Abstract
Thrombotic complications are common in COVID-19 patients, but cerebral venous system involvement, timing after infection, optimal treatment, and long-term outcome are uncertain. We report a case of massive cerebral venous thrombosis and concomitant internal iliac vein thrombosis occurring in the late phase of paucisymptomatic COVID-19 infection. Mild respiratory symptoms, without fever, started 3 weeks before headache and acute neurological deficits. The patient had silent hypoxemia and typical COVID-19 associated interstitial pneumonia. Brain CT scan showed a left parietal hypodense lesion with associated sulcal subarachnoid hemorrhage. CT cerebral venography showed a massive cerebral venous thrombosis involving the right transverse sinus, the right jugular bulb, the superior sagittal sinus, the straight sinus, the vein of Galen, and both internal cerebral veins. Abdominal CT scan showed no malignancy but revealed an asymptomatic right internal iliac vein thrombosis. Both cerebral venous thrombosis and pelvic vein thrombosis were effectively treated with unfractionated heparin started on the day of admission, then shifted to low molecular weight heparin, with a favorable clinical course. Nasopharyngel swab, repeated twice, tested negative for SARS-CoV-2. Serological tests confirmed SARS-CoV-2 infection. Our case supports active surveillance and prevention of thrombotic complications associated with COVID-19, which may affect both peripheral and cerebral venous system. Early initiation of unfractionated heparin may lead to good neurologic outcome.
Keywords: COVID-19, cerebral venous thrombosis, internal iliac vein thrombosis, anticoagulation, thromboinflammation
Introduction
At the time of this writing, health care systems are facing worldwide the pandemic of the coronavirus SARS-CoV-2 and its associated disease, named COVID-19. Although COVID-19 mostly affects the respiratory system, ranging from mild flu-like symptoms to severe pneumonia, coagulopathy is a common feature of the disease and it is highly associated with poor prognosis (1, 2). Here, we report the case of a concomitant massive cerebral venous thrombosis and internal iliac vein thrombosis occurring in the late phase of a paucisymptomatic COVID-19 infection.
Case Description
A 62 years old female patient was referred to the emergency room for acute onset of confusion, dysarthria, and right limbs weakness. In the previous 3 weeks she had dry cough, fatigue, and loss of appetite, without fever. In the last few days, she complained of a headache. No abdominal or pelvic pain was reported. Her medical history was limited to arterial hypertension. Neurological examination showed a moderately agitated patient with global aphasia, right-sided neglect, and a severe hypotonic right hemiparesis (Medical Research Council power scale 1/5 at right upper limb and 0/5 at right lower limb). Arterial blood gases showed hypoxia (paO2 59 mmHg breathing room air), despite she had no dyspnea. D-dimer levels were high (2,768 ng/mL) and reactive C protein was moderately elevated (19.45 mg/dL).
Diagnostic Assessment
Brain CT showed a left parietal hypodense lesion and a sulcal subarachnoid hemorrhage over the left temporal lobe. CT cerebral venography showed a massive cerebral venous thrombosis (CVT) involving the right transverse sinus, the right jugular bulb, the superior sagittal sinus, the straight sinus, the vein of Galen, and both internal cerebral veins (Figure 1A). Chest CT showed multiple bilateral ground-glass opacities and consolidations typical of COVID-19 pneumonia (Figure 2A). Infectious disease specialist was consulted and diagnosed COVID-19 on the base of typical chest CT findings, blood tests and recent history of cough and malaise. Nasopharyngel swab, repeated twice, tested negative for SARS-CoV-2. Serological test confirmed SARS-Cov-2 infection. A diagnosis of massive CVT associated with COVID-19 was made and she was admitted to the Acute Stroke Unit. On the day of admission, anticoagulation therapy was started with full-dose intravenous unfractionated heparin (UFH; 5,000 units bolus, followed by 1,000 units/hour, adjusted to aPTT), then shifted to subcutaneous enoxaparin (1 mg/kg every 12 h) after 5 days. Antibiotics (ceftriaxone and azithromycin), anti-viral therapy (lopinavir + ritonavir), and hydroxychloroquine were also started on the same day. Low dose supplemental oxygen (24–28%) was administered, with no need of further respiratory support. Contrast-enhanced abdominal and pelvic CT scan showed no malignancy but revealed a right internal iliac vein thrombosis (Figure 2B). Both CVT and iliac vein thrombosis responded well to anticoagulation therapy. Clinical course was favorable with gradual improvement in language, cognition, and right motor deficit. Follow-up brain CT and CT angiography showed reduction of venous infarct volume, resolution of subarachnoid hemorrhage and partial venous recanalization (Figure 1B). She was discharged 3 weeks later in a rehabilitation center with the advice to continue anticoagulation therapy and was shifted to warfarin. She regained functional independence and was discharged home 3 months after admission. Thrombophilia screening was negative for antithrombin deficiency, factor II and V mutations, antiphospholipid antibodies, and hyperhomocysteinemia. Protein C and protein S were not assayed, since interference with anticoagulation therapy prevented their interpretation.
Figure 1.
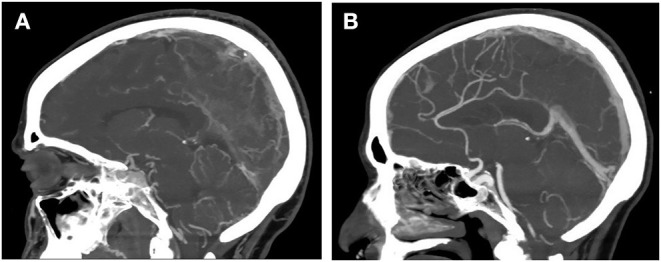
CT cerebral venography showing massive cerebral venous thrombosis associated with COVID-19 infection. (A) At admission. (B) After 3 weeks of anticoagulation therapy.
Figure 2.
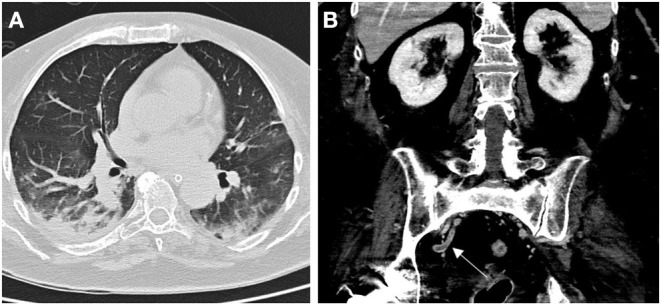
Chest CT scan showing typical COVID-19 associated bilateral pneumonia ad admission (A). Contrast-enhanced abdominal CT scan showing right internal vein thrombosis (arrow; B).
Discussion
We report a probable correlation between COVID-19 infection and concomitant thromboses of cerebral venous sinuses and internal iliac vein, the latter being a rare site for deep vein thrombosis with a significant risk for pulmonary embolism (3). Our case suggests that hypercoagulability and thromboinflammation associated with COVID-19 (4) may cause venous thrombosis at multiple unusual sites, even in the late post-viremic phase of a mild respiratory infection (when nasopharingeal swabs could give a negative result). Since the beginning of the pandemic, rare cases of CVT associated with COVID-19 have been reported (5–12). Individual patient data available are reported in Table 1. Although previous reports lack some clinical details, available data suggests that CVT is more frequent in middle-aged COVID-19 patients, presenting with altered mental status several days after the onset of a mild-to-moderate respiratory syndrome. In most of these cases, short-term outcome was in-hospital mortality (50%) or it was unreported (15%). In-hospital mortality or unreported outcome was even higher (75%) when internal cerebral veins were involved, as in our patient. Concomitant CVT and pelvic vein thromboses have not been previously reported in COVID-19 patients, probably reflecting the difficulty in diagnosis of pelvic thrombosis. However, in such cases detection of pelvic thrombosis is important to guide treatment intensity and duration. In our case, both CVT and internal iliac vein thrombosis were effectively treated with early intravenous UFH followed by enoxaparin and warfarin, leading to an excellent neurological recovery and prevention of pulmonary embolism. Although early initiation of anticoagulation therapy, immediately after diagnosis in the emergency room, partially limited hypercoagulability testing, we believe that early treatment influenced outcome and should not be delayed. Our case further supports active surveillance and prevention of hypercoagulability associated with COVID-19, even in patients with mild symptoms.
Table 1.
Clinical features of reported individual patients with cerebral venous thrombosis related to COVID-19 infection.
| Patient (Ref.) | Age | Sex | Time from COVID-19 symptoms (days) | COVID-19 respiratory syndrome | Altered mental status at presentation | Internal cerebral veins involved | Elevated D-dimer | Initial treatment | Death |
|---|---|---|---|---|---|---|---|---|---|
| 1* | 62 | F | 21 | Moderate | Yes | Yes | Yes | UFH | No |
| 2 (13) | 38 | M | 10 | Severe | Yes | Yes | Yes | LMWH | Yes |
| 3 (13) | 41 | F | 5§ | Mild | Yes | Yes | Yes | UFH | Yes |
| 4 (13) | 23 | M | 7 | Severe | Yes | Yes | Yes | NA | Yes |
| 5 (14) | 81 | M | 18 | Severe | Yes | No | Yes | unAC | Yes |
| 6 (15) | 72 | M | 5§ | NA | Yes | Yes | NA | unAC | Yes |
| 7 (16) | 62 | F | 15 | Moderate | Yes | Yes | Yes | NA | NA |
| 8 (16) | 54 | F | 14 | Moderate | No | No | Yes | NA | NA |
| 9 (17) | 44 | F | 14 | Severe | Yes | Yes | Yes | LMWH | NA |
| 10 (5) | 29 | F | 7§ | Mild | Yes | No | Yes | UFH | No |
| 11 (6) | 59 | M | NA | Mild | No | No | Yes | LMWH | No |
| 12 (7) | 53 | M | 7 | Moderate | No | No | Yes | LMWH | No |
| 13 (8) | 65 | M | NA | Mild | Yes | No | NA | unAC | No |
| 14 (9) | 30s | M | 4 | Mild | No | No | No | Dabigatran | No |
| 15 (9) | 30s | M | NA | Mild | Yes | No | Yes | UFH | Yes |
| 16 (10) | 69 | F | 21 | Moderate | Yes | Yes | Yes | UFH | No |
| 17 (10) | 79 | F | 3 | Mild | Yes | No | Yes | LMWH | No |
| 18 (11) | 63 | F | 12 | Moderate | No | No | NA | UFH | Yes |
| 19 (12) | 30 | M | NA | Mild | No | No | Yes | LMWH | Yes |
| Median (range) or n (%) | 54 (23–81) | 52% (M:F) | 10 (3–21) | 81% | 42% | 94% | 50% |
Ref., reference.
Current case.
Approximation. UFH, unfractionated heparin; LMWH, low molecular weight heparin; unAC, unspecified anticoagulation therapy; n, number; NA, not available.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.
Ethics Statement
Ethical review and approval was not required for the study on human participants in accordance with the local legislation and institutional requirements. The patients/participants provided their written informed consent to participate in this study. Written informed consent was obtained from the individual(s) for the publication of any potentially identifiable images or data included in this article.
Author Contributions
SB and FD had the idea for the paper. SB prepared the first draft with FD and VF. PR performed imaging acquisition and interpretation, prepared the figure, and critically reviewed the manuscript for intellectual content. SB, FD, VF, BS, LF, MP, PS, DC, CC, FP, DM, FB, and FP were involved in the clinical care of the patients and critically reviewed the manuscript for intellectual content. AC performed the virological and serological tests and critically reviewed the manuscript for intellectual content. IA and CF assisted with imaging and case interpretation and critically reviewed the manuscript for intellectual content. All authors contributed to the article and approved the submitted version.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
Funding. This study was supported by the Italian Ministry of University and Research grant PRIN 2017CY3J3W.
References
- 1.Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:844–7. 10.1111/jth.14768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhai Z, Li C, Chen Y, Gerotziafas G, Zhang Z, Wan J, et al. Prevention and treatment of venous thromboembolism associated with coronavirus disease 2019 infection: a consensus statement before guidelines. Thromb Haemost. (2020) 120:937–48. 10.1055/s-0040-1710019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brodmann M, Gary T, Hafner F, Tiesenhausen K, Deutschmann H, Pilger E. Massive pulmonary embolism caused by internal iliac vein thrombosis with free-floating thrombus formation in the inferior vena cava. Ann Vasc Surg. (2012) 26:420.e5–7. 10.1016/j.avsg.2011.07.016 [DOI] [PubMed] [Google Scholar]
- 4.Connors JM, Levy JH. Thromboinflammation and the hypercoagulability of COVID-19. J Thromb Haemost. (2020) 18:1559–61. 10.1111/jth.14849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klein DE, Libman R, Kirsch C, Arora R. Cerebral venous thrombosis: a typical presentation of COVID-19 in the young. J Stroke Cerebrovasc Dis. (2020) 29:104989. 10.1016/j.jstrokecerebrovasdis.2020.104989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hughes C, Nichols T, Pike M, Subbe C, Elghenzai S. Cerebral venous sinus thrombosis as a presentation of COVID-19. Eur J Case Rep Intern Med. (2020) 7:1691. 10.12890/2020_001691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dahl-Cruz F, Guevara-Dalrymple N, López-Hernández N. Trombosis venosa cerebral e infección por SARS-CoV-2 [Cerebral venous thrombosis and SARS-CoV-2 infection]. Rev Neurol. (2020) 70:391–2. 10.33588/rn.7010.2020204 [DOI] [PubMed] [Google Scholar]
- 8.Hemasian H, Ansari B. First case of Covid-19 presented with cerebral venous thrombosis: a rare and dreaded case. Rev Neurol. (2020) 176:521–3. 10.1016/j.neurol.2020.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tu TM, Goh C, Tan YK, Leow AS, Pang YZ, Chien J, et al. Cerebral venous thrombosis in patients with COVID-19 infection: a case series and systematic review. J Stroke Cerebrovasc Dis. (2020) 29:105379. 10.1016/j.jstrokecerebrovasdis.2020.105379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nwajei F, Anand P, Abdalkader M, Andreu Arasa VC, Aparicio HJ, Behbahani S, et al. Cerebral venous sinus thromboses in patients with SARS-CoV-2 infection: three cases and a review of the literature. J Stroke Cerebrovasc Dis. (2020) 29:105412. 10.1016/j.jstrokecerebrovasdis.2020.105412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Roy-Gash F, De Mesmay M, Devys JM, Vespignani H, Blanc R, Engrand NSS. COVID-19-associated acute cerebral venous thrombosis: clinical, CT, MRI and EEG features. Crit Care. (2020) 24:419. 10.1186/s13054-020-03225-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hussain S, Vattoth S, Haroon KH, Muhammad A. A case of coronavirus disease 2019 presenting with seizures secondary to cerebral venous sinus thrombosis. Case Rep Neurol. (2020) 12:260–5. 10.1159/000509505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cavalcanti DD, Raz E, Shapiro M, Dehkharghani S, Yaghi S, Lillemoe K, et al. Cerebral venous thrombosis associated with COVID-19. Am J Neuroradiol. (2020) 41:1370–6. 10.3174/ajnr.A6644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Malentacchi M, Gned D, Angelino V, Demichelis S, Perboni A, Veltri A, et al. Concomitant brain arterial and venous thrombosis in a COVID-19 patient. Eur J Neurol. (2020) 27:e38–9. 10.1111/ene.14380 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chougar L, Mathon B, Weiss N, Degos V, Shor N. Atypical deep cerebral vein thrombosis with hemorrhagic venous infarction in a patient positive for COVID-19. Am J Neuroradiol. (2020). 10.3174/ajnr.A6641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Poillon G, Obadia M, Perrin M, Savatovsky J, Lecler A. Cerebral venous thrombosis associated with COVID-19 infection: causality or coincidence? J Neuroradiol. (2020). 10.1016/j.neurad.2020.05.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garaci F, Di Giuliano F, Picchi E, Da Ros V, Floris R. Venous cerebral thrombosis in COVID-19 patient. J Neurol Sci. (2020) 414:116871 10.1016/j.jns.2020.116871 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The original contributions presented in the study are included in the article/supplementary material, further inquiries can be directed to the corresponding author.


