Abstract
BACKGROUND
Infertility affects 48.5 million couples worldwide with a prevalence estimated at 3.5–16.7% in low- and middle-income countries (LMIC), and as high as 30–40% in Sub-Saharan Africa. ART services are not accessible to the majority of these infertile couples due to the high cost of treatments in addition to cultural, religious and legal barriers. Infertility and childlessness, particularly in LMIC, have devastating consequences, which has resulted in considerable interest in developing affordable IVF procedures. However, there is a paucity of evidence on the safety, efficiency and ability to replicate techniques under different field conditions, and how to integrate more affordable ART options into existing infrastructures.
OBJECTIVE AND RATIONALE
This review was performed to investigate the current availability of IVF in LMIC and which other ART options are under development. This work will unfold the landscape of available and potential ART services in LMIC and is a key element in positioning infertility more broadly in the Global Public Health Agenda.
SEARCH METHODS
A systematic literature search was performed of articles and gray literature on IVF and other ART options in LMIC published between January 2010 and January 2020. We selected studies on IVF and other ART treatments for infertile couples of reproductive age (18–44 years) from LMIC. The review was limited to articles published after 2010, based on the recent evolution in the field of ART practices in LMIC over the last decade. Citations from high-income countries, including data prior to 2010 and focusing on specialized ART procedures, were excluded. The literature search included PubMed, Popline, CINHAL, EMBASE and Global Index Medicus. No restrictions were applied with regard to study design or language. Two reviewers independently screened the titles and abstracts, and extracted data. A search for gray literature was performed using the ‘Google’ search engine and specific databases (worldcat.org, greylit.org). In addition, the reference lists of included studies were assessed.
OUTCOMES
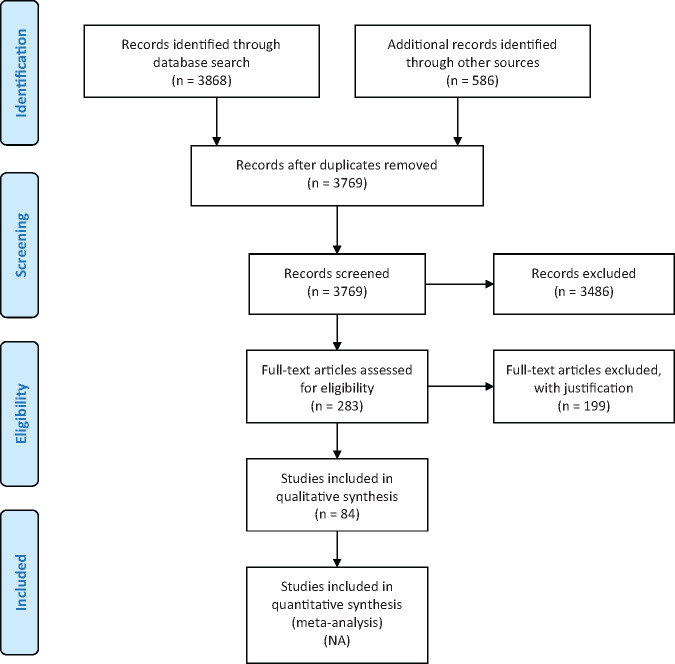
The search of the electronic databases yielded 3769 citations. After review of the titles and abstracts, 283 studies were included. The full texts were reviewed and a further 199 articles were excluded. The gray literature search yielded 586 citations, most of which were excluded after screening the title, and the remaining documents were excluded after full-text assessment due to duplicate entries, not from LMIC, not relevant or no access to the full document. Eighty-four citations were included as part of the review and separated into regions. The majority of the studies were observational and qualitative studies. In general, ART services are available and described in several LMIC, ranging from advanced techniques in China to basic introduction of IVF in some African countries. Efforts to provide affordable ART treatments are described in feasibility studies and efficacy studies; however, most citations were of low to very low quality. We found no studies from LMIC reporting the implementation of low-cost ART that is effective, accessible and affordable to most of those in need of the services.
WIDER IMPLICATIONS
The World Health Organization is in a unique position to provide much needed guidance for infertility management in LMIC. This review provides insight into the landscape of ART in LMIC in various regions worldwide, which will guide efforts to improve the availability, quality, accessibility and acceptability of biomedical infertility care, including ART in these countries.
Keywords: infertility, low- and middle-income countries, IVF, ART, fertility care, fertility coverage, affordable ART, accessible ART
Introduction
The World Health Organization (WHO) defines health as ‘a state of complete physical, mental and social well-being and not merely the absence of disease or infirmity’ (World Health Organization, 1948). In 2010, an estimated 48.5 million couples worldwide were infertile, defined at that time as an inability ‘to have any live birth over a 5-year period’ (Mascarenhas et al., 2012). The overall prevalence of infertility is estimated at 3.5–16.7% in low- and middle-income countries (LMIC), with the prevalence as high as 30–40% in some regions of Sub-Saharan Africa (Ombelet, 2009; Inhorn and Patrizio, 2015). Infertility in LMIC is more than a health problem, it is a social issue and a public health matter that continues to be neglected (Bahamondes and Makuch, 2014).
Infertility is known to cause significant psychological and social effects, such as fear, guilt, depression, self-blame, marital stress, emotional abuse, intimate partner violence, divorce and abandonment of the partner, social isolation, economic deprivation, loss of social status, and in some regions (e.g. Africa and Asia) even starvation, disease, violence-induced suicide and loss of dignity in death (Ombelet et al., 2008; Hammarberg and Kirkman, 2013; Stellar et al., 2016).
The most common etiologies of infertility in LMIC are male factor and tubal disease secondary to sexually transmitted infections, unsafe abortion and complications of childbirth (Ombelet, 2009; van der Poel, 2012). Tubal factor infertility is reported to be as high as 85% in Sub-Saharan Africa compared with 33% worldwide (Ombelet, 2009). The most effective treatment is ART (Sharma et al., 2009; Bahamondes and Makuch, 2014).
Infertility and ART are not considered a priority in many LMIC. The most often used arguments against the use of ART are overpopulation, other health priorities (e.g. family planning, vaccinations, malaria, HIV), limited government budgets and limited experience of providers with inadequate facilities for performing sophisticated procedures (Ombelet and Campo, 2007). Furthermore, in some LMIC, ART is considered to be expensive, only moderately effective, with risks of complications and unknown effects on women and their offspring (Ombelet and Campo, 2007). In 2008, ESHRE published a series of monographs by experts from around the world highlighting the importance of infertility, its prevalence, access to treatment and outcomes in developing countries (ESHRE Special Task Force on ‘Developing Countries and Infertility’, 2008). Along with the WHO and ESHRE, other non-governmental organizations (NGOs) are involved in initiatives aimed at improving access to ART in LMIC including the American Society for Reproductive Medicine, the International Federation of Gynecology and Obstetrics, the International Federation of Fertility Societies and the International Committee for Monitoring Assisted Reproductive Technologies.
Providing ART services in an LMIC requires an understanding of the country-specific magnitude and character of the issue of infertility, as well as identification of pre-existing resources that may be utilized (Sharma et al., 2009; Bahamondes and Makuch, 2014). WHO is in a unique position, with 194 member states worldwide, to assist in evaluating the burden of disease by the systematic assessment of infertility and resources available within various regions.
There is complete absence of affordable and accessible ART services in some LMIC possibly due to high costs of IVF and underdeveloped infrastructure in addition to cultural, religious and legal barriers. This deficiency has led to considerable interest by NGOs, policy makers and ART specialists in developing more affordable IVF protocols such as minimal ovarian stimulation. However, there is a paucity of evidence and systematic reviews on the safety and efficiency profile of low-cost ART, on the ability to replicate various techniques in different laboratories and under various field conditions and on how to integrate ART into existing health systems and infrastructures.
This review investigated the currently available IVF services in LMIC and potential for future development. This work will assist in unfolding the landscape of available services and the potential for ART services in LMIC. This is a key element in positioning infertility more broadly in the Global Public Health Agenda of WHO. This work will also inform future WHO guidelines concerning the provision of ART in LMIC.
Methods
This review was reported in accordance with the PRISMA and GATHER guidelines (Moher et al., 2009; Stevens et al., 2016). The protocol was registered on 24 April 2017 and published with PROSPERO International prospective register of systematic reviews (ID number: CRD42017064413). There were no amendments to the protocol after registration.
Search strategy and selection criteria
The electronic databases searched included PubMed, Popline, CINAHL, EMBASE and Global Index Medicus (regional WHO online databases). Citations were collected from inception until 1 January 2020. An internet search was performed using ‘Google’ search engine with the terms ‘infertility’, ‘low- and middle-income countries’ and ‘in vitro fertilization’ or ‘assisted reproductive technologies’ (limited to results published after 2010). Similar search terms were used for gray literature databases (worldcat.org, greylit.org). In addition, the reference lists of included studies were checked. Experts and professionals within the field of infertility, and the members of the ESHRE Special Interest Group Global and Socio-cultural Aspects of Infertility (n = 221) were contacted to provide information on any unpublished papers or data on the subject of ‘ART in low- and middle-income countries’.
Search strategies were customized for each electronic database according to their individual subject headings and searching structure. The search strategy used for PUBMED is available in Supplementary data. In constructing the search terms, accepted definitions of ART and IVF were used (Zegers-Hochschild et al., 2009; et al., 2017). LMIC were defined according to the World Bank classification of countries by Gross National Income per capita (low-income country (LIC) up to $995, lower-middle-income country (lower MIC) $996 to $3895 and upper-middle-income country (upper MIC) $3896 to $12 055) (World Bank Country and Lending Groups). Upper MIC, which often have ART services on par with high-income countries were labeled to distinguish them from LIC and lower MIC. For this review, no restrictions were applied with regard to study design or language. Reviewers were able to read English, French, German, Italian, Portuguese, Spanish and Russian studies. Reports in other languages were included and authors were asked to provide a translated version, or some of the details of the study in English. Endnote (Version X.8) bibliographic software was used to store the citations and remove duplicates.
For inclusion in the review, we selected citations on ART for adult women and men of reproductive age (18–44 years old) from LMIC (experiencing reproductive difficulties or infertility). All identified citations, irrespective of language, published over the last decade from 1 January 2010 to 1 January 2020 were assessed. The review was limited to articles published after 2010, based on the recent evolution in the field of ART practices in LMIC over the last decade. ART is defined as all interventions that include the in vitro handling of both human oocytes and sperm or of embryos for the purpose of reproduction. This includes, but is not limited to IVF and embryo transfer, ICSI, embryo biopsy, preimplantation genetic testing, assisted hatching, gamete intrafallopian transfer, zygote intrafallopian transfer, gamete and embryo cryopreservation, semen, oocyte and embryo donation, and gestational carrier cycles (Zegers-Hochschild et al., 2017). This review focused on IVF and embryo transfer procedures being performed in LMIC over the last decade. Articles focusing solely on ICSI, specialized ART procedures and that did not discuss IVF were excluded. The main reason for this restriction was these advanced procedures not always being accessible or affordable to the general population in an LMIC, where the cost of ART is estimated to be up to 50% higher than the gross national per capita income of many LMIC (Vayena et al., 2009).
Concerning outcomes, articles were assessed for quantitative outcomes on the efficacy of the ART (mainly pregnancy rate or live birth rate (LBR)), or qualitative and quantitative outcomes on feasibility. We defined feasibility as the process in which low-cost ART are deployed, leading to their acceptability and usability. All citations were evaluated based on the titles and abstracts by two independent reviewers (T.M.C. and N.V.). In the absence of sufficient data in the abstract to assess relevance, the full text was obtained. A list of the excluded reports is available from the authors upon request. The full-text reports were assessed for relevance and the data extracted by two independent reviewers (T.M.C. and N.V.). A third reviewer (I.T.) was available to resolve queries and disagreements. Attempts were made to contact the authors to obtain missing information or clarification whenever necessary.
Risk of bias and data analysis
The protocol for this review included assessment of risk of bias for all individual articles. The majority of included articles did not assess quantitative outcomes (efficacy or others) related to a therapeutic or diagnostic intervention, but merely reported on feasibility (current practice) in a narrative fashion. For studies assessing efficacy of interventions, the majority were either very small feasibility or pilot studies (assessed as high to very high risk of bias), or they were available only as an (conference) abstract. For the remaining interventional studies, risk of bias was assessed with the risk of bias in non-randomized studies of interventions (ROBINs)-1 tool (Sterne et al., 2016). Risk of bias was only assessed when the full-text paper could be retrieved. The collected data were, as expected, highly heterogeneous. Statistical comparison of the data was not possible due to the variable study design and quantitative data in the included citations. The results from included citations were collated in a descriptive fashion and meta-analysis was not feasible.
Results
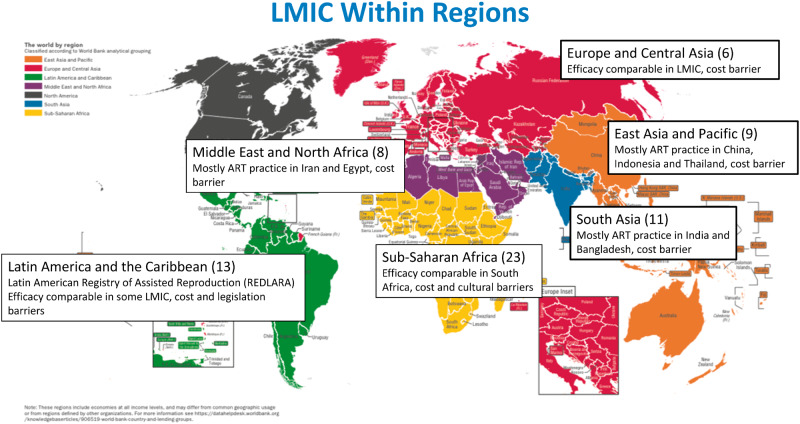
An extensive search of the databases yielded 3769 citations after removal of duplicate entries. After review of the titles and abstracts, 283 articles were included (Fig. 1). The full texts of 283 articles were reviewed, including case reports, review papers, commentaries, gray literature and abstracts from conference proceedings. A further 199 citations were excluded. The gray literature search yielded 586 citations, of which 334 were excluded after screening of the title. Further assessment of the remaining gray literature documents resulted in all citations being excluded. Exclusion of citations was based on duplicate entries from the database searches, not LMIC, not relevant or no access to the full document. Of the 84 included articles, 63 citations, mostly qualitative and observational studies, described an overall picture (efficacy, feasibility and acceptability) of ART in LMIC. These articles were summarized into regions (Supplementary Tables SI-VI, Fig. 2). Fourteen studies reported on the efficacy and feasibility of cost-limiting initiatives aimed at affordable ART in LMIC (Table I). A systematic review of literature was conducted; however, due to the heterogeneity of the included articles, the results are collated and presented here in a narrative fashion.
Figure 1.
PRISMA flow diagram of included and excluded studies of the review.
Figure 2.
Results of studies from LMIC summarized within regions. The numbers in parentheses represent the number of studies found within regions and a summary of their themes is shown. LMIC, low- and middle-income countries. Adapted from SDG Atlas 2018, The World By Region: http://datatopics.worldbank.org/sdgatlas/the-world-by-region.html.
I.
Summary of publications describing the overall picture (efficacy, feasibility and acceptability) of ART in East Asia and the Pacific.
| Reference | Country | Study design | Sample size | ART services | Quantitative outcomes on the efficacy of the ART | Quantitative and qualitative outcomes on the feasibility of the ART | Remarks |
|---|---|---|---|---|---|---|---|
| Audibert and Glass (2015) | China | Qualitative, Quantitative | 363 | IVF, ICSI | ART success in China was comparable to Spain and USA. The percentage of patients with implan embryos was similar across studied countries and ranged from an average of 85% for patients <35 years old to 51% for patients >42 years old. Among patients <35 years old, the fertility specialists based in China reported the highest implantation rate (54%). The implantation rate among patients >42 years old was 27% of patients in China. Among patients <35 years old, the reported PR was 53 % in China. Specialists in China reported an LBR of 47% in women <35 years. For patients >42 years old, the reported LBR was 21% in China. | The main unmet need reported by fertility specialists is better coverage for the cost of IVF. Fertility treatment is a highly technological field and is very costly, indicating it may be difficult to address this unmet need. | |
|
| |||||||
| Bennett et al. (2012) | Indonesia |
Qualitative Survey |
212 | ART | Not reported | Barriers to access include low confidence in infertility treatment and high rates of switching between providers due to perceived treatment failure, the number and location of clinics, lack of a well-established referral system and cost of treatment. Patients also experienced fear of receiving a diagnosis of sterility, vaginal examinations and embarrassment. | |
|
| |||||||
| Ha (2013) | Asia | Qualitative | NA | ART | Many Asian countries aggressively pursue technological development. Weak legislative and administrative regulations have created various problems and controversial cases. This study asserts that risks associated with technology are characterized as social facts not natural ones or mere ‘side effects’, since technological development and risk are closely intertwined. | Not reported | |
|
| |||||||
| Kwek et al. (2018) | Singapore | Retrospective Cohort | 5294 cycles | IVF, ICSI | The mean age of patients undergoing single blastocysts stage embryo transfer was lower than the other two groups. The DET, single and double blastocysts stage embryo transfer groups achieved similar LBR (33.9%, 38.7%, 35.4%, P > 0.05) and CPR (42.4%, 46.2%, 46.9%). | Not reported | |
|
| |||||||
| Li et al. (2018) | Asia-Oceania region | Survey | 24 countries | IUI, IVF | Not reported |
IUI and IVF were available in 23 and 22 countries, respectively. In Macau, only IUI, but not IVF, was officially available, no ART was available in Fiji. The typical cost per IVF cycle was US$2500 or above, the cost for IUI varied widely from less than US$200 to more than US$2500. ART was governed by legislation or national regulations in 12 of the countries, and 15 had a national registry, to which reporting was compulsory in 11 of them. Only Australia, Nepal, New Zealand and Saudi Arabia allowed ART for both single men and women, while only Australia and New Zealand allowed ART for homosexual couples. In Vietnam, ART was allowed only for single women (but not men). In Israel, only single or homosexual women but not men were allowed to receive ART. Government subsidy was available for IUI and IVF in 10 and 9 responding countries, respectively. Compensation to gamete donors and surrogate mothers were allowed in some countries, mostly on the basis of covering the medical treatment cost and compensation for leave from work. |
|
|
| |||||||
| Qiao and Feng (2014) | China |
Qualitative nationwide survey |
178 centers and 13 sperm banks | ART, IUI | Total ART cycle procedures of conventional IVF, ICSI, IVM, PGD, FET during 2005–2011 were 393 538, 168 498, 2596, 2269, 124 501, respectively. | ART cycles cost on average 30 000 Yuan (about $5000), and the average cost of a live birth following fresh autologous cycles was 100 000 Yuan (about $16 666) or more for an ART operation. Demand is not being met and many of infertile couples are on waiting lists. New regulations require assisted reproductive programs or centers to have a minimum of 12 medical staff who have to be trained by one of 10 China’s Ministry of Health (CMOH)-authorized reproductive training centers; facility space must be at least 260 m2 and be equipped with specific equipment. | |
|
| |||||||
| Wahlberg (2016) | China | Qualitative | NA | ART | In 2013, there were a total of 609 009 ART cycles carried out in China (including artificial insemination by husband: 91 725; artificial insemination by donor: 30 229; IVF: 221 025; ICSI : 98 935; and frozen embryo: 167 095). These cycles resulted in 193 863 pregnancies in 2013 and there were 145 108 live ART births in that year too. | The estimated cost per IVF cycle is 20 000–30 000 RMB while for ICSI it is 25 000–35 000 RMB per cycle. Fertility treatment is not covered by public or private insurance, which means that for many infertile couples in China, ART remains out of reach. | |
|
| |||||||
| Whittaker (2016) | Thailand | Qualitative | NA | ART | Not reported | Availability of ART remains limited and inaccessible for most of those who require treatment. With no public insurance available for infertility treatment in Thailand, ART remains an out-of-pocket expense beyond the financial reach of most average Thais. The cost of treatment ranges between US$2900 per cycle in government hospitals to US$5800 in private centers, while the average per capita income of Thais is estimated to be US$240 per month. Three-quarters of the infertility clinics in Thailand are in the urban centers, limiting physical accessibility for rural populations. As more and more children were born through IVF, this has removed some of the early stigma and shame associated with it. | |
|
| |||||||
| Ye et al. (2013) | China | Retrospective cohort | 3178 | IVF, ICSI | LBR per started cycle (34.21% (661/1932) versus 34.19% (426/1246)), LBR per ET cycle (38.30% (661/1726) versus 39.48% (426/1079)), miscarriage rate (13.6% (109/804) versus 16.4% (86/523)), moderate/severe OHSS rate (5.80% (112/1932) versus 7.78% (97/1246)). | Cost of gonadotropins needed for the patients in HP-FSH group was lower than that in rFSH group (4005 ± 1650 versus 6482 ± 2095). | Abstract |
PR, pregnancy rate; LBR, live birth rate; DET, double embryo transfer; CPR, clinical pregnancy rate; FET, frozen embryo transfer; HP-FSH, highly purified urinary FSH; rFSH, recombinant FSH; OHSS, ovarian hyperstimulation syndrome.
ART reports within regions
East Asia and Pacific.
ART in the region of East Asia and Pacific is described as a rapidly growing business (Wahlberg, 2016). A survey reported that in the countries within this region for which information was collected (China, Indonesia, Korea, Malaysia, Mongolia, Myanmar, Philippines, Singapore, Thailand and Vietnam), IVF is available for couples, although subsidized only in Singapore and Korea (Li et al., 2018). Nine citations reported mostly on the current practice in China and Thailand, which are upper MIC, and may not be representative of the practice in LMIC within this region (Supplementary Table SI). China is depicted as having high standards of practice and technological developments are aggressively pursued (Ha, 2013). Efficacy of ART in China was described as similar to efficacy in developed high-income European countries, such as France and Spain (LBR per started cycle of 47% in women <35 years old from ART) (Audibert and Glass, 2015). As expected, China with a high population is also unique in the high number of IVF cycles, and the term ‘scaled up IVF’ has been used with 145 108 live births reported after ART in 2013 (Wahlberg, 2016). Other countries in this region report the use of ART to a lesser extent, but there were no articles describing the efficacy of treatments (Ye et al., 2013).
With regards to the feasibility of ART, a study from Indonesia reported barriers to access which included low confidence in infertility treatment, high rates of switching between providers due to treatment failure, the number and location of clinics, the lack of a well-established referral system, the cost of treatment and patients with fear of receiving the diagnosis of infertility, fear of vaginal examinations or embarrassment (Bennett et al., 2012). In Thailand, ART treatment is considered out of reach for most average-income people. In addition, three-quarters of infertility clinics are located in urban centers, limiting physical accessibility for rural populations (Whittaker, 2016). One report from China found lower cost with recombinant FSH compared to highly purified FSH with similar pregnancy outcomes and LBR between the groups (Ye et al., 2013). Concerning acceptability, it is reported that shame and stigma have decreased over time and ART is now an accepted way to conceive (Whittaker, 2016). Although ART is well developed in China, it is still out of reach for most infertile couples due to an enormous demand for treatment, resulting in long waiting times and costs estimated between US$5000 and US$16 000 per IVF cycle, which is not covered by public or private insurance (Qiao and Feng, 2014; Audibert and Glass, 2015).
Europe and Central Asia.
ART in Europe is widely studied and reported by the European IVF Monitoring Programme. Six citations were included from LMIC, which were all from upper MIC (Supplementary Table SII). Large differences still exist between the number of cycles per 1 million women (aged 15–45 years) in these countries, but overall there is reportedly good access to ART services (European IVF monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology et al., 2017). Regarding efficacy of IVF, the data reported from LMIC are comparable to those in other, higher income, European countries (European IVF monitoring Consortium (EIM) for the European Society of Human Reproduction and Embryology et al., 2017). An LBR of 17.2% per transfer was reported in Bosnia Herzegovina and 26.5% in Serbia, both upper MIC (Balic, 2011; Mitic et al., 2012).
With regard to the feasibility and acceptability, the costs of treatment are most often discussed, along with legislative issues and regulations. In Turkey, government funding is reportedly provided (up to two IVF cycles in women aged 23–39 years old) only if all other options have been exhausted (Urman and Yakin, 2010). Turkish ART centers are required to be licensed by the government (Aytoz, 2012). In contrast, other countries have reported very little regulation and ART is influenced by market forces. For example, in Bulgaria where minimal regulation of ART is described, access and outcomes are poor, with 10 IVF clinics and low financial support for IVF treatments (Balabanova and Simonstein, 2010). Within Europe, a recent collaborative audit between ESHRE and the patient organization Fertility Europe demonstrated clear discrepancies in availability, accessibility and funding support within nine selected European Union countries (2017, Fertility Europe and European Society of Human Reproduction and Embryology (ESHRE), 2017). Only one study reported on the attitudes toward ART: a survey of 136 medical students, nurses and doctors in Russia (upper MIC) reporting that 97.2% of respondents knew enough about ART and had a positive attitude toward it (Khamoshina et al., 2010).
Latin America and the Caribbean.
Data on the number of IVF clinics and treatment cycles are reported in the Latin American Registry of Assisted Reproduction (REDLARA) (Zegers-Hochschild et al., 2013, 2014, 2015, 2016). The number of centers and countries in this region reporting data is increasing, with 13 citations included in the review (Supplementary Table SIII). Countries like Argentina and now Uruguay, with a consistent policy toward recognizing the human right to start a family and ensuring access to care, demonstrated the highest number of ART cycles per population, in contrast to countries where treatment depends on the couple’s capacity to pay (Zegers-Hochschild et al., 2016). An IVF unit in Jamaica reported ART outcomes similar to high-income countries (LBR of 16.8%, comparable to 21.3% in the UK). Furthermore, the paper reports that establishment of this dedicated unit has contributed to educating the public about infertility and ART, with increasing demand from infertile couples and also from less traditional families (Pottinger et al., 2012).
More studies discussed feasibility and accessibility. Health authorities in Brazil (upper MIC) have reported that ‘complex infertility treatments’, referring to ART, are unavailable to infertile couples (Makuch and Bahamondes, 2012). Despite this, a few public institutions are reported to offer infertility evaluation and IVF treatment, with some partially charging for the procedures. Access to care is limited due to high costs, long waiting times, complex scheduling processes and lack of political initiatives to implement more affordable ART. ART in Brazil is reportedly mostly offered in the private medical sector at high cost and health care services are unable to meet the growing demand for infertility treatment (Makuch and Bahamondes, 2012; de Souza, 2014; Corrêa and Loyola, 2015). It is reported there is no specific legislation regulating assisted conception. Political, economic and ethical challenges exist for policy makers to decide on allocation of funds for ART, considering the universal access and free of charge nature of the Brazilian health care system (Garcia and Bellamy, 2015). A recent study showed among the 4275 newborns enrolled in the Pelotas 2015 Birth Cohort Study, 18 births (0.4%) were the result of ART. Most ART was IVF (70.6%) and 90% women had double embryo transfer. All cycles were performed in private clinics with direct out-of-pocket payment. In 2012, the right to start a family was embraced by the Brazilian Unified Health System as a human right. Since then, 12 clinics and hospitals received financial support from the Brazilian government to provide universal access to ART services. Most of these clinics are situated in São Paulo, with no clinics reported in Northern Brazil (Silva et al., 2019). A study also highlighted that for people living with HIV/AIDS who desire to have a child face significant barriers to accessing ART treatment and counseling (Rossi Ada et al., 2011).
In some countries, until recently, laws existed prohibiting IVF. In Costa Rica (upper MIC), the Inter-American Court of Human Rights in 2012 ruled that the Supreme Court of Costa Rica's judgment in 2000 prohibiting IVF violated the human right to private and family life, the human right to found and raise a family, and the human right to non-discrimination on grounds of disability, financial means or gender (Zegers-Hochschild et al., 2013). On the other hand, in Brazil, the government launched a policy in 2012 establishing ART as a universal right within the National Health System (Silva et al., 2019).
REDLARA estimated that Mexico (upper MIC) had the third highest number of reported ART cycles in 2013 (Zegers-Hochschild et al., 2016). In 2015, 52 ART centers were registered with the Federal Commission for the Protection of Sanitary Risk who initiated a campaign of accreditation to verify that ART clinics are working to appropriate standards. Rather than generating a general comprehensive law, assisted reproduction specialists, in conjunction with representatives of government offices (e.g. Ministry of Health), members of private and public hospitals and NGOs are developing standards of practice for assisted reproduction services (González-Santos, 2016).
Middle East and North Africa.
Eight citations from this region were included in the review, most from Iran and Egypt (Supplementary Table SIV). Iran (upper MIC) is reported as the only EMRO country in which gamete donation and surrogacy are practiced. The role of ART has become increasingly important for the state, which views the rise in voluntary childlessness as a national challenge and is facilitating infertility treatment for couples of reproductive age (Tremayne and Akhondi, 2016).
Over 60 infertility clinics operate in the capital Tehran, as well as other major cities in Iran (Tremayne and Akhondi, 2016). An ART center in Iran quoted that 24.1% of IVF/ICSI cycles were successful (Abutorabi et al., 2014). Infertility centers in Iran are reported to operate outside of government-financed health facilities and services are only provided to those who can afford it. Although ART is limited by costs in Iran, the cost is relatively lower than neighboring countries, which encourages foreign infertile couples to travel to Iran to undergo ART. Lack of national auditing, supervision and a registry are cited as the major drawbacks of the quality of care of ART system in Iran (Sadeghi, 2015; Abedini et al., 2016).
Changes to the stimulation protocols to reduce costs were reported from a single unit in Egypt, which performed 3233 IVF and ICSI cycles over 5 years using HMG-only protocols as a practical and more affordable method of stimulation. The authors describe similar clinical pregnancy rates with a mean cost reduction of over US$600 in the HMG-only group (Sallam et al., 2013). One report highlighted the need to develop and implement strategies to improve the management of infertility and ART in Egypt. Suggested strategies included continuous updating of undergraduate and postgraduate education, professional development programs and in-service training (Gibreel et al., 2015).
South Asia.
A survey from this region reported that in the countries for which information was available (Bangladesh, India, Nepal, Pakistan and Sri Lanka), IVF is available for couples, but is not subsidized by the government. Oocyte donation and surrogacy are available and regulated in India, Nepal and Sri Lanka. Of these five countries, only India reported having a national registry for IVF activities, but it is not compulsory. The typical cost per treatment cycle of IVF was US$2500 or above in all responding countries, and IUI varied widely among the responding countries, from less than US$200 to more than US$2500 (Li et al., 2018). Of the 11 citations included in the review from this region, most were from India (Supplementary Table SV). IVF/ICSI, gamete donation and surrogacy are established ART practices in India in both the public and private sectors, allowing a maximum of three embryos per transfer (Widge and Cleland, 2011). The low cost, easy access, availability and economical prices of IVF drugs, along with availability of surrogates and gamete donors have fueled the growth of the ART industry in this region. Over the last decade, there has been a progressive increase in the number of ART clinics in India with the number of voluntary reporting IVF centers increasing. However, many centers in India are still not registered with a regulating body and not reporting their ART cycles (Malhotra et al., 2015).
Mishra (2013) described the drawback of the growing number of centers (now estimated to be over 500 clinics in India) and available treatments is that new ART therapies are often introduced directly from the laboratory to clinical practice and (safety) data are collected as patients are treated with new protocols. The options for infertility treatment in India vary from pharmacotherapy, often clomiphene citrate as the initial approach for most women, to more expensive ART options. Often patients still turn to alternative systems of medicine and faith healing, hoping for a quick and successful outcome (Palatty et al., 2012).
Although considered the most important barrier for ART in most countries, the financial burden was the commonest reason for dropping out of IVF cycles in India. In spite of the financial constraints, the majority of the couples consented to the first IVF cycle, but they had to stop treatment when repeated cycles of IVF were indicated. The authors did not report on LBR (Kulkarni et al., 2014). Efforts have been reported to reduce the costs of treatments (to one-third of the cost of conventional ART) by using minimal stimulation protocols and other cost-cutting measures in an attempt to provide more affordable treatment (Aleyamma et al., 2011; Olofsson et al., 2013). Mild stimulation protocols in use in India have been noted to have a lower pregnancy rate per cycle compared to conventional IVF protocols (17.6% versus 28.6%), although the cumulative LBR after 1 year of treatment was similar (43.4% versus 44.7%) (Mahajan, 2013). Quality management systems are being implemented in Indian ART clinics, and it is acknowledged by practitioners that the critical determinants of a high-quality IVF laboratory are the people, procedure, equipment and the laboratory design (Olofsson et al., 2013).
In Bangladesh, 10 tertiary level infertility centers reported 16 700 new patients per year but only 5% proceeded to ART mainly due to financial constraints (Fatima et al., 2015). Some centers in Pakistan offer minimal stimulation protocols, ICSI at a reduced cost and even free IVF in some cases to meet the demand (Shah Nawaz and Azhar, 2014). Infertility and its challenge and barriers to care can be deduced from evidence on IUI in a survey from Pakistan: 90% of respondents declined IUI because of religious and cultural taboos, and if they received treatment they were not willing to disclose this to their family (Khalid and Qureshi, 2012).
Sub-Saharan Africa.
It is reported that <1.5% of the population of Africa has access to ART (Ombelet and Onofre, 2019). This review included 23 citations from this region (Supplementary Table SVI). South Africa (upper MIC) is the most developed and experienced in provision of ART, and was previously the only country with a published data registry (South African registry for assisted reproductive techniques, SARA) in this region (SARA report, 2014). More recently Dyer et al. (2019) published data from the newly developed African Network and Registry for Assisted Reproductive Technology with voluntary reporting from 40 centers in 13 countries. The Association for Fertility and Reproductive Health of Nigeria is active as a regulatory framework and provides ethical guidelines for ART (Okonta et al., 2018). We found seven studies exploring the efficacy of ART treatments (Eluga et al., 2010; Olukoya et al., 2012; Orhue et al., 2012; De Beer et al., 2016).
One method to increase efficacy of treatment strongly practiced in Sub-Saharan Africa is the transfer of multiple embryos, justified as being for economic reasons and the fear of failure (Onah and Okohue, 2010; Fadare and Adeniyi, 2015). In Nigeria, the LBR has been reported as high as 76%, but with high multiple pregnancy rates of up to 40% (Olukoya et al., 2012). It is reported as common practice to transfer up to five embryos at once in Nigeria, Ghana, Mali and Uganda (Fadare and Adeniyi, 2015; Hörbst, 2016; Horbst and Gerrits, 2016). Furthermore, limited storage facilities and the quality thereof (power supply, access to liquid nitrogen), costs associated with storage, religious concerns about the fate of additional embryos, and the patient’s perspective on multiple pregnancies all support the justification for transfer of more than two embryos in these countries (Fadare and Adeniyi, 2015). African women reportedly wish for and do not mind multiple gestations, the complications notwithstanding, particularly when they are over 35 years old with a long history of infertility (Onah and Okohue, 2010). In contrast, in South Africa (upper MIC) fewer embryos are transferred (up to three) as they have more experience with ART, better technical expertise and legal restrictions (Huyser and Boyd, 2012; Fadare and Adeniyi, 2015).
ART treatments in Sub-Saharan Africa are largely similar to practices in high-income countries. Owing to the lack of local knowledge, guidelines or legislation, clinicians starting an ART clinic often refer to European/American guidelines, organize collaborations and training in European centers, and even use second-hand equipment from European laboratories. A recent case report showed the feasibility of knowledge transfer from high- to low-income settings in the set-up of a fertility clinic in Zimbabwe, resulting in safe and affordable ART with successful outcomes (Hammarberg et al., 2018). Nevertheless, local practices are also implemented, such as extended bed rest and hospitalization in Ghana after embryo transfers (Gerrits, 2016), and egg sharing to reduce costs for patients (Hörbst, 2016).
Regarding feasibility and accessibility, most studies focused on costs, accessibility of clinics/services, public awareness and acceptability of treatment. There were no reports of state-funded ART treatments in this region, but affordable ART services have been introduced in some countries (South Africa, Uganda and Nigeria) (Eluga et al., 2010; Orhue et al., 2012; De Beer et al., 2016). Such affordable alternatives are reported to have an out-of-pocket cost of around US$200 per IVF cycle (Eluga et al., 2010), while other studies quoted costs of up to US$2700 per IVF cycle in Ghana, up to US$4500 in Kenya and up to US$10 000 in Nigeria (Fadare and Adeniyi, 2015; Gerrits, 2016; Ndegwa, 2016). These costs are to be seen in relation to the national monthly minimum wage, which is approximately US$110 in Nigeria (Fadare and Adeniyi, 2015). One in five couples (22%) in South Africa (upper MIC) incurred catastrophic expenditure, defined as an out-of-pocket cost >40% of annual non-food expenditure, and reported coping by reducing expenditure on clothing and food, using of savings, borrowing money and taking on extra work (Dyer et al., 2013). Almost 4 years after ART, couples had not recovered financially from the treatment (Dyer et al., 2017). Costs are considered to be a factor in the low utilization rates of ART services (Omokanye et al., 2017; Botha et al., 2018; Dyer et al., 2019; Ombelet and Onofre, 2019). The accessibility of clinics is another barrier to ART treatment for patients, with only a few clinics reported in Kenya (Murage et al., 2011). Transnational ART is becoming common, with people crossing borders to access treatment in South Africa and Ghana (Gerrits, 2016).
Public awareness and acceptability of ART treatments were studied by surveys in Nigeria, which reported several cultural concerns (e.g. legitimacy of children born, patriarchy, polygyny and value of children) and ethical issues (e.g. decision-making about the use of technologies, discrimination against children born, psychological problems and loss of self-esteem, side effects and costs) related to ART. These issues are largely dependent on the local context (urban and rural) and religion (Catholic, Muslim, Anglican and traditional religions) (Fabamwo and Akinola, 2013; Iliyasu et al., 2013; Bello et al., 2014; Menuba et al., 2014; Fadare and Adeniyi, 2015; Omokanye et al., 2017; Botha et al., 2018; Dyer et al., 2019; Ombelet and Onofre, 2019). We found no studies exploring the acceptability of ART treatment in other African countries.
Cost-limiting initiatives aiming at affordable ART
Several options for lowering the cost of ART have been described in 14 citations and compared to conventional ART, although mostly in small feasibility trials or pilot studies (Table I).
In India, low-cost ART was evaluated in 143 carefully selected patients. A mild stimulation protocol with several cost-cutting measures (e.g. eliminating superfluous investigations), resulted in an LBR per started cycle of 14%, at an average direct cost of US$675 for IVF (Aleyamma et al., 2011). In South Africa, a clinical pregnancy rate of 16.3% per embryo transfer was reported with a low-cost protocol (mild ovarian stimulation, optimum utilization of trained personnel and adapted laboratory procedures) (conference abstract) (De Beer et al., 2016). In another study, low-cost ART using minimal ovarian stimulation, the local laboratory and a locally trained embryologist was assessed in 15 patients in Uganda. All patients proceeded to oocyte retrieval, but pregnancy outcomes were not reported (conference abstract) (Eluga et al., 2010).
Minimal stimulation compared to conventional stimulation was assessed in Turkey (upper MIC) and showed that minimal stimulation resulted in similar clinical pregnancy rates while being more cost effective (Özörnek et al., 2013). A study comparing two minimal stimulation protocols in normal responders with tubal factor infertility reported improved outcomes with clomiphene citrate as compared to letrozole, and concluded that such stimulation is feasible in the Indian context (Nagulapally et al., 2012). Other studies evaluated minor changes to the stimulation drugs to reduce costs. In India, women undergoing treatment for severe male factor infertility with letrozole reduced the total dose of GnRH agonist required, and reduced the cost by 34% while pregnancy rates were comparable with conventional GnRH agonist protocols (Mukherjee et al., 2012). A randomized controlled trial from Pakistan, only accessible as an abstract, compared stimulation with aromatase inhibitors, gonadotrophins and indomethacin to standard stimulation with GnRH analogs and reported similar pregnancy and LBR (Shah Nawaz and Azhar, 2014).
In addition to minimal stimulation protocols and changes in stimulation drugs, novel simplified culture systems have been tested. A study from Colombia assessed the INVOcell® device for intravaginal culture. A mean of 4.2 oocytes was inseminated and cultured in the INVOcell® device, resulting in, on average, 2.6 embryos and a clinical pregnancy rate of 40% per cycle (Lucena et al., 2013). In another study from the same research group, good quality embryos, higher implantation and pregnancy rates were obtained using INVOcell® compared to conventional IVF/ICSI (Navarro-Carbonell et al., 2012; Lucena et al., 2013). A case report from Pakistan reported that using intravaginal culture with the INVOcell® device was successful and accep to the patient (Khan et al., 2013).
International initiatives have been focusing on bringing ART to low-resource settings. The Walking Egg project aims to reach the goal of ‘global access to infertility care’ (Dhont, 2011). As part of the project, feasibility and pilot studies on low-cost ART have been published. In a prospective non-inferiority study, IVF with the simplified culture system, without the need for specialized medical-grade gases or equipment, was evaluated against the routine culture system in 40 patients, of whom 35 reached embryo transfer (Day 3). Fertilization rates, cleavage rates and clinical pregnancy rates (8/12 with simplified versus 2/12 with standard culture) were similar (Van Blerkom et al., 2014). In a next step, a feasibility study of the simplified (t)WE lab system, a closed [same tube] system for fertilization and development until Day 3, resulted in three pregnancies and four live births (Ombelet et al., 2014). All these studies were performed in Belgium. Recently, the same research group published a study on how to implement these systems in low-resource settings (Ombelet and Goossens, 2016), and they reported the birth of the first baby in Ghana (Ombelet and Onofre, 2019).
Other studies have assessed the efficacy and feasibility of improvements to the ART clinic organization. Batching treatment cycles is used in Nigeria as a method of reducing the costs, with a clinical pregnancy rate of 30% per embryo transfer (Orhue et al., 2012). An intensive course with training in hystero-contrast-sonography and one-step IUI was a possible tool as a first step to introduce basic infertility care into resource-poor settings, like Eritrea, before advancing to ART (Gnoth et al., 2013). Despite these studies and efforts, most of the initiatives come from high-income countries, and are still not immediately transferable to all LMIC settings (Bahamondes and Makuch, 2014).
Risk of bias across studies
Risk of bias of individual studies is recorded in I. We found 14 studies focusing on efficacy, feasibility and acceptability of more affordable ART options, of which two provided indirect evidence (moderate risk of bias) as they were conducted as pilot studies in high-income countries (Ombelet et al., 2014; Van Blerkom et al., 2014). Of the remaining 12 studies, five could be accessed as a (conference) abstract only (Eluga et al., 2010; Nagulapally et al., 2012; Özörnek et al., 2013; Shah Nawaz and Azhar, 2014; De Beer et al., 2016), three were scored as at critical risk of bias (Gnoth et al., 2013; Khan et al., 2013; Lucena et al., 2013), two at serious risk (Aleyamma et al., 2011; Orhue et al., 2012) and two at moderate risk of bias (Mukherjee et al., 2012; Navarro-Carbonell et al., 2012).
Discussion
The primary aim of this review was to establish the availability of IVF and other ART services in LMIC, focusing on accessibility, efficacy, feasibility and acceptability. In addition, we summarized citations on the currently available affordable ART services or cost-reducing interventions. While performing the review, it was evident from many studies that high-cost ART treatments are being offered in LMIC, often in private clinics and with the aim of providing ART access to the more economically affluent population. Such ART treatments are not accessible to the low- and middle-income people living in urban areas, as the cost is estimated to be up to 50% higher than the gross national per capita income in many LMIC (Vayena et al., 2009). Affordable ART was considered to be ART that is not cost prohibitive and is accessible to the general population of an LMIC.
There were recurring themes among the citations reviewed. Reports included national data with regard to number of clinics, success rates, costs and drawbacks being faced. Several of the included studies discussed suggestions for increasing access, availability and acceptability of ART within a country. Finally, studies also reported on attitudes toward infertility and ART and a shift in perception that can be made through education of health care providers, patients and communities (Vayena et al., 2009).
Very few papers were found specifically discussing low-cost ART options and most were small feasibility studies or pilot studies performed in developed countries rather than large-scale evaluations of the efficacy, safety and feasibility of these treatments in LMIC. Affordable ART initiatives include the Walking Egg project and the INVOcell® device. These projects are integral in bringing adapted ART services to LMIC, but they should be evaluated through robust research for efficacy and safety, and further adapted to the local infrastructure. Recommendations on how to establish an ART center in a low-resource setting have been published to improve access (Cooke et al., 2008).
When bringing ART to LMIC, the variation in the etiology of infertility should be taken into consideration. Male infertility is more common in some regions and is managed using various ART (for instance sperm extraction along with ICSI), or IUI. However, local culture and stigma in some regions prevents the man of the infertile couple undergoing fertility testing, which significantly affects the female partner, but may also influence availability and research on treatments for male factor infertility (Agarwal et al., 2015). Secondary infertility, often following unsafe abortions and complications at childbirth, is also frequently reported in some LMIC and infertility management should include preventative measures in addition to implementing more affordable ART strategies.
In addition to the varying composition of infertile populations in LMIC, the differences in settings between countries and regions are significant. LMIC is too wide a category to assess and summarize fertility treatments appropriately. There was a vast difference between ART offered in low-income countries and lower MIC, compared to upper MIC. The upper MIC, such as China, implement new cutting edge treatments and technologies aggressively without technical restrictions (Ha, 2013), while at the other end of the spectrum, low-income countries struggle to introduce fertility assessment and low-cost options (Gnoth et al., 2013). The need for ART regulating bodies within countries and regions was universally reported by the studies.
Another aspect of ART in LMIC that raises concern is cross-border reproductive care, where possibly as a consequence of the lack of ART legislation, private clinics offer high-quality ART procedures to foreign infertile couples at a high cost (Abedini et al., 2016). Cross-border reproductive care could be beneficial to local residents as it boosts the economy, as well as bringing resources, technologies and treatments to their country (Sadeghi, 2015). However, the local inhabitants are generally not able to afford the same treatments and are rarely offered cost-saving opportunities such as egg sharing. Young women have the potential to be exploited as egg donors or surrogates for wealthy foreigners.
While performing the review, it was noted that there is basic science and clinical research occurring in LMIC, which is helping to inform and improve outcomes in ART worldwide. In addition, national and regional registries are attempting to collect data on ART treatments, with some success in Latin America and Africa (Zegers-Hochschild et al., 2013; SARA report, 2014; Zegers-Hochschild et al., 2014; et al., 2015; et al., 2016). More rigorous data reporting, collection and verification are needed from LMIC to enable a meta-analysis in the future (Kushnir et al., 2017).
In conclusion, the results of this review demonstrate some degree of availability of IVF and other ART services in LMIC but highlight a need for the development of more affordable and accessible ART overall. Infertility continues to be a global health problem that is still not being adequately addressed worldwide. This review was performed to inform the WHO guidelines and to consider ART services as an important strategy in the management of men and women with infertility in LMIC. These guidelines will hopefully assist policy makers in including the management of infertility, including IVF and other ART services, in the reproductive health agenda and hence to improve overall access to reproductive care in LMIC.
Limitations
Health care systems and populations largely vary among different countries, even within the same geographic region, and information on these variations is not readily available. In addition to this regional variation, the availability, heterogeneity and quality of studies largely influenced the conclusions to be drawn for the different regions. The heterogeneity of studies and reports can also be attributed to the less restrictive inclusion criteria for outcomes, which were set as such to increase the sensitivity of the search strategy.
Regarding the options for affordable ART in LMIC, the most significant limitation was the lack of high-quality studies. Of the 14 studies included in the section ‘Cost limiting initiatives aiming at affordable ART’, only four were of moderate risk of bias, of which two provided indirect evidence. Although attempts were made to contact authors and to find the published trials of references included as an abstract, we may have missed valuable data from studies not available in the included databases or retrievable through the English search terms and unpublished data.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Supplementary Material
Acknowledgements
We would like to thank Tomas Allen (WHO) and Nancy Bianchi (University of Vermont) for their advice and assistance with the search strategies.
Authors’ roles
I.T. was responsible for the concept of this protocol. T.M.C. and K.B. wrote the protocol and designed the search strategy. I.T., R.F. and K.L. reviewed and edited the protocol. K.B. performed the final literature search. T.M.C. and N.V. extracted the data, performed the analysis of the articles and wrote the manuscript. All the authors contributed to the design, interpretation of the results and critical revision of the manuscript.
The authors J.K. and I.T. are staff members of the World Health Organization. The authors alone are responsible for the views expressed in this publication and they do not necessarily represent the views, decisions or policies of the World Health Organization.
Funding
This study was supported by the World Health Organization Department of Reproductive Health and Research. This work was also funded by the UNDP-UNFPA-UNICEF-WHO-World Bank Special Programme of Research, Development and Research Training in Human Reproduction (HRP), a cosponsored program executed by the World Health Organization.
Conflict of interest
None declared.
References
- Abedini M, Ghaheri A, Omani Samani R. Assisted reproductive technology in Iran: the first national report on centers, 2011. Int J Fertil Steril 2016;10:283–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutorabi R, Razavi V, Baghazade S, Sharegh L, Mostafavi FS. Evaluation of the success rate of assisted reproductive techniques (ART) in Shahid Beheshti infertility center, Isfahan, Iran. J Isfahan Med School 2014;32:1767–1781. [Google Scholar]
- Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol 2015;13:37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aleyamma TK, Kamath MS, Muthukumar K, Mangalaraj AM, George K. Affordable ART: a different perspective. Hum Reprod 2011;26:3312–3318. [DOI] [PubMed] [Google Scholar]
- Audibert C, Glass D. A global perspective on assisted reproductive technology fertility treatment: an 8-country fertility specialist survey. Reprod Biol Endocrinol 2015;13:133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytoz A. Female health care professionals in fertility services in Turkey. Hum Reprod 2012;27: ii18. [Google Scholar]
- Bahamondes L, Makuch MY. Infertility care and the introduction of new reproductive technologies in poor resource settings. Reprod Biol Endocrinol 2014;12:87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balabanova E, Simonstein F. Assisted reproduction: a comparative review of IVF policies in two pro-natalist countries. Health Care Anal 2010;18:188–202. [DOI] [PubMed] [Google Scholar]
- Balic D. How to make assisted reproductive technologies (ART) affordable in Bosnia and Herzegovina: experience after the first 105 cycles. Med Arh 2011;65:119–121. [PubMed] [Google Scholar]
- Banerjee K, Singla B. Acceptance of donor eggs, donor sperms, or donor embryos in Indian infertile couples. J Hum Reprod Sci 2018;11:169–171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bello FA, Akinajo OR, Olayemi O. In-vitro fertilization, gamete donation and surrogacy: perceptions of women attending an infertility clinic in Ibadan, Nigeria. Afr J Reprod Health 2014;18:127–133. [PubMed] [Google Scholar]
- Bennett LR, Wiweko B, Hinting A, Adnyana IB, Pangestu M. Indonesian infertility patients' health seeking behaviour and patterns of access to biomedical infertility care: an interviewer administered survey conducted in three clinics. Reprod Health 2012;9:24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Botha B, Shamley D, Dyer S. Availability, effectiveness and safety of ART in sub-Saharan Africa: a systematic review. Hum Reprod Open 2018;2018:hoy003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooke I, Gianaroli L, Hovatta O, Trounson A. Affordable ART and the Third World: difficulties to overcome. Hum Reprod 2008;2008:93–96. [Google Scholar]
- Corrêa MCDV, Loyola MA. Tecnologias de reprodução assistida no Brasil: opções para ampliar o acesso. Physis (Rio J) 2015;25:753–777. [Google Scholar]
- De Beer MW, Matsaseng TC, Erasmus EL, Nel NA, Pillay D, Nosarka S. Affordable ART outcomes at the Tygerberg Fertility Clinic: Tygerberg Academic Hospital (TBAH), South Africa-special reference to tubal factor infertility. Reprod Biomed Online 2016;32:S3. [Google Scholar]
- MdCB de Souza Latin America and access to assisted reproductive techniques: a Brazilian perspective. JBRA Assist Reprod 2014;18:47–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhont N. The Walking Egg non-profit organisation. Facts Views Vis Obgyn 2011;3:253–255. [PMC free article] [PubMed] [Google Scholar]
- Dyer S, Archary P, de Mouzon J, Fiadjoe M, Ashiru O. Assisted reproductive technologies in Africa: first results from the African Network and Registry for Assisted Reproductive Technology, 2013. Reprod Biomed Online 2019;38:216–224. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Sherwood K, McIntyre D, Ataguba JE. Catastrophic payment for assisted reproduction techniques with conventional ovarian stimulation in the public health sector of South Africa: frequency and coping strategies. Hum Reprod 2013;28:2755–2764. [DOI] [PubMed] [Google Scholar]
- Dyer SJ, Vinoos L, Ataguba JE. Poor recovery of households from out-of-pocket payment for assisted reproductive technology. Hum Reprod 2017;32:2431–2436. [DOI] [PubMed] [Google Scholar]
- Eluga M, Tamale Sali E, Desmet B, Albano C, Devroey P, Ombelet W, Platteau P. Controlled ovarian stimulation for in vitro fertilization in a low resource setting: a pilot study in Kampala-Uganda. Hum Reprod 2010;25:i21–i22. [Google Scholar]
- ESHRE special task force on ‘developing countries and infertility’. ESHRE Monographs 2008;2008:1–117. [Google Scholar]
- European IVF monitoring Consortium (EIM); for the European Society of Human Reproduction and Embryology, Calhaz-Jorge C, De Geyter C, Kupka MS, de Mouzon J, Erb K, Mocanu E, Motrenko T, Scaravelli G, Wyns C, Goossens V, et al. Assisted reproductive technology in Europe, 2013: results generated from European registers by ESHRE. Hum Reprod 2017;32:1957–1973. [DOI] [PubMed] [Google Scholar]
- European Policy Audit on Fertility (EPAF). 2017.
- Fabamwo AO, Akinola OI. The understanding and acceptability of assisted reproductive technology (ART) among infertile women in urban Lagos, Nigeria. J Obstet Gynaecol 2013;33:71–74. [DOI] [PubMed] [Google Scholar]
- Fadare JO, Adeniyi AA. Ethical issues in newer assisted reproductive technologies: a view from Nigeria. Niger J Clin Pract 2015;18 Suppl:S57–S61. [DOI] [PubMed] [Google Scholar]
- Fatima P, Ishrat S, Rahman D, Banu J, Deeba F, Begum N, Anwary SA, Hossain HB. Quality and quantity of infertility care in Bangladesh. Mymensingh Med J 2015;24:70–73. [PubMed] [Google Scholar]
- Fertility Europe, European Society of Human Reproduction and Embryology (ESHRE). A Policy Audit on Fertility: Analysis of 9 EU Countries 2017. http://www.fertilityeurope.eu/our-projects/policy-audit/ (5 October 2020, date last accessed).
- Garcia S, Bellamy M. Assisted conception services and regulation within the Brazilian context. JBRA Assist Reprod 2015;19:198–203. [DOI] [PubMed] [Google Scholar]
- Gerrits T. Assisted reproductive technologies in Ghana: transnational undertakings, local practices and ‘more affordable’ IVF. Reprod Biomed Soc Online 2016;2:32–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibreel A, Eladawi N, El-Gilany AH, Allakkany N, Shams M. How do Egyptian gynecologists manage infertility? Cross-sectional study. J Obstet Gynaecol Res 2015;41:1067–1073. [DOI] [PubMed] [Google Scholar]
- Gnoth C, Kaulhausen H, Marzolf S. First steps into gynaecological endocrinology and reproductive medicine in resource-poor countries: an eritrean experience. J Reprod Med Endokrinol 2013;10:44–48. [Google Scholar]
- González-Santos SP. From esterilología to reproductive biology: the story of the Mexican assisted reproduction business. Reprod Biomed Soc Online 2016;2:116–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha JO. Risk disparities in the globalisation of assisted reproductive technology: the case of Asia. Glob Public Health 2013;8:904–925. [DOI] [PubMed] [Google Scholar]
- Hammarberg K, Kirkman M. Infertility in resource-constrained settings: moving towards amelioration. Reprod Biomed Online 2013;26:189–195. [DOI] [PubMed] [Google Scholar]
- Hammarberg K, Trounson A, McBain J, Matthews P, Robertson T, Robertson F, Magli C, Mhlanga T, Makurumure T, Marechera F. Improving access to ART in low-income settings through knowledge transfer: a case study from Zimbabwe. Hum Reprod Open 2018;2018:hoy017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörbst V. ‘ You cannot do IVF in Africa as in Europe’: the making of IVF in Mali and Uganda. Reprod Biomed Soc Online 2016;2:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horbst V, Gerrits T. Transnational connections of health professionals: medicoscapes and assisted reproduction in Ghana and Uganda. Ethn Health 2016;21:357–374. [DOI] [PubMed] [Google Scholar]
- Huyser C, Boyd L. Assisted reproduction laboratory cost-drivers in South Africa: value, virtue and validity. Obstet Gynaecol Forum 2012;22:15–21. [Google Scholar]
- Iliyasu Z, Galadanci HS, Abubakar IS, Bashir FM, Salihu HM, Aliyu MH. Perception of infertility and acceptability of assisted reproduction technology in northern Nigeria. Niger J Med 2013;22:341–347. [PubMed] [Google Scholar]
- Inhorn MC, Patrizio P. Infertility around the globe: new thinking on gender, reproductive technologies and global movements in the 21st century. Hum Reprod Update 2015;21:411–426. [DOI] [PubMed] [Google Scholar]
- Khalid SN, Qureshi IZ. Perceptions of infertile couples regarding infertility and intrauterine insemination (IUI) in a rural population and services at government hospitals in Punjab, Pakistan. Hum Reprod 2012;27: ii7-8. [Google Scholar]
- Khamoshina MB, Arkhipova MP, Rudneva OD, Vostrikova TV, Radzinskaya EV. The level of awareness of ART and the attitude to it of medical students and workers without work experience in IVF clinics. Reprod Biomed Online 2010;20:S89. [Google Scholar]
- Khan M, Zafar S, Syed S. Successful intravaginal culture of human embryos for the first time in Pakistan—an experience at the Sindh Institute of Reproductive Medicine, Karachi. J Pak Med Assoc 2013;63:630–632. [PubMed] [Google Scholar]
- Kulkarni G, Mohanty NC, Mohanty IR, Jadhav P, Boricha BG. Survey of reasons for discontinuation from in vitro fertilization treatment among couples attending infertility clinic. J Hum Reprod Sci 2014;7:249–254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kushnir VA, Barad DH, Albertini DF, Darmon SK, Gleicher N. Systematic review of worldwide trends in assisted reproductive technology 2004-2013. Reprod Biol Endocrinol 2017;15:6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwek LK, SaffariSE, TanHH, ChanJK, Nada S. Comparison between single and double cleavage-stage embryo transfers, single and double blastocyst transfers in a South East Asian In Vitro Fertilisation Centre. Ann Acad Med Singapore 2018;47:451. [PubMed] [Google Scholar]
- Li HWR, Tank J, Haththotuwa R, Asia and Oceania Federation of Obstetrics and Gynaecology. Updated status of assisted reproductive technology activities in the Asia-Oceania region. J Obstet Gynaecol Res 2018;44:1667–1672. [DOI] [PubMed] [Google Scholar]
- Lucena EE, Moran AA, Saa AA, Pulido CC, Lombana OO, Bonilla EE. Invo/vagina complex replaces conventional incubators. Fertil Steril 2013;100:S527. [Google Scholar]
- Mahajan N. Should mild stimulation be the order of the day? J Hum Reprod Sci 2013;6:220–226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makuch MY, Bahamondes L. Barriers to access to infertility care and assisted reproductive technology within the public health sector in Brazil. Facts Views Vis Obgyn 2012;4:221–226. [PMC free article] [PubMed] [Google Scholar]
- Malhotra J, Malhotra N, Pai HD, Goswami D. Retrospective six year analysis of ART directory of India. Hum Reprod 2015;30:i442–i443. [Google Scholar]
- Mascarenhas MN, Flaxman SR, Boerma T, Vanderpoel S, Stevens GA. National, regional, and global trends in infertility prevalence since 1990: a systematic analysis of 277 health surveys. PLoS Med 2012;9:e1001356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menuba IE, Ugwu EO, Obi SN, Lawani LO, Onwuka CI. Clinical management and therapeutic outcome of infertile couples in southeast Nigeria. Ther Clin Risk Manag 2014;10:763–768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mishra V. IVF scenario in India. Int J Fertil Steril 2013;7:20–21. [Google Scholar]
- Mitic D, Kopitovic V, Popovic J, Milatovic S, Basic M, Milojevic M. [ Results of in vitro fertilization cycles at the Clinic for Gynecology and Obstetrics, Clinical Center of Nis]. Med Pregl 2012;65:315–318. [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, Group P. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009;339:b2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Sharma S, Chakravarty BN. Letrozole in a low-cost in vitro fertilization protocol in intracytoplasmic sperm injection cycles for male factor infertility: a randomized controlled trial. J Hum Reprod Sci 2012;5:170–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murage A, Muteshi MC, Githae F. Assisted reproduction services provision in a developing country: time to act? Fertil Steril 2011;96:966–968. [DOI] [PubMed] [Google Scholar]
- Nagulapally S, Mittal S, Malhotra N. A randomized controlled study of minimal stimulation IVF with two different protocols in normal responders. Hum Reprod 2012;27: ii7-8. [Google Scholar]
- Navarro-Carbonell DE, Lucena-Quevedo E, Saa-Madriñán ÁM. Comparación de la calidad embrionaria entre fertilización in vitro (FIV) y cultivo intravaginal de ovocitos (INVO) en el Centro Colombiano de Fertilidad y Esterilidad—CECOLFES, Bogotá, Colombia. Rev Colomb Obstet Ginecol 2012;63:227–233. [Google Scholar]
- Ndegwa SW. Affordable ART in Kenya: the only hope for involuntary childlessness. Facts Views Vis Obgyn 2016;8:128–130. [PMC free article] [PubMed] [Google Scholar]
- Nigam M, Nigam R, Chaturvedi R, Jain A. Ethical and legal aspects of artificial reproductive techniques including surrogacy. Anil Aggrawal's Internet J Forensic Med Toxicol 2011;12:1–21. [Google Scholar]
- Okafor NI, Joe-IkechebeluNN, Ikechebelu JI. Perceptions of infertility andin vitro fertilization treatment among married couples in Anambra State, Nigeria. Afr J Reprod Health 2017;21:55. [DOI] [PubMed] [Google Scholar]
- Okonta PI, Ajayi R, Bamgbopa K, Ogbeche R, Okeke CC, Onwuzurigbo K. Ethical issues in the practice of assisted reproductive technologies in Nigeria: empirical data from fertility practitioners. Afr J Reprod Health 2018;22:51–58. [DOI] [PubMed] [Google Scholar]
- Olofsson JI, Banker MR, Sjoblom LP. Quality management systems for your in vitro fertilization clinic's laboratory: why bother? J Hum Reprod Sci 2013;6:3–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olukoya OY, Okeke CC, Kemi AI, Ogbeche RO, Adewusi AJ, Ashiru OA. Multiple gestations/pregnancies from IVF process in a fertility center in Nigeria, 2009-2011: implementing policy towards fewer (double and single) embryo transfer. Nig Q J Hosp Med 2012;22:80–84. [PubMed] [Google Scholar]
- Ombelet W. Reproductive healthcare systems should include accessible infertility diagnosis and treatment: an important challenge for resource-poor countries. Int J Gynaecol Obstet 2009;106:168–171. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Campo R. Affordable IVF for developing countries. Reprod Biomed Online 2007;15:257–265. [DOI] [PubMed] [Google Scholar]
- Ombelet W, Cooke I, Dyer S, Serour G, Devroey P. Infertility and the provision of infertility medical services in developing countries. Hum Reprod Update 2008;14:605–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, Goossens J. The Walking Egg Project: how to start a TWE centre? Facts Views Vis Obgyn 2016;8:119–124. [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, Onofre J. IVF in Africa: what is it all about? Facts Views Vis Obgyn 2019;11:65–76. [PMC free article] [PubMed] [Google Scholar]
- Ombelet W, Van Blerkom J, Klerkx E, Janssen M, Dhont N, Mestdagh G, Nargund G, Campo R. The (t)WE lab simplified IVF procedure: first births after freezing/thawing. Facts Views Vis Obgyn 2014;6:45–49. [PMC free article] [PubMed] [Google Scholar]
- Omokanye L, Olatinwo A, Durowade K, Raji H, Raji S, Biliaminu S, Ganiyu S. Determinants of utilization of assisted reproductive technology services in Ilorin, Nigeria. J Med Soc 2017;31:109. [Google Scholar]
- Onah HE, Okohue J. Case against single embryo transfer in African IVF clinics. Middle East Fertil Soc J 2010;15:296–297. [Google Scholar]
- Orhue AA, Aziken ME, Osemwenkha AP, Ibadin KO, Odoma G. In vitro fertilization at a public hospital in Nigeria. Int J Gynaecol Obstet 2012;118:56–60. [DOI] [PubMed] [Google Scholar]
- Özörnek H, Özay A, Öztel Z, Atasever E, Turan E, Ergin E. Minimal stimulation is as effective as classical stimulation in a single embryo transfer program in Turkey. Fertil Steril 2013;100:S277. [Google Scholar]
- Palatty PL, Kamble PS, Shirke M, Kamble S, Revankar M, Revankar VM. A clinical round up of the female infertility therapy amongst Indians. J Clin Diagn Res 2012;6:1343–1349. [Google Scholar]
- Pottinger AM, Everett-Keane D, McKenzie C. Evolution of in vitro fertilization at the University of the West Indies, Jamaica. West Ind Med J 2012;61:460–462. [DOI] [PubMed] [Google Scholar]
- Qiao J, Feng HL. Assisted reproductive technology in China: compliance and non-compliance. Transl Pediatr 2014;3:91–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi Ada S, Amaral E, Makuch MY. Access of people living with HIV to infertility services: perspective of Brazilian healthcare professionals. AIDS Care 2011;23:1329–1335. [DOI] [PubMed] [Google Scholar]
- Sadeghi MR. Access to infertility services in middle east. J Reprod Infertil 2015;16:179. [PMC free article] [PubMed] [Google Scholar]
- Sallam H, Ezzeldin F, Sallam N, Agameya A, Sallam S, Farrag A. HMG-only protocol for IVF, a practical and more affordable option for developing countries. Hum Reprod 2013;28:i138. [Google Scholar]
- SARA report. South African Registry for assisted reproductive techniques 2014 report (6th ANNUAL SARA report). 2014. http://anara-africa.com/wp-content/uploads/2017/09/SARA-2014-22.05.2017.pdf (5 October 2020, date last accessed).
- Shah Nawaz S, Azhar A. Amazing low cost IVF/ICSI induction protocol in developing countries. Hum Reprod 2014;29 Suppl 1:i1–i389.26207010 [Google Scholar]
- Sharma S, Mittal S, Aggarwal P. Management of infertility in low resource countries. BJOG 2009;116 Suppl:77–83. [DOI] [PubMed] [Google Scholar]
- Silva SGD, Bertoldi AD, Silveira MFD, Domingues MR, Evenson KR, Santos ISD. Assisted reproductive technology: prevalence and associated factors in Southern Brazil. Rev Saude Publica 2019;53:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stellar C, Garcia-Moreno C, Temmerman M, van der Poel S. A systematic review and narrative report of the relationship between infertility, subfertility, and intimate partner violence. Int J Gynaecol Obstet 2016;133:3–8. [DOI] [PubMed] [Google Scholar]
- Sterne JA, Hernan MA, Reeves BC, Savovic J, Berkman ND, Viswanathan M, Henry D, Altman DG, Ansari MT, Boutron I et al. ROBINS-I: a tool for assessing risk of bias in non-randomised studies of interventions. BMJ 2016;355:i4919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens GA, Alkema L, Black RE, Boerma JT, Collins GS, Ezzati M, Grove JT, Hogan DR, Hogan MC, Horton R et al. Guidelines for accurate and transparent health estimates reporting: the GATHER statement. Lancet 2016;388:e19–e23. [DOI] [PubMed] [Google Scholar]
- Tremayne S, Akhondi MM. Conceiving IVF in Iran. Reprod Biomed Soc Online 2016;2:62–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Urman B, Yakin K. New Turkish legislation on assisted reproductive techniques and centres: a step in the right direction? Reprod Biomed Online 2010;21:729–731. [DOI] [PubMed] [Google Scholar]
- Van Blerkom J, Ombelet W, Klerkx E, Janssen M, Dhont N, Nargund G, Campo R. First births with a simplified culture system for clinical IVF and embryo transfer. Reprod Biomed Online 2014;28:310–320. [DOI] [PubMed] [Google Scholar]
- van der Poel SZ. Historical walk: the HRP Special Programme and infertility. Gynecol Obstet Invest 2012;74:218–227. [DOI] [PubMed] [Google Scholar]
- Vayena E, Peterson HB, Adamson D, Nygren K-G. Assisted reproductive technologies in developing countries: are we caring yet? Fertil Steril 2009;92:413–416. [DOI] [PubMed] [Google Scholar]
- Wahlberg A. The birth and routinization of IVF in China. Reprod Biomed Soc Online 2016;2:97–107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker A. From ‘Mung Ming’ to ‘Baby Gammy’: a local history of assisted reproduction in Thailand. Reprod Biomed Soc Online 2016;2:71–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Widge A, Cleland J. Negotiating boundaries: accessing donor gametes in India. Facts Views Vis Obgyn 2011;3:53–60. [PMC free article] [PubMed] [Google Scholar]
- World Bank Country and Lending Groups. https://datahelpdesk.worldbank.org/knowledgebase/articles/906519-world-bank-country-and-lending-groups (30 January 2020, date last accessed).
- World Health Organization. Preamble to the Constitution of the World Health Organization as adopted by the International Health Conference, New York, 19-22 June, 1946; signed on 22 July 1946 by the representatives of 61 States (Official Records of the World Health Organization, no. 2, p. 100) and entered into force on 7 April 1948. http://www who int/governance/eb/who_constitution_en pdf. 1948. (5 October 2020, date last accessed).
- Ye H, Huang GN, Cao YX, Zhong Y, Huang YH, Zhu GJ, Zhou LM, Chen ZJ, Shi JZ, Zeng Y et al. [Effect of domestic highly purified urinary follicle stimulating hormone on outcomes of in vitro fertilization-embryo transfer in controlled ovarian stimulation]. Zhonghua Fu Chan Ke Za Zhi 2013;48:838–842. [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, Van der Poel S. The international committee for monitoring assisted reproductive technology (ICMART) and the world health organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod 2009;24:2683–2687. Simultaneously Published in Fertil Steril 2009;2692: 1520–2684. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, Dyer S, Racowsky C, de Mouzon J, Sokol R, Rienzi L, Sunde A, Schmidt L, Cooke ID et al. The international glossary on infertility and fertility care, 2017. Hum Reprod 2017;32:1786–1801. Simultaneous Publication in Fertil Steril 2017;1108: 1393–1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Dickens BM, Dughman-Manzur S. Human rights to in vitro fertilization. Int J Gynaecol Obstet 2013;123:86–89. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, de Souza MdCB. Assisted reproductive technologies (ART) in Latin America: the Latin American Registry, 2011. J Brasil Reprod Assist 2013;17:216–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, de Souza MdCB. Assisted reproductive technologies in Latin America: the Latin American Registry, 2012. Reprod Biomed Online 2015;30:43–51. [DOI] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, Souza MdCB. Assisted reproductive technologies (ART) in Latin America: the Latin American Registry, 2012. JBRA Assist Reprod 2014;18:127–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Schwarze JE, Crosby JA, Musri C, Urbina MT; Latin American Network of Assisted Reproduction. Assisted reproductive techniques in Latin America: the Latin American Registry, 2013. Reprod Biomed Online 2016;32:614–625. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.