Abstract
Natural products, which can be isolated from living organisms worldwide, have played a pivotal role in drug discovery since ancient times. However, it has become more challenging to identify a structurally novel molecule with promising biological activity for pharmaceutical development, mainly due to the limited methodologies for their acquisition. In this review, we summarize our recent studies that activate the biosynthetic potential of filamentous fungi by genetic engineering to harness the metabolic flow for the efficient production of unprecedented natural products. The recent revolution in genome sequencing technology enables the accumulation of vast amounts of information on biosynthetic genes, the blueprint of the molecular construction. Utilizing the established heterologous expression system, activation of the pathway-specific transcription factor coupled with a knockout strategy, and manipulating the global regulatory gene, the biosynthetic genes were exploited to activate biosynthetic pathways and decipher the encoded enzyme functions. We show that this methodology was beneficial for acquiring fungal treasures for drug discovery. These studies also enabled the investigation of the molecular function of natural products in fungal development.
Keywords: Biosynthesis, Natural products, Spiro compounds
Introduction
Humans utilize natural products, defined as small organic molecules, originating from natural resources such as plants, animals, and microbes [1]. The prescription of willow tree extract for the treatment of pain, fever, and childbirth by Hippocrates of Kos, the father of all doctors, more than 2000 years ago is an example of nature-driven drug discovery [2]. Although these medications were administered as crude materials since ancient times, the active substance salicin was identified and isolated for the first time in the nineteenth century. Purification of the substance enabled the development of acetylsalicylic acid, aspirin, a painkiller used to reduce pain, fever, and inflammation. Historically, once a compound was isolated as a pure material from natural resources, analysis of its effects on living organisms was greatly accelerated, making it possible to develop novel medicines. Therefore, human beings have been searching for compounds exhibiting useful biological activities from natural resources worldwide since the nineteenth century. At present, no small number of natural products or artificially designed molecules, inspired by the structure of natural products are being used as drugs [3]. Additionally, they are essential as medicines and research tools for unveiling many biological phenomena. For instance, leptomycin B, which was initially identified as an antifungal agent from the soil bacterium Streptomyces sp. [4], has revealed the function of its molecular target, Crm1, as a protein responsible for nuclear transportation in eukaryotes [5, 6]. Today, the molecule is commercially available and used in various biochemical studies such as investigations on site-specific roles of proteins that are mobile through the nuclear membrane. Moreover, the association between cancer and Crm1 has attracted attention, and an alternative Crm1 inhibitor, KPT-330 (Selinexor) [7], has been developed for the treatment of refractory myeloma. In addition, there are many examples of natural products that significantly contribute to opening a door for cryptic biological phenomena. As mentioned above, discovering novel natural products remains meaningful from the insights of both academic and industrial perspectives.
However, it is not easy to find new bioactive natural products using traditional methods [8]. Historically, to effectively identify unique substances, the biological sources searched for natural products have successfully shifted from readily available terrestrial plants to microorganisms (especially actinomycetes and molds) and then to living organisms at places not easily accessible by people, such as deep sea or extreme environments. Therefore, most organisms on Earth have been targeted as sources, with the most easily accessible substances already discovered. Indeed, many pharmaceutical companies that previously established their own natural product-based drug discovery departments and conducted research around the 1980s have since closed down. The focus has shifted to alternative modalities, such as antibodies, oligonucleotides, and regenerative medicines. Of the reasons that could be considered are (1) the discovery of new compounds is more difficult than before and (2) the identification of active compounds, structure determination, structure optimization, and establishment of a sustainable supply system are time-consuming and resource intensive.
In contrast, the revolution in next-generation sequencing technologies in the early twenty-first century sheds light on the revival of natural product-based drug discovery. Low costs and rapid deciphering of microbial genomes and transcriptomes, coupled with advances in genetic engineering technology, have revealed numerous natural product biosynthetic pathways [9]. They reported that structurally complicated natural products are constructed from simple building units such as amino acids and organic acids, so-called primary metabolites, through successive biochemical reactions carried out by multiple enzymes. Therefore, the accumulation of information on various biosynthetic enzymes has made it possible to utilize the biosynthetic genes discovered in the genome sequences as a blueprint for the construction of natural product scaffolds.
In particular, it has become possible to predict the molecular functions of biosynthetic enzymes, such as polyketide synthases (PKSs) [10] and non-ribosomal peptide synthetases (NRPSs) [11] which are responsible for the construction of scaffolds for natural products based on the components of domain architecture. Furthermore, phylogenetic analysis and insight into the conserved catalytic residues retrieved from the amino acid sequence of the enzymes provide information about the catalytic processes [12]. Alternatively, the DNA sequence analysis now allows us to estimate which genes are likely to lead to a novel natural product.
Fortunately, many studies have shown that microbial genomes contain more orphan biosynthetic genes than the number of compounds identified so far. For example, the genome of the filamentous fungus Aspergillus terreus, which is the producer of lovastatin, a treatment for dyslipidemia, contains more than 50 biosynthetic gene clusters (BGCs), many of which have unknown functions [13]. Moreover, transcriptome analysis revealed that a large number of biosynthetic genes are transcriptionally silent under conventional laboratory culture conditions, indicating that they are cryptic and unexploited but have the potential to produce molecules with unprecedented chemical structures [14]. With this background, we focused on the silent genes encoded in the fungal genome and sought to engineer the genes to activate the hidden biosynthetic pathways to produce structurally novel metabolites effectively. Additionally, en route to the aim, we have encountered nature-driven fascinating chemistry prompted by enzyme catalysis, which is also introduced and discussed in this review article.
Engineered biosynthesis of spirotryprostatin
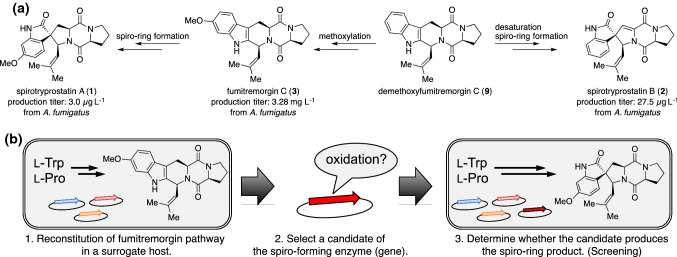
The natural products we initially targeted to be produced by genetic engineering-based activation of the pathway in a microbe were spirotryprostatins A (1) and B (2), originally isolated from the filamentous fungus Aspergillus fumigatus BM939 with extremely low titers (3.0 µg L−1 for 1 and 27.5 µg L−1 for 2) [15]. The compounds were reported to show cell cycle arrest in the mouse breast cancer cells tsFT210 at modest concentrations. Additionally, their complex structures have attracted attention in synthetic chemistry, and achievements of the total synthesis, including the elegant construction of the spiro ring, were reported by several groups [16, 17]. Alternatively, it remains unclear how the microbes build up the spirocycle in their metabolic process. We considered that the drastically decreased production titer of 1 and 2 in A. fumigatus as compared to the related compounds [for example, the titer of fumitremorgin C (3): 3.28 mg L−1] were likely caused by the transcriptionally silent biosynthetic gene that is responsible for spiro ring formation (Fig. 1a). Therefore, we hypothesized that the overexpression of biosynthetic genes will result in the overproduction of molecules.
Fig. 1.
Spirotryprostatins biosynthesis. a Predicted biosynthetic pathway toward spirotryprostatins A and B. b The strategy we applied for the investigation of the spiro-forming enzyme
Our initial attempt to identify the gene(s) responsible for the formation of the spiro ring using a gene-targeting approach was ineffective due to the unobservable productions of 1 and 2 in the cultured A. fumigatus A1159 by LC–MS analysis. This indicated that the reverse genetic strategy was not applicable to sufficiently identify the gene that yields the trace constituents. Therefore, to tackle this obstacle, we employed an alternative approach based on heterologous expression of the biosynthetic genes of the spirotryprostatin pathway in a surrogate host. We hypothesized that this strategy would characterize the spiro-forming enzyme and provide a sustainable production system for the molecules.
The strategy for screening the spiro-ring-forming enzyme is as follows (Fig. 1b). First, the biosynthetic pathway of fumitremorgin C (3), a plausible precursor of 1, is reconstituted in a heterologous host such as Saccharomyces cerevisiae through the introduction and overexpression of biosynthetic genes of 3.[18] Second, a candidate gene that plausibly codes for the enzyme catalyzing the desired reaction is introduced into the host with the fumitremorgin pathway. The production of 1 and 2 in the heterologous host allowed us to investigate the screened gene’s activity as a spiro-ring-forming enzyme.
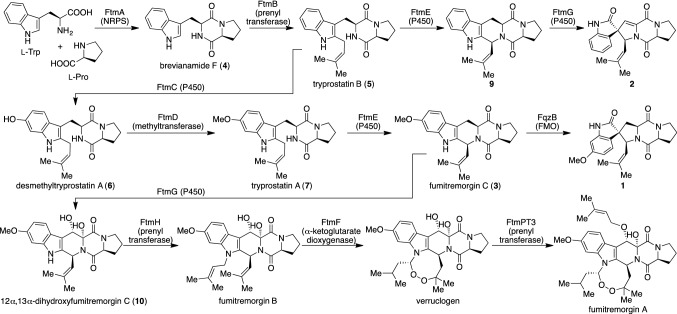
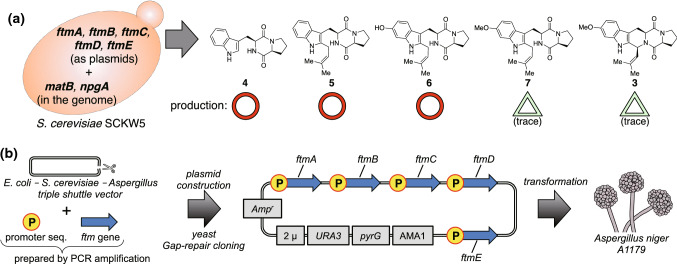
Based on the reported biosynthetic pathway of 3 [19–22], five biosynthetic enzymes are required for the production of 3 (Fig. 2). As the initial step, FtmA, which is a non-ribosomal peptide synthetase, catalyzes the formation of brevianamide F (4) from l-tryptophan and l-proline. Then, a prenyltransferase FtmB provides tryprostatin B (5) by adding a prenyl group to the indole ring of 4. Subsequent aromatic hydroxylation of 5 by a cytochrome P450 monooxygenase FtmC yields desmethyltryprostatin A (6), which is further converted to tryprostatin A (7) by methyltransferase FtmD. Finally, the second cytochrome P450 monooxygenase FtmE allows 7 to afford 3 via radical-prompted intramolecular cyclization. We cloned the open reading frames of these five genes (ftmA–E) tandemly connected with the active promoter for expression in S. cerevisiae. Additionally, to increase metabolite production, we established an engineered yeast strain SCKW5, a derivative of BY4705 containing two exogenous genes, matB (malonyl-CoA synthase from Rhizobium leguminosarum) and npgA (phosphopantetheinyl transferase from Aspergillus nidulans) in the chromosome (Fig. 3a) [23]. We confirmed the production of 4, 5, and 6, whereas 7 and 3 were not observed in this system. Although our attempts toward the mass production of 3, such as optimization of culture conditions, using highly expressed promoters, and adjusting the codon usage of the genes to that of S. cerevisiae, finally provided 3 as a trace amount in the culture, it was insufficient for the subsequent screening of the spiro-forming enzyme. We concluded that the methylation reaction catalyzed by FtmD was not sufficient in this condition, possibly due to either the failure of the production of catalytically active enzyme in the cell or lack of the cofactor S-adenosyl-l-methionine (SAM) for secondary metabolism. Therefore, we next exploited the filamentous fungus Aspergillus niger, a producer of secondary metabolites containing methyl groups, as a heterologous expression host. We chose A. niger A1179 (publicly available from the Fungal Genetics Stock Center, USA), a uridine/uracil auxotrophic strain (pyrG-) capable of being transformed by the protoplast-PEG method with a pyrG selection marker [24]. We modified the commercially available pPTRII expression plasmid [25] to construct a plasmid for the delivery of five ftm genes linked with high expression promoters [26] (the ftmA–E genes to the following promoters, glaA, amyB, gpdA, mbfA, and glaA, respectively). The A. niger transformed with this plasmid showed very high productivity of 3 (25 mg L−1) under starch-induced expression conditions (Fig. 3b).
Fig. 2.
The biosynthetic pathway of tryprostatin/spirotryprostatin/fumitremorgin-class fungal metabolites
Fig. 3.
Reconstitution of the biosynthetic pathway in the surrogate host. a S. cerevisiae-based heterologous expression system for producing tryprostatins. b A. niger-based heterologous expression system
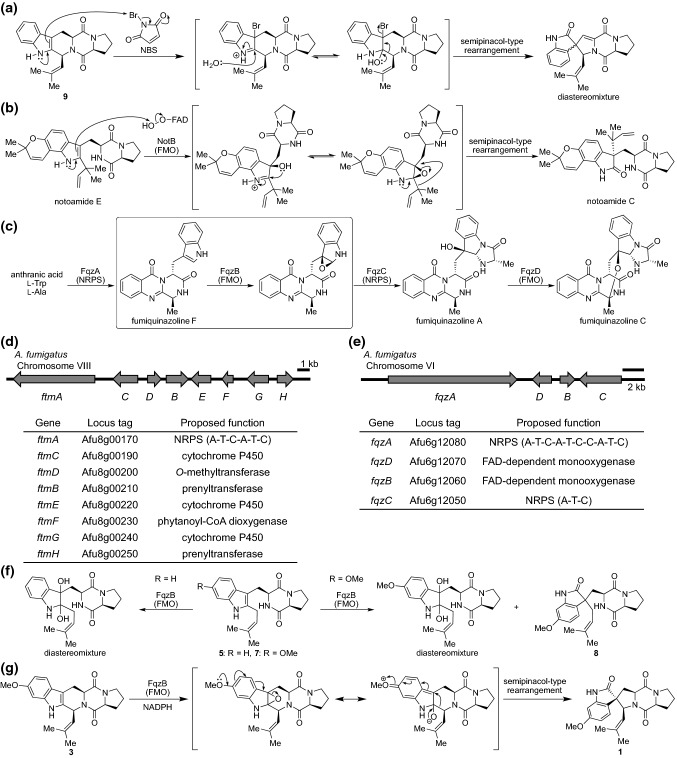
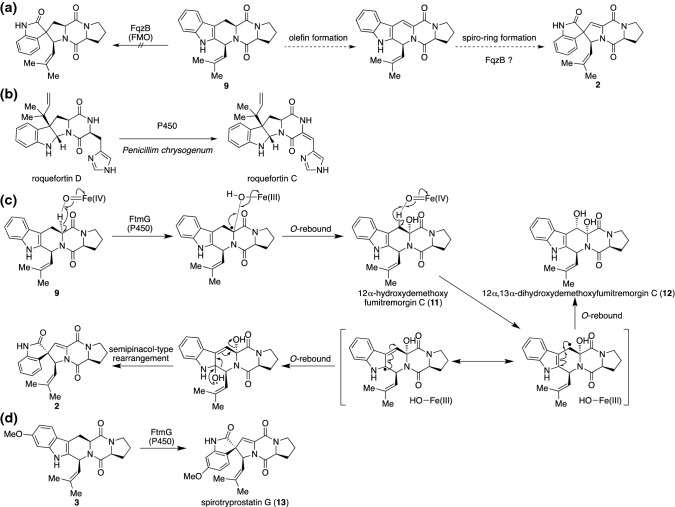
Hence, the situation was set to begin screening of the spiro-forming enzyme. We noticed that “biomimetic total synthesis” could provide valuable insight into how natural products are biosynthesized naturally. For the chemical synthesis of this class of compounds, a crucial step is a semipinacol-type rearrangement via oxidation at the 2,3-position of the indole ring to form spirooxindole using N-bromosuccinimide (NBS) [27]. Spiro-ring structures in spirotryprostatins were constructed synthetically using these methods (Fig. 4a). We predicted that an enzyme responsible for the oxidation at the indole ring was likely involved in the biosynthesis of spirotryprostatins. According to a report showing the enzyme NotB that converts notoamide E to notoamide C via the epoxidation of indole and subsequent semipinacol-type rearrangement (Fig. 4b) [28], we looked for a candidate gene that encodes a similar protein to NotB in the genome of A. fumigatus. We found fqzB (Afu6g12060), which encodes a flavoprotein and has been reported to oxidize an indole ring of fumiquinazoline F to generate a 1,2-epoxyindole intermediate toward the biosynthesis of fumiquinazoline A (Fig. 4c) [29]. Although fqzB was located at a distance to the ftm cluster (Fig. 4d, e), we sought to verify its role in spirotryprostatin biosynthesis because of the substrate’s shared substructure, the tryptophan residue. We introduced fqzB into the previously established plasmid synthesizing 3 in A. niger and successfully determined the production of 1 in the culture. The production titer (1.0 mg L−1) of the heterologous production was 333-fold higher than that of the wild-type strain A. fumigatus BM939 [30].
Fig. 4.
Determination of the spiro-forming enzyme for the biosynthesis of spirotryprostatin A. a A biomimetic synthesis for producing spirotryprostatin scaffold. b NotB-catalyzed semipinacol-type rearrangement in the biosynthesis of notoamide C. c The enzymatic function of FqzB in fumiquinazoline biosynthesis. d The BGC of tryprostatin/fumitremorgin. e The BGC of fumiquinazolines. f In vitro enzymatic reaction of FqzB with tryprostatins A and B. g FqzB-catalyzed ortho-quinonemethide formation is the crucial step in the spiro-ring formation
In addition to the in vivo determination, we verified the catalytic activity of FqzB in vitro. While the reaction yield was less than 1.0%, a recombinant FqzB protein obtained from Escherichia coli was able to transform 3 into 1 in the presence of NADPH, suggesting that the low reaction rate of the enzyme might have caused the trace production of 1 in the culture of wild-type A. fumigatus. It should also be noted that FqzB might be dedicated to the biosynthesis of fumiquinazolines, not to that of spirotryprostatins, due to its occurrence in the fumiquinazoline gene cluster. FqzB was also capable of converting 5 and 7 to the corresponding products by indole oxidation. Interestingly, the 2-oxindole derivative (8) that could be converted via semipinacol-type rearrangement was generated from 7 by FqzB (Fig. 4f), indicating that the generation of a para-quinone methide intermediate from the methoxy indole derivative was able to assist the rearrangement (Fig. 4g). Although FqzB showed a promiscuous substrate scope, it did not transformed demethoxylfumitremorgin C (9) into a spiro-compound, the predicted precursor of 2. The structural differences between 1 and 2, the lack of the methoxy group, and the presence of the unsaturated bond at C-12,13 in 2 led us to predict that the unsaturation reaction would precede the spiro-ring formation in the biosynthesis of 2 (Fig. 5a).
Fig. 5.
Determination of the spiro-forming enzyme for the biosynthesis of spirotryprostatin B. a In vitro analysis revealed the alternative pathway toward spirotryprostatin B. b Proposed pathway toward roquefortin C from roquefortin D. c Proposed mechanism of FtmG-catalyzed spiro-ring formation in the spirotryprostatin B biosynthesis. d FtmG is capable of transforming 3 into spirotryprostatin G (13), a new congener in spirotryprostatins
To decipher how the α,β-unsaturated amide was formed in the diketopiperadine (DKP) scaffold of 2, we investigated for natural products harboring a similar partial structure. We identified that roquefortines originally isolated from the fungus Penicillium chrysogenum carry the olefin at the histidine residue of the DKP, and their BGC was reported [31]. Although the enzyme catalyzing olefin formation was not investigated, we predicted each biosynthetic step toward roquefortine C production. We estimated that cytochrome P450 is likely responsible for olefin formation from the α,β-saturated amide because it promotes C–H activation-initiated oxidation (Fig. 5b). With BLASTP searches against the genome of A. fumigatus using the P450 gene of the roquefortin gene cluster as a query, we identified a cytochrome P450, FtmG, encoded in the ftm gene cluster as the most relevant protein. FtmG has been reported to install a vicinal dihydroxyl group at the C-12,13 of 3 for the formation of 12α,13α-dihydroxyfumitremorgin C (10) [22]. However, it has never been investigated as an olefin-forming enzyme. Thus, we tested whether FtmG catalyzes olefin formation using a yeast-based biotransformation assay. Surprisingly, using 9 as a substrate, we confirmed the generation of 2 as well as 12α-hydroxydemethoxyfumitremorgin C (11) and 12α,13α-dihydroxydemethoxyfumitremorgin C (12) in the culture of ftmG-expressing S. cerevisiae BY4705 but not in the negative controls (Fig. 5c). This unanticipated result showed us that FtmG, not FqzB, is indeed a spiro-ring-forming enzyme for the production of 2. The enzymatic reaction of FtmG was further verified by an in vitro assay with the microsome fraction obtained from the yeast transformant expressing ftmG. The structures of the reaction products were unambiguously elucidated based on NMR spectroscopy. Accordingly, four genes (ftmA, ftmB, ftmE, and ftmG) were introduced into the host A. niger for de novo production of 2, and they successfully afforded 2 with a titer of 4.0 mg L−1 (145-fold increase to that of the wild type) in this system. As seen above, the spiro-ring formation of two structurally similar natural products, 1 and 2, is unexpectedly catalyzed by distinct enzymes, FqzB and FtmG, respectively, with independent reaction mechanisms. For the biosynthesis of 1, the initial step is the epoxidation of the indole ring catalyzed by the hydroperoxide reaction center of the flavoenzyme FqzB. Then, the electron-rich aromatic methoxide accelerated an epoxide opening concomitant with the formation of a labile ortho-quinone-methide intermediate, which immediately undergoes semipinacol-type rearrangement to afford 1 (Fig. 4g). In contrast, biosynthesis of 2 occurs through a radical mechanism in which the Fe(IV) =O catalytic core in the heme of cytochrome P450 FtmG catalyzes stepwise C–H activation. The crucial step for spirocycle formation is the second round of radical generation at C-13 of 11. We proposed that the radical migrated from C-13 to C-2 ahead of rebound to the Fe(IV)–OH center. Finally, semipinacol-type rearrangement of the hemiaminal intermediate provides a spiro-ring with the α,β-unsaturated amide scaffold of 2 (Fig. 5c). Thus, we showed the distinct mechanisms, the epoxide route, or the radical route, for the spiro-carbon formation in the spirotryprostatin biosynthesis. The results further extended to produce a novel natural product, spirotryprostatin G (13), carrying the aromatic methoxide with α,β-unsaturated amide, by utilizing the biocatalyst FtmG with the substrate 3 (Fig. 5d). We demonstrated that the A. niger-based fungal expression system is a powerful tool for identifying the enzyme function and enhancing the production titer for natural products isolated from natural resources as trace components. It should be noted that successive studies have established a modified system using Aspergillus nidulans A1145 as a heterologous expression host, capable of harboring triple plasmids, for the study of fungal natural products [32–34].
Expression of the transcriptional regulator in the silent gene cluster
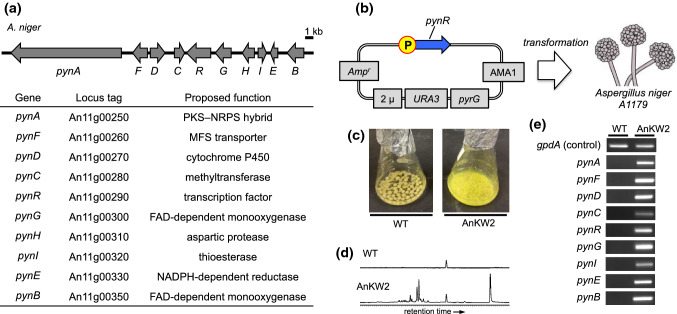
In contrast to the above study, in which biosynthesis of rare natural products was achieved by the overexpression of multiple biosynthetic genes in the heterologous host, we thought that a more rapid, easy-handling, and versatile method needed to be developed. We focused on pathway-specific transcription factor genes that were occasionally located within the BGCs of filamentous fungi [35]. To identify such genes, we exploited a genome mining approach based on the homology search of the desired proteins in the genome database. The transcription factor we examined was the gene embedded in the BGC that was transcriptionally inactive and consisted of a unique combination of auxiliary enzyme genes. We identified pynR (An11g00290) in the genome of A. niger as a candidate because its BGC that contains a hybrid of polyketide synthase–non-ribosomal peptide synthetase (PKS–NRPS) was thoroughly silent by RNA-seq analysis. In addition, they contained multiple proteins with unknown functions, such as PynH, which harbors a fold of aspartic protease that had never been reported as a modification enzyme for the biosynthesis of natural products (Fig. 6a). Therefore, we employed A. niger A1179 to be transformed by the plasmid carrying the pynR gene connected to the inducible glaA promoter (Fig. 6b). Interestingly, the culture broth exhibited a pale yellow color under the condition of pynR expression induction (Fig. 6c). Comparative LC–MS analysis between the wild-type and the mutant revealed a specific production of more than 20 chemical species in the mutant (Fig. 6d). Our RT-PCR analysis indicated that all genes in the pyn gene cluster, not only for pynR, were strongly transcribed in the pynR-expressing mutant (Fig. 6e). Hence, we characterized the produced compounds by purification and following NMR-based structure elucidation, revealing that structurally novel metabolites, pyranonigrins E–G (14–16), were discovered with high production titers (each more than 20 mg L−1, Fig. 7a). Pyranonigrin E is a relative of pyranonigrins A and S, which were also isolated from A. niger and have a γ-pyrone ring in the structure (Fig. 7b) [36]. However, the difference in alkyl chain length and amino acid origin suggested that the distinct enzyme might biosynthesize them. Of note, prior to our report, pyranonigrin E was independently discovered and designated by Awakawa et al. [37] and Riko et al., who reported a different chemical structure [38]. Therefore, to avoid confusion, we propose the former and the latter be referred to as pyranonigrin E1 and pyranonigrin E2, respectively. We were further interested in the biosynthesis of pyranonigrins that contain characteristic substructures such as fused γ-pyrone, amino acid-derived exo-methylene, and cyclobutane [39].
Fig. 6.
Activation of a silent gene cluster (pyn cluster) in A. niger. a The pyn gene cluster for the biosynthesis of pyranonigrin E1. b The experimental design for the overexpression of the transcription factor, pynR. c The culture of the pynR-expressed mutant (AnKW2) and the wild type (WT). d HPLC analysis of AnKW2 and WT. The chromatogram was monitored at λ = 280 nm. e RT-PCR analysis of the pyn cluster
Fig. 7.
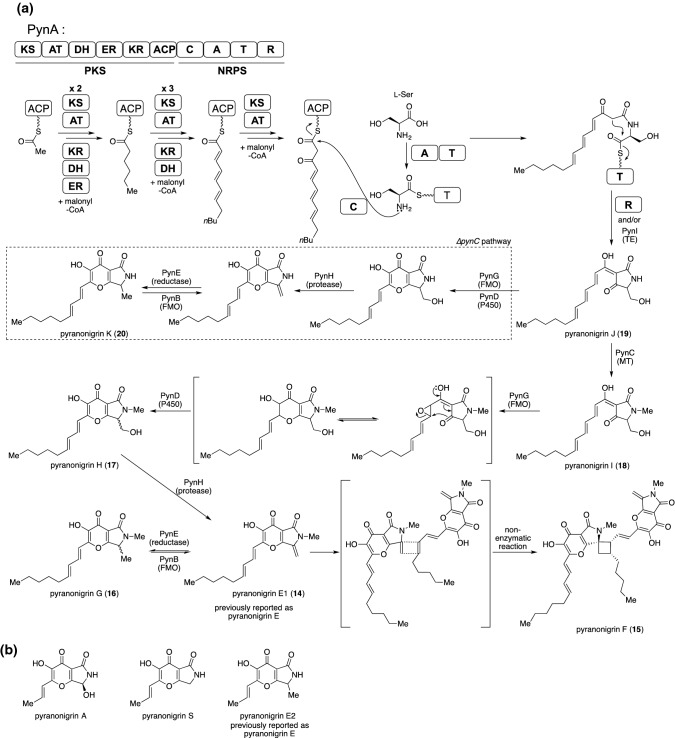
The pyranonigrin biosynthesis. a Proposed biosynthetic pathway of pyranonigrin E1, in which production was activated through the overexpression of pynR. b The chemical structures of the other pyranonigrins
To analyze how each biosynthetic enzyme participates in the biosynthesis of pyranonigrins, we constructed a line of knockout mutant that was deficient of the gene allocated to the pyn cluster. Each knockout strain was produced by homologous recombination with the protoplast-PEG procedure. We identified newly produced metabolites, pyranigrins H–K (17–20), from the mutants, ∆pynH, ∆pynG, ∆pynC, and ∆pynB. The chemical structures of the obtained products suggested the respective enzyme’s role in the biosynthetic pathway, and they were subsequently determined by experiments, such as enzymatic reactions with recombinant proteins or biotransformation assays using heterologous expression systems. Briefly, acetyl-CoA, malonyl-CoA, and l-serine were the starting materials for biosynthesis, and they were assembled to produce a tetramic acid intermediate (19) by PynA, which is a PKS–NRPS hybrid megasynthase. After methylation to 19 was performed by PynC with SAM to produce 18, successive oxidations triggered by PynG (FMO) coupled with PynD (cytochrome P450 monooxygenase) afforded 17 as a γ-pyrone ring-containing product. A protein carrying an aspartic protease fold, PynH, catalyzes the dehydration of l-serine-derived secondary alcohol to produce an exo-methylene, a hallmark of 14. Interestingly, 14 and 16 can be interconverted by the catalysis of PynE (reductase) and PynB (FMO). As similar mutual conversions have also been reported in the biosynthesis of chaetoviridin [40], there might be a shared strategy in fungal metabolite biosynthesis, presumably for effectively conducting a prodrug strategy. Furthermore, we confirmed that the dimeric structure of 15 was sufficiently generated from 14 by spontaneous [2 + 2] electrocyclization, rather than by enzyme catalysis. The identification of 20, which is the demethylated form of 16, from the ∆pynC mutant indicated that the biosynthetic enzymes PynG, PynD, PynH, and PynE have a promiscuous substrate scope. We showed that it is an effective strategy to provide structurally novel natural products through biosynthetic analyses of metabolites that are encoded by the silent gene cluster of fungus. This strategy was further extended to explore other fungal metabolites such as shanorellins [41] from Chaetomium globosum and aspirochlorines [42] from Aspergillus oryzae.
Activation of the biosynthetic pathway in basidiomycete fungi
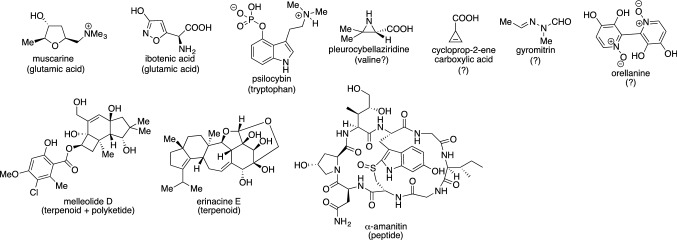
Previously discussed studies intended to activate specific biosynthetic genes of interest that were bioinformatically predicted to be involved in the biosynthesis of natural products. The following section presents a study aimed to activate nonspecific regions in the genome to acquire unexpected natural products. Similar to ascomycetes, basidiomycetes are one of the microorganisms that make up the fungal kingdom. They are widely known to produce fruiting bodies (mushrooms) for sporulation, and many of them have been used as traditional medicines in the world, while no small numbers of species are toxic to humans [43]. Therefore, basidiomycetes have been recognized as a treasure trove of biologically active substances for the development of drugs. Many natural products have been identified through traditional methods such as picking in wild fields and artificial cultivation. Taking a look at the genomes of basidiomycete fungi, it reveals the natural products’ biosynthetic genes with a domain structure similar to that of ascomycetes. Indeed, polyketides (for example, melleolide D), peptides (for example, α-amanitin), and terpenes (for example, erinacine E), in which biosynthesis is well studied, have also been isolated and structurally determined from basidiomycetes (Fig. 8). Moreover, basidiomycetous natural products that are likely produced through unusual biosynthetic pathways have also been identified, such as muscarine, ibotenic acid [44], psilocybin [45], pleurocybellaziridine [46], cycloprop-2-ene carboxylic acid [47], orellanine [48], and gyromitrin [49] (Fig. 8). Of note, many compounds are known to exhibit potent biological activity, while their biosynthetic machinery has remained elusive.
Fig. 8.
The secondary metabolites isolated from basidiomycete fungi. The proposed biosynthetic origin is bracketed
According to the examples of successful activation of biosynthetic pathways from silent BGCs of ascomycetes, phylogenetically related basidiomycetes are also potential candidates for biosynthesizing hidden natural products encrypted in the genome. However, distinct from ascomycetes, limited numbers of basidiomycetes species have been genetically engineered with knockout and overexpression, hampering the biosynthetic analysis of its natural products. Therefore, to establish a way to study the natural products, we selected the mushroom fungus Coprinopsis cinerea [50] (commonly known as gray shag), used as a model organism for studying fruiting body formation with genetics due to its ability to manipulate genes.
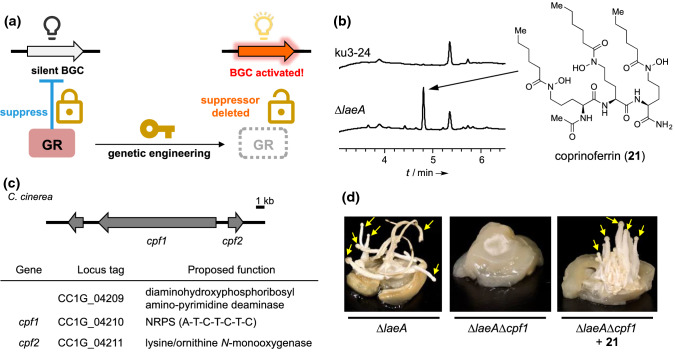
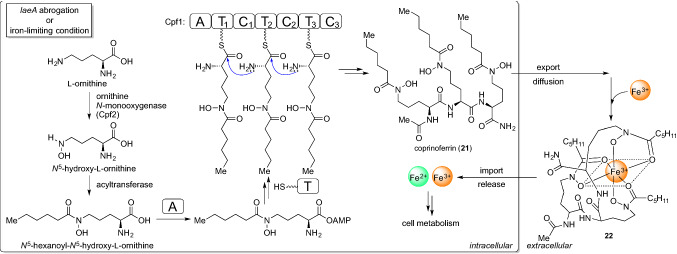
To activate the cryptic pathway in C. cinerea, we focused on laeA homologs encoded in the genome. LaeA is a member of the conserved transcriptional regulatory velvet complex involved in secondary metabolism and sexual development in ascomycetes [51]. Because the loss of laeA decreases the production of secondary metabolites, LaeA is recognized as a global regulator of secondary metabolism in ascomycetes [52]. While the molecular function of LaeA remains unclear, the encoded protein has an SAM-binding motif that is representative of methyltransferase, and is predicted to be involved in epigenetic regulation associated with secondary metabolism [53]. Therefore, we designed to exploit a laeA homologous gene in C. cinerea to induce the production of natural products with novel chemical structures. We selected a gene (CC1G_00498, hereafter laeA) homologous to laeA from A. fumigatus as a candidate (identity = 48%). We engineered C. cinerea ku3-24 strain [54], which was deficient in ku70 for adequate gene replacement, to construct the laeA disruption mutant (C. cinerea ∆laeA) and analyzed the production of the metabolites (Fig. 9a). We observed an increased production of a compound with the molecular formula C35H65N7O10 by LC–HRESIMS analysis in the ∆laeA strain (Fig. 9b). The compound was purified from the large-scale cultivation of ∆laeA, structurally elucidated to be an unidentified molecule, and designated as coprinoferrin (21) [55]. The chemical structure of 21, which carries a trimeric N5-acylated-N5-hydroxyl-l-ornithine residue, suggested that it might form a complex with Fe(III) or other metal ions [56]. We experimentally confirmed the Fe(III)-binding property of 21, and its iron complex (22) was also detectable in the broth of ∆laeA culture. It has been suggested that 21 plays the role of siderophore [57] widely used for incorporating environmental Fe(III) into the cell in various microorganisms. To determine the contribution of 21 to hyphal growth and development in C. cinerea, we analyzed the biosynthesis of 21. Based on its chemical structure, we searched the genome of C. cinerea and identified CC1G_04210 (hereafter cpf1) that encodes NRPS carrying a single adenylation module with triplicated thiolation-condensation didomains, as a candidate gene (Fig. 9c). Gene knockout experiments confirmed that cpf1 is essential for the production of both 21 and 22. Additionally, characteristic phenotypes such as stunning hyphal growth and deficiency in fruiting body formation were observed in the C. cinerea ∆laeA∆cpf1 strain, while these phenotypes were restored by administration of 21 to the culture medium (Fig. 9d). These results suggest that C. cinerea biosynthesizes 21 to effectively acquire environmental Fe(III) and utilize it for their growth and development. Additional experiments determined that C. cinerea ∆laeA∆cpf1 was able to form a fruiting body where excess Fe(III) was accumulated, but 21 did not require a high concentration of Fe(III). This suggests that 21 is able to diffuse into the medium and solubilize and transport Fe(III) into the fungal cells (Fig. 10). We also observed trace production of 21 in the wild type C. cinerea, whereas, the maximum titer was tenfold lower than that of the ∆laeA strain. Although the reason for the higher accumulation of 21 in C. cinerea in response to laeA deficiency remains unexplained, we showed that genetic engineering of the global regulator is a strategy for the activation of secondary metabolism in basidiomycete fungi. Interestingly, the gene cluster containing cpf1 is highly conserved in more than two hundred genome-sequenced basidiomycete fungi. We have recently confirmed that some of the species also produce 21, suggesting that 21 may be a common metabolite among a kind of basidiomycetes, and it is possible to have a role in driving the growth and development of mushroom fungi. Although many fungi produced 21, our strategy enabled the discovery of the molecule for the first time. As such, we showed that genetic engineering is a powerful tool for the discovery of natural products.
Fig. 9.
Activation of the cryptic pathway in C. cinerea. a The strategy for the activation of secondary metabolism by a deletion of the global regulator (GR), laeA. b HPLC analysis determined the increased production of coprinoferrin in ∆laeA. The parent strain (ku3-24) also produces 21 with a few production titer. c The BGC of coprinoferrin. d The fruiting body formation assay. ∆laeA∆cpf1 did not differentiate into the fruiting body. However, the phenotype was restored when 21 was administered to the culture
Fig. 10.
The proposed biosynthetic pathway of coprinoferrin
Closing remarks
In this review, we summarized our recent studies focusing on the activation of biosynthetic pathways in filamentous fungi. We introduced yeast- and A. niger-based heterologous expression systems for the sustainable production of natural products that were limited to the original fungal strain. This synthetic biology strategy was also demonstrated to decipher the specialized biosynthetic pathways of natural products. However, there are still issues to be addressed in these systems. In particular, in the yeast system, we could not obtain the desired products due to several unknown factors. Although we raised some possibilities, such as the loss of enzyme activity in the heterologous host or insufficient supply of the cofactors, we realized that it is not always easy to determine the reasons for the intercepting metabolic process. A systematic understanding of heterologous cells, such as the balance of gene expression, substrate supply level, solubility, or folding of the produced enzyme, will provide a versatile tool for the metabolic engineering of natural products. Second, we showed the genetic engineering of the transcription regulator embedded in the silent BGC of fungus to unveil novel natural products with high titers. In combination with the pathway disruption by knockout strategy, it further allowed us to identify a series of biosynthetic products and provide information on the pathway toward the final product from the starting materials. Subsequent analyses revealed unique biosynthetic systems, including enzymatic function, which catalyze an unprecedented biochemical transformation. Finally, our recent strategy to activate the basidiomycetous biosynthetic pathway was introduced. Genetic engineering of a global regulator laeA in C. cinerea was capable of inducing siderophore biosynthesis. Our reverse genetic approach based on the deletion of cpf1 responsible for coprinoferrin production revealed the significant roles of siderophore in mushroom fungus on hyphal growth and fruiting body formation. Although this strategy does not apply to a wide range of basidiomycete fungi due to the difficulty in genetically transforming them, recent technological advances such as genome editing [58] will help decipher the secondary metabolism of basidiomycetes. In addition, one of our current missions is to use C. cinerea as a heterologous host for the expression of the gene from basidiomycete, and we hope it will be achieved in the near future [59]. In summary, we insist that it is still possible to provide structurally novel secondary metabolites, as there is a vast amount of accumulated information on biosynthetic genes in the database. These valuable resources, obtained worldwide, need to be efficiently utilized for drug discovery and natural product research.
Acknowledgements
I would like to thank Professor Kenji Watanabe of the University of Shizuoka for all his support and his nomination for the Award for the Pharmacognosy Society of Japan (PSJ) Award for Young Scientist 2019. I would like to express my appreciation to Professor Daisuke Uemura of Kanagawa University (also as an emeritus professor at Nagoya University), Professor Hideaki Kakeya of Kyoto University, and Professor Christian Hertweck of Leibniz Institute for Natural Product Research and Infection Biology—Hans Knöll Institute (HKI)—for their supervision and insightful suggestions. Drs. Noriyasu Ishikawa, Tsuyoshi Yamamoto of Watanabe’s laboratory alumni, and Mr. Jun Takanishi of the current member of the laboratory significantly contributed to the research mentioned above. This work was supported in part by a Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (JSPS KAKENHI Grant numbers 17K15265, 20K05866). I would like to thank Editage (www.editage.com) for English language editing. Finally, I sincerely thank the committee members of the Japan Society of Pharmacognosy for their encouragement and nomination for the award.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- 2.Nicolaou KC, Montagnon T (eds) Aspirin, Chap. 4. In: Molecules that changed the world: a brief history of the art and science of synthesis and its impact on society. Wiley-VCH, Weinheim, pp 385 (ISBN 9783527309832)
- 3.Newman DJ, Cragg GM. Natural products as sources of new drugs over the nearly four decades from 01/1981 to 09/2019. J Nat Prod. 2020;83:770–803. doi: 10.1021/acs.jnatprod.9b01285. [DOI] [PubMed] [Google Scholar]
- 4.Hamamoto T, Seto H, Beppu T. Leptomycins A and B, new antifungal antibiotics. II. Structure elucidation. J Antibiot (Tokyo) 1983;36:646–650. doi: 10.7164/antibiotics.36.646. [DOI] [PubMed] [Google Scholar]
- 5.Nishi K, Yoshida M, Fujiwara D, Nishikawa M, Horinouchi S, Beppu T. Leptomycin B targets a regulatory cascade of crm1, a fission yeast nuclear protein, involved in control of higher order chromosome structure and gene expression. J Biol Chem. 1994;269:6320–6324. doi: 10.1016/S0021-9258(17)37374-X. [DOI] [PubMed] [Google Scholar]
- 6.Ferreira BI, Cautain B, Grenho I, Link W. Small molecule inhibitors of CRM1. Front Pharmacol. 2020;11:625. doi: 10.3389/fphar.2020.00625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benkova K, Mihalyova J, Hajek R, Jelinek T. Selinexor, selective inhibitor of nuclear export: unselective bullet for blood cancers. Blood Rev. 2020 doi: 10.1016/j.blre.2020.100758. [DOI] [PubMed] [Google Scholar]
- 8.Tsunematsu Y, Nishimura S, Hattori A, Oishi S, Fujii N, Kakeya H. Isolation, structure elucidation, and total synthesis of tryptopeptins A and B, new TGF-beta signaling modulators from Streptomyces sp. Org Lett. 2015;17:258–261. doi: 10.1021/ol503340k. [DOI] [PubMed] [Google Scholar]
- 9.Walsh CT, Fischbach MA. Natural products version 2.0: connecting genes to molecules. J Am Chem Soc. 2010;132:2469–2493. doi: 10.1021/ja909118a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hertweck C. The biosynthetic logic of polyketide diversity. Angew Chem Int Ed Engl. 2009;48:4688–4716. doi: 10.1002/anie.200806121. [DOI] [PubMed] [Google Scholar]
- 11.Hur GH, Vickery CR, Burkart MD. Explorations of catalytic domains in non-ribosomal peptide synthetase enzymology. Nat Prod Rep. 2012;29:1074–1098. doi: 10.1039/c2np20025b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen F, Tholl D, Bohlmann J, Pichersky E. The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J. 2011;66:212–229. doi: 10.1111/j.1365-313X.2011.04520.x. [DOI] [PubMed] [Google Scholar]
- 13.Romsdahl J, Wang CCC. Recent advances in the genome mining of Aspergillus secondary metabolites (covering 2012–2018) Medchemcomm. 2019;10:840–866. doi: 10.1039/c9md00054b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brakhage AA. Regulation of fungal secondary metabolism. Nat Rev Microbiol. 2013;11:21–32. doi: 10.1038/nrmicro2916. [DOI] [PubMed] [Google Scholar]
- 15.Cui CB, Kakeya H, Osada H. Novel mammalian cell cycle inhibitors, spirotryprostatins A and B, produced by Aspergillus fumigatus, which inhibit mammalian cell cycle at G2/M phase. Tetrahedron. 1996;52:12651–12666. doi: 10.1016/0040-4020(96)00737-5. [DOI] [Google Scholar]
- 16.Sebahar PR, Williams RM. The asymmetric total synthesis of (+)- and (−)-spirotryprostatin B. J Am Chem Soc. 2000;122:5666–5667. doi: 10.1021/ja001133n. [DOI] [Google Scholar]
- 17.Edmondson S, Danishefsky SJ, Sepp-Lorenzino L, Rosen N. Total synthesis of spirotryprostatin A, leading to the discovery of some biologically promising analogues. J Am Chem Soc. 1999;121:2147–2155. doi: 10.1021/ja983788i. [DOI] [Google Scholar]
- 18.Tsunematsu Y, Ishiuchi K, Hotta K, Watanabe K. Yeast-based genome mining, production and mechanistic studies of the biosynthesis of fungal polyketide and peptide natural products. Nat Prod Rep. 2013;30:1139–1149. doi: 10.1039/c3np70037b. [DOI] [PubMed] [Google Scholar]
- 19.Li SM. Genome mining and biosynthesis of fumitremorgin-type alkaloids in ascomycetes. J Antibiot (Tokyo) 2011;64:45–49. doi: 10.1038/ja.2010.128. [DOI] [PubMed] [Google Scholar]
- 20.Maiya S, Grundmann A, Li SM, Turner G. The fumitremorgin gene cluster of Aspergillus fumigatus: identification of a gene encoding brevianamide F synthetase. ChemBioChem. 2006;7:1062–1069. doi: 10.1002/cbic.200600003. [DOI] [PubMed] [Google Scholar]
- 21.Grundmann A, Li SM. Overproduction, purification and characterization of FtmPT1, a brevianamide F prenyltransferase from Aspergillus fumigatus. Microbiology. 2005;151:2199–2207. doi: 10.1099/mic.0.27962-0. [DOI] [PubMed] [Google Scholar]
- 22.Kato N, Suzuki H, Takagi H, Asami Y, Kakeya H, Uramoto M, et al. Identification of cytochrome P450s required for fumitremorgin biosynthesis in Aspergillus fumigatus. ChemBioChem. 2009;10:920–928. doi: 10.1002/cbic.200800787. [DOI] [PubMed] [Google Scholar]
- 23.Ishiuchi K, Nakazawa T, Ookuma T, Sugimoto S, Sato M, Tsunematsu Y, et al. Establishing a new methodology for genome mining and biosynthesis of polyketides and peptides through yeast molecular genetics. ChemBioChem. 2012;13:846–854. doi: 10.1002/cbic.201100798. [DOI] [PubMed] [Google Scholar]
- 24.Meyer V, Arentshorst M, El-Ghezal A, Drews AC, Kooistra R, van den Hondel CA, et al. Highly efficient gene targeting in the Aspergillus nigerkusA mutant. J Biotechnol. 2007;128:770–775. doi: 10.1016/j.jbiotec.2006.12.021. [DOI] [PubMed] [Google Scholar]
- 25.Kubodera T, Yamashita N, Nishimura A. Transformation of Aspergillus sp. and Trichoderma reesei using the pyrithiamine resistance gene (ptrA) of Aspergillus oryzae. Biosci Biotechnol Biochem. 2002;66:404–406. doi: 10.1271/bbb.66.404. [DOI] [PubMed] [Google Scholar]
- 26.Blumhoff M, Steiger MG, Marx H, Mattanovich D, Sauer M. Six novel constitutive promoters for metabolic engineering of Aspergillus niger. Appl Microbiol Biotechnol. 2013;97:259–267. doi: 10.1007/s00253-012-4207-9. [DOI] [PubMed] [Google Scholar]
- 27.Wang H, Ganesan A. A biomimetic total synthesis of (-)-spirotryprostatin B and related studies. J Org Chem. 2000;65:4685–4693. doi: 10.1021/jo000306o. [DOI] [PubMed] [Google Scholar]
- 28.Li S, Finefield JM, Sunderhaus JD, McAfoos TJ, Williams RM, Sherman DH. Biochemical characterization of NotB as an FAD-dependent oxidase in the biosynthesis of notoamide indole alkaloids. J Am Chem Soc. 2012;134:788–791. doi: 10.1021/ja2093212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ames BD, Liu X, Walsh CT. Enzymatic processing of fumiquinazoline F: a tandem oxidative-acylation strategy for the generation of multicyclic scaffolds in fungal indole alkaloid biosynthesis. Biochemistry. 2010;49:8564–8576. doi: 10.1021/bi1012029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tsunematsu Y, Ishikawa N, Wakana D, Goda Y, Noguchi H, Moriya H, et al. Distinct mechanisms for spiro-carbon formation reveal biosynthetic pathway crosstalk. Nat Chem Biol. 2013;9:818–825. doi: 10.1038/nchembio.1366. [DOI] [PubMed] [Google Scholar]
- 31.Garcia-Estrada C, Ullan RV, Albillos SM, Fernandez-Bodega MA, Durek P, von Dohren H, et al. A single cluster of coregulated genes encodes the biosynthesis of the mycotoxins roquefortine C and meleagrin in Penicillium chrysogenum. Chem Biol. 2011;18:1499–1512. doi: 10.1016/j.chembiol.2011.08.012. [DOI] [PubMed] [Google Scholar]
- 32.Kato H, Tsunematsu Y, Yamamoto T, Namiki T, Kishimoto S, Noguchi H, et al. New natural products isolated from Metarhizium robertsii ARSEF 23 by chemical screening and identification of the gene cluster through engineered biosynthesis in Aspergillus nidulans A1145. J Antibiot (Tokyo) 2016;69:561–566. doi: 10.1038/ja.2016.54. [DOI] [PubMed] [Google Scholar]
- 33.Yokoyama M, Hirayama Y, Yamamoto T, Kishimoto S, Tsunematsu Y, Watanabe K. Integration of chemical, genetic, and bioinformatic approaches delineates fungal polyketide-peptide hybrid biosynthesis. Org Lett. 2017;19:2002–2005. doi: 10.1021/acs.orglett.7b00559. [DOI] [PubMed] [Google Scholar]
- 34.Sato M, Yagishita F, Mino T, Uchiyama N, Patel A, Chooi YH, et al. Involvement of lipocalin-like CghA in decalin-forming stereoselective intramolecular [4 + 2] cycloaddition. ChemBioChem. 2015;16:2294–2298. doi: 10.1002/cbic.201500386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lyu HN, Liu HW, Keller NP, Yin WB. Harnessing diverse transcriptional regulators for natural product discovery in fungi. Nat Prod Rep. 2020;37:6–16. doi: 10.1039/c8np00027a. [DOI] [PubMed] [Google Scholar]
- 36.Schlingmann G, Taniguchi T, He H, Bigelis R, Yang HY, Koehn FE, et al. Reassessing the structure of pyranonigrin. J Nat Prod. 2007;70:1180–1187. doi: 10.1021/np070175n. [DOI] [PubMed] [Google Scholar]
- 37.Awakawa T, Yang XL, Wakimoto T, Abe I. Pyranonigrin E: a PKS-NRPS hybrid metabolite from Aspergillus niger identified by genome mining. ChemBioChem. 2013;14:2095–2099. doi: 10.1002/cbic.201300430. [DOI] [PubMed] [Google Scholar]
- 38.Riko R, Nakamura H, Shindo K. Studies on pyranonigrins-isolation of pyranonigrin E and biosynthetic studies on pyranonigrin A. J Antibiot (Tokyo) 2014;67:179–181. doi: 10.1038/ja.2013.91. [DOI] [PubMed] [Google Scholar]
- 39.Yamamoto T, Tsunematsu Y, Noguchi H, Hotta K, Watanabe K. Elucidation of pyranonigrin biosynthetic pathway reveals a mode of tetramic acid, fused gamma-pyrone, and exo-methylene formation. Org Lett. 2015;17:4992–4995. doi: 10.1021/acs.orglett.5b02435. [DOI] [PubMed] [Google Scholar]
- 40.Sato M, Winter JM, Kishimoto S, Noguchi H, Tang Y, Watanabe K. Combinatorial generation of chemical diversity by redox enzymes in chaetoviridin biosynthesis. Org Lett. 2016;18:1446–1449. doi: 10.1021/acs.orglett.6b00380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsunematsu Y, Ichinoseki S, Nakazawa T, Ishikawa N, Noguchi H, Hotta K, et al. Overexpressing transcriptional regulator in Chaetomium globosum activates a silent biosynthetic pathway: evaluation of shanorellin biosynthesis. J Antibiot (Tokyo) 2012;65:377–380. doi: 10.1038/ja.2012.34. [DOI] [PubMed] [Google Scholar]
- 42.Tsunematsu Y, Maeda N, Yokoyama M, Chankhamjon P, Watanabe K, Scherlach K, et al. Enzymatic amide tailoring promotes retro-aldol amino acid conversion to form the antifungal agent aspirochlorine. Angew Chem Int Ed Engl. 2018;57:14051–14054. doi: 10.1002/anie.201806740. [DOI] [PubMed] [Google Scholar]
- 43.Lin HC, Hewage RT, Lu YC, Chooi YH. Biosynthesis of bioactive natural products from Basidiomycota. Org Biomol Chem. 2019;17:1027–1036. doi: 10.1039/c8ob02774a. [DOI] [PubMed] [Google Scholar]
- 44.Obermaier S, Muller M. Ibotenic acid biosynthesis in the fly agaric is initiated by glutamate hydroxylation. Angew Chem Int Ed Engl. 2020 doi: 10.1002/anie.202001870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Fricke J, Blei F, Hoffmeister D. Enzymatic synthesis of psilocybin. Angew Chem Int Ed Engl. 2017;56:12352–12355. doi: 10.1002/anie.201705489. [DOI] [PubMed] [Google Scholar]
- 46.Wakimoto T, Asakawa T, Akahoshi S, Suzuki T, Nagai K, Kawagishi H, et al. Proof of the existence of an unstable amino acid: pleurocybellaziridine in Pleurocybella porrigens. Angew Chem Int Ed Engl. 2011;50:1168–1170. doi: 10.1002/anie.201004646. [DOI] [PubMed] [Google Scholar]
- 47.Matsuura M, Saikawa Y, Inui K, Nakae K, Igarashi M, Hashimoto K, et al. Identification of the toxic trigger in mushroom poisoning. Nat Chem Biol. 2009;5:465–467. doi: 10.1038/nchembio.179. [DOI] [PubMed] [Google Scholar]
- 48.Spiteller P, Spiteller M, Steglich W. Occurrence of the fungal toxin orellanine as a diglucoside and investigation of its biosynthesis. Angew Chem Int Ed Engl. 2003;42:2864–2867. doi: 10.1002/anie.200351066. [DOI] [PubMed] [Google Scholar]
- 49.Karlson-Stiber C, Persson H. Cytotoxic fungi—an overview. Toxicon. 2003;42:339–349. doi: 10.1016/S0041-0101(03)00238-1. [DOI] [PubMed] [Google Scholar]
- 50.Stajich JE, Wilke SK, Ahren D, Au CH, Birren BW, Borodovsky M, et al. Insights into evolution of multicellular fungi from the assembled chromosomes of the mushroom Coprinopsis cinerea (Coprinus cinereus) Proc Natl Acad Sci USA. 2010;107:11889–11894. doi: 10.1073/pnas.1003391107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bayram O, Braus GH. Coordination of secondary metabolism and development in fungi: the velvet family of regulatory proteins. FEMS Microbiol Rev. 2012;36:1–24. doi: 10.1111/j.1574-6976.2011.00285.x. [DOI] [PubMed] [Google Scholar]
- 52.Nakazawa T, Ishiuchi K, Sato M, Tsunematsu Y, Sugimoto S, Gotanda Y, et al. Targeted disruption of transcriptional regulators in Chaetomium globosum activates biosynthetic pathways and reveals transcriptional regulator-like behavior of aureonitol. J Am Chem Soc. 2013;135:13446–13455. doi: 10.1021/ja405128k. [DOI] [PubMed] [Google Scholar]
- 53.Jain S, Keller N. Insights to fungal biology through LaeA sleuthing. Fungal Biol Rev. 2013;27:51–59. doi: 10.1016/j.fbr.2013.05.004. [DOI] [Google Scholar]
- 54.Nakazawa T, Honda Y. Absence of a gene encoding cytosine deaminase in the genome of the agaricomycete Coprinopsis cinerea enables simple marker recycling through 5-fluorocytosine counterselection. FEMS Microbiol Lett. 2015;362:fnv123. doi: 10.1093/femsle/fnv123. [DOI] [PubMed] [Google Scholar]
- 55.Tsunematsu Y, Takanishi J, Asai S, Masuya T, Nakazawa T, Watanabe K. Genomic mushroom hunting decrypts coprinoferrin, a siderophore secondary metabolite vital to fungal cell development. Org Lett. 2019;21:7582–7586. doi: 10.1021/acs.orglett.9b02861. [DOI] [PubMed] [Google Scholar]
- 56.Brandenburger E, Gressler M, Leonhardt R, Lackner G, Habel A, Hertweck C, et al. A highly conserved basidiomycete peptide synthetase produces a trimeric hydroxamate siderophore. Appl Environ Microbiol. 2017;83:e01478–e1517. doi: 10.1128/AEM.01478-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hider RC, Kong X. Chemistry and biology of siderophores. Nat Prod Rep. 2010;27:637–657. doi: 10.1039/b906679a. [DOI] [PubMed] [Google Scholar]
- 58.Sugano SS, Suzuki H, Shimokita E, Chiba H, Noji S, Osakabe Y, et al. Genome editing in the mushroom-forming basidiomycete Coprinopsis cinerea, optimized by a high-throughput transformation system. Sci Rep. 2017;7:1260. doi: 10.1038/s41598-017-00883-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Asai S, Tsunematsu Y, Masuya T, Otaka J, Osada H, Watanabe K. Uncovering hidden sesquiterpene biosynthetic pathway through expression boost area-mediated productivity enhancement in basidiomycete. J Antibiot (Tokyo) 2020;73:721–728. doi: 10.1038/s41429-020-0355-9. [DOI] [PubMed] [Google Scholar]