Abstract
Anaplastic large cell lymphoma (ALCL), ALK negative (ALK−) is an aggressive lymphoproliferative disorder of mature T lymphocytes characterised by hallmark cells, CD30 positivity and lacking ALK protein expression. ALCL, ALK− has to be differentiated from peripheral T-cell lymphoma-not otherwise specified and classical Hodgkin’s lymphoma. ALK− anaplastic large cell leukaemia should be considered in a patient with a history of ALCL, ALK− presenting with new leukaemia. We report a rare presentation of relapsed ALCL, ALK− with leukaemia after autologous stem cell transplantation in a 57-year-old male. Leukaemia, either as primary presentation or secondary transformation confers worse prognosis in ALCL, ALK− with very few cases reported so far. Emergency resuscitation with leukapheresis and treatment of tumour lysis syndrome along with supportive care should be followed by combination chemotherapy. Brentuximab vedotin and stem cell transplantation are the backbone of treatment for relapsed/refractory disease.
Keywords: haematology (incl blood transfusion), oncology, screening (oncology)
Background
Anaplastic large cell lymphomas (ALCLs) are of T-cell origin and can be primarily cutaneous or systemic or related to breast implants. The 2016 revision of WHO classification of lymphoid malignancies differentiated systemic anaplastic large cell lymphoma into ALK+ and ALK− forms and grouped them under nodal peripheral T-cell lymphoma with T follicular helper phenotype.1 In this article, ALCL is used to describe primarily systemic ALCL. ALCLs are malignancies of mature T lymphocytes defined by expression of the lymphocyte activation marker CD30 and presence of large lymphoid cells, frequently with kidney bean shaped nuclei (ie, hallmark cells).1 A subset express anaplastic lymphoma kinase (ALK) protein, as a result of chromosomal rearrangements, most commonly due to a unique, balanced chromosomal translocation t(2;5) (p23;q35). ALCL, ALK negative (ALK−) has similar gene expression profiling signature to ALCL, ALK positive (ALK+) and is distinct from other natural killer/T-cell lymphomas.1 However, ALCL, ALK− is both clinically and biologically heterogeneous compared with ALCL, ALK+.2 At presentation patients with ALCL, ALK− are usually older, often have high stage disease (III–IV), have B symptoms, high International Prognostic Index scores and high lactate dehydrogenase (LDH) levels. In addition, they usually have a more aggressive course.3 Not surprisingly, ALCL, ALK− has a 5 year overall survival rate of consistently <50%, which is worse compared with ALK+ cases (≥70% survival rate).4 Leukaemic presentation of ALCL, ALK− is exceedingly rare.5–8 Here, we describe an adult with leukaemic relapse of primary systemic ALCL, ALK−.
Case presentation
A 57-year-old African-American male in incarceration, presented with chest and back pain of 2 weeks duration in 2018 at our institution. He had become progressively more fatigued with worsening back pain, dyspnoea, cough and hoarseness. He had also experienced worsening abdominal pain associated with diarrhoea.
On examination, he was noted to be in moderate distress due to abdominal pain. Diffuse abdominal tenderness was noted on light palpation. Examination of lungs, heart, spine, and oral cavity was normal.
Five years ago, he was diagnosed with ALCL, ALK−, stage IIIA after undergoing inguinal hernia repair and notes having swollen nodes in face bilaterally but no B symptoms. He was diagnosed and treated for initial episode in outside hospital and full details were not available. He received six cycles of cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) and achieved complete remission by PET-CT scan. After 2 years of initial diagnosis and 3 years prior to current presentation, he had relapsed with right inguinal adenopathy and extensive intra-abdominal adenopathy. He was treated with 9 cycles of brentuximab vedotin (BV). He achieved complete remission based on post-treatment PET-CT scan, and a bone marrow biopsy was clear of any lymphoma involvement. He received autologous stem cell transplant (SCT) after bis-chloroethylnitrosourea, etoposide, cytarabine and melphelan (BEAM) conditioning 3 months prior to this presentation.
He was in remission following chemotherapy and autologous SCT. Three weeks prior to current presentation, his labs were unimpressive, and bone marrow biopsy did not show any evidence of lymphoma/leukaemia. Table 1 shows the evolution of his disease based on peripheral blood and bone marrow studies on presentation compared with that 3 weeks ago.
Table 1.
Peripheral blood and bone marrow findings at presentation compared with 3 weeks ago
| 3 Weeks prior | 3 Weeks after (in 2018) | |
| Total leucocyte count per μL | 9800 | 457 000 |
| Differential | 57% segmented neutrophils, 23% lymphocytes | 4% segmented neutrophils, 11% lymphocytes, 77% atypical lymphocytes |
| Haemoglobin (gm/dL)/Hematocrit (%) | 13.8/39.9 | 9.5/26.3 |
| Platelets per μL | 269 000 | 17 000 |
| Bone marrow | Negative for anaplastic large cell lymphoma with maturing trilineage haematopoiesis | Involved by anaplastic large cell lymphoma, ALK−, 70% of cellularity with reduced maturing trilineage haematopoiesis |
| Flow cytometry | No aberrant immunophenotype | Atypical lymphocyte population expressing CD2, HLA-DR and dim cytoplasmic CD3 |
Investigations
Patient had renal failure with elevated blood urea nitrogen and creatinine at 108 mg/dL and 2.29 mg/dL, respectively. His liver function tests were abnormal with elevated total bilirubin at 10.3 mg/dL, elevated alkaline phosphatase 289 U/L and elevated liver transaminases (aspartate transaminase 113 U/L and alanine transaminase 94 U/L). Uric acid was increased at 16 mg/dL, and LDH was high at 1866 U/L. Testing for Epstein-Barr virus and human T-cell lymphotropic virus type 1 was negative.
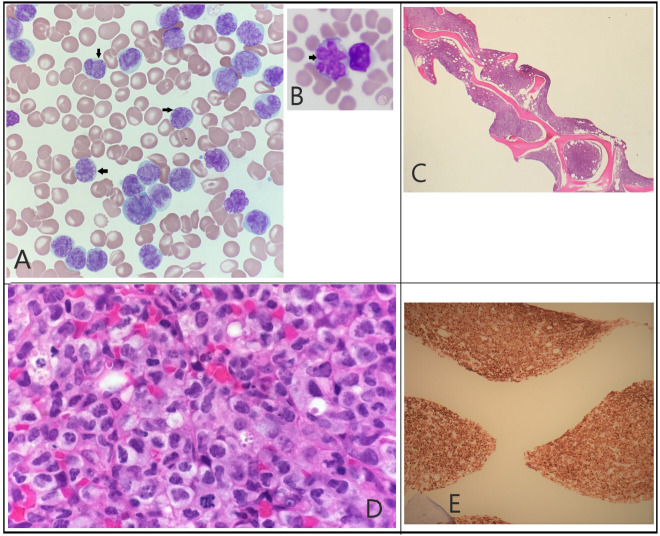
Figure 1A–E illustrates his peripheral smear and bone marrow studies graphically.
Figure 1.
(A) Peripheral smear: atypical lymphocytosis (black arrows) with deeply folded nuclei and condensed chromatin, scant agranular blue cytoplasm. (B) Hallmark cells: hallmark cells (black arrow) with ‘flower like’ nuclei (not from our patient18). (C) Bone marrow biopsy: low power: hypercellular marrow with decreased maturing lineage haematopoiesis. (D) Bone marrow biopsy: high power: hypercellular (>90%) due to atypical, diffuse, sheet-like infiltrate of lymphoid cells, like those seen in peripheral blood. (E) CD30 staining: strong CD30 positivity in bone marrow.
CT scan of thorax and abdomen showed new hepatosplenomegaly, scattered bilateral pulmonary nodular opacities and bone/rib involvement. A diagnosis of leukaemic transformation of patient’s ALCL, ALK− was made.
Treatment
Patient received urgent leukapheresis for leukostasis, hydroxyurea was started for hyperleukocytosis and intravenous steroids was used to control bone pain. Tumour lysis syndrome developed leading to non-oliguric acute tubular necrosis. He was treated with allopurinol and rasburicase for tumour lysis and required intermittent haemodialysis due to acute tubular necrosis. He required platelet and packed red cells transfusions. Cerebrospinal fluid studies showed aberrant population expressing CD2 confirming central nervous system involvement. Induction with two cycles of gemcitabine–oxaliplatin along with intrathecal methotrexate was given while in the hospital. He was treated for pneumonia and clostridium difficile colitis during the hospital stay. He received six cycles of bendamustine and BV after discharge.
Outcome and follow-up
Follow-up PET scan 2 months after the completion of therapy showed excellent partial response. However, progression was noted on PET scan 1 year out from the described presentation. Patient was then started on romidepsin monotherapy as palliative option. He is now 2 years out from leukaemic relapse and has received 10 out of 12 planned cycles of romidepsin. He achieved complete response after six cycles. Of note, patient has been unable to be considered for allogenic SCT primarily because he continues to be incarcerated.
Discussion
ALCL is a CD30 positive peripheral T-cell lymphoma often with null immunophenotype. ALCL represents 2%–3% of non-Hodgkin’s lymphomas (NHLs) and 12% of all T-cell lymphomas.9 ALCL is divided based on the presence or absence of ALK translocation. In cases with ALK translocation, >80% have t(2;5) resulting in NPM–ALK fusion gene, that translates a unique chimeric NPM/ALK protein with altered tyrosine kinase activity.4 ALCL, ALK– may have a more aggressive clinical course and has more heterogeneous genetics when compared with ALCL, ALK+. However, recent studies have shown convergent mutations and kinase fusions that lead to constitutive activation of JAK/STAT3 pathway, which explains morphologic and phenotypic similarities between ALK+ and ALK– ALCL.10
ALCL, ALK– affects older adults with a median age at diagnosis of approximately 55–60 years, and there is a slight male predominance. Lymph nodes are the most common sites of involvement. Extra nodal involvement, when present, is seen in bone, gastrointestinal tract, lungs, soft tissue and skin. ALCL, ALK+ more commonly affects younger individuals and may in part explain the better prognosis.1 Primary leukaemic presentation is rare and confers worse prognosis with relapse within 6 months after standard CHOP therapy.6 No definite risk factors are known.
Morphologically, the tissue architecture is replaced by solid, cohesive sheets of typically large neoplastic cells (figure 1D). Within the lymph node, involvement may be mostly confined to the subcapsular sinus, mimicking metastatic carcinoma. The neoplastic cells are large and pleomorphic, with some characteristic hallmark cells. Hallmark cells are large and have lobulated or irregular nuclear contours, lacy to clumped chromatin and contain moderate to abundant basophilic cytoplasm. The cells impart a ‘kidney bean’ appearance (figure 1B). The neoplastic cells express markers of T-cell differentiation or may have a null immunophenotype, which could cause a diagnostic dilemma. However, the neoplastic cells will stain uniformly for CD30. The one exception is the small cell variant of ALCL, ALK+, where uniform expression of CD30 is not noted. Other T-cell markers such as CD2, CD3, CD4, CD7 and cytotoxic antigens such as granzyme B and perforin are variably positive. However, again the cells may exhibit null cell phenotype, necessitating a broad immunohistochemical panel to render an accurate diagnosis.11
ALCL, ALK− has to be differentiated from peripheral T-cell lymphoma, not otherwise specified (PTCL-NOS) and classical Hodgkin’s lymphoma among other entities. Our case showed positivity for CD2, CD3 and CD30. The immunophenotype and morphology excluded other entities in the differential diagnoses.
Historically, most patients received anthracycline-based combination chemotherapy, CHOP and relapse/refractory ALCL was treated with CD30 antibody drug conjugate, BV.12 High-dose chemotherapy, typically BEAM, followed by autologous SCT is used commonly as a consolidation strategy when remission is achieved with salvage chemotherapy. Allogenic SCT is reserved for medically fit patients with relapsed/refractory disease, especially after autologous SCT. Our patient received the recommended treatments as his disease relapsed with the standard of care therapies at those times. Based on ECHELON-2 trial, Bv-CHP (brentuximab vedotin, cyclophosphamide, doxorubicin and prednisone) is the standard first-line therapy in 2020, but our case was treated in 2013 when, CHOP was in use and BV was commonly used in second line setting.13 Additionally, inclusion of etoposide to CHOP may improve results for front-line therapy.14
Two cases of leukaemic relapse of ALCL, ALK− has been described in the literature. Huang et al described leukaemic relapse of systemic ALCL, ALK− 20 years after remission with chemotherapy and autologous SCT in a 55-year-old woman.15 The relapse was CD30 positive null cell phenotype and she responded to salvage therapy with CHOP. Glynn et al described a case of leukaemic transformation of primary cutaneous ALCL, ALK−, 1 year after autologous SCT with downregulation of CD30.16
For ALCL, ALK−, the overall 5 year failure free survival is 36% and 5 year overall survival is 49%.9 ALCL, ALK− has intermediate prognosis in comparison to ALCL, ALK+ and PTCL-NOS, with ALCL, ALK+ faring better. Broadly, there are three prognostic categories of ALCL, ALK− based on the presence of DUSP22/IRF4 and TP63 rearrangements.17 A subset with rearrangements at the locus containing DUSP22 and IRF4 in chromosome 6p25 is monomorphic, lacks cytotoxic granules and has superior prognosis comparable to that of patients with ALCL, ALK+. A small subset with TP63 rearrangements has very aggressive course. The third subset, also called triple negative group has intermediate prognosis. These FISH probes are not standard yet, and they were not performed for our patient.
Learning points.
ALCL, ALK− is an aggressive lymphoma characterised by hallmark cells, CD30 positivity, and absence of ALK translocation.
It is a rare cause of T-cell leukaemia. Leukaemic presentation, either primary presentation or secondary transformation confers worse prognosis.
Emergency resuscitation with leukapheresis and treatment of tumour lysis syndrome along with supportive care should be followed by combination chemotherapy.
Brentuximab vedotin based therapy and stem cell transplantation (if not done previously) are the mainstay of management for relapsed/refractory disease.
Acknowledgments
The authors thank the Augusta University Medical Center, Augusta, Georgia, USA.
Footnotes
Contributors: NRK drafted the case report and searched literature. NRK, KB and LB were involved in care of the patient. NRK obtained informed consent for publication. NS interpreted the peripheral smear and bone marrow study. KB, LB and NS reviewed and revised the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Obtained.
Provenance and peer review: Not commissioned; externally peer reviewed.
References
- 1.Swerdlow SH, Campo E, Pileri SA, et al. . The 2016 revision of the world Health organization classification of lymphoid neoplasms. Blood 2016;127:2375–90. 10.1182/blood-2016-01-643569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mereu E, Pellegrino E, Scarfò I, et al. . The heterogeneous landscape of ALK negative ALCL. Oncotarget 2017;8:18525–36. 10.18632/oncotarget.14503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferreri AJM, Govi S, Pileri SA, et al. . Anaplastic large cell lymphoma, ALK-negative. Crit Rev Oncol Hematol 2013;85:206–15. 10.1016/j.critrevonc.2012.06.004 [DOI] [PubMed] [Google Scholar]
- 4.Gascoyne RD, Aoun P, Wu D, et al. . Prognostic significance of anaplastic lymphoma kinase (ALK) protein expression in adults with anaplastic large cell lymphoma. Blood 1999;93:3913–21. 10.1182/blood.V93.11.3913 [DOI] [PubMed] [Google Scholar]
- 5.Dalal BI, Chhanabhai M, Horsman DE, et al. . Anaplastic large-cell lymphoma presenting as acute leukemia. Am J Hematol 2005;79:164–5. 10.1002/ajh.20359 [DOI] [PubMed] [Google Scholar]
- 6.Lu Y, Zhao X, Wang E, et al. . ALK-negative anaplastic large cell lymphoma with extensive peripheral blood and bone marrow involvements manifested as “leukemic phase”. Leuk Res 2010;34:475–82. 10.1016/j.leukres.2009.07.034 [DOI] [PubMed] [Google Scholar]
- 7.Wong WS, Liu BWF, Lam FSC, et al. . ALK-negative anaplastic large cell lymphoma in leukemic phase with near-pentaploidy. Leuk Lymphoma 2010;51:1927–30. 10.3109/10428194.2010.502585 [DOI] [PubMed] [Google Scholar]
- 8.Tanaka H, Ohwada C, Hashimoto S, et al. . Leukemic presentation of ALK-negative anaplastic large cell lymphoma in a patient with myelodysplastic syndrome. Intern Med 2012;51:199–203. 10.2169/internalmedicine.51.6146 [DOI] [PubMed] [Google Scholar]
- 9.Savage KJ, Harris NL, Vose JM, et al. . ALK− anaplastic large-cell lymphoma is clinically and immunophenotypically different from both ALK+ ALCL and peripheral T-cell lymphoma, not otherwise specified: report from the International peripheral T-cell lymphoma project. Blood 2008;111:5496–504. 10.1182/blood-2008-01-134270 [DOI] [PubMed] [Google Scholar]
- 10.Crescenzo R, Abate F, Lasorsa E, et al. . Convergent mutations and kinase fusions lead to oncogenic STAT3 activation in anaplastic large cell lymphoma. Cancer Cell 2015;27:516–32. 10.1016/j.ccell.2015.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stein H, Foss HD, Durkop H, et al. . CD30(+) anaplastic large cell lymphoma: a review of its histopathologic, genetic, and clinical features. Blood 2000;96:3681–95. [PubMed] [Google Scholar]
- 12.Chen X, Soma LA, Fromm JR. Targeted therapy for Hodgkin lymphoma and systemic anaplastic large cell lymphoma: focus on brentuximab vedotin. Onco Targets Ther 2013;7:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Horwitz S, O'Connor OA, Pro B, et al. . Brentuximab vedotin with chemotherapy for CD30-positive peripheral T-cell lymphoma (ECHELON-2): a global, double-blind, randomised, phase 3 trial. The Lancet 2019;393:229–40. 10.1016/S0140-6736(18)32984-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sibon D, Nguyen D-P, Schmitz N, et al. . Alk-Positive anaplastic large-cell lymphoma in adults: an individual patient data pooled analysis of 263 patients. Haematologica 2019;104:e562–5. 10.3324/haematol.2018.213512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Huang Q, Gaal KK, Nademanee A. Acute leukemic manifestation of recurrent anaplastic large-cell lymphoma 20 years after autologous bone marrow transplantation. JCO 2012;30:e34–6. 10.1200/JCO.2011.37.4785 [DOI] [PubMed] [Google Scholar]
- 16.Glynn E, Shustov AR, Murphy C, et al. . Leukemic phase of primary cutaneous anaplastic large-cell lymphoma (ALK-negative), with downregulation of CD30. J Hematop 2018;11:45–9. 10.1007/s12308-018-0318-2 [DOI] [Google Scholar]
- 17.Parrilla Castellar ER, Jaffe ES, Said JW, et al. . ALK-negative anaplastic large cell lymphoma is a genetically heterogeneous disease with widely disparate clinical outcomes. Blood 2014;124:1473–80. 10.1182/blood-2014-04-571091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Changlee S, Pang ACH. ALK-negative anaplastic large cell lymphoma, leukemic phase [Internet]. Available: https://imagebank.hematology.org/image/17922/alknegative-anaplastic-large-cell-lymphoma-leukemic-phase [Accessed 7 Jul 2019].