Abstract
Rechargeable solid-state sodium-ion batteries (SSSBs) hold great promise for safer and more energy-dense energy storage. However, the poor electrochemical stability between current sulfide-based solid electrolytes and high-voltage oxide cathodes has limited their long-term cycling performance and practicality. Here, we report the discovery of the ion conductor Na3-xY1-xZrxCl6 (NYZC) that is both electrochemically stable (up to 3.8 V vs. Na/Na+) and chemically compatible with oxide cathodes. Its high ionic conductivity of 6.6 × 10−5 S cm−1 at ambient temperature, several orders of magnitude higher than oxide coatings, is attributed to abundant Na vacancies and cooperative MCl6 rotation, resulting in an extremely low interfacial impedance. A SSSB comprising a NaCrO2 + NYZC composite cathode, Na3PS4 electrolyte, and Na-Sn anode exhibits an exceptional first-cycle Coulombic efficiency of 97.1% at room temperature and can cycle over 1000 cycles with 89.3% capacity retention at 40 °C. These findings highlight the immense potential of halides for SSSB applications.
Subject terms: Batteries, Engineering, Batteries
Rechargeable solid-state sodium-ion batteries hold great promise for safer and more energy-dense energy storage. Here, the authors show a new sodium-based halide, Na3-xY1-xZrxCl6, for sodium-all-solid-state batteries with enhanced ionic conductivity and long-term cycling stability.
Introduction
A solid-state architecture for rechargeable sodium-ion batteries has garnered substantial research interest in recent years1–5. By replacing flammable organic liquid electrolytes with solid electrolytes (SEs), solid-state sodium-ion batteries (SSSB) promise not only higher safety, but also potentially enable higher voltage cathodes, metal anodes, and stacking architectures to achieve higher energy densities. In addition, the higher abundance of sodium relative to lithium makes sodium-ion batteries a more cost-effective alternative, especially for large-scale grid storage applications where low operating costs are more strongly emphasized than a high energy density6. However, an ideal SE has to meet a stringent set of requirements, namely high ionic conductivity, low electronic conductivity, and electrochemical, chemical, and mechanical compatibility with electrodes. While major breakthroughs have been made in achieving liquid-like ionic conductivity values in sulfide SEs, their poor electrochemical and chemical interfacial stability against common electrodes remains a critical bottleneck for practical applications.
Recently, two lithium halide superionic conductors, Li3YCl6 and Li3YBr6, have been reported as promising SEs for solid-state lithium-ion batteries7. Exhibiting reasonable Li+ conductivities in the range of 0.5-0.7 mS cm−1, the most interesting feature of these halide SEs is their electrochemical and chemical stability, demonstrated via compatibility with the 4 V LiCoO2 cathode7. As a result, more studies have since emerged on halide SEs (Li3InCl6 and LixScCl3+x) that also exhibit high Li+ diffusivity, compatibility with LiCoO2, and facile processability8–10. In addition to these, there has also been a report on aliovalent substitution with Zr, yielding the halide Li3−xM1−xZrxCl6 (M = Er, Y), where the introduction of vacancies led to an increase in the ionic conductivity up to the order of 10−3 S/cm at room temperature11. Interestingly, unlike the fast Li-ion conducting sulfides or oxides, fast Li-ion conduction in these halide frameworks do not require a bcc anion sublattice, allowing a much wider selection of compositions when designing halide SE chemistries10. It is important to note that to a first approximation, the oxidative electrochemical stability of SEs are determined by anion chemistry10,12,13, and for halides, it generally follows the trend F > Cl>Br>I10,13.
In contrast to the Li halides mentioned, the Na analogs Na3YCl6 and Na3YBr6 have been relatively less studied; previous studies have reported experimental ionic conductivities on the order of 10−4–10−6 S/cm at 500 K. These materials are therefore expected to have much lower room-temperature ionic conductivities than their lithium counterparts and thus impractical for SE applications14. Here, we report the data-driven development of Na3-xY1-xZrxCl6 (NYZCx) as a new class of sodium SEs exhibiting high ionic conductivities as well as excellent electrochemical and chemical stability up to 3.8 V vs Na/Na+. Using density functional theory (DFT) calculations, it was predicted that aliovalent doping of Y3+ with Zr4+ would improve the Na+ conductivity of Na3YCl6 by three orders of magnitude, while retaining a wide electrochemical window and good chemical stability. A SSSB comprising a NaCrO2:NYZC0.75:vapor grown carbon fibers (VGCF) composite cathode with Na3PS4 (NPS) as the SE and a Na-Sn (2:1) anode exhibited an extremely high first cycle Coulombic efficiency (CE) of 97.6% at room temperature. Even when cycled at 40 °C and a rate of 1 C, the SSSB displayed stable electrochemical performance over 1000 cycles with 89.3% capacity retention.
Results and discussion
Electrochemically stable and conductive Na3-xY1-xZrxCl6
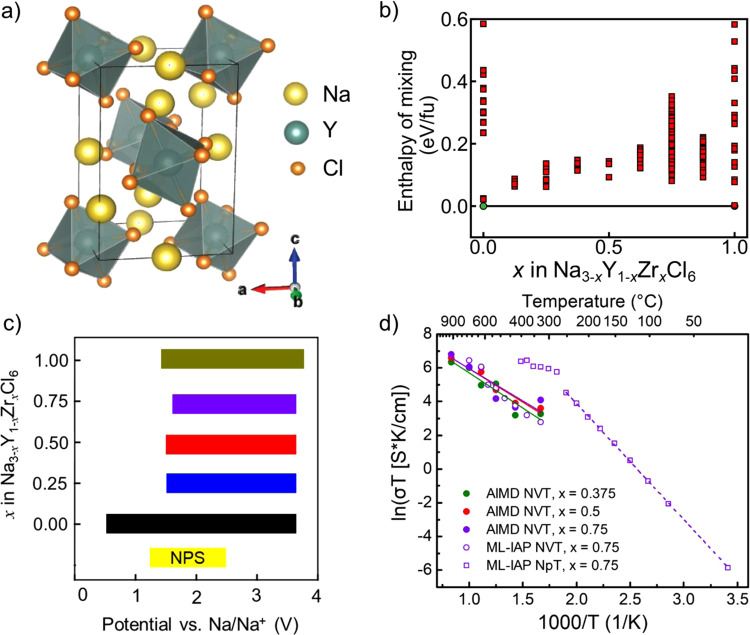
Unlike its lithium counterpart, Na3YCl6 (NYC) (Fig. 1a, space group: P21/n) does not exhibit partial occupancy in the 2d and 4e Na sites, which may explain its lower ionic conductivity. A series of ions (Ti4+, Zr4+, Hf4+, and Ta5+) were evaluated as potential aliovalent dopants for Y3+ to increase the concentration of defects and thus the ionic conductivity of Na3-(z-3)xY3+1-xMz+xCl62,4,15–17. The effect of ionic substitution on the phase stability of NYC is shown in Supplementary Figure 1. Zr4+ is predicted to exhibit a low dopant formation energy and is also low-cost, due to the abundance of Zr. Furthermore, the enthalpies of mixing of the NYC-Na2ZrCl6 (NZC) pseudo-binary system are low, as shown in Fig. 1b.
Fig. 1. Effect of Zr dopants on properties of Na3YCl6.
a Crystal structure of Na3YCl6. b Stability of Na3-xY1-xZrxCl6 after incorporating Zr4+ into Na3YCl6. Each square marker indicates a symmetrically distinct ordering of Na and Y/Zr. c Electrochemical stability window of Na3-xY1-xZrxCl6 (0 ≤ x ≤ 1), with the window of Na3PS4 (NPS) shown as a reference. d Arrhenius plot for Na3-xY1-xZrxCl6 from AIMD simulations (at x = 0.375, 0.5, and 0.75; solid lines and markers) and ML-IAP MD simulations (at x = 0.75; dashed lines and open markers). AIMD simulations were carried out at T = 600–1000 K at 100 K intervals, using a supercell of 150 atoms for up to 200 ps, while the ML-IAP MD simulations were carried out at T = 350 K–650 K using a supercell of 592 atoms for up to 10 ns.
The electrochemical window of Na3-xY1-xZrxCl6 (NYZCx) was investigated using the grand potential phase diagram approach18,19. Consistent with previous studies on the Li analogs, NYZCx SEs exhibit wide electrochemical windows, with a particularly high oxidation limit of ~3.8 V vs Na/Na+ (Fig. 1c). This high oxidation limit is maintained regardless of Zr content. However, the reduction limit narrows (from 0.6 V for NYC to 1.5 V for NYZCx), due to the higher thermodynamic reduction potential of Zr4+ compared to Y3+. The oxidation limit of 3.8 V for NYZCx indicates that it could be compatible with the NaCrO2 cathode, which has an operating voltage window of 2–3.6 V vs Na/Na+20. In contrast, sulfide SEs, such as Na3PS4, have oxidation limits of only ~2.5 V vs Na/Na+21. In addition, the reaction energies of NYZC0.75 with NaCrO2 and with metallic Na were found to be less negative compared to NPS (Supplementary Table 2).
The crystalline form of the end members NYC and NZC exhibit a closed-pack arrangement of [YCl6]3− and [ZrCl6]2− polyanions, respectively. With increasing x in NYZCx, the unit cell volume increases, which results in a widening of the Na+ diffusion channels, as shown in Supplementary Figure 2. NVT ab initio molecular dynamics (AIMD) simulations were carried out at 600−1000 K for NYZCx for x = 0, 0.375, 0.5, and 0.75. For NYC, AIMD simulations indicate no diffusion of Na+ ions even at elevated temperatures (Supplementary Table 1), consistent with its poor ionic conductivity. With Zr4+ doping, Na+ diffusivity increases substantially (Fig. 1d).
Due to the high cost of ab initio methods, NVT AIMD simulations (constrained to the pre-equilibrated volume) were limited to small supercells and temperatures above 500 K to ensure sufficient diffusion statistics. To probe the diffusivity at lower temperatures, a highly accurate ML-IAP based on the moment tensor potential formalism was developed using snapshots extracted from the AIMD trajectories as well as ground state and strained structures of NYC, NZC and the highest conductivity NYZC0.75 (see Methods section for details)22–25. To our knowledge, this is the first work that demonstrates the use of AIMD simulations to fit a ML-IAP. As shown in Fig. 1d, NVT MD simulations of NYZC0.75 carried out using this ML-IAP reproduces the AIMD diffusivities to good accuracy at 600–1000 K. The validated ML-IAP was then applied for NpT MD simulations of NYZC0.75 using a much larger cell (592 atoms) over much longer time scales (up to 10 ns) and under constant atmospheric pressure at 350 to 650 K. Interestingly, a non-Arrhenius behavior is observed; there is a transition between two linear regimes at around 500–550 K. Similar step changes in diffusion characteristics have been previously observed experimentally in NYC and other superionic conductors14,26. The activation barrier for diffusion in the low temperature regime (<500 K) is predicted to be 594 meV, and the room temperature Na+ conductivity is predicted to be 1.4 × 10−5 S cm−1, which is two orders of magnitude higher than that of NYC.
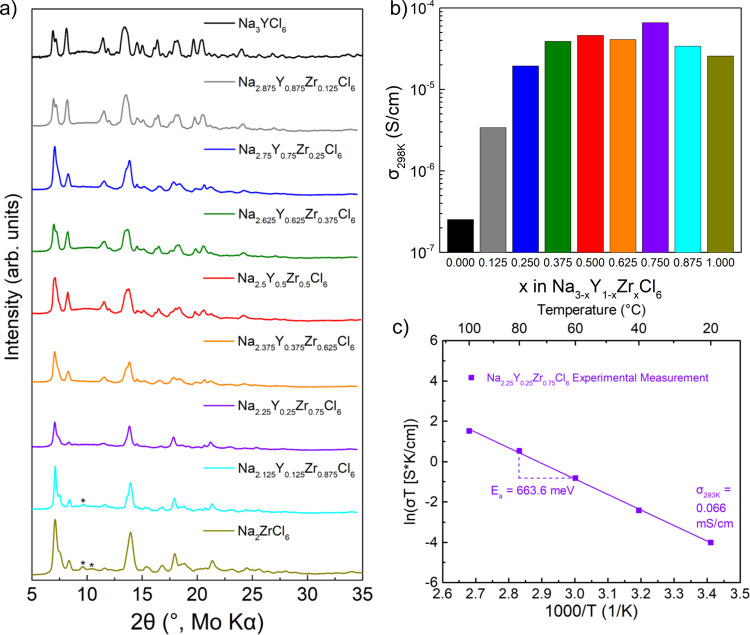
NYZCx compounds were synthesized using stoichiometric amounts of NaCl, YCl3, and ZrCl4 (see Methods). The parent compound NYC was first synthesized (detailed in Supplementary Note 1) and its XRD pattern and corresponding Rietveld Refinement results are shown in Supplementary Fig. 3a and Supplementary Table 3. These results are consistent with previous reports27. The room temperature ionic conductivity of NYC was determined to be 9.5 × 10−8 S cm−1 via electrochemical impedance spectroscopy (EIS) measurements (Supplementary Fig. 3b).
With Zr doping, the P21/n space group of the parent compound NYC is largely retained (Fig. 2a) up to x = 0.875, suggesting a solid solution in this compositional range. For x ≥ 0.875, additional peaks emerge in the XRD patterns at 2θ = 9.6° and 10.5°, indicating the presence of a second, different phase. This particular phase was determined to be crystalline NZC; using DFT calculations (Supplementary Note 2 and Supplementary Fig. 4a), the lowest energy NZC structure was found to be isostructural with Na2TiF6 (hexagonal space group P -3 m 1, structures shown in Supplementary Fig. 4b)28. This structure is consistent with experimental synchrotron XRD (λ = 0.1668 Å) and Rietveld refinement results of NZC measured after heat treatment (Supplementary Fig. 4c and Supplementary Table 4). Furthermore, X-ray photoelectron spectroscopy (XPS) measurements (Supplementary Fig. 5) indicate the presence of both Zr-Cl and Y-Cl bonds (as seen in the Cl 2p region) and thus structural units in the as-prepared NYZC0.5.
Fig. 2. Experimental Characterization of Na3-xY1-xZrxCl6.
a XRD of the Na3-xY1-xZrxCl6 compositions, obtained in x = 0.125 increments. Asterisks indicate the presence of new peaks. b The corresponding room temperature conductivity values. c Arrhenius plot of Na2.25Y0.25Zr0.75Cl6 from experimental measurements. The activation energy (low-temperature regime) and room temperature conductivity values are consistent with the MTP results.
Figure 2b shows the extracted conductivity values (from the corresponding Nyquist plots in Supplementary Fig. 6a), over the entire NYZCx compositional range at x = 0.125 increments. The ionic conductivity for 0.375 ≤ x < 1 is in the range of 2.6−6.6 × 10−5 S cm−1, with NYZC0.75 exhibiting the highest conductivity of 6.6 × 10−5 S cm−1 (the equivalent circuit fitting is shown in Supplementary Fig. 6b). A drop in conductivity was observed for x ≥ 0.875 compositions, attributed to the formation of a small amount of crystalline NZC phase with a much lower room temperature conductivity of 1.4 × 10−7 S/cm (Supplementary Fig. 4d). In addition, crystalline NZC has a relative density of ~79%, which could also contribute to its lower conductivity. In contrast, the relative density of NYZC0.75 is 90%, and a cross-sectional scanning electron microscope (SEM) image of the NYZC0.75 pellet indicates a dense particle morphology (Supplementary Fig. 6b inset). Since NYZC0.75 exhibited the highest conductivity among all compositions explored herein, the activation energy was measured (Fig. 2c) and found to be 663.6 meV, in good agreement with the ML-IAP NpT simulations for the low temperature regime. In addition, the electronic conductivity of NYZC0.75 was determined to be 8.89 × 10−9 S cm−1 via DC polarization (Supplementary Fig. 7), i.e., NYZC0.75 is an ionic conductor and an electronic insulator.
It is important to note that the experimental value of the room temperature conductivity for NYZC0.75 (6.6 × 10−5 S cm−1) is somewhat higher than the ML-IAP value (1.4 × 10−5 S cm−1). While crystalline NYZC0.75 was modeled, the ball milling procedure used in our experiments can introduce disorder and amorphize the sample, and it was previously shown that the relatively less crystalline Li3YCl6 was demonstrated to have a significantly higher conductivity than its highly-crystalline counterpart7. Hence, to further determine the impact of crystallinity on Na+ diffusion, the ionic conductivity before and after ball milling was investigated for NYC and for NYZC0.75, and results are shown in Supplementary Fig. 8 and detailed in Supplementary Note 3. The local Na environments and structural disorder were also probed via 23Na solid-state nuclear magnetic resonance (NMR).
Local Na environments and disorder
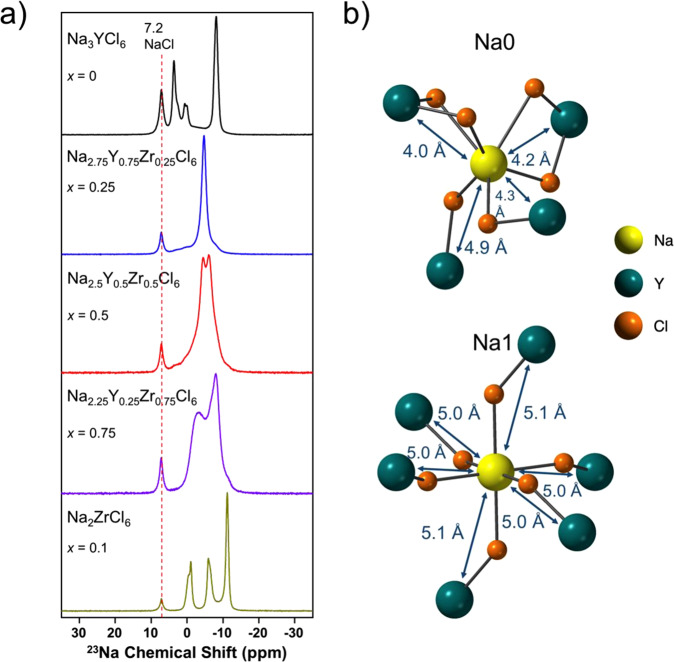
23Na magic angle spinning (MAS) solid-state NMR spectra obtained on NYZCx (x = 0, 0.25, 0.5, 0.75, 1) are presented in Fig. 3a. The signal at about 7.2 ppm is attributed to NaCl(s) present as either an impurity or residual precursor phase in all NYZCx samples29. The NaCl content obtained from 23Na NMR signal integration was found to be 16.0 %, 10.0 %, 5.9 %, 6.2 % and 4.0 % for x = 0, 0.25, 0.5, 0.75 and 1.0, respectively. The decrease in NaCl impurities with increasing Zr content is likely due to the concomitant decrease in Na content in NYZCx relative to NYC, which renders the formation of NaCl less favorable.
Fig. 3. 23Na single-pulse solid-state NMR spectra.
a Spectra collected on Na3-xY1-xZrxCl6 (x = 0, 0.25, 0.5, 0.75 and 1); the data were acquired at 18.8 T and at a MAS rate of 12 kHz and at a set temperature of 298 K. b Schematic of the Na0 and Na1 local environments in Na3YCl6.
While two crystallographically-distinct Na environments are expected in Na3YCl6 (as shown in Fig. 3b), the spectrum obtained on this compound (top spectrum in Fig. 3) exhibits at least five distinct resonances besides the NaCl impurity signal at about 7.2 ppm, which suggests the presence of local structural disorder and/or non-stoichiometry. We have excluded the possibility of additional impurity phases in the sample, due to the lack of candidate Na-containing impurity phases with 23Na resonant frequencies and signal line-shapes matching those observed in the Na3YCl6 spectrum. The presence of well-defined peaks suggests the presence of a range of Na environments with varying numbers of Cl and Y (Na) nuclei in their first and second coordination shells, respectively, as in a non-stoichiometric material or a material with some disorder on the cation lattice. We note that a non-stoichiometric solid electrolyte phase will necessarily result from the presence of a separate NaCl component, unless some YCl3 impurity is also present in the sample. The latter phase is not observed with XRD, but 89Y NMR data would be needed to completely rule out the presence of amorphous YCl3 in the sample (the long relaxation time of 89Y spins, however, makes such experiments prohibitively time consuming). Non-stoichiometry will also lead to a range of bond angles and bond lengths around Na nuclei in the structure, which could partially account for peak broadening in the Na3YCl6 spectrum. Additional NMR experiments are underway, as well as first principles calculations of NMR parameters, to fully assign the data presented here.
Previous NMR studies on solid NaYF430 and on the NaF-YF3 molten system31 have shown that the 23Na chemical shift becomes more negative as the number of Y3+ ions in the second coordination shell increases. In stoichiometric Na3YCl6, the Na0 site shown in Fig. 3b has four YCl6 neighboring octahedra, two of which are edge-sharing with the Y nucleus at a distance of 4.0–4.2 Ǻ from the central Na, and two of which are corner-sharing with Y-Na distances of 4.3 and 4.9 Ǻ. In contrast, the Na1 site has six corner-sharing YCl6 neighboring octahedra, with Y being 5.0–5.1 Ǻ away from the central Na. The presence of non-stoichiometry or disorder on the cation lattice will reduce the number of Y in the vicinity of the central Na and more strongly affect the chemical shift of Na0 (fewer Y3+ neighbors that are closer to the central Na) compared to that of Na1 (larger number of Y3+ neighbors further away from the central Na). It will also lead to additional 23Na NMR signals at more positive ppm values. With this in mind, we tentatively assign the most intense 23Na resonance at −8.1 ppm to Na nuclei in locally stoichiometric Na0 sites, the resonance at 3.6 p.p.m. to locally stoichiometric Na1 sites and the lower intensity peaks in-between these two resonances to Na nuclei in distorted and/or Y-deficient Na0 and Na1 sites. This assignment is consistent with the fact that there are twice as many Na0 sites than Na1 sites in the structure, such that the most populated Na local environment is expected to be the locally stoichiometric Na0 site.
Interestingly, while the substitution of Y by Zr is expected to vastly increase the number of possible Na local environments, the 23Na NMR spectra collected on the mixed Y/Zr samples exhibit either a single major resonance (as for the x = 0.25 sample) or two dominant signals (as for the x = 0.5 and 0.75 samples). In NMR, if two or more environments are in fast chemical exchange on the experimental timescale, their signals coalesce into a single peak at the weighted average of their resonant frequencies. Notably, the exchange rate at which the signals coalesce depends on the chemical shift separation of the individual resonances for the exchanging sites. Hence, the presence of few resonances in the spectra collected on the x = 0.25, 0.5 and 0.75 compounds indicate fast Na+ diffusion in these structures, consistent with the ionic conductivity measurements presented earlier. Given that all three compounds exhibit relatively similar Na diffusion properties, the fact that a single peak is observed in the x = 0.25 sample while two broader peaks are observed for the x = 0.5 and 0.75 samples may stem from a smaller distribution of Na local environments in the former, leading to chemical shifts that are closer together than in the x = 0.5 and 0.75 compounds. Finally, the spectrum collected on NZC indicates the presence of at least three distinct Na sites in the structure, not including the NaCl peak at 7.2 ppm. Since the ideal NZC structure contains a single Na environment, the three distinct 23Na resonances could indicate non-stoichiometry or local structural disorder. In addition, Na+ diffusion in this compound is too slow to lead to coalescence of the 23Na resonances of the exchanging sites.
Mechanism for enhanced conductivity
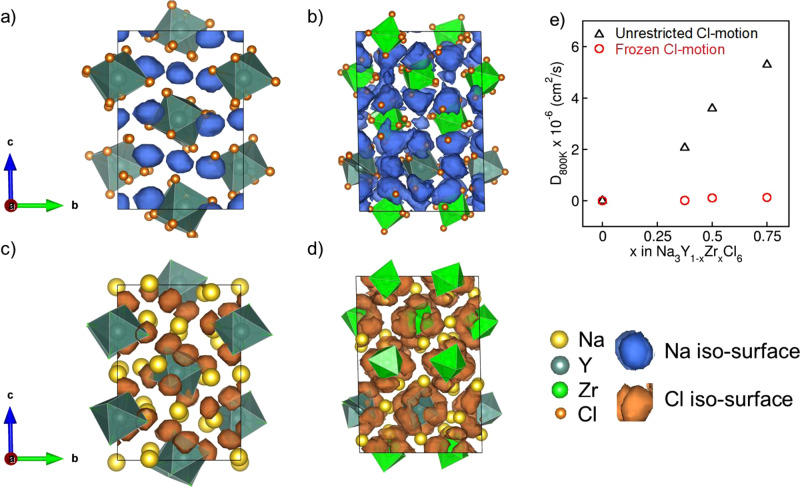
To probe the origins of the greatly enhanced conductivity in NYZCx, we compared the probability distributions for both Na+ and Cl− in NYC and NYZC0.75 extracted from 100 ps of AIMD trajectory simulations at 600 K. In NYC, the Na+ trajectories (Fig. 4a) indicate mostly local Na+ motion with little long-range transport, consistent with the observed low Na+ conductivity. In contrast, fast macroscopic 3D Na+ diffusion is observed for NYZC (Fig. 4b). Interestingly, there are substantial differences in the trajectories of the anion framework as well. While the Cl− remain relatively static in NYC even at these elevated temperatures (Fig. 4c), substantial Cl− motion, corresponding to YCl63−/ZrCl62− octahedra rotations, are observed in NYZC0.75 (Fig. 4d). While similar behavior has been observed in lithium superionic conductors containing borohydride anions B10H102− 32, B12H122− 33, and BH4− 34, and the sulfides β-Li3PS4 and Li3.25Si0.25P0.75S435, this is the first observed instance of rotational motion of Cl− in a halide single ion conductor. It is important to note that while there is polyanionic rotation, there is no signature for Zr(Y)-Cl bond breaking (Supplementary Fig. 9).
Fig. 4. Effect of octahedra rotation on Na+ diffusivity.
Plots of the probability density (isosurface value = 5 × 10−4) of a Na+ in Na3YCl6, b Na+ in Na2.25Y0.25Z0.75Cl6, c Cl− in Na3YCl6 and d Cl− in Na2.25Y0.25Z0.75Cl6, over 100 ps of AIMD simulations at 600 K. The motion of Na+ and Cl− in Na3YCl6 are relatively localized, while macroscopic Na+ diffusion with (Zr/Y)Cl6 octahedral rotation are observed in Na2.25Y0.25Z0.75Cl6. e Na+ diffusivity at 800 K (D800K, in cm2/s) for varying Zr content in Na3−xY1−xZrxCl6, compared with a selective dynamics simulation with Cl− ions frozen in space, which shows negligible Na+ diffusivity.
To investigate the effects of octahedral rotations and lattice volume on Na+ conductivities, two gedankenexperiments were performed with NYZC0.75 where YCl63−/ZrCl62− octahedra were either frozen in their initial positions or the NYZC0.75 lattice was constrained to the lattice volume of NYC. At 800 K, NYZCx with frozen YCl63−/ZrCl62− octahedra does not exhibit significantly higher Na+ diffusivity compared to NYC despite the presence of Na+ vacancies and an increased unit cell volume (Fig. 4e). Nevertheless, an increased cell volume due to Zr4+ doping is necessary for YCl63−/ZrCl62− octahedral rotations to occur; when NYZC0.75 is constrained to have the same unit cell volume as NYC, the magnitude of the octahedral rotations is greatly reduced (Supplementary Fig. 10), as is ionic conductivity. These AIMD calculations therefore indicate that an increase in cell volume or in octahedral motion significantly improves Na+ conductivity - both effects are closely related and most likely responsible for enhanced Na+ diffusion kinetics in NYZC0.75, as compared to NYC.
In addition, an analysis of Na+ motion in NYZC0.75 was carried out using the trajectories from the ML-IAP NpT MD simulations at 500 K and 550 K, i.e., below and above the transition point for the two linear regimes in Fig. 1d, respectively. It was found that the Na+ diffusion topology changes from being quasi-2D to being 3D at the transition temperature, accompanied by a sharp increase in the degree of YCl63-/ZrCl62- octahedra rotation (Supplementary Fig. 11). We may therefore surmise that the much lower barriers for Na+ diffusion in the high-temperature regime compared to the low-temperature regime is due to the activation of additional rotational modes and diffusion pathways above the transition temperature. These results highlight the cooperative interplay between increased lattice volume36 and octahedral rotations in enhancing the long-range Na+ conductivity in this framework.
Cathode composite for a long cycle-life solid-state sodium battery
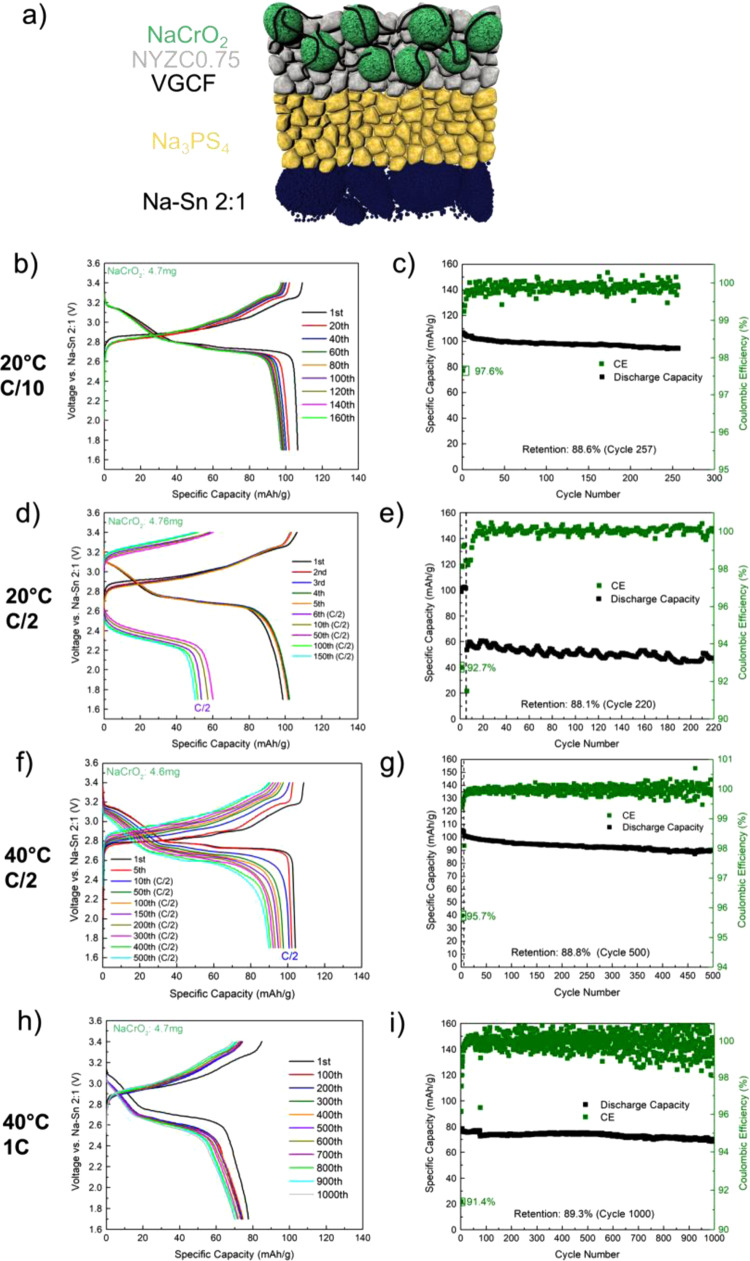
Given the high cathodic stability and conductivity of NYZC0.75, cells comprising NYZC0.75 in a composite with the NaCrO2 cathode and Na3PS4 as the SE were constructed; a schematic is shown in Fig. 5a. For comparison, a control cell using Na3PS4 alone, without NYZC0.75, was also constructed (Supplementary Fig. 12a). At 20 °C at a rate of C/10 (Fig. 5b, c for NYZC0.75 and Supplementary Fig. 12b, c for NPS), it is evident that the first cycle Coulombic efficiency (CE) drastically increased in the NYZC0.75 cell (from 71.9% to 97.6%). This observed first cycle CE for the NYZC0.75 cell is the highest among those reported for Na ASSBs that use NaCrO2 as the cathode5,37–39. We believe that the high cathodic stability of NYZC0.75 protects the Na3PS4 SE from oxidation by NaCrO2, and in turn the Na3PS4 SE forms a stable passivating interface with the Na-Sn anode7. This is consistent with results from symmetric cell experiments carried out with NYZCx and NPS with the Na-Sn alloys Na15Sn4 (0.1 V vs. Na/Na+) and Na-Sn 2:1 (0.3 V vs. Na/Na+), as shown in Supplementary Fig. 1340. Based on the results, Na-Sn 2:1 was chosen for its stability with NPS.
Fig. 5. Electrochemical performance of the NYZC0.75 SSSBs.
a Cell schematic. Voltage profile and specific capacity as a function of cycle number of this cell configuration, respectively, running at: b–c 20 °C and C/10, d–e 20 °C and C/10 for the first 5 cycles and subsequent cycling at C/2, f–g 40 °C and C/10 for the first 5 cycles and subsequent cycling at C/2, and h–i 40 °C and 1 C. In each case, the NYZC0.75 cells exhibit long-term cycling stability, with 89.3% capacity retention at 1000 cycles for the 40 °C 1 C cell.
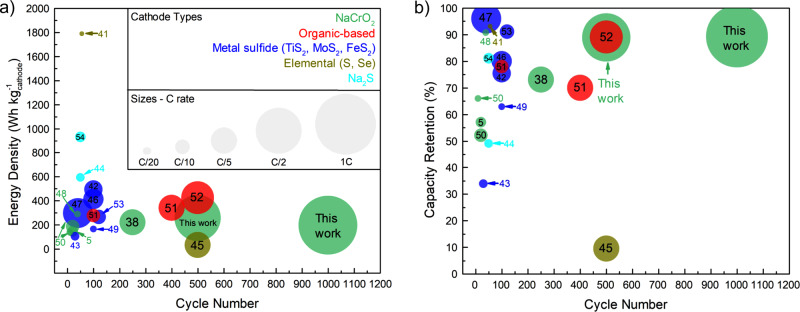
To study the rate capability of the NYZC0.75 cell configuration, additional cells were constructed and tested at C/2 (after the first 5 cycles at C/10) at both 20 °C and 40 °C (Fig. 5d–g, respectively). At 20 °C, there is a noticeable drop in specific capacity (from 101 to 53.7 mAh/g) after switching to a rate of C/2. This is due to several reasons: one, the NPS layer is relatively thick (~800 µm) and the conductivity of NYZC0.75 is in the order of 10−5 S/cm. It is important to note that the cyclical behavior in Fig. 5e is due to temperature variations in the glovebox, as the cell was not inside a temperature-controlled chamber. At 40 °C, where the conductivity of NYZC0.75 is in the range of 1–2 × 10−4 S/cm, thus increasing the kinetics, the drop in capacity is negligible (from 104 to 101 mAh/g) when switching to a rate of C/2. For this particular SSSB, the average CE is 99.96%, which yields a capacity retention of 88% after 500 cycles. Furthermore, another NYZC0.75-NPS SSSB was constructed and cycled at 40 °C and at a rate of 1 C (Fig. 5h, i). Although there is a slight drop in capacity (78 mAh/g) compared to the cell cycled at 40 °C and C/2 (101 mAh/g), the cell cycled at 1 C lasted over 1000 cycles with a capacity retention of 89.3%, highlighting the superior stability of NYZC0.75 when paired with NaCrO2. To date, this is the highest capacity retention obtained for a SSSB, and Fig. 6 compares cycling performance metrics (gravimetric energy density per active mass, cycle life, capacity retention, rate, and cathode type) across various SSSB reports5,38,41–54. It is important to note that the cathode composite contains 39% of active material, NaCrO2, which is far from practical values. However, these results illustrate that a NYZC0.75-NaCrO2-VGCF cathode composite matrix exhibits stable electrochemical performance with prolonged cycling. Increasing the active mass loading is a necessary direction for future work.
Fig. 6. Comparison of SSSB performance metrics.
a Gravimetric energy density (per mass of active material) plotted as a function of cycle number. b Capacity retention as a function of cycle number5,38,41–54. The cycling performance comparison highlights the compatibility and stability of the NaCrO2 + Na2.25Y0.25Zr0.75Cl6 + VGCF composite cathode.
Furthermore, an NPS control cell was also cycled 40 °C and C/10 (Supplementary Fig. 12d, e); the first cycle CE decreased from 71.9% (at 20 °C) to 62.4%, showing that NPS oxidation is exacerbated at 40 °C. This result contrasts with the demonstrated superior electrochemical stability of NYZC0.75 at 40 °C and high rates.
To investigate the effect of reducing the thickness of NPS, a modified cell design with a stainless steel (current collector) support was fabricated, such that the amount of NPS was reduced by half. Additional details can be found in Supplementary Note 4, and the rate capability test and EIS are shown in Supplementary Fig. 14. Based on these results, thinning the electrolyte layer (or reducing the amount of inactive material) and further optimization of the cell configuration is a promising avenue for future work.
To characterize the chemical environments in NPS and NYZC0.75 components after cycling, the SSSBs were disassembled to recover the composite cathodes and XPS measurements were conducted. Supplementary Fig. 15 compares the S 2p and P 2p binding energy regions of pristine NPS and the cycled NPS-containing composite cathode. Consistent with previous reports, when paired with an oxide cathode, NPS oxidizes to form elemental sulfur, other P2Sx compounds, and possibly compounds containing P-O bonds21,55,56. Supplementary Fig. 16 shows the Zr 3d and Y 3d bonds of pristine versus cycled NYZC0.75. Even with cells cycled at elevated temperatures or high rates, the Zr-Cl and Y-Cl bonds are retained in the composite cathode, confirming the electrochemical stability of NYZC0.75 when used with NaCrO2.
To evaluate the chemical stability, temperature-dependent XRD patterns were collected for 1:1 mixtures of NPS:NaCrO2 and NYZC0.75:NaCrO2 (Supplementary Fig. 17). In both the cases, no new additional peaks appeared, indicating no chemical reaction, even at temperatures as high as 220 °C. This is in accordance with Supplementary Table 2, where the reaction energy with NaCrO2 is low for both NPS (−0.18 eV/atom) and NYZC0.75 (−0.14 eV/atom). Thus, the observed superior cycling stability of NYZC0.75 arises from its intrinsic chemical stability in combination with its wide electrochemical window, whereas NPS is electrochemically unstable in the presence of NaCrO2 and undergoes oxidative decomposition during charging.
In summary, we reported on aliovalent substitution in the halide-based ionic crystal Na3YCl6, which leads to phases with enhanced Na+ conduction due to the presence of an interconnected network of Na+ diffusion channels (specifically, substituting Y3+ in Na3YCl6 with Zr4+ to form Na3-xY1-xZrxCl6). Zr4+ substitution was found to increase the volume of the unit cell, which in turn enables polyanion rotation. The synergy between polyanionic rotation and increase in the effective mobile carrier concentration leads to a significant increase in the Na+ diffusivity upon Zr incorporation, which is absent in the parent Na3YCl6 compound. This was confirmed experimentally by an increase in the ionic conductivity by two orders of magnitude upon Zr substitution. Furthermore, the wide oxidative electrochemical window (up to 3.8 V) was retained after substitution, which proved to be beneficial when paired with a NaCrO2 cathode in a model SSSB. In this configuration, no electrochemical decomposition was observed, in contrast with a cell comprising Na3PS4 in the cathode composite, as revealed by XPS. At 40 °C and a rate of 1 C, the cell containing Na2.25Y0.25Zr0.75Cl6 was able to cycle over 1000 cycles with a capacity retention of 89.3%, the highest cycle life for a SSSB to date. Thus, further exploration of halide-based materials, especially in SSSBs, is a worthy area of continued investigation. This methodology of coupling computational and experimental evaluation, verification, and testing of material properties is an effective and necessary strategy toward finding compatible, long-lasting, and high-performing SSSB chemistries.
Methods
Structural relaxations and energy calculations
All density functional theory (DFT) calculations were performed using the projector augmented wave (PAW) approach as implemented in the VASP package57,58. The Perdew-Burke-Ernzerhof (PBE) generalized gradient approximation functional was used59. A plane-wave cut-off of 520 eV was used for DFT relaxations and energy calculations, consistent with the settings used in the Materials Project database60. All input file generation and post-processing analysis were performed using Pymatgen and pymatgen-diffusion packages61. Spin-polarized DFT calculations revealed that the net magnetization is nearly zero (≈ 0.01) for NYZC since both Y3+ and Zr4+ exhibit diamagnetic properties (i.e. noble gas configuration). In addition, NYZC, unlike the electrode, does not undergo any redox process. Thus, the net magnetization for NYZC should always be zero, therefore calculations were carried out without spin polarization.
The pre-relaxed structures of Na3YCl6 (mp-31362) and Na3YBr6 (mp-29080) were extracted from the Materials Project (MP) database62,63. The corresponding ICSD64 IDs are #59886 and #82355, respectively. Aliovalent substitution on the Y3+ sites with charge compensation by Na+ vacancies were performed to generate Na3-(z-3)xY1-xMz+xCl6 (M = Ti4+, Zr4+, Hf4+, Ta5+) structures. DFT calculations were performed on all symmetrically distinct orderings of Y/M and Na/vacancies to identify the lowest energy structure. Candidate structures for the Na2ZrCl6 phase were obtained by performing ionic substitutions of all structures in MP database matching the formula of A2MX6. All candidate structures were fully relaxed using DFT prior to calculating their energies. The computed XRD pattern of the lowest energy candidate was successfully matched to the experimental XRD pattern.
Other than the target phases of interest, the pre-computed energies of all other structures in the Na-Y-Zr-Cl phase space were obtained from the Materials Project database and used in the calculation of the energy above hull (Ehull), electrochemical stability window, and the interfacial reaction products following the methodologies established in prior publications21,65.
Topological analysis of the framework chemistries was performed using Zeo ++, an open source topological analysis package66. The quantity of interest is the largest included sphere radius along the free sphere path Rinc. This gives an estimate of the diffusion channel size which is associated with the ionic conductivity of the material.
Ab initio molecular dynamics
Non-spin polarized ab initio molecular dynamics (AIMD) simulations were carried out in the NVT ensemble. From the spin-polarized DFT calculations, the net magnetization is 0 for all ions, which supports the use of non-spin-polarized calculations for AIMD. A plane-wave energy cutoff of 280 eV, supercells with the minimum dimension larger than 10 Å and a minimal Γ-centered 1 × 1 × 1 k-mesh were used. The time step was set to 2 fs. Simulations were carried out at several temperatures between 500 K and 1200 K and the corresponding diffusivities were extracted using the Nernst-Einstein relationship from the slope of the plot of the mean square displacement of Na ions with time.
Machine learning interatomic potential and molecular dynamic simulations
The moment tensor potential (MTP)23–25 for NYZC0.75 was developed using the open-source Materials Machine Learning (maml) Python package. The training data comprises 800 snapshots extracted at 400 fs intervals from AIMD NVT simulations at 600 K, 800 K, 1000 K, and 1200 K. Static DFT calculations were then performed to obtain accurate energies and forces. A training:test split of 90:10 was used to train the machine learning model. The MTP cutoff radius and the maximum level of basis functions, levmax were chosen to be 5.0 Å and 14, respectively. The mean absolute error (MAE) on the energies and forces were 1 meV atom−1 and 63.5 meV Å−1, respectively (Supplementary Figure 18 and Supplementary Table 5). NPT MD simulations using the MTP were carried out using LAMMPS67. The simulation time was at a least amount of 10 ns with a 2 fs time step. A 4 × 4 × 4 supercell of the NYZC0.75 with 592 atoms was used.
Material synthesis
All fabrication processes were conducted in an Ar-filled glovebox (mBraun 200B, H2O ppm <0.5, O2 ppm < 1), unless otherwise noted.
Stoichiometric amounts of the precursors NaCl (>99%, Sigma Aldrich), YCl3, (99.9%, Sigma Aldrich) were hand-mixed in a mortar and pestle for 10 min and the powder mixture was placed in a 50 mL ZrO2 ball mill jar (Retsch Emax) with eleven 10 mm-diameter Y-ZrO2 milling balls. The mixture was milled for 2 h at 500 r.p.m. The material was extracted from the jars in the glovebox, pelletized at a pressure of 370 MPa with a 13 mm pellet die (Carver), loaded into a quartz tube, flame sealed, and heated in a box furnace (Lindberg Blue M) at 500 °C for 24 h. To homogenize the material, the material was ball milled again after heat treatment using 88 5 mm diameter Y-ZrO2 milling balls for a duration of 4 h. The material was extracted and stored in the glovebox before further testing.
For the Zr substituted compounds, the same procedure was conducted with the addition of ZrCl4 (99.99%, Sigma Aldrich) as a third precursor, and the reagent ratios adjusted according to stoichiometry.
Characterization—XRD
Powder samples were loaded into 0.5 mm-diameter boron-rich capillary tubes (Charles Supper). The tube opening was capped with clay and wrapped in paraffin film before it was brought outside of the glovebox to be flame-sealed with a butane torch. The samples were measured on a Bruker Kappa goniometer equipped with a Bruker Vantec 500 detector. The sample was placed in the Bragg−Brentano θ − θ configuration and the Debye−Scherrer method was used for measurements. XRD data was collected using Cu Kα radiation at 45 kV and 50 mA, over a 2θ range of 5 − 90° with a step size of 0.01°. Rietveld refinement was carried out with the FullProf software suite.
For temperature-dependent capillary XRD, the capillary tubes were heated at a rate of 5 °C/min and held at the target temperature for one hour before the XRD measurement was taken.
For Synchrotron XRD, the samples were prepared by loading the powders into polyimide tubes in the glovebox and were subsequently sealed with epoxy. Measurements were carried out at Beamline 28-ID-1 at NSLS-II.
Characterization—electrochemical
75 mg of powder was pressed at 370 MPa into a 10 mm polyether ether ketone (PEEK die) using two titanium plungers and the dimensions were measured with calipers. The relative density of the pellets was determined by comparing the measured dimensions with the XRD results. On both sides of the pellet, acetylene black (AB) was added for better contact with the current collectors; once added, the AB was also pressed at 370 MPa using the titanium plungers. The cell configuration was secured into a cell holder and connected to a Solartron 1260 impedance analyzer. Impedance measurements were taken with an applied AC potential of 30 mV over a frequency range of 1 MHz to 1 Hz. Temperature-dependent EIS measurements were also conducted within the glovebox; the sample was heated from 20 °C to 100 °C and EIS measurements were recorded at every 20 °C increment. Measurements were taken only after the sample was held at the target temperature for over an hour to allow for equilibration. The heating rate was 2 °C/min. The activation energy (Ea) was calculated from the slope of the resulting Arrhenius plot.
DC polarization was also conducted by the Solartron 1260 impedance analyzer. The cell setup was similar as before; the powder was pressed at 370 MPa into a 10 mm PEEK die using two titanium plungers and subsequently secured into a cell holder. The applied DC potential was 50 mV and the current response was measured over time.
The model SSSB is composed of NaCrO2 as the positive electrode, Na-Sn (2:1) as the negative electrode, and Na3PS4 as the electrolyte. The positive electrode is mixed into a composite with a weight ratio of 11:16:1 of NaCrO2: Na3PS4: VGCF (VGCF from Sigma Aldrich; length 20–200 μm, <100 ppm iron, average diameter: 130 nm, average specific surface area: 24 m2 g−1). The battery is fabricated through mechanical pressing; 75 mg of Na3PS4 powder is pressed first at 370 MPa, then about 12 mg of the composite NaCrO2 powder is placed on one side of the Na3PS4 pellet and pressed at the same pressure, and finally on the opposite side of the Na3PS4, an excess of Na-Sn 2:1 alloy (35 mg) is pressed at the same pressure. After securing the cell in a cell holder, the electrical leads were connected to an electrochemical cycler (Landhe). For a rate of C/10, the current density used was 64 µA cm−2.
To incorporate Na2.25Y0.25Zr0.75Cl6 (NYZC0.75) into the model SSSB, NYZC0.75 replaced Na3PS4 in the composite cathode (still hand-mixed with the same 11:16:1 ratio). For cells cycled at 40 °C, the cell assemblies were placed into a compact box furnace (MTI KSI-1100X) within the Ar-filled glovebox. Current densities ranged from 64 µA cm−2 (C/10) to 640 µA cm−2 (1 C).
After cycling, the cell was disassembled to characterize any material changes.
Characterization—23Na solid-state NMR
All 23Na solid-state NMR experiments were performed on a 3.2 mm HX probe on a Bruker Avance III Ultrashield Plus 800 MHz (18.8 T) NMR spectrometer. A 1 M NaCl aqueous solution, with a reported 23Na chemical shift of 0.04 ppm68 was used as reference sample to calibrate the chemical shift scale.
23Na NMR spectra were collected on NYZCx (x = 0, 0.25, 0.5, 0.75, 1) compounds with a simple pulse and acquire (zg) sequence and at a magic angle spinning (MAS) rate of 12 kHz as no spectral enhancement was observed at higher spinning speeds. The solid electrolyte samples were packed inside 3.2 mm sapphire rotors in an Ar-filled glovebox to avoid contamination with air or moisture. In addition, a flow of N2 gas was used to spin the samples, providing an inert atmosphere during 23Na NMR signal acquisition.
Due to the quadrupolar nature of 23Na nuclei (I = 3/2), the calibrated pulse durations differed for the various 23Na local environments in the samples. Hence, all spectra were obtained using a 30° radiofrequency (RF) excitation pulse in lieu of a standard 90° pulse angle to uniformly excite all 23Na spins in the sample and provide internally quantitative 23Na NMR spectra. The quantitative nature of the so-obtained spectra was confirmed by collecting data using a pulse angle as low as 5°, which showed no change in the relative amounts of each resonance compared to the 30° pulse data. The power level used for all measurements was 100 W (~93 kHz) with a 90° pulse duration of around 2.7 μs, therefore, a 30° pulse duration of either 0.9 or 0.95 μs was used depending on the optimization of each sample. A 30 s delay was applied before each scan when signal averaging in order to allow full relaxation, where the relaxation times of these samples are 2 s or below.
Characterization—XPS
The powders were adhered onto a small metallic sample stub (Shimadzu) with carbon tape. The metallic stub was secured into a metallic canister and sealed inside the glovebox with clamps.
The metallic canister was placed into a N2 glovebox that is attached to the XPS tool (Kratos Axis Supra), where the sample can be transferred into the analysis chamber without any exposure to ambient air. All measurements were taken using 15 kV Al Kα radiation at a chamber pressure less than 5 × 10−8 torr. For the wide survey scans, a pass energy of 160 eV and a dwell time of 100 ms was used, but for specific element regions, a pass energy of 20 eV, a dwell time of 300 ms, and a step size of 0.05 eV was used. The charge neutralizer was enabled during all the measurements. Data calibration and analysis were conducted using the CasaXPS software, and all region spectra were calibrated using the C 1 s peak.
Characterization—focused ion beam (FIB)
The Na3-xY1-xZrxCl6 powders were pressed into a 10mm-diameter pellet at 370 MPa. The pellet was extracted and then mounted onto a SEM sample stage (Ted Pella) and transferred into the FEI Scios DualBeam FIB/SEM using the air-sensitive holder to avoid any ambient air exposure. Cross sections were milled using the FIB and SEM images were taken of the samples.
Supplementary information
Acknowledgements
Funding to support this work was provided by the Energy & Biosciences Institute through the EBI-Shell program, contract number PT78832. Characterization work was performed in part at the San Diego Nanotechnology Infrastructure (SDNI), a member of the National Nanotechnology Coordinated Infrastructure, which is supported by the National Science Foundation under Grant ECCS-1542148. XPS was conducted at the University of California, Irvine Materials Research Institute (IMRI) using instrumentation funded in part by the National Science Foundation Major Research Instrumentation Program under Grant CHE-1338173. Computational work was performed using the Extreme Science and Engineering Discovery Environment (XSEDE, which is supported by National Science Foundation Grant ACI-1053575), in addition to the Triton Super Computer Center (TSCC) at the University of California, San Diego, and the National Energy Research Scientific Computing Center (NERSC). The NMR experimental work used the shared facilities of the UCSB MRSEC (National Science Foundation, NSF DMR 1720256), a member of the Materials Research Facilities Network. This work was supported in part by the National Science Foundation Graduate Research Fellowship under Grant No. 1650114. The work done at Brookhaven National Laboratory used beamline 28-ID-1 of the National Synchrotron Light Source II, a US DOE Office of Science User Facility operated for the DOE Office of Science by Brookhaven National Laboratory under contract No. DE-SC0012704. We acknowledge the support from the USA National Science Foundation under Award Number DMR1608968 for sodium-based chemistries. We thank Dr. Milan Gembicky at the Crystallography Facility at the University of California, San Diego for assisting on the capillary XRD data collection. We thank Ms. Hyeseung Chung and Mrs. Baharak Sayahpour for their assistance in conducting experiments.
Author contributions
E.A.W., S.B., P.M.R., J.Q., and A.B. wrote the manuscript. E.A.W. carried out experimental synthesis and electrochemical measurements and testing. S.B., H.T., Z.Z., and J.Q. conducted the computational work. P.M.R. conducted NMR and E.S. helped with the data analysis and NMR assignments. A.G. and Y.L. conducted synchrotron XRD measurements, and along with J.-M.D. and E.Z., provided input on analysis. E.S. also provided input on the Rietveld refinements. G.D. aided in cell construction. H.N. aided with capillary XRD data collection. R.S. and G.V. managed the project and were involved in experimental planning and project discussions. K.W.C. supervised the work of A.G., R.J.C. supervised the work of P.M.R., Y.S.M. and A.B. supervised the work of E.A.W., J.-M.D., Y.L., E.Z., and G.D., and S.P.O. supervised the work of S.B., H.T., J.Q., and Z.Z. All authors helped with data interpretation and analysis.
Data availability
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
The X-ray crystallographic coordinates for the structures reported in this study, Na3YCl6 and Na2ZrCl6, have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2057626-2057627. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
Code availability
DFT calculations and AIMD simulations were carried out using the open-source Python Materials Genomics (pymatgen) package, which is available at http://pymatgen.org. The moment tensor potential was fitted using the open-source materials machine learning (maml) package, which is available at https://github.com/materialsvirtuallab/maml.
Competing interests
R.S. and G.V. are employees of Shell International Exploration and Production Inc., USA and Shell Global Solutions International BV, Netherlands, respectively. A patent was filed for this work through the UCSD Office of Innovation and Commercialization. The remaining authors declare no competing interests.
Footnotes
Peer review information Nature Communications thanks the anonymous reviewers for their contribution to the peer review of this work.
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Erik A. Wu, Swastika Banerjee, and Hanmei Tang.
Contributor Information
Raphaële J. Clément, Email: rclement@ucsb.edu
Abhik Banerjee, Email: abhik.banerjee@tcgcrest.org.
Ying Shirley Meng, Email: shmeng@ucsd.edu.
Shyue Ping Ong, Email: ongsp@eng.ucsd.edu.
Supplementary information
The online version contains supplementary material available at 10.1038/s41467-021-21488-7.
References
- 1.Kamaya N, et al. A lithium superionic conductor. Nat. Mater. 2011;10:682–686. doi: 10.1038/nmat3066. [DOI] [PubMed] [Google Scholar]
- 2.Hayashi A, et al. A sodium-ion sulfide solid electrolyte with unprecedented conductivity at room temperature. Nat. Commun. 2019;10:1–6. doi: 10.1038/s41467-018-07882-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richards WD, Miara LJ, Wang Y, Kim JC, Ceder G. Interface Stability in Solid-State Batteries. Chem. Mater. 2016;28:266–273. doi: 10.1021/acs.chemmater.5b04082. [DOI] [Google Scholar]
- 4.Kato Y, et al. High-power all-solid-state batteries using sulfide superionic conductors. Nat. Energy. 2016;1:1–7. doi: 10.1038/nenergy.2016.30. [DOI] [Google Scholar]
- 5.Banerjee A, et al. Na3SbS4: a solution processable sodium superionic conductor for all-solid-state sodium-ion batteries. Angew. Chem. Int. Ed. 2016;55:9634–9638. doi: 10.1002/anie.201604158. [DOI] [PubMed] [Google Scholar]
- 6.Wang Y, et al. Development of solid-state electrolytes for sodium-ion battery–a short review. Nano Mater. Sci. 2019;1:91–100. doi: 10.1016/j.nanoms.2019.02.007. [DOI] [Google Scholar]
- 7.Asano T, et al. Solid halide electrolytes with high lithium-ion conductivity for application in 4 V class bulk-type all-solid-state batteries. Adv. Mater. 2018;30:1803075. doi: 10.1002/adma.201803075. [DOI] [PubMed] [Google Scholar]
- 8.Li X, et al. Air-stable Li3InCl6 electrolyte with high voltage compatibility for all-solid-state batteries. Energy Environ. Sci. 2019;12:2665–2671. doi: 10.1039/C9EE02311A. [DOI] [Google Scholar]
- 9.Liang J, et al. Site-occupation-tuned superionic LixScCl3+x halide solid electrolytes for all-solid-state batteries. J. Am. Chem. Soc. 2020;142:7012–7022. doi: 10.1021/jacs.0c00134. [DOI] [PubMed] [Google Scholar]
- 10.Wang S, et al. Lithium chlorides and bromides as promising solid-state chemistries for fast ion conductors with good electrochemical stability. Angew. Chem. Int. Ed. 2019;58:8039–8043. doi: 10.1002/anie.201901938. [DOI] [PubMed] [Google Scholar]
- 11.Park K-H, et al. High-voltage superionic halide solid electrolytes for all-solid-state Li-ion batteries. ACS Energy Lett. 2020;5:533–539. doi: 10.1021/acsenergylett.9b02599. [DOI] [Google Scholar]
- 12.Li X, et al. Progress and perspectives on halide lithium conductors for all-solid-state lithium batteries. Energy Environ. Sci. 2020;13:1429–1461. doi: 10.1039/C9EE03828K. [DOI] [Google Scholar]
- 13.Xu Z, et al. Influence of anion charge on Li ion diffusion in a new solid-state electrolyte, Li3LaI6. Chem. Mater. 2019;31:7425–7433. doi: 10.1021/acs.chemmater.9b02075. [DOI] [Google Scholar]
- 14.Wickleder MS, Meyer G. Ternäre halogenide vom Typ A3MX6. III [1, 2]. synthese, strukturen und Ionenleitfähigkeit der halogenide Na3MX6 (X = Cl, Br) Z. F.ür. Anorg. Allg. Chem. 1995;621:457–463. doi: 10.1002/zaac.19956210321. [DOI] [Google Scholar]
- 15.Sun Y, Suzuki K, Hori S, Hirayama M, Kanno R. Superionic conductors: Li10+δ[SnySi1–y]1+δP2−δS12 with a Li10GeP2S12-type structure in the Li3PS4–Li4SnS4–Li4SiS4 Quasi-ternary system. Chem. Mater. 2017;29:5858–5864. doi: 10.1021/acs.chemmater.7b00886. [DOI] [Google Scholar]
- 16.Zhao W, Yi J, He P, Zhou H. Solid-state electrolytes for lithium-ion batteries: fundamentals, challenges and perspectives. Electrochem. Energy Rev. 2019;2:574–605. doi: 10.1007/s41918-019-00048-0. [DOI] [Google Scholar]
- 17.Jolley AG, Cohn G, Hitz GT, Wachsman ED. Improving the ionic conductivity of NASICON through aliovalent cation substitution of Na3Zr2Si2PO12. Ionics. 2015;21:3031–3038. doi: 10.1007/s11581-015-1498-8. [DOI] [Google Scholar]
- 18.Ong SP, et al. Phase stability, electrochemical stability and ionic conductivity of the Li10±1MP2X12 (M = Ge, Si, Sn, Al or P, and X = O, S or Se) family of superionic conductors. Energy Environ. Sci. 2012;6:148–156. doi: 10.1039/C2EE23355J. [DOI] [Google Scholar]
- 19.Zhu Y, He X, Mo Y. First principles study on electrochemical and chemical stability of solid electrolyte–electrode interfaces in all-solid-state Li-ion batteries. J. Mater. Chem. A. 2016;4:3253–3266. doi: 10.1039/C5TA08574H. [DOI] [Google Scholar]
- 20.Yu C-Y, et al. NaCrO2 cathode for high-rate sodium-ion batteries. Energy Environ. Sci. 2015;8:2019–2026. doi: 10.1039/C5EE00695C. [DOI] [Google Scholar]
- 21.Tang H, et al. Probing solid–solid interfacial reactions in all-solid-state sodium-ion batteries with first-principles calculations. Chem. Mater. 2018;30:163–173. doi: 10.1021/acs.chemmater.7b04096. [DOI] [Google Scholar]
- 22.Zuo Y, et al. Performance and cost assessment of machine learning interatomic potentials. J. Phys. Chem. A. 2020;124:731–745. doi: 10.1021/acs.jpca.9b08723. [DOI] [PubMed] [Google Scholar]
- 23.Shapeev AV. Moment tensor potentials: a class of systematically improvable interatomic potentials. Multiscale Model. Simul. 2016;14:1153–1173. doi: 10.1137/15M1054183. [DOI] [Google Scholar]
- 24.Podryabinkin EV, Shapeev AV. Active learning of linearly parametrized interatomic potentials. Comput. Mater. Sci. 2017;140:171–180. doi: 10.1016/j.commatsci.2017.08.031. [DOI] [Google Scholar]
- 25.Gubaev K, Podryabinkin EV, Hart GLW, Shapeev AV. Accelerating high-throughput searches for new alloys with active learning of interatomic potentials. Comput. Mater. Sci. 2019;156:148–156. doi: 10.1016/j.commatsci.2018.09.031. [DOI] [Google Scholar]
- 26.Inaguma Y, et al. High ionic conductivity in lithium lanthanum titanate. Solid State Commun. 1993;86:689–693. doi: 10.1016/0038-1098(93)90841-A. [DOI] [Google Scholar]
- 27.Liao W, Dronskowski R. Trisodium yttrium(III) hexachloride. Acta Crystallogr. Sect. E Struct. Rep. Online. 2004;60:i72–i73. doi: 10.1107/S1600536804011043. [DOI] [Google Scholar]
- 28.Cipriani C. Ricerche strutturistiche e cristallochimiche sul fluotitanato di sodi, 1955. Period. Mineral. 1955;24:361–375. [Google Scholar]
- 29.Harris RK, Nesbitt GJ. Cross polarization for quadrupolar nuclei—Proton to sodium-23. J. Magn. Reson. 1988;78:245–256. [Google Scholar]
- 30.Arnold AA, et al. Structure of NaYF4 upconverting nanoparticles: a multinuclear solid-state NMR and DFT computational study. J. Phys. Chem. C. 2013;117:25733–25741. doi: 10.1021/jp405813a. [DOI] [Google Scholar]
- 31.Bessada C, Rakhmatullin A, Rollet A-L, Zanghi D. High temperature NMR approach of mixtures of rare earth and alkali fluorides: an insight into the local structure. J. Fluor. Chem. 2009;130:45–52. doi: 10.1016/j.jfluchem.2008.07.010. [DOI] [Google Scholar]
- 32.Udovic TJ, et al. Exceptional superionic conductivity in disordered sodium decahydro-closo-decaborate. Adv. Mater. 2014;26:7622–7626. doi: 10.1002/adma.201403157. [DOI] [PubMed] [Google Scholar]
- 33.Verdal N, et al. Anion reorientations in the superionic conducting phase of Na2B12H12. J. Phys. Chem. C. 2014;118:17483–17489. doi: 10.1021/jp506252c. [DOI] [Google Scholar]
- 34.Martelli P, et al. Rotational motion in LiBH4/LiI solid solutions. J. Phys. Chem. A. 2011;115:5329–5334. doi: 10.1021/jp201372b. [DOI] [PubMed] [Google Scholar]
- 35.Zhang Z, et al. Targeting superionic conductivity by turning on anion rotation at room temperature in fast ion conductors. Matter. 2020;2:1667–1684. doi: 10.1016/j.matt.2020.04.027. [DOI] [Google Scholar]
- 36.Banerjee S, Zhang X, Wang L-W. Motif-based design of an oxysulfide class of lithium superionic conductors: toward improved stability and record-high Li-ion conductivity. Chem. Mater. 2019;31:7265–7276. doi: 10.1021/acs.chemmater.9b01639. [DOI] [Google Scholar]
- 37.Duchêne L, et al. Crystallization of closo-borate electrolytes from solution enabling infiltration into slurry-casted porous electrodes for all-solid-state batteries. Energy Storage Mater. 2020;26:543–549. doi: 10.1016/j.ensm.2019.11.027. [DOI] [Google Scholar]
- 38.Duchêne L, et al. A stable 3 V all-solid-state sodium–ion battery based on a closo-borate electrolyte. Energy Environ. Sci. 2017;10:2609–2615. doi: 10.1039/C7EE02420G. [DOI] [Google Scholar]
- 39.Hayashi A, Noi K, Tanibata N, Nagao M, Tatsumisago M. High sodium ion conductivity of glass–ceramic electrolytes with cubic Na3PS4. J. Power Sources. 2014;258:420–423. doi: 10.1016/j.jpowsour.2014.02.054. [DOI] [Google Scholar]
- 40.Stratford JM, et al. Investigating sodium storage mechanisms in tin anodes: a combined pair distribution function analysis, density functional theory, and solid-state NMR approach. J. Am. Chem. Soc. 2017;139:7273–7286. doi: 10.1021/jacs.7b01398. [DOI] [PubMed] [Google Scholar]
- 41.Ando T, Sakuda A, Tatsumisago M, Hayashi A. All-solid-state sodium-sulfur battery showing full capacity with activated carbon MSP20-sulfur-Na3SbS4 composite. Electrochem. Commun. 2020;116:106741. doi: 10.1016/j.elecom.2020.106741. [DOI] [Google Scholar]
- 42.Wan H, et al. Core–shell Fe1–xS@Na2.9PS3.95Se0.05 nanorods for room temperature all-solid-state sodium batteries with high energy density. ACS Nano. 2018;12:2809–2817. doi: 10.1021/acsnano.8b00073. [DOI] [PubMed] [Google Scholar]
- 43.Zhang S, et al. Gradiently sodiated alucone as an interfacial stabilizing strategy for solid-state Na metal batteries. Adv. Funct. Mater. 2020;30:2001118. doi: 10.1002/adfm.202001118. [DOI] [Google Scholar]
- 44.Yue J, et al. High-performance all-inorganic solid-state sodium–sulfur battery. ACS Nano. 2017;11:4885–4891. doi: 10.1021/acsnano.7b01445. [DOI] [PubMed] [Google Scholar]
- 45.Rao RP, Zhang X, Phuah KC, Adams S. Mechanochemical synthesis of fast sodium ion conductor Na11Sn2PSe12 enables first sodium–selenium all-solid-state battery. J. Mater. Chem. A. 2019;7:20790–20798. doi: 10.1039/C9TA06279C. [DOI] [Google Scholar]
- 46.Wan H, et al. Nanoscaled Na3PS4 solid electrolyte for all-solid-state FeS2/Na batteries with ultrahigh initial coulombic efficiency of 95% and excellent cyclic performances. ACS Appl. Mater. Interfaces. 2018;10:12300–12304. doi: 10.1021/acsami.8b01805. [DOI] [PubMed] [Google Scholar]
- 47.Heo JW, Banerjee A, Park KH, Jung YS, Hong S-T. New Na-ion solid electrolytes Na4−xSn1−xSbxS4 (0.02 ≤ x ≤ 0.33) for all-solid-state Na-ion batteries. Adv. Energy Mater. 2018;8:1702716. doi: 10.1002/aenm.201702716. [DOI] [Google Scholar]
- 48.Murgia F, Brighi M, Černý R. Room-temperature-operating Na solid-state battery with complex hydride as electrolyte. Electrochem. Commun. 2019;106:106534. doi: 10.1016/j.elecom.2019.106534. [DOI] [Google Scholar]
- 49.Santhosha AL, Medenbach L, Palaniselvam T, Adelhelm P. Sodium-storage behavior of exfoliated MoS2 as an electrode material for solid-state batteries with Na3PS4 as the solid electrolyte. J. Phys. Chem. C. 2020;124:10298–10305. doi: 10.1021/acs.jpcc.0c00387. [DOI] [Google Scholar]
- 50.Zhang D, et al. Synthesis of cubic Na3SbS4 solid electrolyte with enhanced ion transport for all-solid-state sodium-ion batteries. Electrochim. Acta. 2018;259:100–109. doi: 10.1016/j.electacta.2017.10.173. [DOI] [Google Scholar]
- 51.Chi X, et al. Tailored organic electrode material compatible with sulfide electrolyte for stable all-solid-state sodium batteries. Angew. Chem. Int. Ed. 2018;57:2630–2634. doi: 10.1002/anie.201712895. [DOI] [PubMed] [Google Scholar]
- 52.Hao F, et al. Taming active material-solid electrolyte interfaces with organic cathode for all-solid-state batteries. Joule. 2019;3:1349–1359. doi: 10.1016/j.joule.2019.03.017. [DOI] [Google Scholar]
- 53.Moon CK, et al. Vacancy-driven Na+ superionic conduction in new Ca-doped Na3PS4 for all-solid-state Na-ion batteries. ACS Energy Lett. 2018;3:2504–2512. doi: 10.1021/acsenergylett.8b01479. [DOI] [Google Scholar]
- 54.Fan X, et al. High-performance all-solid-state Na–S battery enabled by casting–annealing technology. ACS Nano. 2018;12:3360–3368. doi: 10.1021/acsnano.7b08856. [DOI] [PubMed] [Google Scholar]
- 55.Tan DHS, et al. Elucidating reversible electrochemical redox of Li6PS5Cl solid electrolyte. ACS Energy Lett. 2019;4:2418–2427. doi: 10.1021/acsenergylett.9b01693. [DOI] [Google Scholar]
- 56.Tian Y, et al. Compatibility issues between electrodes and electrolytes in solid-state batteries. Energy Environ. Sci. 2017;10:1150–1166. doi: 10.1039/C7EE00534B. [DOI] [Google Scholar]
- 57.Kresse G, Furthmüller J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B. 1996;54:11169–11186. doi: 10.1103/PhysRevB.54.11169. [DOI] [PubMed] [Google Scholar]
- 58.Blöchl PE. Projector augmented-wave method. Phys. Rev. B. 1994;50:17953–17979. doi: 10.1103/PhysRevB.50.17953. [DOI] [PubMed] [Google Scholar]
- 59.Perdew JP, Burke K, Ernzerhof M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996;77:3865–3868. doi: 10.1103/PhysRevLett.77.3865. [DOI] [PubMed] [Google Scholar]
- 60.Ong SP, et al. Python Materials Genomics (pymatgen): a robust, open-source python library for materials analysis. Comput. Mater. Sci. 2013;68:314–319. doi: 10.1016/j.commatsci.2012.10.028. [DOI] [Google Scholar]
- 61.Deng Z, Zhu Z, Chu I-H, Ong SP. Data-driven first-principles methods for the study and design of alkali superionic conductors. Chem. Mater. 2017;29:281–288. doi: 10.1021/acs.chemmater.6b02648. [DOI] [Google Scholar]
- 62.Jain A, et al. Commentary: The materials project: a materials genome approach to accelerating materials innovation. APL Mater. 2013;1:011002. doi: 10.1063/1.4812323. [DOI] [Google Scholar]
- 63.Ong SP, et al. The Materials Application Programming Interface (API): a simple, flexible and efficient API for materials data based on REpresentational State Transfer (REST) principles. Comput. Mater. Sci. 2015;97:209–215. doi: 10.1016/j.commatsci.2014.10.037. [DOI] [Google Scholar]
- 64.Bergerhoff G, Hundt R, Sievers R, Brown ID. The inorganic crystal structure data base. J. Chem. Inf. Comput. Sci. 1983;23:66–69. doi: 10.1021/ci00038a003. [DOI] [Google Scholar]
- 65.Ong SP, Wang L, Kang B, Ceder G. Li−Fe−P−O2 phase diagram from first principles calculations. Chem. Mater. 2008;20:1798–1807. doi: 10.1021/cm702327g. [DOI] [Google Scholar]
- 66.Willems TF, Rycroft CH, Kazi M, Meza JC, Haranczyk M. Algorithms and tools for high-throughput geometry-based analysis of crystalline porous materials. Microporous Mesoporous Mater. 2012;149:134–141. doi: 10.1016/j.micromeso.2011.08.020. [DOI] [Google Scholar]
- 67.Plimpton, S. Fast parallel algorithms for short-range molecular dynamics. https://www.osti.gov/biblio/10176421 (1993) 10.2172/10176421.
- 68.Makulski W. Multinuclear magnetic resonance study of sodium salts in water solutions. Magnetochemistry. 2019;5:68. doi: 10.3390/magnetochemistry5040068. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and/or analyzed during the current study are available from the corresponding author on reasonable request.
The X-ray crystallographic coordinates for the structures reported in this study, Na3YCl6 and Na2ZrCl6, have been deposited at the Cambridge Crystallographic Data Centre (CCDC), under deposition numbers 2057626-2057627. These data can be obtained free of charge from The Cambridge Crystallographic Data Centre via www.ccdc.cam.ac.uk/data_request/cif.
DFT calculations and AIMD simulations were carried out using the open-source Python Materials Genomics (pymatgen) package, which is available at http://pymatgen.org. The moment tensor potential was fitted using the open-source materials machine learning (maml) package, which is available at https://github.com/materialsvirtuallab/maml.