Abstract
Objective
To examine the health services experience of patients with cancer from regional and remote Australia using the Australian National Cancer Control Indicators (NCCI) guidelines as an assessment framework.
Design
Cross-sectional.
Setting
Queensland non-for-profit cancer accommodation lodges.
Participants
Participants were patients with cancer who travelled for treatment from rural and remote Queensland to major urban centres (n=518; age mean=64.6, SD=11.18).
Outcome measures
Assessments included NCCI patient indicators, quality of life (QoL), psychological distress and unmet supportive care needs.
Results
The frequency at which NCCI indicators were met ranged from 37.5% for receiving an assessment and care plan to 97.3% for understanding explanations about diagnosis. Geographical considerations did not impact patient experience, whereas middle school educated participants were more likely than those with senior-level education or higher to receive an assessment and care plan (OR=1.90, 95% CI 1.23 to 2.91) and to report having their views on treatment taken into account (OR=2.22, 95% CI 1.49 to 3.33). Patients with breast or prostate cancer reported better communication and patient involvement and information and services provision (r=p<0.001) compared with those with skin and head and neck cancer. When compared with information and service provision, communication and patient involvement showed stronger positive associations with QoL (z=2.03, p=0.042), psychosocial (z=2.05, p=0.040) and patient care (z=2.00, p=0.046) outcomes.
Conclusion
The patient care experience varies across the NCCI indicators by sociodemographic and clinical factors that likely reflect healthcare system biases. Perceptions about communication and involvement appear most critical for optimal outcomes and should be a priority action area for cancer control.
Keywords: health services administration & management, quality in health care, adult oncology, organisation of health services
Strengths and limitations of this study.
This is the first study to quantitatively measure and test the National Cancer Control Indicators for patient experience.
Findings provide important insight into the patient experience for a regional and remote population who are at risk of fragmented or poorly coordinated care.
This large representative sample was recruited from a state-wide jurisdiction and so likely represents the actual experience of patients from regional and remote Australia.
This study was cross-sectional; therefore, causality cannot be assumed.
This study was aimed at gaining an insight into the experiences of regional and remote patients with cancer; hence, findings are not generalisable to urbanised populations.
Introduction
In Australia, as in other high-income countries, cancer care delivery systems continue to be tested by increasing cancer prevalence due to an ageing population and increasing survival.1 Compounding this, widening socioeconomic and geographical inequities in cancer outcomes,2 increasing healthcare costs and workforce shortages3 4 are all exacerbated by rapidly expanding and complex cancer diagnostic and treatment options.5 In response, national societies and cancer control agencies globally have developed frameworks and guidelines for quality care cancer services that typically include characteristics such as being person-centred and tailored, evidence-based, coordinated, multidisciplinary, quality assured and accountable.6–9 While many of these guidelines focus on treatment, supportive and psychosocial care is also a central feature. For example, the first (of eight) recommendations from the Institute of Medicine 2013 report centres on patients and families receiving understandable information about all aspects of their cancer care.8 The Australian government’s guides to best-practice cancer care, the Optimal Cancer Care Pathways, list access to supportive care, including survivorship, as a key theme across all steps of the care pathway.7 Similarly, risk stratified pathways of cancer care in the UK emphasise assessing and supporting holistic patient needs, including those that are psychosocial and spiritual. While such frameworks are important, the question arises as to how cancer services might best evaluate the extent to which cancer care is meeting these recommendations, where gaps most exist and, crucially who is more vulnerable to underservicing.
A number of groups have developed indicators to reflect the extent to which optimal care is being delivered in terms of information, communication, education and care coordination during diagnosis and treatment.10 11 Thus far, this been for quality assurance purposes within administering jurisdictions, with findings not generally presented within the peer-reviewed literature. A set of items was recently developed by Cancer Australia for monitoring patients with cancer experiences at the national level. The National Cancer Control Indicators (NCCI) patient experiences items are based on the National Health Service (NHS) England Cancer Patient Experience Survey12 and reflect the receipt and understanding of information about diagnosis and side effects of treatment, as well as patient involvement in care and decision making, and the provision of care coordination tools and services. To date, results from the Australian NCCI indicators for patient experience have not been reported.
It is especially important to consider the quality of the patient experience for people who live in geographically remote locations. People with cancer living in remote locations incur the additional burden of having to travel long distances to attend specialist treatment facilities that are not available in sparsely populated and geographically remote areas of the country.13 Patients with cancer who live outside of major cities in Australia are known to experience poorer cancer outcomes14 15 and report poor physical and mental health,16 17 lower quality of life (QoL)18 19 and unmet supportive care needs18 20 21 compared with their urban counterparts.
Accordingly, the present study applied the NCCI guidelines as a framework to examine the health services experience of patients with cancer and their families from regional and remote Australia experiencing geographical dislocation while obtaining cancer treatment. In doing so, (1) the construct validity of the NCCI guidelines was examined; (2) the extent to which these guidelines are currently being met was tested; (3) sociodemographic predictors of underservicing were explored; and (4) how psychosocial outcomes, unmet supportive care needs, satisfaction with healthcare were related to underservice were described.
Methods
Patient and public involvement statement
Patient and public involvement in the design and conduct of the study was sought from community members, research volunteers and pilot study participants. Several community members, including cancer survivors living in rural areas, reviewed interview and questionnaire items providing feedback on the clarity, formatting and time to complete. The research volunteers tested and evaluated materials and protocols, while patients provided written and verbal feedback to researchers regarding clarity, burden and relevance associated with completing study materials. Minor refinements to the study materials were made to increase clarity and ease of delivery based on this feedback.
Participants
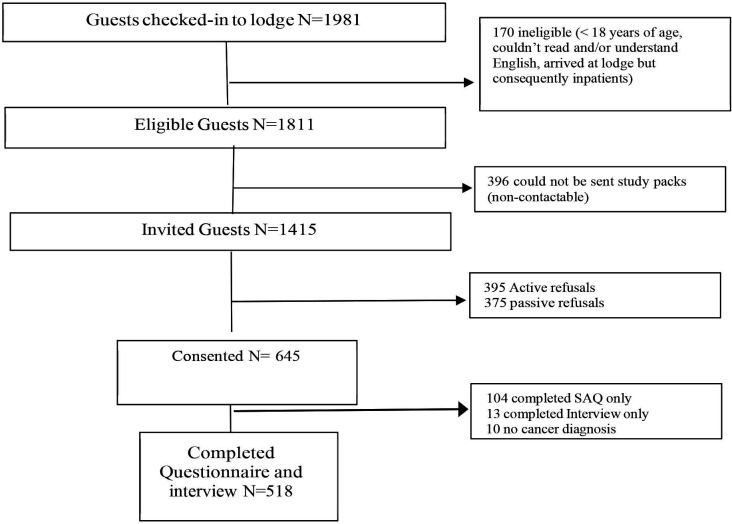
Participants (n=518) were patients with cancer from regional and remote Queensland staying at six Cancer Council Queensland (CCQ) lodges. CCQ is a not for profit organisation offering a range of services to those affected by cancer, one of those being the accommodation lodges which aim to limit out of pocket expenses for patients. People who are diagnosed with cancer, who are required to travel for their treatment, may receive a referral to stay at one of the CCQ lodges from their healthcare team. Accommodation costs are determined in conjunction with the patient’s eligibility for the Queensland government’s Patient Travel Subsidy Scheme, which is designed to assist in the cost of travel to the nearest specialist medical service that is more than 50 km from the patients nearest hospital. Figure 1 depicts participant recruitment flow. Eligibility criteria were 18 years or older, ability to read and understand English, and staying at a CCQ lodge for cancer treatment. A total of 14 015 of 1811 eligible CCQ lodge guests staying between 11 September 2017 and 1 February 2020 were provided with an invitation pack containing study details, consent forms and a questionnaire. Three hundred and ninety-six eligible guests were not approached as contact details were not provided or accurate.
Figure 1.
Recruitment flowchart. SAQ, Self-Administered Questionnaire.
Invitation packs were distributed on arrival by lodge staff or, if this was not possible (eg, after hours check-in), were sent via mail to their home address. Patients were contacted by phone 1 week after pack distribution, offered further details and invited to participate. Assessments included a Self-Administered Questionnaire (SAQ) and face-to-face (or telephone) interview at baseline, followed by SAQs at 3 and 12 months and annually thereafter.
Of the eligible patients who received an invitation pack (n=1415), 645 (45.2%) consented to participate, 395 (28.1%) actively refused, and 375 (26.7%) did not return a consent form and could not be recontacted. This report focusses on data collected at baseline for a sample of 518 consenting participants who had a cancer diagnosis and completed both the questionnaire and interview components of the study. Based on the available names and addresses of non-respondents, it could be estimated that responders and non-responders did not differ significantly according to gender, remoteness or socioeconomic status.
Materials and measures
Questionnaires assessed demographic and patient characteristics, patient experiences according to the NCCI, psychological distress and cognitive adjustment, satisfaction with healthcare, QoL and supportive care needs. Structured interviews assessed diagnostic and treatment pathways.
Demographics and patient characteristics
Site of current cancer, gender, age, country of birth, highest level of education and household income were reported by each participant. Participant’s residential street address at baseline was geocoded and mapped to the 2011 SA2 boundaries using MapMarker Australia V.15.16.0.21 and MapInfo Pro V.5.0 and classified by Remoteness Area22 and Socioeconomic Index for Areas (SEIFA).23 The most recently diagnosed primary cancer site was obtained via self-report and verified against the population-based Queensland Cancer Register (QCR). Self-report data were relied on where diagnosis could not be verified by the QCR (n=39), for example, if the patient had non-melanoma skin cancer (which is not routinely notified to registries in Australia) or the patient’s diagnosis had not yet been notified to the QCR.
NCCI: patient experience
Eight items derived from the NHS England Cancer Patient Experience Survey10 were adapted by Cancer Australia12 as measures of NCCI of patients with cancer experiences. The items captured four key elements, including (1) patient information, communication and education during diagnosis; (2) patient information, communication and education during treatment; (3) patient coordination and integration of care, continuity and transition; and (4) respect for patient preference. Response scales for each item vary, including three-category (eg, yes, no, I don’t know/remember) and four-category (eg, yes, yes to some extent, no, I don’t know/remember) response options. Responses to each NCCI item were collapsed into a yes/no binary response with those responding with ‘I don’t know’ or ‘I don’t remember’ coded as missing. Full-item wording, response categories and method for collapsing responses are available in as supplementary material (online supplemental file 1).
bmjopen-2020-042507supp001.pdf (101KB, pdf)
As the NCCI items have not been validated for use in research, an exploratory factor analysis was conducted for the current sample. One-factor to four-factor solutions were extracted sequentially using Mplus V.8 software.24 The decision on the number of factors to retain was driven by (1) the overall and comparative model fit (determined by χ2 and Δχ2 and their corresponding p values), (2) balancing the trade-off between explanatory power and parsimony (determined by the Bayesian and the Akaike information criteria), and (3) an interpretable pattern of strong and non-cross-loading factor loadings.
For the one-factor to three-factor solutions, overall model fit improved as a function of the number of factors extracted (see table 1). However, the four-factor solution yielded a poorer fit than the three-factor solution according to both χ2 and information criteria values and was not considered a candidate solution. The Bayesian information criterion shows that the three-factor solution exhibited poorer fit compared with the two-factor solution once model complexity was accounted for. Therefore, the two-factor solution represented the best trade-off of explanatory power and parsimony. Finally, the two-factor solution also yielded a simple structure in the pattern of item loadings. Each NCCI item loaded cleanly onto one of each of the two factors (see table 2), the first reflecting effective communication and patient involvement and the second reflecting the provision of information or services. A confirmatory factor analytic approach was used to calculate factor score variables for the communication and patient involvement and provision of information and services. Factor score variables were transformed so that scores ranged from 0 (low) to 1.68 (high) for the communication and patient involvement factor and 0 (low) to 1.84 (high) for the provision of information and services factor.
Table 1.
Comparative fit statistics for one-factor to four-factor exploratory factor analysis solutions
| One-factor | Two-factor | Three-factor | Four-factor | |
| AIC | 3848.70 | 3784.24 | 3775.34 | 3787.09 |
| ΔAIC | – | 64.46 | 8.89 | −11.74 |
| BIC | 3916.70 | 3881.99 | 3898.59 | 3931.59 |
| ΔBIC | – | 34.71 | −16.61 | −32.99 |
| χ2 (p) | 435.96 (<0.001) | 306.55 (.001) | 207.71 (.776) | 225.45 (.368) |
| Δχ2 (p) | – | 84.76 (<0.001) | 40.37 (<0.001) | (Δχ2 is negative) |
AIC, Akaike's information criterion; BIC, Bayesian information criterion.
Table 2.
Exploratory factor analysis item loadings for two-factor solution
| Item | Communication and patient involvement | Provision of information and services |
| Do you think your views were taken into account when the team of doctors and nurses caring for you were discussing which treatment you should have? | 0.884* | 0.005 |
| Were you involved as much as you wanted to be in decisions about your care and treatment? | 0.925* | −0.063 |
| Were the possible side effects of treatment(s) explained in a way you could understand? | 0.507* | 0.249* |
| When you were told you had cancer, did you understand the explanation of what was wrong with you? | 0.633* | 0.059 |
| Have you been offered a written assessment and care plan? | 0.185 | 0.579* |
| When you were told you had cancer, were you given written information about the type of cancer you had? | 0.075 | 0.579* |
| Before you started your treatment, were you given written information about the side effects of treatment(s)? | 0.002 | 0.719* |
| Were you given the name of a clinical nurse specialist who would support you through your treatment? | −0.001 | 0.736* |
Bold indicates the factor on which item loaded most strongly.
*Geomin rotated loadings significant at 5% level.
Psychological distress and adjustment
Stress, anxiety and depression were measured using the 21-Item Depression, Anxiety and Stress Scale.25 The scale asks respondents to indicate the degree to which each statement applied to them over the past week on a 4-point Likert scale ranging from 0=not at all to 3=almost always. Scores for each subscale were summed and multiplied by 2, with higher scores indicating more distress.26 Reliability for the anxiety (α=0.67), stress (α=0.87) and depression (α=0.90) subscales was adequate to excellent.
Psychological adjustment to a cancer diagnosis was assessed using the Constructed Meaning Scale (CMS). The eight-item CMS measures a patients’ cognitive response to being diagnosed with a life-threatening illness27 on a 4-point Likert scale ranging from 1=strongly disagree to 4=strongly agree. Scores on the CMS reflect ability to construct a positive outlook regarding the effect that cancer has or will have on their future, their relationships and their sense of self. Internal consistency in the current study was good (α=0.77).
Satisfaction with healthcare
Nine items were created by the researchers to assess patients’ satisfaction with their healthcare in terms of the referral process, speed of diagnosis, speed of test results, the hospital where they were treated and the doctors and nurses, the emotional and physical support they receive in hospital and finally, and their medical care overall. Degree of satisfaction with each item was reported on a 5-point Likert scale ranging from 1=very dissatisfied to 5=very satisfied. Data and participant feedback from the pilot phase were examined to ensure items were clear and relevant, and excellent internal consistency for the measure was evident (α=0.92). Items were averaged to create a mean score.
Quality of life
Multidimensional QoL was measured using the 35-item Assessment of Quality of Life 8 Dimension instrument (AQoL-8D).28 Participants responded on a 5-point Likert scale based on aspects of QoL during the past week. Responses are coded so that lower scores reflect poorer QoL on two psychometrically derived dimensions reflecting physical and psychological well-being. Internal reliability was evident for physical (α=0.64) and psychological (α=0.92) dimensions in the current study.
Supportive care needs
Unmet need was measured using the Supportive Care Needs Survey Short Form-34.29 The scale assesses patient need for support across five domains, including physical and daily living, psychological, health systems and information, patient care and support and sexuality with a single item regarding financial needs. Responses were coded: 0 (no need/not applicable/need satisfied), 1 (low need), 2 (moderate need) or 3 (high need), and means were calculated, resulting in six continuous variables reflecting the degree of need in each domain. Subscales showed excellent internal reliability (physical and daily living needs, α=0.86; psychological needs, α=0.94; health system and information needs, α=0.95; patient care and support needs, α=0.88; and sexuality needs, α=0.87).
Analysis
Data analyses were carried out in SPSS V.26.30 Frequencies and percentages were calculated for patient responses to each NCCI item. Demographics and area-level characteristic differences in the likelihood of reporting yes to an NCCI item were examined using χ2 statistics. A family-wise error rate adjustment was applied to constrain the chance of type 1 errors to 5%. One-way analyses of variance were used to identify group differences in NCCI factor scores and psychosocial outcomes; Pearson’s point biserial correlations assessed whether age was associated with NCCI items and factors. Where group differences were significant, post hoc contrasts were applied to compare each category against others. ORs and 95% CIs were reported for contrasts involving NCCI items. Associations between factor scores and health/psychosocial variables were assessed using a series of correlations with coefficients graphed and compared using Fisher’s z-test. Missing data were excluded from analyses in a pairwise manner.
Results
Sample characteristics
Participant ages ranged from 26 to 93 (mean (M)=64.6, SD=11.18), and 47.3% of participants identified as female and 52.7% as male. Most participants were born in Australia (80.5%), with the remainder born in the UK (9.9%), New Zealand (4.7%) and other countries (4.9%). Most participants reported low income, with 64.8% reporting a household income under $50 000 a year (ie, the median yearly gross income in Australia). Most patients were not fully covered by private health insurance (81.5%), and the majority lived in inner (44.0%) or outer (42.5%) regional areas marked by high levels of socioeconomic disadvantage (ie, 66.5% were in the lowest socioeconomic quintiles). The most common primary cancers were those of the breast (19.3%), head and neck (14.3%), and skin (12.6%; see table 3A). References to population statistics available through the Queensland Cancer Registry, the current sample was representative of the non-metropolitan Queensland cancer population in terms of gender, age and country of birth. However, patients with skin cancer were under-represented (24.5% in population) and patients with head and neck cancer were over-represented (5.8% in population). At the time of data collection, time since diagnosis for each participant ranged between and 33.7 years and 1 day (Median=211 days), with 64% of participants diagnosed within the previous 12 months.
Table 3.
Participant characteristics and responses to NCCI items with chi-square and ANOVA group comparisons
| Total N (%)* |
Communication and patient involvement | Provision of information and services | |||||||||
| Views on treatment, n (%) | Involved in decisions, n (%) | Side effects explained, n (%) | Understand explanation, n (%) | Factor score M (SD) | Written info on type of cancer, n (%) | Assessment and care plan, n (%) |
Clinical nurse support, n (%) |
Written info on side effects, n (%) |
Factor score M (SD) | ||
| (A) Characteristics | |||||||||||
| Gender | χ2=2.69 | χ2 =1.30 | χ2=0.32 | χ2 =0.10 | F=2.15 | χ2 =7.88† | χ2 =0.23 | χ2 =1.05 | χ2 =1.46 | F=2.92 | |
| Female | 245 (52.7) | 147 (63.4) | 149 (63.4) | 156 (65.5) | 234 (97.5) | 1.17 (0.43) | 163 (70.3) | 73 (36.3) | 157 (71.7) | 199 (86.9) | 1.36 (0.46) |
| Male | 273 (47.3) | 147 (56.1) | 165 (58.4) | 182 (67.9) | 263 (97.0) | 1.11 (0.43) | 152 (58.2) | 86 (38.6) | 160 (66.4) | 209 (82.9) | 1.21 (0.48) |
| Education | χ2=16.61† | 144 (68.2) | χ2=9.70† | χ2=0.84 | F=9.24 | χ2=3.00 | χ2=11.44† | χ2=6.39 | χ2=2.65 | F=7.46† | |
| Middle school (year 10) | 217 (42.3) | 146 (70.2) | 41 (55.4) | 155 (73.1) | 205 (96.7) | 1.23 (0.42) | 141 (68.1) | 85 (47.0) | 141 (75.8) | 176 (86.7) | 1.27 (0.48) |
| Senior school (year 12) | 78 (15.1) | 39 (53.4) | 117 (55.2) | 41 (53.9) | 76 (98.7) | 1.05 (0.42) | 44 (57.9) | 17 (28.3) | 47 (64.4) | 55 (78.6) | 1.07 (0.47) |
| Trade/Tertiary | 218 (42.5) | 107 (51.4) | χ2=0.06 | 139 (65.3) | 211 (97.2) | 1.08 (0.42) | 128 (62.4) | 57 (31.8) | 127 (64.8) | 172 (84.7) | 1.12 (0.46) |
| >median income | χ2=0.03 | 182 (59.7) | χ2=0.06 | χ2=2.17 | F=0.09 | χ2=0.24 | χ2=0.44 | χ2=1.46 | χ2=1.66 | F=0.01 | |
| Yes | 318 (65.3) | 73 (57.5) | 144 (68.2) | 84 (67.2) | 126 (99.2) | 1.14 (0.40) | 81 (65.3) | 37 (34.9) | 77 (66.4) | 103 (88.8) | 1.17 (0.47) |
| No | 169 (34.7) | 172 (58.3) | 78 (60.9) | 204 (66.0) | 301 (96.8) | 1.13 (0.44) | 189 (62.8) | 100 (38.6) | 200 (72.5) | 248 (83.8) | 1.17 (0.48) |
| Born in Australia | χ2=0.01 | χ2=0.15 | χ2 =0.59 | χ2=1.62 | F=0.18 | χ2 =0.55 | χ2=0.15 | χ2 =0.43 | χ2=0.85 | F=0.02 | |
| Yes | 413 (80.5) | 233 (59.6) | 238 (60.3) | 261 (65.6) | 387 (96.8) | 1.32 (0.44) | 246 (63.2) | 127 (38.0) | 248 (69.5) | 322 (85.4) | 1.17 (0.48) |
| No | 100 (19.5) | 58 (59.8) | 63 (62.4) | 71 (69.6) | 104 (99.0) | 1.15 (0.42) | 66 (67.3) | 30 (35.7) | 64 (66.0) | 80 (81.6) | 1.16 (0.45) |
| Full PHI cover | χ2=0.86 | χ2=0.44 | χ2=0.10 | χ2=0.96 | F=0.26 | χ2=0.32 | χ2=0.05 | χ2=3.38 | χ2=0.85 | F=0.01 | |
| Yes | 91 (18.5) | 55 (64.7) | 56 (64.4) | 57 (65.5) | 85 (98.8) | 1.16 (0.24) | 55 (67.1) | 27 (36.0) | 48 (60.0) | 73 (88.0) | 1.17 (0.47) |
| No | 400 (81.5) | 224 (59.3) | 233 (60.5) | 261 (67.3) | 380 (96.9) | 1.37 (0.44) | 242 (63.7) | 120 (37.4) | 247 (70.6) | 308 (83.9) | 1.17 (0.48) |
| Cancer site | χ2=4.97 | χ2=8.65 | χ2=2.59 | χ2=1.37 | F=2.29* | χ2=29.65† | χ2=2.81 | χ2=46.11† | χ2=17.43† | F=8.03† | |
| Breast | 100 (19.3) | 65 (68.4) | 66 (68.0) | 60 (61.9) | 97 (98.0) | 1.23 (0.43) | 78 (80.4) | 33 (41.3) | 87 (92.6) | 84 (92.3) | 1.36 (0.39) |
| Skin | 65 (12.6) | 38 (59.4) | 32 (50.8) | 39 (61.9) | 62 (95.4) | 1.05 (0.46) | 29 (44.6) | 19 (34.5) | 27 (48.2) | 43 (69.4) | 0.97 (0.54) |
| Head and Neck | 74 (14.3) | 39 (54.2) | 48 (66.7) | 51 (68.9) | 71 (97.3) | 1.14 (0.42) | 37 (52.9) | 26 (44.8) | 39 (66.1) | 60 (85.7) | 1.16 (0.48) |
| Prostate | 64 (12.3) | 38 (62.3) | 43 (67.2) | 43 (68.3) | 62 (98.4) | 1.20 (0.41) | 47 (75.8) | 20 (37.7) | 47 (81.0) | 49 (80.3) | 1.25 (0.45) |
| Other | 215 (41.5) | 114 (56.4) | 116 (56.3) | 145 (69.4) | 205 (97.2) | 1.10 (0.24) | 124 (62.3) | 61 (34.3) | 117 (60.6) | 172 (87.3 | 1.12 (0.46) |
| Total | 518 (100) | 294 (59.5) | 305 (60.8) | 338 (66.8) | 498 (97.3) | 1.14 (0.43) | 316 (64.0) | 159 (37.5) | 317 (68.9) | 408 (84.8) | 1.17 (0.47) |
| (B) Area-level charactoristics | |||||||||||
| SEIFA Quintile | χ2=5.29 | χ2=3.45 | χ2=4.04 | χ2=4.59 | F=0.61 | χ2=1.30 | χ2=0.90 | χ2=5.58 | χ2=2.64 | F=0.41 | |
| First (lowest) | 185 (36.0) | 115 (64.2) | 110 (60.8) | 129 (71.3) | 180 (98.4) | 1.17 (0.43) | 113 (63.8) | 56 (37.3) | 118 (71.1) | 154 (88.0) | 1.20 (0.46) |
| Second | 155 (30.3) | 89 (60.5) | 93 (61.2) | 99 (65.6) | 147 (96.1) | 1.12 (0.45) | 98 (65.8) | 49 (38.9) | 90 (65.7) | 122 (82.4) | 1.16 (0.49) |
| Third | 112 (21.9) | 59 (57.3) | 59 (56.75) | 65 (60.2) | 105 (95.5) | 1.09 (0.44) | 66 (63.5) | 36 (40.9) | 71 (72.4) | 82 (83.7) | 1.17 (0.49) |
| Forth | 54 (10.6) | 25 (47.2) | 37 (69.8) | 37 (68.5) | 54 (100.0) | 1.12 (0.39) | 32 (59.3) | 17 (34.7) | 31 (64.6) | 42 (82.4) | 1.13 (0.48) |
| Fifth (highest) | 5 (1.0) | 3 (60.0) | 2 (40.0) | 3 (60.0) | 5 (100.0) | 1.04 (0.43) | 4 (80.0) | 1 (25.0) | 1 (25.0) | 3 (75.0) | 1.02 (0.041) |
| ARIA | χ2=5.22 | χ2=3.35 | χ2=4.55 | χ2=2.66 | F=0.48 | χ2=0.99 | χ2=4.67 | χ2=2.47 | χ2=0.94 | F=0.99 | |
| Major city | 24 (4.7) | 9 (37.5) | 14 (58.3) | 13 (54.2) | 24 (100.0) | 1.03 (0.44) | 17 (70.8) | 10 (41.7) | 14 (63.6) | 20 (90.9) | 1.17 (0.46) |
| Inner regional | 225 (44.0) | 128 (60.7) | 140 (63.6) | 154 (70.3) | 216 (97.3) | 1.15 (0.38) | 139 (64.7) | 61 (33.5) | 133 (68.6) | 177 (83.5) | 1.16 (0.44) |
| Outer regional | 217 (42.5) | 128 (61.0) | 125 (60.4) | 140 (65.7) | 207 (96.3) | 1.12 (0.47) | 129 (62.3) | 76 (43.4) | 134 (68.0) | 172 (85.1) | 1.19 (0.52) |
| Remote | 23 (4.5) | 13 (61.9) | 12 (54.5) | 14 (66.7) | 23 (100.0) | 1.14 (0.44) | 13 (61.9) | 7 (38.9) | 14 (66.7) | 16 (84.2) | 1.17 (0.50) |
| Very remote | 22 (4.3) | 13 (61.9) | 10 (45.5) | 12 (54.5) | 21 (100.0) | 1.08 (0.43) | 15 (68.2) | 5 (27.8) | 16 (84.2) | 18 (85.7) | 1.18 (0.48) |
Mand SD reported for factor scores.
n(%) indicates the number and percentage of participants in each demographic category responding yes to item in section A and the number and percentage of participants in each area-level category responding yes to the item in section B.
*Valid percent calculated based on non-missing responses to this item.
†p<0.05 (applying family-wise errorrate adjustment for multiple χ2tests).
‡p<0.05 (applying family-wise error rate adjustment).
ARIA, Accessibility/Remoteness Index of Australia; NCCI, National Cancer Control Indicators; PHI, private health insurance; SEIFA, Socioeconomic Index for Areas.
Communication and patient involvement
Participants reported that their views were taken into account when their team of doctors and nurses were discussing their treatment 59.5% of the time (table 3A). Those with middle school education or lower were twice as likely to report having their views taken into account in treatment decisions (OR=2.22, 95% CI% 1.49 to 3.33) compared with those with senior high school or trade-level/tertiary-level education. Older patients were slightly more likely to report that their views were taken into account by doctors and nurses when deciding on treatment (r=0.11, p=0.02). Similarly, 60.8% of participants felt they were involved in decisions about their care and treatment as much as they would have liked; however, this did not vary according to individual characteristics.
Most participants reported understanding the explanation of ‘what was wrong with them’ on diagnosis (97.3%), and this did not differ significantly according to individual characteristics. More than half of the patients (66.8%) reported that the possible side effects of their treatment were explained to them in a way they could understand. Those with middle school education or lower were 1.5 times more likely to report having side effects explained to them in a way that they understood compared with those with trade-level/tertiary-level education (OR=1.50, 95% CI 1.14 to 1.96), and those with senior level education were less likely to report this compared with those with trade-level/tertiary-level education (OR=0.65, 95% CI 0.46 to 0.90).
Group differences were evident in participants’ scores on the communication and patient involvement factor with patients possessing middle school-level education (M=1.23, SD=0.42) reporting higher scores than those with trade-level/tertiary-level education (M=1.08, SD=0.42; t (509)=42.75, p<0.001, d=0.36) or those with senior school-level education (M=1.05, SD=0.42; t (509)=21.88, p<0.001, d=0.43). When compared with all other cancer types, patients with breast (M=1.23, SD=0.43; t (512)=28.75, p<0.001, d=0.28) and prostate (M=1.20, SD=0.41; t (512)=22.40, p<0.001, d=0.17) cancers reported higher scores on the communication and patient involvement factor, while those with skin (M=1.05, SD=0.46; t (512)=19.75, p<0.001, d=0.22) and head and neck (M=1.14, SD=0.42; t (512)=22.83, p<0.001, d=0.01) cancers reported lower scores than those with other cancer types.
Provision of information and services
Sixty-four per cent of participants were given written information about the type of cancer they had. Women were 1.69 (95% CI 1.16 to 2.46) times as likely to report receiving this information when compared with men. Patients with breast cancer were 2.41 (95% CI 1.39 to 4.42) times more likely, and those with skin cancer were 0.49 (95% CI 0.28 to 1.18) times as likely to receive written information about the type of cancer they had compared with those with other cancers. The majority of patients reported being given written information about the side effects of treatment (84.8%) with those with skin cancer less likely to receive this information compared with those with other cancer types 0.33 (95% CI 0.17 to 0.65).
Only 37.5% of participants reported receiving a written assessment and care plan. Those with middle school education or lower were almost two times more likely to report receiving an assessment and care plan (OR=1.90, 95% CI 1.23 to 2.91) compared with those with senior high school or trade-level/tertiary-level education. Over two-thirds of patients reported being given the name of a clinical nurse specialist to support them through treatment (68.9%); however, patients with breast cancer were 8.07 (95% CI 3.55 to 18.37) times more likely, and patients with prostate cancer were 2.77 (95% CI 1.36 to 5.68) times more likely to be offered this service compared with participants with other types of cancer.
Those with middle school-level education or lower (M=1.27, SD=0.48) reported higher scores on the provision of service and information factor than those with trade-level/tertiary-level education (M=1.12, SD=0.47; t (509)=39.71, p<0.001, d=0.32) or those with senior school-level education (M=1.07, SD=0.46; t (509)=20.28, p<0.001, d=0.42). When compared with all other cancer types, patients with breast (M=1.36, SD=0.39; t (99)=34.47, p<0.001, d=0.54) or prostate (M=1.25, SD=0.45; t (63)=22.42, p<0.001, d=0.19) cancer reported higher scores on the provision of information and services factor, while those with skin (M=0.97, SD=0.54; t (64)=14.52, p≤0.001, d=0.46) or head and neck (M=1.16, SD=0.48; t (73)=20.73, p<0.001, d=0.03) cancer reported lower scores than those with other cancer types. Area-level characteristics (ie, remoteness or SEIFA) were not significantly associated with single items or factor scores reflecting provision of information and services (see table 3B). Mean levels of physical QoL (F(4)=5.94, p<0.001), anxiety (F(4)=3.42, p=0.009), physical and daily living (F(4)=5.02, p<0.001), sexuality (F(4)=3.34, p=0.010) and supportive care needs were significant different across cancer types. Post hoc comparisons with a Bonferroni adjustment showed that breast cancer survivors (M=4.48, SD=4.10) reported significantly lower levels of anxiety than those in the ‘other’ cancer group (M=6.63, SD=5.94; p=0.015), and those with prostate cancer (M=0.50, SD=0.68) and breast cancer (M=0.59, SD=0.68) reported lower physical and daily living supportive care needs than those in the ‘other’ cancer group (M=0.89, SD=0.82; p=0.004 and p=0.012, respectively). Those with prostate cancer (M=0.64, SD=0.92) also reported significantly higher sexuality supportive care needs than those with skin (M=0.29, SD=0.60.), p=0.039) or head and neck cancer (M=0.28, SD=0.61; p=0.020), and those with breast (M=75.73, SD=10.25) and prostate (M=70.88, SD=10.81) cancers report significantly higher physical QoL compared with those in the ‘other’ cancer group (M=70.88, SD=10.81; both p<0.001).
Associations between NCCI factor scores and health and psychosocial variables
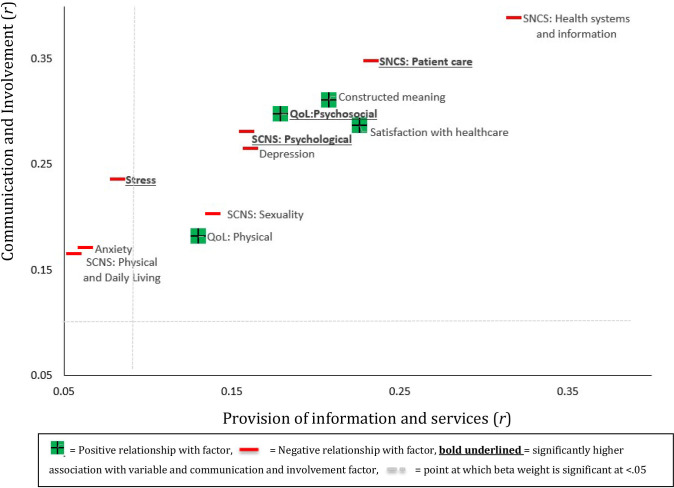
Both communication and patient involvement and provision of information and services factors shared significant positive associations with QoL, satisfaction with healthcare and constructed meaning (see figure 2). They were also both associated with lower supportive care needs in most cases, the strongest associations being with health system and information needs. The communication and patient involvement factor was moderately associated with greater psychosocial QoL (r=0.30, p<0.001) and satisfaction with healthcare (r=0.29, p<0.001) as well as lower levels of unmet ‘health systems and information’ (r=−0.39, p<0.001) and ‘patient care’ (r=0.35, p<0.001) needs and lower cancer threat appraisal (r=0.31, p<0.001). The provision of information and services factor had a weaker pattern of associations though still moderately predicted lower levels of unmet ‘health systems and information’ (r=−0.32, p<0.001) and ‘patient care’ (r=−0.23, p<0.001) needs, as well as greater satisfaction with healthcare (r=0.23, p<0.001). The communication and patient involvement factor shared significantly stronger associations with higher psychosocial QoL (z=2.03, p=0.042) and lower levels of unmet need in terms of psychosocial support (z=2.05, p=0.040) and patient care (z=2.00, p=0.046). Communication and patient involvement was associated with lower stress and anxiety, while provision of information and services was not (see figure 2).
Figure 2.
Visual comparison of the correlation coefficients between each health/psychosocial variable and each factor. QoL, quality of life; SCNS, Supportive Care Needs Scale.
Area-level characteristics (ie, remoteness or SEIFA) were not significantly associated with single items or scores on either NCCI factor (see table 3B).
Discussion
The goal of delivering equitable patient-centred cancer care is a corner stone of cancer control plans and care guidelines.6 7 9 12 The present study suggests that the NCCI patient experience indicators have validity and potential as a tool for monitoring and benchmarking the quality of cancer care relating specifically to patient understanding and involvement. Importantly, this brief tool discriminated between different aspects of patient experience for those dislocated from their home during treatment and identified characteristics associated with a poorer experience. While it was almost universal for patients to recall understanding the explanation of their treatment, and most people reported that treatment side effects were explained and supported with written information, only a minority received a written assessment and care plan. Further, patient experience varied by clinical and sociodemographic characteristics, suggesting that there is work to be done on better understanding what influences care and how we might intervene.
From a construct perspective, the patient experience as measured by the NCCI indicators presented along two key dimensions: (1) communication and patient involvement and (2) provision of information and services. For both dimensions, the strong association with health system and information needs provides evidence of convergent validity. The closer connection between communication and patient involvement and QoL and psychosocial outcomes intuitively makes sense, given the important role of the interpersonal relationships between the healthcare teams and patients, as well as the self-efficacy and personal agency that evolves from the patient’s involvement in their healthcare.31 These associations may be bidirectional. Patients with lower psychological distress have a higher capacity to absorb information, take part in decision making, benefit from communications with healthcare professionals32 33 and are subsequently more likely to report satisfaction with this element of their care.34 In fact, this may be reflected in the current findings that patients with breast cancer in this sample reported both lower levels of anxiety and better patient experiences compared with those with other cancers. Those individuals who are psychologically vulnerable or have poorer QoL likely need stepped up care to achieve optimal outcomes.35
Despite suggestions that remote living is associated with poorer experiences for cancer survivors, area-level factors were not associated with NCCI outcomes. Rather, differences in patient experiences according to cancer were apparent. Notably, patients with breast and prostate cancers were more likely to receive clinical nurse support compared with patients with skin and head and neck cancers. This may reflect the different resources and services available for specific cancers, for example, the introduction of the specialist nurse role for patients with breast cancer,36 and more recently patients with prostate cancer.37 While a specialist nurse appears to greatly enhance patient experience,38 providing this for all cancer types is likely a resourcing challenge especially for regional and remote health services. Models that incorporate telehealth and that span broadly across multiple cancer types or chronic disease may be needed.39 Higher education appeared to be associated with less communication and patient involvement and information and services. The reasons for this are unclear, but it may be that health professionals assume these patients require less support or alternatively that people with more education have greater expectations in this domain of care.
As the aim of the present study was to provide specific insight into the experiences of regional and remote patients with cancer, caution should be in applied in generalising these findings to urbanised populations. Since the survey is cross-sectional we cannot assume causality; and the data are self-reported and were not able to be verified by observational data or care records. However, this large, representative sample was recruited from a statewide jurisdiction and so likely represents the actual experience of patients from regional and remote Australia. Although regional and remote areas tend to be marked by higher socio-economic disadvantage in Australia,23 the particularly low SES status of this sample may be due to recruiting participants through free or low-cost accommodation services. Low levels of variance in area-level disadvantage in this sample may have impeded the detection of significant effects. These alternative hypotheses should be the subject of future research with samples not in receipt of such services. Although the aim of the current research was to assess patient experiences using a metric published by a national governing body, it is important to note that several valid measures of patient experience covering different aspects of patients’ care and support needs exist,10 11 29 and future research will benefit from their inclusion.
The present results outline the patient experience for a rural and regional population who are at risk of fragmented or poorly coordinated care. Patients who report better communication with their healthcare team and more involvement have better QoL, less stress and anxiety, and lower threat. Fulfilling the NCCI indicators connects to lower unmet need in health services and information. Cancer care services that ensure these indicators are met are better placed to provide an optimal cancer experience and improved patient-reported outcomes.
The NCCI presents as a useful and valid tool for assessing the patient experience. The aspect of care that appears most crucial is communication and involvement with the healthcare team. Strategies to optimise this for regional and remote patients need to be a cancer control priority.
Supplementary Material
Acknowledgments
We thank the research participants for taking part in the study and the Cancer Council Queensland volunteers and lodge staff who assisted with recruitment and data collection and entry. We also acknowledge the efforts of research team members Anna Stiller (Pamo Lozang) and Zina Ndugwa in assisting with project and data management.
Footnotes
Twitter: @ChambersInOz
Contributors: JD, BG, JFA, SM, FC-W, MI, NR, LZ, AR and SKC contributed to the study conception and design. Material preparation and data collection were conducted by BG, FC-W, AR and LZ, and analyses were performed by BG and MI. The first draft of the manuscript was written by JD and SKC, and JD, BG, JFA, SM, FC-W, MI, NR, LZ, AR and SKC provided substantial contributions in revising the manuscript and approved the final manuscript.
Funding: The study was funded by The University of Southern Queensland and Cancer Council Queensland. Award/Grant number is not applicable.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: The questionnaire and methodology for this study were approved by the human research ethics committee of the University of Southern Queensland (ethics approval number: ref. H17REA152).
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: Data that support the findings of this study are available from the corresponding author upon reasonable request.
Supplemental material: This content has been supplied by the author(s). It has not been vetted by BMJ Publishing Group Limited (BMJ) and may not have been peer-reviewed. Any opinions or recommendations discussed are solely those of the author(s) and are not endorsed by BMJ. BMJ disclaims all liability and responsibility arising from any reliance placed on the content. Where the content includes any translated material, BMJ does not warrant the accuracy and reliability of the translations (including but not limited to local regulations, clinical guidelines, terminology, drug names and drug dosages), and is not responsible for any error and/or omissions arising from translation and adaptation or otherwise.
References
- 1.Bray F, Ferlay J, Soerjomataram I, et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2018;68:394–424. 10.3322/caac.21492 [DOI] [PubMed] [Google Scholar]
- 2.Dasgupta P, Baade PD, Aitken JF, et al. Geographical variations in prostate cancer outcomes: a systematic review of international evidence. Front Oncol 2019;9:238. 10.3389/fonc.2019.00238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alfano CM, Jefford M, Maher J, et al. Building personalized cancer follow-up care pathways in the United States: lessons learned from implementation in England, Northern Ireland, and Australia. Am Soc Clin Oncol Educ Book 2019;39:625–39. 10.1200/EDBK_238267 [DOI] [PubMed] [Google Scholar]
- 4.Ellison EC, Pawlik TM, Way DP, et al. The impact of the aging population and incidence of cancer on future projections of general surgical workforce needs. Surgery 2018;163:553–9. 10.1016/j.surg.2017.09.035 [DOI] [PubMed] [Google Scholar]
- 5.Jakka S, Rossbach M. An economic perspective on personalized medicine. Hugo J 2013;7 10.1186/1877-6566-7-1 [DOI] [Google Scholar]
- 6.National Health Service, UK Innovation to implementation: stratified pathways of care for people living with or beyond cancer a ‘how to guide’ [online], 2016. Available: https://www.england.nhs.uk/wp-content/uploads/2016/04/stratified-pathways-update.pdf
- 7.Australian Government, Department of Health . Optimal cancer care pathways (OCPs) [online], 2018. Available: https://www1.health.gov.au/internet/main/publishing.nsf/Content/occp
- 8.Ganz PA, Balogh E. Delivering high-quality cancer care: charting a new course for a system in crisis. Wash DC Inst Med Natl Acad 2013. [PubMed] [Google Scholar]
- 9.Bao H, Yang F, Su S, et al. Evaluating the effect of clinical care pathways on quality of cancer care: analysis of breast, colon and rectal cancer pathways. J Cancer Res Clin Oncol 2016;142:1079–89. 10.1007/s00432-015-2106-z [DOI] [PubMed] [Google Scholar]
- 10.Department of Health National cancer patient experience survey 2018. National results summary [online]. National Health Service, United Kingdom, 2018. Available: https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/216682/dh_122520.pdf
- 11.State of Victoria, Department of Health and Human Services Understanding care experiences of people with cancer. Findings from pilot study 2 [online]. Victorian Government, 2016. Available: file:///C:/Users/bella/Downloads/Technical%20paper%20-%20Patient%20experience%20survey.pdf
- 12.Cancer Australia, Australian Government National cancer control indicators [online], 2019. Available: https://ncci.canceraustralia.gov.au/
- 13.Wilkes LM, White K, Mohan S, et al. Accessing metropolitan cancer care services: practical needs of rural families. J Psychosoc Oncol 2006;24:85–101. 10.1300/J077v24n02_06 [DOI] [PubMed] [Google Scholar]
- 14.Ireland MJ, March S, Crawford-Williams F, et al. A systematic review of geographical differences in management and outcomes for colorectal cancer in Australia. BMC Cancer 2017;17:95. 10.1186/s12885-017-3067-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cancer Council Queensland, Queensland University of Technology, Cooperative Research Centre for Spatial Information Australian cancer atlas version 09-2018 [online], 2018. Available: https://atlas.cancer.org.au [Accessed 14 Dec 2018].
- 16.Weaver KE, Geiger AM, Lu L, et al. Rural-urban disparities in health status among US cancer survivors. Cancer 2013;119:1050–7. 10.1002/cncr.27840 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Salehi A, Frommolt V, Coyne E. Factors affecting provision of care services for patients with cancer living in the rural area: an integrative review. Aust J Cancer Nurs 2019;20. [Google Scholar]
- 18.Butow PN, Phillips F, Schweder J, et al. Psychosocial well-being and supportive care needs of cancer patients living in urban and rural/regional areas: a systematic review. Support Care Cancer 2012;20:1–22. 10.1007/s00520-011-1270-1 [DOI] [PubMed] [Google Scholar]
- 19.Burris JL, Andrykowski M. Disparities in mental health between rural and nonrural cancer survivors: a preliminary study. Psychooncology 2010;19:637–45. 10.1002/pon.1600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beesley V, Eakin E, Steginga S, et al. Unmet needs of gynaecological cancer survivors: implications for developing community support services. Psychooncology 2008;17:392–400. 10.1002/pon.1249 [DOI] [PubMed] [Google Scholar]
- 21.Pateman KA, Cockburn NL, Batstone MD, et al. Quality of life of head and neck cancer patients in urban and regional areas: an Australian perspective. Aust J Rural Health 2018;26:157–64. 10.1111/ajr.12340 [DOI] [PubMed] [Google Scholar]
- 22.Australian Bureau of Statistics Australian statistical geography standard (ASGS): Correspondences. Cat. No. 1270.0.55.006 [online]. Canberra: ABS, 2011. Available: https://www.abs.gov.au/AUSSTATS/abs@.nsf/DetailsPage/1270.0.55.006July%202011?OpenDocument [Accessed 7 Nov 2019].
- 23.Australian Bureau of Statistics ABS.Stat SEIFA by local government area (LGA) [online]. Canberra: ABS, 2011. Available: http://stat.data.abs.gov.au/Index.aspx?DataSetCode=ABS_SEIFA_LGA [Accessed 7 Nov 2019].
- 24.Muthén Linda K, Muthén Bengt O. Mplus user’s guide. Los Angel: Muthen Muthen, 2007. [Google Scholar]
- 25.Antony MM, Bieling PJ, Cox BJ, et al. Psychometric properties of the 42-item and 21-item versions of the depression anxiety stress scales in clinical groups and a community sample. Psychol Assess 1998;10:176–81. 10.1037/1040-3590.10.2.176 [DOI] [Google Scholar]
- 26.Gomez F A guide to the depression, anxiety and stress scale (DASS 21). Cent East Syd Prim Health Netw 2016. [Google Scholar]
- 27.Fife BL The measurement of meaning in illness. Soc Sci Med 1995;40:1021–8. 10.1016/0277-9536(94)00174-R [DOI] [PubMed] [Google Scholar]
- 28.Richardson J, Sinha K, Iezzi A, et al. Modelling the utility of health states with the assessment of quality of life (AQoL) 8D instrument: overview and utility scoring algorithm. Res Pap 63 Cent Health Econ 2011. [Google Scholar]
- 29.Boyes A, Girgis A, Lecathelinais C. Brief assessment of adult cancer patients’ perceived needs: development and validation of the 34-item supportive care needs survey (SCNS-SF34). J Eval Clin Pract 2009;15:602–6. 10.1111/j.1365-2753.2008.01057.x [DOI] [PubMed] [Google Scholar]
- 30.IBM Corp IBM SPSS statistics for windows, version 26. Armouk, NY: IBM Corp, 2019. [Google Scholar]
- 31.Prip A, Møller KA, Nielsen DL, et al. The patient-healthcare professional relationship and communication in the oncology outpatient setting: a systematic review. Cancer Nurs 2018;41:E11–22. 10.1097/NCC.0000000000000533 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jansen J, van Weert JCM, de Groot J, et al. Emotional and informational patient cues: the impact of nurses' responses on recall. Patient Educ Couns 2010;79:218–24. 10.1016/j.pec.2009.10.010 [DOI] [PubMed] [Google Scholar]
- 33.van Osch M, Sep M, van Vliet LM, et al. Reducing patients’ anxiety and uncertainty, and improving recall in bad news consultations. Health Psychol 2014;33:1382–90. 10.1037/hea0000097 [DOI] [PubMed] [Google Scholar]
- 34.Kane HL, Halpern MT, Squiers LB, et al. Implementing and evaluating shared decision making in oncology practice. CA Cancer J Clin 2014;64:377–88. 10.3322/caac.21245 [DOI] [PubMed] [Google Scholar]
- 35.Schofield P, Chambers S. Effective, clinically feasible and sustainable: key design features of psycho-educational and supportive care interventions to promote individualised self-management in cancer care. Acta Oncol 2015;54:805–12. 10.3109/0284186X.2015.1010016 [DOI] [PubMed] [Google Scholar]
- 36.Amir Z, Scully J, Borrill C. The professional role of breast cancer nurses in multi-disciplinary breast cancer care teams. Eur J Oncol Nurs 2004;8:306–14. 10.1016/j.ejon.2003.12.011 [DOI] [PubMed] [Google Scholar]
- 37.Ralph N, Green A, Sara S, et al. Prostate cancer survivorship priorities for men and their partners: Delphi consensus from a nurse specialist cohort. J Clin Nurs 2020;29:265–73. 10.1111/jocn.15096 [DOI] [PubMed] [Google Scholar]
- 38.Dunn J, Ralph N, Green A, et al. Contemporary consumer perspectives on prostate cancer survivorship: fifty voices. Psychooncology 2020;29:557–63. 10.1002/pon.5306 [DOI] [PubMed] [Google Scholar]
- 39.Lovell NH, Redmond SJ, Basilakis J. Telehealth technologies for managing chronic disease-experiences from Australia and the UK. in: 2010 annual International Conference of the IEEE engineering in medicine and biology. IEEE 2010:5267–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjopen-2020-042507supp001.pdf (101KB, pdf)