1. INTRODUCTION
Mixed-phenotype acute leukemia (MPAL) is a rare and heterogeneous subtype of leukemias, comprising about 1.5-5.8% of acute leukemia [1], [2], [3], [4]. MPAL can present as one blast population expressing different lineage specific markers or two distinct blast populations each of a different lineage. MPAL is often challenging to diagnose and mange, and the current diagnosis of MPAL mainly relies on morphology and immunophenotyping analysis by Flow cytometry. The identification of Auer rods in the blasts is traditionally considered as one of morphological features of myeloblasts and is diagnostic for acute myeloid leukemia. In this study, we describ 3 cases of MPAL with Auer rods identified in blasts and their clinicopathological features are discussed.
2. CASE REPORT
Case #1 was a 24-year-old woman with a 2-month history of weakness, skin rash (abdomen and face) and splenomegaly (17.8cm in length and 6.1cm in width measured by ultrasonography). Complete blood count showed pancytopenia with hemoglobin of 67 g/L, red cell count of 2.05 × 1012/L, platelet count of 102 × 109/L, and white blood cell count of 1.89 × 109/L. Peripheral blood smears revealed 67% circulating blasts. Bone marrow smears showed a hypercellular bone marrow with two types of blasts (Figure A): one blast population (45% of total cells) was large with moderate to abundant basophilic cytoplasm, sparse to numerous perinuclear azurophilic granules and frequent cytoplasmic vacuolation. Notably, Auer rods that appeared as single sharp rods were found in a subset of blasts (Figure B). Another blast population (42% of total cells) was small to medium sized with scant agranular cytoplasm. Cytochemical stain for myeloperoxidase (MPO) was positive in 9% blasts, among which Auer rods were highlighted. The two distinct blast populations were also confirmed by flow cytometric analysis which showed two populations with a different immunophenotype: one blast population showed a myeloid phenotype, uniformly positive for CD33, CD34, HLA-DR, CD123, and CD11c, dimly positive for CD38, CD7, CD13, CD15, MPO, CD117, and CD64. They did not express cytoCD3, CD4, CD10, CD11b, CD14, CD19, CD56, cCD79a, CD300e and lysozyme. The second blast population showed a T-cell immunophenotype with high expression of CD2, cytoCD3, CD7, CD10, CD34, CD38, and CD123, dim expression of HLA-DR, CD33, CD13, CD5, and CD99. Other markers including sCS3, MPO, CD4, CD1a, CD8 and CD16 were negative. Cytogenetic analysis by G- and R-banding showed 46, XX [20]. No specific fusion genes were detected. Fluorescence in situ hybridization (FISH) was negative for KMT2A/MLL gene rearrangement and P53 gene deletion. Next generation sequencing (NGS) showed mutations in RELN Exon55 p.R2955C (variant allele frequency, VAF, 48.79%), TET2 Exon3 p.Q324H (VAF, 6.81%) and TP53 Exon9 p.N331D (VAF, 8.02%). Skin biopsy of abdominal erythema showed infiltration of T lymphoblasts, consistent with leukemia cutis. The patient was diagnosed as MPAL, T/myeloid with skin involvement. She was treated with VDCP+ Ara-C regimen (Vincristine, Daunorubicin, Cyclophosphamide, Prednisone, Cytarabine) with a poor response.
Figure 1.
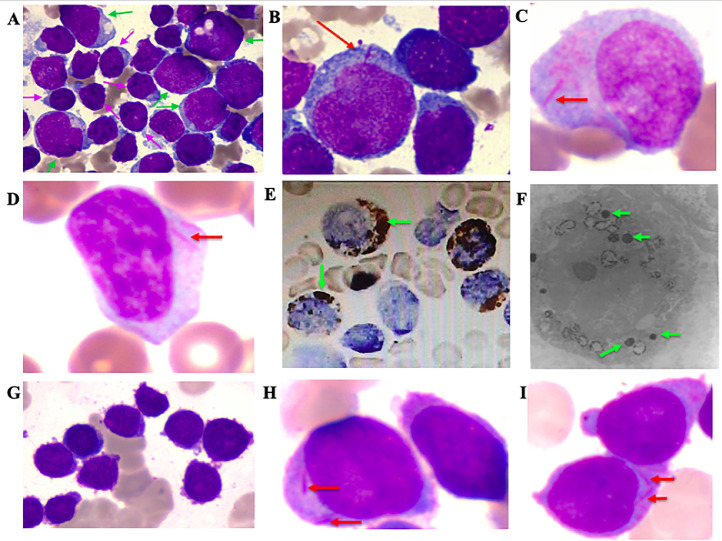
The morphologic features of blasts in cases of MPAL with Auer rods. A: There are two blast populations from case #1: the first population is large sized (green arrow) with cytoplasmic granules. The second population is small to medium sized with scanty cytoplasm without granules (pink arrow). B: Auer rods are identified in the first blast population (red arrow) in case #1. C-D: Some blasts have distinct Auer rods (red arrow) in the bone marrow and peripheral blood smears respectively (Wright-Giemsa stain) from case #2. E: Cytochemical stains demonstrate strong myeloperoxidase activity among these blasts (green arrow) in case #2. F: Myeloperoxidase granules are identified in the blast cells from case #2 by electronic microscopy (green arrow). G: Blasts in case #3 are intermediate to large sized with round nuclei, dispersed to variably condensed chromatin and small amounts of cytoplasm. H-I: Auer rods are identified in a subset of blasts in Case #3.
Case #2 was a 34-years-old man with a reported history of T-lymphoblastic leukemia (T-ALL) diagnosed 15 months ago at an outside hospital. Fifteen months ago, he presented with “fever and coughing” and complete blood count showed leukocytosis with a white blood cell count of 100 × 109/L. Bone marrow study showed T-ALL with blasts positive for cytoCD3, CD5, CD7, CD8, CD13, CD33, CD34, CD38, HLA-DR, TdT and CD56 (partial). Cytogenetic karyotype was 46, XY [20]. He was diagnosed with T-ALL and treated with Hyper-CVAD regimen. The patient did not respond to the therapy and then switched to HD-MTX +Ara-C regimen and achieved morphological remission with 3% blast in bone marrow. He then received consolidation therapy composed of EA, TA, HD-MTX, VDCLP regimens (EA: Etoposide + Cytarabine, TA: Pirarubicin + Cytarabine, HD-MTX: high-dose Methotrexate, VDCLP: Vincristine, Daunorubicin, Cyclophosphamide, L-asparaginase, Prednisone). Nine months after the initial diagnosis, the patient had relapsed disease and treated with VCP,MA and EA regimens (VCP: Vincristine, Cyclophosphamide, Prednisone, MA: Mitoxantrone + Cytarabine, EA: Etoposide +Cytarabine) with a limited response. He was transferred to our hospital for further management. Physical examination showed multiple superficial lymphadenopathy. Bone marrow and peripheral blood smears showed numerous blasts that were intermediate or large sized with obvious Auer rods in a subset of blasts (Figure C-D). Immunophenotyping by flow cytometry showed one blast population, positive for cytoCD3, CD7, CD123, CD33, CD34, CD38, HLA-DR, TdT, CD56, CD13, and CD11b, negative for CD2, CD4, CD8, CD5, and MPO. Although MPO was negative by flow cytometric analysis, special cytochemical stain showed 28% blasts positive for MPO (Figure E). To further confirm MPO positivity, electron microscopic study was performed and showed that 38% of blasts were positive for MPO (Figure F). Given that blasts expressed both T (cytoCD3) and myeloid (MPO) lineage defining markers, the diagnosis of MPAL, T/myeloid was rendered. Additional studies showed no BCR/ABL fusion or FLT3-ITD/TKD. No T-cell receptor or immunoglobulin heavy chain rearrangements was detected. The patient was treated with one cycle of HAD+VP regimen (HAD+VP: Homoharringtonine + Cytarabine + Daunorubicin + Vincristine + Prednisone) and showed no response.
Case #3 was a 51-years-old man presented with sore throat and weakness, Peripheral blood showed leukopenia with white blood cell count of 1.55 × 109/L, Bone marrow biopsy and smears showed acute leukemia with 80% blasts. Blasts were intermediate to large sized and about 20% of the blasts were identified to have Auer rods (Figure G-I). Flow cytometric analysis identified two blast populations: one predominant population (66% of nucleated cells) showed a myeloid immunophenotype, positive for CD117, CD34, CD38, HLA-DR, CD13, CD33, MPO, TdT (partial), CD123 (dim) and CD71 (dim), and negative for CD16, CD15, CD64, CD11b, CD56, CD36, CD14, CD19, cCD79a, and cytoCD3. Another minor blast population (5% of nucleated cells) showed a T-cell immunophenotype, positive for CD2 (partial), cytoCD3, CD7, CD34, CD38, HLA-DR, TdT, CD13, CD10, CD99, CD117 (dim) and CD123 (dim), and negative for CD16, CD15, CD33, CD11b, CD56, CD19, cCD79a, MPO, CD1a, CD4, CD8, CD5, and sCD3. Conventional karyotyping analysis showed 46, X, -Y, +mar [11]/46, XY [9]. Molecular study by NGS showed mutations in the following genes: NF1, Exon40 p.R1968X; RUNX1, Exon5 p.K152Gfs*25; NOTCH1, Exon26 p.L1574P; PHF6,Exon4 p.L109Pfs*4. The patient was diagnosed with MPAL, myeloid/T. He had severe sepsis and died before the initiation of chemotherapy.
3. DISCUSSION
In this study, we described 3 cases of MPAL with Auer rods and all were diagnosed as T/myeloid subtype following the criteria proposed in 2016 World Health Organization (WHO) classification and the European Group for Immunological Characterization of Acute Leukemias (EGIL). According to the WHO 2016, MPAL is a subtype of acute leukemia of ambiguous lineage, and its accurate diagnosis is often challenging, mainly relying on morphology and immunophenotyping performance by Flow cytometry. The differential diagnosis for MPAL is broad; AML with t(8;21) often expresses MPO as well as CD19 and other B cell markers such as PAX5, raising a possible diagnosis of MPAL, myeloid/B. The morphology and cytogenetic study to demonstrate RUNX1/RUNX1T1 translocation will clarify the diagnosis. For MPAL, T/myeloid, an important differential diagnosis is Early T-precursor lymphoblastic leukemia (ETP-ALL) as ETP-ALL often expresses myeloid markers. But by definition, ETP-ALL is negative for MPO. Thus positive MPO in blasts will rule out ETP-ALL.
Of these three cases, two (cases #1 and 3) showed two separate blast populations, myeloid and T respectively and Auer rods were identified in the myeloblasts. The remaining case (case #2) showed one blast population expressing both T and myeloid markers. All three cases showed non-complex karyotypes. Mutation analysis by NGS was done in two cases and both showed mutations associated with a poor prognosis. Two patients received induction chemotherapy and neither achieved a complete remission. The management of patients with MPAL is challenging and currently there is no standard treatment regimen for MPAL. Previous studies indicated that acute lymphoblastic leukemia-like therapeutic approach followed by allogeneic stem cell transplant after first remission was favored in these patients [5], [6], [7], [8], but novel therapeutic strategies need to be explored in the future.
The presence of Auer rods in MPAL had been very rarely reported in literatures [9], [10], [11], [12], [13]. Among these reported cases, all were Myeloid/T with the exception of one case being Myeloid/B. The 3 cases described in our study were also myeloid/T subtype. Thus, Auer rods are more commonly seen in myeloid/T of MPAL than myeloid/B subtype. The diagnostic pitfall is that the detection of Auer rods in acute leukemia does not warrant a diagnosis of AML. Careful morphologic evaluation as well as flow cytometric analysis is recommended to rule out MPAL, especially T/myeloid subtype.
Trial registration
There is no trial registration in this study.
Authorship Statement and Disclosures
Yan Li, Wenrui Yang, Huijun Wang, Hui Wei, Ying Wang, Bingcheng Liu, Jianxiang Wang and Yingchang Mi collected the data and wrote the paper. Jigang Xiao took the morphologic pictures of bone marrow smears. Yongxin Ru and Shuxu Dong took the electronic picture. Yan Li, Wenrui Yang and Dong Lin were involved in patient management and clinical data collection. Wei Wang revised the manuscript and contributed valuable advice. All the authors [[1], [2], [3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]] have reviewed and approved the manuscript and do not have any disclosures/conflicts of interest.
Acknowledgments
The authors would like to appreciate the funding of National Key Research and Development Program of China (2019YFC0840605), National Science and Technology Major Project (2017ZX09304024), National Natural Science Foundation of China (81830005, 81770181).
The authors would also like to thank Dr. Huijun Wang for performing flow cytometric analysis.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.lrr.2021.100236.
Contributor Information
Jianxiang Wang, Email: wangjx@ihcams.ac.cn.
Yingchang Mi, Email: ychmi@ihcams.ac.cn.
Appendix. Supplementary materials
References
- 1.Bene MC, Castoldi G, Knapp W, Ludwig WD, Matutes E, Orfao A, van't Veer MB. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL) Leukemia. 1995;9:1783–1786. [PubMed] [Google Scholar]
- 2.Weinberg OK, Arber DA. Mixed-phenotype acute leukemia: historical overview and a new definition. Leukemia. 2010;24:1844–1851. doi: 10.1038/leu.2010.202. [DOI] [PubMed] [Google Scholar]
- 3.Yan L, Ping N, Zhu M, Sun A, Xue Y, Ruan C, Drexler HG, Macleod RA, Wu D, Chen S. Clinical, immunophenotypic, cytogenetic, and molecular genetic features in 117 adult patients with mixed-phenotype acute leukemia defined by WHO-2008 classification. Haematologica. 2012;97:1708–1712. doi: 10.3324/haematol.2012.064485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van den Ancker W, Terwijn M, Westers TM, Merle PA, van Beckhoven E, Dräger AM, Ossenkoppele GJ, van de Loosdrecht AA. Acute leukemias of ambiguous lineage: diagnostic consequences of the WHO2008 classification. Leukemia. 2010;24:1392–1396. doi: 10.1038/leu.2010.119. [DOI] [PubMed] [Google Scholar]
- 5.Lee JH, Min YH, Chung CW, Kim BK, Yoon HJ, Jo DY, Shin HJ, Bang SM, Won JH, Zang DY. Prognostic implications of the immunophenotype in biphenotypic acute leukemia. Leuk Lymphoma. 2008;49:700–709. doi: 10.1080/10428190701843247. [DOI] [PubMed] [Google Scholar]
- 6.Xu XQ, Wang JM, Lü SQ, Chen L, Yang JM, Zhang WP, Song XM, Hou J, Ni X, Qiu HY. Clinical and biological characteristics of adult biphenotypic acute leukemia in comparison with that of acute myeloid leukemia and acute lymphoblastic leukemia: a case series of a Chinese population. Haematologica. 2009;94:919–927. doi: 10.3324/haematol.2008.003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liu QF, Fan ZP, Wu MQ, Sun J, Wu XL, Xu D, Jiang QL, Zhang Y, Huang F, Wei YQ. Allo-HSCT for acute leukemia of ambiguous lineage in adults: the comparison between standard conditioning and intensified conditioning regimens. Ann Hematol. 2013;92:679–687. doi: 10.1007/s00277-012-1662-4. [DOI] [PubMed] [Google Scholar]
- 8.Weir EG, Ali Ansari-Lari M, Batista DA, Griffin CA, Fuller S, Smith BD, Borowitz MJ. Acute bilineal leukemia: a rare disease with poor outcome. Leukemia. 2007;21:2264–2270. doi: 10.1038/sj.leu.2404848. [DOI] [PubMed] [Google Scholar]
- 9.Zucker ML, Plapp FV, Rachel JM, Murphy CA, Bohn BA, Boeschen JL, McCann LM, Peterson JT. An adult case of acute biphenotypic leukemia with characteristic mixed morphology. Mo Med. 1993;90:601–604. [PubMed] [Google Scholar]
- 10.Saragas E, Spector I, Chita G, Dukes IA, Bernstein R, Keene P, Mendelow BV. Acute mixed-lineage leukaemia involving myeloid and T-cell phenotypes. A case report. S Afr Med J. 1987;71:529–530. [PubMed] [Google Scholar]
- 11.Reizenstein P, Beksac M, Biberfeld P, Christensson B, Lagerlöf B, Laurén L, Ost A, Porwit A. Leukemic myeloblasts expressing lymphoid markers. Acta Haematol. 1985;74:148–150. doi: 10.1159/000206191. [DOI] [PubMed] [Google Scholar]
- 12.Cross AH, Goorha RM, Nuss R, Behm FG, Murphy SB, Kalwinsky DK, Raimondi S, Kitchingman GR, Mirro J., Jr Acute myeloid leukemia with T-lymphoid features: a distinct biologic and clinical entity. Blood. 1988;72:579–587. [PubMed] [Google Scholar]
- 13.Ferrara F, Finizio O, Rosa CD, Mele G, Mettivier V, Rametta V, Spada OA, Vecchio LD. Acute Myeloid Leukemia Expressing T-Cell Antigens: Clinico-Hematological Report on Six Cases. Leuk Lymphoma. 1990;3:217–222. doi: 10.3109/10428199009050999. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.