Highlights
-
•
Proton beam therapy is likely to be safe for liver hemangiomas because, in our experience.
-
•
Proton beam therapy achieves good local control for liver hemangiomas without causing severe hepatic damage.
-
•
Proton beam therapy expected to minimize the risk of late adverse events.
Keywords: Radiotherapy, Proton beam therapy, Hemangioma, Liver, Secondary cancer
Abstract
Background
Hepatic hemangiomas are benign tumors with a favorable prognosis, but giant hepatic hemangiomas can cause abdominal symptoms and are indicated for treatment. Most cases are treated with surgery, but radiotherapy has also been used. However, to date, there have been no reports of proton beam therapy for a hepatic hemangioma.
Case presentation
A 46-year-old woman had a tumor of 80 × 80 mm in the left medial lobe of the liver, which was diagnosed as a giant hemangioma based on the contrast pattern. Therapy was required for a giant hepatic hemangioma with symptoms, but the patient refused blood transfusion due to religious reasons, which made surgical resection difficult. Therefore, she was referred to our hospital for proton beam therapy. At her first visit, liver function was Child-Pugh A (5 points) and there was no elevation of tumor markers. Proton beam therapy of 28.6 Gy (RBE) given in 13 fractions was performed without interruption. The only observed acute radiation toxicity was Grade 1 dermatitis. One year after proton beam therapy, the hemangioma had significantly decreased, and a complete response has been maintained for 15 years based on ultrasound and MRI.
Conclusion
This case is the first reported use of proton beam therapy for a hepatic hemangioma. The outcome suggests that this treatment may be effective for a giant liver hemangioma.
1. Background
Hepatic hemangiomas are the most common benign tumors of the liver and are reported to account for 73% of benign liver tumors. Among all liver tumors, hemangiomas are the second most common after metastatic liver tumors [1], [2], [3], [4]. A hemangioma is generally small, asymptomatic, undetectable and may be found at autopsy [5], [6], [7]. In recent years, the frequency of detection of hepatic hemangiomas has increased due to more use of imaging such as ultrasound, computed tomography (CT), magnetic resonance imaging (MRI), positron emission tomography-CT (PET-CT), and angiography [6], [7], [8], [9], [10]. Although hepatic hemangiomas are benign, some of these tumors (<40%) may increase in size [11], [12], [13]. Lesions larger than 4 cm in diameter are referred to as giant hemangiomas [14].
The recommended management of a hemangioma after discovery is monitoring by follow-up of changes of size. Hemangiomas rarely cause intraperitoneal hemorrhage due to spontaneous rupture, but careful follow-up is necessary for tumors larger than 5 cm, and especially for giant hemangiomas larger than 10 cm, as the risk of rupture is higher than that for smaller tumors. Clinical symptoms such as abdominal pain, a rapid increase of the tumor diameter, bleeding due to spontaneous or traumatic rupture, and blood coagulation abnormalities may occur, and such cases require treatment. Surgery is commonly performed, but there are a few reports of use of hepatic artery embolization or radiotherapy [15]. Herein, we describe the first case of use of proton beam therapy for a giant hemangioma.
2. Case report
The patient was a 46-year-old woman with no liver function abnormalities in whom a medical examination 3 years earlier had revealed increases of hepatobiliary enzymes (AST 62 IU/L, ALT 68 IU/L, ALP 458 IU/L and γ-GTP 195 IU/L) and detection of a liver mass on abdominal ultrasound. Abdominal CT revealed a tumor of 80 × 80 mm in the left medial lobe of the liver, which was diagnosed as a giant hemangioma based on the contrast pattern (Fig. 2). Similarly, MRI showed a typical image of a hepatic hemangioma with a clear border. The homogenous lesion, which was hypointense on T1-weighted images and hyperintense on T2-weighted images, presented with a “cotton-wool” pattern. In addition, dilatation of the peripheral bile duct, and compression of the bile duct and blood vessels were observed. A physical examination revealed a flat and soft abdomen and tenderness of the right rib.
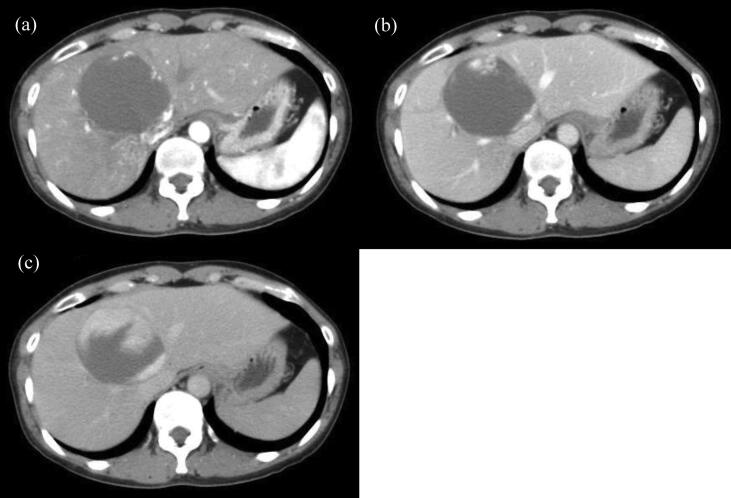
Fig. 2.
CT showed a giant hepatic hemangioma in the left medial lobe before proton beam therapy. (a) Hepatoarterial phase (b) Portal venous phase (c) Equilibrium phase.
Therapy for the giant hepatic hemangioma was considered to be required due to the symptoms. However, the patient was unable to undergo a blood transfusion for religious reasons, making surgical resection difficult. Therefore, she was referred to our hospital for proton beam therapy. At her first visit, liver function was Child-Pugh A (Alb: 4.5 g/dl, T-Bil: 1.1 mg/dl, PT%: 103%, no hepatic encephalopathy, no abdominal dropsy) and there was no elevation of tumor markers. We proceeded proton beam therapy. Acute radiation toxicity was observed as Grade 1 dermatitis only.
The patient refused follow-up by CT imaging, so we basically follow up with ultrasound examination. Tumor size was gradually decreased 50 × 50 mm diameter one year after proton beam therapy, 44 × 33 mm after 1 year and half, 40 × 26 mm after 2 years, 36 × 26 mm after 3 years, respectively. Follow up by the ultrasound examination once a year was continued and the tumor was slightly decreased. The hemangioma had decreased in size by ultrasound. 11 years later, T2-weighted MRI image showed a low signal area which had reduced to 29 × 15 mm and the size was maintained (Fig. 3). It was difficult to be recognized by ultrasound. And there was no deformation of liver. Except for the atrophy of the irradiated area of the tumor, the rest of the liver did not deform and remained intact. In addition, there were no increase of hepatobiliary enzymes in a blood test and no symptoms in an abdominal examination. PS was 0 and the patient was healthy. Changes in blood chemistry are shown in Table 1.
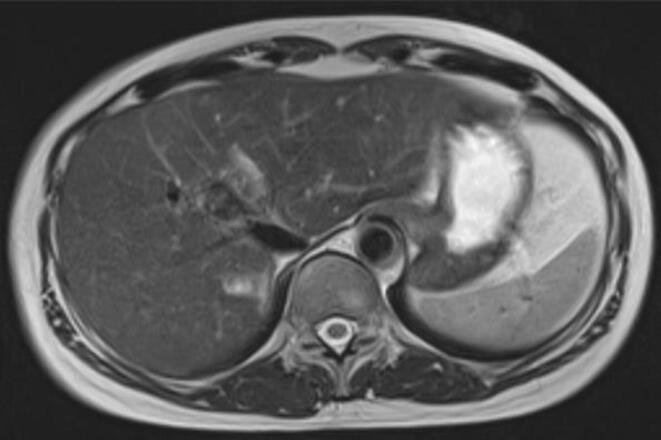
Fig. 3.
T2-weighted MRI showed that the size of the giant hepatic hemangioma had reduced to 29 × 15 mm and maintained for 15 years after proton beam therapy.
Table 1.
Changes in hepatobiliary enzymes before and after PBT.
| Before PBT | At the end of PBT | 1 year after PBT | 1.5 years after PBT | 2 years after PBT | 3 years after PBT | |
|---|---|---|---|---|---|---|
| AST (U/l) | 27 | 48 | 29 | 27 | 27 | 26 |
| ALT (U/l) | 33 | 51 | 29 | 25 | 22 | 18 |
| LDH (U/l) | 158 | 162 | 147 | 158 | 153 | 162 |
| ALP (U/l) | 362 | 405 | 275 | 218 | 191 | 277 |
| γ-GTP (U/l) | 162 | 179 | 73 | 51 | 32 | 22 |
| CHE (U/l) | 287 | 272 | 277 | 265 | 299 | 275 |
| T-Bil (mg/dl) | 1.1 | 0.7 | 0.6 | 0.6 | 0.8 | 0.5 |
| Alb (g/dl) | 4.5 | 4.6 | 4.0 | 4.2 | 4.2 | 4.2 |
3. Proton beam therapy
Before treatment planning for hepatocellular carcinoma (HCC) or intrahepatic cholangiocarcinoma, patients typically have a metal fiducial marker (an iridium seed of 0.8 mm in diameter and 2 mm in length) implanted near the tumor to aid in positioning. Patient set-up is performed as follows, using respiratory synchronization and a 5-mm margin for respiratory motion: the laser is applied to the body prior to treatment, the patient's position is adjusted based on a body mark on the skin, and orthogonal fluoroscopy is then used to match the bone structure, with reference to the DRR prior to treatment. Online correction is performed and then final adjustments are used around the position of the fiducial marker. Additionally, a respiration sensor and 3D tumor motion are used. The terminal stage is then acquired using an imaging system, and irradiation with the proton beam is performed only at this stage. By using this system, the error is <5 mm [16].
In a case of HCC, the GTV is irradiated with a margin of about 10 mm and an additional leaf margin of 5–10 mm to cover microfiltration of the tumor. However, the present case was a benign tumor and no margin was added to the GTV. Also, the patient refused to have a metal fiducial marker implanted, so a total leaf margin of 10 mm was added while using respiratory synchronization to cover the setup error. For HCC, the gastrointestinal tract is normally avoided as much as possible after 40–50 Gy (RBE) to maximize the non-irradiated area of the liver. However, since the total dose for hemangioma is low, at risk organs were not a concern in the present case. To minimize the risk of secondary cancers, proton therapy was started with 28.6 Gy (RBE) in one field from a 320° direction in 13 fractions (Fig. 1) and was completed without interruption.
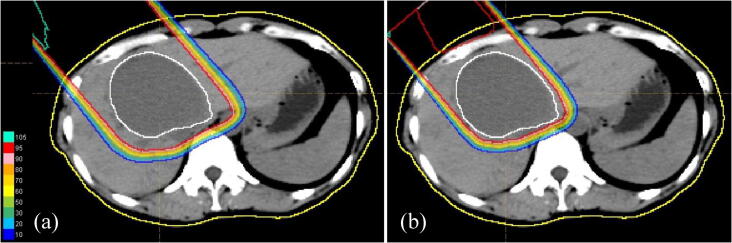
Fig. 1.
Dose-distribution chart for proton beam therapy, showing isodose curves representing 100% to 10% of the prescribed dose at 10% intervals. Normal liver outside the blue line was not irradiated. The treatment plan was changed after ten rounds to gradually reduce the treatment margin. (a) 0–22 Gy (RBE)/10 Fr. (b) 24.2–28.6 Gy (RBE)/11–13 Fr. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
4. Discussion
There are no reports of hepatic hemangiomas becoming malignant [6], [14], and asymptomatic cases only require general follow-up once every 6 months to 1 year [6]. Indications for treatment include pain, persistent increase in size, effects on surrounding organs, rupture, and complication of Kasabach-Merritt syndrome [12]. Surgery is the first choice for symptomatic hepatic hemangiomas [10], [14], [15], and other options include arterial embolization and radiofrequency ablation [12], [15]. If these treatments are difficult due to the size, number, site, or general condition, radiotherapy may be an option. However, information is limited [10]. Searching in PubMed for articles with “Radiotherapy, Hemangiomas” as the keywords in the past 30 years, there are only three reports about treatment results of radiotherapy for hepatic hemangiomas.
Radiotherapy is not recommended as the first treatment for hepatic hemangiomas because it can cause radiation hepatitis and secondary cancer [14], [15]. Gaspar et al. reported radiotherapy for 7 cases of symptomatic inoperable hepatic hemangiomas. The total dose at the isocenter calculated according to ICRU recommendations varied from 15 to 30 Gy given in 15–22 fractions. The observation period was 40–67 months, and all 7 cases showed improvement of symptoms [10]. At the time of final follow-up, there were 3 cases with abdominal symptoms and one with abnormal liver function. Biswal et al. treated 4 patients aged 42–55 years with 30 Gy given in 15 fractions and obtained reduction and relief from symptoms during an observation period of 12–36 months [15]. Lee et al. used 3D-CRT of 30 Gy given in 15 fractions in a 79-year-old patient and found that reduction of size was maintained after 15 months of follow-up [17]. Adverse events of Grade 1 (elevation of AST, ALT) occurred in these treatments, but there were no severe late effects. However, in longer-term follow-up, Moore et al. and Okazaki et al. found emergence of secondary HCC 20 years after treatment of hepatic hemangiomas [18], [19], and Weshler et al. reported one case of squamous cell carcinoma of the renal pelvis 20 years after right hemi-abdominal irradiation for a cavernous hemangioma of the liver [20].
Sanford et al. reported that proton therapy was associated with significantly fewer post-irradiation liver function exacerbations compared to X-ray therapy. In addition, the incidence of radiation hepatitis was significantly higher in patients with sub-average liver doses higher than the median dose of 21.6 Gy, indicating that minimizing this dose is clinically meaningful [21]. In our case, even though the patient had a benign liver tumor, the total dose was 28.6 Gy, suggesting that radiation exposure to the normal liver should have been reduced. Taddei et al. compared the DVH of irradiation for HCC using a dose distribution chart, and showed that proton therapy lowered the dose to the normal liver compared to X-ray therapy [22]. These results suggest that proton therapy not only minimizes the effect on liver function, but may also reduce the risk of second cancers.
There have been no reports of use of proton beam therapy for hepatic hemangiomas. However, this therapy is likely to be safe for liver hemangiomas because, in our experience, proton beam therapy achieves good local control for other liver tumors without causing severe hepatic damage [23], [24], [25]. Hemangiomas require lower doses (20–30 Gy) and smaller margins (about 0–5 mm) compared to malignant liver tumors (≥70 Gy, ≥10 mm), which suggests that toxicity effects after proton beam therapy for hemangiomas are likely to be mild. Proton beam therapy may significantly reduce the risk of secondary cancer [26], [27], [28], which is important because hepatic hemangiomas are benign tumors that occur in young people, and the long-term prognosis after treatment is important. Proton beam therapy for hemangiomas may reduce risks of subsequent hepatic failure and secondary cancer.
Searching in PubMed for articles with “Surgery, Hemangiomas” as the keywords in the past 15 years, there are only five reports about treatment results of surgery for hepatic hemangiomas. Wang et al. reported the laparoscopic surgery of 44 cases of hepatic hemangioma and reported that the median hemorrhage volume was 335 ml and the blood transfusion rate was 9.1% [29]. Wahab et al. operated in 144 patients with giant hepatic hemangioma (median diameter of 10 (5–31) cm) from January 2000 to April 2017. 92 cases were enucleated and hepatectomy was performed in 52 cases [30]. The hemorrhage volume was 400 (50–10,000) ml in the median value of hepatic hemangioma >10 cm (300 pairs 575 ml, P = 0.007), and the blood transfusion was 18.1% in less than 10 cm. 51.4% at 10 cm or more. Liu et al. reported that they performed surgery on 141 cases (>20 cm, 36 cases, >10 cm but <20 cm, 105 cases) of huge hemangiomas over 10 cm [31]. And the mean value of hemorrhage was 838.4 ± 998.4 ml, and blood transfusion was 31.2%. In the report of Yang et al., forty-one patients underwent hepatectomy and 53 underwent laparoscopic hepatectomy [32]. The tumor diameter was 8.32 ± 2.49 cm, 7.95 ± 2.01 cm, respectively. Although there was no description of the blood transfusion, the average bleeding quantity was 437.81 ml and 361.69 ml, respectively. Hu et al. reported 19, 13, and 25 cases of robotic hemi-hepatectomy, hemi-hepatectomy, and open hemi-hepatectomy, respectively [33]. Mean volume of the tumor was 553.2 ± 122.3 cm3, 556.2 ± 0.2178 cm3, 667.5 ± 202.6 cm3, respectively. And blood transfusion was 26.3%, 30.8% and 32%, respectively.
Searching in PubMed for articles with the keyword “RFA, Hemangiomas” over the past 15 years, there are only five reports about treatment results of RFA for hepatic hemangiomas. The search resulted in 4 case series and 1 case report [34], [35], [36], [37], [38]. The success rate of hemangioma treatment with RFA was around 71–86.5% [34], [35]. Hemoglobinuria, renal failure, pleural effusion and hyperbilirubinemia were reported as toxicities by RFA for hemangioma. Risk of blood transfusion looks lower compared to surgery, but the risk of bleeding cannot be reduced to zero. Although, surgery and RFA are common treatment strategy for hemangioma. The risk of bleeding and blood transfusion is inevitable. So the patient that surgery or RFA is difficult (complications, elderly, refuse blood transfusion, etc.) may be a good indication for proton beam therapy.
5. Conclusion
This case is the first reported use of proton beam therapy for a hepatic hemangioma. The successful long-term outcome indicates that this therapy may be useful for a giant liver hemangioma.
Financial disclosure
All authors have no financial relationships associated with the content of the manuscript.
Availability of data and materials
Please contact the corresponding author for data requests.
Ethics approval and consent to participate
The patients signed a consent form prior to initiation of treatment and collection of data.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Ishak K.G., Rabin L. Benign tumors of the liver. Med Clin North Am. 1975;59:995–1013. doi: 10.1016/s0025-7125(16)31998-8. [DOI] [PubMed] [Google Scholar]
- 2.Yang Z., Tan H., Liu X., Sun Y. Extremely giant liver hemangioma (50 cm) with Kasabach-Merritt syndrome. J Gastrointest Surg. 2017;21:1748–1749. doi: 10.1007/s11605-017-3429-7. [DOI] [PubMed] [Google Scholar]
- 3.Reddy K.R., Kligerman S., Levi J., Livingstone A., Molina E., Franceschi D. Benign and solid tumors of the liver: relationship to sex, age, size of tumors, and outcome. Am Surg. 2001;67:173–178. [PubMed] [Google Scholar]
- 4.Hasan H.Y., Hinshaw J.L., Borman E.J., Gegios A., Leverson G., Winslow E.R. Assessing normal growth of hepatic hemangiomas during long-term follow-up. JAMA Surg. 2014;149:1266–1271. doi: 10.1001/jamasurg.2014.477. [DOI] [PubMed] [Google Scholar]
- 5.Bioulac-Sage P., Laumonier H., Laurent C., Blanc J., Balabaud C. Benign and malignant vasculartumors of the liver in adults. Semin Liver Dis. 2008;28:302–314. doi: 10.1055/s-0028-1085098. [DOI] [PubMed] [Google Scholar]
- 6.Bajenaru N. Hepatic hemangioma – review. J Med Life. 2015;8(special issue):4–11. [PMC free article] [PubMed] [Google Scholar]
- 7.Okano H. Natural course of cavernous hepatic hemangioma. Oncol Rep. 2001;8(2):411–414. doi: 10.3892/or.8.2.411. [DOI] [PubMed] [Google Scholar]
- 8.Mungovan J.A., Cronan J.J., Vacarro J. Hepatic cavernous hemangiomas: lack of enlargement over time. Radiology. 1994;191(1):111–113. doi: 10.1148/radiology.191.1.8134554. [DOI] [PubMed] [Google Scholar]
- 9.Takagi H. Diagnosis and management of cavernous hemangioma of the liver. Semin Surg Oncol. 1985;1(1):12–22. doi: 10.1002/ssu.2980010104. [DOI] [PubMed] [Google Scholar]
- 10.Gaspar L., Mascarenhas F., da Costa M.S. Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol. 1993;29:45–50. doi: 10.1016/0167-8140(93)90172-5. [DOI] [PubMed] [Google Scholar]
- 11.Reading N.G., Forbes A., Nunnerley H.B., Williams R. Hepatic haemangioma: a critical review of diagnosis and management. Q J Med. 1988;67(253):431–445. [PubMed] [Google Scholar]
- 12.Leon M., Chavez L., Surani S. Hepatic hemangioma: what internists need to know. World J Gastroenterol. 2020;26(1):11–20. doi: 10.3748/wjg.v26.i1.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Corigliano N., Mercantini P., Amodio P.M. Hemoperitoneum from a spontaneous rupture of a giant hemangioma of the liver: report of a case. Surg Today. 2003;33:459–463. doi: 10.1007/s10595-002-2514-z. [DOI] [PubMed] [Google Scholar]
- 14.Biswal B.M., Sandhu M., Lal P., Bal C.S. Role of radiotherapy in cavernous hemangioma liver. Indian J Gastroenterol. 1995;14(3):95–98. [PubMed] [Google Scholar]
- 15.Toro A., Mahfouz A.E., Ardiri A. What is changing in indications and treatment of hepatic hemangiomas. A review. Ann Hepatol. 2014;13(4):327–339. [PubMed] [Google Scholar]
- 16.Oshiro Y., Okumura T., Ishida M. Displacement of hepatic tumor at time to exposure in end-expiratory-triggered-pulse proton therapy. Radiother Oncol. 2011;99(2):124–130. doi: 10.1016/j.radonc.2011.05.009. [DOI] [PubMed] [Google Scholar]
- 17.Lee H.L. CT and MRI findings correlate with the time-course of unresectable cavernous haemangioma of the liver after fractionated radiotherapy. Br J Radiol. 2012;85(1011):e49–e52. doi: 10.1259/bjr/74795623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moore T.A. Hepatoma occurring two decades after hepatic irradiation. Gastroenterology. 1976;71(1):128–132. [PubMed] [Google Scholar]
- 19.Okazaki N., Yoshino M., Yoshida T., Ohno T., Kitagawa T. Radiotherapy of hemangioma cavernosum of the liver. Gastroenterology. 1977;73(2):353–356. [PubMed] [Google Scholar]
- 20.Weshler Z., Sulkes A., Kopolovic J. Squamous cell carcinoma of the renal pelvis as a late complication of hepatic irradiation: a case report. J Surg Oncol. 1983;22(2):84–86. doi: 10.1002/jso.2930220205. [DOI] [PubMed] [Google Scholar]
- 21.Sanford Nina N., Pursley Jennifer. Protons versus photons for unresectable hepatocellular carcinoma: liver decompensation and overall survival. Int J Radiat Oncol Biol Phys. 2019;105(1):64–72. doi: 10.1016/j.ijrobp.2019.01.076. Phys Med Biol. 2010 December 7; 55(23): 7055–7065. [DOI] [PubMed] [Google Scholar]
- 22.Taddei Phillip J, Howell Rebecca M, et al. Risk of second malignant neoplasm following proton versus intensity-modulated photon radiotherapies for hepatocellular carcinoma. [DOI] [PMC free article] [PubMed]
- 23.Mizumoto M., Okumura T., Hashimoto T. Proton beam therapy for hepatocellular carcinoma: a comparison of three treatment protocols. Int J Radiat Oncol Biol Phys. 2011;81(4):1039–1045. doi: 10.1016/j.ijrobp.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 24.Mizumoto M., Okumura T., Hashimoto T. Evaluation of liver function after proton beam therapy for hepatocellular carcinoma. Int J Radiat Oncol Biol Phys. 2012;82(3):e529–e535. doi: 10.1016/j.ijrobp.2011.05.056. [DOI] [PubMed] [Google Scholar]
- 25.Shosei S., Toshiyuki O., Yoshiko O. Clinical outcomes of previously untreated patients with unresectable intrahepatic cholangiocarcinoma following proton beam therapy. Radiat Oncol. 2019;14(1):241. doi: 10.1186/s13014-019-1451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sethi R.V., Shih H.A., Yeap B.Y. Second nonocular tumors among survivors of retinoblastoma treated with contemporary photon and proton radiotherapy. Cancer. 2014;120(1):126–133. doi: 10.1002/cncr.28387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lautenschlaeger S., Iancu G., Flatten V. Advantage of proton-radiotherapy for pediatric patients and adolescents with Hodgkin's disease. Radiat Oncol. 2019;14(1):157. doi: 10.1186/s13014-019-1360-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tamura M., Sakurai H., Mizumoto M. Lifetime attributable risk of radiation-induced secondary cancer from proton beam therapy compared with that of intensity-modulated X-ray therapy in randomly sampled pediatric cancer patients. J Radiat Res. 2017;58(3):363–371. doi: 10.1093/jrr/rrw088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang Y., Ji W. Laparoscopic liver resection and enucleation of liver hemangioma with selective hepatic vascular occlusion: technique and indications. J Laparoendosc Adv Surg Tech A. 2017;27(9):944–950. doi: 10.1089/lap.2016.0432. [DOI] [PubMed] [Google Scholar]
- 30.Wahab M.A., Nakeeb N.E. Surgical management of giant hepatic hemangioma: single center's experience with 144 patients. J Gastrointest Surg. 2018;22(5):849–858. doi: 10.1007/s11605-018-3696-y. [DOI] [PubMed] [Google Scholar]
- 31.Liu X., Yang Z. Characteristics and operative treatment of extremely giant liver hemangioma >20 cm. Surgery. 2017;161(6):1514–1524. doi: 10.1016/j.surg.2016.12.015. [DOI] [PubMed] [Google Scholar]
- 32.Yan C., Li B. Laparoscopic hepatectomy is superior to open procedures for hepatic hemangioma. Hepatobiliary Pancreat Dis Int. 2020 doi: 10.1016/j.hbpd.2020.09.001. S1499-3872(20)30198-3. [DOI] [PubMed] [Google Scholar]
- 33.Hu M., Chen K. Robotic, laparoscopic or open hemihepatectomy for giant liver haemangiomas over 10 cm in diameter. BMC Surg. 2020;20(1):93. doi: 10.1186/s12893-020-00760-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Qu C., Liu H. Percutaneous ultrasound-guided 'three-step' radiofrequency ablation for giant hepatic hemangioma (5–15 cm): a safe and effective new technique. Int J Hyperthermia. 2020;37(1):212–219. doi: 10.1080/02656736.2020.1732484. [DOI] [PubMed] [Google Scholar]
- 35.Zou H., Yan J. The new technology of enhanced radiofrequency ablation is safe and effective for treating giant hepatic hemangioma. Zhonghua Gan Zang Bing Za Zhi. 2012;20(4):261–265. doi: 10.3760/cma.j.issn.1007-3418.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 36.Yang X., Liu J. Heme is involved in the systemic inflammatory response following radiofrequency ablation of hepatic hemangiomas. Eur J Gastroenterol Hepatol. 2020;32(9):1200–1206. doi: 10.1097/MEG.0000000000001636. [DOI] [PubMed] [Google Scholar]
- 37.Park S.Y., Tak W.Y. Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol. 2011;54(3):559–565. doi: 10.1016/j.jhep.2010.07.024. [DOI] [PubMed] [Google Scholar]
- 38.van Tilborg A.A.J.M., Dresselaars D.H. RF ablation of giant hemangiomas inducing acute renal failure: a report of two cases. Cardiovasc Intervent Radiol. 2016;39(11):1644–1648. doi: 10.1007/s00270-016-1415-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Please contact the corresponding author for data requests.