Abstract
There is a growing trend to explore microalgae as an alternative resource for the food, feed, pharmaceutical, cosmetic and fuel industry. Moreover, the polar lipidome of microalgae is interesting because of the reports of bioactive polar lipids which could foster new applications for microalgae. In this work, we identified for the first time the Chlorococcum amblystomatis lipidome using hydrophilic interaction liquid chromatography-high resolution electrospray ionization- tandem mass spectrometry (HILIC–HR–ESI–MS/MS). The Chlorococcum amblystomatis strain had a lipid content of 20.77% and the fatty acid profile, determined by gas chromatography-mass spectrometry, has shown that this microalga contains high amounts of omega-3 polyunsaturated fatty acids (PUFAs). The lipidome identified included 245 molecular ions and 350 lipid species comprising 15 different classes of glycolipids (6), phospholipids (7) and betaine lipids (2). Of these, 157 lipid species and the main lipid species of each class were esterified with omega-3 PUFAs. The lipid extract has shown antioxidant activity and anti-inflammatory potential. Lipid extracts also had low values of atherogenic (0.54) and thrombogenic index (0.27). In conclusion, the lipid extracts of Chlorococcum amblystomatis have been found to be a source of lipids rich in omega-3 PUFAs for of great value for the food, feed, cosmetic, nutraceutical and pharmaceutical industries.
Subject terms: Lipidomics, Lipids
Introduction
Reducing the impact of climate change is one of the main challenges of this decade, which has increased the demand to move to more environmental and sustainable resources1,2. Microalgae represent a sustainable3,4 and versatile5,6 resource with exploitation potential for different industries (for example food, feed, nutraceutical, cosmetic, pharmaceutical)7. These unicellular organisms are found not only in aquatic systems but also in terrestrial systems, with a wide range of catalogued species8,9. Microalgae are valued for their photosynthetic efficiency, low water requirement, fast growth and high biomass production10, which allows efficient biomass production with lower resource consumption that meet industry’s needs. Also, microalgae grow with greater photosynthetic efficiency than higher plants and do not require arable land to be cultivated11. Microalgae have been used for different applications, namely, as direct food, as food nutrients, for animal feed, as sources of bioactive products for the pharmaceutical industry, and as a source of lipids for the production of biodiesel5. For example, microalgae can consume nutrients that are in excess in the agri-food industry waste streams, such as nitrogen and phosphorous12.. In terms of nutrients, they are a rich source of essential amino acids, vitamins, minerals, pigments and lipids, namely polyunsaturated fatty acids (PUFAs)13. They are considered a promising source of bioactive components due to their antioxidant, anti-inflammatory, anti-obesity, anti-tumour, antiviral and anti-bacterial properties14. Among the bioactive compounds described, phospholipids (PL) seem to be interesting ingredients as the main vector of omega-3 fatty acids (FA)15 and glycolipids (GL) as promising bioactive phytochemicals16. There is a large body of evidence suggesting that omega-3 PUFAs esterified in polar lipids have important physiological properties. For example, PL and GL containing omega-3 FA, were associated with antioxidant and anti-inflammatory properties, and with cardiovascular protection17 and also that PLs esterified in omega-3 PUFAs are excellent delivery vehicles18,19. In addition, GLs with PUFAs have shown anti-inflammatory potential with nitric oxide release inhibitory activity20,21 while DGDGs and SQDGs from algae showed chemotherapeutic potential22 and SQDG esterified in EPA also displays an anti-proliferative effect23. Very recent studies have focused on the identification of polar lipids from the most commercially used microalgae, such as Chlorella sp. and Chlorella vulgaris24,25, Nannochloropsis oceanica and Nannochloropsis oculata26,27, and Phaeodactylum tricornutum28. However, polar lipids are scarcely studied compared to other ingredients derived from microalgae, an even larger number of microalgae lipidome remains unknown, unexplored or poorly studied, hampering the full biotechnological exploitation of this resource.
Chlorococcum sp. is a green microalga which belongs to the phylum Chlorophyta, class Chlorophyceae, order Chlorococcaceae and family Chlorococcaceae, and it can be cultivated under photoautotrophic and mixotrophic conditions29. The biotechnological potential of this microalga remains poorly explored29. Chlorococcum sp. has been described as a useful tool in carbon sequestration, lipid flocculation in wastewater30 and carotenoid production31, a good source for the production of biodiesel32 and useful for the food industry33,34. The production of lutein from Chlorococcum citriforme has already been patented35. Regarding its biochemical composition, a total biomass dry weight content of proteins of 13.08–40%32,36, carbohydrates of 3.42–40%32,36, chlorophylls of 0.4–1.5%32, carotenoids of 3.2–12.6 mg/g37 and lipids of 8.71–32.3%32,36,38–40 has been described. In addition to its high lipid content, the FA composition of the Chlorococcum strains studied to date, included good amounts of omega-3 fatty acids33 suggesting the possibility of added-value lipids that needs to be explored.
The interest of knowing the lipidome of green microalgae, either to optimize the production of biodiesel or to produce lipids with added value, has led to the complete profiling of several microalgae16,27. As such, the complete lipidome has been reported for certain green microalgae belonging to the phylum Chlorophyta, such as Haematococcus sp.27, Chlamydomonas sp.41, Scenedesmus sp.24, Chlorella sp.42 and Chlorella kessleri43. Microalgal lipids have received much attention due to their high abundance of omega-3 and omega-6 PUFAs, and, more recently, due to their polar lipids content (e.g. monogalactosyldiacylglycerol [MGDG], digalactosyldiacylglycerol [DGDGs]), in which the PUFAs are esterified. These glycolipids are plastid lipids, therefore abundant in green microalgae. Also, these lipids were reported to have desired bioactive properties, such as antibacterial, antioxidant and anti-inflammatory activities16. PUFAs were already described in Chlorococcum sp.32,33,38,39, however, the polar lipidome of this microalga remains to be described, preventing its full exploitation. Thus, to fill this gap, in the present work, we have analysed and characterized in detail the polar lipidome of Chlorococcum amblystomatis, cultivated in autotrophic conditions, using a lipidomic approach based on the hydrophilic interaction liquid chromatography-electrospray ionization-high resolution mass spectrometry (HILIC–HR–ESI–MS). We also explored the bioactive potential of lipid extracts of Chlorococcum amblystomatis to inhibit COX-2 activity and its antioxidant properties, using commercial kits, to promote valorisation of this microalga as a source of bioactive lipids for the food, feed, cosmetics or pharmaceutical industries.
Results
Total lipid content and fatty acid composition of Chlorococcum amblystomatis
The lipid extracts of Chlorococcum amblystomatis corresponded to an average yield of 20.77 ± 0.57% of microalgal biomass. The fatty acid composition of Chlorococcum amblystomatis identified by GC–MS showed that C16:0 was the most abundant FA (23.0 ± 0.8%), followed by C18:3 n-3 (19.4 ± 0.6%), C18:0 (13.9 ± 2.3%), C16:4 n-3 (11.1 ± 0.6%), C20:5 n-3 (8.9 ± 0.6%) and C16:1 n-9 (7.5 ± 0.3%) (Table 1). The remaining identified FAs had relative abundances of less than 5%. This microalga had a total omega-3 fatty acid content of 43.2%.
Table 1.
Fatty acid profile identified in the total lipid extract of Chlorococcum amblystomatis by GC–MS.
| Fatty acids | Relative abundance (%) ± SD |
|---|---|
| C12:0 | 0.1 ± 0.0 |
| C14:0 | 1.5 ± 0.2 |
| C15:0-iso | 0.0 ± 0.0 |
| C15:0 | 0.2 ± 0.0 |
| C16:0 | 23.0 ± 0.8 |
| C16:1 Δ9 | 7.5 ± 0.3 |
| C16:1 Δ11 | 0.1 ± 0.0 |
| C16:2 Δ7.10 (n−6) | 0.3 ± 0.0 |
| C16:3 Δ4,7,10 (n−6) | 0.6 ± 0.1 |
| C17:1 | 0.1 ± 0.0 |
| C16:3 Δ7,10,13 (n−3) | 0.6 ± 0.1 |
| C16:4 Δ4,7,10,13 (n−3) | 11.1 ± 0.6 |
| C18:0 | 13.9 ± 2.3 |
| C18:1 Δ9 | 1.6 ± 0.1 |
| C18:1 Δ11 | 3.6 ± 0.2 |
| C18:2 Δ9,12 (n−6) | 2.3 ± 0.2 |
| C18:3 Δ6,9,12 (n−6) | 1.0 ± 0.1 |
| C18:3 Δ9,12,15 (n−3) | 19.4 ± 0.6 |
| C18:4 Δ6,9,12,15 (n−3) | 3.2 ± 0.2 |
| C20:0 | 0.2 ± 0.0 |
| C20:4 Δ5,8,11,14 (n−6) | 1.1 ± 0.1 |
| C20:5 Δ5,8,11,14,17 (n−3) | 8.9 ± 0.6 |
| Σ SFA | 38.8 |
| Σ MUFAs | 12.9 |
| Σ PUFAs | 48.4 |
| Σ (n−3) | 43.2 |
| Σ (n−6) | 5.2 |
| n−6/n−3 ratio | 0.1 |
| AI | 0.5 |
| TI | 0.3 |
| (h/H) | 1.4 |
Values are expressed in relative abundance (%) and represent the mean of five analytical samples ± standard deviation (SD).
SFA saturated fatty acids, MUFAs monounsaturated fatty acids, PUFAs polyunsaturated fatty acids, AI atherosclerotic index, TI thrombogenic index, (h/H) (hypocholesterolemic/hypercholesterolemic) ratio.
To assess the potential health benefits of Chlorococcum amblystomatis, the atherogenic (AI), thrombogenic (TI), hypocholesterolemic/hypercholesterolemic (h/H) and the PUFA n-6/ PUFA n-3 indices were calculated. The AI and TI of the lipid extracts were 0.5 and 0.3, respectively, while the h/H ratio was 1.4 and the n-6/n-3 ratio was 0.1.
Polar lipid composition of Chlorococcum amblystomatis
The polar lipid profile was revealed by hydrophilic interaction liquid chromatography-high resolution electrospray mass spectrometry (HILIC–HR–ESI–MS and HILIC–HR–ESI–MS/MS) (Supplementary file S1). Using this approach, a total of 245 molecular ions, with a minimum of 350 molecular species, belonging to 15 classes of lipids were identified. Of these, 101 molecular ions were glycolipids (GL), 54 molecular ions were betaine lipids (BL), 89 molecular ions were phospholipids (PL), and 1 molecular ion was an inositol phosphoceramide (PI-Cer) (Tables 2 and 3). Figures explaining how the MS/MS data were interpreted can be found in the Supplementary Figures S1–S13. The m/z of the fragment ions used to identify each molecular ion and each molecular species can be found in Supplementary Table S1 and S2.
Table 2.
Polar lipid classes identified in the total lipid extract of Chlorococcum amblystomatis by HILIC–HR–ESI–MS and MS/MS.
| Lipid classes | Chlorococcum amblystomatis | ||
|---|---|---|---|
| Molecular ions number | Lipid species number | Major species | |
| Glycolipids | 101 | 158 | – |
| MGDG | 30 | 47 | MGDG(34:7) |
| MGMG | 11 | 11 | MGMG(16:4) |
| DGDG | 27 | 48 | DGDG(34:3) |
| DGMG | 11 | 11 | DGMG(18:3) |
| SQDG | 21 | 40 | SQDG(32:1) |
| SQMG | 1 | 1 | SQMG(16:0) |
| Phospholipids | 89 | 107 | – |
| LPC | 11 | 11 | LPC(16:1) |
| PC | 32 | 39 | PC(34:2) |
| LPE | 9 | 9 | LPE(16:0) |
| PE | 13 | 14 | PE(34:4) |
| PG | 22 | 32 | PG(34:3) |
| PI | 2 | 2 | PI(32:1) |
| Betaine lipids | 54 | 84 | |
| DGTS | 38 | 68 | DGTS(34:4) |
| MGTS | 16 | 16 | MGTS(18:4) |
| Sphingolipids | 1 | 1 | |
| PI-Cer | 1 | 1 | PI-Cer(d18:1/14:0) |
| Total | 245 | 350 | – |
The total number of molecular ions, lipid species identified in each class and lipid species per class is shown.
MGDG monogalactosyldiacylglycerol, MGMG monogalactosylmonoacylglycerol, DGDG digalactosyldiacylglycerol, DGMG digalactosylmonoacylglycerol, SQDG sulfoquinovosyl diacylglycerol, SQMG sulfoquinovosyl monoacylglycerol, LPC lysophosphatidylcholine, PC phosphatidylcholine, LPE lysophosphatidylethanolamine, PE phosphatidylethanolamine, PG phosphatidylglycerol, PI phosphatidylinositol, DGTS diacylglycerol-trimethylhomoserine, MGTS monoacylglycerol-trimethylhomoserine, PI-Cer inositol phosphoceramide.
Table 3.
Phospholipid species identified in the total lipid extract of Chlorococcum amblystomatis by HILIC–HR–ESI–MS and MS/MS.
| Lipid species (C:N) | Calculated m/z | Observed m/z | Error (ppm) | Fatty acyl chains (C:N) | Formula |
|---|---|---|---|---|---|
| LPC identified as [M+H]+ | |||||
| LPC14:0) | 468.3090 | 468.3086 | − 0.9 | ** | C22H47NO7P |
| LPC(16:0) | 496.3403 | 496.3404 | 0.2 | ** | C24H51NO7P |
| LPC(16:1) | 494.3247 | 494.3244 | − 0.5 | 16:1 | C24H49NO7P |
| LPC(16:2) | 492.3090 | 492.3083 | − 1.5 | ** | C24H47NO7P |
| LPC(18:1) | 522.3560 | 522.3561 | 0.3 | 18:1 | C26H53NO7P |
| LPC(18:2) | 520.3403 | 520.3402 | − 0.2 | 18:2 | C26H51NO7P |
| LPC(18:3) | 518.3247 | 518.3233 | − 2.6 | * | C26H49NO7P |
| LPC(18:4) | 516.3090 | 516.3067 | − 4.5 | * | C26H47NO7P |
| LPC(20:3) | 546.3560 | 546.3560 | 0.1 | ** | C28H53NO7P |
| LPC(20:4) | 544.3403 | 544.3398 | − 0.9 | ** | C28H51NO7P |
| LPC(20:5) | 542.3247 | 542.3241 | − 1.0 | * | C28H49NO7P |
| PC identified as [M+H]+ | |||||
| PC(28:1) | 676.4917 | 676.4905 | − 1.8 | * | C36H71NO8P |
| PC(30:0) | 706.5387 | 706.5371 | − 2.2 | ** | C38H77NO8P |
| PC(30:1) | 704.5230 | 704.5250 | 2.8 | 16:1–14:0 | C38H75NO8P |
| PC(30:3) | 700.4917 | 700.4895 | − 3.2 | * | C38H71NO8P |
| PC(32:1) | 732.5543 | 732.5539 | − 0.6 | 16:1–16:0 | C40H79NO8P |
| PC(32:2) | 730.5387 | 730.5384 | − 0.4 | 16:1–16:1 | C40H77NO8P |
| PC(32:3) | 728.5230 | 728.5230 | 0.0 | 16:1–16:2 | C40H75NO8P |
| PC(32:4) | 726.5074 | 726.5058 | − 2.2 | * | C40H73NO8P |
| PC(32:5) | 724.4917 | 724.4908 | − 1.3 | ** | C40H71NO8P |
| PC(34:1) | 760.5856 | 760.5842 | − 1.9 | 16:0–18:1 and 16:1–18:0 | C42H83NO8P |
| PC(34:2) | 758.5700 | 758.5696 | − 0.5 | 16:1–18:1 and 16:0–18:2 | C42H81NO8P |
| PC(34:3) | 756.5543 | 756.5542 | − 0.2 | 16:1–18:2 | C42H79NO8P |
| PC(34:4) | 754.5387 | 754.5371 | − 2.1 | 16:1–18:3 and 16:2–18:2 | C42H77NO8P |
| PC(34:5) | 752.5230 | 752.5210 | − 2.7 | * | C42H75NO8P |
| PC(34:6) | 750.5074 | 750.5064 | − 1.3 | * | C42H73NO8P |
| PC(34:7) | 748.4917 | 748.4899 | − 2.4 | ** | C42H71NO8P |
| PC(36:2) | 786.6013 | 786.6004 | − 1.1 | ** | C44H85NO8P |
| PC(36:3) | 784.5856 | 784.5850 | − 0.8 | 18:1–18:2 | C44H83NO8P |
| PC(36:4) | 782.5700 | 782.5682 | − 2.3 | 18:2–18:2 and 16:0–20:4 | C44H81NO8P |
| PC(36:5) | 780.5543 | 780.5528 | − 2.0 | 18:3–18:2, 16:0–20:5 and 16:1–20:4 | C44H79NO8P |
| PC(36:6) | 778.5387 | 778.5379 | − 1.0 | 16:1–20:5 | C44H77NO8P |
| PC(36:7) | 776.5230 | 776.5212 | − 2.4 | * | C44H75NO8P |
| PC(38:5) | 808.5856 | 808.5843 | − 1.6 | 18:1–20:4 | C46H83NO8P |
| PC(38:6) | 806.5700 | 806.5690 | − 1.2 | 18:2–20:4 and 18:1–20:5 | C46H81NO8P |
| PC(38:7) | 804.5543 | 804.5525 | − 2.3 | 18:2–20:5 | C46H79NO8P |
| PC(38:8) | 802.5387 | 802.5351 | − 4.5 | ** | C46H77NO8P |
| PC(38:9) | 800.5230 | 800.5204 | − 3.3 | * | C46H75NO8P |
| PC(40:10) | 826.5387 | 826.5369 | − 2.2 | * | C48H77NO8P |
| PC(40:5) | 836.6169 | 836.6180 | 1.3 | * | C48H87NO8P |
| PC(40:7) | 832.5856 | 832.5831 | − 3.0 | ** | C48H83NO8P |
| PC(40:8) | 830.5700 | 830.5674 | − 3.1 | * | C48H81NO8P |
| PC(40:9) | 828.5543 | 828.5520 | − 2.8 | * | C48H79NO8P |
| LPE identified as [M+H]+ | |||||
| LPE(14:0) | 426.2621 | 426.2615 | − 1.3 | ** | C19H41NO7P |
| LPE(16:0) | 454.2934 | 454.2928 | − 1.2 | 16:0 | C21H45NO7P |
| LPE(16:1) | 452.2777 | 452.2774 | − 0.7 | * | C21H43NO7P |
| LPE(16:4) | 446.2308 | 446.2303 | − 1t.0 | ** | C21H37NO7P |
| LPE(18:1) | 480.3090 | 480.3092 | 0.4 | 18:1 | C23H47NO7P |
| LPE(18:2) | 478.2934 | 478.2926 | − 1.6 | ** | C23H45NO7P |
| LPE(18:3) | 476.2777 | 476.2774 | − 0.7 | * | C23H43NO7P |
| LPE(18:4) | 474.2621 | 474.2618 | − 0.6 | 18:4 | C23H41NO7P |
| LPE(20:5) | 500.2777 | 500.2781 | 0.8 | ** | C25H43NO7P |
| PE identified as [M+H]+ | |||||
| PE(30:1) | 662.4761 | 662.4754 | − 1.0 | 14:0–16:1 | C35H69NO8P |
| PE(30:3) | 658.4448 | 658.4422 | − 3.9 | * | C35H65NO8P |
| PE(32:1) | 690.5074 | 690.5058 | − 2.3 | * | C37H73NO8P |
| PE(32:2) | 688.4917 | 688.4925 | 1.1 | 16:1–16:1 | C37H71O8NP |
| PE(32:4) | 684.4604 | 684.4593 | − 1.7 | 16:4–16:0 | C37H67NO8P |
| PE(34:1) | 718.5387 | 718.5353 | − 4.7 | 16:0–18:1 | C39H77NO8P |
| PE(34:2) | 716.5230 | 716.5219 | − 1.6 | 16:1–18:1 and 16:0–18:2 | C39H75NO8P |
| PE(34:3) | 714.5074 | 714.5044 | − 4.2 | * | C39H73O8NP |
| PE(34:4) | 712.4917 | 712.4901 | − 2.3 | 16:0–18:4 | C39H71NO8P |
| PE(34:5) | 710.4761 | 710.4754 | − 1.0 | 16:1–18:4 | C39H69NO8P |
| PE(36:2) | 744.5543 | 744.5535 | − 1.1 | 18:1–18:1 | C41H79O8NP |
| PE(36:5) | 738.5074 | 738.5075 | 0.2 | 18:4–18:1 | C41H73O8NP |
| PE(36:6) | 736.4917 | 736.4917 | 0.0 | * | C41H71NO8P |
| PG identified as [M−H]− | |||||
| PG(30:0) | 693.4707 | 693.4700 | − 1.0 | 14:0–16:0 | C36H70O10P |
| PG(30:1) | 691.4550 | 691.4545 | − 0.7 | ** | C36H68O10P |
| PG(32:0) | 721.5020 | 721.5026 | 0.8 | ** | C38H74O10P |
| PG(32:1) | 719.4863 | 719.4866 | 0.4 | 16:1–16:0 and 14:0–18:1 | C38H72O10P |
| PG(32:2) | 717.4707 | 717.4703 | − 0.6 | 16:1–16:1 | C38H70O10P |
| PG(34:1) | 747.5176 | 747.5181 | 0.7 | 16:0–18:1 | C40H76O10P |
| PG(34:2) | 745.5020 | 745.5011 | − 1.2 | ** | C40H74O10P |
| PG(34:3) | 743.4867 | 743.4868 | 0.1 | 16:0–18:3 | C40H72O10P |
| PG(34:4) | 741.4707 | 741.4711 | 0.5 | 16:0–18:4 and 16:1–18:3 | C40H70O10P |
| PG(34:5) | 739.4550 | 739.4534 | − 2.2 | 14:0–20:5 and 16:2–18:3 | C40H68O10P |
| PG(36:2) | 773.5333 | 773.5337 | 0.5 | 18:1–18:1 | C42H78O10P |
| PG(36:5) | 767.4864 | 767.4863 | − 0.1 | 16:0–20:5 | C42H72O10P |
| PG(36:6) | 765.4707 | 765.4715 | 1.0 | 16:1–20:5 | C42H70O10P |
| PG(38:5) | 795.5176 | 795.5197 | 2.6 | ** | C44H76O10P |
| PG(32:2-OH) | 733.4656 | 733.4653 | − 0.4 | 16:0-OH-16:2 | C38H70O11P |
| PG(34:1-OH) | 763.5125 | 763.5115 | − 1.3 | 18:0-OH-16:1 | C40H76O11P |
| PG(34:2-OH) | 761.4969 | 761.4971 | 0.2 | 18:0-OH-16:2 | C40H74O11P |
| PG(34:3-OH) | 759.4812 | 759.4808 | − 0.5 | 18:3-OH-16:0, 18:2-OH-16:1, 18:1-OH-16:2 and 18:0-OH-16:3 | C40H72O11P |
| PG(34:4-OH) | 757.4656 | 757.4653 | − 0.4 | 18:4-OH-16:0, 18:3-OH-16:1 and 18:2-OH-16:2 | C40H70O11P |
| PG(34:5-OH) | 755.4499 | 755.4480 | − 2.5 | 18:3-OH-16:2 and 18:4-OH-16:1 | C40H68O11P |
| PG(36:5-OH) | 783.4812 | 783.4828 | 2.0 | 16:0-OH-20:5 and 20:5-OH-16:0 | C42H72O11P |
| PG(36:6-OH) | 781.4656 | 781.4665 | 1.1 | 20:5-OH-16:1 | C42H70O11P |
| PI identified as [M−H]− | |||||
| PI(32:1) | 807.5024 | 807.5018 | − 0.7 | 16:0–16:1 | C41H76O13P |
| PI(34:1) | 835.5318 | 835.5302 | − 1.9 | 16:0–18:1 | C43H80O13P |
| PI-Cer identified as [M−H]− | |||||
| PI-Cer(d18:1/14:0) | 750.4921 | 750.4921 | 0.0 | * | C38H73NO11P |
C number of carbon atoms, N number of double bonds.
*Identified based on the polar head fragmentation, calculated mass, and retention time.
**Identified according to the calculated mass and the retention time.
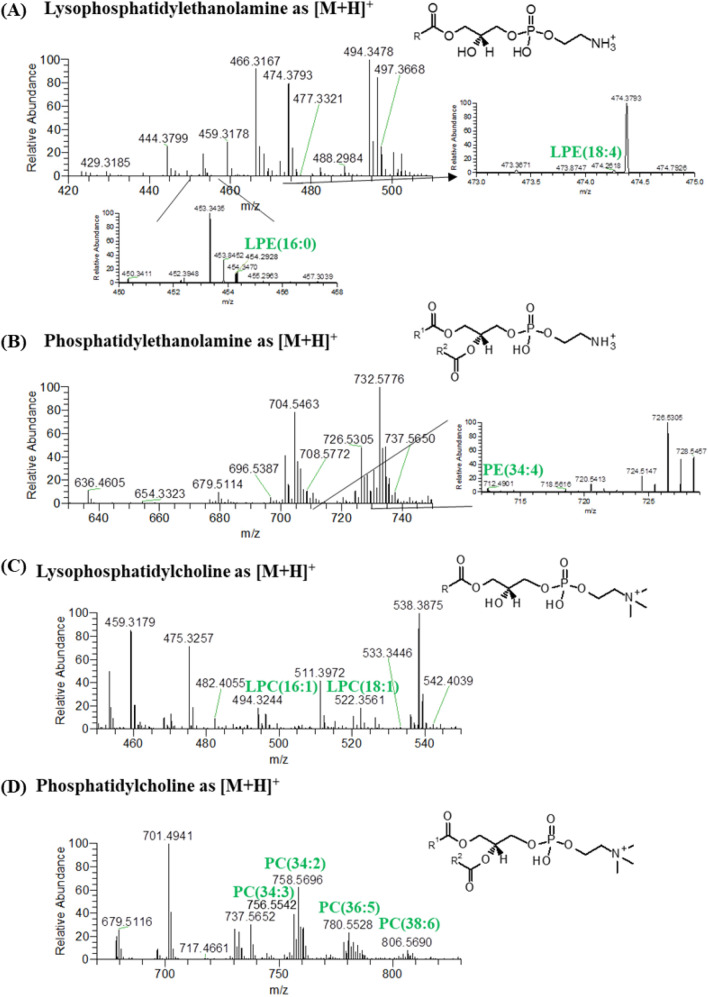
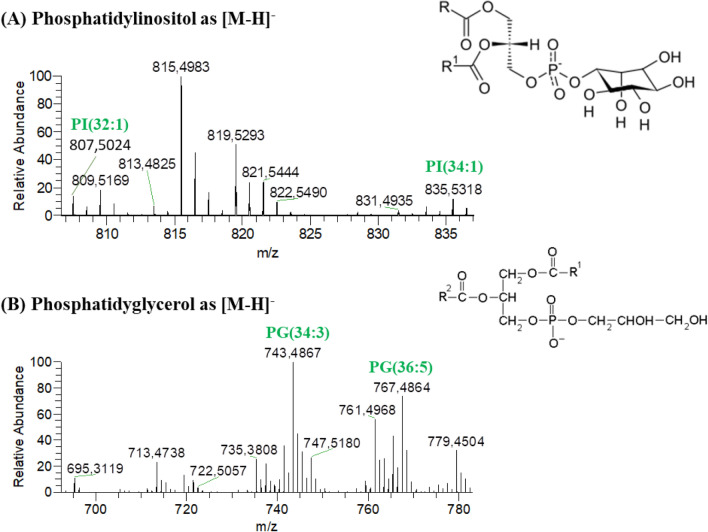
The PL classes identified included phosphatidylcholine (PC), lysophosphatidylcholine (LPC), phosphatidylethanolamine (PE) and lysophosphatidylethanolamine (LPE), identified in the HILIC–HR–ESI–MS analysis as the molecular ion [M+H]+ (Fig. 1); and phosphatidylglycerol (PG) and phosphatidylinositol (PI) identified as the negative molecular ion, [M−H]− (Fig. 2). The only sphingolipid species found in Chlorococcum amblystomatis was a PI-Cer, which has been identified in the analysis on the negative ion mode. A total of 32 molecular ions of PC, 11 of LPC, 13 of PE, 9 of LPE, 22 of PG, 2 of PI and 1 of PI-Cer were recognized. Analysis of the MS/MS data allowed to recognize that each ion can correspond to several molecular species, with different combinations of fatty acyl chains, but with the same (C:N) (Supplementary Table S2). The molecular species identified per class were of 39 PC, 11 LPC, 14 PE, 9 LPE, 32 PG, 2 PI, and 1 PI-Cer.
Figure 1.
HILIC–HR–ESI–MS spectra of the classes of phospholipids (PL) identified in positive ion mode as [M+H]+. These classes were (A) lysophosphatidylethanolamine (LPE), (B) phosphatidylethanolamine (PE), (C) lysophosphatidylcholine (LPC) and (D) phosphatidylcholine (PC).
Figure 2.
HILIC–HR–ESI–MS spectra of classes of phospholipids (PL) identified in negative ion mode as [M−H]−. These classes were (A) phosphatidylinositol (PI) and (B) phosphatidylglycerol (PG).
As previously indicated, the identification of the lipid species was based on the mass accuracy, retention time and interpretation of MS/MS data. The interpretation of the MS/MS data of the molecular ions of the PC, LPC, PE and LPE species allowed to confirm their identity (Supplementary Table S2). This was done by confirming for each species the presence of the polar head groups, in particular by the presence of the product ion at m/z 184 (for PC and LPC) and fragment ions arising from the neutral loss of 141 Da (for PE and LPE)16,44–48. On the other hand, the complementary analysis of the molecular ions on the HILIC–HR–ESI–MS/MS spectra acquired in negative ion mode, observed as [M+CH3COO]− ions for PC and LPC and [M−H]− ions for PE and LPE, allowed to assign the fatty acyl composition. This was done by observing the presence of the RCOO−carboxylate fragment anions of the fatty acids esterified in the glycerol backbone16,44–48.
The HILIC–HR–ESI–MS/MS spectra of the negative molecular ions belonging to the PG, PI and PI-Cer classes allowed to confirm the presence of the corresponding polar heads by observing for each species the product ions at m/z 171 (for PG) and m/z 241 (for PI and PI-Cer), and identify the fatty acyl chains by the presence of RCOO−carboxylate anions (for PG and PI)16,44–48 (Supplementary Table S2). The only PI-Cer detected was identified by the accurate mass measurement, the retention time, and the presence of the polar head at m/z 241. The most abundant species in each phospholipid class were: PC(34:2) identified as PC(16:1–18:1) and PC(16:0–18:2); LPC(16:1); PE(34:4) identified as PE(16:0–18:4); LPE(16:0); PG(34:3) identified as PG(16:0–18:3); and PI(32:1) identified as PI(16:0–16:1).
Among the PG molecular ions identified, a few oxidized PG species were found (Table 3), the most abundant being PG(34:2-OH), identified as PG(18:0-OH-16:2). All the oxidized PG species were identified based on exact mass measurements, retention time and MS/MS spectra analysis (Supplementary Table S3). In the MS/MS spectra, the presence of mass shifts of + 16 Da relative to the [M−H]− ions and of RCOO−carboxylate hydroxyl anions was indicative of the formation of hydroxy derivatives49,50. No product ions with oxidized polar head groups were observed 45.
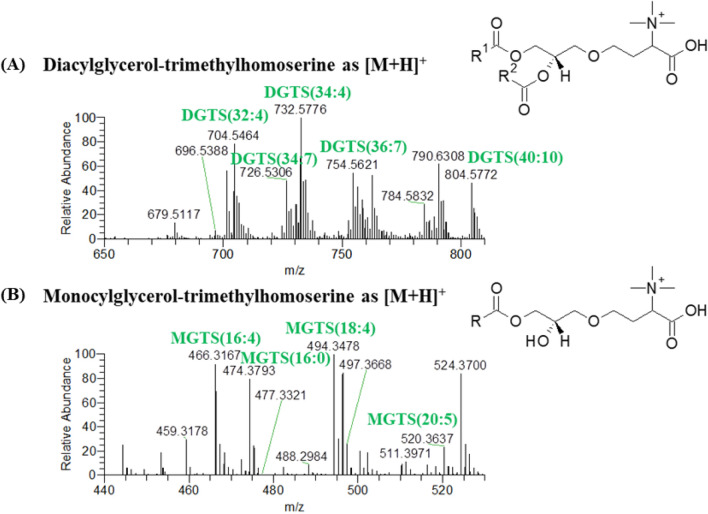
The betaine lipids identified in the HILIC–HR–ESI–MS data included the classes of monoacylglycerol-trimethylhomoserine (MGTS) and diacylglycerol-trimethylhomoserine (DGTS), and were identified in positive ion mode as [M+H]+ ions (Table 4, Fig. 3). A total of 38 molecular ions of DGTS and 16 molecular ions of MGTS were identified, corresponding to 68 molecular species of DGTS and 16 molecular species of MGTS. In the HILIC–HR–ESI–MS/MS spectra of MGTS and DGTS, as [M+H]+ ions, the presence of the polar head group in each species was confirmed by observation of the product ion at m/z 23644,47, and the fatty acyl composition was determined by observation of the fragment ions resulting from the neutral losses of the fatty acyl chains as an acid (–RCOOH) and ketene (–R=C=O) derivatives44,47 (Supplementary Table S2).
Table 4.
Betaine lipids identified in the polar lipidome of Chlorococcum amblystomatis by HILIC–HR–ESI–MS and MS/MS.
| Lipid species (C:N) | Calculated m/z | Observed m/z | Error (ppm) | Fatty acyl chains (C:N) | Formula |
|---|---|---|---|---|---|
| MGTS identified as [M+H]+ | |||||
| MGTS(14:0) | 446.3482 | 446.3479 | − 0.6 | 14:0 | C24H48O6N |
| MGTS(14:1) | 444.3325 | 444.3323 | − 0.5 | 14:1 | C24H46O6N |
| MGTS(16:0) | 474.3795 | 474.3793 | − 0.4 | 16:0 | C26H52O6N |
| MGTS(16:1) | 472.3638 | 472.3635 | − 0.7 | 16:1 | C26H50O6N |
| MGTS(16:2) | 470.3482 | 470.3485 | 0.7 | 16:2 | C26H48O6N |
| MGTS(16:3) | 468.3325 | 468.3335 | 2.1 | ** | C26H46O6N |
| MGTS(16:4) | 466.3169 | 466.3167 | − 0.4 | 16:4 | C26H44O6N |
| MGTS(18:0) | 502.4108 | 502.4115 | 1.5 | 18:0 | C28H56O6N |
| MGTS(18:1) | 500.3951 | 500.3948 | − 0.6 | 18:1 | C28H54O6N |
| MGTS(18:2) | 498.3795 | 498.3789 | − 1.1 | ** | C28H52O6N |
| MGTS(18:3) | 496.3638 | 496.3636 | − 0.4 | ** | C28H50O6N |
| MGTS(18:4) | 494.3482 | 494.3478 | − 0.7 | 18:4 | C28H48O6N |
| MGTS(18:5) | 492.3325 | 492.3318 | − 1.5 | 18:5 | C28H46O6N |
| MGTS(20:0) | 530.4421 | 530.4402 | − 3.5 | 20:0 | C30H60O6N |
| MGTS(20:4) | 522.3795 | 522.3798 | 0.6 | ** | C30H52O6N |
| MGTS(20:5) | 520.3638 | 520.3637 | − 0.2 | 20:5 | C30H50O6N |
| DGTS identified as [M+H]+ | |||||
| DGTS(28:1) | 654.5309 | 654.5325 | 2.4 | * | C38H72O7N |
| DGTS(30:0) | 684.5778 | 684.5759 | − 2.8 | 16:0–14:0 | C40H78O7N |
| DGTS(30:1) | 682.5622 | 682.5619 | − 0.4 | 16:1–14:0 | C40H76O7N |
| DGTS(30:2) | 680.5465 | 680.5444 | − 3.1 | 16:2–14:0 | C40H74O7N |
| DGTS(30:3) | 678.5309 | 678.5306 | − 0.4 | ** | C40H72O7N |
| DGTS(30:4) | 676.5152 | 676.5151 | − 0.1 | 16:4–16:0 | C40H70O7N |
| DGTS(30:5) | 674.4996 | 674.5002 | 0.9 | 16:4–14:1 | C40H68O7N |
| DGTS(32:0) | 712.6091 | 712.6067 | − 3.4 | 16:0–16:0 | C42H82O7N |
| DGTS(32:1) | 710.5935 | 710.5933 | − 0.3 | 16:1–16:0 | C42H80O7N |
| DGTS(32:2) | 708.5778 | 708.5772 | − 0.8 | 16:0–16:2 and 16:1–16:1 | C42H78O7N |
| DGTS(32:3) | 706.5622 | 706.5610 | − 1.7 | 16:3–16:0 | C42H76O7N |
| DGTS(32:4) | 704.5465 | 704.5464 | − 0.1 | 14:0–18:4, 16:4–16:0 and 16:3–16:1 | C42H74O7N |
| DGTS(32:5) | 702.5309 | 702.5306 | − 0.4 | ** | C42H72O7N |
| DGTS(32:6) | 700.5152 | 700.5152 | 0.0 | 16:4–16:2 and 16:3–16:3 | C42H70O7N |
| DGTS(32:7) | 698.4996 | 698.4993 | − 0.4 | 16:4–16:3 | C42H68O7N |
| DGTS(32:8) | 696.4839 | 696.4844 | 0.7 | 16:4–16:4 | C42H66O7N |
| DGTS(34:1) | 738.6248 | 738.6251 | 0.4 | 16:0–18:1 | C44H84O7N |
| DGTS(34:2) | 736.6091 | 736.6056 | − 4.8 | 16:1–18:1, 16:0–18:2 and 20:2–14:0 | C44H82O7N |
| DGTS(34:3) | 734.5935 | 734.5929 | − 0.8 | 16:2–18:1, 16:1–18:2 and 20:3–14:0 | C44H80O7N |
| DGTS(34:4) | 732.5778 | 732.5776 | − 0.3 | 18:4–16:0 and 18:3–16:1 | C44H78O7N |
| DGTS(34:5) | 730.5622 | 730.5616 | − 0.8 | 18:4–16:1, 18:1–16:4 and 18:2–16:3 | C44H76O7N |
| DGTS(34:5-OH) | 746.5571 | 746.5575 | 0.5 | 16:4-OH-18:1, 16:1-OH-18:4 and 16:0-OH-18:5 | C44H76O8N |
| DGTS(34:6) | 728.5465 | 728.5458 | − 1.0 | ** | C44H74O7N |
| DGTS(34:7) | 726.5309 | 726.5306 | − 0.4 | 16:4–18:3 and 16:3–18:4 | C44H72O7N |
| DGTS(34:7-OH) | 742.5258 | 742.5258 | 0.0 | 16:4-OH-18:3 and 16:3-OH-18:4 | C44H72O8N |
| DGTS(34:8) | 724.5152 | 724.5149 | − 0.5 | 16:4–18:4 | C44H70O7N |
| DGTS(36:10) | 748.5152 | 748.5140 | − 1.6 | 18:5–18:5 | C46H70O7N |
| DGTS(36:4) | 760.6091 | 760.6098 | 0.9 | 16:0–20:4, 18:3–18:1, 18:2–18:2, 20:3–16:1, 20:2–16:2, 20:1–16:3 and 20:0–16:4 | C46H82O7N |
| DGTS(36:5) | 758.5935 | 758.5933 | − 0.3 | 18:4–18:1, 20:5–16:0, 20:4–16:1, 20:3–16:2, 20:2–16:3, 20:1–16:4 and 16:1–20:4 | C46H80O7N |
| DGTS(36:6) | 756.5778 | 756.5775 | − 0.4 | 16:1–20:5, 20:3–16:3, 18:2–18:4 and 18:3–18:3 | C46H78O7N |
| DGTS(36:7) | 754.5622 | 754.5621 | − 0.1 | 18:4–18:3 | C46H76O7N |
| DGTS(36:8) | 752.5465 | 752.5462 | − 0.4 | 18:4–18:4 | C46H74O7N |
| DGTS(36:9) | 750.5309 | 750.5281 | − 3.7 | 18:5–18.4 | C46H72O7N |
| DGTS(38:10) | 776.5465 | 776.5464 | − 0.2 | 20:5–18:5 | C48H74O7N |
| DGTS(38:7) | 782.5935 | 782.5929 | − 0.8 | 20:5–18:2 | C48H80O7N |
| DGTS(38:8) | 780.5778 | 780.5743 | − 4.5 | 20:5–18:3 | C48H78O7N |
| DGTS(40:10) | 804.5778 | 804.5772 | − 0.7 | 20:5–20:5 | C50H78O7N |
| DGTS(40:9) | 806.5935 | 806.5905 | − 3.7 | 20:4–20:5 | C50H80O7N |
C number of carbon atoms, N number of double bonds.
*Identified based on the polar head fragmentation, calculated mass, and retention time.
**Identified according to the calculated mass and the retention time.
Figure 3.
HILIC–HR–ESI–MS spectra of the classes of betaine lipids (BL) identified in positive ion mode as [M+H]+. These classes were (A) diacylglycerol-trimethylhomoserine (DGTS) and (B) monoacylglycerol-trimethylhomoserine (MGTS).
The most abundant ions in each class of betaine lipids were MGTS (18:4) and DGTS (34:4) assigned as DGTS (18:4–16:0) and DGTS (18:3–16:1) (Table 4). Interestingly, among the identified DGTS molecular ions, oxidized DGTS molecular ions were also found, the most abundant being DGTS (34:7-OH), identified as a combination of two molecular species of DGTS (16:4-OH-18:3) and DGTS (16:3-OH-18:4). These oxidized ions were identified based on exact mass measurements, retention times, and by analysis of MS/MS spectra (Supplementary Table S3). The MS/MS of the oxidized DGTS molecular ions showed a neutral loss of H2O and the product ions formed due to the loss of oxidized fatty acyl chains (acid and keto derivatives). Also, the product ion characteristic of betaine lipids at m/z 236 was present in all MS/MS spectra, therefore no product ions with oxidized polar head groups were observed.
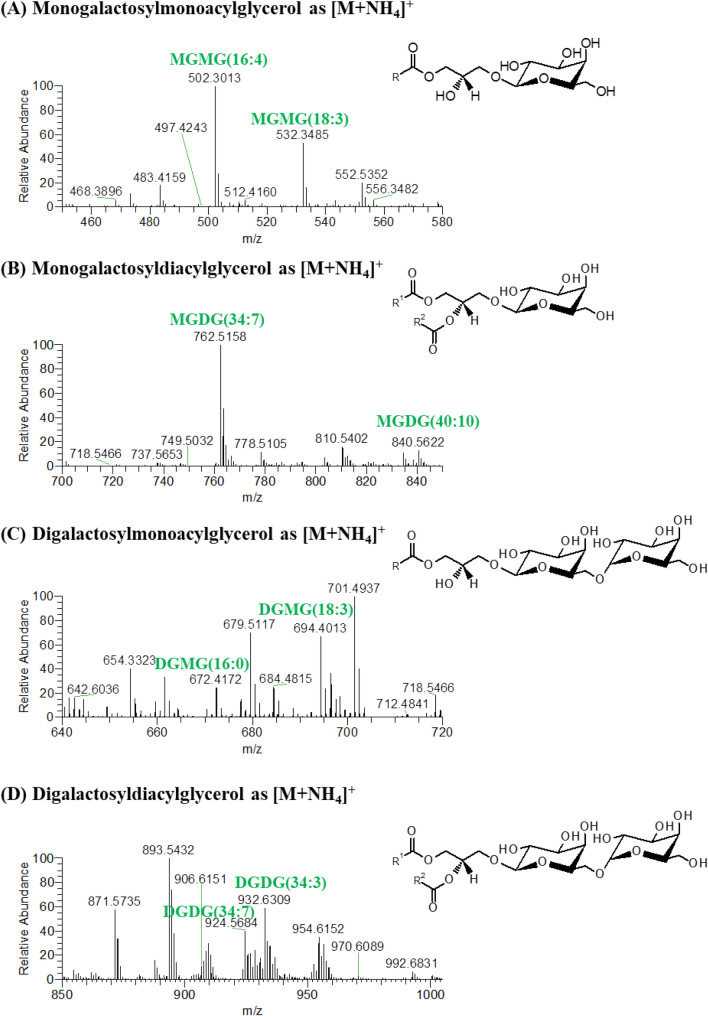
The glycolipids identified in the Chlorococcum amblystomatis samples included monogalactosyldiacylglycerol (MGDG), monogalactosylmonoacylglycerol (MGMG), digalactosyldiacylglycerol (DGDG) and digalactosylmonoacylglycerol (DGMG), identified as [M+NH4]+ ions (Fig. 4, Table 5). We also identified sulfoquinovosyl diacylglycerol lipids (SQDG) and a sulfoquinovosyl monoacylglycerol lipid (SQMG), identified as [M−H]− ions (Fig. 5, Table 5). Analysis of the HILIC–HR–ESI–MS and MS/MS spectra identified the following number of molecular ions: 30 MGDG, 11 MGMG, 27 DGDG, 11 DGMG, 21 SQDG and 1 SQMG (Supplementary Table S2). From these molecular ions, we identified the following number of molecular species using MS/MS data: 47 MGDG, 11 MGMG, 48 DGDG, 11 DGMG, 40 SQDG and 1 SQMG.
Figure 4.
HILIC–HR–ESI–MS spectra of the classes of glycolipids (GL) identified in positive ion mode as [M+NH4]+. These classes were (A) monogalactosylmonoacylglycerol (MGMG), (B) monogalactosyldiacylglycerol (MGDG), (C) digalactosylmonoacylglycerol (DGMG) and (D) digalactosyldiacylglycerol (DGDG).
Table 5.
Glycolipids identified in the polar lipidome of Chlorococcum amblystomatis by HILIC–HR–ESI–MS and MS/MS.
| Lipid species (C:N) | Calculated m/z | Observed m/z | Error (ppm) | Fatty acyl chains (C:N) | Formula |
|---|---|---|---|---|---|
| MGMG identified as [M+NH4]+ | |||||
| MGMG(14:0) | 482.3329 | 482.3324 | − 1.1 | 14:0 | C23H48NO9 |
| MGMG(16:0) | 510.3642 | 510.3639 | − 0.6 | 16:0 | C25H52NO9 |
| MGMG(16:1) | 508.3486 | 508.3482 | − 0.7 | 16:1 | C25H50NO9 |
| MGMG(16:3) | 504.3173 | 504.3174 | 0.3 | ** | C25H46NO9 |
| MGMG(16:4) | 502.3016 | 502.3013 | − 0.6 | 16:4 | C25H44NO9 |
| MGMG(16:4-OH) | 518.2965 | 518.2963 | − 0.4 | 16:4-OH | C25H44NO9O |
| MGMG(18:1) | 536.3799 | 536.3793 | − 1.0 | 18:1 | C27H54NO9 |
| MGMG(18:3) | 532.3486 | 532.3485 | − 0.1 | 18:3 | C27H50NO9 |
| MGMG(18:4) | 530.3329 | 530.3330 | 0.2 | * | C27H48NO9 |
| MGMG(20:4) | 558.3642 | 558.3636 | − 1.1 | ** | C29H52NO9 |
| MGMG(20:5) | 556.3486 | 556.3482 | − 0.6 | ** | C29H50NO9 |
| MGDG identified as [M+NH4]+ | |||||
| MGDG(30:0) | 720.5625 | 720.5593 | − 4.4 | ** | C39H78NO10 |
| MGDG(30:1) | 718.5464 | 718.5466 | 0.3 | 16:1–14:0 | C39H76NO10 |
| MGDG(32:0) | 748.5939 | 748.5910 | − 3.8 | 16:0–16:0 | C41H82NO10 |
| MGDG(32:1) | 746.5777 | 746.5780 | 0.4 | 16:1–16:0 | C41H80NO10 |
| MGDG(32:2) | 744.5626 | 744.5624 | − 0.3 | 16:1–16:1, 14:0–18:2 and 16:0–16:2 | C41H78NO10 |
| MGDG(32:3) | 742.5464 | 742.5467 | 0.4 | ** | C41H76NO10 |
| MGDG(32:4) | 740.5307 | 740.5305 | − 0.3 | ** | C41H74NO10 |
| MGDG(32:5) | 738.5156 | 738.5158 | 0.2 | ** | C41H72NO10 |
| MGDG(32:6) | 736.4994 | 736.4999 | 0.7 | ** | C41H70NO10 |
| MGDG(32:7) | 734.4843 | 734.4835 | − 1.1 | ** | C41H68NO10 |
| MGDG(32:8) | 732.4687 | 732.4686 | − 0.1 | 16:4–16:4 | C41H66NO10 |
| MGDG(34:1) | 774.6090 | 774.6073 | − 2.2 | ** | C43H84NO10 |
| MGDG(34:2) | 772.5933 | 772.5926 | − 0.9 | 18:2–16:0 | C43H82NO10 |
| MGDG(34:3) | 770.5782 | 770.5782 | 0.0 | 18:3–16:0 and 18:2–16:1 | C43H80NO10 |
| MGDG(34:4) | 768.5626 | 768.5645 | 2.5 | 16:2–18:2, 16:1–18:3, 16:0–18:4, 16:3–18:1 and 16:4–18:0 | C43H78NO10 |
| MGDG(34:5) | 766.5469 | 766.5474 | 0.6 | * | C43H76NO10 |
| MGDG(34:7) | 762.5156 | 762.5158 | 0.2 | 16:4–18:3 and 18:4–16:3 | C43H72NO10 |
| MGDG(34:8) | 760.5000 | 760.4999 | − 0.1 | ** | C43H70NO10 |
| MGDG(36:3) | 798.6095 | 798.6055 | − 5.0 | ** | C45H84NO10 |
| MGDG(36:4) | 796.5933 | 796.5892 | − 5.1 | ** | C45H82NO10 |
| MGDG(36:5) | 794.5782 | 794.5778 | − 0.5 | 18:3–18:2, 18:1–18:4, 20:5–16:0, 16:4–20:2 and 16:1–20:4 | C45H80NO10 |
| MGDG(36:6) | 792.5625 | 792.5623 | − 0.3 | 18:3–18:3, 16:1–20:5 and 16:4–20:2 | C45H78NO10 |
| MGDG(36:7) | 790.5469 | 790.5470 | 0.1 | ** | C45H76NO10 |
| MGDG(36:8) | 788.5313 | 788.5339 | 3.3 | ** | C45H74NO10 |
| MGDG(38:6) | 820.5939 | 820.5931 | − 1.0 | 18:1–20:5, 18:2–20:4 and 18:3–20:3 | C47H82NO10 |
| MGDG(38:7) | 818.5782 | 818.5777 | − 0.6 | 20:5–18:2 and 18:3–20:4 | C47H80NO10 |
| MGDG(38:8) | 816.5626 | 816.5614 | − 1.4 | ** | C47H78NO10 |
| MGDG(40:10) | 840.5626 | 840.5622 | − 0.4 | 20:5–20:5 | C49H78NO10 |
| MGDG(40:8) | 844.5939 | 844.5916 | − 2.7 | 20:4–20:4 | C49H82NO10 |
| MGDG(40:7) | 846.6095 | 846.6097 | 0.2 | ** | C49H84NO10 |
| DGMG identified as [M+NH4]+ | |||||
| DGMG(14:0) | 644.3857 | 644.3848 | − 1.4 | ** | C29H58NO14 |
| DGMG(16:0) | 672.4170 | 672.4172 | 0.3 | 16:0 | C31H62NO14 |
| DGMG(16:1) | 670.4014 | 670.4012 | − 0.3 | 16:1 | C31H60NO14 |
| DGMG(16:2) | 668.3857 | 668.3854 | − 0.5 | ** | C31H58NO14 |
| DGMG(16:3) | 666.3701 | 666.3699 | − 0.3 | ** | C31H56NO14 |
| DGMG(16:4) | 664.3544 | 664.3541 | − 0.5 | ** | C31H54NO14 |
| DGMG(18:1) | 698.4327 | 698.4327 | 0.0 | 18:1 | C33H64NO14 |
| DGMG(18:2) | 696.4170 | 696.4149 | − 3.0 | ** | C33H62NO14 |
| DGMG(18:3) | 694.4014 | 694.4013 | − 0.1 | 18:3 | C33H60NO14 |
| DGMG(18:4) | 692.3857 | 692.3856 | − 0.2 | ** | C33H58NO14 |
| DGMG(20:5) | 718.4014 | 718.4009 | − 0.7 | ** | C35H60NO14 |
| DGDG identified as [M+NH4]+ | |||||
| DGDG(30:0) | 882.6154 | 882.6111 | − 4.9 | ** | C45H88O15N |
| DGDG(30:1) | 880.5997 | 880.5990 | − 0.8 | 16:1–14:0 | C45H86O15N |
| DGDG(32:1) | 908.6310 | 908.6306 | − 0.4 | 16:0–16:1 | C47H90O15N |
| DGDG(32:2) | 906.6154 | 906.6151 | − 0.3 | 16:0–16:2 and 16:1–16:1 | C47H88O15N |
| DGDG(32:3) | 904.5997 | 904.6011 | 1.5 | 18:3–14:0, 16:0–16:3 and 16:1–16:2 | C47H86O15N |
| DGDG(32:4) | 902.5841 | 902.5846 | 0.6 | 18:3–14:1, 16:0–16:4 and 14:0–18:4 | C47H84O15N |
| DGDG(32:5) | 900.5684 | 900.5678 | − 0.7 | 16:1–16:4 | C47H82O15N |
| DGDG(32:6) | 898.5528 | 898.5512 | − 1.8 | ** | C47H80O15N |
| DGDG(34:1) | 936.6623 | 936.6615 | − 0.9 | 18:1–16:0 | C49H94O15N |
| DGDG(34:2) | 934.6467 | 934.6443 | − 2.6 | 18:1–16:1 and 18:2–16:0 | C49H92O15N |
| DGDG(34:3) | 932.6310 | 932.6309 | − 0.1 | 16:0–18:3 and 18:1–16:2 | C49H90O15N |
| DGDG(34:4) | 930.6154 | 930.6143 | − 1.2 | 18:1–16:3, 18:2–16:2, 18:3–16:1, 18:4–16:0 and 14:0–20:4 | C49H88O15N |
| DGDG(34:5) | 928.5997 | 928.5991 | − 0.6 | 18:3–16:2, 18:2–16:3, 18:1–16:4, 16:1–18:4 and 14:0–20:5 | C49H86O15N |
| DGDG(34:6) | 926.5841 | 926.5820 | − 2.3 | ** | C49H84O15N |
| DGDG(34:7) | 924.5684 | 924.5684 | 0.0 | 18:3–16:4 and 18:4–16:3 | C49H82O15N |
| DGDG(34:8) | 922.5528 | 922.5501 | − 2.9 | ** | C49H80O15N |
| DGDG(36:2) | 962.6780 | 962.6751 | − 3.0 | ** | C51H96O15N |
| DGDG(36:3) | 960.6623 | 960.6606 | − 1.8 | 18:3–18:0 and 18:1–18:2 | C51H94O15N |
| DGDG(36:4) | 958.6467 | 958.6433 | − 3.5 | ** | C51H92O15N |
| DGDG(36:5) | 956.6310 | 956.6301 | − 0.9 | 20:5–16:0, 18:1–18:4 and 20:1–16:4 | C51H90O15N |
| DGDG(36:6) | 954.6154 | 954.6152 | − 0.2 | 18:3–18:3, 20:5–16:1 and 18:4–18:2 | C51H88O15N |
| DGDG(36:7) | 952.5997 | 952.5999 | 0.2 | 18:3–18:4 | C51H86O15N |
| DGDG(36:8) | 950.5841 | 950.5835 | − 0.6 | 18:4–18:4 | C51H84O15N |
| DGDG(38:6) | 982.6467 | 982.6421 | − 4.7 | ** | C53H92O15N |
| DGDG(38:7) | 980.6310 | 980.6312 | 0.2 | 18:2–20:5 | C53H90O15N |
| DGDG(40:10) | 1002.6154 | 1002.6148 | − 0.6 | 20:5–20:5 | C55H88O15N |
| DGDG(40:9) | 1004.6310 | 1004.6323 | 1.3 | ** | C55H90O15N |
| SQDG identified as [M−H]− | |||||
| SQDG(28:0) | 737.4510 | 737.4492 | − 2.4 | ** | C37H69O12S |
| SQDG(30:0) | 765.4823 | 765.4818 | − 0.6 | 14:0–16:0 | C39H73O12S |
| SQDG(30:1) | 763.4666 | 763.4667 | 0.1 | 14:0–16:1 | C39H71O12S |
| SQDG(32:0) | 793.5136 | 793.5135 | − 0.1 | ** | C41H77O12S |
| SQDG(32:1) | 791.4979 | 791.4982 | 0.3 | 16:0–16:1 | C41H75O12S |
| SQDG(32:2) | 789.4823 | 789.4821 | − 0.2 | 16:1–16:1 and 16:0–16:2 | C41H73O12S |
| SQDG(32:3) | 787.4666 | 787.4668 | 0.2 | 16:1–16:2, 16:0–16:3 and 18:1–14:1 | C41H71O12S |
| SQDG(32:4) | 785.4510 | 785.4517 | 0.9 | 16:0–16:4, 16:1–16:3 and 16:2–16:2 | C41H69O12S |
| SQDG(34:0) | 821.5449 | 821.5446 | − 0.3 | 16:0–18:0 | C43H81O12S |
| SQDG(34:1) | 819.5292 | 819.5296 | 0.5 | 16:0–18:1 | C43H79O12S |
| SQDG(34:3) | 815.4979 | 815.4982 | 0.3 | 16:0–18:3 and 16:2–18:1 | C43H75O12S |
| SQDG(34:4) | 813.4823 | 813.4829 | 0.8 | 16:0–18:4, 16:1–18:3, 16:2–18:2, 18:1–16:3 and 14:0–20:4 | C43H73O12S |
| SQDG(34:5) | 811.4666 | 811.4667 | 0.1 | 16:0–18:5, 16:1–18:4, 16:2–18:3, 16:3–18:2 and 16:4–18:1 | C43H71O12S |
| SQDG(36:0) | 849.5762 | 849.5761 | − 0.1 | 16:0–20:0 | C45H85O12S |
| SQDG(36:3) | 843.5292 | 843.5287 | − 0.6 | ** | C45H79O12S |
| SQDG(36:4) | 841.5136 | 841.5135 | − 0.1 | 16:0–20:4 | C45H77O12S |
| SQDG(36:5) | 839.4979 | 839.4987 | 0.9 | ** | C45H75O12S |
| SQDG(36:6) | 837.4823 | 837.4823 | 0.0 | 16:1–20:5, 20:4–16:2, 18:2–18:4 and 18:3–18:3 | C45H73O12S |
| SQDG(36:7) | 835.4666 | 835.4676 | 1.2 | 18:3–18:4, 20:4–16:3 and 20:5–16:2 | C45H71O12S |
| SQDG(34:3-OH) | 831.4928 | 831.4930 | 0.2 | – | C43H75O13S |
| SQDG(34:4-OH) | 829.4772 | 829.4752 | − 2.4 | – | C43H73O13S |
| SQMG identified as [M−H]− | |||||
| SQMG(16:0) | 555.2839 | 555.2838 | − 0.2 | 16:0 | C25H47O11S |
C carbons, N number of double bonds.
*Identified based on the polar head fragment, calculated mass and retention time.
**Identified based on calculated mass and retention time.
Figure 5.
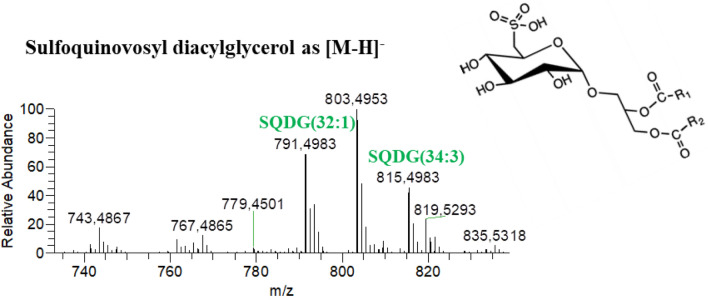
HILIC–HR–ESI–MS spectrum of the classes of glycolipids (GL) identified in negative ion mode as [M−H]−. This class was sulfoquinovosyl diacylglycerol (SQDG).
The typical fragmentation observed in the HILIC–HR–ESI–MS/MS spectra of neutral glycolipids, as [M+NH4]+ ions, allowed to confirm the polar head group by the neutral loss of 197 Da (for MGDG) or 359 Da (for DGDG), which corresponds to the loss of the carbohydrate moiety combined with the loss of NH344,47. The assignment of the fatty acyl composition was corroborated by the presence of product ions corresponding to each fatty acyl group as acylium plus 74 [RCO+74]+ ion44,47 (Supplementary Table S2). On the other hand, the HILIC–HR–ESI–MS/MS spectra of the [M−H]− ions of the acidic glycolipids (SQDG and SQMG), when available, showed the product ion of the sulfoquinovosyl polar head group at m/z 225 and the fatty acyl composition was confirmed by the neutral loss of fatty acyl chains as carboxylic acid (-RCOOH) and by the presence of carboxylate RCOO− anions (Supplementary Table S2).
The most abundant species in each class of glycolipids were: MGDG (34:7), identified as MGDG (16:4–18:3) and MGDG (18:4–16:3); MGMG (16:4); DGDG (34:3), identified as DGDG (16:0–18:3) and DGDG (18:1–16:2); DGMG (18:3); and SQDG (32:1), identified as SQDG (16:0–16:1). The only identified glycolipid species belonging to the SQMG class was identified by accurate mass measurements and by the presence on the MS/MS spectrum of the product ion corresponding to the polar head at m/z 225. Among the MGMG and SQDG species identified, we observed three oxidized species, MGMG (16:4-OH), SQDG (34:3-OH) identified as SQDG (18:3-OH-16:0) and SQDG (34:4-OH) identified as SQDG (18:4-OH-16:0). HILIC–HR–ESI–MS/MS analysis of all oxidized GL species suggested that a hydroxyl group was present in the fatty acyl group (Supplementary Table S3).
The relative quantification of the identified species was carried out as described in the methods section. Figure 6 shows the most abundant lipid species, identified as molecular ions, in the polar lipidome of Chlorococcum amblystomatis, with a relative amount greater than or equal to 1.5%. Glycolipids were the group of lipids with the largest number of species (101). The most abundant species was MGDG (34:7), assigned as MGDG (16:3–18:4) and MGDG (16:4–18:3), and the second most abundant species was SQDG (16:0–16:1).
Figure 6.
The most abundant lipid species, identified as molecular ions, in the polar lipidome of Chlorococcum amblystomatis, with a relative amount ≥ 1.5%. The results are represented by an average of five samples ± standard deviations.
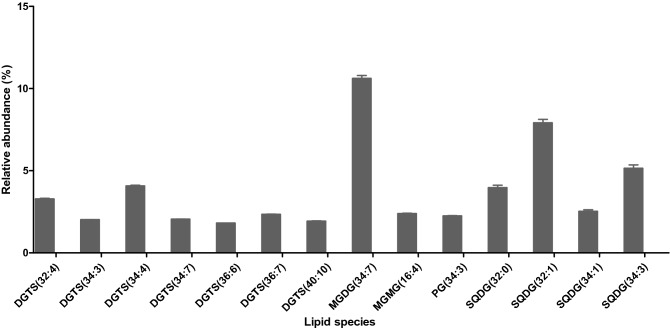
LC–MS data was acquired using an internal standard for each class of phospholipids. LC–MS data were normalized against their assigned internal standard, to semi-quantify each molecular ion. Due to the lack of commercially available GL and BL standards, internal standards from classes with a retention time closer to those identified for GL and BL were used to normalize their respective data, as performed in our laboratory45,52–54. The use of one internal standard is acceptable for semi-quantification because the ionization efficiency of polar lipids depends mainly on the polar head, while the length of the chain and the degree of unsaturation contribute little55,56. However, we would like to point out the limitations of our inter-class quantitation approach, because despite the similar retention time, the response factors for each class, even between phospholipids, tend to differ in ESI–MS, which prevents a robust and accurate quantification51–53. Normalized data were then used to semi-quantify the abundances of the identified molecular ions. For a clearer overview of the composition of the polar lipidome of Chlorococcum amblystomatis, we have summed up the relative percentage of all the detected lipid species belonging to glycolipids, betaines and phospholipids (Fig. 7A). According to the largest number of molecular ions, the highest cumulative levels were observed for glycolipids (51.9%), followed by betaine lipids (30.1%) and phospholipids (17.9%). The sum of the relative percentage of all lipid species belonging to each class of lipids was also calculated. The highest cumulative levels were observed for DGTS (27.6%) followed by SQDG (23.7%) (Fig. 7B).
Figure 7.
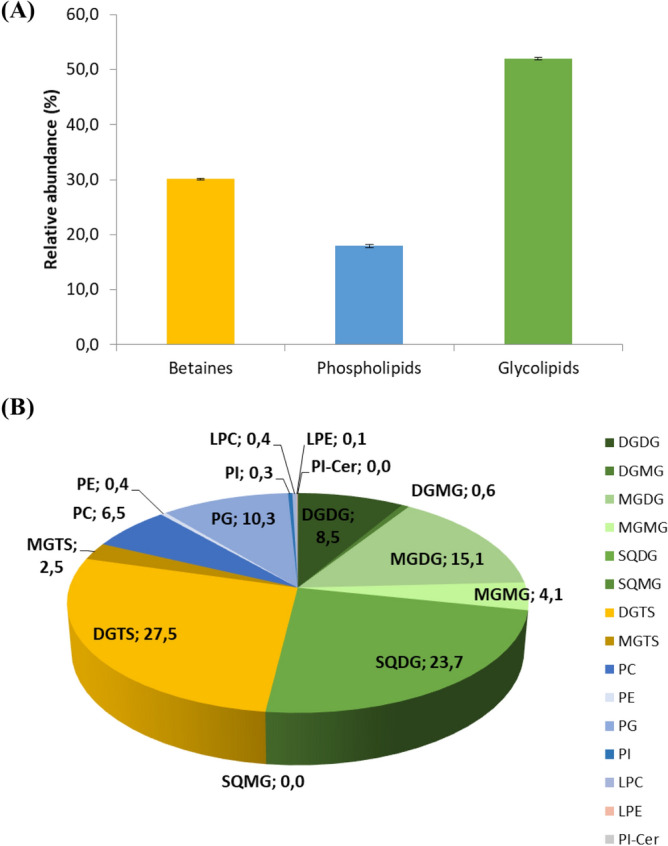
(A) Representation of the relative percentage of betaines, phospholipids and glycolipids present in Chlorococcum amblystomatis. Values are represented by an average of five replicates ± standard deviation. (B) The relative percentage of each class of lipids.
Evaluation of in vitro anti-inflammatory and antioxidant properties of Chlorococcum amblystomatis lipid extracts
The anti-inflammatory potential of lipid extracts from Chlorococcum amblystomatis were evaluated using a test kit for screening for inhibition of human COX-2. With 10 µl of an extract of 50 µg mL−1 (5 µg), we have observed an inhibition of 87.5 ± 0.1 of COX-2 activity (Fig. 8). These results suggest that the lipid extracts of Chlorococcum amblystomatis have anti-inflammatory potential.
Figure 8.
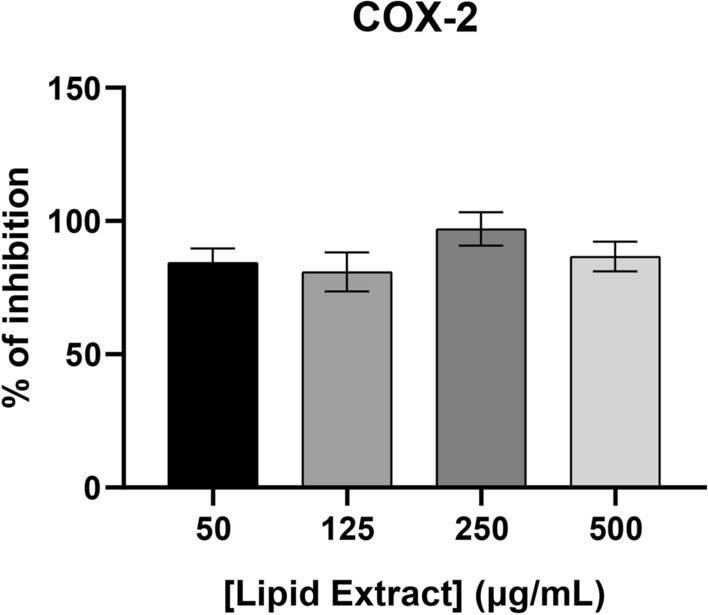
Percent inhibition of COx-2 as a function of the concentration of the lipid extracts of Chlorococcum amblystomatis.
The antioxidant potential of lipid extracts from Chlorococcum amblystomatis were evaluated using free radical DPPH• and ABTS•+ scavenging assays (Fig. 9), as described in the methods section. The results obtained for the DPPH assay revealed that the concentration of the extract resulting in a 40% inhibition (IC40) of DPPH• was at an estimated concentration of 226.81 ± 2.99 µg mL−1. The average antioxidant activity (Trolox equivalent, TE) was 76.25 ± 1.02 Trolox µmol g−1 of lipid extract. In contrast, the lipid extract which resulted in a 50% inhibition (IC50) of ABTS•+ was 37.69 ± 4.44 µg mL−1 and the expressed TE activity of 428.81 ± 54.32 Trolox µmol g−1 of lipid extract.
Figure 9.
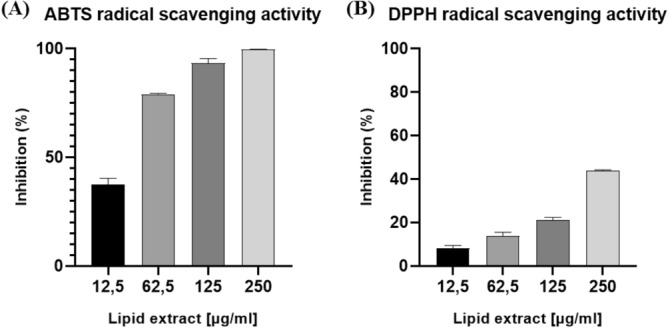
Percent inhibition as a function of the concentration of the lipid extracts of Chlorococcum amblystomatis after 120 min of ABTS•+ (A) and DPPH• (B) radical scavenging activity.
Discussion
Chlorococcum amblystomatis is a green microalga with great potential to be a sustainable resource of commercially important essential lipids1,2,29, with promising applications in several industrial sectors32,33. In our work, the lipid extracts represented 20.77 ± 0.57%, of the dry weight of the biomass, which is consistent with the lipid content reported for Chlorococcum (8.71–32.3%)10,11,18–20. This wide range of lipid content determined by different studies is consistent with the variation in lipid content due to growing conditions32,36,38–40 and the use of different extraction methods54.
The FA profile identified by GC–MS included as the most abundant FA: C16:0, C18:3 (n-3), C18:0, C16:4 (n-3), C20:5 (n-3) and C16:1–9, representing respectively 23%, 19%, 14%, 11%, 9% and 7% of the total FA pool. It is important to note that this is the first time that FA C16:4 (n-3) and C20:5 (n-3) have been reported in Chlorococcum amblystomatis. The values of relative abundance collected in the present work are different from those reported in the literature to Chlorococcum sp32,33,38,39. Such differences could result from differences in growth conditions 55, different strains, different species, different derivatization methods or even GC detectors (FID or MS) 56. Chlorococcum amblystomatis extracts were rich in omega-3 PUFAs, representing 43.2%, with a high contribution of the most abundant FA, C18:3 (n-3), C16:4 (n-3) and C20:5 (n-3). Omega-3 PUFAs are known to have a variety of health benefits, such as preventing chronic diseases, as they are associated with anti-inflammatory and antioxidant protection, benefits for the cardiovascular system, prevention of breast cancer, improvement of neurological capacities and visual development57–59. To understand the beneficial impact on health and assess the nutritional quality of Chlorococcum amblystomatis, we calculated the values of AI (0.5), TI (0.3), h/H (1.4), and n-6/n-3 (0.1). The AI and TI indexes are commonly used to assess the potential of the matrix to stimulate platelet aggregation, and the lower the indexes the more beneficial it is in reducing the prevalence of heart disease 60, and have already been used to assess these benefits in microalgae, seaweeds and fish61–65. Our results show that these indexes of the lipid extract of Chlorococcum amblystomatis were similar to those observed in fish oils66,67. Compared to Spirulina platensis, Nannochloropsis gaditana, Nannochloropsis oculata and Porphyridium tricornutum, Chlorococcum amblystomatis had lower AI values. Their AI values ranged between 0.6 and 1.7 61. As for the TI index, Chlorococcum amblystomatis had lower values than Spirulina platensis, Nannochloropsis gaditana and Porphyridium Cruentum 61, which were 1.5, 3.8 and 0.6, respectively. The higher values for h/H and the lower n-6/n-3 ratio (1.4 and 0.1, respectively) reinforce the nutritional potential of the lipid extracts of Chlorococcum amblystomatis.
The profiling of the total lipid extract using high-resolution HILIC–ESI–MS and MS–MS approaches, made it possible, for the first time, to describe the polar lipid composition, in Chlorococcum amblystomatis. The polar lipids identified included glycolipids (GL), phospholipids (PL) and betaine lipids (BL). Data from the literature indicate that algae are a valuable and promising source of polar lipids with bioactive properties, namely, polar lipids esterified to commercially important PUFAs, e.g. eicosapentaenoic acid (EPA). Our profiling made it possible to identify 245 molecular ions, corresponding to a minimum of 350 molecular species: 89 molecular ions of PL, 101 molecular ions of GL (sulfo- and galactolipids), 54 molecular ions of BL and 1 inositol phosphoceramide. These ions were distributed by 15 classes of lipids: PL classes were LPC, PC, LPE, PE, PG and PI; sphingolipid classes were PI-Cer, BL classes were MGTS and DGTS; and GL classes were MGMG, MGDG, DGMG, DGDG, SQDG, and SQDG.
About 45% of the lipid species detected were esterified to omega-3 fatty acids. Some of these species caught our attention because of their richness in omega-3 PUFAs. The PG (34:3) and the PG (36:5), respectively assigned to PG (16:0–18:3) and PG (16:0–20:5) are two species of PL among the most abundant of Chlorococcum amblystomatis, and both carried omega-3 fatty acids, including EPA. In a previous study in which anti-inflammatory activity was addressed, PG (16:0–20:5) was suggested to promote down-regulated iNOS activity in LPS-stimulated macrophages21,68.
Since in Chlorococcum amblystomatis there are high amounts of PLs esterified to omega-3 PUFAs and it has been suggested that the PLs are an excellent delivery vehicle for PUFAs18,19, this will favour its valorization for food and nutraceutical formulations, where they can be used as value-added ingredients, and also in the cosmetic industry, as they can be used to create moisturizing emulsions 69. Interestingly, oxidized phosphatidylglycerols (PG) with bonded oxylipins have been identified in this study. Some studies have suggested the anti-inflammatory potential of oxylipins 70, as reported for Nannochloropsis gaditana and Chlamydomonas debaryana 71. Indeed, we observed a COX-2 inhibitory activity from the lipid extracts of Chlorcococcum amblystomatis, to which these oxylipins could have contributed. Consequently, extracts rich in polar lipids from Chlorococcum amblystomatis could constitute an interesting opportunity for the nutraceutical or pharmaceutical industries.
The BL identified in the polar lipidome of Chlorococcum amblystomatis, include as the most abundant species, DGTS (34:4), DGTS (32:4), DGTS (36:7), DGTS (34:7), DGTS (34:3), DGTS (40:10) and DGTS (36:6). Moreover, DGTS (40:10) was identified as DGTS (20:5–20:5), a molecular species associated with anti-inflammatory activity, by reducing the production of nitric oxide (NO) 72. However, BL remains poorly studied to date, and little is known about its bioactive potential.
The polar lipidome of Chlorococcum amblystomatis was particularly rich in glycolipids (MGDG, DGDG and SQDG) which represented approximately half of the polar lipids content (Fig. 7). There is an interest in characterizing GL because of their reported bioactive properties 16. For example, SQDG of the microalga Porphyridium purpureum have been associated with antioxidant activity, due to their inhibitory effect on the generation of superoxide in peritoneal mononuclear cells 73. GL with PUFAs have shown anti-inflammatory activity by inhibiting the release of nitric oxide by macrophages20,21. There is also a patent for MGDG with EPA to be used as an anti-inflammatory compound 74. Another interesting application for glycolipids comes from the use of DGDG and SQDG from seaweeds as chemotherapy agents 22. SQDG esterified in EPA appears to have an anti-proliferative effect by inhibiting the key enzymes telomerase 23 and DNA polymerase-α and -β 75. GL species with known bioactivity, such as MGDG (20:5–16:0), MGDG (20:5–16:1), MGDG (20:5–18:2), MGDG (20:5–20:5), DGDG (20:5–16:0), DGDG (20:5–18:2), DGDG (18:4–16:0) and MGDG (16:4–18:3), were also identified in the Chlorococcum amblystomatis lipidome. These GL species were associated with anti-inflammatory activity by down-regulating the iNOS46,76–78.
Regarding sulfur-containing lipids, SQDG (34:3), assigned as SQDG (16:0–18:3), and SQDG (32:0), were among the most abundant SQDG identified in Chlorococcum amblystomatis, and were linked to anti-inflammatory 79 and antiviral activities 80, respectively. Also, SQDG (16:0–20:4) and SQDG (16:0–18:4), identified in extracts of Chlorococcum amblystomatis, have been reported for their antiproliferative properties81,82. Finally, SQMG (16:0), although detected in low abundance, has been reported to have antimicrobial and antitumor activities 83.
The polar lipids of Chlorococcum amblystomatis may contribute to the antioxidant activity observed in the DPPH and ABTS radical scavenging assays, as some of the reported polar lipids were previously associated with antioxidant activity 73. Natural antioxidants are highly sought after for their biological effects 84 and their wide applications in the food and pharmaceutical sectors 85. As such, microalgal biomass extracts have been widely explored regarding their potential antioxidant activity86,87. Ethanolic and aqueous extracts are mainly composed of phenolic compounds, while methanolic extracts are rich in polar lipids 88. In a recent study, the DPPH assay was employed to evaluate antioxidant activity on 9 microalgae. The methanolic extracts of the different microalgae strains showed antioxidant activity, with percentage inhibition of DPPH ranging from 15 to 45% (IC15 to IC45; at 200 μg mL−1 extracts concentration) 86. In this work, the dichloromethane:methanol extracts of Chlorococcum amblystomatis resulted in a percentage of inhibition of 36% at 200 µg mL−1, displaying a value better compared to 7 strains of microalgae from the reported work.
COX-2 is an important component of inflammation, associated with pro-inflammatory activity, responsible for the production of prostaglandin E2 89. In the present work, the lipid extracts of Chlorococcum amblystomatis exhibit a COX-2 inhibiting activity. It is the first time that such a response has been described in Chlorococcum sp. However, recent work, also measuring COX-2 inhibition, compared the anti-inflammatory potential of aqueous and ethanolic extracts of two Tetraselmis sp. strains and a Skeletonema sp. strain 81. The highest value of anti-inflammatory activity (82 ± 2%) was measured for the ethanolic extract of Skeletonema sp. at a concentration of 1 mg mL-1. Our results showed that the COX inhibition of dichloromethane: methanol extracts of Chlorococcum amblystomatis was 87.5 ± 0.1%, at a concentration of 50 μg mL−1. These results are consistent with the hypothesis that Chlorococcum amblystomatis has a higher COX-2 inhibitory power than Tetraselmis sp. and Skeletonema sp. The high COX-2 inhibitory activity of Chlorococcum amblystomatis extracts results in promising anti-inflammatory potential. Future studies should explore the use of lipid extracts of Chlorococcum amblystomatis in inflammatory cells to explore this anti-inflammatory potential.
Conclusions
The polar lipidome of Chlorococcum amblystomatis was characterized for the first time in this study. The Chlorococcum amblystomatis strain used revealed a high content of omega-3 PUFAs. PUFAs are associated with several health benefits, such as the prevention of cardiovascular disease. The HILIC–MS/MS lipidomic approach identified 245 molecular ions of polar lipids, in Chlorococcum amblystomatis, revealing to be a microalga rich in glyco- and betaine lipids. Some of the identified polar lipids have already been reported with biological activity, for example, DGTS (20:5–20:5), SQDG (16:0–18:3), MGDG (20:5–18:2) and DGDG (20:5–18:2) which were associated with anti-inflammatory activity. In addition, extracts rich in polar lipids had COX-2 inhibiting activity and antioxidant activity. In conclusion, due to its chemical, biochemical, bioactive, and health-promoting properties, the lipid extracts of Chlorococcum amblystomatis have been found to be of high value for application in food, feed, cosmetic, nutraceutical, and pharmaceutical applications.
Materials and methods
Reagents
HPLC grade methanol (MeOH), ethanol absolute, and dichloromethane (CH2Cl2), were purchased from Fisher Scientific Ltd. (Loughborough, UK). All other reagents were purchased from major commercial sources. Milli-Q water (Synergy, Millipore Corporation, Billerica, MA, USA) was used. Phospholipid internal standards 1,2-dimyristoyl-sn-glycero-3-phosphocholine (dMPC), 1,2-dimyristoyl-sn-glycero-3-phosphoethanolamine (dMPE), 1,2-dimyristoyl-sn-glycero-3-phospho-(10-rac-glycerol) (dMPG), 1,2-dimyristoyl-sn-glycero-3-phospho-l-serine (dMPS), 1,2-dipalmitoyl-sn-glycero-3-phosphatidylinositol (dPPI), N-palmitoyl-D-erythro-sphingosylphosphorylcholine (SM), 1-nonadecanoyl-2-hydroxy-sn-glycero-3-phosphocholine (LPC) were purchased from Avanti Polar Lipids, Inc. (Alabaster, AL), according to the methodology previously described34,35. DPPH• was purchased from Aldrich (Milwaukee, WI). 2,20-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt (ABTS•+) was obtained from Fluka (Buchs, Switzerland). The 6-hydroxy-2,5,7,8-tetramethylchromane-2-carboxylic acid (Trolox) were purchased from Sigma-Aldrich (St Louis, MO, USA). The cyclooxygenase (COX-2) inhibitory screening assay was performed using a commercial kit, Cayman test kit-701080 from Cayman Chemical Company (Ann Arbor, MI, USA). All the other reagents and chemicals used were of the highest grade of purity commercially available.
Microalgae material
The spray-dried biomass of Chlorococcum amblystomatis was supplied by Allmicroalgae, Natural products S.A. located in Pataias, Portugal, Fábrica Cibra Pataias, 2445-287 Pataias. The strain was isolated locally and is deposited in the Allmicroalgae culture collection under the code 0066CA 34.
Portugal Chlorococcum amblystomatis 0066 CA was cultivated autotrophically in Guillard’s F2 culture medium, whose composition was adapted to local water, using nitrates as the source of nitrogen 91. Briefly, 5 L flask reactors were cultivated from 7 to 15 days, under continuous exposure to light. Five 5 L flask reactors were used to inoculate one 0.1 m3 L outdoor Flat Panel (FP) reactor, which was later sequentially scaled as follows: 0.25 m3 L to a 0.5 m3 L to a 1 m3 Flat Panels. Four of the later reactors were used as inoculum of a 10 m3 photobioreactor (PBR). The reactor was operated for 21 days, exposed to the environmental light and temperature conditions, at an average temperature of 15.5 °C and light irradiance of 20.10 MJ m−2 day−1. pH was maintained constant, 7.0–8.0, by pulse injections of CO2 and the temperature was kept under 28 °C by a sprinkler-like irrigation system. After growing period, the biomass was industrially collected by centrifugation and further spray-drying.
Lipid extraction procedure
Lipid extraction was carried out using a mixture of dichloromethane: methanol solvents (DM) (2:1, v/v). The lipids were extracted from 25 mg of lyophilized Chlorococcum amblystomatis biomass using the solvent mixture. The suspension was centrifuged (Selecta JP Mixtasel, Abrera, Barcelona, Spain) at 2000 rpm for 10 min and the supernatant was collected in a new pre-weighed glass tube. This process was repeated four times until the extraction solvent lost the green colour. The combined supernatants were dried under a stream of nitrogen.
The Folch extraction method was used to the obtained dried supernatants54,92. The extracts were redissolved in 2 mL of dichloromethane, and 1 mL of methanol and 0.75 mL of Milli-Q water were added. The mixture was vortexed for 2 min followed by phase separation by centrifugation at 2000 rpm for 10 min. The organic phase was collected in a new pre-weighed tube, and the aqueous phase was reextracted with 2 mL of dichloromethane, two more times. The combined organic phases were dried under a stream of nitrogen and weighted.
Each series of extracts was repeated five times and the total lipid content was determined by gravimetry. The yield of lipids extracted from dry biomass extracts (DW) was calculated as follows (Eq. 1):
| 1 |
COX-2 inhibition assay
A commercial cyclooxygenase (COX-2) inhibitory screening assay kit—Cayman test kit-701080 (Cayman Chemical Company, Ann Arbor, MI, USA)—was used to assess their anti-inflammatory potential 93. This screening assay directly measures the amount of prostaglandin F2α generated from arachidonic acid (AA, C20:4 [n-6]) in the cyclooxygenase reaction. This assay was carried out according to the instructions described by the supplier of the assay kit, using an aliquot of test extract or DMSO. For this assay, lipid extracts of Chlorococcum amblystomatis (500 μg, 250 μg, 125 μg and 50 μg) were dissolved in DMSO and the final volume of reaction was 1000 μl. Positive and negative controls were provided by the assay kit protocol. The positive control used inactivated COX-2 enzyme, and negative control used the enzyme with 100% initial activity without any inhibitor. The assay was performed in three replicates. Interferences were considered by subtracting COX-2 inhibition from the blank assays. The results were expressed as a percentage of inhibited COX-2.
DPPH Radical Scavenging Assay
The antioxidant scavenging activity against the α,α-diphenyl-β-picrylhydrazyl radical (DPPH•) was evaluated as described previously94,95 with some modifications. Briefly, 150 µL of an ethanolic dilution of the extracts (25, 125, 250, 500 µg mL−1) or 150 µL of the Trolox standard solution (5, 12.5, 25, 37.5 µmol L−1 in ethanol) were mixed in triplicate with 150 µL of a DPPH• working solution in ethanol (absorbance ~ 0.9, 517 nm). The mixture was incubated for 120 min and the absorbance was measured at 517 nm every 5 min (Multiskan GO 1.00.38, Thermo Scientific, Hudson, NH, USA). A control was prepared by replacing the DPPH• solution with ethanol. The antioxidant activity, expressed as a percentage of inhibition of the DPPH radical, was calculated using the following equation (Eq. 2):
| 2 |
The activity is expressed in Trolox Equivalents, which were calculated using Eq. 2, where IC40 values are the concentration of sample or of Trolox that induces the reduction the DPPH• radical to 40%) (Eq. 3):
| 3 |
ABTS radical cation scavenging assay
The antioxidant scavenging activity against the 2,20-azino-bis-3-ethylbenzothiazoline-6-sulfonic acid radical cation (ABTS•+) was evaluated using a method previously described94,96 with some modifications. Briefly, 150 µL of an ethanolic dilution of the extracts (25, 125, 250, 500 µg mL−1) or 150 µL of the Trolox standard solution (5, 12.5, 25, 37.5 µmol L−1 in ethanol) were mixed in triplicate with 150 µL of an ABTS•+ working solution in ethanol (absorbance ~ 0.9, 734 nm). The mixture was incubated for 120 min and the absorbance was measured at 734 nm every 5 min (Multiskan GO 1.00.38, Thermo Scientific, Hudson, NH, USA). A control was prepared by replacing the ABTS•+ solution with ethanol. The antioxidant activity, expressed as a percentage of inhibition of the ABTS radical (IC40 substituted by IC50), was calculated using Eq. (2) (AbsDPPH• substituted by AbsABTS•+) and expressed in Trolox Equivalents (Eq. 3).
Analysis of fatty acids by gas chromatography–mass spectrometry (GC–MS)
The fatty acid methyl esters (FAMEs) were prepared from total lipid extracts of Chlorococcum amblystomatis by transmethylation reaction using a methanolic solution of potassium hydroxide (2.0 M) according to the methodology previously described 90. A volume of 2.0 μL of a hexane solution containing FAMEs and 1.03 μg mL−1 of methyl nonadecanoate (Sigma, St. Louis, MO, USA) as internal standard was injected in a chromatography-mass spectrometry (GC–MS) (Agilent Technologies 8860 GC System, Santa Clara, CA, USA) equipped with a DB-FFAP column with the following specifications: 30 m long, 0.32 mm internal diameter, and 0.25 μm film thickness (J & W Scientific, Folsom, CA, USA). The GC equipment was connected to an Agilent 5977B Mass Selective Detector operating with electron impact ionization at 70 eV and a scanning range of m/z 50–550 (1 s cycle in a full scan mode). The following conditions were used: helium as carrier gas (constant flow 1.4 mL min−1), inlet temperature 220 °C, detector temperature 230 °C, injection volume 2 μL (splitless). The oven temperature was programmed as follows: 58 °C for 2 min, 25 °C min−1 to 160 °C, 2 °C min−1 to 210 °C, 30 °C min−1 to 225 °C (held for 20 min). The data acquisition software used was GCMS5977B/Enhanced MassHunter. Data were analysed using Agilent MassHunter Qualitative Analysis 10.0 software. The identification of FA was carried out by comparison of the MS spectrum with the NIST chemical database library and confirmed with the literature reports. Five independent replicates were injected.
Atherogenic, thrombogenic and hypocholesterolemic/hypercholesterolemic indices
The atherogenic, thrombogenic and hypocholesterolemic/hypercholesterolemic indices (h/H) were calculated as the content ratio of saturated fatty acids (SFA)/unsaturated FA, as monounsaturated fatty acids (MUFAs) and omega-3 and omega-6 PUFAs, using the following formula (Eqs. 4, 5 and 6), as proposed by Ulbricht and Southgate 97
| 4 |
| 5 |
| 6 |
Polar lipidome analysis by hydrophilic interaction liquid chromatography coupled to high-resolution tandem mass spectrometry (HILIC–HR–MS/MS)
The polar lipidome was determined according to the methodology previously described34,35. The dried samples were dissolved in CH2Cl2 to a final concentration of 1 ug uL-1. From each sample, a volume of 10 µL (10 µg of lipid extract) was taken and transferred to an appropriate vial, followed by the addition of 86 µL of a solvent system composed of two mobile phases in a proportion of 90% v/v eluent B and 10% v/v eluent A and 4 µL of a mixture of internal standards (dMPC—0.02 μg, dMPE—0.02 μg, SM (17:0/d18:1)—0.02 μg, LPC—0.02 μg, dPPI—0.08 μg, dMPG—0.012 μg, dMPS—0.04 μg). The composition of eluent B was 60% v/v of acetronitrile, 40% v/v of methanol, and 5 mM of ammonium acetate and the composition of eluent A was 50% v/v of acetonitrile, 25% v/v of methanol, 25% v/v of water, and 5 mM ammonium acetate.
The lipids were separated by hydrophilic interaction liquid chromatography (HILIC) using a microbore Ascentis Si column (10 cm × 1.0 mm, 3 µm; Sigma-Aldrich) and a high performance-liquid chromatography (HPLC) system (Ultimate 3000 Dionex, Thermo Fisher Scientific, Bremen, Germany) with an autosampler coupled to the Q-Exactive hybrid quadrupole Orbitrap mass spectrometer (Thermo Fisher Scientific, Bremen, Germany). A 5 µL aliquot of each sample mixture was injected into the HPLC column, at a flow rate of 50 µL min−1 and a temperature of 35 °C. The following gradient was applied: 10% A (0–2 min), 10–90% A (2–15 min), 90% A (15–17 min). The mass spectrometer with Orbitrap technology was operated simultaneously in positive (electrospray voltage 3.0 kV) and negative (electrospray voltage − 2.7 kV) modes, with the following configuration: high resolution with 70,000, AGC target of 1 × 106, capillary temperature 250 °C, and sheath gas flow of 15 U. The tandem mass spectrometry experiments were performed according to the following configuration: resolution of 17,500, AGC target of 1 × 105, with one full scan mass spectrum and 10 data-dependent MS/MS scans. The cycles were repeated continuously throughout the experiments with the dynamic exclusion of 60 s and an intensity threshold of 1 × 104. Normalized collision energy (CE) ranged between 25, 30, and 35 eV. Data acquisition was performed using the Xcalibur data system (V3.3, Thermo Fisher Scientific, USA). Five independent biological replicas were carried out.
Data analysis
To identify the classes of the polar lipids in the lipid extracts acquired spectra were analysed using Xcalibur v3.3 (Thermo Fisher Scientific, USA). The identification was carried out according to a standard approach in our laboratory 98: this approach consists of the localization of the species according to the retention time of internal standards (information related to internal standards can be found in Supplementary Table S1), accurate mass measurements (5 ppm) and identification of recurring fragmentation patterns (Supplementary Figures S1–S13) and their comparison with those of internal standards and published information on fragmentation patterns98–101. After identification, the quantification of molecular species was carried out by integrating the chromatographic peaks using the MZmine v2.42 software. The software allows filtering and smoothing, peak detection, peak processing and assignment against an internal database 102. All peaks of raw intensity bellow 1 × 104 were excluded.
Relative quantification was performed by exporting the values of the peak areas to a computer spreadsheet (Excel, Microsoft, Redmond, WA). To normalize the data, the peak areas of the extracted ion chromatograms (EIC) of each lipid molecular species were divided by the EIC peak areas of the selected internal standards. Relative abundances were calculated using dMPC, dMPE, SM(17:0/d18:1), LPC, dPPI, dMPG and dMPS as internal standards. To normalize DGTS and MGTS we used PE as internal standard, for SQDG we used PG as internal standard, and for MGDG, DGDG, DGMG and MGMG, we used Ceramide as internal standard. For clear visualization, normalized data was transformed in percentage. The normalized data was calculated as follows (Eq. 7):
| 7 |
The relative percentage of betaines, phospholipids and glycolipids was calculated as follows (Eq. 8):
| 8 |
The relative percentage of the distribution of omega-3 and omega-6 fatty acids across the different main classes of polar lipids (betaines, phospholipids and glycolipids) was calculated as follows (Eq. 9):
| 9 |
Raw abundances, normalized data and relative quantification can be found on the additional spreadsheet (Supplementary file S1).
Supplementary Information
Acknowledgements
The authors would like to acknowledge all staff members of Allmicroalgae Natural Products, S.A. for the kind support in microalgae growth. Thanks are due to the University of Aveiro and FCT / MCT for the financial support to QOPNA ((FCT UID/QUI/00062/2019) and LAQV/REQUIMTE (UIDB/50006/2020), CESAM (UIDB/50017/2020 + UIDP/50017/2020) and to RNEM, Portuguese Mass Spectrometry Network, (LISBOA-01-0145-FEDER-402-022125) through national funds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement. Tiago Alexandre Conde (2020.05678.BD) to FCT for his grant. Daniela Salomé Moderno do Couto (SFRH/BD/138992/2018) is grateful to FCT for her grant. Tânia Melo thanks the research contract under the project Omics 4 Algae: Lipidomic tools for chemical phenotyping, traceability and valorisation of seaweeds from aquaculture as a sustainable source of high added-value compounds (POCI-01-0145-FEDER-030962), funded by Centro2020, through FEDER and PT2020. This is a contribution of Marine Lipidomics laboratory. This work has received funding under the project AlgaValor, from the Portugal 2020 program (Grant agreement nos. POCI-01-0247-FEDER-035234; LISBOA-01-0247-FEDER-035234; ALG-01-0247-FEDER-035234).
Author contributions
Conceptualization and design: T.A.C, D.C., P.D.; and M.R.D.; methodology and formal analysis: T.A.C, D.C., P.D., M.R.D. and T.M.; validation, T.A.C., D.C., P.D., M.R.D. and T.M.; collection and assembly of data: T.A.C., D.C., P.D., M.R.D. and T.M.; writing—original draft preparation: T.A.C., D.C., P.D., M.R.D. and T.M.; All authors reviewed, read and approved the published version of the manuscript.
Data availability
Raw datasets generated during this study are available from the corresponding authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1038/s41598-021-83455-y.
References
- 1.Zhu LD, Li ZH, Hiltunen E. Strategies for lipid production improvement in microalgae as a biodiesel feedstock. Biomed. Res. Int. 2016;2016:1–8. doi: 10.1155/2016/8792548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nations U. Transforming Our World: United Nations the 2030 Agenda for Sustainable Development. In: United Nations Sustainable Development Summit. 2015.
- 3.Khan MI, Shin JH, Kim JD. The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb. Cell Fact. 2018;17(1):1–21. doi: 10.1186/s12934-018-0879-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ravindran B, Gupta SK, Cho WM, Kim JK, Lee SR, Jeong KH, Lee DJ, Choi HC. Microalgae potential and multiple roles-current progress and future prospects-an overview. Sustainability. 2016;8(12):1–16. doi: 10.3390/su8121215. [DOI] [Google Scholar]
- 5.Hamed I. The evolution and versatility of microalgal biotechnology: A review. Compr. Rev. Food Sci. Food Saf. 2016;15(6):1104–1123. doi: 10.1111/1541-4337.12227. [DOI] [PubMed] [Google Scholar]
- 6.Koller M, Muhr A, Braunegg G. Microalgae as versatile cellular factories for valued products. Algal Res. 2014;6:52–63. doi: 10.1016/j.algal.2014.09.002. [DOI] [Google Scholar]
- 7.Afzal, I., Shahid, A., Ibrahim, M., Liu, T., Nawaz, M., & Mehmood, M.A. Microalgae: A promising feedstock for energy and high-value products. Algae based polymers, blends, and composites: chemistry, biotechnology and materials science. Elsevier, pp. 55–75 (2017).
- 8.Mata TM, Martins AA, Caetano NS. Microalgae for biodiesel production and other applications: A review. Renew. Sustain. Energy Rev. 2010;14(1):217–232. doi: 10.1016/j.rser.2009.07.020. [DOI] [Google Scholar]
- 9.Pulz O, Gross W. Valuable products from biotechnology of microalgae. Appl. Microbiol. Biotechnol. 2004;65:635–648. doi: 10.1007/s00253-004-1647-x. [DOI] [PubMed] [Google Scholar]
- 10.Patil V, Tran KQ, Giselrød HR. Towards sustainable production of biofuels from microalgae. Int. J. Mol. Sci. 2008;9(7):1188–1195. doi: 10.3390/ijms9071188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ganesan VSH. Biomass from microalgae: an overview. Oceanogr. Open Access. 2014;2(1):1–7. [Google Scholar]
- 12.Acién Fernández FG, Gómez-Serrano C, Fernández-Sevilla JM. Recovery of nutrients from wastewaters using microalgae. Front. Sustain. Food Syst. 2018;2(59):1–13. [Google Scholar]
- 13.Kay RA, Barton LL. Microalgae as Food and Supplement. Crit. Rev. Food Sci. Nutr. 1991;30(6):555–573. doi: 10.1080/10408399109527556. [DOI] [PubMed] [Google Scholar]
- 14.da Vaz B, S, Moreira JB, Morais MG de, Costa JAV, Microalgae as a new source of bioactive compounds in food supplements. Curr. Opin. Food Sci. 2016;7:73–77. doi: 10.1016/j.cofs.2015.12.006. [DOI] [Google Scholar]
- 15.Ahmmed MK, Ahmmed F, Tian H, Carne A, Bekhit AED. Marine omega-3 (n-3) phospholipids: A comprehensive review of their properties, sources, bioavailability, and relation to brain health. Compr. Rev. Food. Sci. Food Saf. 2020;19(1):64–123. doi: 10.1111/1541-4337.12510. [DOI] [PubMed] [Google Scholar]
- 16.da Costa E, Silva J, Mendonça SH, Abreu MH, Domingues MR. Lipidomic approaches towards deciphering glycolipids from microalgae as a reservoir of bioactive lipids. Mar. Drugs. 2016;14(5):1. doi: 10.3390/md14050101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schneider H, Braun A, Füllekrug J, Stremmel W, Ehehalt R. Lipid based therapy for ulcerative colitis-modulation of intestinal mucus membrane phospholipids as a tool to influence inflammation. Int. J. Mol. Sci. 2010;11(10):4149–4164. doi: 10.3390/ijms11104149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lagarde M, Bernoud N, Thiès F, Brossard N, Lemaitre-Delaunay D, Croset M, Lecerf J. Lysophosphatidylcholine as a carrier of docosahexaenoic acid to target tissues. World Rev. Nutr. Diet. 2001;88:173–177. doi: 10.1159/000059750. [DOI] [PubMed] [Google Scholar]
- 19.Picq M, Chen P, Perez M, Michaud M, Véricel E, Guichardant M, Lagarde M. DHA metabolism: Targeting the brain and lipoxygenation. Mol. Neurobiol. 2010;42(1):48–51. doi: 10.1007/s12035-010-8131-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lopes G, Daletos G, Proksch P, Andrade PB, Valentão P. Anti-inflammatory potential of monogalactosyl diacylglycerols and a monoacylglycerol from the edible brown seaweed Fucus spiralis linnaeus. Mar Drugs. 2014;12(3):1406–1418. doi: 10.3390/md12031406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Banskota AH, Stefanova R, Sperker S, Lall SP, Craigie JS, Hafting JT, Critchley AT. Polar lipids from the marine macroalga Palmaria palmata inhibit lipopolysaccharide-induced nitric oxide production in RAW264.7 macrophage cells. Phytochemistry. 2014;101:101–108. doi: 10.1016/j.phytochem.2014.02.004. [DOI] [PubMed] [Google Scholar]
- 22.Hossain Z, Kurihara H, Hosokawa M, Takahashi K. Growth inhibition and induction of differentiation and apoptosis mediated by sodium butyrate in Caco-2 cells with algal glycolipids. Vitr Cell Dev. Biol. Anim. 2005;41(5–6):154–159. doi: 10.1290/0409058.1. [DOI] [PubMed] [Google Scholar]
- 23.Eitsuka T, Nakagawa K, Igarashi M, Miyazawa T. Telomerase inhibition by sulfoquinovosyldiacylglycerol from edible purple laver (Porphyra yezoensis) Cancer Lett. 2004;212(1):15–20. doi: 10.1016/j.canlet.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 24.Yao L, Gerde JA, Lee SL, Wang T, Harrata KA. Microalgae lipid characterization. J Agric Food Chem. 2015;63(6):1773–1787. doi: 10.1021/jf5050603. [DOI] [PubMed] [Google Scholar]
- 25.Li S, Xu J, Chen J, Chen J, Zhou C, Yan X. The major lipid changes of some important diet microalgae during the entire growth phase. Aquaculture. 2014;428–429:104–110. doi: 10.1016/j.aquaculture.2014.02.032. [DOI] [Google Scholar]
- 26.Meng Y, Cao X, Yao C, Xue S, Yang Q. Identification of the role of polar glycerolipids in lipid metabolism and their acyl attribution for TAG accumulation in Nannochloropsis oceanica. Algal Res. 2017;24:122–129. doi: 10.1016/j.algal.2017.03.004. [DOI] [Google Scholar]
- 27.Liu P, Corilo YE, Marshall AG. Polar lipid composition of biodiesel algae candidates nannochloropsis oculata and haematococcus pluvialis from nano liquid chromatography coupled with negative electrospray ionization 14.5 T fourier transform ion cyclotron resonance mass spe. Energy Fuels. 2016;30(10):8270–8276. doi: 10.1021/acs.energyfuels.6b01514. [DOI] [Google Scholar]
- 28.Jouhet J, Lupette J, Clerc O, Magneschi L, Bedhomme M, Collin S, Roy S, Maréchal E, Rébeillé F. LC–MS/MS versus TLC plus GC methods: Consistency of glycerolipid and fatty acid profiles in microalgae and higher plant cells and effect of a nitrogen starvation. PLoS ONE. 2017;12(8):e0182423. doi: 10.1371/journal.pone.0182423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hasan CMM, Aftabuddin S, Sharif M, Khan M. Triacylglycerol profile of a microalga Chlorococcum sp. as a potential biofuel feedstock. J. Bangladesh Acad. Sci. 2016;40(2):147–153. doi: 10.3329/jbas.v40i2.30770. [DOI] [Google Scholar]
- 30.Lv J, Wang X, Liu W, Feng J, Liu Q, Nan F, Jiao X, Xie S. The performance of a self-flocculating microalga Chlorococcum sp GD in wastewater with different ammonia concentrations. Int. J. Environ. Res. Public Health. 2018;15(3):434. doi: 10.3390/ijerph15030434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ambati RR, Gogisetty D, Aswathanarayana RG, Ravi S, Bikkina PN, Bo L, Yuepeng S. Industrial potential of carotenoid pigments from microalgae: Current trends and future prospects. Crit. Rev. Food Sci. Nutr. 2019;59(12):1880–1902. doi: 10.1080/10408398.2018.1432561. [DOI] [PubMed] [Google Scholar]
- 32.Ghosh A, Khanra S, Mondal M, Devi TI, Halder G, Tiwari ON, Bhowmick TK, Gayen K. Biochemical characterization of microalgae collected from north east region of India advancing towards the algae-based commercial production. Asia Pacific J. Chem. Eng. 2017;12(5):745–754. doi: 10.1002/apj.2114. [DOI] [Google Scholar]
- 33.Sassi KKB, da Silva JA, Calixto CD, Sassi R, da Sassi CF. Metabolites of interest for food technology produced by microalgae from the Northeast Brazil. Rev Ciência Agronômica. 2019;50(1):54–65. [Google Scholar]
- 34.Correia N, Pereira H, Silva JT, Soares M, Sousa CB, Schüler LM, Costa M, Pereira L. Isolation, identification and biotechnological applications of a novel, robust, free-living Chlorococcum (Oophila) amblystomatis strain isolated from a local pond. Appl. Sci. 2020;10(9):3040–3054. doi: 10.3390/app10093040. [DOI] [Google Scholar]
- 35.Weiss, A., Johannisbauer, W., Gutsche, B., Cordero, B., Martin, L., Rodriguez, H., & Vargas, M.A. Obraztsova I. Process for obtaining lutein from algae. US; p. U.S. Patent No. 20070196893A1 12 january 2006.
- 36.Idrissi Abdelkhalek, E. A., Mohamed, B., Mohammed, A., & Lotfi, A. Growth performance and biochemical composition of nineteen microalgae collected from different Moroccan reservoirs. Mediterr Mar. Sci. 17(1):323 (2016).
- 37.Liu, B.H., & Lee, Y.K. Secondary carotenoids formation by the green alga Chlorococcum sp. J. Appl. Phycol.1, 301–307 (2000).
- 38.Prabakaran P, Tech DR-CJ. Selection of microalgae for accumulation of lipid production under different growth conditions. Chemistry (Easton) 1:131–7 (2013).
- 39.Mahapatra DM, Ramachandra TV. Algal biofuel: Bountiful lipid from chlorococcum sp. proliferating in municipal wastewater. Curr. Sci. 2013;105(1):47–55. [Google Scholar]
- 40.Harwati TU, Willke T, Vorlop KD. Characterization of the lipid accumulation in a tropical freshwater microalgae Chlorococcum sp. Bioresour. Technol. 2012;121:54–60. doi: 10.1016/j.biortech.2012.06.098. [DOI] [PubMed] [Google Scholar]
- 41.Yang M, Meng Y, Chu Y, Fan Y, Cao X, Xue S, Chi Z. Triacylglycerol accumulates exclusively outside the chloroplast in short-term nitrogen-deprived Chlamydomonas reinhardtii. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863(12):1478–1487. doi: 10.1016/j.bbalip.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 42.White D, Rooks P, Kimmance S, Tait K, Jones M, Tarran G, Cook C, Llewellyn C. Modulation of polar lipid profiles in Chlorella sp. response to nutrient limitation. Metabolites. 2019;9(3):39. doi: 10.3390/metabo9030039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Řezanka T, Podojil M. Preparative separation of algal polar lipids and of individual molecular species by high-performance liquid chromatography and their identification by gas chromatography—mass spectrometry. J. Chromatogr. A. 1989;463:397–408. doi: 10.1016/S0021-9673(01)84492-7. [DOI] [PubMed] [Google Scholar]
- 44.da Costa E, Melo T, Moreira ASP, Alves E, Domingues P, Calado R, Abreu MH, Domingues MR. Decoding bioactive polar lipid profile of the macroalgae Codium tomentosum from a sustainable IMTA system using a lipidomic approach. Algal Res. 2015;12:388–397. doi: 10.1016/j.algal.2015.09.020. [DOI] [Google Scholar]
- 45.Melo T, Alves E, Azevedo V, Martins AS, Neves B, Domingues P, Calado R, Abreu MH, Domingues MR. Lipidomics as a new approach for the bioprospecting of marine macroalgae: Unraveling the polar lipid and fatty acid composition of chondrus crispus. Algal Res. 2015;8:181–191. doi: 10.1016/j.algal.2015.02.016. [DOI] [Google Scholar]
- 46.da Costa E, Domingues P, Melo T, Coelho E, Pereira R, Calado R, Abreu MH, Domingues MR. Lipidomic Signatures Reveal Seasonal Shifts on the Relative Abundance of High-Valued Lipids from the Brown Algae Fucus vesiculosus. Mar Drugs. 2019;17(6):335. doi: 10.3390/md17060335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lopes D, Moreira ASP, Rey F, da Costa E, Melo T, Maciel E, Rego A, Abreu MH, Domingues P, Calado R, Lillebø AI, Rosário Domingues M. Lipidomic signature of the green macroalgae Ulva rigida farmed in a sustainable integrated multi-trophic aquaculture. J. Appl. Phycol. 2018;1:1–13. [Google Scholar]
- 48.Rey F, Lopes D, Maciel E, Monteiro J, Skjermo J, Funderud J, Raposo D, Domingues P, Calado R, Domingues MR Polar lipid profile of Saccharina latissima, a functional food from the sea. Algal Res. 2019;39:101473. doi: 10.1016/j.algal.2019.101473. [DOI] [Google Scholar]
- 49.Couto D, Santinha D, Melo T, Ferreira-Fernandes E, Videira RA, Campos A, Fardilha M, Domingues P, Domingues MRM. Glycosphingolipids and oxidative stress: Evaluation of hydroxyl radical oxidation of galactosyl and lactosylceramides using mass spectrometry. Chem. Phys. Lipids. 2015;191:106–114. doi: 10.1016/j.chemphyslip.2015.08.014. [DOI] [PubMed] [Google Scholar]
- 50.Couto D, Melo T, Maciel E, Campos A, Alves E, Guedes S, Domingues MRM, Domingues P. New insights on non-enzymatic oxidation of ganglioside GM1 using mass spectrometry. J. Am. Soc. Mass Spectrom. 2016;27(12):1. doi: 10.1007/s13361-016-1474-1. [DOI] [PubMed] [Google Scholar]
- 51.Han X, Gross RW. Electrospray ionization mass spectroscopic analysis of human erythrocyte plasma membrane phospholipids. Proc. Natl. Acad. Sci. USA. 1994;91(22):10635–10639. doi: 10.1073/pnas.91.22.10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hermansson M, Uphoff A, Käkelä R, Somerharju P. Automated quantitative analysis of complex lipidomes by liquid chromatography/mass spectrometry. Anal. Chem. 2005;77(7):2166–2175. doi: 10.1021/ac048489s. [DOI] [PubMed] [Google Scholar]
- 53.Ejsing CS, Duchoslav E, Sampaio J, Simons K, Bonner R, Thiele C, Ekroos K, Shevchenko A. Automated identification and quantification of glycerophospholipid molecular species by multiple precursor ion scanning. Anal. Chem. 2006;78(17):6202–6214. doi: 10.1021/ac060545x. [DOI] [PubMed] [Google Scholar]
- 54.Figueiredo ARP, da Costa E, Silva J, Domingues MR, Domingues P. The effects of different extraction methods of lipids from Nannochloropsis oceanica on the contents of omega-3 fatty acids. Algal Res. 2019;41(12):1. [Google Scholar]
- 55.Olaizola, M., Grewe, C. Commercial microalgal cultivation systems. In: Grand challenges in algae biotechnology. p. 3–34 (2019).
- 56.Do NH, Ramli A, Kee LM. A review on methods used in analysis of microalgae lipid composition. J. Japan Inst. Energy. 2017;96(12):532–537. doi: 10.3775/jie.96.532. [DOI] [Google Scholar]
- 57.Mankad D, Dupuis A, Smile S, Roberts W, Brian J, Lui T, Genore L, Zaghloul D, Iaboni A, Marcon PMA, Anagnostou E. A randomized, placebo controlled trial of omega-3 fatty acids in the treatment of young children with autism. Mol. Autism. 2015;6(1):1–11. doi: 10.1186/s13229-015-0010-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Barros R, Moreira A, Fonseca J, Delgado L, Graça Castel-Branco M, Haahtela T, Lopes C, Moreira P. Dietary intake of α-linolenic acid and low ratio of n-6:n-3 PUFA are associated with decreased exhaled NO and improved asthma control. Br. J. Nutr. 2011;106(3):441–450. doi: 10.1017/S0007114511000328. [DOI] [PubMed] [Google Scholar]
- 59.Rennie KL, Hughes J, Lang R, Jebb SA. Nutritional management of rheumatoid arthritis: A review of the evidence. J. Hum. Nutr. Diet. 2003;16(2):97–109. doi: 10.1046/j.1365-277X.2003.00423.x. [DOI] [PubMed] [Google Scholar]
- 60.Šimat V, Bogdanović T, Poljak V, Petričević S. Changes in fatty acid composition, atherogenic and thrombogenic health lipid indices and lipid stability of bogue (Boops boops Linnaeus, 1758) during storage on ice: Effect of fish farming activities. J. Food Compos. Anal. 2015;40:120–125. doi: 10.1016/j.jfca.2014.12.026. [DOI] [Google Scholar]
- 61.Matos ÂP, Feller R, Moecke EHS, de Oliveira JV, Junior AF, Derner RB. Chemical characterization of six microalgae with potential utility for food application. J. Am. Oil Chem. Soc. 2016;93(7):963–972. doi: 10.1007/s11746-016-2849-y. [DOI] [Google Scholar]
- 62.Marques B, Lillebø AI, Domingues M. Effect of high-pressure processing (HPP) on the fatty acid profile of different sized Ragworms (Hediste diversicolor) cultured in an integrated multi-trophic aquaculture (IMTA) system. Molecules. 2019;24(24):1–11. doi: 10.3390/molecules24244503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lopes, D., Melo, T., Rey, F., Meneses, J., Monteira, F.L., Helguero, L.A., Abreu, M.H., Lillebø, A.I., Calado, R., & Domingues, M.R. Valuing bioactive lipids from green, red and brown macroalgae from aquaculture, to foster functionality and biotechnological applications. Molecules (2020). [DOI] [PMC free article] [PubMed]
- 64.Łuczyńska J, Paszczyk B, Nowosad J, Łuczyński MJ. Mercury, fatty acids content and lipid quality indexes in muscles of freshwater and marine fish on the polish market: Risk assessment of fish consumption. Int. J. Environ. Res. Public Health. 2017;14(10):1–17. doi: 10.3390/ijerph14101120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Magdugo RP, Terme N, Lang M, Pliego-Cortés H, Marty C, Hurtado AQ, Bedoux G, Bourgougnon N. An analysis of the nutritional and health values of Caulerpa racemosa (Forsskål) and Ulva fasciata (Delile)—Two chlorophyta collected from the Philippines. Molecules. 2020;25(12):1–23. doi: 10.3390/molecules25122901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Rueda FM, Hernández MD, Egea MA, Aguado F, García B, Martínez FJ. Differences in tissue fatty acid composition between reared and wild sharpsnout sea bream, Diplodus puntazzo (Cetti, 1777) Br. J. Nutr. 2001;86(5):617–622. doi: 10.1079/BJN2001438. [DOI] [PubMed] [Google Scholar]
- 67.Valfré F, Caprino F, Turchini GM. The health benefit of seafood. Vet. Res. Commun. 2003;27(1):507–512. doi: 10.1023/B:VERC.0000014208.47984.8c. [DOI] [PubMed] [Google Scholar]
- 68.Maciel E, Costa Leal M, Lillebø AI, Domingues P, Domingues MR, Calado R. Bioprospecting of marine macrophytes using MS-based lipidomics as a new approach. Mar. Drugs. 2016;14(3):1. doi: 10.3390/md14030049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Burri L, Hoem N, Banni S, Berge K. Marine Omega-3 phospholipids: Metabolism and biological activities. Int. J. Mol. Sci. 2012;13(11):15401–15419. doi: 10.3390/ijms131115401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gabbs M, Leng S, Devassy JG, Aukema HM. Advances in our understanding of oxylipins. Am. Soc. Nutr. 2015;6:513–540. doi: 10.3945/an.114.007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.De Los RC, Ávila-Román J, Ortega MJ, De La Jara A, García-Mauriño S, Motilva V, Zubía E. Oxylipins from the microalgae Chlamydomonas debaryana and Nannochloropsis gaditana and their activity as TNF-α inhibitors. Phytochemistry. 2014;102:152–161. doi: 10.1016/j.phytochem.2014.03.011. [DOI] [PubMed] [Google Scholar]
- 72.Banskota AH, Stefanova R, Sperker S, McGinn PJ. New diacylglyceryltrimethylhomoserines from the marine microalga Nannochloropsis granulata and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013;25(5):1513–1521. doi: 10.1007/s10811-012-9967-1. [DOI] [Google Scholar]
- 73.Bergé JP, Debiton E, Dumay J, Durand P, Barthomeuf C. In vitro anti-inflammatory and anti-proliferative activity of sulfolipids from the red alga Porphyridium cruentum. J. Agric. Food Chem. 2002;50(21):6227–6232. doi: 10.1021/jf020290y. [DOI] [PubMed] [Google Scholar]
- 74.Winget, R.R. Anti-inflammatory compositions containing monogalactosyl dieicosapentaenoyl glycerol and methods relating thereto. United States of America; 5,767,095.
- 75.Ohta K, Mizushina Y, Hirata N, Takemura M, Sugawara F, Matsukage A, Yoshida S, Sakaguchi K. Action of a new mammalian DNA polymerase inhibitor, sulfoquinovosyldiacylglycerol. Biol. Pharm. Bull. 1999;22(2):111–116. doi: 10.1248/bpb.22.111. [DOI] [PubMed] [Google Scholar]
- 76.Banskota AH, Stefanova R, Gallant P, McGinn PJ. Mono- and digalactosyldiacylglycerols: Potent nitric oxide inhibitors from the marine microalga Nannochloropsis granulata. J. Appl. Phycol. 2013;25(2):349–357. doi: 10.1007/s10811-012-9869-2. [DOI] [Google Scholar]
- 77.Banskota AH, Gallant P, Stefanova R, Melanson R, O’Leary SJB. Monogalactosyldiacylglycerols, potent nitric oxide inhibitors from the marine microalga Tetraselmis chui. Nat. Prod. Res. 2013;27(12):1084–1090. doi: 10.1080/14786419.2012.717285. [DOI] [PubMed] [Google Scholar]
- 78.Banskota AH, Stefanova R, Sperker S, Melanson R, Osborne JA, O’Leary SJB. Five new galactolipids from the freshwater microalga Porphyridium aerugineum and their nitric oxide inhibitory activity. J. Appl. Phycol. 2013;25(4):951–960. doi: 10.1007/s10811-012-9935-9. [DOI] [Google Scholar]
- 79.Bruno A, Rossi C, Marcolongo G, Di Lena A, Venzo A, Berrie CP, Corda D. Selective in vivo anti-inflammatory action of the galactolipid monogalactosyldiacylglycerol. Eur. J. Pharmacol. 2005;524(1–3):159–168. doi: 10.1016/j.ejphar.2005.09.023. [DOI] [PubMed] [Google Scholar]
- 80.Wang H, Li YL, Shen WZ, Rui W, Ma XJ, Cen YZ. Antiviral activity of a sulfoquinovosyldiacylglycerol (SQDG) compound isolated from the green alga Caulerpa racemosa. Bot. Mar. 2007;50(3):185–190. [Google Scholar]
- 81.Maeda N, Kokai Y, Ohtani S, Sahara H, Hada T, Ishimaru C, Kuriyama I, Yonezawa Y, Iijima H, Yoshida H, Sato N, Mizushina Y. Anti-tumor effects of the glycolipids fraction from spinach which inhibited DNA polymerase activity. Nutr. Cancer. 2007;57(2):216–223. doi: 10.1080/01635580701277908. [DOI] [PubMed] [Google Scholar]
- 82.Tsai CJ, Sun PB. Identification of sulfoglycolipid bioactivities and characteristic fatty acids of marine macroalgae. J. Agric. Food Chem. 2012;60(34):8404–8410. doi: 10.1021/jf302241d. [DOI] [PubMed] [Google Scholar]
- 83.El Baz FK, El Baroty GS, Abd El Baky HH, Abd El-Salam OI, Ibrahim EA. Structural characterization and biological activity of sulfolipids from selected marine algae. Grasas Aceites. 2013;64(5):561–571. doi: 10.3989/gya.050213. [DOI] [Google Scholar]
- 84.Xu D-P, Li Y, Meng X, Zhou T, Zhou Y, Zheng J, Zhang J-J, Li H-B. Natural antioxidants in foods and medicinal plants: Extraction, assessment and resources. Int. J. Mol. Sci. 2017;18(1):1. doi: 10.3390/ijms18010096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Antioxidant MS, Applications I. Res. Rev. J. Pharmacol. Toxicol. Stud. 2016;4(4):1–10. [Google Scholar]
- 86.Banskota AH, Sperker S, Stefanova R, McGinn PJ, O’Leary SJB. Antioxidant properties and lipid composition of selected microalgae. J. Appl. Phycol. 2019;31(1):309–318. doi: 10.1007/s10811-018-1523-1. [DOI] [Google Scholar]
- 87.Cardoso C, Pereira H, Franca J, Matos J, Monteiro I, Pousão-Ferreira P, Gomes A, Barreira L, Varela J, Neng N, Nogueira JM, Afonso C, Bandarra NM. Lipid composition and some bioactivities of 3 newly isolated microalgae (Tetraselmis sp. IMP3, Tetraselmis sp. CTP4, and Skeletonema sp.) Aquac Int. 2020;28(2):711–727. doi: 10.1007/s10499-019-00489-w. [DOI] [Google Scholar]
- 88.Esmaeili A, Khakpoor M. Biological activities and chemical composition of solvent extracts of Stoechospermum marginatum (C. Agardh) Acta Biochim. Pol. 2012;59(4):581–585. doi: 10.18388/abp.2012_2095. [DOI] [PubMed] [Google Scholar]
- 89.Ricciotti E, Fitzgerald GA. Prostaglandins and inflammation. Arterioscler. Thromb. Vasc. Biol. 2011;31(5):986–1000. doi: 10.1161/ATVBAHA.110.207449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Aued-Pimentel S, Lago JHG, Chaves MH, Kumagai EE. Evaluation of a methylation procedure to determine cyclopropenoids fatty acids from Sterculia striata St Hil. Et Nauds seed oil. J. Chromatogr. A. 2004;1054(1–2):235–239. doi: 10.1016/j.chroma.2004.07.090. [DOI] [PubMed] [Google Scholar]
- 91.Barros A, Guerra LT, Simões M, Santos E, Fonseca D, Silva J, Costa L, Navalho J. Mass balance analysis of carbon and nitrogen in industrial scale mixotrophic microalgae cultures. Algal Res. 2017;21:35–41. doi: 10.1016/j.algal.2016.10.014. [DOI] [Google Scholar]
- 92.Folch J, Lees M, Sloane SG. A simple method for the isolation and purification of total lipids from animal tissues. J. Biol. Chem. 1957;226:497–509. doi: 10.1016/S0021-9258(18)64849-5. [DOI] [PubMed] [Google Scholar]
- 93.Bonfanti C, Cardoso C, Afonso C, Matos J, Garcia T, Tanni S, Bandarra NM. Potential of microalga Isochrysis galbana: Bioactivity and bioaccessibility. Algal. Res. 2017;2018(29):242–248. [Google Scholar]
- 94.Magalhães LM, Segundo MA, Reis S, Lima JLFC. Automatic method for determination of total antioxidant capacity using 2,2-diphenyl-1-picrylhydrazyl assay. Anal. Chim. Acta. 2006;558(1–2):310–318. doi: 10.1016/j.aca.2005.11.013. [DOI] [Google Scholar]
- 95.Melo T, Marques SS, Ferreira I, Cruz MT, Domingues P, Segundo MA, Domingues MRM. New insights into the anti-inflammatory and antioxidant properties of nitrated phospholipids. Lipids. 2018;53(1):117–131. doi: 10.1002/lipd.12007. [DOI] [PubMed] [Google Scholar]
- 96.Magalhães LM, Barreiros L, Maia MA, Reis S, Segundo MA. Rapid assessment of endpoint antioxidant capacity of red wines through microchemical methods using a kinetic matching approach. Talanta. 2012;97:473–483. doi: 10.1016/j.talanta.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 97.Ulbricht T. V., & Southgate, D. A. T. Coronary heart disease and dietary factors. Lancet338, 985–992. [DOI] [PubMed]
- 98.Lopes, D., Melo, T., Meneses, J., Abreu, M.H., Pereira, R., Domingues, P., Lillebø, A. I., Calado, R., & Rosário Domingues M. A new look for the red macroalga palmaria palmata: a seafood with polar lipids rich in EPA and with antioxidant properties. Mar. Drugs. 17(9) (2019). [DOI] [PMC free article] [PubMed]
- 99.Moreira ASP, da Costa E, Melo T, Sulpice R, Cardoso SM, Pitarma B, Pereira R, Abreu MH, Domingues P, Calado R, Domingues MR. Seasonal plasticity of the polar lipidome of Ulva rigida cultivated in a sustainable integrated multi-trophic aquaculture. Algal Res. 2020;49:101958. doi: 10.1016/j.algal.2020.101958. [DOI] [Google Scholar]
- 100.Monteiro JP, Rey F, Melo T, Moreira ASP, Arbona JF, Skjermo J, Forbord S, Funderud J, Raposo D, Kerrison PD, Perrineau MM, Gachon C, Domingues P, Calado R, Domingues MR The unique lipidomic signatures of saccharina latissima can be used to pinpoint their geographic origin. Biomolecules. 2020;10(1):1–17. doi: 10.3390/biom10010107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.da Costa, E., Amaro, H. M., Melo, T., Guedes, A. C., & Domingues, M.R. Screening for polar lipids, antioxidant, and anti-inflammatory activities of Gloeothece sp. lipid extracts pursuing new phytochemicals from cyanobacteria. J. Appl. Phycol. (2020).
- 102.Pluskal T, Castillo S, Villar-Briones A, Orešič M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinf. 2010;11(395):1–11. doi: 10.1186/1471-2105-11-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Raw datasets generated during this study are available from the corresponding authors upon reasonable request.