Abstract
Perivascular adipose tissue (PVAT) has recently entered in the realm of cardiovascular diseases as a putative target for intervention. Notwithstanding its relevance, there is still a long way before the role of PVAT in physiology and pathology is fully understood. The general idea that PVAT anti-contractile effect is beneficial and its pro-contractile effect is harmful is being questioned by several reports. The role of some PVAT important products or systems such as nitric oxide (NO), reactive oxygen species (ROS), and RAS may vary depending on the context, disease, place of production, etc., which adds doubts on how mediators of PVAT anti- and pro-contractile effects are called to action and their final result. This short review will address some points regarding NO, ROS, and RAS in the beneficial and harmful roles of PVAT.
Keywords: perivascular adipose tissue, nitric oxide, vascular dysfunction, angiotensin, superoxide, sepsis
Introduction
Since the pioneering work of Soltis and Cassis (1991), perivascular adipose tissue (PVAT) has been recognized as an active player in vascular physiology and pathology. Besides adipocytes that are its main cellular component, PVAT also contains fibroblasts, endothelial and immune cells (macrophages, lymphocytes, and eosinophils), extracellular matrix, and adrenergic nerves endings (see Figure 1). Depending on the vessel type, PVAT may have white or brown adipose tissue. In addition, the same vessel may have the two types in different segments (such as the aorta) or even a mixture of both (renal artery, for example; Padilla et al., 2013). Although there are excellent reviews (Ayala-Lopez and Watts, 2017; Fernandez-Alfonso et al., 2017; Saxton et al., 2019) concerning several aspects of PVAT, some components of the vast array of PVAT products have received less attention, namely, the renin–angiotensin system (RAS), nitric oxide (NO), and reactive oxygen species (ROS). This short review will address findings concerning these systems in the beneficial and harmful roles of PVAT.
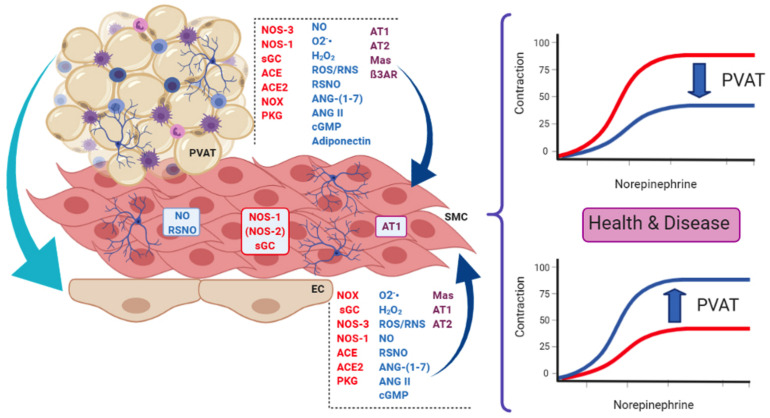
FIGURE 1.
Some of the main actors present in endothelial cells (EC) and perivascular adipose tissue (PVAT) that influence smooth muscle cells (SMC) in the context of the present review. PVAT can also modulate endothelial function and influence the vascular tone. These actors are involved in the dual role of PVAT, namely, its anti-contractile (upper curves) and pro-contractile (lower curves) effects on vessel response, both relevant in health and disease. Enzymes are in red letters, mediators in blue, and receptors/effectors in purple. PVAT contains adipocytes, innervation (neurons) and immune cells (lymphocytes, macrophages, and eosinophils), and vessels (not shown). NOS-3, NOS endothelial isoform; NOS-1, NOS neuronal isoform; (NOS-2), NOS inducible isoform; NOX, NADPH oxidases; sGC, soluble guanylate cyclase; ACE, angiotensin-converting enzyme; ACE2, ACE isoform 2; NO, nitric oxide; O2-, superoxide anion; H2O2, hydrogen peroxide; ROS/RNS, reactive oxygen and nitrogen species, respectively; RSNO, low-molecular weight and protein S-nitrosothiols; ANG-(1–7), angiotensin 1–7; ANG II, angiotensin II; cGMP, cyclic GMP; AT1, angiotensin AT1 receptor; AT2, angiotensin AT2 receptor; Mas, Mas receptor; ß3AR, beta-3 adrenergic receptor; PKG, protein kinase G.
PVAT and Renin–Angiotensin System
Renin–angiotensin system has been described as a hormonal system involved in blood pressure regulation and water balance. In this context, the main elements of this system are represented by the angiotensin-converting enzyme (ACE), angiotensin II (ANG II), and AT1 and AT2 receptors (Schiavone et al., 1988; Campagnole-Santos et al., 1989). Later, it has been demonstrated the existence of a counterregulatory RAS axis composed by ACE-2 (Donoghue et al., 2000; Tipnis et al., 2000), the biologically active peptide ANG-(1–7), and Mas receptor (Santos et al., 2003a,b, 2004; Mercure et al., 2008; Santiago et al., 2010).
There are few studies about the function of RAS in PVAT, but it has been shown that it contributes to vascular tone, structural and functional alterations, as well as local inflammatory process (Ferreira et al., 2010). RAS was first described in PVAT when a substantial amount of angiotensinogen mRNA was found in PVAT (Cassis et al., 1988). Later, ACE and ANG II were detected by immunohistochemistry in mesenteric arteries PVAT (Lu et al., 2010). Mas receptor and ANG-(1–7) have also been found in PVAT (Lee et al., 2009; Nobrega et al., 2019). Interestingly, although all RAS components, except renin, were expressed in both adipose tissue types present in PVAT, the expression of renin/prorenin receptor, angiotensinogen, and AT1 and AT2 receptor was higher in PVAT containing white adipose tissue (Gálvez-Prieto et al., 2008).
The involvement of RAS from PVAT on vascular tone is dual. The pro-contractile effect was observed in mesenteric arteries stimulated by electrical field, where PVAT generated ANG II, enhancing the contraction (Lu et al., 2010). In non-physiological situations, such as hypoxia, PVAT from small arteries lost its anti-contractile activity due to PVAT-mediated ANG II secretion (Greenstein et al., 2009; Rosei et al., 2015). In the same line, PVAT from thoracic aorta of rats subjected to myocardial infarction-induced heart failure becomes dysfunctional due to overactivation of RAS characterized by increased activity/levels of ACE1, angiotensin II, and AT1 and AT2 receptors, thus enhancing oxidative stress and, consequently, reducing NO bioavailability (Fontes et al., 2020). On the other hand, studies performed in large arteries and veins showed that ANG-(1–7) is one of the PVAT-derived relaxing factors (PVDRFs). For example, ANG-(1–7) levels released by PVAT were reduced in hypertensive rats (SHR model) (Lu et al., 2011a). As for the mechanisms involved in PVAT RAS anti-contractile effects, ANG-(1–7) released by PVAT acts by Mas receptor on endothelial cells, releasing NO and leading to relaxation of the blood vessels (Lee et al., 2009, 2011; Lu et al., 2011b). These findings have been expanded by showing that aorta associated PVAT displays Mas and AT2 receptors, PI3k/Akt pathway, as well as endothelial (eNOS; NOS-3) and neuronal (nNOS; NOS-1) NO synthase (NOS) isoforms. The presence of these receptors and enzymes in PVAT is responsible for NO and hydrogen peroxide production due to NOS-3 and NOS-1 isoforms’ activation, respectively (Nobrega et al., 2019).
Besides directly affecting vessel tonus, RAS is also involved in PVAT inflammatory and immune actions on vessel function. For example, PVAT releases adipokines, such as adiponectin (Chatterjee et al., 2009) that plays a cardiovascular protection role which is related to its anti-inflammatory (Matsuzawa, 2006) and anti-proliferative effects (Wang et al., 2005). Treatment of hypertensive patients with ACE inhibitor or AT1 receptor antagonist increased adiponectin concentrations (Furuhashi et al., 2003), suggesting that PVAT RAS may indeed have a proinflammatory effect. Furthermore, when perivascular fat is submitted to proinflammatory insults, such as aldosterone and hypoxia, macrophages are activated leading to the loss of anti-contractile activity of healthy PVAT (Greenstein et al., 2009; Withers et al., 2011). Moreover, AT1 receptors from PVAT shift macrophage to proinflammatory (M1) polarization and this effect is involved in local inflammation and matrix metalloproteinase (MMP) activation, contributing to the pathological environment, such as for aneurysm formation (Sakaue et al., 2017).
In summary, PVAT RAS is involved in vascular tonus maintenance in physiological and pathophysiological situations, as well as in the inflammatory and immune aspects affecting vessel function.
PVAT and NO/ROS
Perivascular adipose tissue adipocytes express NOS-3, but the enzyme is also expressed in PVAT endothelial cells (Ribiere et al., 1996; Araujo et al., 2011; Xia et al., 2016). NOS-1 has also been found in PVAT (Nobrega et al., 2019). The expression of PVAT NOS-3 seems to be variable between vessels and it can vary even in the same vessel (Victorio et al., 2016). NOS-3 from PVAT shares several characteristics with the endothelial isoform such as the need of dimerization for catalysis, chaperone dependency, L-arginine concentrations, and the need of BH4 (Victorio and Davel, 2020). NOS enzymes and in particular PVAT NOS-3 produce superoxide anion when uncoupled (Vasquez-Vivar et al., 2003; Margaritis et al., 2013; Nobrega et al., 2019). Although several conditions are known to reduce NOS-3 activity in PVAT (lack of L-arginine, reduction in serine 1177 phosphorylation and acetylation; Man et al., 2020), BH4 oxidation and its reduced availability probably are the main cause for NOS-3 uncoupling in endothelial cells and most likely in PVAT as well (Vasquez-Vivar et al., 2003; Marchesi et al., 2009). Since not all NOS molecules are uncoupled at the same time, some of them produce NO while some produce superoxide anion. Superoxide anion and NO can react stoichiometrically to produce peroxynitrite, a powerful oxidant (reviewed in Beckman, 2009). Adiponectin, one of the main PVAT products, can recouple NOS-3, improve redox state via PI3/Akt-mediated phosphorylation of NOS-3, and increase BH4 bioavailability (Margaritis et al., 2013).
NO produced by PVAT indeed has important physiological and pathological effects and inhibition or increase in its production affects vessel response (see, for example, the excellent reviews by Zaborska et al., 2017; Daiber et al., 2019; Nava and Llorens, 2019). NO produced in PVAT uses canonical pathways to evoke its effect on vessels such as calcium-dependent potassium channels (Gao et al., 2007; Lee et al., 2009) and cyclic GMP-dependent protein kinase (PKG) (Withers et al., 2014). PVAT adipocytes also express beta-3 adrenergic receptors and its stimulation increases cAMP levels, activates voltage-dependent potassium channels isoform 7 (Kv7), and induces NO release (Bussey et al., 2018). However, PVAT also releases a transferable relaxing factor that acts by tyrosine kinase-dependent activation of potassium channels that are not NO (Lohn et al., 2002).
Although NOS uncoupling is an important ROS producer, PVAT also generates ROS from other sources such as mitochondria and NOX (Gao et al., 2006; Nosalski and Guzik, 2017). Mitochondrial contribution comes mainly from electron transport chain by generating superoxide anions (Costa et al., 2016). Whereas small amounts of ROS are important for inter and intracellular signals for cell physiology, higher amounts of them can be detrimental (Garcia-Redondo et al., 2016; Krylatov et al., 2018). Notwithstanding the importance of superoxide, its short half-life and radius of diffusion (Fridovich, 1995) make its stable metabolite hydrogen peroxide more relevant for direct ROS effects in PVAT (Ardanaz and Pagano, 2006; Gao et al., 2007). Hydrogen peroxide can be formed by superoxide dismutation carried out by SOD or, particularly relevant for PVAT containing brown adipose tissue, by the activity of NOX4 which produces hydrogen peroxide directly (Friederich-Persson et al., 2017).
Whereas the effect and role of NO in PVAT anti-contractile effect are relatively well established, the effects of ROS produced by PVAT are still unsettled. For instance, it is widely accepted that superoxide anion produced in excess may reduce NO bioavailability, thus decreasing PVAT anti-contractile effect (Marchesi et al., 2009). However, ROS may directly act as mediators of PVAT anti-contractile effects (Costa et al., 2016; Chang et al., 2020). For example, electrical field stimulation and perivascular nerve activation enhance PVAT pro-contractile effect due to superoxide release and MAPK/ERK activation (Gao et al., 2006). Hydrogen peroxide in turn relaxes vessels via potassium channel activation and/or via soluble guanylate cyclase/PKG activation (Gao et al., 2007; Withers et al., 2014; Friederich-Persson et al., 2017). Moreover, specific ROS can originate opposing effects depending on its local of production. For instance, hydrogen peroxide produced by the vessel induces vasoconstriction in hypertension and cardiovascular disease, but the same species exhibit vasorelaxant properties when produced by PVAT (Ardanaz and Pagano, 2006; Gao et al., 2007).
Perivascular adipose tissue from mouse thoracic aorta expresses Mas and AT2 receptors as well as NOS-3 and NOS-1, being the products NO and hydrogen peroxide relevant effectors for PVAT anti-contractile effect toward phenylephrine. Interestingly, this PVAT anti-contractile effect is only verified in the absence of vascular endothelium (Nobrega et al., 2019). Since it has been suggested that PVAT has both endothelium-dependent and -independent pathways affecting vascular tone (Gao et al., 2007), collectively these findings raise important questions on the relevance of endothelium to PVAT effects, what is the role of PVAT in endothelial dysfunction and if this interaction is the same in different species.
In summary, the role of NO and ROS (and their potential interaction) in PVAT pathological role still needs more studies since antioxidant approaches as therapeutic options have failed to improve PVAT actions in cardiovascular diseases.
Beneficial Effects of PVAT
In physiological situations, PVAT has a global anti-contractile effect that can be seen in different vessels such as the aorta and mesentery arteries, as well as in veins. These PVAT anti-contractile effects are thought to contribute to the vascular tonus maintenance (Soltis and Cassis, 1991; Gao et al., 2005; Lu et al., 2011b). However, PVAT influence on vessel tonus may vary depending on its adipose tissue composition and/or vessel location. For instance, in the thoracic aorta, PVAT adipose tissue is composed by brown adipose tissue (BAT), whereas in the abdominal aorta and mesenteric arteries, it is mostly composed by white adipose tissue (WAT; Padilla et al., 2013; Brown et al., 2014). This difference does not concern only to the phenotype, but also to PVAT paracrine actions, since the influence of PVAT on contractility of the abdominal aorta is smaller compared to thoracic aorta. The different quantitative anti-contractile effect is thought to be related to a lower expression of NOS-3 in the abdominal segment (Victorio et al., 2016).
The activation of endothelial Mas receptor by ANG-(1–7) released by PVAT has also been found to be a relevant anti-contractile mechanism in the aorta (Lee et al., 2011). NO acts as a key downstream effector not only for ANG-(1–7), but it is relevant for the anti-contractile effect of PVAT-derived adipokines as well, in both arterial and venous vascular networks. For example, endothelium-dependent dilation induced by adiponectin (Chen et al., 2003; Lynch et al., 2013) and leptin (Vecchione et al., 2002; Gálvez-Prieto et al., 2012) also relies, at least in part, on the NO production through phosphorylation and activation of NOS-3.
Perivascular adipose tissue surrounding veins also affects their tonus via NO and ROS production (Lu et al., 2011b). For example, in rat-isolated vena cava, PVAT reduced the contraction elicited by phenylephrine, serotonin, and thromboxane-A2 mimetic vasoconstrictor (U46619), indicating that PVAT anti-contractile effect is agonist independent. Interestingly, the contraction in vena cava is only attenuated by PVAT when in the presence of endothelium and this effect is related to Mas receptors activation and subsequent NO production (Lu et al., 2011b).
Besides being an important player in physiological situations, PVAT can show beneficial actions in cardiovascular diseases, such as atherosclerosis. For instance, PVAT displays an endothelial protective effect in the LDLr-KO model of atherosclerosis through the compensatory increase in PVAT NOS-3 expression (in sharp contrast to the lack of NOS-3 expression in the endothelium), thus leading to the recovery of acetylcholine relaxation in the early stages of atherosclerosis development (Baltieri et al., 2018). Another PVAT protective effect relates to the increase in adiponectin levels leading to prevention of plaque formation by recoupling NOS-3 and increasing NO bioavailability, and also through macrophage autophagy induction via suppression of Akt/FOXO3 (Margaritis et al., 2013; Li et al., 2015). Of note, increases in adiponectin gene expression can be induced by vascular superoxide and by products of lipid peroxidation, thus representing a local mechanism to control oxidative stress (Margaritis et al., 2013). Furthermore, Mas receptor activation with the agonist AVE0991 induced anti-atherosclerotic and anti-inflammatory actions by reducing monocyte/macrophage differentiation and recruitment to PVAT during early stages of atherosclerosis in ApoE−/− mice (Skiba et al., 2017).
Harmful Effects of PVAT
Akin to the endothelial dysfunction, PVAT dysfunction can be an early marker of vascular disease. Experiments in aorta rings from pre-hypertensive SHR rats have shown that the reduction in the PVAT anti-contractile effect precedes the establishment of hypertension (Gálvez-Prieto et al., 2008). As for the mechanisms involved, the lack of PVAT anti-contractile response during hypertension is caused, at least in part, by a reduction in leptin production and impaired activation of NOS-3 (Gálvez-Prieto et al., 2012). The anti-contractile effect of PVAT is completely abolished in models of obesity induced by high-fat diet (HFD) and the New Zealand genetic model (NZO), and significantly reduced in the ob/ob genetic model (Marchesi et al., 2009; Ketonen et al., 2010; Agabiti-Rosei et al., 2014). Animals treated with HFD showed uncoupling of NOS-3 and decreased availability of its substrate arginine, leading to a reduction in NO production and increasing superoxide anion production, well known for its pro-contractile action (Gil-Ortega et al., 2014; Xia et al., 2016). In addition to NO and ROS, other local changes are known to shift the PVAT effect in obesity, including changes in the size and mass of the adipocytes, changes in the secretory profile of the PVAT adipose tissue, and the reduction in density and formation of capillaries, providing a hypoxic environment (Marchesi et al., 2009). Hypoxia, in turn, increases the production and release of proinflammatory cytokines, chemokines (MIP-1α and MCP-1), and leptin, inducing the infiltration of immune cells and decreasing local adiponectin production with consequent downregulation of NOS-3, thus favoring endothelial dysfunction (Chatterjee et al., 2009; Greenstein et al., 2009; Ketonen et al., 2010). As stated above, immune cells, such as macrophages, have an important influence on PVAT dysfunction, as they induce NOX and NOS-2 (iNOS; inducible isoform) activity, resulting in an increased production of superoxide anion and other ROS, such as peroxynitrite. The local increase of NOX goes along with the increased expression of its p67phox subunit in the PVAT of obese animals (Ketonen et al., 2010; DeVallance et al., 2018). This communication between hypoxia-inflammation-oxidative stress leads to proinflammatory cytokine and adipokine production, such as leptin, TNF-α, and IL-6 and the negative regulation of anti-inflammatory mediators, such as adiponectin and IL-10, thus aggravating endothelial dysfunction, resulting in the loss or attenuation of the anti-contractile action of PVAT in obesity (Xia and Li, 2017).
In addition to hypertension and obesity, a shift in PVAT genes and proteins content/expression associated with oxidative damage and inflammation in experimental models of atherosclerosis has been observed. This local inflammatory profile includes increases in MCP-1, IL-6, and angiopoietin-like protein 2 (Angptl2), supporting immune cell migration and accelerating neointima hyperplasia (Viedt et al., 2002; Tian et al., 2013; Manka et al., 2014; Quesada et al., 2018). Moreover, both components of RAS and macrophage markers were upregulated in PVAT in ApoE−/− mice fed with a high-cholesterol diet. PVAT transplantation from these animals into ApoE−/− recipient mice fed a normal chow diet induced an increase in atherosclerosis development. This effect was significantly reduced by blocking ANG II receptors or by transplanting PVAT from mice lacking AT1 receptors, pointing to a RAS-dependent mechanism of PVAT inflammation during atherosclerosis (Irie et al., 2015).
A hitherto unknown involvement of PVAT in vascular dysfunction of septic shock has been recently addressed. Sepsis vascular dysfunction is characterized by the loss of response to vasoconstrictors and profound hypotension. Paradoxically, in this pathological condition, the anti-contractile effect of PVAT is increased. Ex vivo experiments using aorta rings with intact PVAT showed a worsening in the response to norepinephrine, phenylephrine, and serotonin (Awata et al., 2019; Barp et al., 2020). The increased PVAT anti-contractile effect in sepsis could be entirely attributed to PVAT, since PVAT was taken from septic aorta and mesenteric artery and then incubated with healthy vessel rings stripped of their own PVAT reduced norepinephrine effects (Barp et al., 2020). The mechanisms seem to be dependent on the PVAT location/phenotype. In septic thoracic aorta PVAT (brown adipose tissue phenotype), the mechanism is related to an increase in beta-3 adrenergic receptor (known to induce adiponectin and to increase NO production) density and also in NOS-1 expression, leading to an increase in NO production which acts through soluble guanylate cyclase and S-nitrosylation (Awata et al., 2019; Barp et al., 2020). However, in superior mesentery (white adipose tissue phenotype), ROS production probably from mitochondrial dysfunction seems to be the main mechanism of vascular dysfunction in this vessel type during sepsis progression (Barp et al., 2020). These reports shed light on the fact that even the accepted beneficial anti-contractile effect of PVAT may turn against the host, evidencing that PVAT contribution to vascular (dys)function is far more complicated.
The communication between the PVAT and the underlying vasculature occurs bidirectionally, and both NO/ROS and RAS systems contribute to vascular homeostasis and to different cardiovascular diseases. However, confirmation of PVAT as a potential target to improve vascular function still demands more studies to understand the mechanisms involved and then enabling new therapeutic approaches.
Discussion
During last decades, important knowledge about the structure and function of PVAT in cardiovascular maintenance and disease was achieved but there are still many unanswered points such as:
-
(a)
What are the most relevant mechanisms of crosstalk between PVAT and the endothelium?
-
(b)
What are and where are the sensor mechanisms that trigger PVAT anti-contractile and pro-contractile effects?
-
(c)
What is the contribution of NO, ROS, and RAS to the divergent roles of PVAT (e.g., pro- and anti-contractile)?
The real beneficial and harmful effects of PVAT in the vascular systems are far from being fully comprehended. A short compilation (Table 1) taken only from works cited here clearly shows that the variety of protocols, vessel types, stimulus, and species make difficult to see a clear pattern of PVAT influences in vascular physiology and pathology. In particular, the “dual” role of PVAT cannot be simply ascribed to its anti- and pro-contractile effects as each of these effects can be called upon in both physiological and pathological situations. However, and notwithstanding the lack of several crucial information on the mechanisms, PVAT is a putative target for the treatment of cardiovascular diseases.
TABLE 1.
Type of vessel containing PVAT, observed effect, type of stimulus, source of vessels and/or PVAT, and condition or model in which the study was conducted.
| References | Vessel(s) | Effect | Stimulus | Species | Condition/model |
| Agabiti-Rosei et al., 2014 | MA | AC | NE | Mouse | Obesity |
| Awata et al., 2019 | TA | AC | Phe; 5-HT | Rat | Sepsis |
| Baltieri et al., 2018 | TA | AC | ACh; insulin | Mouse | Hypercholesterolemia |
| Barp et al., 2020 | TA; MA | AC | NE | Rat | Sepsis |
| Bussey et al., 2018 | MA | AC | NE | Rat | Physiological |
| Costa et al., 2016 | TA | AC | Phe | Rat | Physiological |
| DeVallance et al., 2018 | TA | PC | ACh; Phe | Rat | Metabolic syndrome |
| Fontes et al., 2020 | TA | PC | Phe | Rat | Heart failure |
| Friederich-Persson et al., 2017 | MA | AC | NE | Mouse | Physiological |
| Gálvez-Prieto et al., 2012 | Aorta | AC | AII | Rat | Hypertension |
| Gao et al., 2005 | TA | AC | U4; Phe | Human | Coronary artery disease |
| Gao et al., 2006 | MA | PC | EFS | Rat | Physiological |
| Gao et al., 2007 | Aorta | AC | Phe; 5-HT | Rat | Physiological |
| Gil-Ortega et al., 2014 | MA | AC | NE | Mouse | Obesity |
| Greenstein et al., 2009 | Sm. arteries | AC | NE | Human | Obesity |
| Greenstein et al., 2009 | MA | AC | NE | Rat | Obesity |
| Ketonen et al., 2010 | Abd. Aorta | PC | Phe | Mouse | Obesity |
| Lee et al., 2009 | Aorta | AC | Phe | Rat | Physiological |
| Lee et al., 2011 | TA | AC | Phe | Mouse | Physiological |
| Lohn et al., 2002 | Aorta | AC | Phe; AII; 5-HT | Rat | Physiological |
| Lu et al., 2010 | MA | PC | EFS; AII | Rat | Physiological |
| Lu et al., 2011a | Aorta | AC | Phe | Rat | Hypertension |
| Lu et al., 2011b | IVC | AC | Phe; U4; 5-HT | Rat | Physiological |
| Lynch et al., 2013 | MA | AC | NE | Mouse | Physiological |
| Marchesi et al., 2009 | MA | AC | NE | Mouse | Metabolic syndrome |
| Margaritis et al., 2013 | SV; IMA | AC | ACh; SNP | Human | Coronary artery disease |
| Nobrega et al., 2019 | TA | AC | Phe | Mouse | Physiological |
| Rosei et al., 2015 | MA | AC | NE | Rat | Hypoxia |
| Soltis and Cassis, 1991 | TA | AC | NE | Rat | Physiological |
| Victorio et al., 2016 | TA | AC | Phe | Rat | Physiological |
| Withers et al., 2011 | MA | AC | NE | Mouse | Inflammation |
| Withers et al., 2014 | MA | AC | Phe | Mouse | Physiological/hypoxia |
MA, mesenteric artery; TA, thoracic aorta or thoracic arteries, in human case; Sm. Arteries, small arteries; Abd. Aorta, abdominal aorta; IVC, inferior vena cava; SV, saphenous vein; IMA, internal mammary artery; AC, anti-contractile; PC, pro-contractile; NE, norepinephrine; Phe, phenylephrine; 5-HT, serotonin; Ach, acetylcholine; AII, angiotensin II; EFS, electrical field stimulation; SNP, sodium nitroprusside; U4, U46619.
Author Contributions
CB, DB, and JA wrote the manuscript. All authors equally contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by CNPq (Conselho Nacional de Desenvolvimento Científico e Tecnológico; Grant 306082/2014-4 to JA), CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior; Grant 1966/2016 to JA and Grant 1424569 to DB), and FAPEMIG (Fundação de Amparo à Pesquisa do Estado de Minas Gerais; Grant CBB-APQ0202014 to DB), Brazil.
References
- Agabiti-Rosei C., De Ciuceis C., Rossini C., Porteri E., Rodella L. F., Withers S. B., et al. (2014). Anticontractile activity of perivascular fat in obese mice and the effect of long-term treatment with melatonin. J. Hypertens 32 1264–1274. 10.1097/hjh.0000000000000178 [DOI] [PubMed] [Google Scholar]
- Araujo A. V., Ferezin C. Z., Rodrigues G. J., Lunardi C. N., Vercesi J. A., Grando M. D., et al. (2011). Prostacyclin, not only nitric oxide, is a mediator of the vasorelaxation induced by acetylcholine in aortas from rats submitted to cecal ligation and perforation (CLP). Vascul. Pharmacol. 54 44–51. 10.1016/j.vph.2010.12.002 [DOI] [PubMed] [Google Scholar]
- Ardanaz N., Pagano P. J. (2006). Hydrogen peroxide as a paracrine vascular mediator: regulation and signaling leading to dysfunction. Exp. Biol. Med. (Maywood) 231 237–251. 10.1177/153537020623100302 [DOI] [PubMed] [Google Scholar]
- Awata W. M. C., Gonzaga N. A., Borges V. F., Silva C. B. P., Tanus-Santos J. E., Cunha F. Q., et al. (2019). Perivascular adipose tissue contributes to lethal sepsis-induced vasoplegia in rats. Eur. J. Pharmacol. 863:172706. 10.1016/j.ejphar.2019.172706 [DOI] [PubMed] [Google Scholar]
- Ayala-Lopez N., Watts S. W. (2017). New actions of an old friend: perivascular adipose tissue’s adrenergic mechanisms. Br. J. Pharmacol. 174 3454–3465. 10.1111/bph.13663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltieri N., Guizoni D. M., Victorio J. A., Davel A. P. (2018). Protective role of perivascular adipose tissue in endothelial dysfunction and insulin-induced vasodilatation of hypercholesterolemic LDL receptor-deficient mice. Front. Physiol. 9:229. 10.3389/fphys.2018.00229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barp C. G., Benedet P. O., Assreuy J. (2020). Perivascular adipose tissue phenotype and sepsis vascular dysfunction: differential contribution of NO, ROS and beta 3-adrenergic receptor. Life Sci. 254:117819. 10.1016/j.lfs.2020.117819 [DOI] [PubMed] [Google Scholar]
- Beckman J. S. (2009). Understanding peroxynitrite biochemistry and its potential for treating human diseases. Arch. Biochem. Biophys. 484 114–116. 10.1016/j.abb.2009.03.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown N. K., Zhou Z., Zhang J., Zeng R., Wu J., Eitzman D. T., et al. (2014). Perivascular adipose tissue in vascular function and disease: a review of current research and animal models. Arterioscler. Thromb. Vasc. Biol. 34 1621–1630. 10.1161/atvbaha.114.303029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussey C. E., Withers S. B., Saxton S. N., Bodagh N., Aldous R. G., Heagerty A. M. (2018). beta3 -Adrenoceptor stimulation of perivascular adipocytes leads to increased fat cell-derived NO and vascular relaxation in small arteries. Br. J. Pharmacol. 175 3685–3698. 10.1111/bph.14433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagnole-Santos M. J., Diz D. I., Santos R. A., Khosla M. C., Brosnihan K. B., Ferrario C. M. (1989). Cardiovascular effects of angiotensin-(1-7) injected into the dorsal medulla of rats. Am. J. Physiol. 257 H324–H329. [DOI] [PubMed] [Google Scholar]
- Cassis L. A., Lynch K. R., Peach M. J. (1988). Localization of angiotensinogen messenger RNA in rat aorta. Circ. Res. 62 1259–1262. 10.1161/01.res.62.6.1259 [DOI] [PubMed] [Google Scholar]
- Chang L., Garcia-Barrio M. T., Chen Y. E. (2020). Perivascular adipose tissue regulates vascular function by targeting vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 40 1094–1109. 10.1161/atvbaha.120.312464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee T. K., Stoll L. L., Denning G. M., Harrelson A., Blomkalns A. L., Idelman G., et al. (2009). Proinflammatory phenotype of perivascular adipocytes: influence of high-fat feeding. Circ. Res. 104 541–549. 10.1161/circresaha.108.182998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H., Montagnani M., Funahashi T., Shimomura I., Quon M. J. (2003). Adiponectin stimulates production of nitric oxide in vascular endothelial cells. J. Biol. Chem. 278 45021–45026. 10.1074/jbc.m307878200 [DOI] [PubMed] [Google Scholar]
- Costa R. M., Filgueira F. P., Tostes R. C., Carvalho M. H., Akamine E. H., Lobato N. S. (2016). H2O2 generated from mitochondrial electron transport chain in thoracic perivascular adipose tissue is crucial for modulation of vascular smooth muscle contraction. Vascul. Pharmacol. 84 28–37. 10.1016/j.vph.2016.05.008 [DOI] [PubMed] [Google Scholar]
- Daiber A., Xia N., Steven S., Oelze M., Hanf A., Kroller-Schon S., et al. (2019). New Therapeutic Implications of Endothelial Nitric Oxide Synthase (eNOS) Function/Dysfunction in Cardiovascular Disease. Int. J. Mol. Sci. 20:187. 10.3390/ijms20010187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeVallance E., Branyan K. W., Lemaster K., Olfert I. M., Smith D. M., Pistilli E. E., et al. (2018). Aortic dysfunction in metabolic syndrome mediated by perivascular adipose tissue TNFalpha- and NOX2-dependent pathway. Exp. Physiol. 103 590–603. 10.1113/ep086818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoghue M., Hsieh F., Baronas E., Godbout K., Gosselin M., Stagliano N., et al. (2000). A novel angiotensin-converting enzyme-related carboxypeptidase (ACE2) converts angiotensin I to angiotensin 1-9. Circ. Res. 87 E1–E9. [DOI] [PubMed] [Google Scholar]
- Fernandez-Alfonso M. S., Somoza B., Tsvetkov D., Kuczmanski A., Dashwood M., Gil-Ortega M. (2017). Role of perivascular adipose tissue in health and disease. Compr. Physiol. 8 23–59. 10.1002/cphy.c170004 [DOI] [PubMed] [Google Scholar]
- Ferreira A. J., Castro C. H., Guatimosim S., Almeida P. W., Gomes E. R., Dias-Peixoto M. F., et al. (2010). Attenuation of isoproterenol-induced cardiac fibrosis in transgenic rats harboring an angiotensin-(1-7)-producing fusion protein in the heart. Ther. Adv. Cardiovasc. Dis. 4 83–96. 10.1177/1753944709353426 [DOI] [PubMed] [Google Scholar]
- Fontes M. T., Paula S. M., Lino C. A., Senger N., Couto G. K., Barreto-Chaves M. L. M., et al. (2020). Renin-angiotensin system overactivation in perivascular adipose tissue contributes to vascular dysfunction in heart failure. Clin. Sci. (Lond) 134 3195–3211. 10.1042/cs20201099 [DOI] [PubMed] [Google Scholar]
- Fridovich I. (1995). Superoxide radical and superoxide dismutases. Annu. Rev. Biochem. 64 97–112. 10.1146/annurev.bi.64.070195.000525 [DOI] [PubMed] [Google Scholar]
- Friederich-Persson M., Nguyen Dinh Cat A., Persson P., Montezano A. C., Touyz R. M. (2017). Brown adipose tissue regulates small artery function through NADPH oxidase 4-Derived hydrogen peroxide and redox-sensitive protein kinase G-1alpha. Arterioscler. Thromb. Vasc. Biol. 37 455–465. 10.1161/atvbaha.116.308659 [DOI] [PubMed] [Google Scholar]
- Furuhashi M., Ura N., Higashiura K., Murakami H., Tanaka M., Moniwa N., et al. (2003). Blockade of the renin-angiotensin system increases adiponectin concentrations in patients with essential hypertension. Hypertension 42 76–81. 10.1161/01.hyp.0000078490.59735.6e [DOI] [PubMed] [Google Scholar]
- Gálvez-Prieto B., Bolbrinker J., Stucchi P., De Las Heras A. I., Merino B., Arribas S., et al. (2008). Comparative expression analysis of the renin-angiotensin system components between white and brown perivascular adipose tissue. J. Endocrinol. 197 55–64. 10.1677/joe-07-0284 [DOI] [PubMed] [Google Scholar]
- Gálvez-Prieto B., Somoza B., Gil-Ortega M., Garcia-Prieto C. F., De Las Heras A. I., Gonzalez M. C., et al. (2012). Anticontractile effect of perivascular adipose tissue and leptin are reduced in hypertension. Front. Pharmacol. 3:103. 10.3389/fphar.2012.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. J., Lu C., Su L. Y., Sharma A. M., Lee R. M. (2007). Modulation of vascular function by perivascular adipose tissue: the role of endothelium and hydrogen peroxide. Br. J. Pharmacol. 151 323–331. 10.1038/sj.bjp.0707228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao Y. J., Takemori K., Su L. Y., An W. S., Lu C., Sharma A. M., et al. (2006). Perivascular adipose tissue promotes vasoconstriction: the role of superoxide anion. Cardiovasc. Res. 71 363–373. 10.1016/j.cardiores.2006.03.013 [DOI] [PubMed] [Google Scholar]
- Gao Y. J., Zeng Z. H., Teoh K., Sharma A. M., Abouzahr L., Cybulsky I., et al. (2005). Perivascular adipose tissue modulates vascular function in the human internal thoracic artery. J. Thorac. Cardiovasc. Surg. 130 1130–1136. 10.1016/j.jtcvs.2005.05.028 [DOI] [PubMed] [Google Scholar]
- Garcia-Redondo A. B., Aguado A., Briones A. M., Salaices M. (2016). NADPH oxidases and vascular remodeling in cardiovascular diseases. Pharmacol. Res. 114 110–120. 10.1016/j.phrs.2016.10.015 [DOI] [PubMed] [Google Scholar]
- Gil-Ortega M., Condezo-Hoyos L., Garcia-Prieto C. F., Arribas S. M., Gonzalez M. C., Aranguez I., et al. (2014). Imbalance between pro and anti-oxidant mechanisms in perivascular adipose tissue aggravates long-term high-fat diet-derived endothelial dysfunction. PLoS One 9:e95312. 10.1371/journal.pone.0095312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein A. S., Khavandi K., Withers S. B., Sonoyama K., Clancy O., Jeziorska M., et al. (2009). Local inflammation and hypoxia abolish the protective anticontractile properties of perivascular fat in obese patients. Circulation 119 1661–1670. 10.1161/circulationaha.108.821181 [DOI] [PubMed] [Google Scholar]
- Irie D., Kawahito H., Wakana N., Kato T., Kishida S., Kikai M., et al. (2015). Transplantation of periaortic adipose tissue from angiotensin receptor blocker-treated mice markedly ameliorates atherosclerosis development in apoE-/- mice. J. Renin Angiotensin Aldosterone Syst. 16 67–78. 10.1177/1470320314552434 [DOI] [PubMed] [Google Scholar]
- Ketonen J., Shi J., Martonen E., Mervaala E. (2010). Periadventitial adipose tissue promotes endothelial dysfunction via oxidative stress in diet-induced obese C57Bl/6 mice. Circ. J. 74 1479–1487. 10.1253/circj.cj-09-0661 [DOI] [PubMed] [Google Scholar]
- Krylatov A. V., Maslov L. N., Voronkov N. S., Boshchenko A. A., Popov S. V., Gomez L., et al. (2018). Reactive oxygen species as intracellular signaling molecules in the cardiovascular system. Curr. Cardiol. Rev. 14 290–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R. M., Bader M., Alenina N., Santos R. A., Gao Y. J., Lu C. (2011). Mas receptors in modulating relaxation induced by perivascular adipose tissue. Life Sci. 89 467–472. 10.1016/j.lfs.2011.07.016 [DOI] [PubMed] [Google Scholar]
- Lee R. M., Lu C., Su L. Y., Gao Y. J. (2009). Endothelium-dependent relaxation factor released by perivascular adipose tissue. J. Hypertens 27 782–790. 10.1097/hjh.0b013e328324ed86 [DOI] [PubMed] [Google Scholar]
- Li C., Wang Z., Wang C., Ma Q., Zhao Y. (2015). Perivascular adipose tissue-derived adiponectin inhibits collar-induced carotid atherosclerosis by promoting macrophage autophagy. PLoS One 10:e0124031. 10.1371/journal.pone.0124031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohn M., Dubrovska G., Lauterbach B., Luft F. C., Gollasch M., Sharma A. M. (2002). Periadventitial fat releases a vascular relaxing factor. FASEB J. 16 1057–1063. 10.1096/fj.02-0024com [DOI] [PubMed] [Google Scholar]
- Lu C., Su L. Y., Lee R. M., Gao Y. J. (2010). Mechanisms for perivascular adipose tissue-mediated potentiation of vascular contraction to perivascular neuronal stimulation: the role of adipocyte-derived angiotensin II. Eur. J. Pharmacol. 634 107–112. 10.1016/j.ejphar.2010.02.006 [DOI] [PubMed] [Google Scholar]
- Lu C., Su L. Y., Lee R. M., Gao Y. J. (2011a). Alterations in perivascular adipose tissue structure and function in hypertension. Eur. J. Pharmacol. 656 68–73. 10.1016/j.ejphar.2011.01.023 [DOI] [PubMed] [Google Scholar]
- Lu C., Zhao A. X., Gao Y. J., Lee R. M. (2011b). Modulation of vein function by perivascular adipose tissue. Eur. J. Pharmacol. 657 111–116. 10.1016/j.ejphar.2010.12.028 [DOI] [PubMed] [Google Scholar]
- Lynch F. M., Withers S. B., Yao Z., Werner M. E., Edwards G., Weston A. H., et al. (2013). Perivascular adipose tissue-derived adiponectin activates BK(Ca) channels to induce anticontractile responses. Am. J. Physiol. Heart. Circ. Physiol. 304 H786–H795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Man A. W. C., Zhou Y., Xia N., Li H. (2020). Perivascular adipose tissue as a target for antioxidant therapy for cardiovascular complications. Antioxidants (Basel) 9:574. 10.3390/antiox9070574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manka D., Chatterjee T. K., Stoll L. L., Basford J. E., Konaniah E. S., Srinivasan R., et al. (2014). Transplanted perivascular adipose tissue accelerates injury-induced neointimal hyperplasia: role of monocyte chemoattractant protein-1. Arterioscler. Thromb. Vasc. Biol. 34 1723–1730. 10.1161/atvbaha.114.303983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchesi C., Ebrahimian T., Angulo O., Paradis P., Schiffrin E. L. (2009). Endothelial nitric oxide synthase uncoupling and perivascular adipose oxidative stress and inflammation contribute to vascular dysfunction in a rodent model of metabolic syndrome. Hypertension 54 1384–1392. 10.1161/hypertensionaha.109.138305 [DOI] [PubMed] [Google Scholar]
- Margaritis M., Antonopoulos A. S., Digby J., Lee R., Reilly S., Coutinho P., et al. (2013). Interactions between vascular wall and perivascular adipose tissue reveal novel roles for adiponectin in the regulation of endothelial nitric oxide synthase function in human vessels. Circulation 127 2209–2221. 10.1161/circulationaha.112.001133 [DOI] [PubMed] [Google Scholar]
- Matsuzawa Y. (2006). Therapy Insight: adipocytokines in metabolic syndrome and related cardiovascular disease. Nat. Clin. Pract. Cardiovasc. Med. 3 35–42. 10.1038/ncpcardio0380 [DOI] [PubMed] [Google Scholar]
- Mercure C., Yogi A., Callera G. E., Aranha A. B., Bader M., Ferreira A. J., et al. (2008). Angiotensin(1-7) blunts hypertensive cardiac remodeling by a direct effect on the heart. Circ. Res. 103 1319–1326. 10.1161/circresaha.108.184911 [DOI] [PubMed] [Google Scholar]
- Nava E., Llorens S. (2019). The local regulation of vascular function: from an inside-outside to an outside-inside model. Front. Physiol. 10:729. 10.3389/fphys.2019.00729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nobrega N., Araujo N. F., Reis D., Facine L. M., Miranda C. A. S., Mota G. C., et al. (2019). Hydrogen peroxide and nitric oxide induce anticontractile effect of perivascular adipose tissue via renin angiotensin system activation. Nitric. Oxide 84 50–59. 10.1016/j.niox.2018.12.011 [DOI] [PubMed] [Google Scholar]
- Nosalski R., Guzik T. J. (2017). Perivascular adipose tissue inflammation in vascular disease. Br. J. Pharmacol. 174 3496–3513. 10.1111/bph.13705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padilla J., Jenkins N. T., Vieira-Potter V. J., Laughlin M. H. (2013). Divergent phenotype of rat thoracic and abdominal perivascular adipose tissues. Am. J. Physiol. Regul. Integr. Comp. Physiol. 304 R543–R552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quesada I., Cejas J., Garcia R., Cannizzo B., Redondo A., Castro C. (2018). Vascular dysfunction elicited by a cross talk between periaortic adipose tissue and the vascular wall is reversed by pioglitazone. Cardiovasc. Ther. 36 e12322. 10.1111/1755-5922.12322 [DOI] [PubMed] [Google Scholar]
- Ribiere C., Jaubert A. M., Gaudiot N., Sabourault D., Marcus M. L., Boucher J. L., et al. (1996). White adipose tissue nitric oxide synthase: a potential source for NO production. Biochem. Biophys. Res. Commun. 222 706–712. 10.1006/bbrc.1996.0824 [DOI] [PubMed] [Google Scholar]
- Rosei C. A., Withers S. B., Belcaid L., De Ciuceis C., Rizzoni D., Heagerty A. M. (2015). Blockade of the renin-angiotensin system in small arteries and anticontractile function of perivascular adipose tissue. J. Hypertens 33 1039–1045. 10.1097/hjh.0000000000000506 [DOI] [PubMed] [Google Scholar]
- Sakaue T., Suzuki J., Hamaguchi M., Suehiro C., Tanino A., Nagao T., et al. (2017). Perivascular adipose tissue angiotensin II type 1 receptor promotes vascular inflammation and aneurysm formation. Hypertension 70 780–789. 10.1161/hypertensionaha.117.09512 [DOI] [PubMed] [Google Scholar]
- Santiago N. M., Guimaraes P. S., Sirvente R. A., Oliveira L. A., Irigoyen M. C., Santos R. A., et al. (2010). Lifetime overproduction of circulating Angiotensin-(1-7) attenuates deoxycorticosterone acetate-salt hypertension-induced cardiac dysfunction and remodeling. Hypertension 55 889–896. 10.1161/hypertensionaha.110.149815 [DOI] [PubMed] [Google Scholar]
- Santos R. A., Ferreira A. J., Nadu A. P., Braga A. N., De Almeida A. P., Campagnole-Santos M. J., et al. (2004). Expression of an angiotensin-(1-7)-producing fusion protein produces cardioprotective effects in rats. Physiol. Genom. 17 292–299. 10.1152/physiolgenomics.00227.2003 [DOI] [PubMed] [Google Scholar]
- Santos R. A., Haibara A. S., Campagnole-Santos M. J., Simoes E., Silva A. C., Paula R. D., et al. (2003a). Characterization of a new selective antagonist for angiotensin-(1-7), D-pro7-angiotensin-(1-7). Hypertension 41 737–743. 10.1161/01.hyp.0000052947.60363.24 [DOI] [PubMed] [Google Scholar]
- Santos R. A., Simoes E., Silva A. C., Maric C., Silva D. M., Machado R. P., et al. (2003b). Angiotensin-(1-7) is an endogenous ligand for the G protein-coupled receptor Mas. Proc. Natl. Acad. Sci. U.S.A. 100 8258–8263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxton S. N., Clark B. J., Withers S. B., Eringa E. C., Heagerty A. M. (2019). Mechanistic links between obesity, diabetes, and blood pressure: role of perivascular adipose tissue. Physiol. Rev. 99 1701–1763. 10.1152/physrev.00034.2018 [DOI] [PubMed] [Google Scholar]
- Schiavone M. T., Santos R. A., Brosnihan K. B., Khosla M. C., Ferrario C. M. (1988). Release of vasopressin from the rat hypothalamo-neurohypophysial system by angiotensin-(1-7) heptapeptide. Proc. Natl. Acad. Sci. U.S.A. 85 4095–4098. 10.1073/pnas.85.11.4095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skiba D. S., Nosalski R., Mikolajczyk T. P., Siedlinski M., Rios F. J., Montezano A. C., et al. (2017). Anti-atherosclerotic effect of the angiotensin 1-7 mimetic AVE0991 is mediated by inhibition of perivascular and plaque inflammation in early atherosclerosis. Br. J. Pharmacol. 174 4055–4069. 10.1111/bph.13685 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltis E. E., Cassis L. A. (1991). Influence of perivascular adipose tissue on rat aortic smooth muscle responsiveness. Clin. Exp. Hypertens A 13 277–296. 10.3109/10641969109042063 [DOI] [PubMed] [Google Scholar]
- Tian Z., Miyata K., Tazume H., Sakaguchi H., Kadomatsu T., Horio E., et al. (2013). Perivascular adipose tissue-secreted angiopoietin-like protein 2 (Angptl2) accelerates neointimal hyperplasia after endovascular injury. J. Mol. Cell Cardiol. 57 1–12. 10.1016/j.yjmcc.2013.01.004 [DOI] [PubMed] [Google Scholar]
- Tipnis S. R., Hooper N. M., Hyde R., Karran E., Christie G., Turner A. J. (2000). A human homolog of angiotensin-converting enzyme. Cloning and functional expression as a captopril-insensitive carboxypeptidase. J. Biol. Chem. 275 33238–33243. [DOI] [PubMed] [Google Scholar]
- Vasquez-Vivar J., Kalyanaraman B., Martasek P. (2003). The role of tetrahydrobiopterin in superoxide generation from eNOS: enzymology and physiological implications. Free Radic. Res. 37 121–127. 10.1080/1071576021000040655 [DOI] [PubMed] [Google Scholar]
- Vecchione C., Maffei A., Colella S., Aretini A., Poulet R., Frati G., et al. (2002). Leptin effect on endothelial nitric oxide is mediated through Akt-endothelial nitric oxide synthase phosphorylation pathway. Diabetes 51 168–173. 10.2337/diabetes.51.1.168 [DOI] [PubMed] [Google Scholar]
- Victorio J. A., Davel A. P. (2020). Perivascular adipose tissue oxidative stress on the pathophysiology of cardiometabolic diseases. Curr. Hypertens Rev. 16 192–200. 10.2174/1573402115666190410153634 [DOI] [PubMed] [Google Scholar]
- Victorio J. A., Fontes M. T., Rossoni L. V., Davel A. P. (2016). Different anti-contractile function and nitric oxide production of thoracic and abdominal perivascular adipose tissues. Front. Physiol. 7:295. 10.3389/fphys.2016.00295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viedt C., Vogel J., Athanasiou T., Shen W., Orth S. R., Kubler W., et al. (2002). Monocyte chemoattractant protein-1 induces proliferation and interleukin-6 production in human smooth muscle cells by differential activation of nuclear factor-kappaB and activator protein-1. Arterioscler. Thromb. Vasc. Biol. 22 914–920. 10.1161/01.atv.0000019009.73586.7f [DOI] [PubMed] [Google Scholar]
- Wang Y., Lam K. S., Xu J. Y., Lu G., Xu L. Y., Cooper G. J., et al. (2005). Adiponectin inhibits cell proliferation by interacting with several growth factors in an oligomerization-dependent manner. J. Biol. Chem. 280 18341–18347. 10.1074/jbc.m501149200 [DOI] [PubMed] [Google Scholar]
- Withers S. B., Agabiti-Rosei C., Livingstone D. M., Little M. C., Aslam R., Malik R. A., et al. (2011). Macrophage activation is responsible for loss of anticontractile function in inflamed perivascular fat. Arterioscler. Thromb. Vasc. Biol. 31 908–913. 10.1161/atvbaha.110.221705 [DOI] [PubMed] [Google Scholar]
- Withers S. B., Simpson L., Fattah S., Werner M. E., Heagerty A. M. (2014). cGMP-dependent protein kinase (PKG) mediates the anticontractile capacity of perivascular adipose tissue. Cardiovasc. Res. 101 130–137. 10.1093/cvr/cvt229 [DOI] [PubMed] [Google Scholar]
- Xia N., Horke S., Habermeier A., Closs E. I., Reifenberg G., Gericke A., et al. (2016). Uncoupling of Endothelial Nitric Oxide Synthase in Perivascular Adipose Tissue of Diet-Induced Obese Mice. Arterioscler. Thromb. Vasc. Biol. 36 78–85. 10.1161/atvbaha.115.306263 [DOI] [PubMed] [Google Scholar]
- Xia N., Li H. (2017). The role of perivascular adipose tissue in obesity-induced vascular dysfunction. Br. J. Pharmacol. 174 3425–3442. 10.1111/bph.13650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaborska K. E., Wareing M., Austin C. (2017). Comparisons between perivascular adipose tissue and the endothelium in their modulation of vascular tone. Br. J. Pharmacol. 174 3388–3397. 10.1111/bph.13648 [DOI] [PMC free article] [PubMed] [Google Scholar]