Highlights
-
•
Adolescents conform to peers’ prosocial behavior.
-
•
High testosterone and low cortisol are associated with prosocial conformity.
-
•
Prosocial conformity is linked to activation in social-reward brain regions.
-
•
Brain activation during prosocial conformity is linked to high testosterone and low cortisol.
Keywords: Dual hormone hypothesis, Prosocial, fMRI, Peer influence, Testosterone, Cortisol
Abstract
The dual hormone hypothesis, which centers on the interaction between testosterone and cortisol on social behavior, offers a compelling framework for examining the role of hormones on the neural correlates of adolescent peer conformity. Expanding on this hypothesis, the present study explored the interaction between testosterone and cortisol via hair concentrations on adolescents’ conformity to peers. During fMRI, 136 adolescents (51 % female) ages 11–14 years (M = 12.32; SD = 0.6) completed a prosocial decision-making task. Participants chose how much of their time to donate to charity before and after observing a low- or high-prosocial peer. Conformity was measured as change in behavior pre- to post-observation. High testosterone with low cortisol was associated with greater conformity to high-prosocial peers but not low prosocial peers. Focusing on high prosocial peers, whole-brain analyses indicated greater activation post- vs. pre-observation as a function of high testosterone and low cortisol in regions implicated in social cognition, salience detection, and reward processing: pSTS/TPJ, insula, OFC, and caudate nucleus. Results highlight the relevance of hormones for understanding the neural correlates of adolescents’ conformity to prosocial peers.
1. Introduction
Adolescents are inherently motivated to emulate the attitudes and behaviors of their peers. Such peer conformity (Kosten et al., 2012) helps adolescents form friendships and attain status within their peer networks (Brechwald and Prinstein, 2013). While adolescents’ propensity for peer conformity can result in maladaptive behaviors, adolescents also conform to prosocial behaviors such as volunteering and helping others (Choukas-Bradley et al., 2015; for a review, see Guroglu, 2020). Engagement in prosocial behaviors increases during adolescence (Do et al., 2019) and has been associated with various positive social and health outcomes (Eisenberg et al., 2006). Increases in neural sensitivity to social information and social rewards during adolescence support changes in peer conformity and prosocial behavior (Blakemore and Mills, 2014; Telzer et al., 2018). Further, dual hormone theories postulate that the interaction between cortisol and testosterone modulates status seeking behaviors (Mehta and Josephs, 2010; Sinclair et al., 2014). Applying a dual hormone perspective to examinations of the neural processes undergirding peer conformity during adolescence affords a more comprehensive understanding of the biological processes supporting adolescent social behavior. To this end, the present study used a dual hormone framework to explore individual differences in the neural correlates of adolescents’ conformity to prosocial peers.
In adolescence, status-seeking involves attempts to be liked and accepted by peers or to achieve social prestige (Li and Wright, 2014). One way to achieve acceptance and status is by conforming to peers (Brechwald and Prinstein, 2013). Based on social learning theories (Akers, 2001; Bandura and Walters, 1977), individuals endorse social norms through observation and learning. When observing a discrepancy between their own behavior and that of their peers’, adolescents are motivated to change their behavior (Cialdini and Goldstein, 2004). Critically, which behaviors are endorsed to achieve status depends on context. For example, prosocial behavior is more likely to be endorsed when status is defined by respect, admiration (Lease et al., 2002), or generosity (Halevy et al., 2012).
Adolescence is a key developmental period for studying peer conformity due to maturational changes in the developing brain. Maturation of brain regions supporting social cognition (e.g., dorsal medial prefrontal cortex (dmPFC), temporoparietal junction (TPJ), and posterior superior temporal sulcus (pSTS)), reward processing (e.g., orbitofrontal cortex (OFC), ventral striatum (VS)), and affective salience (e.g., caudate, nucleus, amygdala) undergo reorganization around the time of puberty, and activation in these regions is associated with greater peer orientation (Blakemore and Mills, 2014; Nelson et al., 2016) and social-reward sensitivity (Foulkes and Blakemore, 2016; Perino et al., 2016). Consistent with this, adolescents evince heightened activation in the mPFC, TPJ, and STS when making prosocial decisions in the presence of peers (van Hoorn et al., 2016). Heightened TPJ activation in response to prosocial stimuli is associated with greater prosocial decision-making among adolescents (Tashjian et al., 2018), which is in turn associated with greater activation in regions supporting socio-emotional (e.g., insula; Wang et al., 2019) and reward (e.g., VS; Braams and Crone, 2017) processing.
Puberty-induced rises in testosterone lead to heightened activation of reward-sensitive brain regions such as the VS (Hermans et al., 2010; Op de Macks et al., 2011), resulting in greater social-reward sensitivity (De Lorme et al., 2013), approach behaviors (Platje et al., 2015), and status-seeking (e.g., Rowe et al., 2004). In contrast, cortisol down-regulates activity in the ventral striatum (Montoya et al., 2014); thus, it is associated with social withdrawal at high levels (Van der Westhuizen and Solms, 2015) and approach behaviors at low levels (Terburg et al., 2009). Further, testosterone and cortisol are jointly involved in connectivity between prefrontal (e.g., mPFC and OFC) and subcortical regions (e.g., amygdala) (Veer et al., 2012). Accordingly, the brain’s reward circuitry may be one mechanism by which testosterone and cortisol modulate status-seeking behaviors (Knight et al., 2020).
The dual hormone hypothesis (Mehta and Josephs, 2010) offers a useful framework for exploring individual differences in adolescents’ conformity to peers. The hypothesis is informed by the co-regulation of the hypothalamic-pituitary-gonadal (HPG) and hypothalamic-pituitary-adrenal (HPA) axes, which control the release of testosterone and cortisol, respectively (Viau, 2002). According to this hypothesis, high testosterone is associated with greater status-seeking when cortisol is low (Terburg et al., 2009), not high (Prasad et al., 2017; Sherman et al., 2016). However, support for the dual hormone hypothesis is still mixed (for reviews, see Dekkers et al., 2019; Grebe et al., 2019; Knight et al., 2020). For example, in some studies, high testosterone is associated with prosocial characteristics when cortisol is high (Zilioli et al., 2014) whereas other studies demonstrate this association when cortisol is low (Ponzi et al., 2016). Some scholars have speculated that the inconsistent support for the dual hormone hypothesis is partly because status is defined differently across contexts (e.g. Knight et al., 2020). For instance, when prosocial behavior is expected to lead to high status, high testosterone with low cortisol should be related to greater prosocial behavior (see Casto et al., 2019; Prasad et al., 2019), whereas this pattern would be reversed in contexts in which low prosocial behavior is expected to lead to high status (Knight et al., 2020). However, these assertions have yet to be empirically tested. Thus, the present study explored how the interaction between testosterone and cortisol relates to adolescents’ conformity to peers who endorse different degrees of prosocial behavior.
To understand the role of hormones on adolescent conformity, we examined the neural correlates of peer conformity and links with testosterone and cortisol concentrations. Cortisol and testosterone were measured via hair samples, which are indicative of cumulative hormone concentrations in the body that are less susceptible than saliva to diurnal variations in hormone secretions (Kirschbaum et al., 2009). To measure peer conformity, we developed a novel prosocial peer influence fMRI task during which adolescents made decisions to donate their time to various charities, observed a peer endorsing either high or low prosocial behavior, and then made decisions to donate again. Changes in adolescents’ donations after observing the peer indexed peer conformity. Our between-subjects design with low- and high-prosocial peer conditions allowed us to test whether the social context interacts with peer conformity and whether the interaction between testosterone and cortisol is associated with status seeking across contexts (i.e., increases in prosocial behavior in the high-prosocial condition and decreases in prosocial behavior in the low-prosocial condition). Considering mixed empirical evidence for the dual hormone hypothesis (Knight et al., 2020), we hypothesized that high testosterone could be associated with prosocial conformity when cortisol is low (e.g., Ponzi et al., 2016) or high (e.g., Zilioli et al., 2015). We further hypothesized that the interaction between testosterone and cortisol at the behavioral level is paralleled by increased activation in brain regions involved in social cognition, affective salience, and reward processing.
2. Materials and methods
2.1. Participants
One hundred thirty six participants (51 % female) ages 11–14 years (M = 12.32; SD = 0.6) from diverse racial/ethnic backgrounds (36 % Hispanic/Latinx, 29 % White, 22 % Black, and 12 % Mixed/Other) completed the study. Of these 136 participants, 32 did not have usable hormone data (further details provided in the methods) and an additional seven did not have usable fMRI data due to movement or incidentals. Thus, the sample included 136 participants with usable behavioral data, 129 participants with usable fMRI data, 104 participants with usable hormone data, and 97 participants with usable data for analyses testing associations between hormone concentrations and neural activation during prosocial conformity.
Participants were screened for neurological disorders, psychotropic medications, or any MRI contraindications. Parent reports identified nine teens with clinical diagnoses, primarily AD(H)D. Participants who were taking AD(H)D medication were asked to refrain from taking their medication 24 h prior to the scan. Participants were accompanied to the scan by their primary caregiver. All participants and their caregivers provided written assent and consent, and the Institutional Review Board approved all procedures. The study lasted approximately 4 h. Adolescents were compensated up to $90 for their time.
2.2. Experimental design
2.2.1. Prosocial influence task
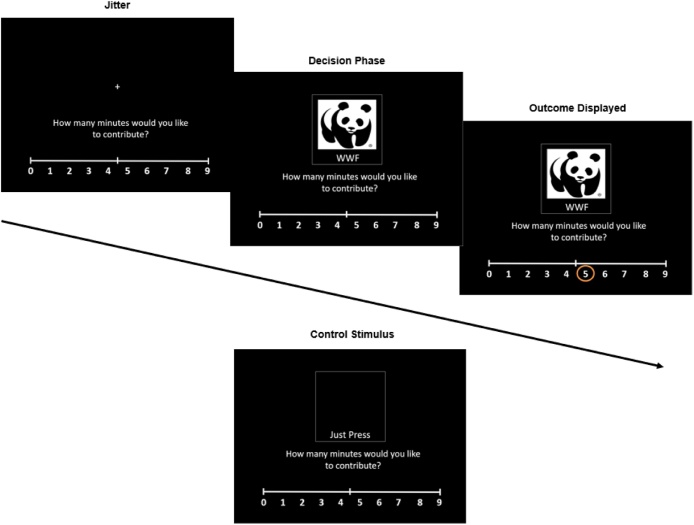
A novel, experimental fMRI task measured peer influence on prosocial decision-making. Participants were told a cover story that the research lab had teamed up with ten local charities from their community. Prior to the scan, participants were shown a logo and description of each charity and learned about the charities’ goals. Following, participants indicated how much they cared about each charity and ranked their top-three favorite charities. Participants were told they had the opportunity to support the charities by donating their time to filling envelopes with donation letters after the scan. Their donated time would come at the cost of their 10-minute break during the session, during which they could otherwise participate in fun activities (e.g., jewelry kits, a basketball hoop, an iPad with games). Participants were also told that another participant had completed the same game. In reality, this participant was a confederate. Prior to the scan, participants viewed the confederate peer’s profile, which was matched to their gender, age, grade, and school.
During the fMRI scan, participants played three rounds of the prosocial influence task. In Round 1, participants chose how much time they wanted to donate, between zero and nine minutes, to each charity. In Round 2, participants observed the choices of the peer confederate and were instructed to input the same response as the peer for each trial (e.g., if the peer responded “7” participants would also enter “7” for that trial). The task used a between-subjects design. Half of the participants (N = 69) observed a high prosocial peer who donated an average of 8 min; the other half of participants (N = 67) observed a low prosocial peer who donated an average of 2 min. We chose an active observation condition to equate decision-making processes across rounds and to assess participants’ attentiveness. Finally, in Round 3, participants again independently chose how much time to donate to each charity.
Each round started with an instruction screen that stated “Play” for Rounds 1 and 3 and “Evaluate Peer” for Round 2. For each round, 40 trials were presented in random order. Each decision screen presented the logo of the charity, charity name, and a rating scale showing 0−9 min. Charities were presented three times per round and were intermixed with 10 control trials, which were included to disentangle prosocial from general decision-making. The display screen for control trials was identical to that of charity trials except there was no logo and the charity name was replaced with the phrase, “Just Press”. During these trials, participants were instructed to press any button.
The decision screen was displayed for a maximum of 3000 ms or until the participant made a decision. Participants’ decisions were highlighted on the screen for 500 ms. During the peer observation round, the decision screen was displayed for an average of 500 ms, followed by the highlighted peer’s decision, which was presented for up to 3000 ms or until the participant responded. The highlighted peer’s decision stayed on the screen for 500 ms after participants’ response. Trials were separated with a randomized jitter based on a Gaussian distribution (M = 2300 ms; range 507−4217 ms). If participants did not respond within 3000 ms, they saw “Too Late” for 1000 ms and proceeded to the next trial. See Fig. 1 for a visual display of the trial sequence.
Fig. 1.
Time Game trial sequence. Ten control stimuli were intermixed with the charity trials in each round.
Participants were instructed that the number of minutes they donated would be randomly selected from one of the trials and that amount of time would be deducted from their 10-minute break. After the scan, participants were given letters for the randomly selected charity and asked to place them in envelopes. A timer was set for the number of minutes they donated on the randomly selected trial. The researcher stepped out of the room during this time. If participants donated 0 min or had time remaining in their 10-minute break, the researcher returned to the room and gave the participants access to activities for the remainder of the 10 min. This procedure was implemented to maintain the believability of the cover story but was not relevant for the analyses.
2.3. Hair hormone acquisition
Three hair segments, 3 mm in diameter and at least 2 cm in length, were cut as close as possible to the scalp from left, center, and right side of the posterior vertex located at the center of the back of the head. The most proximal 2−3 cm hair segment was analyzed using liquid chromatography coupled with tandem mass spectrometry for testosterone and cortisol concentrations. Wash and steroid extraction procedures are described elsewhere (Gao et al., 2013 and Stalder et al., 2013). The majority of subjects (93 %) had at least 3 cm of hair, representing 3-month cumulative hormone concentrations. Hair samples weighing at least 7.5 mg are considered suitable for reliable analysis (Gao et al., 2013). Hair samples from seven participants were less than 7.5 mg in total weight. Sensitivity analyses with and without these seven subjects indicated no change in the results of the primary analyses. Thus, all participants providing hair samples were included in the analyses. Hair samples were not collected for 30 subjects because participants did not wish to provide a hair sample, the hair was too short (less than 2 cm in length), or the hairstyle was not amenable to cutting. Additionally, outliers exceeding 3 standard deviations (n = 4) were excluded from analyses. This resulted in a sample of 104 participants with usable hormone data. In this sample, testosterone and cortisol were not significantly correlated (r = .118, ns). There were no differences in performance on the prosocial task between individuals missing and not missing hormone data (all t(134) < 1.056, all p > .293).
2.3.1. Covariates
There are several biological and environmental factors that can influence hair hormone concentrations. Biologically, testosterone and cortisol concentrations change dramatically over the course of adolescence, with males evincing higher levels of testosterone than females. Thus, we examined associations between pubertal development (Petersen et al., 1988) and biological sex with hormone concentrations. Higher levels of hair cortisol were associated with a slightly earlier pubertal stage (r = -.192, p = .048), but testosterone was not associated with pubertal development (r = -.085, p = .383). Cortisol and testosterone were not significantly associated with age (rcortisol = .01, p = .915; rtestosterone = .111, p = .254). Additionally, there were no significant differences in cortisol (t(105) = -1.28, p = .203) or testosterone (t(105) = -1.199, p = .233) concentrations between biological males and females.
Medications including steroids, oral contraceptives, and hair treatments have all been shown to influence hair hormone concentrations (for a review, see Stalder and Kirschbaum, 2012). Thus, participants reported on their medication use and whether they had bleached or chemically straightened their hair. There were no significant differences in cortisol or testosterone concentrations among participants who bleached (n = 15) or chemically straightened (n = 9) their hair from those who did not (t(104) < 1.05, all p > .29). For participants using relevant medications (n = 12), there was evidence for significantly higher cortisol hair concentrations (t(102) = 3.124, p = .002) but no differences in testosterone hair concentrations (t(102) = .663, p = .509) than participants who were not on medications.
2.4. fMRI data acquisition
Data were collected with a 3-T Siemens Prisma MRI scanner using a 32-channel head coil. We obtained the functional data using T2*-weighted echoplanar images (EPI) (slice thickness = 3 mm; 38 slices; TR = 2 s; TE = 25msec; matrix = 92 × 92; FOV = 230 mm; voxel size 2.5 × 2.5 × 3mm3). For anatomical reference, structural scans were also acquired, including a T2*weighted, matched-bandwidth (MBW; TR = 4 s; TE = 64msec; FOV = 230; matrix = 192 × 192; slice thickness = 3 mm; 38 slices) and a T1* magnetization-prepared rapid-acquisition gradient echo (MPRAGE; TR = 1.9 s; TE = 2.32 msec; FOV = 230; matrix = 256 × 256; sagittal acquisition plane; slice thickness = 0.9 mm; 192 slices). MBW and EPI scans were collected with an oblique axial orientation to maximize brain coverage.
2.5. fMRI data preprocessing and analysis
Standard preprocessing was conducted using the FSL FMRIBs Software Library (FSL v6.0; https://fsl.fmrib.ox.ac.uk/fsl/). We corrected for slice-to-slice head motion using MCFLIRT (Jenkinson et al., 2002). Data were skull-stripped with BET (Smith, 2002), spatially smoothed with a 6-mm FWHM Gaussian kernel, and a high-pass temporal filtering with a 128 s cutoff was applied to remove low-frequency drift across time (Gaussian-weighted least squares straight line fitting; sigma = 64 s). Image co-registration was done using a three-step registration procedure (EPI to T2 to T1), and each functional image was resampled to 2 × 2 × 2 mm and warped to the standard Montreal Neurological Institute 2-mm brain using FLIRT (Jenkinson and Smith, 2001; Jenkinson et al., 2002). To remove artifact signals such as motion and physiological noise, we applied an independent component analysis (ICA) denoising procedure using MELODIC (Beckmann and Smith, 2004) combined with an automated signal classification toolbox (Neyman-Pearson threshold = 0.3; Tohka et al., 2008). Data for 7 participants were excluded due to excessive motion (> 2 mm inter-slice movement on ≥ 10 % of slices) and incidental findings.
After preprocessing, statistical analyses were conducted on individual subject’s data using general linear modelling in the Statistical Parametric Mapping software package (SPM8; Wellcome Centre for Human Neuroimaging, London). The prosocial influence task was modeled as an event-related design. Each trial was convolved with the canonical hemodynamic response function. In the fixed-effects model, we included 6 regressors: charity decisions pre-peer observation, charity decisions post-peer observation, charity decisions during peer observation, and control decisions for each of the three rounds. The decision phase was modeled from the onset of each decision screen with a duration of participants’ response times. At the trial level, the number of minutes donated on each trial was included as a parametric modulator to control for trial-level differences in minutes donated. Controlling for the parametric modulator allowed us to isolate differences in mean activation between the pre- and post-peer observation blocks while holding constant the number of minutes participants donated in each trial. Trials with no response were modeled as a separate junk regressor along with volumes containing excessive motion. The decision regressors were estimated separately for each round to distinguish observing from decision-making and to compare activation before and after peer observation.
The individual-level contrast images were submitted to random-effects group-level analyses. In the current study, our contrast of interest was charity decisions post-peer observation > charity decisions pre-peer observation. We first regressed condition (high-prosocial, low-prosocial) on the post-observation > pre-observation contrast. Next, we examined the post-observation > pre-observation contrast within each condition separately. To test the dual-hormone hypothesis, we conducted whole-brain multiple regression analyses, in which we included the cortisol x testosterone interaction as a regressor, controlling for the main effects of pre-observation donations, cortisol, and testosterone. Group-level analyses were conducted using GLMFlex, which removes outliers and sudden activation changes, partitions error terms, analyzes all voxels containing data, and corrects for variance-covariance inequality (http://mrtools.mgh.harvard.edu/index.php/GLM_Flex). We corrected all analyses for multiple comparisons using Monte Carlo simulations through 3DClustSim (updated version November 2016) in the software package AFNI (Ward, 2000), and accounted for the intrinsic smoothness of the data with the -acf function within the 3dFWHMx command. We used a voxel-wise threshold of p < .005, corresponding to p < .05, FWE cluster-corrected. All reported results are available on NeuroVault (Gorgolewski et al., 2015; see https://neurovault.org/collections/PDZHKDRI/).
3. Results
3.1. Behavioral results
All behavioral analyses were conducted using SPSS 26. The primary study variables were peer conformity, operationalized as the change in minutes donated post- versus pre-peer observation (round 3 min donated – round 1 min donated) and cortisol and testosterone hair concentrations. Considering hormone concentrations typically differ between males and females, descriptive information for the primary variables is presented for the full sample and for males and females separately in Table 1. However, as we note in section 2.3.1, comparisons of hair hormone concentrations yielded no significant differences between males and females in cortisol or testosterone.
Table 1.
Descriptive statistics for primary study variables across females, males, and the full sample.
| N | Min. | Max. | M | SD | ||
|---|---|---|---|---|---|---|
| Hair Cortisol (pg/mg) | Females | 60 | 0.54 | 23.79 | 4.40 | 4.14 |
| Males | 44 | 0.27 | 22.16 | 5.29 | 4.69 | |
| Total | 104 | 0.27 | 23.79 | 4.78 | 4.38 | |
| Hair Testosterone (pg/mg) | Females | 61 | 0 | 1.91 | 0.28 | 0.44 |
| Males | 43 | 0 | 6.45 | 0.46 | 1.08 | |
| Total | 104 | 0 | 6.45 | 0.36 | 0.77 | |
| Pre-Observation Mins. | Females | 70 | 0 | 8.53 | 5.60 | 2.21 |
| Males | 66 | 0 | 8.89 | 5.43 | 2.38 | |
| Total | 136 | 0 | 8.89 | 5.52 | 2.29 | |
| Post-Observation Mins. | Females | 70 | 0 | 8.69 | 5.51 | 2.21 |
| Males | 66 | 0 | 8.70 | 5.34 | 2.59 | |
| Total | 136 | 0 | 8.70 | 5.43 | 2.40 |
Pre-Observation and Post-Observation Mins. represent minutes donated in the pre-peer observation and post-peer observation rounds, respectively.
3.1.1. Peer influence on prosocial behavior
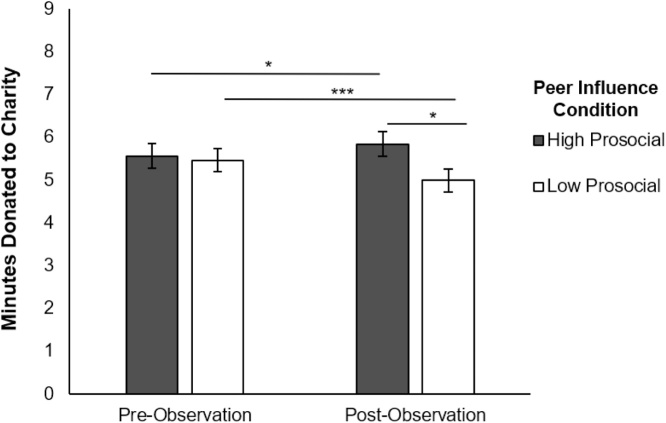
We first examined whether the peer observation manipulation (low vs. high prosocial) yielded changes in participants’ behavior. We conducted a 2 × 2 repeated measures ANOVA for condition (low prosocial, high prosocial) x round (pre-peer influence, post-peer influence) to compare the number of minutes donated. Results indicated a significant interaction between condition and round (F(1, 134) = 20.009, p < .001; see Fig. 2). Follow-up independent samples t-tests indicated no significant differences in the number of minutes donated between the low prosocial and high prosocial groups in the pre-peer observation round (t(134) .246, ns), whereas in the post-peer observation round, adolescents in the high prosocial condition donated significantly more minutes than those in the low prosocial condition (t(134) = 2.085, p = .039). Furthermore, adolescents in the high prosocial condition donated significantly more minutes post-peer observation (t(68) = 2.151, p = .035), whereas adolescents in the low prosocial condition donated significantly fewer minutes post-peer observation (t(66) = -4.42, p < .001). Together, findings suggest that the low and high peer observation manipulation was successful in changing adolescents’ behaviors.
Fig. 2.
Number of minutes donated to charity in the pre- and post- peer observation rounds between groups (high prosocial vs. low prosocial peer observation).
*** p < .001; * p < .05.
3.1.2. Test of the dual-hormone hypothesis
We conducted a linear regression to test the dual-hormone hypothesis in the prediction of peer influence on prosocial behavior. Our outcome measure for this analysis was calculated as the change in minutes donated between post- and pre-peer observation rounds. The total number of minutes donated in the pre-peer observation round was included as a covariate in the first step of the analysis to control for baseline behavior. In the second step, task condition (low prosocial = 1), cortisol concentrations, and testosterone concentrations were included. Pre-observation minutes, cortisol, and testosterone were grand-mean centered. All two-way interactions among task condition, cortisol, and testosterone were included in the third step, and the three-way interaction among condition, cortisol, and testosterone was included in the final step.
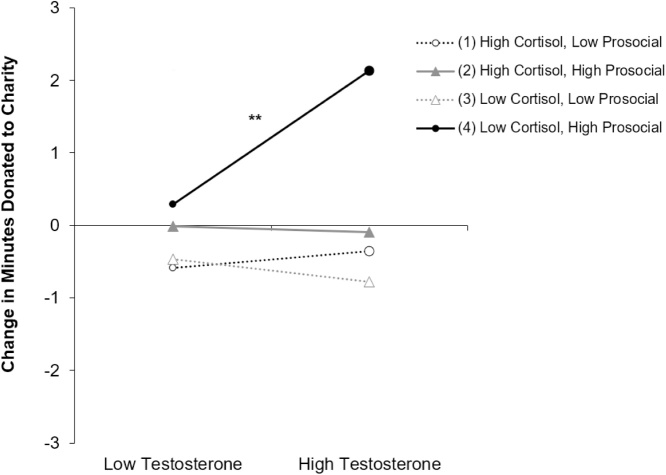
Results indicated a significant three-way interaction among condition, testosterone, and cortisol (see Table 2)1 . To probe this interaction, we conducted two separate regression analyses testing the two-way interaction between cortisol and testosterone in the high prosocial and low prosocial conditions, separately. Results indicated a significant two-way interaction between cortisol and testosterone in the high prosocial (B = -.27, SE (B) = .113, β = -.358, p = .02, 95 % CI = -.496, -.044), but not low prosocial (B = .072, SE (B) = .059, β = .254, p = .231, 95 % CI = -.048, .192) condition. We probed the interaction by plotting the change in minutes donated as a function of cortisol and testosterone at the bottom and top tertiles of their respective distributions in the high prosocial and low prosocial conditions separately (see Fig. 3). Simple slopes analysis indicated that, among adolescents who observed a highly prosocial peer, those with high testosterone and low cortisol demonstrated a significant increase in minutes donated post-peer observation (B = 2.35, p = .005). All other slopes were non-significant. For the marginal effects plot including a marginal rug of observed data, please see Fig. S1 in the supplement. To ensure that biological (sex and puberty) and environmental (medication use and hair treatment) variables did not influence these effects, we conducted a sensitivity analysis controlling for each of these covariates. Behavioral results were unchanged both in terms of statistical significance and effect size. Results from the sensitivity analyses are available in Table S1 of the supplement.
Table 2.
Results from regression analyses predicting prosocial peer conformity as a function of condition (low prosocial, high prosocial) and hormone concentration (cortisol, testosterone).
| 95 % CI |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Model | df | Variable | B | S.E. (B) | LB | UB | Std. β | t | R2 |
| 1 | 100 | Intercept | −.054 | .109 | −0.271 | 0.163 | −.497 | .009 | |
| Pre-Obs. Mins. | −.065 | .046 | −0.157 | 0.027 | −.139 | −1.4 | |||
| 2 | 97 | Condition | −.897*** | .205 | −1.304 | −0.49 | −.407 | −4.374 | .173*** |
| Cortisol | −.031 | .023 | −0.076 | 0.015 | −.122 | −1.325 | |||
| Testosterone | .081 | .131 | −0.179 | 0.342 | .057 | .621 | |||
| 3 | 94 | Cort. x Test. | −.011 | .055 | −0.12 | 0.099 | −.024 | 2.07 | .189 |
| Cond. x Cort. | .107* | .051 | 0.004 | 0.209 | .35 | −1.228 | |||
| Cond. x Test. | −.537 | .437 | −1.406 | 0.331 | −.354 | −.192 | |||
| 4 | 93 | Cond. x Cort. x Test. | .34** | .124 | 0.093 | 0.587 | .685 | 2.73 | .241** |
All variables were centered at the grand mean. Pre-Obs. Mins: minutes donated in the pre-peer observation round; Condition: task condition (1= Low Prosocial); Cortisol and Testosterone: hair concentrations with outliers > 3 S.D. removed; (Cond. x Cort. x Test): interactions among task condition with cortisol and testosterone hair concentrations. LB and UB: lower and upper bounds of the 95 % confidence interval (CI) for the unstandardized coefficient. Significant R2 indicates significant change from the previous model.
p < .001.
p < .01.
p < .05.
Fig. 3.
Estimated slopes for the three-way interaction among task condition, cortisol, and testosterone. Participants could donate up to 9 min to charity. Testosterone and cortisol were centered at the grand mean. Low and high values reflect bottom and top tertiles of the distribution.
** p < .01.
3.2. fMRI results
3.2.1. Peer influence on neural correlates of prosocial behavior
At the whole brain level, we explored changes in neural activation post- versus pre-peer observation as a function of task condition (high prosocial, low prosocial). Results yielded no significant differences in activation across the two conditions.
Because our behavioral results yielded significant differences in the high prosocial but not the low prosocial peer conditions, we next examined changes in neural activation post- versus pre-peer observation among participants in the high prosocial peer condition, controlling for the total number of minutes donated at the trial level. When making greater prosocial decisions after observing a high prosocial peer relative to before peer observation, participants showed significantly greater activation in brain regions implicated in the salience network, including the bilateral insula, fusiform, and dorsal anterior cingulate cortex (dACC) (see Table 3)2 .
Table 3.
Neural regions showing significant change in activation post- versus pre-peer observation among participants (N = 69) in the high prosocial peer condition.
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Region | k | t-value | x | y | z |
| L Insula Lobe | 109 | −4.071 | −36 | 8 | −6 |
| R Insula Lobe | 76 | −3.484 | 46 | 0 | 10 |
| R Anterior Cingulate Cortex | 192 | −3.338 | 8 | 12 | 40 |
| R Fusiform Gyrus | 78 | −3.337 | 34 | −32 | −16 |
| L Inferior Temporal Gyrus | 3047 | −4.283 | −48 | −60 | −8 |
| R Inferior Temporal Gyrus | 234 | −3.998 | 54 | −66 | −12 |
| R Postcentral Gyrus | 169 | −3.728 | 66 | −16 | 34 |
| R Cuneus | 237 | −4.472 | 14 | −94 | 26 |
| L Middle Occipital Gyrus | 181 | −3.637 | −38 | −86 | 28 |
The map was thresholded at p < .005. Monte Carlo Simulation yielded a minimum cluster size of 67 contiguous voxels for whole-brain analysis.
3.2.2. Test of the dual-hormone hypothesis
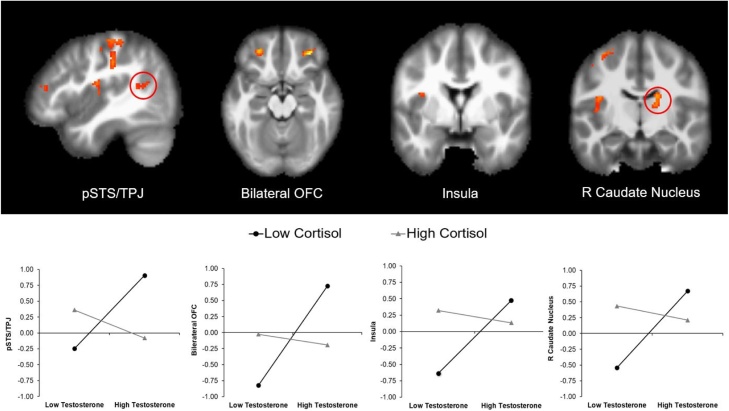
Results from the behavioral analysis indicated a significant cortisol x testosterone interaction only among participants in the high prosocial peer condition but no differences among participants in the low prosocial peer condition. To examine the neural corelates of this interaction, we therefore focused specifically on the high prosocial condition to examine whether the cortisol x testosterone interaction was associated with changes in neural activation when making prosocial decisions post- versus pre-peer observation. Results yielded significant changes in activation in regions such as the posterior superior temporal sulcus/temporoparietal junction (pSTS/TPJ), bilateral orbitofrontal cortex (OFC), caudate, and insula (see Table 4 for full list of activations). For illustrative purposes, we extracted parameter estimates of signal intensity from the pSTS/TPJ, OFC, caudate, and insula and plotted the cortisol x testosterone interaction by plotting the change in neural activation as a function of cortisol and testosterone at the bottom and top tertiles of their respective distributions (see Fig. 4). Examination of the figures suggests that among participants with high testosterone, low cortisol was associated with greater activation post-peer observation than pre-peer observation for all four regions. Note that the plots are illustrative only and are not meant to be interpreted for significance.
Table 4.
Neural regions showing significant change in activation post- versus pre-peer observation among participants (N = 48) in the high prosocial peer observation condition as a function of testosterone and cortisol.
| MNI Coordinates |
|||||
|---|---|---|---|---|---|
| Region | k | t-value | x | y | z |
| pSTS/TPJ | 91 | −2.742 | −44 | −52 | 16 |
| Bilateral Orbitofrontal Cortex | 62 | −4.115 | −28 | 42 | −16 |
| Insula | 158 | −3.082 | −36 | 0 | 18 |
| R Caudate Nucleus | 74 | −3.846 | 16 | −12 | 24 |
| Posterior Cingulate Cortex | 126 | −3.591 | 14 | −50 | 30 |
| L Middle Frontal Gyrus | 68 | −3.17 | −38 | 42 | 18 |
| R Middle Temporal Gyrus | 238 | −4.18 | 50 | −74 | 24 |
| L Precentral Gyrus | 806 | −4.532 | −40 | −28 | 64 |
| Cerebellum | 216 | −5.148 | 10 | −78 | −44 |
| L Cerebellum (Crus 2) | 103 | −3.948 | −6 | −84 | −34 |
| R Superior Orbital Gyrus | 94 | −6.402 | 18 | 38 | −20 |
| L Rolandic Operculum | 158 | −3.629 | −42 | −10 | 20 |
pSTS/TPJ refers to the posterior superior temporal sulcus and temporoparietal junction. Model adjusted for pre-observation donations and the main effects of cortisol and testosterone. All regions are significant at p < .005. Monte Carlo Simulation yielded a minimum cluster size of 60 contiguous voxels for whole-brain analysis.
Fig. 4.
Cortisol x Testosterone interaction correlated with changes in activation in the bilateral OFC, pSTS/TPJ, insula, and caudate nucleus when donating time Post-Observation > Pre-Observation in the high prosocial condition. For descriptive purposes, we extracted parameter estimates of signal intensity from each region (significant at p < .005) and plotted them at low and high values (i.e., bottom and top tertiles) of the distribution for cortisol and testosterone. The plots are for illustrative purposes only and are not meant to be interpreted for significance.
4. Discussion
The present study endeavored to expand the field’s understanding of the associations between hormones and social behavior during adolescence. The dual hormone hypothesis (Mehta and Josephs, 2010) states that high testosterone is associated with status-seeking behaviors when cortisol is low. Applying this framework to adolescent social behavior, we examined the interaction between testosterone and cortisol on adolescents’ conformity to peers on a prosocial fMRI task. The endocrine profile of high testosterone and low cortisol was associated with greater prosocial conformity among adolescents in high-prosocial contexts. This behavioral pattern was paralleled by increased activation in brain regions implicated in social cognition, reward learning, and salience processing. Overall, findings highlight the relevance of hormones for understanding peer conformity during adolescence.
4.1. Dual hormone links to prosocial conformity
Most prior work on the dual hormone hypothesis has focused on behaviors such as dominance and aggression (for a review, see Dekkers et al., 2019), but scholars have recently proposed the possibility that high testosterone and low cortisol may be associated with prosocial behavior in contexts in which prosocial behavior confers status (Knight et al., 2020; Prasad et al., 2019). The present study is one of the first to empirically test this proposition. Findings highlight the importance of context and opportunity for considering the role of hormones in adolescent decision-making. Interestingly, we found that the social context interacts with the dual hormone hypothesis to determine peer conformity. Adolescents with high testosterone and low cortisol showed significant increases in prosocial behavior after observing a highly prosocial peer. When observing a peer engaging in low levels of prosocial behavior, adolescents’ prosocial behavior did not change as a function of cortisol and testosterone Thus, high testosterone and low cortisol may be associated with a general propensity for status seeking that is manifested differently depending on the decision-making context. Although the rise in peer conformity during adolescence is often described as a vulnerability, findings from this study highlight the positive potential of peers (Guroglu, 2020). One implication of this work is the potential to improve adolescent decision-making by providing youth with opportunities to interact with peers who exert positive influences.
Based on the behavioral findings showing a significant interaction in the high-prosocial peer condition, we focused the neural analyses on this condition. The behavioral association between high testosterone and low cortisol with prosocial conformity was paralleled by our neural results. When making prosocial decisions after observing a high prosocial peer, adolescents with high testosterone and low cortisol evinced increased activation in the pSTS/TPJ, insula, OFC, and caudate, consistent with prior studies of prosocial decision-making among adolescents (Do et al., 2019; Spaans et al., 2019; van Hoorn et al., 2016). The pSTS and TPJ are implicated in social cognition, mentalizing, and other-oriented behaviors (Blakemore, 2008). One potential interpretation of these findings is that high testosterone with low cortisol is associated with greater activation in brain regions involved in perspective-taking, potentially motivating prosocial conformity. As perspective-taking and brain regions supporting perspective-taking continue developing across adolescence (Blankenstein et al., 2020; Mills et al., 2014), future research should seek to examine whether age moderates these associations.
Similarly, adolescents with high testosterone and low cortisol evinced heightened activation in the insula when conforming to prosocial peers, a region within the brain’s salience network (Seeley et al., 2007). Heightened insula activation has been observed during prosocial (Telzer et al., 2013; van Hoorn et al., 2016) and empathic (de Vignemont and Singer, 2006; Masten et al., 2011) decision-making. Although previous studies have linked high cortisol with greater empathy (e.g., Shirtcliff et al., 2009; Zilioli et al., 2014), our findings suggest that the role of cortisol varies based on the level of testosterone, and perhaps as a function of whether the context supports status-seeking. For example, high cortisol may be associated with empathy in a submissive context (Zilioli et al., 2014), whereas low cortisol may be associated with empathy if it is used to achieve social goals, such as winning the favor of other peers (Hawley, 2003).
Finally, adolescents with high testosterone and low cortisol evinced increased activation in the OFC and caudate during prosocial conformity. The OFC and caudate have been implicated in reward-based learning (Silverman et al., 2015), social decision-making (Cascio et al., 2015; Chein et al., 2011), and cooperation (Tabibnia and Lieberman, 2007). Adolescents have also evinced heightened caudate activation when winning money for a friend or parent (Braams and Crone, 2017). Given associations among testosterone and cortisol with neural sensitivity to social rewards (De Lorme et al., 2013), our results suggest that the rewards associated with prosocial behavior may be particularly salient among adolescents with high testosterone and low cortisol.
4.2. Contributions, limitations and future directions
The results of our study contribute to the neurodevelopmental literature and expand the application of the dual hormone hypothesis in several ways. First, findings from previous dual hormone studies have been mixed with respect to whether high testosterone is associated with prosocial behaviors when cortisol is high (e.g., Zilioli et al., 2015) or low (e.g., Ponzi et al., 2016). Some scientists have speculated that high cortisol elicits internal distress that supports empathic processing (Shirtcliff et al., 2009), whereas low cortisol is associated with approach orientation and reward sensitivity (Mehta et al., 2015; Terburg et al., 2009). Thus, perhaps low cortisol supports prosocial behaviors serving a personal goal (e.g., status-seeking) or requiring behavioral activation (e.g., changing behavior to align with a peer’s behavior). Scientists have additionally speculated that inconsistent findings with respect to associations between high testosterone and low cortisol with prosocial versus aggressive behaviors arise because what is considered a status-seeking behavior varies across social contexts (e.g., Knight et al., 2020). We have partial support for this theory, demonstrating that high testosterone with low cortisol is associated with high prosocial conformity (or a negative testosterone x cortisol slope as proposed by Knight et al., 2020). Since our task design did not explicitly measure antisocial behavior, it is not possible to confirm whether high testosterone with low cortisol is associated with antisocial behavior in the presence of an antisocial peer. However, results from the low-prosocial condition in our study trend in the anticipated direction, wherein high testosterone and low cortisol trend toward less prosocial behavior.
Furthermore, most prior examinations of the dual hormone hypothesis have used salivary hormones, whereas our study used hair assays. Hair assays offer a novel and promising approach to measuring stable hormone concentrations, ideal for study designs aimed at minimizing the effects of diurnal and menstrual variations in hormone concentrations as well as momentary and saltatory hormonal bursts (Laudenslager et al., 2012). Additionally, our use of liquid chromatography coupled with mass spectrometry to extract the hair hormone concentrations is thought to provide a highly valid measure of hormone concentrations, particularly for cases in which there are extremely low levels of hormones (Welker et al., 2016). The issue of low hormone levels is particularly relevant to our developmental population, as younger individuals tend to have low concentrations testosterone (Grotzinger et al., 2018).
One methodological strength of our study is our use of time as a metric of prosocial behavior rather than money, which is arguably more ecologically valid in that time is a more salient and available resource for most youth. Finally, our study is one of few to consider hormones, brain function, and behavior together (but see Braams et al., 2015). Considering the vast changes in hormonal concentrations during adolescence and the implications of these changes for both brain function and behavior, the developmental literature would benefit from a more comprehensive understanding of links between biology and behavior.
Although the results of this study suggest a promising link between the dual hormone hypothesis and peer conformity in adolescence, it is important to consider several key limitations. First, support for the dual hormone hypothesis in the literature is quite inconsistent (Dekkers et al., 2019), indicating that this theory may be limited to certain individuals in specific contexts rather than a general pattern for all. Additionally, it is important to recognize that, guided by the results of our behavioral analyses, we used a more parsimonious method for the neural analyses by focusing on the high-prosocial peer condition alone, rather than conducting a more complex 3-way interaction at the neural level. Thus, our method of analysis does not provide statistical evidence for significant or meaningful differences between the conditions (Nieuwenhuis et al., 2011). This flexibility in our neural analyses warrants caution when interpreting our results. Nonetheless, the neural findings parallel the pattern of effects found in the 2-way interactions we identified in the behavioral analyses for the high prosocial condition, providing some confidence in the neural results.
Additionally, although our sample size is relatively large for an fMRI study, we recognize the sample size impedes our power to test complex interactions, therefore limiting the interpretability of our findings. Relatedly, we were not powered to examine the moderating role of gender, which is relevant to individual differences in hormone concentrations and prosocial behavior during adolescence (e.g., Braams et al., 2015; Carlo et al., 2012; Zilioli et al., 2015). Furthermore, future research should consider the role of puberty. Although testosterone levels change across puberty, hair testosterone and pubertal status seem to be poorly correlated (e.g., Grotzinger et al., 2018) as they were in this study. There may also be differences in prosocial behavior across puberty, as prior work has shown that early-maturing males are more prosocial than late-maturing males or females (Carlo et al., 2012). Individual differences in personality characteristics such as competitiveness and prosociality may also be relevant to the discussion of prosocial peer conformity (e.g., Pfattheicher, 2017). For example, whereas some adolescents may have conformed to prosocial peers because they were intrinsically motivated to be prosocial, others may have conformed out of the competitive drive to be more prosocial than the peer they observed (Hawley, 2003). Finally, it is not clear whether our findings on hormonal interactions and prosocial influence are unique to adolescence, a time during which testosterone and cortisol levels change dramatically. Thus, future work should take a developmental approach to understanding the dual hormone hypothesis by comparing these findings across children, adolescents, and adults. Further, as hair is a cumulative index of hormone concentrations over time, it may be interesting to examine whether the interaction between testosterone and cortisol changes within individuals over time, thereby resulting in distinct associations between hormones and behavior across development.
4.3. Conclusions
Altogether, this study provides evidence for links between hormone concentrations and the neural correlates of adolescents’ conformity to prosocial peers. Given the rapid changes in hormone concentrations taking place during adolescence, it is important to consider the role of hormones in modulating adolescents’ behavior and the neural mechanisms undergirding such behavior. Furthermore, the importance of context cannot be overstated (van Hoorn et al., 2019). The kinds of peers adolescents surround themselves with influence the behaviors they emulate. If adolescents are surrounded by peers who make adaptive decisions such as engaging in prosocial behaviors, our findings suggest that adolescents are motivated to conform. This finding is in line with a growing body of literature emphasizing the potential for peers to have positive, protective effects on the decisions adolescents make (Telzer et al., 2018).
Data statement
Behavioral and hormonal data from this study are unavailable to access because the study participants did not consent to the public use of their data. However, un-thresholded brain maps from the fMRI analyses are available on NeuroVault at the following link: https://neurovault.org/collections/PDZHKDRI/.
CRediT authorship contribution statement
Natasha Duell: Conceptualization, Formal analysis, Writing - original draft, Writing - review & editing. Jorien van Hoorn: Conceptualization, Methodology. Ethan M. McCormick: Software, Formal analysis. Mitchell J. Prinstein: Supervision, Writing - review & editing. Eva H. Telzer: Funding acquisition, Supervision, Writing - review & editing.
Declaration of Competing Interest
The authors report no declarations of interest.
Acknowledgements
This research was supported by the National Institutes of Health (R01DA039923 to E.H.T.) and the National Science Foundation (BCS 1539651 to E.H.T.). The writing of this article was supported, in part, by a postdoctoral fellowship provided by the Eunice Kennedy Shriver National Institute of Child Health and Development [T32-HD07376] through the Frank Porter Graham Child Development Institute, University of North Carolina at Chapel Hill, to Natasha Duell. We greatly appreciate the assistance of the Biomedical Research Imaging Center at the University of North Carolina at Chapel Hill, as well as Carina Fowler, Susannah Ivory, Amanda Benjamin, Virnaliz Jimenez, Kathy Do, Paul Sharp, Lynda Lin, Christina Rogers, and Tae-Ho Lee for assistance with study design, data collection, and analysis.
Footnotes
This analysis excludes data for four subjects whose hormone data exceeded 3 standard deviations. In a sensitivity analysis wherein the data for the four outliers were winsorized to 3 standard deviations rather than excluded, the three-way interaction was not significant.
Participants in the low prosocial condition did not show significant differences in neural activation when making prosocial decisions after peer observation.
Supplementary material related to this article can be found, in the online version, at doi:https://doi.org/10.1016/j.dcn.2021.100936.
Appendix A. Supplementary data
The following is Supplementary data to this article:
References
- Akers R.L. Social learning theory. In: Paternoster R., Bachman R., editors. Explaining Criminals and Crime: Essays in Contemporary Criminological Theory. Roxbury; Los Angeles, CA: 2001. pp. 192–210. [Google Scholar]
- Bandura A., Walters R.H. General Learning Press; 1977. Social Learning Theory. [Google Scholar]
- Beckmann C.F., Smith S.M. Probabilistic independent component analysis for functional magnetic resonance imaging. IEEE Trans. Med. Imaging. 2004;23:137–152. doi: 10.1109/TMI.2003.822821. [DOI] [PubMed] [Google Scholar]
- Blakemore S.J. The social brain in adolescence. Nat. Rev. Neurosci. 2008;9:267–277. doi: 10.1038/nrn2353. [DOI] [PubMed] [Google Scholar]
- Blakemore S.-J., Mills K.L. Is adolescence a sensitive period for sociocultural processing? Annu. Rev. Psychol. 2014;65:187–207. doi: 10.1146/annurev-psych-010213-115202. [DOI] [PubMed] [Google Scholar]
- Blankenstein N.E., Telzer E.H., Do K.T., van Duijvenvoorde A.C.K., Crone E.A. Behavioral and neural pathways supporting the development of prosocial and risk-taking behavior across adolescence. Child Dev. 2020;91:e665–e681. doi: 10.1111/cdev.13292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Crone E.A. Peers and parents: a comparison between neural activation when winning for friends and mothers in adolescence. Soc. Cogn. Affect. Neurosci. 2017;12:417–426. doi: 10.1093/scan/nsw136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braams B.R., Van Duijvenvoorde A.C.K., Peper J.S., Crone E.A. Longitudinal changes in adolescent risk taking: a comprehensive study on neural responses to rewards, pubertal development and risk taking behaviour. J. Neurosci. 2015;35:7226–7238. doi: 10.1523/JNEUROSCI.4764-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brechwald W.A., Prinstein M.J. Beyond homophily: a decade of advances in understanding peer influences. J. Res. Adolesc. 2013;21:166–179. doi: 10.1111/j.1532-7795.2010.00721.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlo G., Crockett L.J., Wolff J.M., Beal S.J. The role of emotional reactivity, self-regulation, and puberty in adolescents’ prosocial behaviors. Soc. Dev. 2012;21:667–685. doi: 10.1111/j.1467-9507.2012.00660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cascio C.N., Carp J., Brook O’Donnell M., Tinney F.J., Jr., Bingham C.R., Shope J.T., Claude Ouimet M., Pradhan A.K., Simons-Morton B.G., Falk E.B. Buffering social influence: neural correlates of response inhibition predict driving safety in the presence of a peer. J. Cogn. Neurosci. 2015;27:83–95. doi: 10.1162/jocn_a_00693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casto K.V., Hamilton D.K., Edwards D.A. Testosterone and cortisol interact to predict within-team social status hierarchy among Olympic-level women athletes. Adapt. Human Behav. Physiol. 2019;5:237–250. doi: 10.1007/s40750-019-00115-2. [DOI] [Google Scholar]
- Chein J., Albert D., O’Brien L., Uckert K., Steinberg L. Peers increase adolescent risk taking by enhancing activity in the brain’s reward circuitry. Dev. Sci. 2011;14:F1–F10. doi: 10.1111/j.1467-7687.2010.01035.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choukas-Bradley S., Giletta M., Cohen G.L., Prinstein M.J. Peer influence, peer status, and prosocial behavior: an experimental investigation of peer socialization of adolescents’ intentions to volunteer. J. Youth Adolesc. 2015;44:2197–2210. doi: 10.1007/s10964-015-0373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cialdini R.B., Goldstein N.J. Social influence: compliance and conformity. Annu. Rev. Psychol. 2004;55:591–621. doi: 10.1146/annurev.psych.55.090902.142015. [DOI] [PubMed] [Google Scholar]
- De Lorme K., Bell M.R., Sisk C.L. The teenage brain: social reorientation and the adolescent brain—the role of gonadal hormones in the male Syrian hamster. Curr. Dir. Psychol. Sci. 2013;22:128–133. doi: 10.1177/0963721413479607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vignemont F., Singer T. The empathic brain: how, when, and why? Trends Cogn. Sci. 2006;10:435–441. doi: 10.1016/j.tics.2006.08.008. [DOI] [PubMed] [Google Scholar]
- Dekkers T.J., Agelink van Rentergem J.A., Meijer B., Popma A., Wagemaker E., Huizenga H.M. A meta-analytical evaluation of the dual-hormone hypothesis: does cortisol moderate the relationship between testosterone and status, dominance, risk taking, aggression, and psychopathy? Neurosci. Biobehav. Rev. 2019;96:250–271. doi: 10.1016/j.neubiorev.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Do K.T., McCormick E.M., Telzer E.H. The neural development of prosocial behavior from childhood to adolescence. Soc. Cogn. Affect. Neurosci. 2019;14:129–139. doi: 10.1093/scan/nsy117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg N., Fabes R.A., Spinrad T.L. In: Handbook of Child Psychology: Vol. 3. Social, Emotional, and Personality Development. 6th ed. Eisenberg N., Damon W., Lerner R.M., editors. John Wiley & Sons Inc.; Hoboken, NJ, USA: 2006. [Google Scholar]
- Foulkes L., Blakemore S.-J. Is there heightened sensitivity to social reward in adolescence? Current Opinions in Neurobiology. 2016;40:81–85. doi: 10.1016/j.conb.2016.06.016. [DOI] [PubMed] [Google Scholar]
- Gao W., Talder T., Foley P., Rauh M., Deng H., Kirschbaum C. Quantitative analysis of steroid hormones in human hair using a column-switching LC-APCI-MS/MS assay. J. Chromatogr. B. 2013;928:1–8. doi: 10.1016/j.jchromb.2013.03.008. [DOI] [PubMed] [Google Scholar]
- Gorgolewski K.J., Varoquaux G., Rivera G., Schwarz Y., Ghosh S.S., Maumet C., Yarkoni T. NeuroVault.org: a web-based repository for collecting and sharing unthresholded statistical maps of the human brain. Front. Neuroinform. 2015;9:8. doi: 10.3389/fninf.2015.00008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe N.M., Del Giudice M., Thompson M.E., Nickels N., Ponzi D., Zilioli S., Maestripieri D., Gangestad S.W. Testosterone, cortisol, and status-striving personality features: a review and empirical evaluation of the Dual Hormone hypothesis. Horm. Behav. 2019;109:25–37. doi: 10.1016/j.yhbeh.2019.01.006. [DOI] [PubMed] [Google Scholar]
- Grotzinger A.D., Briley D.A., Engelhardt L.E., Mann F.D., Patterson M.W., Tackett J.L., Tucker-Drob E.M., Harden K.P. Genetic and environmental influences on pubertal hormones in human hair across development. Psychoneuroendocrinology. 2018;90:76–84. doi: 10.1016/j.psyneuen.2018.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guroglu B. Adolescent brain in a social world: unravelling the positive power of peers from a neurobehavioral perspective. Eur. J. Dev. Psychol. 2020 doi: 10.1080/17405629.2020.1813101. [DOI] [Google Scholar]
- Halevy N., Chou E.Y., Cohen T.R., Livingston R.W. Status conferral in intergroup social dilemmas: behavioral antecedents and consequences of prestige and dominance. J. Pers. Soc. Psychol. 2012;102:351–366. doi: 10.1037/a0025515. [DOI] [PubMed] [Google Scholar]
- Hawley P.H. Prosocial and coercive configurations of resources control in early adolescence: a case for the well-adapted Machiavellian. Merrill. Q. 2003;49:279–309. doi: 10.1353/mpq.2003.0013. [DOI] [Google Scholar]
- Hermans E.J., Bos a P., Ossewaarde L., Ramsey N.F., Fernandez G., van Honk J. Effects of exogenous testosterone on the ventral striatal BOLD response during reward anticipation in healthy women. NeuroImage. 2010;52:277–283. doi: 10.1016/j.neuroimage.2010.04.019. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Smith S. A global optimisation method for robust affine registration of brain images. Med. Image Anal. 2001;5(2):143–156. doi: 10.1016/S1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17:825–841. doi: 10.1006/nimg.2002.1132. [DOI] [PubMed] [Google Scholar]
- Kirschbaum C., Tietze A., Skoluda N., Dettenborn L. Hair as a retrospective calendar of cortisol production-Increased cortisol incorporation into hair in the third trimester of pregnancy. Psychoneuroendocrinology. 2009;34:32–37. doi: 10.1016/j.psyneuen.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Knight E.L., Sarkar A., Prasad S., Mehta P.H. Beyond the challenge hypothesis: the emergence of the dual-hormone hypothesis and recommendations for future research. Horm. Behav. 2020;123 doi: 10.1016/j.yhbeh.2019.104657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosten P.A., Scheier L.M., Grenard J.L. Latent class analysis of peer conformity: Who is yielding to pressure and why? Youth Soc. 2012;45:565–590. doi: 10.1177/0044118X12454307. [DOI] [Google Scholar]
- Laudenslager M.L., Jorgensen M.J., Fairbanks L.A. Developmental patterns of hair cortisol in male and female nonhuman primates: lower hair cortisol levels in vervet males emerge at puberty. Psychoneuroendocrinology. 2012;37:1736–1739. doi: 10.1016/j.psyneuen.2012.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lease M., Musgrove K., Axelrod J. Dimensions of social status in preadolescent peer groups: likeability, perceived popularity and social dominance. Soc. Dev. 2002;11:508–533. doi: 10.1111/1467-9507.00213. [DOI] [Google Scholar]
- Li Y., Wright M.F. Adolescents’ social status goals: relationships to social status, insecurity, aggression, and prosocial behavior. J. Youth Adolesc. 2014;43:146–160. doi: 10.1007/s10964-013-9939-z. [DOI] [PubMed] [Google Scholar]
- Masten C.L., Morelli S.A., Eisenberger N.I. An fMRI investigation of empathy for ‘social pain’ and subsequent prosocial behavior. NeuroImage. 2011;55:381–388. doi: 10.1016/j.neuroimage.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Mehta P.H., Josephs R.A. Testosterone and cortisol jointly regulate dominance: evidence for a dual-hormone hypothesis. Horm. Behav. 2010;58:898–906. doi: 10.1016/j.yhbeh.2010.08.020. [DOI] [PubMed] [Google Scholar]
- Mehta P.J., Welker K.M., Zilioli S., Carre J.M. Testosterone and cortisol jointly modulate risk-taking. Psychoneuroendocrinology. 2015;56:88–99. doi: 10.1016/j.psyneuen.2015.02.023. [DOI] [PubMed] [Google Scholar]
- Mills K.L., Lalonde F., Clasen L.S., Giedd J.N., Blakemore S.-J. Developmental changes in the structure of the social brain in late childhood and adolescence. Soc. Cogn. Affect. Neurosci. 2014;9:123–131. doi: 10.1093/scan/nss113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya E.R., Bos P.A., Terburg D., Rosenberger L.A., van Honk J. ortisol administration induces global down-regulation of the brain’s reward circuitry. Psychoneuroendocrinology. 2014;47:31–42. doi: 10.1016/j.psyneuen.2014.04.022. [DOI] [PubMed] [Google Scholar]
- Nelson E.E., Jarcho J.M., Guyer A.E. Social re-orientation and brain development: an expanded and updated view. Dev. Cogn. Neurosci. 2016;17:118–127. doi: 10.1016/j.dcn.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieuwenhuis S., Forstmann B.U., Wagenmakers E.J. Erroneous analyses of interactions in neuroscience: a problem of significance. Nat. Neurosci. 2011;14:1105–1107. doi: 10.1038/nn.2886. [DOI] [PubMed] [Google Scholar]
- Op de Macks Z.A., Gunther Moor B., Overgaauw S., Guroglu B., Dahl R.E., Crone E.A. Testosterone levels correspond with increased ventral striatum activation in response to monetary rewards in adolescents. Dev. Cogn. Neurosci. 2011;1:506–516. doi: 10.1016/j.dcn.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perino M.T., Miernicki M.E., Telzer E.H. Letting the good times roll: adolescence as a period of reduced inhibition to appetitive social cues. Soc. Cogn. Affect. Neurosci. 2016;11:1762–1771. doi: 10.1093/scan/nsw096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen A., Crockett L., Richards M., Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Pfattheicher S. Illuminating the dual-hormone hypothesis: about chronic dominance and the interaction of cortisol and testosterone. Aggress. Behav. 2017;43:85–92. doi: 10.1002/ab.21665. [DOI] [PubMed] [Google Scholar]
- Platje E., Popma A., Vermeiren R.R.J.M., Doreleijers T.A.H., Meeus W.H.J., van Lier P.A.C., Koot H.M., Branje S.J.T., Jansen L.M.C. Testosterone and cortisol in relation to aggression in a non-clinical sample of boys and girls. Aggress. Behav. 2015;4:478–487. doi: 10.1002/ab.21585. [DOI] [PubMed] [Google Scholar]
- Ponzi D., Zilioli S., Mehta P.H., Maslov A., Watson N.V. Social network centrality and hormones: the interaction of testosterone and cortisol. Psychoneuroendocrinology. 2016;68:6–13. doi: 10.1016/j.psyneuen.2016.02.014. [DOI] [PubMed] [Google Scholar]
- Prasad S., Knight E.L., Mehta P.H. Basal testosterone’s relationship with dictator game decision-making depends on cortisol reactivity to acute stress: a dual-hormone perspective on dominant behavior during resource allocation. Psychoneuroendocrinology. 2019;101:150–159. doi: 10.1016/j.psyneuen.2018.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowe R., Maughan B., Worthman C.M., Costello E.J., Angold A. Testosterone, antisocial behavior, and social dominance in boys: pubertal development and biosocial interaction. Biol. Psychiatry. 2004;55:546–552. doi: 10.1016/j.biopsych.2003.10.010. [DOI] [PubMed] [Google Scholar]
- Seeley W.W., Menon V., Schatzberg A.F., Keller J., Glover G.H., Kenna H., Reiss A.L., Greicius M.D. Dissociable intrinsic connectivity networks for salience processing and executive control. J. Neurosci. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman G.D., Lerner J.S., Josephs R.A., Renshon J., Gross J.J. The interaction of testosterone and cortisol is associated with attained status in male executives. J. Pers. Soc. Psychol. 2016;110:921–929. doi: 10.1037/pspp0000063. [DOI] [PubMed] [Google Scholar]
- Shirtcliff E.A., Vitacco M.J., Graf A.R., Gostisha A.J., Merz J.L., Zahn-Waxler C. Neurobiology of empathy and callousness: implications for the development of antisocial behavior. Behavioral Science and the Law. 2009;27:137–171. doi: 10.1002/bsl.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silverman M.H., Jedd K., Luciana M. Neural networks involved in adolescent reward processing: an activation likelihood estimation meta-analysis of functional neuroimaging studies. NeuroImage. 2015;122:427–439. doi: 10.1016/j.neuroimage.2015.07.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair D., Purves-Tyson T.D., Allen K.M., Shannon Weickert C. Impacts of stress and sex hormones on dopamine neurotransmission in the adolescent brain. Psychopharmacology. 2014;231:1581–1599. doi: 10.1007/s00213-013-3415-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Hum. Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaans J.P., Peters S., Crone E.A. Neural reward-related reactions to monetary gains for self and charity. Cogn. Affect. Behav. Neurosci. 2019;19:845–858. doi: 10.3758/s13415-018-00672-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T., Kirschbaum C. Analysis of cortisol in hair – state of the art and future directions. Brain Behav. Immun. 2012;26:1019–1029. doi: 10.1016/j.bbi.2012.02.002. [DOI] [PubMed] [Google Scholar]
- Tabibnia G., Lieberman M.D. Fairness and cooperation are rewarding: evidence from social cognitive neuroscience. Ann. N. Y. Acad. Sci. 2007;1118:90–101. doi: 10.1196/annals.1412.001. [DOI] [PubMed] [Google Scholar]
- Tashjian S.M., Weissman D.G., Guyer A.E., Galvan A. Neural response to prosocial scenes relates to subsequent giving behavior in adolescents: a pilot study. Cogn. Affect. Behav. Neurosci. 2018;18:342–352. doi: 10.3758/s13415-018-0573-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., Fuligni A.J., Lieberman M.D., Galvan A. Ventral striatum activation to prosocial rewards predicts longitudinal declines in adolescent risk taking. Dev. Cogn. Neurosci. 2013;3:45–52. doi: 10.1016/j.dcn.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telzer E.H., van Hoorn J., Rogers C.R., Do K.T. Social influence on positive youth development: a developmental neuroscience perspective. Adv. Child Dev. Behav. 2018;54:215–258. doi: 10.1016/bs.acdb.2017.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terburg D., Morgan B., van Honk J. The testosterone-cortisol ratio: a hormonal marker for proneness to social aggression. Int. J. Law Psychiatry. 2009;32:216–223. doi: 10.1016/j.ijlp.2009.04.008. [DOI] [PubMed] [Google Scholar]
- Tohka J., Foerde K., Aron A.R., Tom S.M., Toga A.W., Poldrack R.A. Automatic independent component labeling for artifact removal in fMRI. NeuroImage. 2008;39:1227–1245. doi: 10.1016/j.neuroimage.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van der Westhuizen D., Solms M. Social dominance and the affective neuroscience personality scales. Conscious. Cogn. 2015;33:90–111. doi: 10.1016/j.concog.2014.12.005. [DOI] [PubMed] [Google Scholar]
- van Hoorn J., van Dijk E., Guroglu B., Crone E.A. Neural correlates of prosocial peer influence on public goods game donations during adolescence. Soc. Cogn. Affect. Neurosci. 2016;11:923–933. doi: 10.1093/scan/nsw013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hoorn J., Shablack H., Lindquist K., Telzer E.H. Incorporating the social context into neurocognitive models of adolescent decision-making: a neuroimaging meta-analysis. Neurosci. Biobehav. Rev. 2019;101:129–142. doi: 10.1016/j.neubiorev.2018.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veer I.M., Oei N.Y.L., Spinhoven P., van Buchem M.A., Elzinga B.M., Rombouts S.A.R.B. Endogenous cortisol is associated with functional connectivity between the amygdala and medial prefrontal cortex. Psychoneuroendocrinology. 2012;37:1039–1047. doi: 10.1016/j.psyneuen.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Viau V. Functional cross-talk between the hypothalamic-pituitary-gonadal and - adrenal axes. J. Neuroendocrinol. 2002;14:506–513. doi: 10.1046/j.1365-2826.2002.00798.x. [DOI] [PubMed] [Google Scholar]
- Wang X., Wu Q., Egan L., Gu X., Liu P., Gu H., Yang Y., Luo J., Wu Y., Gao Z., Fan J. Anterior insular cortex plays a critical role in interoceptive attention. Neurosci. eLife. 2019;15:e42265. doi: 10.7554/eLife.42265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilioli S., Ponzi D., Henry A., Paestripieri D. estosterone, cortisol, and empathy: Evidence for the dual-hormone hypothesis. Adapt. Hum. Behav. Physiol. 2014;1:421–433. doi: 10.1007/s40750-014-0017-x. [DOI] [Google Scholar]
- Zilioli S., Ponzi D., Henry A., Maestripieri D. Testosterone, cortisol, and empathy: evidence for the dual-hormone hypothesis. Adapt. Human Behav. Physiol. 2015;1:421–433. doi: 10.1007/s40750-014-0017-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.