Abstract
Background
Kidney replacement therapy (KRT) is a lifesaving but costly treatment for patients with end-stage kidney disease (ESKD). The objective of this study was to review full economic evaluations comparing KRT modalities specified as hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT) for patients with ESKD.
Methods
We conducted a systematic review of the literature from PubMed, Embase, EconLit (EBSCO), Web of Science, Cochrane Library, National Health Service Economic Evaluation Database (NHS EED), Centre for Reviews and Dissemination (CRD) Database of Abstracts of Reviews of Effects (DARE), and CRD Health Technology Assessment Database from inception until 5 January 2020. Full economic evaluations were included if they compared three forms of KRT specified as PD, HD, and KT. The reporting quality of included studies was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist.
Results
Ten studies were identified in the review. The majority of the studies were model-based evaluations and included a cost-utility analysis. Four studies were conducted from a public healthcare perspective, three from a societal perspective, and three from a third-party payer perspective. None of the studies adequately addressed all the applicable items of the CHEERS checklist. The most infrequently reported items were characterizing heterogeneity, target population, and characterizing uncertainty. There is a lack of studies that conduct from a societal perspective and take into account characterizing heterogeneity. All included studies indicate that KT is the most cost-effective KRT modality, with either a dominant position over HD and PD or an incremental cost-effectiveness ratio well below the accepted willingness-to-pay threshold. The majority of studies suggest that PD is less costly and offers comparable or better health outcomes than HD.
Conclusions
Our systematic review suggests that KT is the most cost-effective KRT modality, but there is no firm conclusion about the cost-effectiveness of HD and PD. Further economic evaluations can be conducted from a societal perspective and detail the evidence for subsets of patients with different characteristics.
Electronic supplementary material
The online version of this article (10.1007/s40258-020-00614-4) contains supplementary material, which is available to authorized users.
Key Points for Decision Makers
| Kidney transplantation is the most cost-effective kidney replacement therapy modality, but there is no firm conclusion about the cost-effectiveness of hemodialysis and peritoneal dialysis. |
| Future economic evaluations of the kidney replacement therapy modality can be conducted from a societal perspective and take into account characterizing heterogeneity. |
Introduction
End-stage kidney disease (ESKD) is the final and permanent stage of chronic kidney disease (CKD) [1]. The increasing prevalence of ESKD has been a significant public healthcare challenge worldwide [2]. From 2003 to 2016, the ESKD prevalence has steadily increased worldwide, with a median percent increase of 43% [3]. In 2016, ESKD prevalence ranging from 600 to 999 per million general population (PMP) was reported in approximately 45% of countries [3]. Driven by population aging and the rising incidence of diabetes and hypertension, the prevalence of ESKD is expected to rise to an even greater extent in the next few decades [1, 4, 5].
Kidney replacement therapy (KRT) is a lifesaving but costly treatment for patients with ESKD [6]. There are three primary forms of KRT, namely hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation (KT) [2]. HD is principally performed in dialysis centers by healthcare workers but also can be carried out independently by patients at home [7]. PD is predominantly performed by patients at home without assistance from clinical staff [8]. Globally, the number of people receiving KRT was more than 2.6 million in 2010 and this number is projected to more than double to 5.4 million by 2030 [6]. The use of the different KRT modalities varies considerably across countries and regions. In 2016, KT for patients with ESKD ranged from less than 20% in roughly one-third of countries to 50–70% in approximately one-quarter of countries [3]. In-center HD was the predominant KRT modality in most countries and it accounted for more than 80% of dialysis in 79% of countries in 2016 [3]. Several countries and regions have initiated PD-First policies or PD-Favored policies to encourage the use of PD as the first treatment modality for appropriate patients because PD is deemed to have lower costs than HD, comparable survival and improved quality of life (QOL) [9–12]. Accordingly, higher rates of PD uptake were reported in those countries and regions, such as Hong Kong (71%), the Jalisco region of Mexico (61%), and Guatemala (57%) [3].
The rising prevalence of ESKD and extensive use of KRT have challenged the economic capacity of many countries, especially in low-income countries where there are limited resources and a large gap in access to KRT [7]. Approximately 64% of countries provided public funding for KRT [13]. It is estimated that total ESKD expenditure accounts for 0.91–7.1% of national health system expenditure in countries with a high prevalence of ESKD even though ESKD patients contribute a relatively small proportion of the entire CKD population [14].
As the burden of ESKD on society and healthcare budgets has increased considerably, it is essential to assess the cost-effectiveness of the different KRT modalities to establish cost-effective service provision strategies. An economic evaluation is a comparative analysis that examines two or more interventions in terms of their costs and consequences. It can provide useful insight for prioritizing the programs in the healthcare sector and for optimal resource allocation [15]. Several economic evaluations have been conducted regionally. Nevertheless, there have been few systematic reviews of economic evaluations of all KRT modalities. Previous systematic studies in this area have focused on subsets of KRT modalities, such as comparing alternative dialysis modalities [8, 16] or dialysis modalities with different intensities [17]. Therefore, this study aims to review full economic evaluations comparing KRT modalities specified as PD, HD, and KT for patients with ESKD.
Methods
Data Sources and Search Strategies
This review was conducted according to the guidelines of the Preferred Reporting Items for Systematic reviews and Meta-Analyses (PRISMA) (see Resource 1, Online Supplemental Material (OSM)) [18]. Studies were identified by searching electronic databases and scanning reference lists of relevant studies. The electronic databases, including PubMed, Embase, EconLit (EBSCO), Web of Science, Cochrane Library, National Health Service Economic Evaluation Database (NHS EED), Centre for Reviews and Dissemination (CRD) Database of Abstracts of Reviews of Effects (DARE), and CRD Health Technology Assessment Database, were systematically searched from inception until 5 January 2020. The search strategy used Medical Subject Headings (MeSH) terms and a range of text words pertinent to the identification of studies that included KRT modalities of PD, HD, and KT, and text words relevant to economic evaluations. The search strategies are provided in Resource 2 (OSM).
Eligibility Criteria
For inclusion, the following criteria had to be fulfilled: (1) full economic evaluations that considered both costs and effects, including cost–benefit analysis (CBA), cost-utility analysis (CUA), cost-effectiveness analysis (CEA), and cost-minimization analysis (CMA); (2) comparing three forms of KRT specified as PD, HD, and KT; (3) studies in the English language. All alternative modalities of PD (e.g., automated PD, continuous ambulatory PD), HD (e.g., home HD, in-center HD, nocturnal home HD), and KT (e.g., living-donor transplantation (LT), deceased-donor transplantation (DT)) were considered for inclusion. Exclusion criteria were: (1) partial economic evaluations, such as outcome description, cost description, cost-outcome description, effectiveness evaluation, and cost analysis; (2) systematic reviews, commentaries, congress abstracts; (3) studies without at least three comparators of PD, HD, and KT. No limits were applied for country, publication date, and publication status.
Study Selection
Two reviewers independently performed the study selection in an unblended standardized manner. The titles and abstracts of all citations were evaluated to identify articles for full-text review. Any citations without electronically available abstracts were discarded unless the title was convincing of the study’s relevance. Full-text publications were reviewed by two reviewers using the pre-defined eligibility criteria. All disagreements between reviewers were resolved by discussion and consensus.
Data Extraction and Risk of Bias Assessment
We extracted the following items from each included study: general study characteristics (author, publication year, country, study population, type of economic evaluations, study design, study perspective), model design and structure (model type, cycle length, time horizon, discount rate, number and names of key states/pathways), parameter sources and values (health outcomes, utility values, data sources for utility values, methods of measurement of utility, type and category of included costs, country and reference year of costs, cost values, data sources for cost values), summary of cost-effectiveness results (incremental cost-effectiveness ratios (ICERs), main results, type of sensitivity analyses, outcomes of sensitivity analyses, author’s/authors’ conclusions). One reviewer completed the extraction and the second reviewer checked the extracted data with disagreement resolved by consensus. The risk of bias of included studies was assessed using the Consolidated Health Economic Evaluation Reporting Standards (CHEERS) checklist [19]. All assessments were performed and agreed upon by two reviewers.
Results
Study Selection
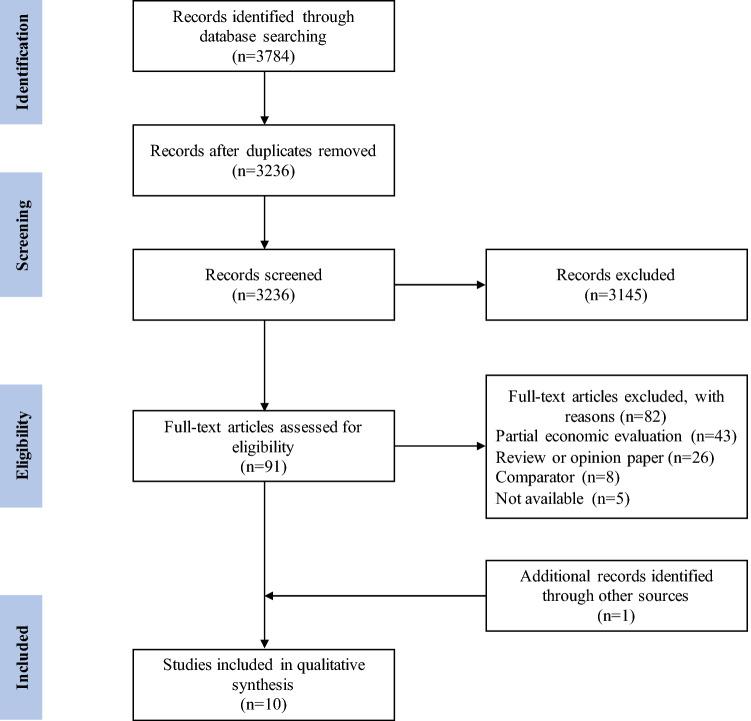
The initial search of electronic databases provided a total of 3784 citations. After adjusting for duplicates, 3236 abstracts were screened and the full-text articles of 91 citations were examined in more detail. Of these, nine studies met the inclusion criteria and were included in the review. One additional study that met the inclusion criteria was identified by scanning reference lists of relevant studies and review articles. A total of ten studies were identified for inclusion in the systematic review. Figure 1 shows the flow diagram of the study selection.
Fig. 1.
Flow diagram showing study selection
Study Characteristics
Table 1 provides a summary of the included studies. The included studies were from upper-middle-income countries and high-income countries. The study population of all studies were patients with ESKD, but only four studies described characteristics of the base case population [20, 24, 27, 29]. Five of the ten studies conducted a CUA [20–24], one conducted a CEA [25], and four conducted both a CEA and CUA [26–29]. Eight studies were model-based economic evaluations using Markov models [20–23, 26–29] and the remaining two studies were a multicenter cross-section study [24] and a retrospective cohort study [25], respectively. Four studies were conducted from a public healthcare perspective [21, 22, 24, 27], three from a societal perspective [20, 23, 29] and three from a third-party payer perspective [25, 26, 28].
Table 1.
Study characteristics
| Author (year) | Country | Study population | Type of EEs | Study design | Study perspective |
|---|---|---|---|---|---|
| Moradpour et al. (2020) [20] | Iran | Adult patients with ESKD | CUA | Model-based EE | Societal perspective |
| Rosselli et al. (2015) [26] | Colombia | Adult patients with ESKD | CEA, CUA | Model-based EE | Third-party payer perspective |
| Jensen et al. (2014) [21] | Denmark | Danish ESKD population | CUA | Model-based EE | Public healthcare perspective |
| Shimizu et al. (2012) [22] | Japan | New patients with ESKD | CUA | Model-based EE | Public healthcare perspective |
| Villa et al. (2012) [23] | Spain | Spanish ESKD population | CUA | Model-based EE | Societal perspective |
| Haller et al. (2011) [27] | Austria | Austrian ESRD population | CEA, CUA | Model-based EE | Public healthcare perspective |
| Howard et al. (2009) [28] | Australia | New ESKD patients in Australia over 2005–2010 | CEA, CUA | Model-based EE | Third-party payer perspective |
| Kontodimopoulos et al. (2008) [24] | Greece | ESKD patients | CUA | Multicenter cross-sectional study | Public healthcare perspective |
| de Wit et al. (1998) [29] | Netherlands | Adult patients with ESKD | CEA, CUA | Model-based EE | Societal perspective |
| Sesso et al. (1990) [25] | Brazil | Nondiabetic patients (aged 15–50 years) who initiated treatment for ESKD | CEA | Retrospective cohort study | Third-party payer perspective |
EE economic evaluation, CUA cost-utility analysis, CEA cost-effectiveness analysis, ESKD end-stage kidney disease
Quality of Reporting and Risk of Bias within Studies
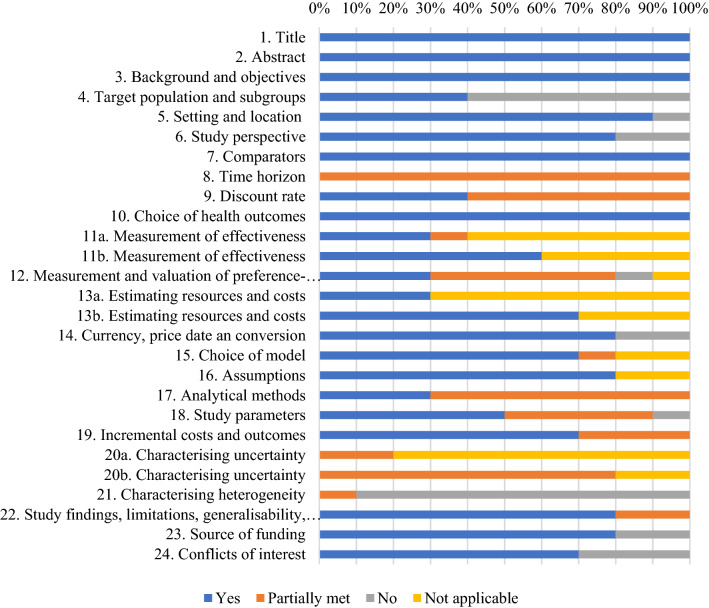
Figure 2 shows the proportions of included studies that complied with applicable items of the CHEERS checklist. None of the included studies addressed all the applicable items of the CHEERS checklist. The most infrequently reported items were characterizing heterogeneity, target population and subgroups, and characterizing uncertainty. About 60% of the studies failed to describe characteristics of the base-case population. All studies stated the time horizon, but none of them clearly described the rationale for choosing the specified time horizon. Most of the studies failed to describe the population used to elicit preference-based outcomes, even though they reported methods used to derive utility values. Four studies did not report value ranges or probability distributions for parameters. In terms of characterizing uncertainty, five studies were restricted to exploring the effects of a few input parameters and none of the studies explored the uncertainty related to the structure of the model and assumptions. One criterion that may have the potential for a higher risk of bias was the characterizing heterogeneity. Most studies did not conduct subgroup analyses, while the study that had conducted subset analyses did not report differences in outcomes between subgroups [29]. The performance of each included study for the CHEERS checklist can be found in Resource 3 (OSM).
Fig. 2.
Proportion of included studies that complied with applicable items of the Consolidated Health Economic Evaluation Reporting Standard (CHEERS) checklist
Model Design and Structure
Table 2 shows the characteristics of the model design and structure. A variety of time horizons were employed in the included studies, varying from 2, 5, 6, 10, and 15 years to lifetime horizons. The cycle length in the models was either 1 month or 1 year, aside from one model that did not specify the cycle length [29]. The discounting is the same for costs and outcomes within each study, with discounting rates varying from 3%, 3.5%, 5% to 6%, aside from one study that explicitly ruled out discounting on the basis of their 2-year time horizon [25]. The majority of model-based economic evaluations used a four-state Markov model approach and the remaining studies developed the Markov model with eight, 12, and 38 states. Moradpour et al. [20] and Villa et al. [23] used the same four-state model structure, but the model by Moradpour et al. [20] applied the 1-month cycle length and the model by Villa et al. [23] applied the 1-year cycle length. Haller et al. [27] defined 12 different states depending on time of initiation of a certain treatment modality. The study by de Wit et al. [29] combined six treatment modalities, three age-groups, and two treatment stages to define 36 states.
Table 2.
Model design and structure
| Author (year) | Model type | Cycle length | Time horizon | Discount rate | Number and names of key states/pathways |
|---|---|---|---|---|---|
| Moradpour et al. (2020) [20] | Markov model | 1 month | Lifetime | 6% costs and benefits | 4 states: HD, PD, KT, death |
| Rosselli et al. (2015) [26] | Markov model | 1 month | 5 years | 3% costs and benefits | 8 states: HD, PD, dialysis, acute rejection, healthy graft, chronic rejection, second line, death |
| Jensen et al. (2014) [21] | Markov model | 1 year | 6 years | 3.5% costs and benefits | 4 states: HD, PD, KT, death |
| Shimizu et al. (2012) [22] | Markov model | 1 year | 15 years | 3% costs and benefits | 8 states: ESKD, HD, PD, PD and HD combination therapy (PD + HD), a living donor transplant, a deceased donor transplant, resumption of dialysis (after transplant), death |
| Villa et al. (2012) [23] | Markov model | 1 year | Three temporal horizons (5, 10, and 15 years) | 3.5% costs and benefits | 4 states: HD, PD, KT, death |
| Haller et al. (2011) [27] | Markov model | 1 month | 10 years | 3% costs and benefits | 12 states: the initial state of a newly diagnosed kidney disease, HD during the first 12 months, HD between 13 and 24 months, HD beyond 25 months, PD during the first 12 months, PD between 13 and 24 months, PD beyond 25 months, KT from a deceased donor during the first 12 months, KT from a living donor during the first 12 months, KT between 13 and 24 months, KT beyond 25 months, death |
| Howard et al. (2009) [28] | Dynamic population-based Markov model | 1 year | 5 years | 5% costs and benefits | 4 states: dialysis, pre-emptive transplant, KT, death |
| Kontodimopoulos et al. (2008) [24] | NA | NA | Lifetime | 5% costs and benefits | NA |
| de Wit et al. (1998) [29] | Markov model | NR | 5 years | 5% costs and benefits | 38 states: combinations of six treatment modalities (center HD, limited care HD, home HD, CAPD, CCPD and KT), three age-groups (0–44 years, 45–64 years, and 65 years and older) and two treatment stages (the first year and subsequent years on the same treatment modality), death, recovery of kidney function |
| Sesso et al. (1990) [25] | NA | NA | 2 years | No discounting | NA |
NA not applicable, NR not reported, ESKD end-stage kidney disease, HD haemodialysis, PD peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, CCPD continuous cycling peritoneal dialysis, KT kidney transplantation, LT living-donor transplantation, DT deceased-donor transplantation
Parameter Sources and Values
Parameter Sources and Values for Utility
In terms of effectiveness outcomes, nine out of ten studies used QALYs as the main outcome measure. Therefore, this review focuses on the derivation of utility values.
Table 3 presents the parameter sources and values. Data sources for utility values include observational surveys and previous literature. The methods used to measure utility values include Time Trade-Off (TTO), Standard Gamble (SG), EuroQol five-dimension scale (EQ-5D), Health Utilities Index (HUI), and SF-6D derived from SF-36 health survey. Moradpour et al. [20], Kontodimopoulos et al. [24], and de Wit et al. [29] obtained their utility scores from their observational surveys, and the remainder used utility values from previous literature. None of those studies obtaining utility values from previous literature fully describe the population characteristics of their cited studies. In the study by Jensen et al. [21], the utility values were derived from Sennfält et al. [30], who used the EQ-5D instrument to investigate a matched population in Sweden. Haller et al. [27] used the utility values directly from de Wit et al. [29]. The study by de Wit et al. [29] used SG, TTO, and EQ-5D to assess dialysis patients’ health states. It is worth noting that de Wit et al. [29] estimated the utility for KT based on three previous studies rather than by interview. Moradpour et al. [20] investigated a total of 214 ESKD patients using the Euro QOL EQ-5D-5L Persian version and they failed to report the main patient characteristics according to the KRT modality. Kontodimopoulos et al. [24] surveyed a total sample of 874 nationally representative ESKD patients and obtained their SF-6D utilities derived from SF-36. It should be noted that, in the study by Kontodimopoulos et al. [24], KT patients were significantly younger and had less co-morbidities than PD and HD patients, which may influence the utilities measured.
Table 3.
Parameter sources and values
| Author (year) | Measure of benefit | Utility values | Data sources for utility values | Methods of measurement of utility | Type and category of included costs | Country and reference year of costs | Cost values | Data sources for cost values |
|---|---|---|---|---|---|---|---|---|
| Moradpour et al. (2020) [20] | QALYs | HD utility 0.72; PD utility 0.75; KT utility 0.82 | Their observational study (n = 214) | EQ-5D-5L Persian version | Direct medical, direct non-medical (costs consumed by patients and caregivers) and indirect costs (income loss of the caregiver in a year) | In 2017 US dollars | Mean annual costs: PD $13,477; HD $12,865; KT $16,450 | Direct medical costs from hospital billing and medical records and direct non-medical costs, and indirect costs derived from interviews with patients (n = 214) |
| Rosselli et al. (2015) [26] | Deaths averted (per 1000 patients); months of life gained; months of dialysis averted; QALYs | HD utility 0.576; PD utility 0.668; KT utility 0.796 | A literature review using the Tufts University database of cost-effectiveness studies | NR | Direct medical costs: vascular access, medical and nursing care, medications, complications, surgical procedure | In 2012 US dollars | Monthly costs: PD $1,307; HD $1,321; KT $17,798 | From different local sources [case studies, official tariffs (ISS 2001 + 35%) for procedures and SISMED for medications] |
| Jensen et al. (2014) [21] | QALYs | HD utility 0.44; PD utility 0.65; KT utility 0.86 | From Sennfält et al. [30] using a matched population in Sweden (n = 81) | EQ-5D | Direct costs: treatment, check-ups, training, costs of removing organs for donation, transplantation, immunosuppressive treatment | In 2012 Danish Krone | Mean annual costs: hospital HD 358,176 DKK; home HD 165,636 DKK; PD 103,888 DKK; normal KT 180,722 DKK; complicated KT 511,899 DKK | From the Danish case-mix system 2012 and the Danish Registry of Medical Products Statistics |
| Shimizu et al. (2012) [22] | QALYs | HD utility 0.44; PD utility 0.53; LT utility 0.71; DT utility 0.57 | Utilities of HD, PD and LT from Lee et al. [51] using EQ-5D (n = 416); DT utility from Groome et al. [52] using SG (n = 66) | EQ-5D, SG | Direct health service costs: administrative fees for treatment, therapeutic agents, examinations, and outpatient care | In 2010 US dollars |
Mean annual costs: First-year: HD $7,294; PD $8684; LT $57,383; DT $76,184; Subsequent-year: HD $40,065; PD $68,989; LT $20,898; DT $20,898 |
The costs of dialysis from doctor’s certificates and the costs of KT estimated from studies by Higashiyama |
| Villa et al. (2012) [23] | QALYs | HD utility 0.69; PD utility 0.69; KT utility 0.81 | A literature review based on a proprietary database obtained from the Sanitary Research Fund Project 96/1327 [uncited] | SF-6D utilities derived from SF-36 | Direct and indirect costs | In 2010 Euros |
Mean annual costs: HD €47,825; PD €35,063; KT €50,079 |
From previous study by Villa et al. [53] |
| Haller et al. (2011) [27] | LYs; QALYs | HD utility 0.66; PD utility 0.81; KT utility 0.90 | From de Wit et al. [29] (n = 165) | EQ-5D, SG, TTO | Direct healthcare costs: inpatient and outpatient treatments, management of complications, investigations, blood tests, medications, radiological imaging procedures, consultations, nursing, all overhead costs such as costs for maintenance, physician and nurse fees, hospital administration, laundry, equipment and building acquisition, transportation, prescribed pharmaceuticals for outpatient treatment, non-ESKD-related admissions | NR |
Mean annual costs: First year: HD €43,600; PD €25,900; LT €50,900; DT €51,000; Second year: HD €40,000; PD €15,300; KT €17,200 Subsequent year: HD €40,600; PD €20,500; KT €12,900 |
From the Upper Austrian Health Insurance and the Elisabethinen Hospital Linz, including all patients who underwent chronic KRT at Elisabethinen Hospital Linz between 1 January 2001 and 31 December 2008 |
| Howard et al. (2009) [28] | LYs; QALYs | HD utility 0.55; PD utility 0.55; First-year KT utility 0.73; Second-year KT utility 0.70 | From Laupacis et al. [57] conducted at three University-based Canadian hospitals, a before and after study of utility based QOL in transplant recipients (n = 168) | TTO | Direct healthcare costs: dialysis equipment, buildings, maintenance, salaries and wages, consumables, costs of initial access, revision of access, drugs, hospitalizations, specialist consultations, review and work-up costs associated with the transplant waiting list, surgery, immunosuppressive therapy | In 2004 Australian dollars |
Mean annual costs: Initial HD access $9,766; Hospital HD $82,764; Home HD $44,739; Satellite HD $48,630; Initial PD access $9,259; CAPD $56,828; First-year LT $70,553; First-year DT $65,375; Subsequent-year LT $44,777; Subsequent-year DT $39,599 |
The costs of home and satellite HD from Agar et al. [54]; the cost of hospital HD costs and all inpatient healthcare from Australian Refined Diagnosis Related Group Version 4.2 codes and corresponding National Hospital Cost Data Collection Round 8 public sector costs [55]; PD costs from Western Australian health data [56] |
| Kontodimopoulos et al. (2008) [24] | QALYs | HD utility 0.639; PD utility 0.599; KT utility 0.716 | Their cross-sectional study (n = 874) | SF-6D utilities derived from SF-36 | Direct medical costs: equipment and infrastructure, diagnostic services, medications and consumables, salaries, operational costs, training costs | NR |
Mean annual costs: HD €36,247; PD €30,719; First-year KT €31,714; Three-year KT €43,275 |
Cost data for dialysis care from the annual accounts of three public and two private dialysis facilities; KT costs estimated from a report by the Hellenic National Transplant Organization [58] |
| de Wit et al. (1998) [29] | Life-years gained; QALYs | home HD utility 0.66; limited care HD utility 0.81; CAPD utility 0.71; CCPD utility 0.81; KT utility 0.90 | Dialysis utilities from the NECOSAD study (n = 165); KT utility based on author’s assumptions | EQ-5D, SG and TTO | Direct and indirect cost (excluding time costs and indirect costs resulting from work loss and inefficiency at work): hospitalizations, medication, work force, labor costs, materials, equipment, meals, housing, energy, laboratory services, diagnostic services, vascular access surgery, travel costs, transplantation operation | In 1996 Dutch Guilders |
Mean annual costs: first-year: center HD 152,666; limited care HD 134,531; home HD 129,456; CAPD 102,839; CCPD 129,951; KT 90,000; subsequent-year: center HD 145,757; limited care HD 127,622; home HD 114,547; CAPD 94,699; CCPD 121,811; KT 18,000 |
The costs of dialysis obtained from the NECOSAD study (n = 165); KT costs obtained from Hilbrands et al. [59] (n = 127) |
| Sesso et al. (1990) [25] |
Life-year of survival; First-year survival rates: HD 100%; CAPD 90%; LT 92%; cadaver KT 70%; Second-year survival rates: HD 91%; CAPD 90%; LT 81%; cadaver KT 70%; |
NA | NA | NA | Direct cost and induced direct costs due to side effects of the treatment: labor, equipment, supplies, transplant surgery, physician fees, hospital fees, laboratory costs, medications, costs related to changing the treatment modality | In 1985 US dollars |
Mean annual costs: First-year: HD $516,112; CAPD $282,996; LT $131,691; cadaver KT $76,468; Second-year: HD $911,913; CAPD $527,814; LT $196,112; cadaver KT $143,040 |
From patient charts and from the hospital accounting system (n = 121) |
NA not applicable, NR not reported, QALY quality-adjusted life year, LYs life-years, ESKD end-stage kidney disease, KRT kidney replacement therapy, HD haemodialysis, PD peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, CCPD continuous cycling peritoneal dialysis, KT kidney transplantation, LT living-donor transplantation, DT deceased-donor transplantation, EQ-5D EuroQol five-dimension scale, TTO Time Trade Off, SG Standard Gamble, HUI Health Utilities Index
There is a wide range of utility values across the included studies. The utility values for HD ranged from 0.44 to 0.72; for PD from 0.53 to 0.81; for KT from 0.57 to 0.90. In seven of the nine studies, KT utility was higher than PD utility, and PD utility was higher than HD utility [20–22, 24, 26]. In two of the nine studies, KT utility was higher than PD and HD utility, with PD and HD utility being equal [23, 28]. The study by de Wit et al. [29] suggests that conflicting results of utility valuations existed among different valuation methods. For example, continuous ambulatory PD patients’ EQ-5D scores were higher than those of center HD patients, while continuous ambulatory PD patients’ SG and TTO scores were lower than those of center HD patients.
Parameter Sources and Cost Inputs
Cost inputs were generally obtained from a combination of multiple sources, including the study hospitals, national institutions, published studies, expert opinion, and interviews with patients. Most of the studies reported adequate information on the key data sources, methods of cost estimation, the breakdown of cost items, currency, and the price year. However, it is not clearly reported in most studies whether resource use was valued at charges or real costs. Four studies [20, 24, 25, 27] obtained some of their resources from databases reflecting actual patient healthcare use, which facilitates the accuracy of the costs associated with each treatment modality. Moradpour et al. [20] and de Wit et al. [29] obtained their data on direct non-medical costs and indirect costs from interviews with patients.
There was a wide variety of types and categories of included costs, with the majority of studies including only the direct healthcare costs (such as diagnostic costs, outpatient routine costs, and hospitalization costs). Three of the ten studies claimed to have been conducted from a societal perspective [20, 23, 29], but only one study considered the productivity losses of patients and caregivers [20]. Although de Wit et al. [29] claimed to have adopted a societal perspective, they excluded time costs and indirect costs (productivity loss) in the cost analysis. Six of the ten studies differentiated between costs in the first year and subsequent years after initiation of KRT [22, 24, 25, 27–29]. This cost differentiation is necessary given that KT costs accumulate primarily in the first year and decrease substantially in subsequent years.
Summary of Cost-Effectiveness Results
Cost-Effectiveness Results
Table 4 summarizes the main results of the included studies. The variability in study design and study perspective hampers our ability to conduct a quantitative statistical analysis to combine the results of included studies. Overall, the majority of studies showed that KT was the most cost-effective KRT modality, and PD was more cost-effective than HD. Most studies suggested that KT held a dominant position over HD and PD with both lower costs and higher effectiveness [21, 22, 24, 26–29]. Studies conducted by Moradpour et al. [20] and Sesso et al. [25] indicated that KT did not dominate but was cost-effective compared with dialysis at the given willingness-to-pay (WTP) thresholds. All but the study by Sesso et al. [25] suggested that PD was more cost-effective than HD. Five out of ten studies evaluated the cost-effectiveness of different KRT assignment strategies [22, 23, 27–29]. These studies suggested that increased uptake of KT and PD by new ESKD patients would reduce costs and improve health outcomes or would be more cost-effective than current practice patterns.
Table 4.
Summary of cost-effectiveness results
| Author (year) | ICER/main results | Type of sensitivity analyses | Outcomes of sensitivity analyses | Authors’ conclusions |
|---|---|---|---|---|
| Moradpour et al. (2020) [20] | PD was dominant over HD; ICER for KT vs. PD: $1,446/QALY | Parameter SA: one-way SA and PSA using variation range of 20% for all input parameters |
The most influential parameters: the utility of KT, discount rate for outcomes, utility of HD, and cost of KT; The probability of KT being more cost-effective at a WTP threshold of $12,380 was 54.5%; The analysis result was not sensitive to parameter changes |
KT is cost-effective compared with PD at a WTP threshold of $12,400, and HD was dominated |
| Rosselli et al. (2015) [26] | KT was a cost-effective alternative from the second year and became the dominant alternative after the fourth year | Parameter SA: one-way SA, multivariate SA and PSA for all input parameters |
The most influential parameters: monthly cost of immunosuppression and monthly cost of dialysis; The probability of KT being more cost-effective at a WTP threshold of $20,000 was 76%; The analysis result was not sensitive to parameter changes |
KT improves the overall survival rates and quality of life and is a cost-saving alternative compared with dialysis |
| Jensen et al. (2014) [21] | KT holds a dominant position over dialysis with both lower costs (810,516 DKK versus 1,032,934 DKK) and higher effects (4.4 QALY versus 1.7 QALY) | Parameter SA: PSA for all input parameters |
KT has a 99.93% likelihood of being cost-effective at a WTP value of 0 DKK per QALY; The analysis result was not sensitive to parameter changes |
KT was the dominant treatment when compared with dialysis |
| Shimizu et al. (2012) [22] |
Base scenario (current composition of KRT) was dominated by Scenario 2 (likelihood of a pre-emptive LT increased by 2.4-times), Scenario 3 (likelihood of a LT increased by 2.4 times) and Scenario 4 (likelihood of a DT increased by 22 times); The ICER of Scenario 1 (likelihood of starting with PD increased by 2.3 times) over Base scenario was $5458/QALY |
Parameter SA: one-way SA for 95% confidence intervals of utilities and costs |
The most influential parameters: cost of HD and LT in subsequent years; ICERs were all less than $50,000/QALY, except in the case of a living donor transplant in subsequent years for Alternative 1 (likelihood of starting with PD increased by 2.3 times) |
Increased rate of KT and PD can reduce costs and improve health outcomes |
| Villa et al. (2012) [23] | Scenario 1 (57% of scheduled incident patients on any RRT modality) was dominated by all the proposed scenarios: Scenario 2 (increased proportion of overall scheduled incident patient to 75% from 57%), Scenario 3 (increased proportion of scheduled patients on PD to 30% from 10%), and Scenario 4 (combined scenarios 2 and 3) | Parameter SA: one-way SA for all parameters with variation rates of ± 10% |
The most influential parameters: utility and costs of PD; For scenario 4: a possible impact on the results from changes in the utility and costs of PD |
An increase in the overall scheduled incidence of KRT on PD should be promoted |
| Haller et al. (2011) [27] | Scenario 1 (current policy of assigning 90.6% of incident ESKD patients to HD, 7.2% to PD, 0.1% to LT and 2.1% to DT) was dominated by Scenario 2 (increasing PD to 20%) and Scenario 3 (increasing PD to 20% and increasing KT to 10%) | Parameter SA: one-way SA for policy parameters and model parameters |
The most influential parameters: PD costs, costs and transition probabilities for KT beyond 25 months after engraftment; The cost savings and gains in QALYs increase steadily in both the proportion of PD assignments and the proportion of LT |
KT and PD are more cost-effective than HD |
| Howard et al. (2009) [28] |
Base scenario (current practice) was dominated by Scenario 1 (an annual incremental increase in transplants to reach an extra 10% by 2010) and Scenario 2 (an annual incremental increase in transplants to reach an extra 50% by 2010) Scenario 3 (50% of patients commencing KRT on PD) and Scenario 4 (optimizing uptake of home HD by the second year on dialysis, 35% uptake in 25–44 years, 25% in 45–64 years; 10% in 65–74 years, 2% in 75 + years) is less costly and at least as effective compared with Base scenario |
Parameter SA: one-way SA for discount rate over the range 2.5–7.5% | Changes in the discount rate did not substantially influence the results | Increasing KT rates and moving towards home-based dialysis (PD and home HD) compared with hospital HD, can reduce costs and improve health outcomes |
| Kontodimopoulos et al. (2008) [24] | ICER not reported; the ratio of cost to QALY was higher in HD (€ 60,353) compared to PD (€ 54,504) and first year KT (€ 45,523) | Parameter SA: one-way SA using discount rates 3–10% for costs and 0–5% for QALYs | Changes in the discount rate did not substantially influence the results | KT is the most cost-effective KRT method, followed in order by PD and HD |
| de Wit et al. (1998) [29] | ICER not reported; the ratio of cost to life year gained for the 5 dialysis modalities was Dfl 133,100 versus Dfl 25,000 for transplantation; the ratio of cost to life years gained and cost to QALY was most favourable for CAPD and least favorable for center HD | Parameter SA: one-way SA for several scenarios of changing patients to less expensive modalities | The influence of the substitutive policies (policies directed towards substitution of patients from the center HD treatment modality to one of CAPD, limited care CHD, and home HD) considered in the sensitivity study was found to be modest in the Dutch context | Centre HD was found to be the least cost-effective treatment, while KT and CAPD were the most cost-effective treatments |
| Sesso et al. (1990) [25] | ICER of HD versus CAPD = $8,155; HD versus cadaver KT = $10,968; HD versus LT = $27,852; CAPD versus cadaver KT = $16,729; LT versus cadaver KT = $ 1,195 | Parameter SA: one-way SA for cost and effectiveness parameters | NR | CAPD is less cost-effective than HD and both are less cost-effective than KT |
NR not reported, QALY quality-adjusted life year, ESKD end-stage kidney disease, KRT kidney replacement therapy, HD haemodialysis, PD peritoneal dialysis, CAPD continuous ambulatory peritoneal dialysis, KT kidney transplantation, LT living-donor transplantation, DT deceased-donor transplantation, ICER incremental cost-effectiveness ratio, SA sensitivity analysis, PAS probabilistic sensitivity analysis, WTP willingness-to-pay
Analyses of Uncertainty
The issue of uncertainty was only partially explored in deterministic sensitivity analyses and probabilistic sensitivity analyses (PSA). All of the reviewed studies conducted sensitivity analyses to explore the implications of parameter uncertainty for the results of economic evaluations. However, none of the model-based economic evaluations dealt with the uncertainty related to the structure of the model. Moradpour et al. [20], Rosselli et al. [26], and Jensen et al. [21] performed PSA, while the remaining seven studies only performed deterministic one-way sensitivity analysis. Half of the included studies [20, 21, 23, 26, 27] performed sensitivity analyses for all input parameters, including transition probabilities, costs, and utilities. There was no key input parameter that was consistently influential across the included studies. In the majority of the studies, sensitivity analyses suggested that parameter changes did not substantially influence the results.
Discussion
Statement of Principal Findings
Model Design and Structure
All included model-based studies used a Markov model, indicating that these studies considered this approach appropriate for modelling ESKD. The structure of each model seemed adequate for reflecting the ESKD-specific health processes. All but one study [25] used time horizons of greater than 5 years, which seems suitable to reflect the long-term treatment outcomes. The study by Howard et al. [28] is the only study that contained time-dependent probabilities. Given that a patient characteristic may determine future patient pathways, the use of individual-level microsimulation could be of value in this condition. In addition, guidelines on good modeling practice advocate the need to explore structural uncertainty, which include simplifications and scientific judgments regarding the construction and interpretation of a model [44]. Nevertheless, none of the studies justified their approach in a sensitivity analysis.
Parameter Sources and Measurement
To some degree, there is a lack of availability of utility data sources with high quality for ESKD patients. The included studies used utility values from previous literature or observational surveys. Rosselli et al. [26] and Villa et al. [23] conducted a literature review to obtained utility values. However, this review was not systematic and they did not provide a critical appraisal of the primary studies included in their review. Several included studies derived their utility values from different sources, which makes it difficult to assess the validity of the results. In addition, utility values derived from observational studies are likely to suffer from selection bias given that study subjects were not randomly assigned to a treatment modality. Jensen et al. [21] obtained utility values from the previous literature by Sennfält et al. [30], which is the only study that aimed to minimize selection bias by matching on selected covariates, such as age, diabetes, heart disease, and living situation. However, this study had a relatively small sample size that comprised 27 matched triplets, thereby affecting the reliability of the results. In study by Haller et al. [27], there were differences in patient characteristics among HD, PD, and KT groups, with HD patients on average being more than 10 years older than PD and KT patients, which might impact the effectiveness of the three modalities. Nonetheless, Haller et al. [27] failed to explicitly discuss issues related to these differences and the impact that they could have on the results.
This raises a concern that the differences in utility values of three KRT modalities might be partly determined by the differences in patient characteristics and conditions. Sculpher points out that [45] the subgroup differences in cost-effectiveness can be attributed to different types of heterogeneity that can be associated with the treatment, the underlying disease, or patient preference. Previous studies suggest that patients commencing PD are usually younger and self-determined healthier individuals, while those commencing HD are in worse conditions and of older age [7, 27]. Given that most included studies did not report the population used to derive utility values or perform subgroup analyses, it remains unclear whether the differences in utility values can be explained by variations between subgroups of patients with different characteristics. Furthermore, it is unclear to what extent the cost-effectiveness results will be impacted by the selection bias.
In terms of cost inputs, the data sources for cost inputs in most reviewed studies were appropriate as they included the most relevant data sources in their setting. Most of the studies provided adequate details on the data sources, methods of cost estimation, and the breakdown of cost items, which enhances the validity of their cost measurement. The variation in the source and type of costs confounds evaluation of costs and makes it challenging to compare across studies.
The study perspective essentially determines the scope of costs that need to be accounted for in the economic evaluations [15]. Nonetheless, it is worth noting that two studies attempted societal perspective analyses but showed no evidence of indirect costs, especially productivity loss, in their analyses. Compared with the third-party payer and public healthcare perspective, the societal perspective considers a broader scope of healthcare costs and other costs associated with the relevant stakeholders in the society, such as time costs of informal caregivers and productivity loss of patients. The societal perspective allows for the identification of the transfer costs from the healthcare system to the household and society [8]. Hence, growing importance has been attached to considering the indirect costs borne by patients and their families [15, 36]. HD is primarily administered in ambulant dialysis units, while PD is predominantly performed at home [37]. The adoption of home-based HD and PD can shift some burden onto family members [27]. Moreover, previous studies show that KT patients have significantly higher employment rates compared with dialysis patients, with employment rates of 11–32% in HD patients, 28–50% in PD patients, and 30–70% in KT recipients [38–43]. The societal perspective can provide decision makers with a more comprehensive understanding of the cost-effectiveness of alternative options and may result in an optimal resource allocation in decision making.
Implications from Cost-Effectiveness Results
Overall, evidence collected from included studies indicates that KT is the most cost-effective KRT modality, with either a dominant position over HD and PD or an ICER well below the accepted WTP threshold. Although the data on costs and health outcomes are country-specific in each study, the comparative costs and effectiveness of KT relative to dialysis modalities are similar across studies. This finding remains consistent over time even though the clinical practice of KRT is likely to evolve. In addition, the majority of included studies suggest that PD is less costly and offers comparable or better health outcomes than HD. We interpret this finding with caution, as the evidence regarding this issue was inconclusive. In terms of cost differences between PD and HD, the majority of studies indicate that PD is a cost-saving therapy compared to HD. The lower cost of PD may be attributable to the minimal use of equipment, infrastructure, and staff time since PD is a primarily home-based treatment option [20]. Nonetheless, the study by Howard et al. [28] indicates that dialysis cost is highly health system-specific and their professional staff expenditure can vary widely from country to country. In terms of health outcomes, several studies assumed equal survival for HD and PD patients [20, 23, 27]. In terms of QOL, HD patients are inclined to have better social interaction and less isolation fear [31, 32]. In contrast, PD patients tend to report less illness intrusion, higher satisfaction, and better renal care [33, 34]. Overall, however, previous studies suggest that no significant difference in QOL exists between PD and HD patients [9, 29, 35]. Therefore, there is limited ability to generalize the finding that PD is more favorable than HD beyond the specific settings and assumptions.
Most of the included studies suggest that KT and PD should be prioritized and encouraged as a primary strategy for KRT for eligible patients when planning KRT services. Nonetheless, it should be noted that modality selection is a complex issue that can be driven by both medical and non-medical factors. Medical factors, such as contraindications, may disqualify patients from choosing a certain modality [46]. Under this circumstance, the cost-effectiveness findings may not be able to override the medical determinants of the modality selection. The cost-effectiveness findings can be of value in situations where non-medical factors play a significant role in influencing modality selection. Financial reimbursement policies have been identified as the most important non-medical factor contributing to modality selection [10]. In several countries that have initiated PD-First policies, efforts have been made to revise the reimbursement policy in a way to encourage utilization of PD [10]. In this sense, the cost-effectiveness findings that KT and PD are more favorable KRT modalities have essential policymaking implications.
Although it has been shown consistently across studies that KT is the optimal KRT modality, the expansion of KT may be challenging to achieve given the limited availability of donor organs. It is estimated that less than 20% of treated ESKD patients are living with a KT in approximately one-third of countries [3]. Therefore, in order to promote cost-effective management of ESKD, it is of great importance to implement specific strategies aimed at increasing kidney donations, such as reducing financial disincentive to living donors, improving donor transplant coordination, and increasing the use of expanded criteria donors [47, 48].
Strengths and Limitations
To our knowledge, this is the first systematic review that has focused on economic evaluations that compared three forms of KRT modalities specified as HD, PD, and KT. We included numerous electronic databases and manually searched the reference lists of relevant studies to ensure the sensitivity of the search strategy. The studies included in our review were conducted in several countries and represented various healthcare systems. Moreover, two reviewers independently carried out the study screening, data extraction and study appraisal, which reduced potential bias in the review process.
Our review has several limitations. First of all, we could not combine data across studies to draw any definitive conclusions regarding the cost-effectiveness of KRT modalities because of the heterogeneity in study designs. As described, all studies varied in their study designs, such as study perspective, cost category and estimations, time horizons, and model structure. Hence, it is impossible to convert findings across studies into a common estimation for comparison. Second, we included studies that compare HD, PD, and KT and excluded studies that compare only one modality or a mix of two different types of dialysis because other researchers have already carried out a systematic review or meta-analysis on them, while studies comparing all three modalities exist but nobody has reviewed them. Third, we excluded studies published in non-English languages and thus there is a potential omission of relevant articles from some cultures or ethnic backgrounds. Fourth, our review focused solely on the published literature, which represents a potential publication bias. Finally, we used the CHEERS checklist to appraise the included studies, which is prone to subjective reviewer judgments.
Considerations for Future Research
Our review has important implications for future research. First, considering the productivity costs associated with the KRT modality, more economic evaluations can be conducted from a societal perspective to quantify indirect costs such as productivity loss borne by patients and their families. Second, though most established models seemed adequate for reflecting the disease and its progression, many studies assumed time-independent probabilities for switching between modalities. Future research might expand more on complicated models to reflect the real-world disease progression and treatment patterns. Third, evidence on the cost-effectiveness of KRT modality in different subgroups of patients is scarce, with no included study having addressed the item of characterizing heterogeneity in the CHEERS checklist. The results may be confounded by differences between subgroups other than the KRT modality. Therefore, if feasible, further studies should also detail the evidence for subsets of patients with different characteristics. Finally, as evidence from future studies becomes available, it is necessary to update the assessment of the cost-effectiveness of KRT modality.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Author contributions
FY and ML designed the study. FY and ML developed the search strategy and screened the studies for eligibility. ML and FY extracted data from the included studies and reviewed reporting against CHEERS. ML and FY drafted the manuscript, with input from PW, ZY and YL. All authors read and approved the final manuscript.
Declarations
Funding
This study was funded by the Clinical Cultivation Project Foundation of Southern Medical University [LC2016PY029] and Shenzhen Key Research Base of Humanities and Social Sciences.
Conflict of interest
Fei Yang, Meixia Liao, Pusheng Wang, Zheng Yang, and Yongguang Liu declare that they have no conflicts of interest.
Ethics approval
Not applicable.
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Availability of data and material
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Code availability
Not applicable.
Footnotes
Fei Yang and Meixia Liao are co-first authors.
References
- 1.GBD Chronic Kidney Disease Collaboration Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2020;395:709–733. doi: 10.1016/S0140-6736(20)30045-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vanholder R, Annemans L, Brown E, Gansevoort R, Gout-Zwart JJ, Lameire N, et al. Reducing the costs of chronic kidney disease while delivering quality health care: a call to action. Nat Rev Nephrol. 2017;13:393–409. doi: 10.1038/nrneph.2017.63. [DOI] [PubMed] [Google Scholar]
- 3.United States Renal Data System . 2018 USRDS annual data report: Epidemiology of kidney disease in the United States. Bethesda: National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases; 2018. [Google Scholar]
- 4.Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380(9859):2095–2128. doi: 10.1016/S0140-6736(12)61728-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jha V, Garcia-Garcia G, Iseki K, Li Z, Naicker S, Plattner B, et al. Chronic kidney disease: global dimension and perspectives. Lancet. 2013;382(9888):260–272. doi: 10.1016/S0140-6736(13)60687-X. [DOI] [PubMed] [Google Scholar]
- 6.Liyanage T, Ninomiya T, Jha V, Jha V, Neal B, Patrice HM, et al. Worldwide access to treatment for end-stage kidney disease: a systematic review. Lancet. 2015;385:1975–1982. doi: 10.1016/S0140-6736(14)61601-9. [DOI] [PubMed] [Google Scholar]
- 7.Mowatt G, Vale L, Perez J, Wyness L, Fraser C, MacLeod A, et al. Systematic review of the effectiveness and cost-effectiveness, and economic evaluation, of home versus hospital or satellite unit haemodialysis for people with end-stage renal failure. Health Technol Assess. 2003;7(2):1–174. doi: 10.3310/hta7020. [DOI] [PubMed] [Google Scholar]
- 8.Howell M, Walker RC, Howard K. Cost effectiveness of dialysis modalities: a systematic review of economic evaluations. Appl Health Econ Health Policy. 2019;17:315–330. doi: 10.1007/s40258-018-00455-2. [DOI] [PubMed] [Google Scholar]
- 9.Chaudhary K, Sangha H, Khanna R. Peritoneal dialysis first: rationale. Clin J Am Soc Nephrol. 2011;6(2):447–456. doi: 10.2215/CJN.07920910. [DOI] [PubMed] [Google Scholar]
- 10.Liu FX, Gao X, Inglese G, Chuengsaman P, Pecoits-Filho R, Yu A. A global overview of the impact of peritoneal dialysis first or favored policies: an opinion. Perit Dial Int. 2015;35(4):406–420. doi: 10.3747/pdi.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yang CW, Harris DCH, Luyckx VA, Nangaku M, Hou FF, Garcia GG, et al. Global case studies for chronic kidney disease/end-stage kidney disease care. Kidney Int Suppl. 2020;10(1):e24–e48. doi: 10.1016/j.kisu.2019.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li PK-T, Chow KM. Peritoneal dialysis-first policy made successful: perspectives and actions. Am J Kidney Dis. 2013;62(5):993–1005. doi: 10.1053/j.ajkd.2013.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Bello AK, Levin A, Lunney M, Osman MA, Ye F, Ashuntantang GE, et al. Status of care for end stage kidney disease in countries and regions worldwide: international cross sectional survey. BMJ. 2019;367:l5873. doi: 10.1136/bmj.l5873. [DOI] [PubMed] [Google Scholar]
- 14.Ismail H, Abdul Manaf MR, Abdul Gafor AH, Mohamad Zaher ZM, Nur Ibrahim AI. International comparisons of economic burden of end-stage renal disease to the national healthcare systems. IIUM Med J Malays. 2019;18(3):188–196. [Google Scholar]
- 15.Drummond MF, Sculpher MJ, Claxton K, Stoddart GL, Torrance GW. Methods for the economic evaluation of health care programmes. 4. Oxford: Oxford University Press; 2015. [Google Scholar]
- 16.Walker R, Marshall MR, Morton RL, McFarlane P, Howard K. The cost-effectiveness of contemporary home haemodialysis modalities compared with facility haemodialysis: a systematic review of full economic evaluations. Nephrology. 2014;19(8):459–470. doi: 10.1111/nep.12269. [DOI] [PubMed] [Google Scholar]
- 17.Laplante S, Liu FX, Culleton B, Bernardo A, King D, Hudson P. The cost effectiveness of high-dose versus conventional haemodialysis: a systematic review. Appl Health Econ Health Policy. 2016;14:185–193. doi: 10.1007/s40258-015-0212-3. [DOI] [PubMed] [Google Scholar]
- 18.Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ. 2009;339:2535. [PMC free article] [PubMed] [Google Scholar]
- 19.Husereau D, Drummond M, Petrou S, Carswell C, Moher D, Greenberg D, et al. Consolidated health economic evaluation reporting standards (CHEERS) statement. Value Health. 2013;16(2):e1–5. doi: 10.1016/j.jval.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Moradpour A, Hadian M, Tavakkoli M. Economic evaluation of end stage renal disease treatments in Iran. Clin Epidemiol Glob Health. 2020;8(1):199–204. [Google Scholar]
- 21.Jensen CE, Sorensen P, Petersen KD. In Denmark kidney transplantation is more cost-effective than dialysis. Dan Med J. 2014;61(3):A4796. [PubMed] [Google Scholar]
- 22.Shimizu U, Saito S, Lings Y, Iino N, Kazama JJ, Akazawa K. Cost-effectiveness achieved through changing the composition of renal replacement therapy in Japan. J Med Econ. 2012;15(3):444–453. doi: 10.3111/13696998.2011.653512. [DOI] [PubMed] [Google Scholar]
- 23.Villa G, Fernandez-Ortiz L, Cuervo J, Rebollo O, Selgas R, Gonzalez T, et al. Cost-effectiveness analysis of the Spanish renal replacement therapy program. Perit Dial Int. 2012;32(2):192–199. doi: 10.3747/pdi.2011.00037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kontodimopoulos N, Niakas D. An estimate of lifelong costs and QALYs in renal replacement therapy based on patients’ life expectancy. Health Policy. 2008;86(1):85–96. doi: 10.1016/j.healthpol.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 25.Sesso R, Eisenberg JM, Stabile C, Draibe S, Ajzen H. Cost-effectiveness analysis of the treatment of end-stage renal disease in Brazil. Int J Technol Assess Health Care. 1990;6(1):107–114. doi: 10.1017/s0266462300008965. [DOI] [PubMed] [Google Scholar]
- 26.Rosselli D, Rueda JD, Diaz CE. Cost-effectiveness of kidney transplantation compared with chronic dialysis in end-stage renal disease. Saudi J Kidney Dis Transpl. 2015;26(4):733–738. doi: 10.4103/1319-2442.160175. [DOI] [PubMed] [Google Scholar]
- 27.Haller M, Gutjahr G, Kramar R, Harnoncourt F, Oberbauer R. Cost-effectiveness analysis of renal replacement therapy in Austria. Nephrol Dial Transpl. 2011;26(9):2988–2995. doi: 10.1093/ndt/gfq780. [DOI] [PubMed] [Google Scholar]
- 28.Howard K, Salkeld G, White S, McDonald S, Chadban S, Craig JC, et al. The cost-effectiveness of increasing kidney transplantation and home-based dialysis. Nephrology. 2009;14(1):123–132. doi: 10.1111/j.1440-1797.2008.01073.x. [DOI] [PubMed] [Google Scholar]
- 29.de Wit GA, Ramsteijn PG, de Charro FT. Economic evaluation of end stage renal disease treatment. Health Policy. 1998;44(3):215–232. doi: 10.1016/s0168-8510(98)00017-7. [DOI] [PubMed] [Google Scholar]
- 30.Sennfält K, Magnusson M, Carlsson P. Comparison of hemodialysis and peritoneal dialysis—a cost-utility analysis. Peritoneal Dialysis Int. 2002;22:39–47. [PubMed] [Google Scholar]
- 31.Juergensen E, Wuerth D, Finkelstein SH, Juergensen PH, Bekui A, Finkelstein FO. Hemodialysis and peritoneal dialysis: patients’ assessment of their satisfaction with therapy and the impact of the therapy on their lives. Clin J Am Soc Nephrol. 2006;1(6):1191–1196. doi: 10.2215/CJN.01220406. [DOI] [PubMed] [Google Scholar]
- 32.McLaughlin K, Manns B, Mortis G, Hons R, Taub K. Why patients with ESRD do not select self-care dialysis as a treatment option. Am J Kidney Dis. 2003;41(2):380–385. doi: 10.1053/ajkd.2003.50047. [DOI] [PubMed] [Google Scholar]
- 33.Brown EA, Johansson L, Farrington K, Gallagher H, Sensky T, Gordon F, et al. Broadening options for long-term dialysis in the elderly (BOLDE): differences in quality of life on peritoneal dialysis compared to haemodialysis for older patients. Nephrol Dial Transplant. 2010;25(11):3755–3763. doi: 10.1093/ndt/gfq212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Goodlad C, Brown E. The role of peritoneal dialysis in modern renal replacement therapy. Postgraduate Med J. 2013;89(1056):584–590. doi: 10.1136/postgradmedj-2012-131406. [DOI] [PubMed] [Google Scholar]
- 35.Liem YS, Bosch JL, Arends LR, Heijenbrok-Kal MH, Hunink MG. Quality of life assessed with the Medical Outcomes Study Short Form 36-Item Health Survey of patients on renal replacement therapy: a systematic review and meta- analysis. Value Health. 2007;10(5):390–397. doi: 10.1111/j.1524-4733.2007.00193.x. [DOI] [PubMed] [Google Scholar]
- 36.Gilbertson EL, Krishnasamy R, Foote C, Kennard AL, Jardine MJ, Gray NA. Burden of care and quality of life among caregivers for adults receiving maintenance dialysis: a systematic review. Am J Kidney Dis. 2019;73(3):332–343. doi: 10.1053/j.ajkd.2018.09.006. [DOI] [PubMed] [Google Scholar]
- 37.Scholten N, Ohnhaeuser T, Schellartz I, von Gersdorff G, Hellmich M, Karbach U, et al. Multidimensional analysis of factors responsible for the low prevalence of ambulatory peritoneal dialysis in Germany (MAU-PD): a cross-sectional Mixed-Methods Study Protocol. BMJ Open. 2019;9:e025451. doi: 10.1136/bmjopen-2018-025451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Manen JG, Korevaar JC, Dekker FW, Reuselaars MC, Boeschoten EW, Krediet RT, NECOSAD Study Group Netherlands cooperative study on adequacy of dialysis: changes in employment status in end-stage renal disease patients during their first year of dialysis. Perit Dial Int. 2001;21(6):595–601. [PubMed] [Google Scholar]
- 39.Curtin RB, Oberley ET, Sacksteder P, Friedman A. Differences between employed and nonemployed dialysis patients. Am J Kidney Dis. 1996;27(4):533–540. doi: 10.1016/s0272-6386(96)90164-x. [DOI] [PubMed] [Google Scholar]
- 40.Hirth RA, Chernew ME, Turenne MN, Pauly MV, Orzol SM, Held PJ. Chronic illness, treatment choice and workforce participation. Int J Health Care Finance Econ. 2003;3(3):167–181. doi: 10.1023/a:1025332802736. [DOI] [PubMed] [Google Scholar]
- 41.Rubin HR, Fink NE, Plantinga LC, Sadler JH, Kliger AS, Powe NR. Patient ratings of dialysis care with peritoneal dialysis vs hemodialysis. JAMA. 2004;291(6):697–703. doi: 10.1001/jama.291.6.697. [DOI] [PubMed] [Google Scholar]
- 42.Helanterä I, Haapio M, Koskinen P, Grönhagen-Riska C, Finne P. Employment of patients receiving maintenance dialysis and after kidney transplant: a cross-sectional study from Finland. Am J Kidney Dis. 2012;59(5):700–706. doi: 10.1053/j.ajkd.2011.08.025. [DOI] [PubMed] [Google Scholar]
- 43.van der Mei SF, Krol B, van Son WJ, de Jong PE, Groothoff JW, van den Heuvel WJ. Social participation and employment status after kidney transplantation: a systematic review. Qual Life Res. 2006;15(6):979–994. doi: 10.1007/s11136-006-0045-5. [DOI] [PubMed] [Google Scholar]
- 44.Bojke L, Claxton K, Sculpher M, et al. Characterizing structural uncertainty in decision analytic models: a review and application of methods. Value in Health. 2009;12(5):739–749. doi: 10.1111/j.1524-4733.2008.00502.x. [DOI] [PubMed] [Google Scholar]
- 45.Sculpher M. Subgroups and heterogeneity in cost-effectiveness analysis. Pharmacoeconomics. 2008;26(9):799–806. doi: 10.2165/00019053-200826090-00009. [DOI] [PubMed] [Google Scholar]
- 46.Blake PG, Quinn RR, Oliver MJ. Peritoneal dialysis and the process of modality selection. Perit Dial Int. 2013;33(3):233–241. doi: 10.3747/pdi.2012.00119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mathew T, Faull R, Snelling P. The shortage of kidneys for transplantation in Australia. Med J Aust. 2005;182:204–205. [PubMed] [Google Scholar]
- 48.Hamm D, Tizzard J. Presumed consent for organ donation is an ethical and effective way of dealing with organ donation shortages. BMJ. 2008;336:230. doi: 10.1136/bmj.39475.498090.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Higashiyama A, Okamura T, Watanabe M, et al. Effect of chronic kidney disease on individual and population medical expenditures in the Japanese population. Hypertens Res. 2009;32:450–454. doi: 10.1038/hr.2009.51. [DOI] [PubMed] [Google Scholar]
- 50.Nakatani T, Uchida J, Naganuma T. Health economics of kidney transplant. Jpn J Transplant. 2009;44:18–25. [Google Scholar]
- 51.Lee AJ, Morgan CL, Conway P, et al. Characterisation and comparison of health-related quality of life for patients with renal failure. Curr Med Res Opin. 2005;21:1777–1783. doi: 10.1185/030079905X65277. [DOI] [PubMed] [Google Scholar]
- 52.Groome PA, Hutchinson TA, Tousignant P, et al. The repeatability of three methods for measuring prospective patients’ values in the context of treatment choice for end-stage renal disease. J Clin Epidemiol. 1999;52:849–860. [PubMed] [Google Scholar]
- 53.Villa G, Rodríguez-Carmona A, Fernández-Ortiz L, Cuervo J, Rebollo P, Otero A, et al. Cost analysis of the Spanish renal replacement therapy programme. Nephrol Dial Transplant. 2011;26:3709–3714. doi: 10.1093/ndt/gfr088. [DOI] [PubMed] [Google Scholar]
- 54.Agar JW, Knight RJ, Simmonds RE, Boddington JM, Waldron CM, Somerville CA. Nocturnal haemodialysis: an Australian cost comparison with conventional satellite haemodialysis. Nephrology (Carlton) 2005;10:557–570. doi: 10.1111/j.1440-1797.2005.00471.x. [DOI] [PubMed] [Google Scholar]
- 55.Commonwealth Department of Health and Ageing . National Hospital Cost Data Collection. Cost Report Round 8 (2003–2004) AR-DRG v4.2. Canberra: Commonwealth Department of Health and Ageing; 2005. [Google Scholar]
- 56.Accountants BCC. Costing analysis of the renal dialysis services funded by the Health Department of Western Australia. Perth: Health Department of Western Australia; 1999. [Google Scholar]
- 57.Laupacis A, Keown P, Pus N, et al. A study of the quality of life and cost-utility of renal transplantation. Kidney Int. 1996;50:235–242. doi: 10.1038/ki.1996.307. [DOI] [PubMed] [Google Scholar]
- 58.Hellenic National Transplant Organization . Interim report on the proposed reimbursement of organ transplants in Greece. Athens: Hellenic National Transplant Organization; 2003. [Google Scholar]
- 59.Hilbrands LB, Hoitsma AJ, Koene RP. Randomized, prospective trial of cyclosporine monotherapy versus azathioprine-prednisone from three months after renal transplantation. Transplantation. 1996;61:1038–1046. doi: 10.1097/00007890-199604150-00009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.