Abstract
Background
The purpose of our study was to develop an online calculator to estimate the effect of docetaxel triplets (DPF) in first line of advanced gastric cancer (AGC), and to assess the external validity of docetaxel trials in individual patients.
Methods
The study includes patients with HER2(-) AGC treated with platin and fluoropyrimidine (PF) or with DPF in first line. Treatment effect and interactions were assessed using Bayesian accelerated failure time models.
Result
The series comprises 1376 patients; 238 treated with DPF and 1138 with PF between 2008 and 2019. DPF was associated with increased progression-free survival (PFS) and overall survival (OS) with time ratio (TR) 1.27 (95% credible interval [CrI], 1.15–1.40), and TR 1.19 (95% CrI, 1.09–1.27), respectively. Serious adverse events were more common with DPF, particularly hematological effects (32% vs 22%). Younger participants received greater DPF dose density without achieving greater disease control, while severe toxicity was likewise higher. DPF yielded superior OS in Lauren intestinal (TR 1.27, 95% CrI, 1.08–1.11) vs diffuse subtype (TR 1.17, 95% CrI, 1.09–1.24) and the probability of increasing OS > 15% was 90% vs 67% in each subtype, respectively. The effect dwindles over time, which can be attributed to pathological changes and clinical practice changes.
Conclusion
Our study confirms the effect of DPF is highly dependent on several clinical–pathological variables, with discreet and gradually declining benefit over platinum doublets in later years, at the expense of increased toxicity. These results may help to underpin the idea that external validity of AGC trials should be revised regularly.
Electronic supplementary material
The online version of this article (10.1007/s10120-020-01116-x) contains supplementary material, which is available to authorized users.
Keywords: Bayesian model, Chemotherapy, Gastric cancer, Docetaxel, Survival
Introduction
External validity or the inferential generalizability of randomized clinical trials (RCTs) refers to the appropriateness of extrapolating trial outcomes from one population to another. It is a complex concept whose determinants are based more on clinical rather than statistical expertise [1] and on factors such as selection criteria, pathological traits, or access to new therapies [2]. Therefore, in light of the ever-changing condition of clinical practice and target populations, this inferential generalizability of past RCTs should be revisited every so often [3–5]. The apparent dwindling effect of docetaxel-containing triple-agent regimens (DPF) in the first-line treatment of advanced gastric cancer (AGC) may be illustrative of this situation.
AGC continues to be a common neoplasm with high mortality, for which palliative chemotherapy is recommended [6]. In 2006, the international TAX325 phase III RCT demonstrated the benefit of docetaxel combined with platin-fluoropyrimidine (DPF) in first line [7]. Nonetheless, the effect on overall survival (OS) of DPF over platin-fluoropyrimidine was modest (median 9.2 vs 8.6 months, hazard ratio (HR) 0.77, P value = 0.02) and was attained at the cost of substantially increased severe toxicity (69% vs 59%), respectively. This discreet benefit profile has diminished in successive RCTs [8, 9]. In this regard, the 2017 update of Wagner’s meta-analysis, with eight comparative studies, lowered the prospect of benefit (HR 0.86, 95% confidence interval (CI) 0.78–0.95) [9]. More recently, adding docetaxel to cisplatin and S-1, appraised in the Japanese JCOG1013 phase III RCT, did not enhance OS (HR 0.99, 95% CI, 0.85–1.16), although it did increase adverse events (e.g., grade 3–4 neutropenia, 59% vs 32%) [8].
This series of results revealing progressively smaller magnitude poses the clinician with a twofold question. The first is whether the effect of docetaxel varies on the basis of time factors, geographical or epidemiological variables. In the interim between these studies, changes in clinical practice have been reported that may have modified the effect. Chief among these changes is the introduction of trastuzumab in tumors that amplify or overexpress human epidermal growth factor receptor-2 (HER2) in 2010 [10]. Given that these neoplasms are no longer treated with docetaxel-based triplet, candidates for DPF include ever since an abundance of diffuse histological subtype—precisely the ones that are most chemo-refractory [10–12]. In addition to this, in 2014, the RAINBOW RCT confirmed the benefit of paclitaxel and ramucirumab in second line, which could dilute the advantage of the docetaxel-based triplet in first line [13]. Thus, clinical guidelines now tend to recommend the dual-agent platin and fluoropyrimidine regimen for HER2-negative AGC, with DPF as an option in fit individuals who require tumor response [14, 15].
The second question is whether there are still any patients who could benefit from the DPF strategy in first line. This is germane, given the paucity of data regarding effect-modifying factors based on key variables such as histopathologic subtype or age [6]. Our study seeks to shed light on both questions. We have, therefore, attempted to reproduce the trends observed in RCTs in a national registry of AGC. Moreover, we have constructed an online calculator that depicts how individual characteristics or clinical–pathological variables have modified the effect that the addition of docetaxel has had on survival endpoints.
Method
Patients and study design
The participants are from the AGAMENON registry in which 34 Spanish and one Chilean center participate and that recruit consecutive cases of locally advanced, unresectable or metastatic adenocarcinoma of the stomach, gastroesophageal junction, or distal esophagus [12, 16–24].
Eligibility criteria for this analysis include being > 18 years of age, cancer that does not overexpress HER2, and first-line treatment with at least one cycle of platin and 5FU (PF) with or without docetaxel [14]. Patients who had completed a prior neoadjuvant or adjuvant treatment in the previous 6 months and those who had received taxanes as part of perioperative schedules were excluded.
The data are managed through a website (http://www.agamenonstudy.com/) that consists of filters and a system of queries to guarantee data reliability and control for missing and inconsistent data, with telephone and online monitoring (PJF).
The study was approved by a multicenter Research Ethics Committee of all the Autonomous Communities and participating hospitals and was classified as a prospective, postmarketing surveillance study by the Spanish Agency of Medicines and Medical Devices (AEMPS), and was not otherwise involved in it. All participants still alive at the time of data collection provided written, signed, informed consent.
Variables
The main endpoint, OS, was defined as the time between treatment initiation and demise from any cause, censoring patients lost to follow-up. Progression-free survival (PFS) was defined as the interval between start of treatment and progression, as per the RECIST 1.1 criteria, death, or last follow-up. Relative dose intensity (RDI) was expressed in percentages and defined as the dose intensity (the amount of drug per unit of time, expressed as mg/m2 weekly) administered with respect to the planned amount for each schedule. To limit confounding bias, nine clinical, pathological, and laboratory confounding factors were selected, on the basis of theoretical criteria and the group’s experience from previous studies [25]. These covariates were performance status (ECOG-PS), Lauren histological classification subtype, histological grade, ascites, stage (III unresectable vs IV), liver tumor burden, type of platin (cisplatin vs oxaliplatin), neutrophil/lymphocyte ratio, age, and year of treatment [26].
To limit the confounding bias, two measures of tumor load have been contemplated: the number of organs involved and liver tumor burden. Patients’ baseline computerized tomographies were re-evaluated by the clinicians and liver tumor burden was categorized as: 0 metastasis, < 25%, 25–50%, 51–75%, and > 75% of liver volume affected by tumor tissue. The number of organs involved was defined as the number of organs (e.g., liver, lung, skeleton, lymph nodes, unresected gastric primary, etc.) affected by the disease, regardless of the number of metastases contained in each organ. Lymph metastases in distant basins were considered different organs.
Statistics
Therapeutic effect was evaluated via a Bayesian parametric accelerated failure time (AFT) model with lognormal distribution; this model assumes that the effect of the covariates is to accelerate or decelerate the course of illness, making them suitable when the assumption of proportional hazards is not met [23]. Its coefficients have an intuitive, direct interpretation in its exponentiated form, as time ratios (TR). Thus, a time ratio equal to 2 for a binary predictor means that the median of time-to-event is doubled in the presence of this variable. With this model, the interaction between DPF, age, histopathologic subtype, and year of treatment was examined. This approach also enables historical external data to be incorporated as priors [27–30]. The prior for the therapeutic effect was based on Wagner’s meta-analysis, for OS ~ N(0.15, 0.045) and for PFS ~ N(0.27, 0.07) [9]. The interactions were appraised skeptically, e.g., ~ N(0, 0.05) for the histological subtype, to discourage subgroup effects deemed extreme [7]. An online calculator was built to obtain the model’s predictions. The trace and density plots for Markov chain Monte Carlo (MCMC) samples denoted adequate convergence (Gelman–Rubin Rhat measure < 1.1 for all the parameters). A frequentist AFT model for OS is also shown in Annex Figure 1 as comparison.
Continuous variables were assessed by restricted cubic splines. Covariates with > 25% missing data were discarded and multiple imputation was applied (fully conditional specification, on 20 imputed datasets) in the rest. The probability of dichotomous outcomes was appraised by logistic regression. All analyses were performed with the R v3.1.6 software package, with the mice, splines, and brms libraries [31–34].
Results
Patients
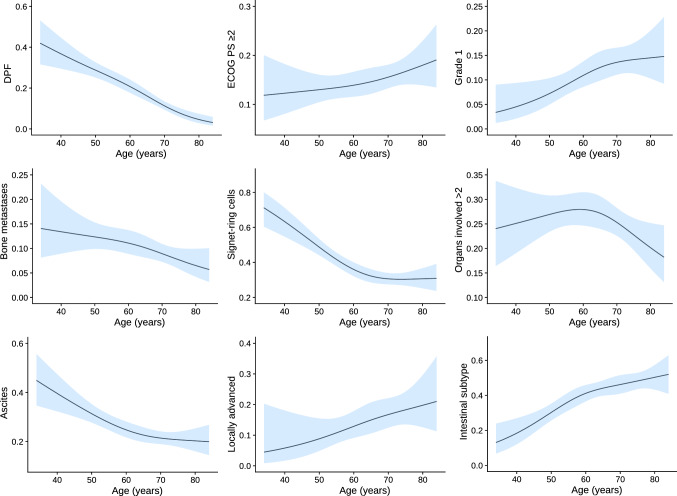
The series comprises 1376 patients; 238 treated with DPF and 1138 with PF between 2008 and 2019. Annex Table 1 details the chemotherapy schedules used. Baseline characteristics are displayed in Table 1. The most salient differences include the fact that patients treated with PF (vs DPF) tend to be older (median 66 vs 58 years), with worse functional status (ECOG-PS ≥ 2, 17% vs 7%), predominance of intestinal subtype (34% vs 26%), and less of a propensity toward peritoneal disease (45% vs 53%). Figure 1 illustrates how younger individuals are more likely to receive DPF and have tumors with distinct clinical–pathological traits (higher percentage of diffuse tumors, signet ring cells, ascites, and greater tumor burden).
Table 1.
Baseline characteristics of patients
| Baseline characteristics | Total, n = 1376 | PF, n = 1138 | DPF, n = 238 |
|---|---|---|---|
| Age, median (range) | 65 (20–89) | 66 (20–89) | 58 (22–88) |
| Sex, female | 450 (33%) | 367 (32%) | 83 (35%) |
| ECOG-PS | |||
| 0 | 282 (20%) | 217 (19%) | 65 (27%) |
| 1 | 889 (65%) | 732 (64%) | 157 (66%) |
| ≥ 2 | 205 (15%) | 189 (17%) | 16 (7%) |
| Primary tumor site | |||
| Esophagus | 114 (9%) | 102 (9%) | 12 (5%) |
| GEJ | 158 (11%) | 132 (12%) | 26 (11%) |
| Stomach | 1104 (80%) | 904 (79%) | 200 (84%) |
| Histological grade | |||
| 1 | 120 (9%) | 110 (10%) | 10 (4%) |
| 2 | 328 (25%) | 337 (29%) | 44 (18%) |
| 3 | 563 (42%) | 450 (40%) | 113 (48%) |
| Not available | 312 (24%) | 241 (21%) | 71 (30%) |
| Lauren classification | |||
| Diffuse | 641 (47%) | 526 (46%) | 115 (49%) |
| Intestinal | 444 (32%) | 383 (34%) | 61 (26%) |
| Not available | 291 (21%) | 229 (20%) | 62 (25%) |
| Signet ring cells | 433 (31%) | 362 (32%) | 71 (30%) |
| Tumor stage at diagnosis, locally advanced unresectable | 59 (4%) | 41 (4%) | 18 (7%) |
| Metastases sites | |||
| Ascites | 344 (25%) | 288 (25%) | 56 (24%) |
| Peritoneal | 634 (46%) | 508 (45%) | 126 (53%) |
| Bone | 137 (10%) | 112 (10%) | 25 (11%) |
| Lung | 167 (12%) | 158 (14%) | 9 (4%) |
| Liver | 486 (35%) | 430 (38%) | 56 (24%) |
| Burden of liver disease > 50% | 238 (17%) | 207 (18%) | 31 (13%) |
| Number of metastatic sites > 2 | 349 (25%) | 291 (26%) | 58 (24%) |
| Platin | |||
| Oxaliplatin | 894 (65%) | 818 (72%) | 76 (32%) |
| Cisplatin | 482 (35%) | 320 (28%) | 162 (68%) |
| Primary tumor resection | 441 (32%) | 360 (32%) | 81 (34%) |
| CEA, ng/ml | |||
| < 5 | 653 (47%) | 521 (46%) | 132 (55%) |
| 5–10 | 131 (10%) | 111 (10%) | 20 (8%) |
| 10–30 | 121 (9%) | 102 (9%) | 29 (12%) |
| > 30 | 232 (17%) | 197 (17%) | 35 (16%) |
| No available | 239 (17%) | 207 (18%) | 22 (9%) |
| Albumin, g/dl | |||
| > 35 g/dl | 921 (67%) | 757 (67%) | 164 (69%) |
| 30–35 | 218 (16%) | 187 (16%) | 31 (13%) |
| < 30 | 114 (8%) | 99 (9%) | 15 (6%) |
| No available | 123 (9%) | 95 (8%) | 28 (12%) |
DPF, docetaxel, platinum, fluoropyrimidine; ECOG-PS, Eastern Cooperative Group Performance Status; GEJ, gastroesophageal junction; PF, platinum, fluoropyrimidine
Fig. 1.
Probability of clinical–pathological variables and treatment pattern based on age. DPF, docetaxel, platinum, fluoropyrimidine; ECOG-PS, Eastern Cooperative Group Performance Status. NOTE: The estimations are derived from logistic regressions that evaluate the non-linear effect of age (restricted cubic splines with 3 knots) on binary variables
At the time of analysis, with a median follow-up of 35.3 months, 1208 progression events and 1128 deaths had been reported. Median PFS and OS for the entire sample were 5.8 (95% CI, 5.5–6.1) and 10.2 (95% CI, 9.6–10.8) months, respectively.
Evaluation of the main effect, dose intensity, and safety profile
Median PFS was 6.5 (95% CI, 5.6–7.3) vs 5.7 months (5.4–6.12) and median OS was 11 (95% CI, 9.8–10.4) vs 10.1 months (9.4–10.7) for DPF vs PF, respectively. In the multivariable model, DPF was associated with increased PFS with TR 1.27 (95% credible interval [CrI], 1.15–1.40) and also improved OS with TR 1.19 (95% CrI, 1.09–1.27). In a sensitivity analysis, the results of the Bayesian model are robust, regardless of geographical location.
The analysis of administered doses indicates that incorporating docetaxel was at the expense of a discreet decrease in RDI for platins and fluoropyrimidines, although the mean dose intensity remained above 80% in most cases (Annex Table 2). This occurred more intensely in the elderly. Thus, in DCX/DCF (docetaxel, cisplatin, capecitabine/5FU) schemes, cisplatin RDI was 81% vs 72%, and docetaxel RDI was 81% vs 72% in individuals <75 vs> 75 years, respectively. The use of cisplatin-based regimens is less common with age; the turning point occurs around the age of 65 (Annex Figure 2).
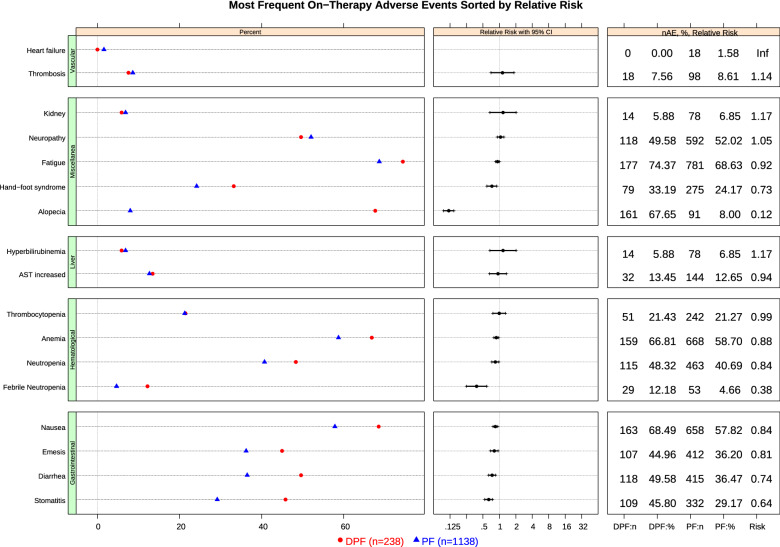
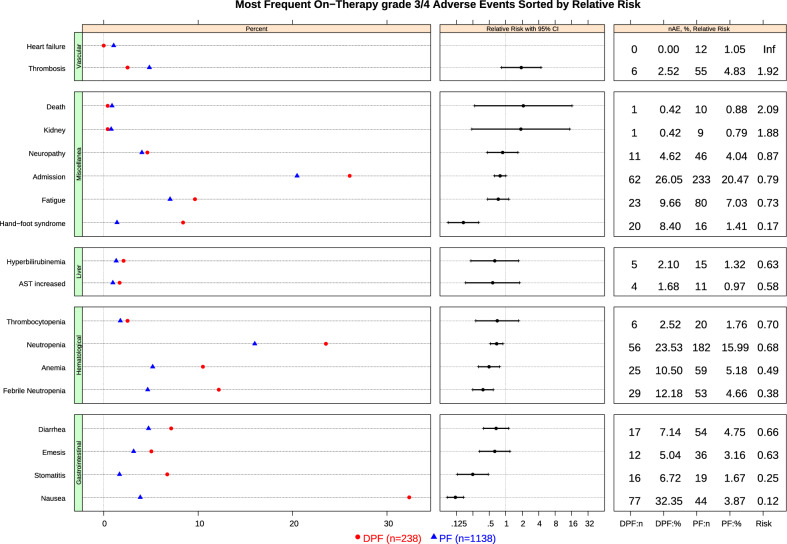
Insofar as safety is concerned, DPF increased serious adverse events compared to PF, particularly hematological events (32% vs 22%), although it was also associated with more diarrhea, stomatitis, alopecia, or asthenia (Figs. 2 & 3). The attempt to intensify therapy in younger subjects yielded the highest rates of severe toxicity with DPF across the board. Accordingly, the probability of grade 3/4 toxicity for DPF was 40%, 37%, and 34%, for people aged 40, 60, and 80 years. By contrast, for PF, it was 31%, 31%, and 35%, respectively. There were fewer PF dose reductions, with 83% vs 79% oxaliplatin RDI in participants < vs ≥ 75 years, respectively.
Fig. 2.
Most Frequent On − Therapy Adverse Events Sorted by Relative Risk. AE, adverse event; AST, Aspartate Aminotransferase; CI, confidence interval; DPF, docetaxel, platinum, fluoropyrimidine; n, number; PF, platinum, fluoropyrimidine
Fig. 3.
Most Frequent On − Therapy grade 3/4 Adverse Events Sorted by Relative Risk. AE, adverse event; AST, Aspartate Aminotransferase; CI, confidence interval; DPF, docetaxel, platinum, fluoropyrimidine; n, number; PF, platinum, fluoropyrimidine
Conditional effects based on individual traits
We then fitted an AFT model to estimate the probability of the effect of DPF depending on individual traits. An online calculator has been designed to obtain these estimations gradually (see the online calculator: https://www.prognostictools.es/AgamenonTriplet/inicio.aspx). Annex Figure 3 shows three examples of the use of this calculator under Wagner’s meta-analysis prior.
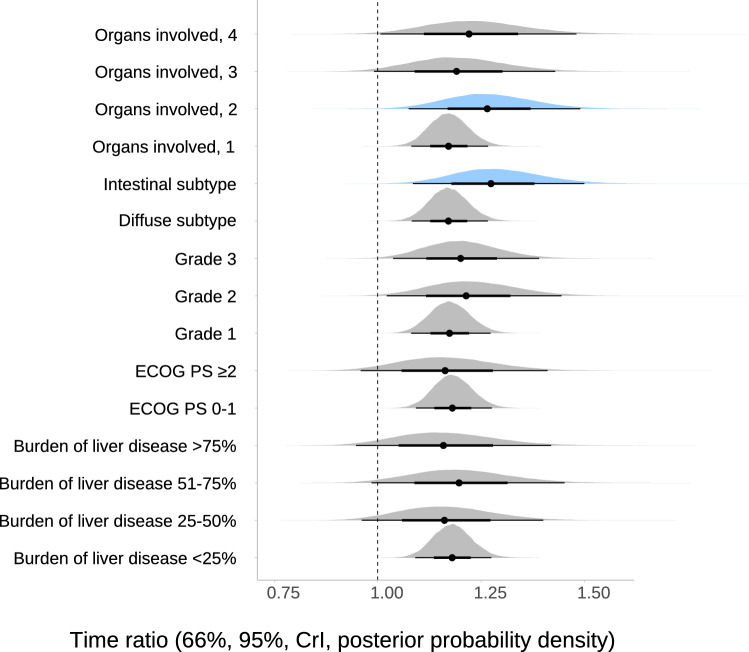
The model suggests a subgroup effect based on histopathological subtype. Thus, the posterior probability of benefit differs depending on histology. Nevertheless, a discreet benefit from DPF in any of the subtypes cannot be ruled out under this supposition (Fig. 4 and Table 2).
Fig. 4.
Posterior probabilities for the therapeutic effect of DPF vs PF on overall survival depending on clinical–pathological variables. CrI, credible interval. We applied specific priors for the main effect ~ N(0.15, 0.045), and skeptical priors for interactions (see methods). The density plots must be interpreted as posterior probability of the effect under each prior. Thus, the more to the right the area, the greater the benefit and the narrower it becomes, the greater the accuracy of the estimate. The results are derived from multivariable AFT models with therapeutic effect by covariate interactions. Color coding on the panels highlights the subgroups with greater effect (blue). A time ratio of more than 1 for the covariate implies that this slows or prolongs the time to event, whereas a time ratio of less than 1 indicates that an event is more likely to occur earlier. Thus, a time ratio equal to 2 would mean that the median of time to event is doubled in patients treated with DPF
Table 2.
Evaluation of potentially age-dependent effect-modifying factors for OS
| Variables | Specific informative prior | ||
|---|---|---|---|
| Time ratio (95%, CrI) | Posterior probability of effect size > 15% (TR > 1.15) | Posterior probability of effect size > 30% (TR > 1.30) | |
| ECOG-PS ≥ 2 | 1.16 (0.99–1.36) | 54% | 1% |
| ECOG-PS 0-1 | 1.18 (1.10–1.25) | 75% | 13% |
| Grade 3 | 1.19 (1.06–1.36) | 72% | 14% |
| Grade 2 | 1.20 (1.05–1.40) | 73% | 22% |
| Grade 1 | 1.17 (1.09–1.25) | 69% | 1% |
| Intestinal subtype | 1.27 (1.08–1.11) | 90% | 40% |
| Diffuse subtype | 1.17 (1.09–1.24) | 67% | 0 |
| Organs involved, 4 | 1.22 (1.04–1.43) | 73% | 26% |
| Organs involved, 3 | 1.18 (1.02–1.39) | 65% | 17% |
| Organs involved, 2 | 1.27 (1.10–1.44) | 88% | 37% |
| Organs involved, 1 | 1.17 (1.09–1.24) | 67% | 0 |
| Burden of liver disease > 75% | 1.16 (0.98–1.37) | 53% | 13% |
| Burden of liver disease 51–75% | 1.19 (1.02–1.40) | 66% | 20% |
| Burden of liver disease 25–50% | 1.16 (0.99–1.36) | 54% | 12% |
| Burden of liver disease < 25% | 1.18 (1.10–1.25) | 74% | 1% |
CrI, Bayesian credible interval; ECOG-PS, Eastern Cooperative Group performance status; ROPE, region of practical equivalence (effect = 0 ± 10%); TR, time ratio; OS = overall survival. The time ratios were derived from bayesian AFT lognormal models with treatment-by-covariate interactions; these models are multivariable (14 confounding factors, see Methods). The specific informative prior encompasses using the adapted evidence from Wagner’s 2017 meta-analysis (normal prior with mean = 0.15, standard deviation = 0.045 for therapeutic effect) with moderately skeptical priors for interactions (see “Methods”)
A time ratio of more than 1 for the covariate implies that this slows or prolongs the time to event, whereas a time ratio of less than 1 indicates that an event is more likely to occur earlier. Thus, a time ratio equal to 2 would mean that the median of time to event is doubled in patients treated with DPF
The posterior probability of effect sizes > 15% or 30% (TR > 1.15 or 1.30) denotes the actual probability of achieving a benefit of that magnitude (15 or 30%) or greater
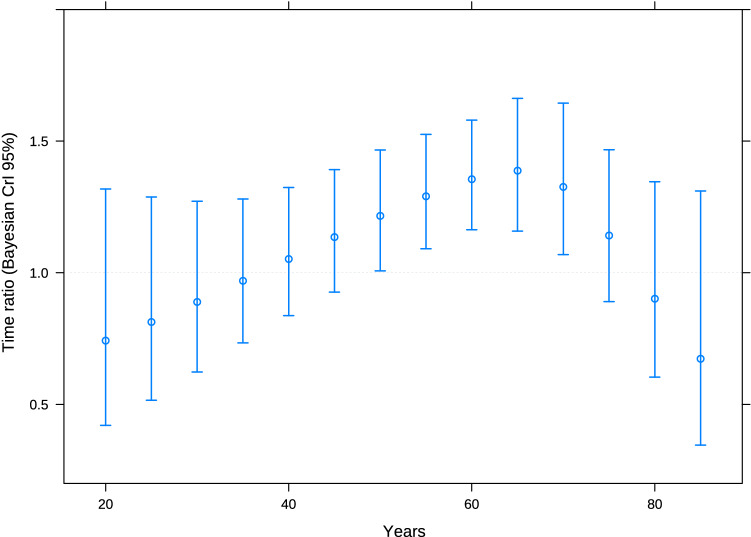
Insofar as age is concerned, middle-aged individuals (range 50–70 years) were those who benefitted from DPF (Fig. 5). Regardless of treatment, the elderly had greater probabilities of tumor control with any treatment at 3 months, but benefited less from DPF in terms of PFS. Nor is PFS increased in the younger range of ages with docetaxel-containing triplets, given that they have more chemo-refractory tumors (Fig. 1).
Fig. 5.
Therapeutic effect on the basis of age. CrI, credible interval. Note: Results are derived from a multivariable AFT Bayesian model, with effect by age interaction
Frequentist and Bayesian models based on weakly informative priors are shown in Annex Figure 1 and Annex Table 3.
Analysis of timing on therapeutic effect
The magnitude of effect of DPF on OS has fallen over the years. In 2013, TR was 1.21 (95% CrI, 1.09–1.33) as opposed to 1.13 (95% CrI, 0.97–1.32) in 2019 (neutral prior). PFS analyses revealed similar trends. Over time, DPF is a less popular option in this series, particularly for the treatment of intestinal subtype tumors (Annex Figure 4). Since 2014, this registry is gradually collecting more treatments with paclitaxel, with or without ramucirumab, as second-line therapy (23% vs 8% after and before 2014, respectively, Annex Figure 5).
Discussion
We have updated in a national AGC registry the external validity of the international bibliography regarding first-line therapies containing docetaxel [7–9]. Overall, we have observed that the use of triplets with docetaxel increased PFS by 27% and OS by 19% in patients with AGC. This improvement in survival endpoints is consistent with those of the data from the TAX325 trial and Wagner’s meta-analysis [7, 9], although it has come about at the expense of increased severe toxicity.
The AGAMENON registry reproduces the temporal trend seen in successive RCTs with DPF in first line [8, 9], making it a worthwhile instrument to explore the reasons for this weakening of effect and ascertain those patient groups for whom DPF combinations are still effective. Our results appear to confirm a reduction of therapeutic effect associated with DPF from 2015 onward, and contributes explanatory information regarding the fluctuation of those effects over time [7, 8]. While our data indicate that the most plausible explanation is the introduction of taxanes in second line, other aspects must be born in mind, such as the increased popularity of the oxaliplatin-containing doublet, which is observed in the literature [35, 36]. Moreover, in recent years, DPF is administered more in diffuse, treatment-resistant tumors, which may be due in part to the use of PF and trastuzumab in HER2 + cancers (most of which are intestinal subtype). In fact, the Japanese JCOG1013 RCT recruited patients with HER2-negative AGC, which possibly increased the rate of diffuse tumors, whereas theTAX325 RCT did not factor in HER2 status [7, 8].
We have elaborated an online calculator to offer an intuitive depiction of the baseline prognosis and the effect of docetaxel-containing triple-agent regimens depending on time variation and clinical–pathological factors. Due to the existence of heterogeneity of effects, the calculator aims to estimate the most likely effect of DPF as a function of covariates. The model we have adjusted here points toward the clinical profile with greater possibilities of gaining from DPF being fit subjects, in particular, aged 50–70, with intestinal tumors, and intermediate tumor burden, as the online calculator illustrates. However, the data from our registry show that younger individuals failed to attain better outcomes with DPF, as can be seen in the calculator, but did suffer a higher rate of serious adverse events. The reason can be found in the higher prevalence of aggressive, chemo-refractory tumor phenotypes in this age range (e.g., diffuse tumors, signet ring cells, peritoneal carcinomatosis, etc.). In these cases, DPF did not seemingly help to prevent progression and the result in terms of survival endpoints was more modest than in subjects aged 50–70 years who benefitted the most with this regimen [26]. Indeed, the various histopathological subtypes of gastric cancer respond differently to chemotherapy [11, 12]. Thus, the intestinal subtype, more sensitive to DPF, is more frequent in elderly individuals, paradoxically the group that tolerates this therapy worse when administered at standard recommended doses. Conversely, diffuse tumors are typical of young patients [11, 26]. Interestingly, most AGC RCTs have not stratified for histology, nor have they appraised interactions between therapeutic effect and different clinical–pathological variables [7]. For this reason, the online calculator is useful for the clinician, and adds evidence to what we already know about these combinations.
In light of this information, the AGAMENON registry points to the value of regularly re-examining and updating the external validity of RCTs, as well as to conduct geographical validations of the outcomes, particularly when the target population changes over time [10–12] or efficacious off-trial therapies entail rethinking treatment strategy [13, 37].
Among elderly subjects in the AGAMENON registry, DPF was seen to be administered with dose reductions and modifications. This mitigated the risk of toxicity in the elderly and revealed that most of the adaptations took place at the beginning, in line with the published clinical trials that have sought to modify the triplet to enhance its tolerability [38–41]. A single-phase II randomized trial (NCT00737373) has compared a docetaxel-containing triplet (FLOT) with 5FU and oxaliplatin (FLO) in an elderly population. It detected significantly increased toxicity and worse quality of life, without evidence of a gain in OS in this context [42]. On the whole, these data led to the assumption that the reduction of benefit in older ages was related to tolerance [14, 43]. In our study, modifications in elderly patients were associated with diminished therapeutic effect, similar to the AIO group’s trial, casting doubt on the usefulness of this strategy.
Our study has certain limitations inherent in a registry study, including missing values for histological subtype in 21% of the cases. The procedures of multiple imputation decrease bias, but in this case, they are associated with more conservative estimations. Second, one of the criticisms of Bayesian models is the subjectivism in selecting priors. However, the use of a perspective based on objective external data lessens this problem, and allows gradual, pragmatic answers to be attained. In this regard, Bayesian analyses have added advantages over the classical frequentist models [28], including greater accuracy of estimations and less bias. Based on the Wagner’s previous results [9], our analysis is capable of capturing a discreet effect in the diffuse subtype, as well as a result for the intestinal subtype in line with the conclusions of the TAX325 RCT [7]. However, the hypothesis about the specific effect of DPF on diffuse tumors would have to be elucidated in further RCTs, which we believe to be unlikely at this time. With respect to the validity of our results, the reader must be aware that the registry still contains a limited number of subjects treated with FLOT, which is also a specific limitation in Wagner’s meta-analysis, used as a prior in our Bayesian model [9]. Despite the fact that the impact of FLOT in seniors is as yet uncertain, the Bayesian model adjusted here contemplates the type of platin, age, and year of treatment, among other factors. While more experience with this scheme in advanced tumors is still needed, the data currently available in perioperative disease point to FLOT being less active in tumors with diffuse histology, which is consistent with our results [11].
In short, our data confirm the need to update the applicability of RCTs, such as TAX325 [7], from time to time, or others that will fall behind as clinical practice changes [44], especially in view of the biological diversity of this disease. Ultimately, our study confirms the benefit of the docetaxel triple-drug regimen as first-line treatment in the real-world setting, attesting to the greater applicability in middle-aged individuals with non-diffuse tumors. However, in the best-case scenario, the benefit is modest and comes at the expense of increased toxicity. Current clinical practice guidelines endorse the use of dual-agent schedules, whereas triplets with docetaxel are deemed a useful alternative for fit patients in the event that an urgent tumor response is needed or in locally advanced, unresectable tumors [14, 45, 46]. The need to design new RCTs in AGC separating the evaluation of effects based on clinical–pathological variables is crucial given these data. This information should be taken into account when choosing treatment strategy in the context of the growing recommendation of dual-agent schedules in first line and the continuum of care of AGC.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgments
Priscilla Chase Duran for editing and translating the manuscript. Miguel Vaquero, Natalia Cateriano and the IRICOM S.L. team for the support of the website registry.
Authors’ contributions
PJF, ACB and JG developed the project, analyzed the data and drafted the manuscript. The other authors recruited patients and provided clinical information, comments, and improvements to the manuscript. All authors participated in the interpretation and discussion of data, and the critical review of the manuscript.
Funding
None to declare; this is an academic study. The study was supported by the authors themselves.
Availability of data and materials
All the data generated or analyzed in this study are included in the manuscript or the supplementary information.
Compliance with ethical standards
Conflict of interest
The authors state that they have no conflict of interest related to this study.
Ethic approval and consent to participate
All procedures followed were in accordance with the ethical standards of the committee responsible for human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. Informed consent was obtained from all patients before being included in the study. This work is original and has not been previously presented elsewhere.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Rothwell PM. Commentary: external validity of results of randomized trials: disentangling a complex concept. Int J Epidemiol. 2010;39:94–96. doi: 10.1093/ije/dyp305. [DOI] [PubMed] [Google Scholar]
- 2.Rothwell PM. Factors that can affect the external validity of randomised controlled trials. PLOS Clin Trial. 2006;1(1):e9. doi: 10.1371/journal.pctr.0010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rothwell PM. External validity of randomised controlled trials:“to whom do the results of this trial apply?”. Lancet. 2005;365:82–93. doi: 10.1016/S0140-6736(04)17670-8. [DOI] [PubMed] [Google Scholar]
- 4.Royston P, Parmar MKB, Altman DG. External validation and updating of a prognostic survival model. Hub Trials Methodol. Res. 2010. https://www.semanticscholar.org/paper/External-validation-and-updating-of-a-prognostic-1-Royston-Parmar/5ba4a569ffa416f740a106e491560837074ce44b.
- 5.Dekkers OM, von Elm E, Algra A, Romijn JA, Vandenbroucke JP. How to assess the external validity of therapeutic trials: a conceptual approach. Int J Epidemiol. 2010;39:89–94. doi: 10.1093/ije/dyp174. [DOI] [PubMed] [Google Scholar]
- 6.Rawla P, Barsouk A. Epidemiology of gastric cancer: global trends, risk factors and prevention. Prz Gastroenterol. 2019;14:26. doi: 10.5114/pg.2018.80001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: a report of the V325 Study Group. J Clin Oncol. 2006;24:4991–4997. doi: 10.1200/JCO.2006.06.8429. [DOI] [PubMed] [Google Scholar]
- 8.Yamada Y, Boku N, Mizusawa J, Iwasa S, Kadowaki S, Nakayama N, et al. Docetaxel plus cisplatin and S-1 versus cisplatin and S-1 in patients with advanced gastric cancer (JCOG1013): an open-label, phase 3, randomised controlled trial. Lancet Gastroenterol Hepatol. 2019;4:501–510. doi: 10.1016/S2468-1253(19)30083-4. [DOI] [PubMed] [Google Scholar]
- 9.Wagner AD, Syn NLX, Moehler M, Grothe W, Yong WP, Tai B, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev. 2017;8:CD004064. doi: 10.1002/14651858.CD004064.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 11.Al-Batran S-E, Hofheinz RD, Pauligk C, Kopp H-G, Haag GM, Luley KB, et al. Histopathological regression after neoadjuvant docetaxel, oxaliplatin, fluorouracil, and leucovorin versus epirubicin, cisplatin, and fluorouracil or capecitabine in patients with resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4-AIO) Lancet Oncol. 2016;17:1697–1708. doi: 10.1016/S1470-2045(16)30531-9. [DOI] [PubMed] [Google Scholar]
- 12.Jiménez Fonseca P, Carmona-Bayonas A, Hernández R, Custodio A, Cano JM, Lacalle A, et al. Lauren subtypes of advanced gastric cancer influence survival and response to chemotherapy: real-world data from the AGAMENON National Cancer Registry. Br J Cancer. 2017;117:775–782. doi: 10.1038/bjc.2017.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wilke H, Muro K, Van Cutsem E, Oh S-C, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): a double-blind, randomised phase 3 trial. Lancet Oncol. 2014;15:1224–1235. doi: 10.1016/S1470-2045(14)70420-6. [DOI] [PubMed] [Google Scholar]
- 14.National Comprehensive Cancer Network. NCCN clinical practice guidelines for gastric cancer. NCCN Guidel. (cited 2019 Mar 11). p. Varsion 2.2020. https://www.nccn.org/professionals/physician_gls/pdf/gastric.pdf.
- 15.Muro K, Van Cutsem E, Narita Y, Pentheroudakis G, Baba E, Li J, et al. Pan-Asian adapted ESMO Clinical Practice Guidelines for the management of patients with metastatic gastric cancer: a JSMO–ESMO initiative endorsed by CSCO, KSMO, MOS, SSO and TOS. Ann Oncol. 2018;30:19–33. doi: 10.1093/annonc/mdy502. [DOI] [PubMed] [Google Scholar]
- 16.Carmona-Bayonas A, Jiménez-Fonseca P, Custodio A, Sánchez Cánovas M, Hernández R, Pericay C, et al. Anthracycline-based triplets do not improve the efficacy of platinum-fluoropyrimidine doublets in first-line treatment of advanced gastric cancer: real-world data from the AGAMENON National Cancer Registry. Gastric Cancer. 2017;14:1379–1388. doi: 10.1007/s10120-017-0718-5. [DOI] [PubMed] [Google Scholar]
- 17.Carmona-Bayonas A, Jiménez-Fonseca P, Lorenzo MLS, Ramchandani A, Martínez EA, Custodio A, et al. On the effect of triplet or doublet chemotherapy in advanced gastric cancer: results from a National Cancer Registry. J Natl Compr Cancer Netw. 2016;14:1379–1388. doi: 10.6004/jnccn.2016.0148. [DOI] [PubMed] [Google Scholar]
- 18.Jiménez-Fonseca P, Carmona-Bayonas A, Lorenzo MLS, Plazas JG, Custodio A, Hernández R, et al. Prognostic significance of performing universal HER2 testing in cases of advanced gastric cancer. Gastric Cancer. 2016;20:465–474. doi: 10.1007/s10120-016-0639-8. [DOI] [PubMed] [Google Scholar]
- 19.Custodio A, Carmona-Bayonas A, Fonseca PJ, Sánchez ML, Antonio Viudez R, Hernández JM, et al. Nomogram-based prediction of survival in patients with advanced oesophagogastric adenocarcinoma receiving first-line chemotherapy: a multicenter prospective study in the era of trastuzumab. Br J Cancer. 2017;116:1526–1535. doi: 10.1038/bjc.2017.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Visa L, Jiménez-Fonseca P, Martínez EA, Hernández R, Custodio A, Garrido M, et al. Efficacy and safety of chemotherapy in older versus non-older patients with advanced gastric cancer: a real-world data, non-inferiority analysis. J Geriatr Oncol. 2017;9:254–264. doi: 10.1016/j.jgo.2017.11.008. [DOI] [PubMed] [Google Scholar]
- 21.Carmona-Bayonas A, Jiménez-Fonseca P, Echavarria I, Cánovas MS, Aguado G, Gallego J, et al. Surgery for metastases for esophageal-gastric cancer in the real world: data from the AGAMENON national registry. Eur J Surg Oncol. 2018;44:1191–1198. doi: 10.1016/j.ejso.2018.03.019. [DOI] [PubMed] [Google Scholar]
- 22.Viúdez A, Carmona-Bayonas A, Gallego J, Lacalle A, Hernández R, Cano JM, et al. Optimal duration of first-line chemotherapy for advanced gastric cancer: data from the AGAMENON registry. Clin Transl Oncol. 2020;22:734–750. doi: 10.1007/s12094-019-02183-y. [DOI] [PubMed] [Google Scholar]
- 23.Carmona-Bayonas A, Jiménez-Fonseca P, et al. Multistate models: accurate and dynamic methods to improve predictions of thrombotic risk in patients with cancer. Thromb Haemost. 2019;119:1849–1859. doi: 10.1055/s-0039-1694012. [DOI] [PubMed] [Google Scholar]
- 24.Cotes Sanchís A, Gallego J, Hernandez R, Arrazubi V, Custodio A, Cano JM, et al. Second-line treatment in advanced gastric cancer: data from the Spanish AGAMENON registry. PLoS One. 2020;15:e0235848. doi: 10.1371/journal.pone.0235848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harrell F. Regression modeling strategies: with applications to linear models, logistic and ordinal regression, and survival analysis. 2. New York: Springer; 2015. [Google Scholar]
- 26.Crew KD, Neugut AI. Epidemiology of gastric cancer. World J Gastroenterol. 2006;12:354. doi: 10.3748/wjg.v12.i3.354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wijeysundera DN, Austin PC, Hux JE, Beattie WS, Laupacis A. Bayesian statistical inference enhances the interpretation of contemporary randomized controlled trials. J Clin Epidemiol. 2009;62:13–21. doi: 10.1016/j.jclinepi.2008.07.006. [DOI] [PubMed] [Google Scholar]
- 28.Van de Schoot R, Kaplan D, Denissen J, Asendorpf JB, Neyer FJ, Van Aken MAG. A gentle introduction to Bayesian analysis: applications to developmental research. Child Dev. 2014;85:842–860. doi: 10.1111/cdev.12169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bland JM, Altman DG. Bayesians and frequentists. Br Med J. 1998;317:1151–1160. doi: 10.1136/bmj.317.7166.1151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bendtsen M. A gentle introduction to the comparison between null hypothesis testing and Bayesian analysis: reanalysis of two randomized controlled trials. J Med Internet Res. 2018;20:e10873. doi: 10.2196/10873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Harrell Jr F, Frank E, Maintaner Frank E. Package ‘rms’. 2015 (cited 2020 Jan 1). p. 229. http://cran.r-project.org/web/packages/rms/index.html.
- 32.Bürkner P-C. Brms: An R package for Bayesian multilevel models using Stan. J Stat Softw Foundation Open Access Stat. 2017;80:1–28. [Google Scholar]
- 33.Stoyan G. A Practical Guide to Splines. Applied Mathematical Sciences. Springer‐Verlag, editor. Berlin‐Heidelberg‐New York; 1980.
- 34.Buuren S van, Groothuis-Oudshoorn K. Mice: multivariate imputation by chained equations in R. J Stat Softw. 2010;1–68
- 35.Cunningham D, Starling N, Rao S, Iveson T, Nicolson M, Coxon F, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 36.Al-Batran S-E, Hartmann JT, Probst S, Schmalenberg H, Hollerbach S, Hofheinz R, et al. Phase III trial in metastatic gastroesophageal adenocarcinoma with fluorouracil, leucovorin plus either oxaliplatin or cisplatin: a study of the Arbeitsgemeinschaft Internistische Onkologie. J Clin Oncol. 2008;26:1435–1442. doi: 10.1200/JCO.2007.13.9378. [DOI] [PubMed] [Google Scholar]
- 37.Moons KGM, Kengne AP, Grobbee DE, Royston P, Vergouwe Y, Altman DG, et al. Risk prediction models: II. External validation, model updating, and impact assessment. Heart. 2012;98:691–698. doi: 10.1136/heartjnl-2011-301247. [DOI] [PubMed] [Google Scholar]
- 38.Shah MA, Janjigian YY, Stoller R, Shibata S, Kemeny M, Krishnamurthi S, et al. Randomized multicenter phase II study of modified docetaxel, cisplatin, and fluorouracil (DCF) versus DCF plus growth factor support in patients with metastatic gastric adenocarcinoma: a study of the US Gastric Cancer Consortium. J Clin Oncol. 2015;33:3874–3879. doi: 10.1200/JCO.2015.60.7465. [DOI] [PubMed] [Google Scholar]
- 39.Al-Batran S-E, Hartmann JT, Hofheinz R, Homann N, Rethwisch V, Probst S, et al. Biweekly fluorouracil, leucovorin, oxaliplatin, and docetaxel (FLOT) for patients with metastatic adenocarcinoma of the stomach or esophagogastric junction: a phase II trial of the Arbeitsgemeinschaft Internistische Onkologie. Ann Oncol. 2008;19:1882–1887. doi: 10.1093/annonc/mdn403. [DOI] [PubMed] [Google Scholar]
- 40.Van Cutsem E, Boni C, Tabernero J, Massuti B, Middleton G, Dane F, et al. Docetaxel plus oxaliplatin with or without fluorouracil or capecitabine in metastatic or locally recurrent gastric cancer: a randomized phase II study. Ann Oncol. 2014;26:149–156. doi: 10.1093/annonc/mdu496. [DOI] [PubMed] [Google Scholar]
- 41.Rosenberg AJ, Rademaker A, Hochster HS, Ryan T, Hensing T, Shankaran V, et al. Docetaxel, oxaliplatin, and 5-fluorouracil (DOF) in metastatic and unresectable gastric/gastroesophageal junction adenocarcinoma: a phase ii study with long-term follow-up. Oncologist. 2019;24:1039–e642. doi: 10.1634/theoncologist.2019-0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Batran S-E, Pauligk C, Homann N, Hartmann JT, Moehler M, Probst S, et al. The feasibility of triple-drug chemotherapy combination in older adult patients with oesophagogastric cancer: a randomised trial of the Arbeitsgemeinschaft Internistische Onkologie (FLOT65+) Eur J Cancer. 2013;49:835–842. doi: 10.1016/j.ejca.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 43.Lorenzen S, Stahl M, Hofheinz R-D, Al-Batran S-E, Lordick F. Influence of taxanes on treatment sequence in gastric cancer. Oncol Res Treat. 2019;43(1–2):1–6. doi: 10.1159/000503428. [DOI] [PubMed] [Google Scholar]
- 44.Tabernero J, Van Cutsem E, Bang Y-J, Fuchs CS, Wyrwicz L, Lee KW, et al. Pembrolizumab with or without chemotherapy versus chemotherapy for advanced gastric or gastroesophageal junction (G/GEJ) adenocarcinoma: The phase III KEYNOTE-062 study. American Society of Clinical Oncology; 2019.
- 45.Al-Batran S-E, Homann N, Pauligk C, Illerhaus G, Martens UM, Stoehlmacher J, et al. Effect of neoadjuvant chemotherapy followed by surgical resection on survival in patients with limited metastatic gastric or gastroesophageal junction cancer: the AIO-FLOT3 trial. JAMA Oncol. 2017;3:1237–1244. doi: 10.1001/jamaoncol.2017.0515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smyth EC, Verheij M, Allum W, Cunningham D, Cervantes A, Arnold D. Gastric cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2016;27:v38–v49. doi: 10.1093/annonc/mdw350. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All the data generated or analyzed in this study are included in the manuscript or the supplementary information.