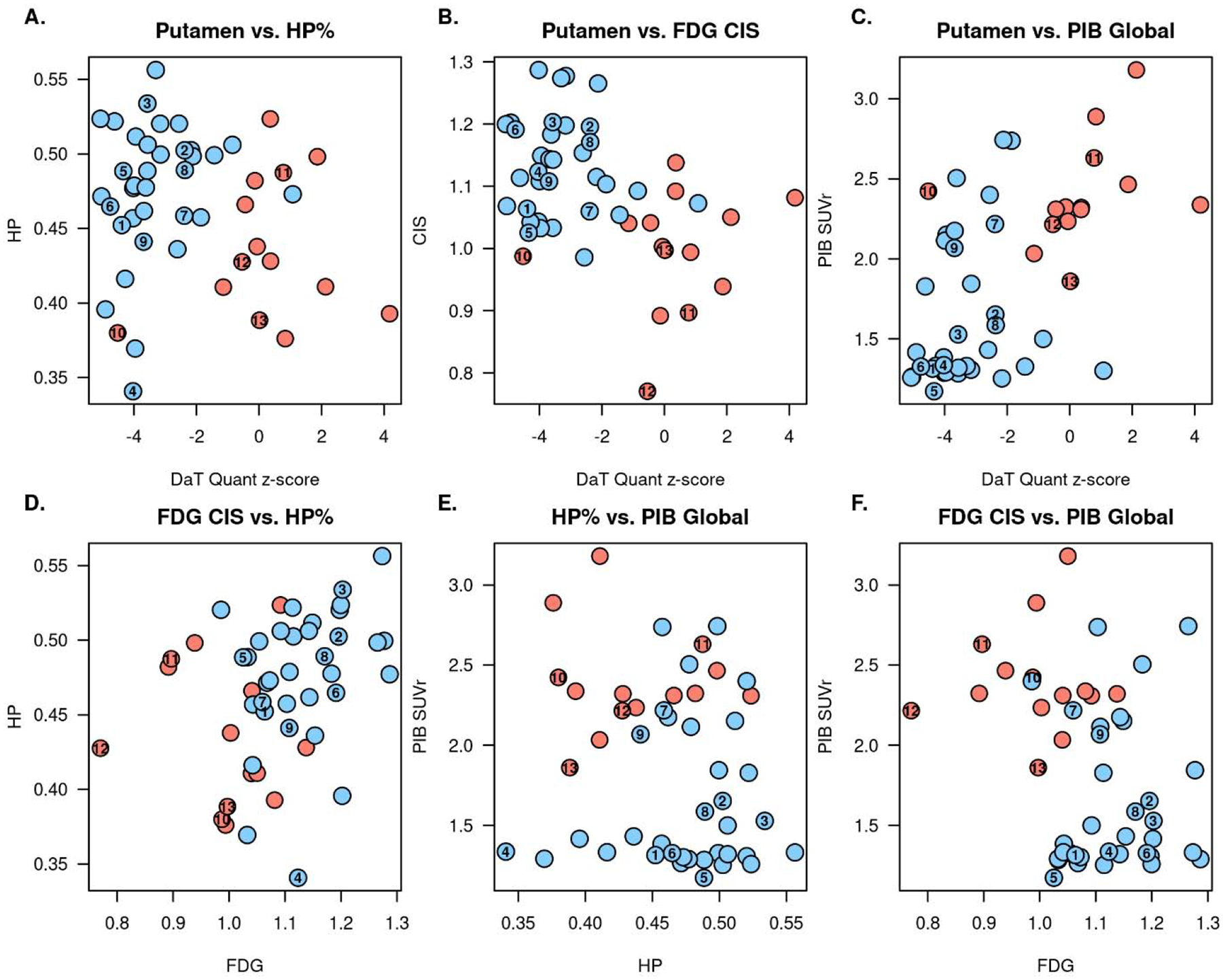
The plots (Blue: DLB, Red: ADem) represent distribution of the combination of the two imaging biomarkers from the followings: DaTQUANT putamen z-score, hippocampal volumes by MRI, CIS ratio by FDG-PET, and global PiB SUVr by PiB-PET.
(A) DaTQUANT + MRI, (B) DaTQUANT + FDG, (C) DaTQUANT + PiB, (D) FDG + MRI, (E) MRI + PiB, (F) FDG + PiB
The cases with autopsy confirmation were numbered on the plots by the same case number as Table 3. Underlying pathology for Cases 1–7 were LBD, Cases 8–11 were LBD+AD, and Cases 12–13 were AD.
Figure 3. Three dimensional ellipsoid plots of the three imaging biomarkers differentiating DLB from ADem.
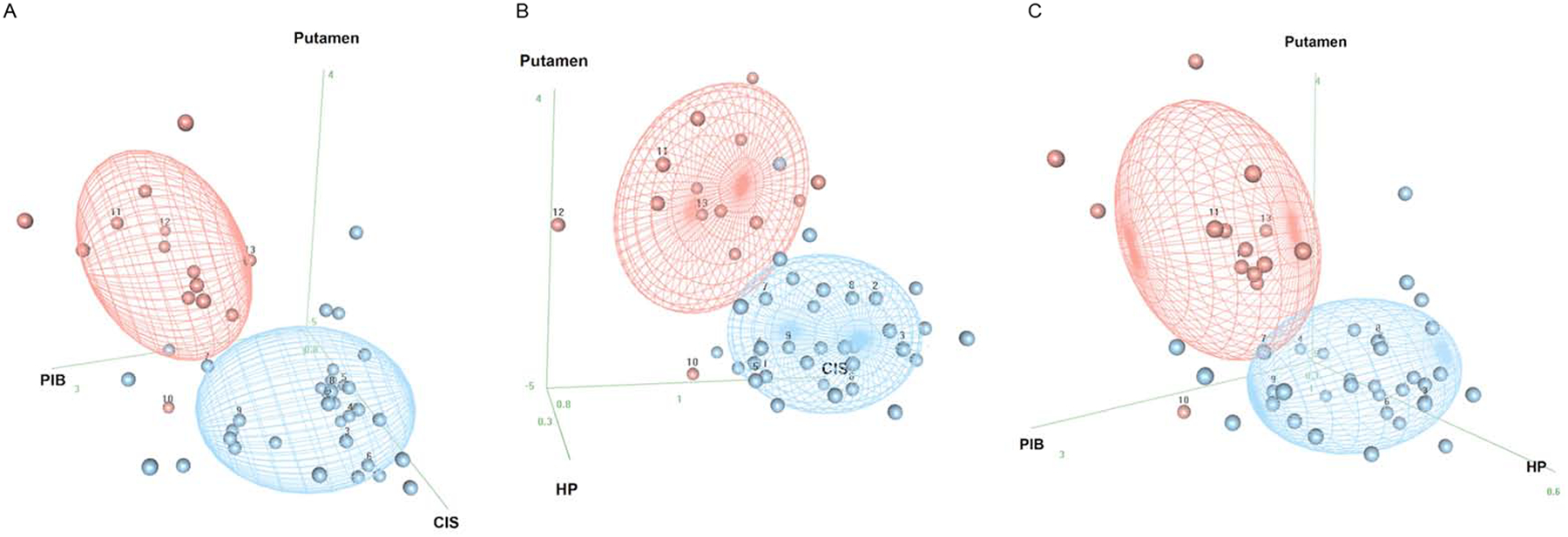
A. 123I-FP-CIT SPECT + FDG-PET + PiB-PET; B. 123I-FP-CIT SPECT + MRI + FDG-PET; C. 123I-FP-CIT SPECT + MRI + PiB-PET The plots show the distribution of the findings from the combination of the three imaging biomarkers from the following: DaTQUANT putamen z-score (“Putamen”), hippocampal volumes by MRI (“HP”), CIS ratio by FDG-PET (“CIS”), and global PiB SUVr by PiB-PET (“PIB”). Concentration ellipsoids represent 50% of the expected proportion of bivariate normal observations in each diagnostic group. A larger spherical ball represents imaging data that are closer to the viewer while smaller spheres represent data farther from the viewer. Red signifies ADem and blue LBD by diagnostic group prediction (concentration ellipsoids) and by individual diagnoses (balls). The cases with autopsy confirmation were numbered on the plots by the same case number as Table 3. Underlying pathology for Cases 1–7 were LBD, Cases 8–11 were LBD+AD, and Cases 12–13 were AD.
Autopsies were performed in thirteen (nine DLB and four ADem) cases, of which eleven cases were included in our prior report (Maltais et al., 2020). Individual autopsy cases (Cases 1–13) with clinical and imaging information are listed in Table 3, and labeled on the 123I-FP-CIT SPECT box-and-whisker plots (Figure 1), scatter plots showing two modality imaging model (Figure 2), and three-dimensional plots showing three modality imaging model (Figure 3). Among the nine DLB cases, three cases had all of the four core clinical features of DLB, four cases had three core clinical features, and two cases had two core clinical features. No autopsy case had conflicting autopsy findings vs. clinical diagnosis; all of the nine DLB cases had underlying diffuse-neocortical LBD pathology; and all of the four ADem cases had intermediate or high AD neuropathologic change. Four cases (two cases for DLB and two cases for ADem) were found to have both diffuse-neocortical LBD pathology and intermediate or high AD neuropathologic change. All of the nine DLB cases (Cases 1–9) had putamen DaTQUANT z-score <−2.0, and both of the ADem cases without substantial LBD pathology (Case 12, 13; none or amygdala-predominant LBD pathology) presented putamen DaTQUANT z-score >−0.82, which was the proposed cutoff z-score value in detecting the presence of LBD in our previous report (Maltais et al., 2020). All of the DLB cases regardless of presence or absence of AD neuropathologic change (Cases 1–9) could be distinguished from ADem by combining the abnormal 123I-FP-CIT SPECT scan with any of the other imaging modality finding (Figure 2 A–C). The ADem cases without LBD pathology (Case 12, 13) were able to be distinguished from DLB by combining the normal 123I-FP-CIT SPECT scan and any of the other imaging modality finding (Figure 2 A–C). There were two ADem cases which turned out to have diffuse-neocortical LBD pathology in addition to high AD neuropathologic change (Case 10, 11). Since Case 10 did not have any core clinical features of DLB, case 10 could not be diagnosed as DLB even with adding indicative biomarkers of DLB including the 123I-FP-CIT SPECT abnormality. Though Case 10 was clinically diagnosed as ADem, the putamen DaTQUANT z-score <−2.0 indicated the presence of underlying widespread LBD pathology as confirmed at autopsy. Despite the abnormal 123I-FP-CIT SPECT scan, Case 10 could be differentiated from DLB by combining any two of the PiB-PET, FDG-PET, and MRI findings (Figure 2 D–F). Unlike in other 10 LBD pathology confirmed cases, putamen DaTQUANT z-score was >−2.0 in Case 11 and could be differentiated from DLB by combining the normal 123I-FP-CIT SPECT scan with PiB-PET or FDG-PET finding (Figure 2 B–C).
Table 3.
Multimodal imaging and pathologic findings in 13 autopsy cases
| Case | Clinical diagnosis | Pathologic group | Sex | Age at scan, yrs | Scan to death, yrs | Core clinical features of DLB | DaTQUANT putamen z-score | PiB SUVr | FDG CIS ratio | MRI HP/TIV | Lewy related pathology | AD neuropathological change | Braak NFT staging |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DLB | LBD | M | 77 | 4 | 3 | −4.39 | 1.31 | 1.06 | 0.452 | Neocortical (diffuse) | Low | III |
| 2 | DLB | LBD | M | 77 | 4 | 3 | −2.38 | 1.65 | 1.20 | 0.502 | Neocortical (diffuse) | Low | II |
| 3 | DLB | LBD | M | 75 | 5 | 4 | −3.57 | 1.53 | 1.20 | 0.534 | Neocortical (diffuse) | Low | II |
| 4 | DLB | LBD | M | 89 | 6 | 4 | −4.03 | 1.34 | 1.12 | 0.341 | Neocortical (diffuse) | Not | II |
| 5 | DLB | LBD | M | 61 | 6 | 4 | −4.34 | 1.17 | 1.03 | 0.488 | Neocortical (diffuse) | Not | None |
| 6 | DLB | LBD | M | 71 | 5 | 2 | −4.76 | 1.33 | 1.19 | 0.465 | Neocortical (diffuse) | Low | II |
| 7 | DLB | LBD | M | 74 | 2 | 3 | −2.39 | 2.22 | 1.06 | 0.459 | Neocortical (diffuse) | Low | III |
| 8 | DLB | LBD + AD | M | 63 | 6 | 3 | −2.37 | 1.59 | 1.17 | 0.489 | Neocortical (diffuse) | Intermediate | V |
| 9 | DLB | LBD + AD | M | 74 | 1 | 2 | −3.69 | 2.07 | 1.11 | 0.441 | Neocortical (diffuse) | Intermediate | III |
| 10 | ADem | LBD + AD | F | 75 | 2 | 0 | −4.52 | 2.42 | 0.99 | 0.380 | Neocortical (diffuse) | High | V |
| 11 | ADem | LBD + AD | F | 63 | 3 | 0 | 0.78 | 2.63 | 0.90 | 0.487 | Neocortical (diffuse) | High | V |
| 12 | ADem | AD | M | 59 | 3 | 0 | −0.54 | 2.22 | 0.77 | 0.428 | None | Intermediate | IV |
| 13 | ADem | AD | M | 74 | 5 | 0 | 0.02 | 1.86 | 1.00 | 0.388 | Amygdala-predominant | High | VI |
4. Discussion
In this study, we demonstrated that 123I-FP-CIT SPECT using semi-quantitative DaTQUANT analysis of the putamen served as one of the most discriminative imaging modalities in distinguishing DLB and ADem. The 123I-FP-CIT SPECT showed the highest c-statistic among the imaging biomarkers analyzed, regardless of whether there was clinical evidence of parkinsonism; however, there wasn’t sufficient power to detect any statistical differences between the c-statistics. In our previous study (Maltais et al., 2020), we found that abnormal 123I-FP-CIT SPECT scan strongly indicated the presence of underlying widespread Lewy-related pathology. Since it is not rare for clinically diagnosed DLB or ADem to have both Lewy-related pathology and AD pathology, overlap observed between DLB and ADem when the 123I-FP-CIT SPECT finding alone was used as an imaging biomarker was consistent with pathology.
Adding another imaging modality to 123I-FP-CIT SPECT enhanced the c-statistics between DLB and ADem with each imaging modality contributing to improve the model. Although hippocampal volume calculated by MRI had a lower c-statistic than FDG-PET or PiB-PET as a single imaging marker, adding it to the 123I-FP-CIT SPECT as dual imaging modalities model demonstrated comparable c-statistics as observed with the 123I-FP-CIT SPECT with FDG-PET or PiB-PET models. There was no correlation found between any of the two imaging biomarkers in DLB participants, indicating each imaging biomarker assessed different biophysiological aspect of DLB and/or ADem and worked complementarily when used in combination. Furthermore, since it is common for DLB and ADem to have both Lewy-related pathology and AD neuropathologic change to varying degrees, it is beneficial to combine multimodal imaging modalities to evaluate pleiotropic pathophysiological features of DLB and ADem. Our findings suggest that multimodal imaging with 123I-FP-CIT SPECT might help clinically discriminating DLB and ADem, as well as detect presence of concurrent Lewy-related and AD pathology in DLB and ADem patients.
In clinical settings, it is common to have MRI examined when 123I-FP-CIT SPECT is performed. With the excellent discriminatory power shown in this study, and considering the availability and cost, simply having 123I-FP-CIT SPECT and MRI with clinical information would be practical. Further adding FDG-PET or PiB-PET to the 123I-FP-CIT SPECT and MRI model would provide additional biophysiological and neuropathological information such as high global PiB retention in PiB-PET indicating presence of comorbid AD pathology in DLB patients.
The primary strength of our study is that multimodal imaging of 123I-FP-CIT SPECT, FDG-PET, PiB-PET, and MRI was performed in DLB and ADem participants with a moderate number with autopsy confirmation. Its limitations include relatively small sample size especially for ADem cohort, and only slightly more than a quarter of the participants had autopsy confirmation to verify the clinical diagnosis of DLB and ADem. The ADem cases were similar in ages and education to the DLB cases, but there were differences in other demographic or clinical features. Although MMSE and CDR® sum of boxes are more sensitive in detecting ADem features such as memory impairment and disorientation, these cognitive and functional scales reflected more severe impairment in ADem participants. Due to relatively small sample size and excellent discriminatory power just by 123I-FP-CIT SPECT alone, multimodal imaging with 123I-FP-CIT SPECT had higher c-statistics, however, there wasn’t sufficient power to detect statistical differences. Nevertheless, we demonstrate that reduced nigrostriatal uptake on 123I-FP-CIT SPECT reflects nigrostriatal dopamine dysfunction with underlying Lewy-related pathology, and other imaging modalities assess different pathophysiological aspects of DLB and/or AD, which potentially work complementarily when used together. It would be informative to have multimodal imaging biomarkers not only to recruit reliable DLB participants but also to evaluate presence/absence of comorbid AD pathology for screening or analyzing data in the future clinical trials on DLB with disease-modifying therapy.
Supplementary Material
Highlights.
DaTQUANT software for 123I-FP-CIT SPECT quantitively discerns DLB and AD well
123I-FP-CIT SPECT showed the best discrimination among the imaging modalities
Having another imaging modality with 123I-FP-CIT SPECT improves diagnostic accuracy
Acknowledgements
We extend our appreciation to the staff of the Mayo Clinic ADRC program for recruiting and following the patients, and particularly to our patients and their families for their participation in neurodegenerative disease research. The staff at GE Healthcare was not involved in the analysis, interpretation of the data, nor preparation of the manuscript. No other potential conflict of interest was reported.
Funding
This work is supported by NIH grants (P50 AG016574, U01 AG006786, AG015866, NS100620, P30 AG062677), grant from GE Healthcare, the Mayo Clinic Dorothy and Harry T. Mangurian Jr. Lewy Body Dementia Program, and the Little Family Foundation, and the Lewy Body Dementia Functional Genomics Program.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
Declarations of interest: none
References
- Albin RL, Minoshima S, D’Amato CJ, Frey KA, Kuhl DA, Sima AA, 1996. Fluoro-deoxyglucose positron emission tomography in diffuse Lewy body disease. Neurology 47(2), 462–466. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ, 2005. Unified segmentation. Neuroimage 26(3), 839–851. [DOI] [PubMed] [Google Scholar]
- Augimeri A, Cherubini A, Cascini GL, Galea D, Caligiuri ME, Barbagallo G, Arabia G, Quattrone A, 2016. CADA-computer-aided DaTSCAN analysis. EJNMMI physics 3(1), 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avants BB, Epstein CL, Grossman M, Gee JC, 2008. Symmetric diffeomorphic image registration with cross-correlation: evaluating automated labeling of elderly and neurodegenerative brain. Med. Image Anal 12(1), 26–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber R, Ballard C, McKeith IG, Gholkar A, O’Brien JT, 2000. MRI volumetric study of dementia with Lewy bodies: a comparison with AD and vascular dementia. Neurology 54(6), 1304–1309. [DOI] [PubMed] [Google Scholar]
- Braak H, Alafuzoff I, Arzberger T, Kretzschmar H, Del Tredici K, 2006. Staging of Alzheimer disease-associated neurofibrillary pathology using paraffin sections and immunocytochemistry. Acta Neuropathol. 112(4), 389–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E, 1991. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 82(4), 239–259. [DOI] [PubMed] [Google Scholar]
- Brogley JE, 2019. DaTQUANT: The Future of Diagnosing Parkinson Disease. J. Nucl. Med. Technol 47(1), 21–26. [DOI] [PubMed] [Google Scholar]
- Buck PO, Wilson RE, Seeberger LC, Conner JB, Castelli-Haley J, 2011. Examination of the UPDRS bradykinesia subscale: equivalence, reliability and validity. J. Parkinsons Dis 1(3), 253–258. [DOI] [PubMed] [Google Scholar]
- Burton EJ, Karas G, Paling SM, Barber R, Williams ED, Ballard CG, McKeith IG, Scheltens P, Barkhof F, O’Brien JT, 2002. Patterns of cerebral atrophy in dementia with Lewy bodies using voxel-based morphometry. Neuroimage 17(2), 618–630. [PubMed] [Google Scholar]
- Colloby SJ, McParland S, O’Brien JT, Attems J, 2012. Neuropathological correlates of dopaminergic imaging in Alzheimer’s disease and Lewy body dementias. Brain 135(Pt 9), 2798–2808. [DOI] [PubMed] [Google Scholar]
- Colloby SJ, O’Brien JT, Fenwick JD, Firbank MJ, Burn DJ, McKeith IG, Williams ED, 2004. The application of statistical parametric mapping to 123I-FP-CIT SPECT in dementia with Lewy bodies, Alzheimer’s disease and Parkinson’s disease. Neuroimage 23(3), 956–966. [DOI] [PubMed] [Google Scholar]
- Djang DS, Janssen MJ, Bohnen N, Booij J, Henderson TA, Herholz K, Minoshima S, Rowe CC, Sabri O, Seibyl J, Van Berckel BN, Wanner M, 2012. SNM practice guideline for dopamine transporter imaging with 123I-ioflupane SPECT 1.0. J. Nucl. Med 53(1), 154–163. [DOI] [PubMed] [Google Scholar]
- Edison P, Rowe CC, Rinne JO, Ng S, Ahmed I, Kemppainen N, Villemagne VL, O’Keefe G, Nagren K, Chaudhury KR, Masters CL, Brooks DJ, 2008. Amyloid load in Parkinson’s disease dementia and Lewy body dementia measured with [11C]PIB positron emission tomography. J. Neurol. Neurosurg. Psychiatry 79(12), 1331–1338. [DOI] [PubMed] [Google Scholar]
- Fahn SER, Members of the UPDRS Development Committee., 1987. Recent Developments in Parkinson’s Disease, Vol 2 Florham Park NJ Macmillan Health Care Information pp 153–163, 293–304. [Google Scholar]
- Ferman TJ, Aoki N, Boeve BF, Aakre JA, Kantarci K, Graff-Radford J, Parisi JE, Van Gerpen JA, Graff-Radford NR, Uitti RJ, Pedraza O, Murray ME, Wszolek ZK, Reichard RR, Fields JA, Ross OA, Knopman DS, Petersen RC, Dickson DW, 2020. Subtypes of dementia with Lewy bodies are associated with α-synuclein and tau distribution. 95(2), e155–e165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferman TJ, Smith GE, Boeve BF, Ivnik RJ, Petersen RC, Knopman D, Graff-Radford N, Parisi J, Dickson DW, 2004. DLB fluctuations: specific features that reliably differentiate DLB from AD and normal aging. Neurology 62(2), 181–187. [DOI] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR, 1975. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J. Psychiatr. Res 12(3), 189–198. [DOI] [PubMed] [Google Scholar]
- Foster ER, Campbell MC, Burack MA, Hartlein J, Flores HP, Cairns NJ, Hershey T, Perlmutter JS, 2010. Amyloid imaging of Lewy body-associated disorders. Mov. Disord 25(15), 2516–2523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garibotto V, Montandon ML, Viaud CT, Allaoua M, Assal F, Burkhard PR, Ratib O, Zaidi H, 2013. Regions of interest-based discriminant analysis of DaTSCAN SPECT and FDG-PET for the classification of dementia. Clin. Nucl. Med 38(3), e112–117. [DOI] [PubMed] [Google Scholar]
- Gomperts SN, Rentz DM, Moran E, Becker JA, Locascio JJ, Klunk WE, Mathis CA, Elmaleh DR, Shoup T, Fischman AJ, Hyman BT, Growdon JH, Johnson KA, 2008. Imaging amyloid deposition in Lewy body diseases. Neurology 71(12), 903–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graff-Radford J, Murray ME, Lowe VJ, Boeve BF, Ferman TJ, Przybelski SA, Lesnick TG, Senjem ML, Gunter JL, Smith GE, Knopman DS, Jack CR Jr., Dickson DW, Petersen RC, Kantarci K, 2014. Dementia with Lewy bodies: basis of cingulate island sign. Neurology 83(9), 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton RL, 2000. Lewy bodies in Alzheimer’s disease: a neuropathological review of 145 cases using alpha-synuclein immunohistochemistry. Brain Pathol. 10(3), 378–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi M, Tashiro M, Arai H, Okamura N, Hara S, Higuchi S, Itoh M, Shin RW, Trojanowski JQ, Sasaki H, 2000. Glucose hypometabolism and neuropathological correlates in brains of dementia with Lewy bodies. Exp. Neurol 162(2), 247–256. [DOI] [PubMed] [Google Scholar]
- Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL, 1982. A new clinical scale for the staging of dementia. Br. J. Psychiatry 140, 566–572. [DOI] [PubMed] [Google Scholar]
- Hyman BT, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Carrillo MC, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Thies B, Trojanowski JQ, Vinters HV, Montine TJ, 2012. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 8(1), 1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii K, Imamura T, Sasaki M, Yamaji S, Sakamoto S, Kitagaki H, Hashimoto M, Hirono N, Shimomura T, Mori E, 1998. Regional cerebral glucose metabolism in dementia with Lewy bodies and Alzheimer’s disease. Neurology 51(1), 125–130. [DOI] [PubMed] [Google Scholar]
- Jack CR Jr., Lowe VJ, Senjem ML, Weigand SD, Kemp BJ, Shiung MM, Knopman DS, Boeve BF, Klunk WE, Mathis CA, Petersen RC, 2008. 11C PiB and structural MRI provide complementary information in imaging of Alzheimer’s disease and amnestic mild cognitive impairment. Brain 131(Pt 3), 665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jellinger KA, Attems J, 2008. Prevalence and impact of vascular and Alzheimer pathologies in Lewy body disease. Acta Neuropathol. 115(4), 427–436. [DOI] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Boeve BF, Weigand SD, Senjem ML, Przybelski SA, Dickson DW, Parisi JE, Knopman DS, Smith GE, Ferman TJ, Petersen RC, Jack CR Jr., 2012a. Multimodality imaging characteristics of dementia with Lewy bodies. Neurobiol. Aging 33(9), 2091–2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Lowe VJ, Chen Q, Przybelski SA, Lesnick TG, Schwarz CG, Senjem ML, Gunter JL, Jack CR Jr., Graff-Radford J, Jones DT, Knopman DS, Graff-Radford N, Ferman TJ, Parisi JE, Dickson DW, Petersen RC, Boeve BF, Murray ME, 2020. β-Amyloid PET and neuropathology in dementia with Lewy bodies. Neurology 94(3), e282–e291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantarci K, Yang C, Schneider JA, Senjem ML, Reyes DA, Lowe VJ, Barnes LL, Aggarwal NT, Bennett DA, Smith GE, Petersen RC, Jack CR Jr., Boeve BF, 2012b. Antemortem amyloid imaging and beta-amyloid pathology in a case with dementia with Lewy bodies. Neurobiol. Aging 33(5), 878–885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim SM, Katsifis A, Villemagne VL, Best R, Jones G, Saling M, Bradshaw J, Merory J, Woodward M, Hopwood M, Rowe CC, 2009. The 18F-FDG PET cingulate island sign and comparison to 123I-beta-CIT SPECT for diagnosis of dementia with Lewy bodies. J. Nucl. Med 50(10), 1638–1645. [DOI] [PubMed] [Google Scholar]
- Lowe VJ, Kemp BJ, Jack CR Jr., Senjem M, Weigand S, Shiung M, Smith G, Knopman D, Boeve B, Mullan B, Petersen RC, 2009. Comparison of 18F-FDG and PiB PET in cognitive impairment. J. Nucl. Med 50(6), 878–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maltais DD, Jordan LG 3rd, Min HK, Miyagawa T, Pryzbleski S, Lesnick TG, Reichard RR, Dickson DW, Murray ME, Kantarci K, Boeve BF, Lowe V, 2020. Confirmation of (123)I-FP-CIT-SPECT (ioflupane) quantification methods in dementia with Lewy body and other neurodegenerative disorders. J. Nucl. Med [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith I, O’Brien J, Walker Z, Tatsch K, Booij J, Darcourt J, Padovani A, Giubbini R, Bonuccelli U, Volterrani D, Holmes C, Kemp P, Tabet N, Meyer I, Reininger C, 2007. Sensitivity and specificity of dopamine transporter imaging with 123I-FP-CIT SPECT in dementia with Lewy bodies: a phase III, multicentre study. The Lancet Neurology 6(4), 305–313. [DOI] [PubMed] [Google Scholar]
- McKeith IG, Boeve BF, Dickson DW, Halliday G, Taylor JP, Weintraub D, Aarsland D, Galvin J, Attems J, Ballard CG, Bayston A, Beach TG, Blanc F, Bohnen N, Bonanni L, Bras J, Brundin P, Burn D, Chen-Plotkin A, Duda JE, El-Agnaf O, Feldman H, Ferman TJ, Ffytche D, Fujishiro H, Galasko D, Goldman JG, Gomperts SN, Graff-Radford NR, Honig LS, Iranzo A, Kantarci K, Kaufer D, Kukull W, Lee VMY, Leverenz JB, Lewis S, Lippa C, Lunde A, Masellis M, Masliah E, McLean P, Mollenhauer B, Montine TJ, Moreno E, Mori E, Murray M, O’Brien JT, Orimo S, Postuma RB, Ramaswamy S, Ross OA, Salmon DP, Singleton A, Taylor A, Thomas A, Tiraboschi P, Toledo JB, Trojanowski JQ, Tsuang D, Walker Z, Yamada M, Kosaka K, 2017. Diagnosis and management of dementia with Lewy bodies: Fourth consensus report of the DLB Consortium. Neurology 89(1), 88–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKeith IG, Dickson DW, Lowe J, Emre M, O’Brien JT, Feldman H, Cummings J, Duda JE, Lippa C, Perry EK, Aarsland D, Arai H, Ballard CG, Boeve B, Burn DJ, Costa D, Del Ser T, Dubois B, Galasko D, Gauthier S, Goetz CG, Gomez-Tortosa E, Halliday G, Hansen LA, Hardy J, Iwatsubo T, Kalaria RN, Kaufer D, Kenny RA, Korczyn A, Kosaka K, Lee VM, Lees A, Litvan I, Londos E, Lopez OL, Minoshima S, Mizuno Y, Molina JA, Mukaetova-Ladinska EB, Pasquier F, Perry RH, Schulz JB, Trojanowski JQ, Yamada M, 2005. Diagnosis and management of dementia with Lewy bodies: third report of the DLB Consortium. Neurology 65(12), 1863–1872. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Knopman DS, Chertkow H, Hyman BT, Jack CR Jr., Kawas CH, Klunk WE, Koroshetz WJ, Manly JJ, Mayeux R, Mohs RC, Morris JC, Rossor MN, Scheltens P, Carrillo MC, Thies B, Weintraub S, Phelps CH, 2011. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s & dementia : the journal of the Alzheimer’s Association 7(3), 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minoshima S, Foster NL, Sima AA, Frey KA, Albin RL, Kuhl DE, 2001. Alzheimer’s disease versus dementia with Lewy bodies: cerebral metabolic distinction with autopsy confirmation. Ann. Neurol 50(3), 358–365. [DOI] [PubMed] [Google Scholar]
- Mirra SS, Heyman A, McKeel D, Sumi SM, Crain BJ, Brownlee LM, Vogel FS, Hughes JP, van Belle G, Berg L, 1991. The Consortium to Establish a Registry for Alzheimer’s Disease (CERAD). Part II. [DOI] [PubMed] [Google Scholar]
- Standardization of the neuropathologic assessment of Alzheimer’s disease. Neurology 41(4), 479–486. [DOI] [PubMed] [Google Scholar]
- Montine TJ, Phelps CH, Beach TG, Bigio EH, Cairns NJ, Dickson DW, Duyckaerts C, Frosch MP, Masliah E, Mirra SS, Nelson PT, Schneider JA, Thal DR, Trojanowski JQ, Vinters HV, Hyman BT, National Institute on A, Alzheimer’s A, 1982. National Institute on Aging-Alzheimer’s Association guidelines for the neuropathologic assessment of Alzheimer’s disease: a practical approach. Acta Neuropathol. 139(1), 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JC, 1993. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology 43(11), 2412–2414. [DOI] [PubMed] [Google Scholar]
- Mosconi L, Tsui WH, Herholz K, Pupi A, Drzezga A, Lucignani G, Reiman EM, Holthoff V, Kalbe E, Sorbi S, Diehl-Schmid J, Perneczky R, Clerici F, Caselli R, Beuthien-Baumann B, Kurz A, Minoshima S, de Leon MJ, 2008. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J. Nucl. Med 49(3), 390–398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piggott MA, Marshall EF, Thomas N, Lloyd S, Court JA, Jaros E, Burn D, Johnson M, Perry RH, McKeith IG, Ballard C, Perry EK, 1999. Striatal dopaminergic markers in dementia with Lewy bodies, Alzheimer’s and Parkinson’s diseases: rostrocaudal distribution. Brain 122 (Pt 8), 1449–1468. [DOI] [PubMed] [Google Scholar]
- Reisberg B, Ferris SH, de Leon MJ, Crook T, 1982. The Global Deterioration Scale for assessment of primary degenerative dementia. Am. J. Psychiatry 139(9), 1136–1139. [DOI] [PubMed] [Google Scholar]
- Schwarz CG, Gunter JL, Wiste HJ, Przybelski SA, Weigand SD, Ward CP, Senjem ML, Vemuri P, Murray ME, Dickson DW, Parisi JE, Kantarci K, Weiner MW, Petersen RC, Jack CR Jr., 2016. A large-scale comparison of cortical thickness and volume methods for measuring Alzheimer’s disease severity. NeuroImage. Clinical 11, 802–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu S, Namioka N, Hirose D, Kanetaka H, Hirao K, Hatanaka H, Takenoshita N, Kaneko Y, Ogawa Y, Tsugawa A, Umahara T, Sakurai H, Hanyu H, 2017. Comparison of diagnostic utility of semi-quantitative analysis for DAT-SPECT for distinguishing DLB from AD. J. Neurol. Sci 377, 50–54. [DOI] [PubMed] [Google Scholar]
- Thal DR, Rub U, Orantes M, Braak H, 2002. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology 58(12), 1791–1800. [DOI] [PubMed] [Google Scholar]
- Thomas AJ, Attems J, Colloby SJ, O’Brien JT, McKeith I, Walker R, Lee L, Burn D, Lett DJ, Walker Z, 2017. Autopsy validation of 123I-FP-CIT dopaminergic neuroimaging for the diagnosis of DLB. Neurology 88(3), 276–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vann Jones SA, O’Brien JT, 2014. The prevalence and incidence of dementia with Lewy bodies: a systematic review of population and clinical studies. Psychol. Med 44(4), 673–683. [DOI] [PubMed] [Google Scholar]
- Walker Z, Costa DC, Walker RW, Shaw K, Gacinovic S, Stevens T, Livingston G, Ince P, McKeith IG, Katona CL, 2002. Differentiation of dementia with Lewy bodies from Alzheimer’s disease using a dopaminergic presynaptic ligand. J. Neurol. Neurosurg. Psychiatry 73(2), 134–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker Z, Jaros E, Walker RW, Lee L, Costa DC, Livingston G, Ince PG, Perry R, McKeith I, Katona CL, 2007. Dementia with Lewy bodies: a comparison of clinical diagnosis, FP-CIT single photon emission computed tomography imaging and autopsy. J. Neurol. Neurosurg. Psychiatry 78(11), 1176–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


