Abstract
Objective
Few studies have examined the effects of participants’ diet and activity prior to sample collection on metabolomics profiles, and results have been conflicting. We compared the effects of overnight fasting with or without 3 days of standardized diet and restricted physical activity on the human blood metabolome, and examined the effects of these protocols on our ability to detect differences in metabolomics profiles in adolescent girls with obesity and polycystic ovary syndrome (PCOS) vs. sex and BMI-matched controls.
Methods
This was a cross-sectional study of 16 adolescent girls with obesity and PCOS and 5 sex and BMI-matched controls. Fasting plasma metabolomic profiles were measured twice in each participant: once without preceding restriction of physical activity or control of macronutrient content (“typical fasting visit”), and again after 12 h of monitored inpatient fasting with 3 days of standardized diet and avoidance of vigorous exercise (“controlled fasting visit”). Moderated paired t-tests with FDR correction for multiple testing and multilevel sparse partial least-squares discriminant analysis (sPLS-DA) were used to examine differences between the 2 visits and to compare the PCOS and control groups with the 2 visits combined and again after stratifying by visit.
Results
Twenty-three known metabolites were significantly different between the controlled fasting and typical fasting visits. Hypoxanthine and glycochenodeoxycholic acid had the largest increases in relative abundance at the controlled fasting visit compared to the typical fasting visit, while oleoyl-glycerol and oleamide had the largest increases in relative abundance at the typical fasting visit compared to the controlled fasting visit. sPLS-DA showed excellent discrimination between the 2 visits; however, when the samples from the 2 visits were combined, differences between the PCOS and control groups could not be detected. After stratifying by visit, discrimination of PCOS status was improved.
Conclusions
There were differences in fasting metabolomic profiles following typical fasting vs monitored fasting with preceding restriction of physical activity and control of macronutrient content, and combining samples from the two visits obscured differences by PCOS status. In studies performing metabolomics analysis, careful attention should be paid to acute diet and activity history. Depending on the sample size of the study and the expected effect size of the outcomes of interest, control of diet and physical activity beyond typical outpatient fasting may not be required.
Keywords: Metabolomics, Study design, Diet, Physical activity, Polycystic ovary syndrome
Abbreviations:
- PCOS
polycystic ovary syndrome
- AIRS
Androgens and Insulin Resistance Study
- BMI
body mass index
- CTRC
Colorado Clinical Translational Research Center
- EDTA
ethylenediaminetetraacetic acid
- LC-MS
liquid chromatography-mass spectrometry
- FDR
false discovery rate
- sPLS-DA
sparse partial least-squares discriminant analysis
- AUC
area under the receiver operating characteristic curve
L.PM.C.GA.-M.CH.R.Y.G.-RB.C.BK.J.N
1. Introduction
Metabolomics, the analysis of metabolites in cells, tissues, and biofluids, can be used to identify biomarkers of disease and to study physiological mechanisms of metabolism; however, the large inter- and intra-individual variability inherent in metabolomic measures remains a major challenge for the field [1]. A carefully designed study, including consideration of aspects related to biological sample collection, sample preparation, instrumental analysis, data processing and analysis, and interpretation, is needed to avoid biased and misleading results, to reduce measurement variability and to increase statistical power [2].
There is an extensive body of literature on the relationships between dietary and activity factors and serum and plasma metabolomics profiles [[3], [4], [5], [6], [7], [8], [9], [10], [11], [12], [13]]. However, relatively little research has been done on the effect of pre-sample collection standardization of study participant diet and activity on the variability in metabolite profiles, and the results are conflicting. Walsh et al. [14] studied the intra- and inter-individual variability in metabolomics profiles and found that a standardized diet and avoidance of vigorous physical activity reduced variability in metabolites in urine samples, but not in plasma or saliva. In another inpatient study with participants admitted twice for 3 days each, technical aspects (e.g., sample preparation and analysis) were the largest source of variability in blood metabolomes, although timing of collection relative to meals did affect variability [15]. However, in a 2-week inpatient study, Winnike et al. [16] demonstrated that differences in the fasting serum metabolome could be detected after 1 day of standardized diet, but that variability did not decrease with increased duration of diet. In another study of standardized protocols for reproducible metabolomics profiling, amino acids increased postprandially, acylcarnitines were generally higher in samples collected during fasting, and certain amino acids and acylcarnitines were significantly affected by strenuous exercise [17]. Finally, Sampson et al. [18] found that fasting status was associated with 34% of metabolites, although the overall proportion of variability explained by these metabolites was relatively small.
If typical outpatient fasting with no preceding control of physical activity or diet is sufficient to control variability in metabolomics measures, this would reduce study costs and participant burden relative to a standardized diet. We are unaware of any studies that directly compared the effects of typical fasting to fasting following a standardized diet and restricted physical activity on the variability in blood metabolomics and the resulting ability to detect other differences between biological groupings of participants. Therefore, the purpose of this study was to compare the effects of typical outpatient fasting to a more rigorously controlled pre-sample collection protocol (3 days of standardized diet and restricted physical activity, followed by 12 h of monitored inpatient fasting) on the fasting human blood metabolome, and to examine the effects of these protocols on our ability to detect differences in metabolomics profiles between adolescent girls with obesity and PCOS and sex and BMI-matched controls. We hypothesized that fasting followed by standardized diet and restricted exercise would be more effective than typical fasting in reducing variability in metabolomics profiles and allowing discrimination of differences by PCOS status.
2. Material and methods
2.1. Participant population
Participants were selected from the polycystic ovary syndrome (PCOS) and control groups of the AIRS (Androgens and Insulin Resistance Study, prior to NCT numbers) study, a cross-sectional study that examined the relationship between testosterone and insulin resistance in adolescent girls with obesity and PCOS [[19], [20], [21]]. Enrollment took place during 2011–2014. Adolescents with PCOS were recruited from specialty clinics and the general adolescent clinics at Children’s Hospital Colorado as well as from the community via advertisement; adolescents without PCOS were recruited from the general adolescent clinic as well as from the community via advertisement. AIRS inclusion criteria included: female sex, obesity (body mass index [BMI] 95th percentile for age and sex), age 12–20 years, and physical inactivity (<3 h per week of habitual exercise, per questionnaire). Exclusion criteria included: diabetes mellitus, alanine aminotransferase >80 IU/mL, blood pressure >140/90 mmHg, hemoglobin <9 mg/dL, serum creatinine >1.5 mg/dL, smoking, medications affecting insulin sensitivity or to treat hypertension or lipids pregnancy, and breastfeeding. PCOS was diagnosed using the National Institute of Health (NIH) criteria of oligomenorrhea, being 18 months post-menarche, and having hyperandrogenism [22,23]. The study was conducted at the University of Colorado and Children’s Hospital Colorado, and was approved by the University of Colorado Anschutz Institutional Review Board in addition to Children’s Hospital Colorado’s Scientific Advisory Review Committee. All participants provided written informed consent. For participants younger than 18 years old of age, parents and participants provided consent and assent, respectively. Samples were selected for metabolomics analysis from AIRS participants with complete insulin clamp data and samples available for analysis. They were then matched on BMI between PCOS and controls, with a ratio of 3 girls with PCOS for each control due to limited availability of stored control samples. The original sample size for the metabolomics analysis consisted of 18 girls with obesity and PCOS and 6 sex and BMI-matched controls; due to the fact that some participants were missing either the typical fasting sample or the controlled fasting sample, the final sample size for this analysis was 16 girls with obesity and PCOS and 5 sex and BMI-matched controls.
2.2. Overall study design
The protocol included two study visits as previously described [19]. Briefly, the typical fasting screening visit included consent, screening labs, a physical examination and an optional oral glucose tolerance test. Participants were instructed to fast for at least 10 h prior to the typical fasting visit. At the controlled fasting visit 2–4 weeks later, the remaining study outcomes, including the hyperinsulinemic-euglycemic clamp, were measured. Prior to the controlled fasting visit, participants were provided a 3-day standardized, isocaloric, weight maintenance diet (55% carbohydrate, 30% fat, 15% protein) from the University of Colorado Clinical Translational Research Center (CTRC) metabolic kitchen, and were asked to avoid vigorous exercise 3 days prior to the controlled fasting visit. Participants wore an ambulatory GT3x accelerometer (Actigraph Corp., Pensacola, FL) for seven days prior to the visit to confirm sedentary activity. Following the 3 days of study diet and restricted physical activity, participants were admitted overnight for a 12-h fast and fasting AM samples were collected prior to undergoing a hyperinsulinemic euglycemic clamp.
2.3. Metabolomics
Untargeted plasma metabolomics analysis was performed at the Michigan Regional Comprehensive Metabolomics Resource Core. Plasma samples were drawn in EDTA tubes, with the plasma separated and frozen immediately at −80.0 °C. LC-MS chromatography was used for chromatographic separation and mass chromatography was used for mass detection. This analysis yielded both identified and unidentified compounds. Identification was made comparing masses and retention times from samples to an in-house library. Quality of analysis, assessed by visual inspection of the chromatographic traces and relative quantification of internal standards, showed that the analysis methods were both stable and reproducible across all samples.
2.4. Statistics
Descriptive statistics for demographics and anthropometrics are presented as mean ± standard deviation or frequencies and percentages, and PCOS and control groups were compared using t-tests and Fisher’s Exact Test. Metabolites that were detected in <3 samples were excluded from analysis, and missing data were imputed using the kth nearest neighbor algorithm. Data were log transformed prior to analysis. Known metabolites were compared between the two visits using paired moderated t-tests with the Benjamini and Hochberg’s p-value correction methods to correct for multiple testing. False discovery rate (FDR)-adjusted p-values (q-values) are reported. Multilevel sparse partial least-squares discriminant analysis (sPLS-DA) [24,25] was used to perform supervised classification using all metabolites and to determine which metabolites were best able to discriminate between the two visits. The performance of the sPLS-DA model was evaluated using cross-validation and the maximum distance as the prediction distance, with the error rate and area under the receiver operating characteristic curve (AUC) averaged across 10 repetitions. To examine the effect of differing diet conditions on the ability to discriminate between participants with PCOS and controls, similar sPLS-DA analyses were performed using PCOS status as the outcome, including two samples, one from each visit, for each participant. Finally, sPLS-DA analyses with PCOS status as the outcome were performed after stratifying by visit.
All statistical analyses were performed using R version 3.6 (R Core Team, Vienna). sPLS-DA was performed using the mixOmics package [26,27].
3. Results
This analysis included 21 girls with obesity: 16 with PCOS and 5 without PCOS. Participant descriptors are presented in Table 1. Girls with PCOS were older (14.6 ± 1.5 vs. 12.8 ± 0.8 years, p = 0.022), although in both groups, all participants were Tanner stage V. Otherwise, girls with PCOS were similar to obese controls.
Table 1.
Participant characteristics.
| Controls | PCOS | P-value | |
|---|---|---|---|
| Na | 5 | 16 | |
| Age (mean (SD)) | 12.8 (0.8) | 14.6 (1.5) | 0.022 |
| Ethnicity (%) | 0.079 | ||
| NHW | 1 (20.0) | 8 (50.0) | |
| Hispanic | 2 (40.0) | 8 (50.0) | |
| Black | 2 (40.0) | 0 (0.0) | |
| Tanner 5 (%) | 5 (100.0) | 16 (100.0) | NA |
| BMI percentile (mean (SD)) | 98.02 (1.19) | 97.97 (1.88) | 0.956 |
All participants had complete data.
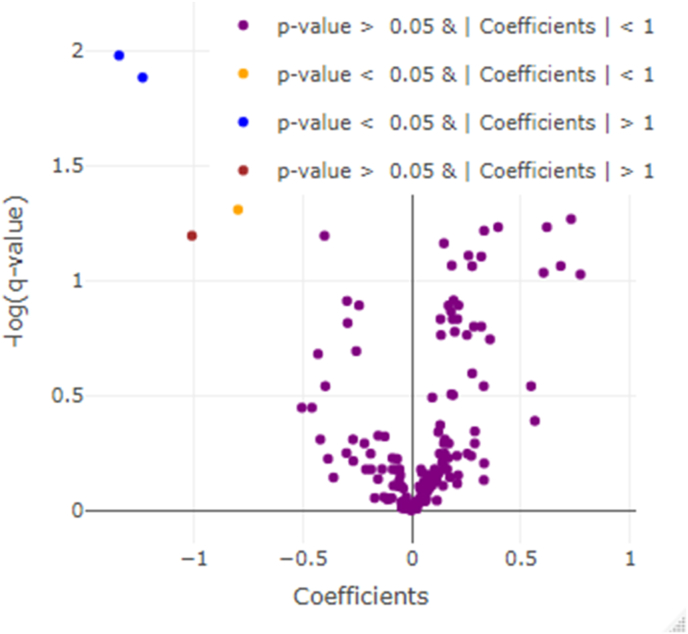
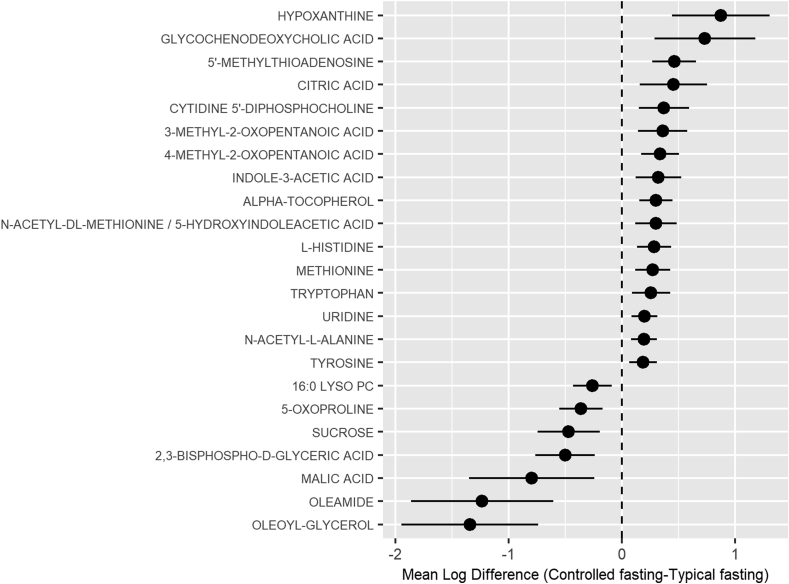
Initial untargeted analysis identified 6747 compounds, 177 of which were known metabolites. Twenty-three known metabolites were significantly different (q < 0.05) between the typical fasting and controlled fasting visits (Fig. 1). Hypoxanthine, a purine derivative, was the metabolite with the largest increase in relative abundance (0.8 fold change) at the controlled fasting visit compared to the typical fasting visit (Fig. 2), with glycochenodeoxycholic acid, a bile salt, having the second largest increase (0.7 fold change). Oleoyl-glycerol, a monoacylglycerol, had the largest increase in relative abundance (1.3 fold change) at the typical fasting visit compared to the controlled fasting visit, with oleamide having the second largest increase in relative abundance (1.2 fold change). The 23 known compounds with q < 0.05 are described in Table 2.
Fig. 1.
Volcano plot of statistical significance vs. the mean difference in relative abundance (i.e., model coefficient) of known metabolites between the controlled fasting and typical fasting visits. A positive coefficient indicates that the relative abundance of the metabolite was higher in the controlled fasting visit.
Fig. 2.
Mean difference in log relative abundance for known metabolites with q < 0.05. A positive coefficient indicates that the relative abundance of the metabolite was higher in the controlled fasting visit.
Table 2.
Characteristics of the 23 known metabolites with q < 0.05, from most significant to least.
| Metabolite | Category | HMDB number | Notes |
|---|---|---|---|
| 5′-METHYLTHIOADENOSINE | Nucleotides, nucleosides, and derivatives | HMDB0001173 | Found in beans, squash, chocolate |
| OLEOYL-GLYCEROL | Monoacylglycerol | HMDB0011537 | End product of intestinal digestion of dietary fats |
| ALPHA-TOCOPHEROL | Tocopherol | HMDB0001893 | Most active form of vitamin E in humans |
| 4-METHYL-2-OXOPENTANOIC ACID | Keto acids and derivatives | HMDB0000695 | Abnormal metabolite that arises from the incomplete breakdown of branched-chain amino acids |
| HYPOXANTHINE | Purine and purine derivatives | HMDB0000157 | Naturally occurring purine derivative |
| OLEAMIDE | Fatty amide | HMDB0002117 | Occurs naturally in the body of animals |
| 2,3-BISPHOSPHO-d-GLYCERIC ACID | Sugar acids and derivatives | HMDB0001294 | Present at high levels in the human red blood cell |
| 5-OXOPROLINE | Amino acid derivative | HMDB0000267 | Elevated blood levels may be associated with problems of glutamine or glutathione metabolism |
| l-HISTIDINE | Amino acid | HMDB0000177 | Essential amino acid |
| METHIONINE | Amino acid | HMDB00696 | Found in meat, fish, dairy |
| URIDINE | Nucleotides, nucleosides, and derivatives | HMDB0000296 | Uridine is found in many foods (anything containing RNA) but is destroyed in the liver and gastrointestinal tract, and so no food, when consumed, has ever been reliably shown to elevate blood uridine levels |
| N-ACETYL-l-ALANINE | Amino acids, peptides, and analogues | HMDB0000766 | Human and fungal metabolite |
| SUCROSE | Disaccharide | HMDB0000258 | Sugar |
| CYTIDINE 5′-DIPHOSPHOCHOLINE | Nucleotides, nucleosides, and derivatives | HMDB0001413 | Essential intermediate in the biosynthetic pathway of structural phospholipids in cell membranes, particularly phosphatidylcholine |
| 3-METHYL-2-OXOPENTANOIC ACID | Keto acids and derivatives | HMDB0000491 | Abnormal metabolite that arises from the incomplete breakdown of branched-chain amino acids |
| GLYCOCHENODEOXYCHOLIC ACID | Bile salt | Acts as a detergent to solubilize fats for absorption | |
| N-ACETYL-dl-METHIONINE/5-HYDROXYINDOLEACETIC ACID | Indoles and derivatives | HMDB0000763 | Breakdown product of serotonin |
| INDOLE-3-ACETIC ACID | Indoles and derivatives | HMDB0000197 | Breakdown product of tryptophan metabolism and is often produced by the action of bacteria in the mammalian gut. |
| TYROSINE | Amino acid | HMDB0000158 | Non-essential amino acid |
| TRYPTOPHAN | Amino acid | HMDB0030396 | Essential amino acid |
| CITRIC ACID | Carboxylic acide and derivatives | HMDB0000094 | Weak acid that is formed in the tricarboxylic acid cycle or that may be introduced with diet |
| 16:0 LYSO PC | Glycerophospholipid | HMDB0010382 | Derived from fish oils, milk fats, vegetable oils and animal fats |
| MALIC ACID | Beta hydroxy acids and derivatives | HMDB0000744 | Found in a variety of foods |
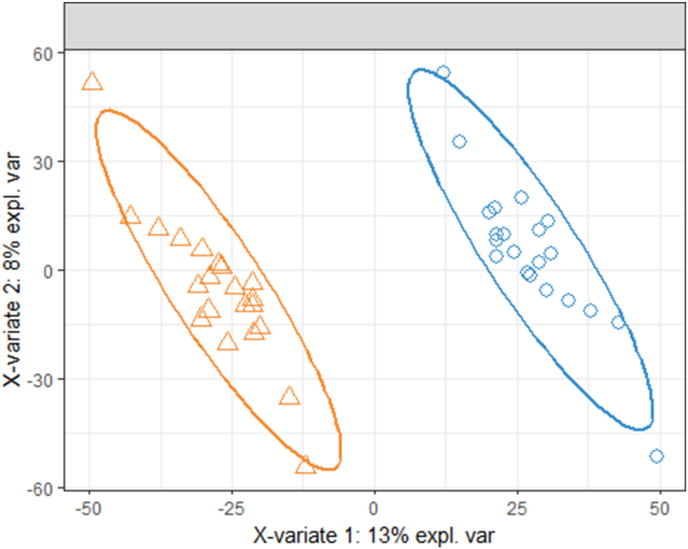
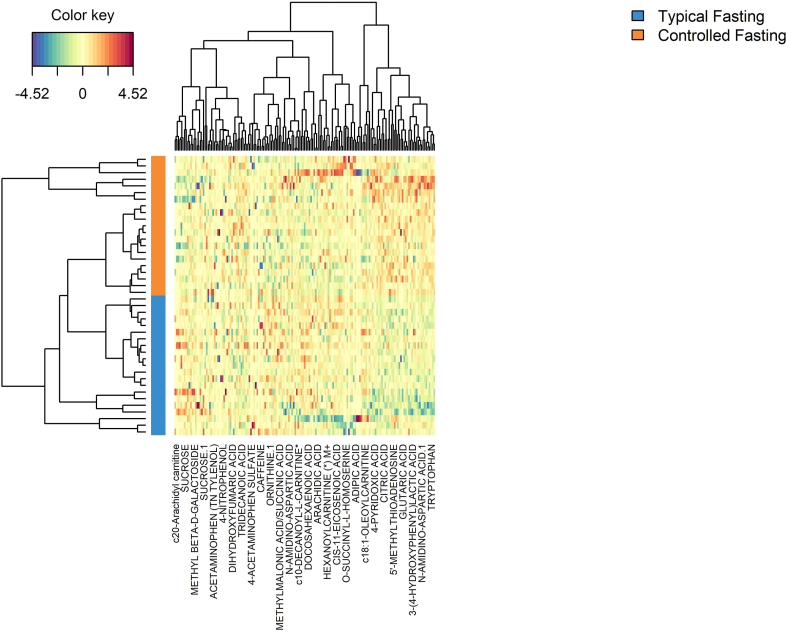
The sPLS-DA model showed excellent discrimination between the typical fasting and controlled fasting visits (Fig. 3). The overall classification error rate was 6.7% for the first sPLS-DA component and 4.8% for the second component. Fig. 4 is a heat map of the 30 known metabolites that were most important in discriminating between visits in the sPLS-DA model. The controlled fasting visit samples had higher relative abundances of c18:1-oleoylcarnitine, 4-pyridoxic acid, citric acid, 5′-methylthioadenosine, glutaric acid, 3-(4-hydroxyphenyl)lactic acid, n-amidino-aspartic acid, and tryptophan compared to the typical fasting visit, while the typical fasting visit samples had higher relative abundances of c20-arachidyl carnitine, sucrose, and methyl beta-d-galactoside.
Fig. 3.
Sample plot from the sPLS-DA using all metabolites, with 95% confidence ellipses for the controlled fasting (orange triangles) and typical fasting (blue circles) samples. Each sample is represented as a point according to its projection on the first two components of the sPLS-DA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 4.
A clustered image map or heat map of the top 30 known metabolites from the sPLS-DA comparing the controlled fasting and typical fasting visits. Rows represent samples and columns represent metabolites.
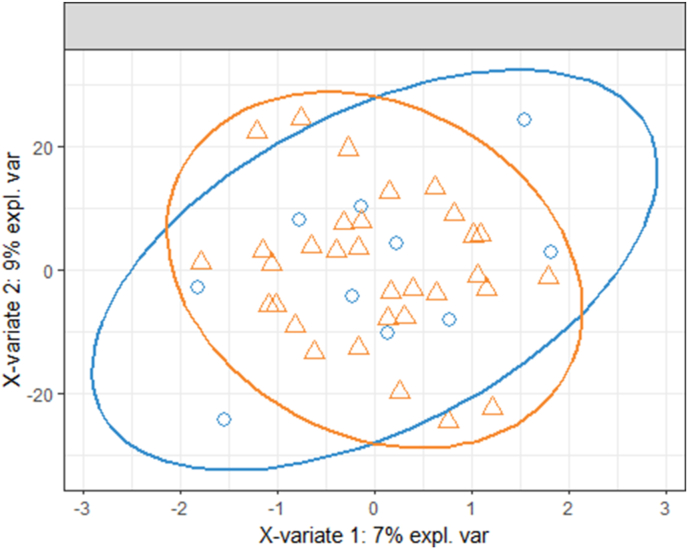
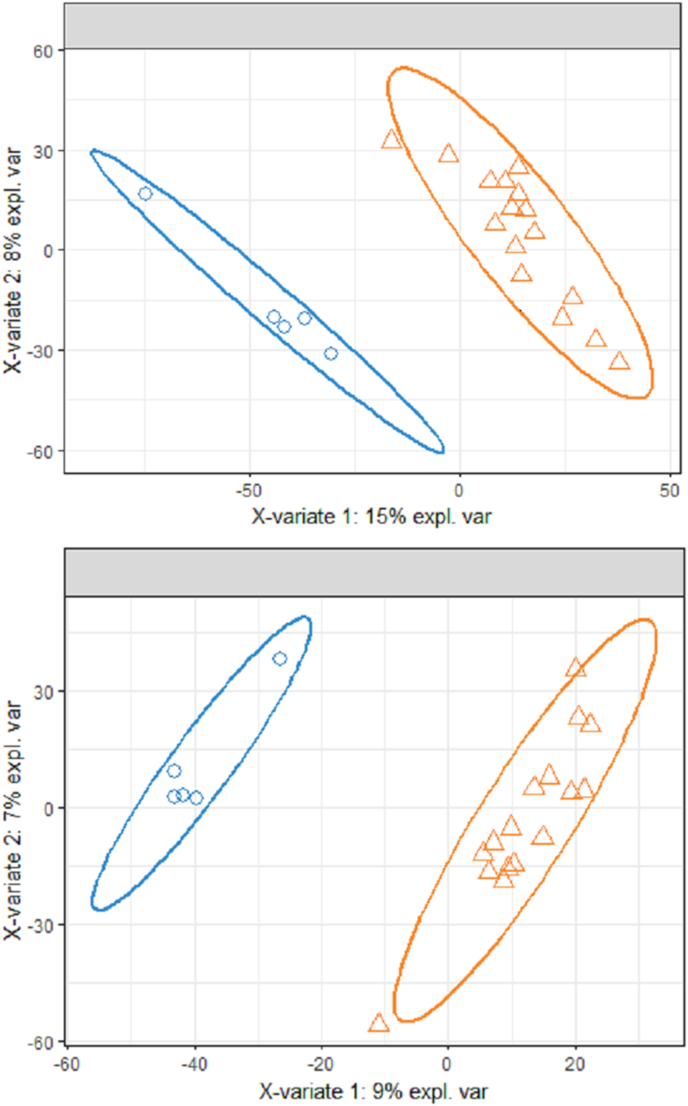
The sPLS-DA model for PCOS vs. control participants, which included the samples from the typical fasting and controlled fasting visits for each participant, was not able to discriminate between the two groups (Fig. 5). The error rate for both the first and second sPLS-DA components was 23.8%. After stratifying by visit, discrimination of PCOS status was improved (Fig. 6), although the amount of variability explained by the first component was higher in the controlled fasting visit samples compared to the fasting visit samples (15% vs. 9%). The classification error rate for the controlled fasting visit was 17.1% and 18.1% for the first two components, respectively. The error rate for the typical fasting visit was 19.5% and 18.1% for the first two components respectively.
Fig. 5.
Sample plot from the sPLS-DA with the controlled fasting and typical fasting visits combined, with 95% confidence ellipses for PCOS (orange triangles) and control (blue circles) samples. Each sample is represented as a point according to its projection on the first two components of the sPLS-DA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
Fig. 6.
Sample plot from the sPLS-DA using all metabolites, with 95% confidence ellipses for PCOS (orange triangles) and control (blue circles) samples. The top panel includes controlled fasting visit samples only, and the bottom panel includes typical fasting visit samples only. Each sample is represented as a point according to its projection on the first two components of the sPLS-DA. (For interpretation of the references to colour in this figure legend, the reader is referred to the Web version of this article.)
4. Discussion
Our results demonstrate that different pre-sample collection protocols for study participants’ diet and physical activity can significantly affect the blood metabolome, and induce variability that obscures other differences of interest (in our case, PCOS vs. control). Once we stratified the analysis by visit, we could discriminate differences by PCOS status, in both the controlled fasting samples and the typical fasting samples. The amount of variability explained by the first component of the sPLS-DA was 15% for the controlled fasting visit, compared to 9% for the typical fasting samples, suggesting that the standardized diet and restricted physical activity may have reduced the variability in metabolites compared to simply fasting. However, the AUC for the first and second components were not drastically different (0.9463 and 0.9475 for controlled fasting visit and 0.9412 and 0.9400 for the typical fasting visit), nor were the cross-validated prediction error rates for the first and second components (17.1% and 18.1% for the controlled fasting visit and 19.5% and 18.1% for the typical fasting visit), which leads us to conclude that typical overnight outpatient fasting may be sufficient to control variability in metabolomics measures and the added burden and cost of a standardized diet may not be justified.
There have been few studies that have investigated the effects of various pre-sample collection protocols on metabolomics profiles, and none that have directly compared the effects of typical fasting alone vs. standardized diet and restricted physical activity followed by monitored inpatient fasting on the ability to detect other biological associations. Walsh et al. [14] found that a standardized diet did not reduce variability in blood metabolomics profiles; however, in their study, diet was standardized by having each participant consume the same foods that they did on the prior visit, and was not standardized across participants. However, in their PCA scores plot, the two visits at which participants consumed the same foods did not cluster together more tightly than at other visits. Similarly, Kim et al. [15] concluded that meal effects were more pronounced in urine metabolomics profiles compared to blood, and, although they stated that it would “be prudent” to utilize fasting samples, random pre-meal samples were recommended if this was not feasible. However, neither of these studies tested whether group differences of interest could be discriminated in the presence of other sources of variability; instead, they examined metrics such as the proportion of variability explained by each source of variation. Our findings are consistent with those of Winnike et al. [16], who demonstrated a change in the serum metabolome after 1 day of standardized diet compared to fasting samples. Although they qualitatively compared the effects of fasting and standardized diet by examining the location of these samples on PCA score plots, they did not examine how the different pre-sample collection protocols affected detection of other effects of interest.
There are several limitations to our study. The sample size used in this analysis was relatively small, and was limited to adolescent females with obesity with and without PCOS, and therefore, the results may not be generalizable to other populations or scientific differences of interest. For example, if the effect size of a between-group difference of interest is larger than that observed in our study between the PCOS group and the control group, it may be distinguishable despite differences in pre-sample collection protocols. We did not collect non-fasting samples, so we cannot conclude whether typical fasting reduced variability compared to non-fasting. The generalizability of our findings to other populations, as well as to other metabolomics platforms, should be confirmed in future studies. Finally, although we provided the standardized diet, participants were not admitted to the hospital during the entire 3 days prior to the controlled fasting visit, only the 12 h prior to the hyperinsulinemic euglycemic clamp, and so we cannot be sure of the level of adherence to the diet, nor can we be sure that participants were truly fasting at the typical fasting visit.
5. Conclusions
The metabolomic profiles associated with samples obtained after typical outpatient fasting and samples obtained after 3 days of a standardized diet and reduced physical activity followed by 12 h of monitored inpatient fasting can be discriminated. Combining these two types of samples obscured differences in participants with PCOS and BMI-matched controls. In studies that plan to perform metabolomics analyses, careful attention should be paid to standardize acute dietary and activity history. Depending on the sample size of the study and the expected effect size of the outcomes of interest, control of diet and physical activity beyond typical outpatient fasting may not be required.
Funding
This work was supported by the following funding: K.J.N.: NCRR K23 RR020038-01, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, NIH/NIDDK 1R56DK088971-01, JDRF 5-2008-291, ADA 7-11-CD-08. M.C.G.: American Heart Association 13CRP 14120015, Thrasher Pediatric Research Foundation Mentored Pilot Grant, NIH/NCRR Colorado CTSI Co-Pilot Grant TL1 RR025778, Pediatric Endocrinology Society Fellowship, NIDDK T32 DK063687, BIRCWH K12HD057022, NIDDK K23DK107871, Doris Duke Foundation 2015212, MRC2: Michigan Regional Comprehensive Metabolomics pilot grant DK097153. This research was also supported by NIH/NCATS Colorado CTSA Grant Number UL1 TR001082.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgments
We thank the participants and their families, the CTRC nursing staff, and the MRC2: Michigan Regional Comprehensive Metabolomics Resource Core, in particular Drs. Charles Burant and Maureen Kachman.
References
- 1.Johnson C.H., Ivanisevic J., Siuzdak G. Metabolomics: beyond biomarkers and towards mechanisms. Nat Rev Mol Cell Biol. 2016;17(7):451–459. doi: 10.1038/nrm.2016.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yin P., Lehmann R., Xu G. Effects of pre-analytical processes on blood samples used in metabolomics studies. Anal Bioanal Chem. 2015;407(17):4879–4892. doi: 10.1007/s00216-015-8565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bouchard-Mercier A., Rudkowska I., Lemieux S., Couture P., Vohl M.-C. The metabolic signature associated with the Western dietary pattern: a cross-sectional study. Nutr J. 2013;12(1):158. doi: 10.1186/1475-2891-12-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Menni C., Zhai G., MacGregor A. Targeted metabolomics profiles are strongly correlated with nutritional patterns in women. Metabolomics. 2013;9(2):506–514. doi: 10.1007/s11306-012-0469-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guertin K.A., Moore S.C., Sampson J.N. Metabolomics in nutritional epidemiology: identifying metabolites associated with diet and quantifying their potential to uncover diet-disease relations in populations. Am J Clin Nutr. 2014;100(1):208–217. doi: 10.3945/ajcn.113.078758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zheng Y., Yu B., Alexander D., Steffen L.M., Boerwinkle E. Human metabolome associates with dietary intake habits among african Americans in the atherosclerosis risk in communities study. Am J Epidemiol. 2014;179(12):1424–1433. doi: 10.1093/aje/kwu073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Esko T., Hirschhorn J.N., Feldman H.A. Metabolomic profiles as reliable biomarkers of dietary composition. Am J Clin Nutr. 2017;105(3):547–554. doi: 10.3945/ajcn.116.144428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Garcia-Perez I., Posma J.M., Gibson R. Objective assessment of dietary patterns by use of metabolic phenotyping: a randomised, controlled, crossover trial. Lancet Diabetes Endocrinol. 2017;5(3):184–195. doi: 10.1016/S2213-8587(16)30419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Playdon M.C., Moore S.C., Derkach A. Identifying biomarkers of dietary patterns by using metabolomics. Am J Clin Nutr. 2017;105(2):450–465. doi: 10.3945/ajcn.116.144501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gonzalez-Granda A., Damms-Machado A., Basrai M., Bischoff S. Changes in plasma acylcarnitine and lysophosphatidylcholine levels following a high-fructose diet: a targeted metabolomics study in healthy women. Nutrients. 2018;10(9):1254. doi: 10.3390/nu10091254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kujala U.M., Mäkinen V.-P., Heinonen I. Long-term leisure-time physical activity and serum metabolome. Circulation. 2013;127(3):340–348. doi: 10.1161/CIRCULATIONAHA.112.105551. [DOI] [PubMed] [Google Scholar]
- 12.Xiao Q., Moore S.C., Keadle S.K. Objectively measured physical activity and plasma metabolomics in the Shanghai Physical Activity Study. Int J Epidemiol. 2016;45(5):1433–1444. doi: 10.1093/ije/dyw033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bell J.A., Hamer M., Richmond R.C., Timpson N.J., Carslake D., Davey Smith G. Associations of device-measured physical activity across adolescence with metabolic traits: prospective cohort study. In: Basu S., editor. vol. 15. 2018. (PLOS med). 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Walsh M.C., Brennan L., Malthouse J.P.G., Roche H.M., Gibney M.J. Effect of acute dietary standardization on the urinary, plasma, and salivary metabolomic profiles of healthy humans. Am J Clin Nutr. 2006;84(3):531–539. doi: 10.1093/ajcn/84.3.531. [DOI] [PubMed] [Google Scholar]
- 15.Kim K., Mall C., Taylor S.L. Mealtime, temporal, and daily variability of the human urinary and plasma metabolomes in a tightly controlled environment. In: Brennan L., editor. vol. 9. 2014. (PLoS ONE). 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Winnike J.H., Busby M.G., Watkins P.B., O’Connell T.M. Effects of a prolonged standardized diet on normalizing the human metabolome. Am J Clin Nutr. 2009;90(6):1496–1501. doi: 10.3945/ajcn.2009.28234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brauer R., Leichtle A.B., Fiedler G.M., Thiery J., Ceglarek U. Preanalytical standardization of amino acid and acylcarnitine metabolite profiling in human blood using tandem mass spectrometry. Metabolomics. 2011;7(3):344–352. doi: 10.1007/s11306-010-0256-1. [DOI] [Google Scholar]
- 18.Sampson J.N., Boca S.M., Shu X.O. Metabolomics in epidemiology: sources of variability in metabolite measurements and implications. Cancer Epidemiol Biomark Prev. 2013;22(4):631–640. doi: 10.1158/1055-9965.EPI-12-1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cree-Green M., Newcomer B.R., Coe G. Peripheral insulin resistance in obese girls with hyperandrogenism is related to oxidative phosphorylation and elevated serum free fatty acids. Am J Physiol Endocrinol Metab. 2015;308(9):E726–E733. doi: 10.1152/ajpendo.00619.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cree-Green M., Rahat H., Newcomer B.R. Insulin resistance, hyperinsulinemia, and mitochondria dysfunction in nonobese girls with polycystic ovarian syndrome. J Endocr Soc. 2017;1(7):931–944. doi: 10.1210/js.2017-00192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cree-Green M., Bergman B.C., Coe G.V. Hepatic steatosis is common in adolescents with obesity and PCOS and relates to de novo lipogenesis but not insulin resistance. Obesity. 2016;24(11):2399–2406. doi: 10.1002/oby.21651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Legro R.S., Arslanian S.A., Ehrmann D.A. Diagnosis and treatment of polycystic ovary syndrome: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2013;98(12):4565–4592. doi: 10.1210/jc.2013-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Witchel S.F., Oberfield S., Rosenfield R.L. The diagnosis of polycystic ovary syndrome during adolescence. Horm Res Paediatr. 2015;83(6):376–389. doi: 10.1159/000375530. [DOI] [PubMed] [Google Scholar]
- 24.Liquet B., Cao K.-A.L., Hocini H., Thiébaut R. A novel approach for biomarker selection and the integration of repeated measures experiments from two assays. BMC Bioinf. 2012;13(1):325. doi: 10.1186/1471-2105-13-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lê Cao K.-A., Boitard S., Besse P. Sparse PLS discriminant analysis: biologically relevant feature selection and graphical displays for multiclass problems. BMC Bioinf. 2011;12(1):253. doi: 10.1186/1471-2105-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rohart F., Gautier B., Singh A., Lê Cao K.-A. mixOmics: an R package for ‘omics feature selection and multiple data integration. In: Schneidman D., editor. vol. 13. 2017. (PLOS comput biol). 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Le Cao K.-A., Rohart F., Gonzalez I. 2016. MixOmics: omics data integration project.https://CRAN.R-project.org/package=mixOmics [Google Scholar]