Abstract
Pruritus represents one of the most common symptoms in dermatology and general medicine. Chronic pruritus severely impairs the quality of life of affected patients. During the last two decades a number of modulators and mediator of pruritus have been identified. Recently, Interleukin (IL)-31 and its receptor complex attracted significant interest, as clinical phase two studies demonstrated therapeutic efficacy of the neutralizing IL-31 receptor A (IL-31RA) antibody nemolizumab in patients suffering from atopic dermatitis or prurigo nodularis. IL-31 has also been shown to play relevant roles in allergic contact dermatitis, urticaria, mastocytosis, allergic rhinitis and asthma. Here, we summarize the current knowledge of the novel cytokine IL-31 and its receptor regarding cellular origin, regulation, signaling pathways and their involvement in biological processes such as pruritus, neuronal growth, inflammation, barrier dysfunction and tissue remodeling.
Keywords: atopic dematitis, interleukin-31, interleukin-31 receptor, neuroinflammation, pruritus
Introduction
Pruritus represents an important archaic sensation, and its evolutionary role is to sensitize the host to a distinct body site in order to remove invading parasites or plant matter. Chronic pruritus severely impairs the quality of life of affected patients and represents a significant unmet medical need. In 2004, Dillon et al. first demonstrated the involvement of IL-31 signaling in the development of pruritus and atopic dermatitis-like skin lesions (1). Subsequently an emerging body of evidence supported a central role of IL-31 and its receptor in bridging the immune system with neurons, epithelial surfaces and connective tissue. Recently, phase two clinical trials demonstrated therapeutic efficacy of the neutralizing IL-31RA antibody nemolizumab in patients suffering from atopic dermatitis or prurigo nodularis (2, 3). In addition, IL-31 also plays a role in TH2-driven and autoimmune diseases such as contact dermatitis, urticaria, mastocytosis, allergic rhinitis; but also systemic sclerosis, dermatomyositis, and lupus erythematosus (4–12). Here, we summarize the current knowledge on the novel cytokine IL-31 and its receptor regarding cellular origin, regulation, signaling pathways and their involvement in biological processes such as pruritus, neuronal growth, inflammation, barrier dysfunction and tissue remodeling.
Interleukin-31
IL-31 represents a member of the IL-6 family of cytokines, which share a four-helical structure and the majority signals through receptor complexes containing glycoprotein 130 (gp130). This family consists of nine members including IL-6, IL-11, ciliary neurotrophic factor (CNTF), leukemia inhibitory factor (LIF), oncostatin M (OSM), cardiotrophin 1 (CT-1), cardiotrophin-like cytokine (CLC), IL-27 and IL-31. Members of the IL-6 family are predominantly expressed under proinflammatory conditions and realize pleiotropic functions in immune-related processes (13). The IL31 gene is located on chromosome 12q24.31 (1). Recent studies support the production of IL-31 in a variety of leukocyte subsets including T cells, eosinophils, basophils, mast cells, monocytes and dendritic cells. However, it is widely accepted that effector memory T cells with a TH2 phenotype represent the major source of IL-31 (4, 14–18). Interestingly, findings linking IL-31 expression with patients suffering from Dedicator of cytokinesis protein 8 (DOCK8) deficiency-related hyper IgE syndrome pointed to an important upstream regulation pathway. DOCK8 loss-of-function mutations lead to a combined immunodeficiency with elevated serum levels of IgE, eosinophilia, decreased number of B and T cells as well as severe atopic dermatitis with increased IL-31 expression (19). Subsequent in vitro- as well as in vivo-studies demonstrated that DOCK8 is a negative regulator of the nuclear translocation of Endothelial PAS domain-containing protein 1 (EPAS1). This function is dependent of STK4 (MST1), a serine threonine kinase involved in apoptosis (20, 21). Knockdown of the MST1 gene led to an increased translocation of EPAS1 to the nucleus in alignment with DOCK8 knockout models showing elevated IL-31 expression (21). Consequently, clinical symptoms of patients with STK4 (MST1) mutations leading to deficiency at the protein level are resembling those with DOCK8 deficiency (22). EPAS1 is regulated by IL-4-mediated signal transducer and activator of transcription 6 (STAT6) signaling in CD4+ T cells (23). Jabara et al. reported that DOCK8 is constitutively associated with myeloid differentiation primary response protein (MyD88), an adaptor protein of Toll-like receptors (TLR) (24). Hence it is interesting to speculate whether microbes such as S. aureus may influence IL-31 production through TLR engagement.
Interleukin-31 Receptor
Within the IL-6 family IL-31 is special, since it shares the four helical structure but does not signal through a receptor complex containing gp130. Instead, it binds to a heterodimeric receptor composed of the IL-31RA chain and the oncostatin M receptor (OSMR) β chain. The IL31RA gene is located on chromosome 5q11.2, 24 kb downstream of IL6ST (25, 26). From a phylogenetical view, IL-31RA is paralogous to gp130, although they share only 28% amino acid identity. It has five fibronectin type III (FNIII)-like domains and shares the WSxWS motif and the conserved cysteines with other type I cytokine receptors within the cytokine binding domain [as reviewed in (27)]. Horejs-Hoeck et al. showed that STAT1 is a relevant transcription factor to activate the promoter region of the IL31RA gene following IFN-γ stimulation and this regulation pathway was confirmed in several studies and cell types (28). Cytokine effects are based on their capacity to assemble receptor complexes to bring the associated kinases in spatial proximity for phosphorylation. Therefore, the expression pattern of relevant receptor chains in target cells determines their ability to respond to specific cytokine signals. The OSMRβ chain is considered to be widely expressed (29). Hence the limiting factor for IL-31 signal transduction appears to be the expression of the IL-31RA chain. Recent studies demonstrate that multiple leukocyte subsets, as well as epithelial and stromal cells express IL-31RA in steady state or more importantly under activated conditions (14, 17, 28, 30, 31). At first, the expression of IL-31RA on itch-conducting dorsal root ganglia (DRG) neurons attracted significant attention (4). Non-immune cells such as keratinocytes, fibroblasts and a distinct subset of DRG neurons also express and signal via IL-31RA (18, 31, 32). Binding of IL-31 to the receptor complex leads to phosphorylation of STAT1, STAT3 and STAT5 via the associated Janus kinase (JAK) 1 and JAK2 (33, 34). Besides JAK/STAT signaling the IL-31 receptor complex activates MEK/ERK and PI3K/Akt pathways as well as the JNK pathway (33, 35–37). Negative feedback mechanisms of IL-31RA signaling include suppressor of cytokine signaling (SOCS)1- and SOCS3-dependent inhibition of STAT3 activation (34). Interestingly, OSMR is a shared subunit of the receptor complexes of IL-31 and OSM, although their biological functions differ. While IL-31 is involved in many TH2-driven diseases as mentioned above, OSM plays an important role in hematopoiesis and cancer development (38). It will be of interest to elucidate the distinct roles of IL-31 and OSM. Taken together, the diverse distribution of its receptor enables IL-31 to target the nervous system, immune functions, epithelial surfaces and stromal cells.
Nervous System
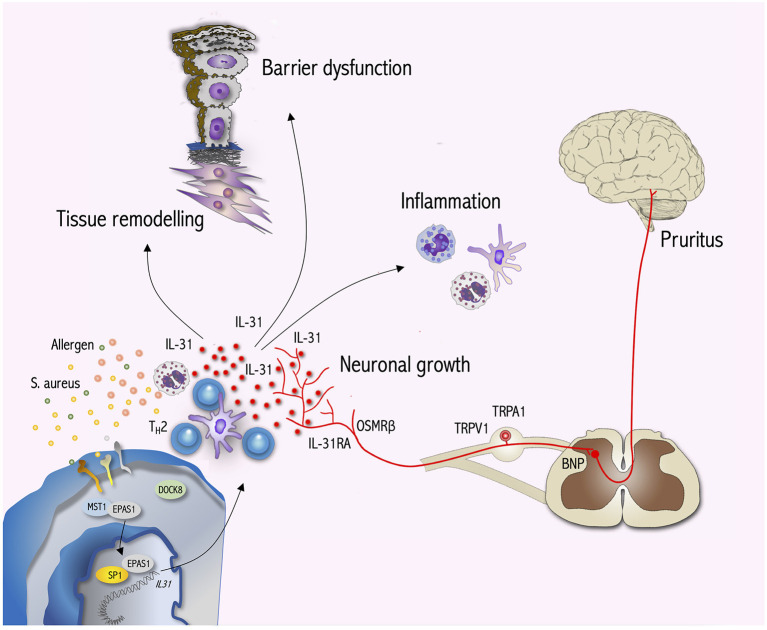
Within the cytokine superfamily IL-31 has a unique position, because it bridges the gap between the immune and the peripheral nervous systems (see Figure 1). During recent years, several independent studies confirmed the expression and signaling of IL-31RA and OSMRβ in a subset of murine as well as human DRG neurons (4, 18, 39–41). These findings stimulated further research on IL-31 targeting sensory neurons. Cevikbas et al. demonstrated in murine behavioral studies that IL-31 induces itch but not pain and mediates its effects independent of mast cells by activating the ion channels TRPV1 and TRPA1. In DRG neurons IL-31 induces intracellular Ca2+ mobilization as well as STAT3 and ERK phosphorylation (18). Following the activation of afferent DRG neurons, neurotransmitters such as natriuretic polypeptide b (Nppb) forward the signal further to the dorsal horn of the spinal cord, where the gastrin-releasing peptide receptor (Grpr) system is subsequently activated transmitting the signal further to projection neurons that transport the information to the brain (42–45). Recently, Meng et al. showed that IL-31 stimulation increased Nppb in DRG neurons in vitro and in vivo and induced soluble N-ethylmaleimide-sensitive-factor attachment receptor (SNARE)–dependent brain natriuretic peptide (BNP) release (46). In pharmacological studies, Ma et al. demonstrated that activation of the spinal neuropeptide Y system dampens IL-31-induced scratching behavior through activation of neuropeptide Y2 receptor on DRG neurons (47). Notably, noxious signals activate neuropeptide Y interneurons, and this may explain, how the infliction of pain, e.g., through scratching, heat, cold, etc., may reduce itch perception in atopic dermatitis patients. Next to the initiation of pruritus signals, Feld et al. recently demonstrated that IL-31 also induces a distinct transcriptional program in sensory neurons, leading to nerve elongation and branching both in vitro and in vivo. Hence the increased density of neuronal networks in the skin may help us understand why atopic dermatitis patients experience increased sensitivity to minimal stimuli inducing sustained itch (48).
Figure 1.
IL-31 signaling bridges the gap between the immune system, neurons and epithelial surfaces. During T cell activation DOCK8 dissociates from EPAS1 enabling EPAS1 to translocate to the nucleus. Within the nucleus EPAS1 forms a complex with SP1 initiating IL31 transcription. TH2 cells are a major source of IL-31 production. In IL-31RA/OSMRß-expressing sensory neurons IL-31 induces the activation of ion channels (TRPV1, TRPA1) and transmits pruritus signals via BNP to the CNS. Moreover, IL-31 stimulates neuronal growth and the branching of sensory nerves. Furthermore, IL-31 targets immune cells such as mast cells, eosinophils, basophils and monocytes/dendritic cells to induce inflammation. Within the skin, IL-31 impairs keratinocyte differentiation as well as barrier function and in turn activates keratinocytes to produce cytokines, chemokines and pruritus mediators amplifying skin inflammation and itch. Interestingly, IL-31 also interacts with dermal fibroblasts initiating tissue remodeling by inducing collagen production and cytokine as well as chemokine expression. Hence, IL-31 signaling exerts pleiotropic effects beyond pruritus.
Immune Functions
Since the IL-31 receptor heterodimer is expressed on a variety of different leukocyte subsets including monocytes, macrophages, dendritic cells, eosinophils, mast cells and basophils, it is interesting to have a closer look at immune functions that are targeted by IL-31. Recently, Raap et al. demonstrated that basophils upon IL-31 stimulation do not release histamine but secrete large amounts of IL-4 and IL-13 (14). This is of particular importance since IL-4 is a critical factor for the differentiation of T cells into a TH2 phenotype and the source of IL-4 in this dendritic cell-driven process is still debated (49, 50). Thus, IL-31 secretion may serve as an early upstream signal during the development of type 2 skin inflammation. In eosinophils and dendritic cells IL-31 induced a set of proinflammatory cytokines and chemokines including TNF-α, IL-1β, IL-6, CXCL1, CXCL8, CCL2 and CCL5 and CCL22 (16, 51). Through these molecules, IL-31 may recruit neutrophils (CXCL1, CXCL8), dendritic cells (CCL2), TH1 (CCL5) and TH2 (CCL22) cells to sites of inflammation or promote angiogenesis (CXCL1, CXCL8) and tissue remodeling (CCL2, IL-6) (52, 53). On the other hand, cytokines such as TNF-α, IL-1β, and IL-6 may directly affect T cell, B cell and dendritic cell functions as well as activate surrounding stromal or epithelial cells (54). Hence, IL-31 signaling is able to amplify inflammation via different self-reinforcing loops.
Epithelial Surfaces
In 2004, Dillon et al. demonstrated for the first time the expression of IL-31RA and OSMRß in epidermal keratinocytes and IL-31 stimulation resulted in chemokine (CXCL1, CCL1, CCL4, CCL17, CCL19, CCL22, and CCL23) expression (1). Subsequently, a number of studies confirmed IL-31 receptor expression on keratinocytes and showed downstream signaling leading to STAT3 and ERK phosphorylation. Recent findings in 2D and 3D keratinocyte culture systems unravel, that IL-31 stimulation also modulates keratinocyte differentiation and disrupts epithelial barrier function (55–57). Cornelissen et al. demonstrated that IL-31 induced cell cycle arrest in keratinocytes and inhibited proliferation (55). Moreover, IL-31 elicited a differentiation defect with decreased filaggrin expression and impaired barrier functions facilitating transepidermal allergen penetration in organotypic keratinocyte cultures (55–57). Next to the production of chemokines, IL-31-stimulated keratinocytes contribute to skin inflammation through the expression of key proinflammatory mediators including IL-1α, IL-1β, IL-6, S100A7, S100A8, S100A9, β-defensin-2, β-defensin-3 (57). Moreover, IL-31-induced BNP release from sensory neurons may activate keratinocytes to produce proinflammatory cytokines and chemokines (46). Hence, inflammation circuits between epithelial surfaces, nerves and immune cells are connected and amplified via IL-31 signaling.
More recently, another keratinocyte-driven circuit potentially amplifying pruritus has been proposed. Andoh et al. demonstrated that intradermal injection of IL-31 induced thromboxane synthase in epidermal keratinocytes and significantly increased the concentration of thromboxane B2, a metabolite of the pruritus mediator thromboxane A2 (58). Moreover, keratinocytes produced the pruritus mediator leukotriene B4 (LTB4) following IL-31 treatment and the LTB4 receptor antagonist CMHVA as well as the 5-lipoxygenase inhibitor, zileuton, suppressed the scratching behavior of mice intradermally injected with IL-31 (59).
Thus, next to the direct engagement of peripheral sensory neurons, IL-31 may sustain pruritus via keratinocyte activation and the release of other pruritus mediators (58–60). Notably, besides epidermal keratinocytes, bronchial and gut epithelial cells have been shown to be a target of IL-31 (61, 62).
Tissue Remodeling
Given the pleiotropic functions of IL-6 family members it has been reasonable to also investigate the role of IL-31 in tissue remodeling. Several studies report a direct effect of IL-31 on fibroblasts (17, 63). IL-31 signaling resulted in STAT3 phosphorylation and the activation of ERK, JNK and AKT (17). It is important to note that pro-fibrotic processes often follow STAT3 signaling pathways representing a considerable checkpoint for tissue fibrosis (64). Indeed, high levels of IL-31 were reported in plasma, fibrotic skin and lung lesions of systemic sclerosis (SSc) patients (10). Moreover, IL-31RA was upregulated in fibrotic skin and lung fibroblasts. Gene expression analysis of IL-31-treated dermal fibroblasts revealed a total of 561 differentially expressed genes with 200 genes involved in processes such as cell proliferation and growth. Furthermore, the authors showed that IL-31 stimulated dermal fibroblast activated STAT3 and PI3K/Akt pathways and induced collagen I production (10). Several expression studies in fibroblasts also support that IL-31 stimulation promotes inflammation and tissue remodeling through the induction of IL-6, IL-16, IL-32, CCL2, CCL13, CCL15, CXCL1, CXCL3, CXCL8, and CXCL10 and matrix metalloproteinases (MMP-1, MMP-3, MMP-7 and MMP-25) (63).
Taken together, IL-31 represents a master regulator of neuroimmune inflammation and bridges the gap between immune cells, the nervous system and epithelial tissues.
Discussion
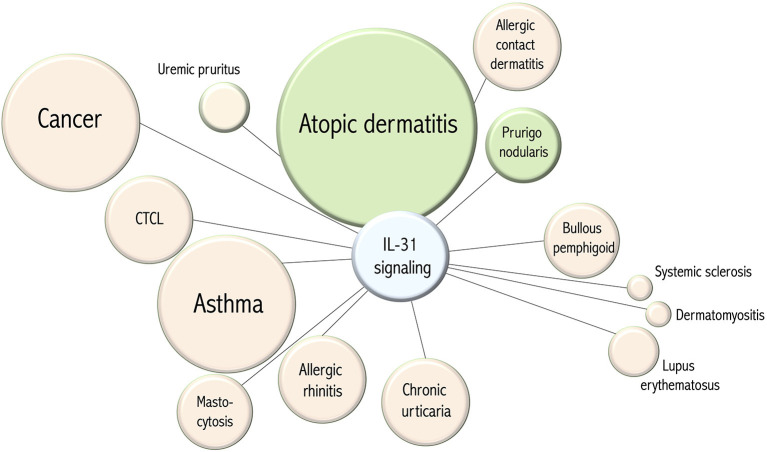
During recent years a variety of diseases have been associated with IL-31 signaling (see Figure 2). An initial focus was directed on processes accompanied with pruritus and following, at least partly, concepts of type 2 inflammation. These included atopic dermatitis, allergic contact dermatitis, urticaria, mastocytosis, allergic rhinitis and asthma (4–9). Given the role of IL-31 signaling in the development of itch it is interesting to speculate whether IL-31 may also be involved in the stimulation of sneezing, coughing or bronchial hyperreactivity. In this context, other epithelial surfaces such as the gut and conditions such as irritable bowel syndrome also come to mind. STAT1 related regulation of IL-31RA may link this pathway also with autoimmune diseases such as systemic sclerosis, dermatomyositis and lupus erythematosus (10–12). A subset of affected patients experience severe pruritus but IL-31 signaling in autoimmune inflammation may also facilitate fibrosis and amplify inflammatory circuits. These are interesting aspects that need to be further explored in the future. Among autoimmune skin diseases, bullous pemphigoid has a unique position since patients develop autoantibodies against hemidesmosomes (BP180, BP230), eosinophilia, urticarial skin lesions, blisters and suffer from severe pruritus. A number of studies demonstrated the expression of IL-31 and its receptor in this condition and bullous pemphigoid appears to be a very interesting candidate for clinical studies targeting IL-31 signaling (65–68). Early on IL-31 expression and serum levels have been investigated in patients suffering from cutaneous T cell lymphoma (69–71). Notably, an increasing body of literature links IL-31 with malignant diseases such as endometrial carcinoma, lung cancer, myeloproliferative disorders, mastocytosis, cutaneous T cell lymphoma and follicular B cell lymphoma (7, 69, 71–76). The role of IL-31 in malignant diseases remains largely obscure but this aspect is worth to closely follow in the future. Taken together, IL-31 is a neuroimmune cytokine and IL-31RA signaling represents a master regulator of inflammation that bridges the gap between immune cells, the nervous system and epithelial tissues.
Figure 2.
Disease associations of IL-31 signaling. The circle diameter of each item correlates to the number of disease-associated publications listed in PubMed (pubmed.ncbi.nlm.nih.gov). The green color of an item corresponds to the therapeutic efficacy in clinical trials of targeting IL-31 signaling. The distance of an item from the center indicates whether IL-31 signaling hypothetically could serve as a therapeutic target.
Author Contributions
JN, MK, AD, BH, and VJ conceptualization and writing. UR and BH critical revision of manuscript. BH and PO editing. All authors contributed to the article and approved the submitted version.
Conflict of Interest
BH has received research funding from Galderma Pharmaceuticals and participates in studies with nemolizumab. VJ is an employee of Galderma Pharmaceuticals and is involved in nemolizumab development. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study received funding from the German Research Foundation DFG HO2092/7-1 (FOR2690-PruSEARCH Translational Pruritus Research) and the European Union (Grant 821511, BIOMAP IMI2).
References
- 1.Dillon SR, Sprecher C, Hammond A, Bilsborough J, Rosenfeld-Franklin M, Presnell SR, et al. Interleukin 31, a cytokine produced by activated T cells, induces dermatitis in mice. Nat Immunol. (2004) 5:752–60. 10.1038/ni1084 [DOI] [PubMed] [Google Scholar]
- 2.Ruzicka T, Hanifin JM, Furue M, Pulka G, Mlynarczyk I, Wollenberg A, et al. Anti-interleukin-31 receptor A antibody for atopic dermatitis. N Engl J Med. (2017) 376:826–35. 10.1056/NEJMoa1606490 [DOI] [PubMed] [Google Scholar]
- 3.Ständer S, Yosipovitch G, Legat FJ, Lacour JP, Paul C, Narbutt J, et al. Trial of nemolizumab in moderate-to-severe prurigo nodularis. N Engl J Med. (2020) 382:706–16. 10.1056/NEJMoa1908316 [DOI] [PubMed] [Google Scholar]
- 4.Sonkoly E, Muller A, Lauerma AI, Pivarcsi A, Soto H, Kemeny L, et al. IL-31: a new link between T cells and pruritus in atopic skin inflammation. J Allergy Clin Immunol. (2006) 117:411–7. 10.1016/j.jaci.2005.10.033 [DOI] [PubMed] [Google Scholar]
- 5.Neis MM, Peters B, Dreuw A, Wenzel J, Bieber T, Mauch C, et al. Enhanced expression levels of IL-31 correlate with IL-4 and IL-13 in atopic and allergic contact dermatitis. J Allergy Clin Immunol. (2006) 118:930–7. 10.1016/j.jaci.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 6.Raap U, Wieczorek D, Gehring M, Pauls I, Ständer S, Kapp A, et al. Increased levels of serum IL-31 in chronic spontaneous urticaria. Exp Dermatol. (2010) 19:464–6. 10.1111/j.1600-0625.2010.01067.x [DOI] [PubMed] [Google Scholar]
- 7.Hartmann K, Wagner N, Rabenhorst A, Pflanz L, Leja S, Förster A, et al. Serum IL-31 levels are increased in a subset of patients with mastocytosis and correlate with disease severity in adult patients. J Allergy Clin Immunol. (2013) 132:232–5. 10.1016/j.jaci.2012.11.008 [DOI] [PubMed] [Google Scholar]
- 8.Chai R, Liu B, Qi F. The significance of the levels of IL-4, IL-31 and TLSP in patients with asthma and/or rhinitis. Immunotherapy. (2017) 9:331–7. 10.2217/imt-2016-0131 [DOI] [PubMed] [Google Scholar]
- 9.Lei Z, Liu G, Huang Q, Lv M, Zu R, Zhang GM, et al. SCF and IL-31 rather than IL-17 and BAFF are potential indicators in patients with allergic asthma. Allergy. (2008) 63:327–32. 10.1111/j.1398-9995.2007.01566.x [DOI] [PubMed] [Google Scholar]
- 10.Yaseen B, Lopez H, Taki Z, Zafar S, Rosario H, Abdi BA, et al. Interleukin-31 promotes pathogenic mechanisms underlying skin and lung fibrosis in scleroderma. Rheumatology. (2020) 59:2625–36. 10.1093/rheumatology/keaa195 [DOI] [PubMed] [Google Scholar]
- 11.Kim HJ, Zeidi M, Bonciani D, Pena SM, Tiao J, Sahu S, et al. Itch in dermatomyositis: the role of increased skin interleukin-31. Br J Dermatol. (2018) 179:669–78. 10.1111/bjd.16498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Huang HT, Chen JM, Guo J, Lan Y, Wei YS. The association of interleukin-31 polymorphisms with interleukin-31 serum levels and risk of systemic lupus erythematosus. Rheumatol Int. (2016) 36:799–805. 10.1007/s00296-016-3422-6 [DOI] [PubMed] [Google Scholar]
- 13.Garbers C, Hermanns HM, Schaper F, Müller-Newen G, Grötzinger J, Rose-John S, et al. Plasticity and cross-talk of interleukin 6-type cytokines. Cytokine Growth Factor Rev. (2012) 23:85–97. 10.1016/j.cytogfr.2012.04.001 [DOI] [PubMed] [Google Scholar]
- 14.Raap U, Gehring M, Kleiner S, Rüdrich U, Eiz-Vesper B, Haas H, et al. Human basophils are a source of - and are differentially activated by - IL-31. Clin Exp Allergy. (2017) 47:499–508. 10.1111/cea.12875 [DOI] [PubMed] [Google Scholar]
- 15.Niyonsaba F, Ushio H, Hara M, Yokoi H, Tominaga M, Takamori K, et al. Antimicrobial peptides human beta-defensins and cathelicidin LL-37 induce the secretion of a pruritogenic cytokine IL-31 by human mast cells. J Immunol. (2010) 184:3526–34. 10.4049/jimmunol.0900712 [DOI] [PubMed] [Google Scholar]
- 16.Cheung PF, Wong CK, Ho AW, Hu S, Chen DP, Lam CW. Activation of human eosinophils and epidermal keratinocytes by Th2 cytokine IL-31: implication for the immunopathogenesis of atopic dermatitis. Int Immunol. (2010) 22:453–67. 10.1093/intimm/dxq027 [DOI] [PubMed] [Google Scholar]
- 17.Wong CK, Leung KM, Qiu HN, Chow JY, Choi AO, Lam CW. Activation of eosinophils interacting with dermal fibroblasts by pruritogenic cytokine IL-31 and alarmin IL-33: implications in atopic dermatitis. PLoS ONE. (2012) 7:e29815. 10.1371/journal.pone.0029815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cevikbas F, Wang X, Akiyama T, Kempkes C, Savinko T, Antal A, et al. A sensory neuron-expressed IL-31 receptor mediates T helper cell-dependent itch: Involvement of TRPV1 and TRPA1. J Allergy Clin Immunol. (2014) 133:448–60. 10.1016/j.jaci.2013.10.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang Q, Davis JC, Lamborn IT, Freeman AF, Jing H, Favreau AJ. Combined immunodeficiency associated with DOCK8 mutations. N Engl J Med. (2009) 361:2046–55. 10.1056/NEJMoa0905506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Graves JD, Draves KE, Gotoh Y, Krebs EG, Clark EA. Both phosphorylation and caspase-mediated cleavage contribute to regulation of the Ste20-like protein kinase Mst1 during CD95/Fas-induced apoptosis. J Biol Chem. (2001) 276:14909–15. 10.1074/jbc.M010905200 [DOI] [PubMed] [Google Scholar]
- 21.Yamamura K, Uruno T, Shiraishi A, Tanaka Y, Ushijima M, Nakahara T, et al. The transcription factor EPAS1 links DOCK8 deficiency to atopic skin inflammation via IL-31 induction. Nat Commun. (2017) 8:13946. 10.1038/ncomms13946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Halacli SO, Ayvaz DC, Sun-Tan C, Erman B, Uz E, Yilmaz DY, et al. STK4 (MST1) deficiency in two siblings with autoimmune cytopenias: a novel mutation. Clin Immunol. (2015) 161:316–23. 10.1016/j.clim.2015.06.010 [DOI] [PubMed] [Google Scholar]
- 23.Elo LL, Järvenpää H, Tuomela S, Raghav S, Ahlfors H, Laurila K, et al. Genome-wide profiling of interleukin-4 and STAT6 transcription factor regulation of human Th2 cell programming. Immunity. (2010) 32:852–62. 10.1016/j.immuni.2010.06.011 [DOI] [PubMed] [Google Scholar]
- 24.Jabara HH, McDonald DR, Janssen E, Massaad MJ, Ramesh N, Borzutzky A, et al. DOCK8 functions as an adaptor that links TLR-MyD88 signaling to B cell activation. Nat Immunol. (2012) 13:612–20. 10.1038/ni.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Diveu C, Lelièvre E, Perret D, Lak-Hal AH, Froger J, Guillet C, et al. GPL, a novel cytokine receptor related to GP130 and leukemia inhibitory factor receptor. J Biol Chem. (2003) 278:49850–9. 10.1074/jbc.M307286200 [DOI] [PubMed] [Google Scholar]
- 26.Ghilardi N, Li J, Hongo JA, Yi S, Gurney A, de Sauvage FJ. A novel type I cytokine receptor is expressed on monocytes, signals proliferation, and activates STAT-3 and STAT-5. J Biol Chem. (2002) 277:16831–6. 10.1074/jbc.M201140200 [DOI] [PubMed] [Google Scholar]
- 27.Zhang Q, Putheti P, Zhou Q, Liu Q, Gao W. Structures and biological functions of IL-31 and IL-31 receptors. Cytokine Growth Factor Rev. (2008) 19:347–56. 10.1016/j.cytogfr.2008.08.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Horejs-Hoeck J, Schwarz H, Lamprecht S, Maier E, Hainzl S, Schmittner M, et al. Dendritic cells activated by IFN-γ/STAT1 express IL-31 receptor and release proinflammatory mediators upon IL-31 treatment. J Immunol. (2012) 188:5319–26. 10.4049/jimmunol.1101044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.GTEx Portal - OSMR. (2020). Available online at: https://www.gtexportal.org/home/gene/OSMR (accessed december 6, 2020).
- 30.Bilsborough J, Leung DY, Maurer M, Howell M, Boguniewicz M, Yao L, et al. IL-31 is associated with cutaneous lymphocyte antigen-positive skin homing T cells in patients with atopic dermatitis. J Allergy Clin Immunol. (2006) 117:418–25. 10.1016/j.jaci.2005.10.046 [DOI] [PubMed] [Google Scholar]
- 31.Jawa RS, Chattopadhyay S, Tracy E, Wang Y, Huntoon K, Dayton MT, et al. Regulated expression of the IL-31 receptor in bronchial and alveolar epithelial cells, pulmonary fibroblasts, and pulmonary macrophages. J Interferon Cytokine Res. (2008) 28:207–19. 10.1089/jir.2007.0057 [DOI] [PubMed] [Google Scholar]
- 32.Kasraie S, Niebuhr M, Baumert K, Werfel T. Functional effects of interleukin 31 in human primary keratinocytes. Allergy. (2011) 66:845–52. 10.1111/j.1398-9995.2011.02545.x [DOI] [PubMed] [Google Scholar]
- 33.Kasraie S, Niebuhr M, Werfel T. Interleukin (IL)-31 activates signal transducer and activator of transcription (STAT)-1, STAT-5 and extracellular signal-regulated kinase 1/2 and down-regulates IL-12p40 production in activated human macrophages. Allergy. (2013) 68:739–47. 10.1111/all.12152 [DOI] [PubMed] [Google Scholar]
- 34.Maier E, Mittermeir M, Ess S, Neuper T, Schmiedlechner A, Duschl A, et al. Prerequisites for functional interleukin 31 signaling and its feedback regulation by suppressor of cytokine signaling 3 (SOCS3). J Biol Chem. (2015) 290:24747–59. 10.1074/jbc.M115.661306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dambacher J, Beigel F, Seiderer J, Haller D, Göke B, Auernhammer CJ, et al. Interleukin 31 mediates MAP kinase and STAT1/3 activation in intestinal epithelial cells and its expression is upregulated in inflammatory bowel disease. Gut. (2007) 56:1257–65. 10.1136/gut.2006.118679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diveu C, Lak-Hal AH, Froger J, Ravon E, Grimaud L, Barbier F, et al. Predominant expression of the long isoform of GP130-like (GPL) receptor is required for interleukin-31 signaling. Eur Cytokine Netw. (2004) 15:291–302. [PubMed] [Google Scholar]
- 37.Chattopadhyay S, Tracy E, Liang P, Robledo O, Rose-John S, Baumann H. Interleukin-31 and oncostatin-M mediate distinct signaling reactions and response patterns in lung epithelial cells. J Biol Chem. (2007) 282:3014–26. 10.1074/jbc.M609655200 [DOI] [PubMed] [Google Scholar]
- 38.Stephens JM, Elks CM. Oncostatin M: potential implications for malignancy and metabolism. Curr Pharm Des. (2017) 23:3645–57. 10.2174/1381612823666170704122559 [DOI] [PubMed] [Google Scholar]
- 39.Bando T, Morikawa Y, Komori T, Senba E. Complete overlap of interleukin-31 receptor A and oncostatin M receptor beta in the adult dorsal root ganglia with distinct developmental expression patterns. Neuroscience. (2006) 142:1263–71. 10.1016/j.neuroscience.2006.07.009 [DOI] [PubMed] [Google Scholar]
- 40.Tamura S, Morikawa Y, Miyajima A, Senba E. Expression of oncostatin M receptor beta in a specific subset of nociceptive sensory neurons. Eur J Neurosci. (2003) 17:2287–98. 10.1046/j.1460-9568.2003.02681.x [DOI] [PubMed] [Google Scholar]
- 41.Arai I, Tsuji M, Miyagawa K, Takeda H, Akiyama N, Saito S. Repeated administration of IL-31 upregulates IL-31 receptor A (IL-31RA) in dorsal root ganglia and causes severe itch-associated scratching behaviour in mice. Exp Dermatol. (2015) 24:75–8. 10.1111/exd.12587 [DOI] [PubMed] [Google Scholar]
- 42.Mishra SK, Hoon MA. The cells and circuitry for itch responses in mice. Science. (2013) 340:968–71. 10.1126/science.1233765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sun YG, Zhao ZQ, Meng XL, Yin J, Liu XY, Chen ZF. Cellular basis of itch sensation. Science. (2009) 325:1531–4. 10.1126/science.1174868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Aresh B, Freitag FB, Perry S, Blümel E, Lau J, Franck MCM, et al. Spinal cord interneurons expressing the gastrin-releasing peptide receptor convey itch through VGLUT2-mediated signaling. Pain. (2017) 158:945–61. 10.1097/j.pain.0000000000000861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mu D, Deng J, Liu KF, Wu ZY, Shi YF, Guo WM, et al. A central neural circuit for itch sensation. Science. (2017) 357:695–9. 10.1126/science.aaf4918 [DOI] [PubMed] [Google Scholar]
- 46.Meng J, Moriyama M, Feld M, Buddenkotte J, Buhl T, Szöllösi A, et al. New mechanism underlying IL-31-induced atopic dermatitis. J Allergy Clin Immunol. (2018) 141:1677–89.e8. 10.1016/j.jaci.2017.12.1002 [DOI] [PubMed] [Google Scholar]
- 47.Ma H, Gao T, Jakobsson JET, Weman HM, Xu B, Larhammar D, et al. The neuropeptide Y Y(2) receptor is coexpressed with Nppb in primary afferent neurons and Y(2) activation reduces histaminergic and IL-31-induced itch. J Pharmacol Exp Ther. (2020) 372:73–82. 10.1124/jpet.119.262584 [DOI] [PubMed] [Google Scholar]
- 48.Feld M, Garcia R, Buddenkotte J, Katayama S, Lewis K, Muirhead G, et al. The pruritus- and TH2-associated cytokine IL-31 promotes growth of sensory nerves. J Allergy Clin Immunol. (2016) 138:500–8.e24. 10.1016/j.jaci.2016.02.020 [DOI] [PubMed] [Google Scholar]
- 49.Yoshimoto T. The hunt for the source of primary interleukin-4: how we discovered that natural killer T cells and basophils determine T helper type 2 cell differentiation in vivo. Front Immunol. (2018) 9:716. 10.3389/fimmu.2018.00716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sokol CL, Medzhitov R. Role of basophils in the initiation of Th2 responses. Curr Opin Immunol. (2010) 22:73–7. 10.1016/j.coi.2010.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kunsleben N, Rüdrich U, Gehring M, Novak N, Kapp A, Raap U. IL-31 induces chemotaxis, calcium mobilization, release of reactive oxygen species, and CCL26 in eosinophils, which are capable to release IL-31. J Invest Dermatol. (2015) 135:1908–11. 10.1038/jid.2015.106 [DOI] [PubMed] [Google Scholar]
- 52.Zlotnik A, Burkhardt AM, Homey B. Homeostatic chemokine receptors and organ-specific metastasis. Nat Rev Immunol. (2011) 11:597–606. 10.1038/nri3049 [DOI] [PubMed] [Google Scholar]
- 53.Griffith JW, Sokol CL, Luster AD. Chemokines and chemokine receptors: positioning cells for host defense and immunity. Annu Rev Immunol. (2014) 32:659–702. 10.1146/annurev-immunol-032713-120145 [DOI] [PubMed] [Google Scholar]
- 54.Jones SA, Jenkins BJ. Recent insights into targeting the IL-6 cytokine family in inflammatory diseases and cancer. Nat Rev Immunol. (2018) 18:773–89. 10.1038/s41577-018-0066-7 [DOI] [PubMed] [Google Scholar]
- 55.Cornelissen C, Lüscher-Firzlaff J, Baron JM, Lüscher B. Signaling by IL-31 and functional consequences. Eur J Cell Biol. (2012) 91:552–66. 10.1016/j.ejcb.2011.07.006 [DOI] [PubMed] [Google Scholar]
- 56.Singh B, Jegga AG, Shanmukhappa KS, Edukulla R, Khurana Hershey GH, Medvedovic M, et al. IL-31-driven skin remodeling involves epidermal cell proliferation and thickening that lead to impaired skin-barrier function. PLoS ONE. (2016) 11:e0161877. 10.1371/journal.pone.0161877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hänel KH, Pfaff CM, Cornelissen C, Amann PM, Marquardt Y, Czaja K, et al. Control of the physical and antimicrobial skin barrier by an IL-31-IL-1 signaling network. J Immunol. (2016) 196:3233–44. 10.4049/jimmunol.1402943 [DOI] [PubMed] [Google Scholar]
- 58.Andoh T, Li S, Uta D. Involvement of thromboxane A(2) in interleukin-31-induced itch-associated response in mice. Pharmacol Rep. (2018) 70:251–7. 10.1016/j.pharep.2017.10.001 [DOI] [PubMed] [Google Scholar]
- 59.Andoh T, Harada A, Kuraishi Y. Involvement of leukotriene B4 released from keratinocytes in itch-associated response to intradermal interleukin-31 in mice. Acta Derm Venereol. (2017) 97:922–7. 10.2340/00015555-2697 [DOI] [PubMed] [Google Scholar]
- 60.Kahremany S, Hofmann L, Gruzman A, Cohen G. Advances in understanding the initial steps of pruritoceptive itch: how the itch hits the switch. Int J Mol Sci. (2020) 21:4883. 10.3390/ijms21144883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Ip WK, Wong CK, Li ML, Li PW, Cheung PF, Lam CW. Interleukin-31 induces cytokine and chemokine production from human bronchial epithelial cells through activation of mitogen-activated protein kinase signalling pathways: implications for the allergic response. Immunology. (2007) 122:532–41. 10.1111/j.1365-2567.2007.02668.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Perrigoue JG, Zaph C, Guild K, Du Y, Artis D. IL-31-IL-31R interactions limit the magnitude of Th2 cytokine-dependent immunity and inflammation following intestinal helminth infection. J Immunol. (2009) 182:6088–94. 10.4049/jimmunol.0802459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yagi Y, Andoh A, Nishida A, Shioya M, Nishimura T, Hashimoto T, et al. Interleukin-31 stimulates production of inflammatory mediators from human colonic subepithelial myofibroblasts. Int J Mol Med. (2007) 19:941–6. 10.3892/ijmm.19.6.941 [DOI] [PubMed] [Google Scholar]
- 64.Chakraborty D, Šumová B, Mallano T, Chen CW, Distler A, Bergmann C, et al. Activation of STAT3 integrates common profibrotic pathways to promote fibroblast activation and tissue fibrosis. Nat Commun. (2017) 8:1130. 10.1038/s41467-017-01236-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kulczycka-Siennicka L, Cynkier A, Waszczykowska E, Wozniacka A, Zebrowska A. The role of intereukin-31 in pathogenesis of itch and its intensity in a course of bullous pemphigoid and dermatitis herpetiformis. Biomed Res Int. (2017) 2017:5965492. 10.1155/2017/5965492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Salz M, Haeberle S, Hoffmann J, Enk AH, Hadaschik EN. Elevated IL-31 serum levels in bullous pemphigoid patients correlate with eosinophil numbers and are associated with BP180-IgE. J Dermatol Sci. (2017) 87:309–11. 10.1016/j.jdermsci.2017.07.019 [DOI] [PubMed] [Google Scholar]
- 67.Rüdrich U, Gehring M, Papakonstantinou E, Illerhaus A, Engmann J, Kapp A, et al. Eosinophils are a major source of interleukin-31 in bullous pemphigoid. Acta Derm Venereol. (2018) 98:766–71. 10.2340/00015555-2951 [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Pathophysiologic mechanisms of itch in bullous pemphigoid. J Am Acad Dermatol. (2020) 83:53–62. 10.1016/j.jaad.2019.07.060 [DOI] [PubMed] [Google Scholar]
- 69.Ohmatsu H, Sugaya M, Suga H, Morimura S, Miyagaki T, Kai H, et al. Serum IL-31 levels are increased in patients with cutaneous T-cell lymphoma. Acta Derm Venereol. (2012) 92:282–3. 10.2340/00015555-1345 [DOI] [PubMed] [Google Scholar]
- 70.Nattkemper LA, Martinez-Escala ME, Gelman AB, Singer EM, Rook AH, Guitart J, et al. Cutaneous T-cell lymphoma and pruritus: the expression of IL-31 and its receptors in the skin. Acta Derm Venereol. (2016) 96:894–8. 10.2340/00015555-2417 [DOI] [PubMed] [Google Scholar]
- 71.Singer EM, Shin DB, Nattkemper LA, Benoit BM, Klein RS, Didigu CA, et al. IL-31 is produced by the malignant T-cell population in cutaneous T-Cell lymphoma and correlates with CTCL pruritus. J Invest Dermatol. (2013) 133:2783–5. 10.1038/jid.2013.227 [DOI] [PubMed] [Google Scholar]
- 72.Zeng X, Zhang Z, Gao QQ, Wang YY, Yu XZ, Zhou B, et al. Clinical significance of serum interleukin-31 and interleukin-33 levels in patients of endometrial cancer: a case control study. Dis Markers. (2016) 2016:9262919. 10.1155/2016/9262919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Naumnik W, Naumnik B, Niewiarowska K, Ossolinska M, Chyczewska E. Novel cytokines: IL-27, IL-29, IL-31 and IL-33. Can they be useful in clinical practice at the time diagnosis of lung cancer? Exp Oncol. (2012) 34:348–53. [PubMed] [Google Scholar]
- 74.Lange M, Gleń J, Zabłotna M, Nedoszytko B, Sokołowska-Wojdyło M, Rebała K, et al. Interleukin-31 polymorphisms and serum IL-31 level in patients with mastocytosis: correlation with clinical presen-tation and pruritus. Acta Derm Venereol. (2017) 97:47–53. 10.2340/00015555-2474 [DOI] [PubMed] [Google Scholar]
- 75.Ferretti E, Tripodo C, Pagnan G, Guarnotta C, Marimpietri D, Corrias MV, et al. The interleukin (IL)-31/IL-31R axis contributes to tumor growth in human follicular lymphoma. Leukemia. (2015) 29:958–67. 10.1038/leu.2014.291 [DOI] [PubMed] [Google Scholar]
- 76.Ferretti E, Corcione A, Pistoia V. The IL-31/IL-31 receptor axis: general features and role in tumor microenvironment. J Leukoc Biol. (2017) 102:711–7. 10.1189/jlb.3MR0117-033R [DOI] [PubMed] [Google Scholar]