Abstract
BACKGROUND
Despite increasing regulation, exposure to persistent organic pollutants (POPs) remains a serious public health concern due to their accumulation in the environment and ability to biomagnify up the food chain. POPs are associated with endocrine-disrupting effects including adverse reproductive outcomes that could affect fecundability, i.e. the capacity to conceive a pregnancy, quantified as time to pregnancy (TTP).
OBJECTIVE AND RATIONALE
Results of epidemiologic studies that examine the impact of various chemical classes of POPs on TTP have not been synthesised. We undertook a systematic review to summarise the strength of evidence for associations of four common groups of POPs with couple fecundability and to identify gaps and limitations in the literature in order to inform policy decisions and future research.
SEARCH METHODS
We performed an electronic search of literature published between 1 January 2007 and 6 August 2019 in MEDLINE, EMBASE.com, Global Health, DART/TOXLINE and POPLINE. We included empirical research papers that examined human exposure to organochlorine (OC) pesticides, brominated flame retardants, polychlorinated organic compounds and/or per- and polyfluoroalkyl substances (PFAS) and considered TTP or fecundability as an outcome. Standardised forms for screening, data extraction and study quality were developed using DistillerSR software, and all reviews were completed in duplicate. We used the Newcastle-Ottawa Scale to assess risk of bias and devised additional quality metrics based on specific methodological features of fecundability studies.
OUTCOMES
The search returned 4573 articles, and 28 papers from 19 different studies met inclusion criteria. Among them, four studies measured TTP prospectively, three had data on participants’ prenatal exposure, three examined associations in both male and female partners and one focused exclusively on males. Analyses varied widely in terms of exposure characterisation, precluding a meta-analytic approach. Evidence was strongest for adverse associations of female exposure to polychlorinated biphenyls with TTP, with some additional support for associations of female exposure to polybrominated diphenyl ethers and PFAS with longer TTP. Our review provided little or no support for associations between female exposure to OC pesticides or male exposure to any of the POP groups and TTP.
WIDER IMPLICATIONS
Evidence suggests that female exposure to at least some POPs may reduce fecundability. Although many of these chemicals are no longer in production, they are still detectable in human biosamples because of their persistence in the environment. Replacement chemicals that are being introduced as older ones are restricted may have similar reproductive consequences. Future studies should examine these newer POPs, assess interactions between POPs and other chemical and non-chemical exposures, investigate how POPs are distributed in and metabolised by the human body and focus on populations that may be disproportionately exposed.
Keywords: time to pregnancy, couple fecundability, systematic review, environmental effects, endocrine-disrupting chemicals, persistent organic pollutants, organochlorine pesticides, brominated flame retardants, polychlorinated biphenyls, perfluoroalkyl substances
Introduction
Persistent organic pollutants (POPs) are stable compounds that were first linked to impaired reproductive function by Rachel Carson, who described breeding failure among birds exposed to dichlorodiphenyltrichloroethane (DDT) in her landmark book, Silent Spring (Carson, 1962). Since then, studies have examined associations of various POPs, including organochlorine (OC) pesticides, brominated flame retardants (BFRs), polychlorinated biphenyls (PCBs) and per- and polyfluoroalkyl substances (PFAS), with a range of reproductive outcomes in animals and humans (Gore et al., 2015; Kahn et al., 2020). Ecological data showing a decline in sperm count in industrialised countries coincident with the spread of POP use in the decades following World War II (Carlsen et al., 1992; Swan et al., 2000; Levine et al., 2017) led to the hypothesis that these chemicals may affect male reproductive potential by impairing foetal testicular development (Skakkebaek et al., 2001). Subsequent epidemiologic studies have documented associations of PCBs with reduced semen quality (Meeker and Hauser, 2010), although evidence for associations with other POPs is sparse or conflicting (Vested et al., 2014). It is theorised that environmental chemicals may also alter female reproductive processes, impeding the ability to produce viable oocytes and to establish and maintain a pregnancy (Buck Louis et al., 2011).
Couple fecundity, or the biological ability to reproduce, is a product of both male and female factors. It is most commonly measured as fecundability, or the per-cycle probability of conception when a couple has unprotected intercourse (Baird et al., 1986; Joffe, 1997; Smarr et al., 2017), and is operationalised as time to pregnancy (TTP). Identifying predictors of longer TTP has both clinical and public health relevance. Clinical infertility is defined as TTP >12 months (Zegers-Hochschild et al., 2009) and couples with suspected infertility often seek medical intervention, despite associated personal (Galhardo et al., 2011; Luk and Loke, 2015) and financial costs (Wu et al., 2013) and potential health risks to women and children (Qin et al., 2016). According to 2011–2015 data, 23.6% of currently married, childless women age 15–44 years in the USA have impaired fecundity, i.e. have not successfully carried a pregnancy to term (National Center for Health Statistics, 2020), and the use of assisted reproductive technologies and the number of fertility clinics providing these services continues to increase (Sunderam et al., 2019). While factors such as higher average age at first pregnancy attempt partially explain this increase (Ely and Hamilton, 2018), exposure to endocrine-disrupting chemicals such as POPs may also contribute to the trend.
Because of their stability and predominantly lipophilic properties, POPs persist in soil, air and water as well as in animal tissue, where they can accumulate and biomagnify. Individual POPs may have half-lives from a few weeks to a dozen years or more depending on environmental or physiological conditions. Since the 1970s, a number of POPs have been banned from production, especially in developed countries. OC pesticides were largely restricted in the USA in the 1970s, although they continue to be used in some countries for malaria control (Agency for Toxic Substances and Disease Registry, 2019; Kleanthi et al., 2008). Among the BFRs, polybrominated biphenyls (PBBs) were banned by the USA in 1976 and production of polybrominated diphenyl ethers (PBDEs) was ceased in 2013 (U.S. Environmental Protection Agency, 2017). PCBs were banned in the USA in 1979. PFAS, used to create coatings that resist heat, oil, stains, grease and water, are still widely used, although some are being regulated and voluntary phase-outs have begun. In 2008, the Stockholm Convention on Persistent Organic Pollutants identified a ‘dirty dozen’ chemicals that pose severe enough risk to human health to warrant worldwide restriction (United Nations Environment Programme, 2008). Despite the growing environmental and health concerns about POPs, many of these chemicals persist in the food supply, in consumer products and in the built environment. Even those that have been banned are still detectable in most people, albeit at lower concentrations (Wattigney et al., 2015).
Although the biological mechanisms are only partially understood, OC pesticides, BFRs, PCBs and PFAS all exhibit properties that allow them to disrupt endocrine and reproductive processes. The OC pesticide DDT has been shown to bind and activate both alpha and beta oestrogen receptors, and its metabolite, dichlorodiphenyldichloroethylene (DDE), has been shown to be a potent androgen receptor agonist in animal and in-vitro studies (Kelce et al., 1995; Klotz et al., 1996; Kuiper et al., 1998). DDT, DDE and dichlorodiphenyldichloroethane, another DDT metabolite, have been associated with testicular (McGlynn et al., 2008), endometrial (Hardell et al., 2004a), breast (Wolff et al., 1993; Safe and Zacharewski, 1997) and pancreatic (Porta et al., 2008) cancers, as well as type 2 diabetes (Codru et al., 2007).
With respect to BFRs, PBDEs have been found to disrupt thyroid hormone homeostasis, perturb expression and oestrogen sensitivity of sex hormone-regulated genes, impair spermatogenesis and alter sexual behaviours in rodents (Kuriyama et al., 2004; Lichtensteiger et al., 2004; Talsness et al., 2007). In humans, certain PBDEs have been associated with decreased semen quality (Abdelouahab et al., 2011; Mumford et al., 2015), altered reproductive hormones in both women (Gao et al., 2016) and men (Meeker et al., 2009; Makey et al., 2016) and poor IVF outcomes (Johnson et al., 2012). Certain PBBs have been linked to prolonged menstrual cycles, extended periods of implantation bleeding, altered testosterone and progesterone metabolism and poor IVF outcomes in animal models (Allen and Lambrecht, 1978; Newton et al., 1982; Kholkute et al., 1994), as well as perturbed menstrual cycle hormones and impaired ovarian function in humans (Davis et al., 2005; Howards et al., 2019).
In-vitro research (Kovacevic et al., 1995; Schrader and Cooke, 2003), animal models (Bergeron et al., 1994; Hany et al., 1999; Kaya et al., 2002) and epidemiological studies (Gore et al., 2015) have all found evidence linking PCBs to endocrine disruption, a plausible conclusion, as certain PCB congeners bear stereochemical resemblance to steroid hormones including oestrogen and testosterone (Connor et al., 1997). In addition to human studies showing cross-sectional associations of higher serum PCB concentrations with lower sperm motility and serum testosterone (Goncharov et al., 2009; Meeker and Hauser, 2010), some evidence suggests that prenatal PCB exposure may result in testicular cancer in males (Hardell et al., 2004b). In women, PCBs have been associated with breast cancer (Recio-Vega et al., 2008; Morgan et al., 2017; Wielsoe et al., 2017) and endometriosis (Cano-Sancho et al., 2019; Wen et al., 2019).
Based on laboratory findings, PFAS could interfere with reproductive function via multiple pathways. In male rodents, PFAS delay Leydig cell maturation, damage seminiferous tubules, increase spermatagonal and Leydig cell apoptosis and decrease testosterone levels. In-vitro oocyte studies indicate that PFAS may diminish ovarian reserve and reduce endogenous hormone synthesis by activating peroxisome proliferator-activated receptors, disrupting gap junction intercellular communication between oocyte and granulosa cells, inducing thyroid hormone deficiency, antagonising ovarian enzyme activities involved in ovarian steroidogenesis or inhibiting kisspeptin signalling in the hypothalamus (Ding et al., 2020; Gonsioroski et al., 2020). In humans, perfluorooctane sulphonic acid (PFOS), a surfactant PFAS, was found to be associated with higher levels of sex hormone-binding globulin (SHBG) and luteinising hormone but not testosterone in highly exposed men (Petersen et al., 2018). PFAS were associated with increased odds of premature ovarian insufficiency in women (Zhang et al., 2018) and with lower SHBG, follicle-stimulating hormone and testosterone in adolescents (Tsai et al., 2015). In a study of contaminated drinking water supplies, PFOS was associated with lower oestradiol and testosterone in boys and with lower testosterone in girls, and perfluorooctanoic acid (PFOA), another surfactant PFAS, was associated with lower testosterone among boys (Lopez-Espinosa et al., 2016).
Given the range of chemicals included in this review, a list of chemical abbreviations is provided in Table I.
Table I.
Summary of chemicals and abbreviations.
| Organochlorine (OC) pesticides |
| Aldrin |
| DDE: dichlorodiphenyldichloroethylene |
| DDT: dichlorociphenyltrichloroethane |
| Dieldrin |
| Endosulfan |
| HCB: hexachlorobenzene |
| HCH: hexachlorohexane |
| Heptachlor |
| Heptachlor epoxide |
| Mirex |
| Oxychlordane |
| Trans-nonachlor |
| Halogenated flame retardants |
| HBCDD: hexabromocyclododecane |
| PBBs: polybrominated biphenyls |
| PBDEs/BDEs: polybrominated diphenyl ethers |
| Polychlorinated organic compounds |
| PCBs: polychlorinated biphenyls |
| PCDFs: polychlorinated dibenzofurans |
| TCDD: 2,3,7,8-tetrachlorodibenzo-p-dioxin (dioxin) |
| PFAS: per- and polyfluoroalkyl substances |
| EtFOSAA: ethyl perfluorooctane sulphonamido acetic acid |
| MeFOSAA: methyl perfluorooctane sulphonamido acetic acid |
| PFDA: perfluorodecanoic acid |
| PFDeA: perfluordecanoic acid |
| PFDoDA: perfluorododecanoic acid |
| PFHpS: perfluoroheptane sulphonic acid |
| PFHxS: perfluorohexane sulphonic acid |
| PFNA: perfluorononanoic acid |
| PFOA: perfluorooctanoic acid |
| PFOS: perfluorooctane sulphonic acid |
| PFOSA: perfluorooctane sulphonamide |
| PFTrDA: perfluorotridecanoic acid |
| PFUnA/PFUnDA: perfluoroundecanoic acid |
Objectives
The aim of this systematic review was to summarise the strength of evidence for associations of POPs with couple fecundability (operationalised as TTP) and to identify gaps and limitations in the literature, with the goal of informing policy decisions and future research programs. Our systematic review focuses on chemicals targeted for elimination or restriction by the Stockholm Convention on Persistent Organic Pollutants (United Nations Environment Programme, 2008) (not currently ratified by the USA). Although the use and production of POPs are increasingly restricted, even chemicals that have been banned continue to impact human health because of their persistence in the environment and food chain. Understanding the association between POPs and TTP is important from a public health perspective not only because TTP is the main diagnostic indicator of couple infertility and subsequent use of assisted reproductive technologies, but also because of accumulating evidence that subfertility itself is associated with adverse health outcomes both for those attempting to conceive (Senapati, 2018; Choy and Eisenberg, 2020) and for their resulting children (Hwang et al., 2018; Robinson et al., 2020). Where the strength of evidence is limited, we highlight improvements in study design that will help guide future research and provide more conclusive results.
Methods
This review followed the Preferred Reporting Items for Systematic review and Meta-Analysis (PRISMA) statement (Moher et al., 2009). The protocol is registered (CRD42019146534) on PROSPERO (www.crd.york.ac.uk/prospero) and we adhered to Preferred Reporting Items for Systematic review and Meta-Analysis Protocols (PRISMA-P) (Moher et al., 2015; Shamseer et al., 2015).
Search strategy
A professional health sciences librarian (MK-F) developed and executed two bibliographic searches that were combined for the current review. The first search identified papers published between 1 January 2007 and 25 August 2017 in MEDLINE on the PubMed platform, EMBASE.com, Global Health (OvidSP), DART (Developmental and Reproductive Toxicology)/TOXLINE (National Library of Medicine, US) and POPLINE (popline.org) (Hipwell et al., 2019). In the second search, MK-F updated the strategy after checking for new controlled vocabulary terms and adding one deemed relevant, then searched the same five databases for articles published from 31 July 2017 until 6 August 2019. Dates covered overlapped slightly with the original search in order not to miss any added but not previously indexed articles. Subject thesaurus vocabulary (e.g. MeSH, EMTREE), text words and author keywords all contributed to both search builds with the exception of one MeSH term (‘Heavy Metal Poisoning’), which was introduced in 2018 and therefore only included in the search update. Retrievals were limited to human studies in English. Animal, plant and soil fertility terms were removed before downloading to EndNote (Thomson Reuters). Duplicates among the new citations and from the original search were deleted. The full search strategy is reproduced in the Supplementary Table SI.
Screening and eligibility
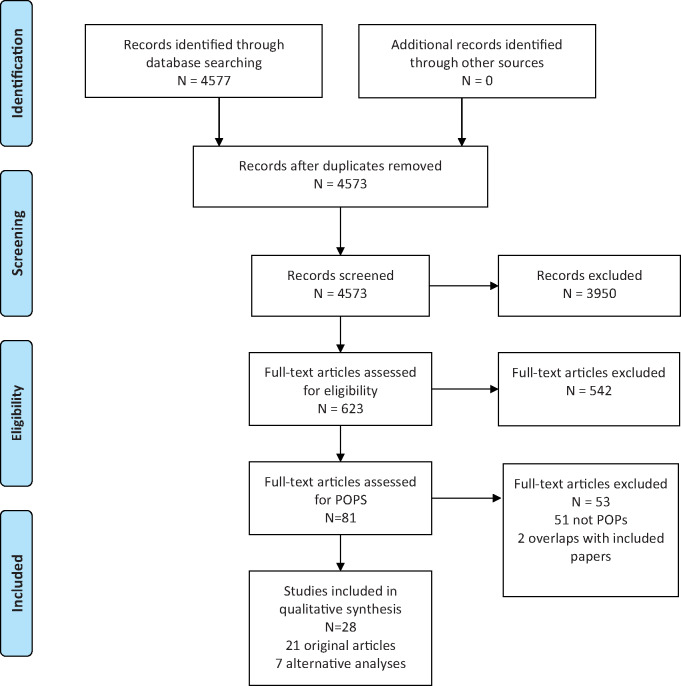
Article screening was conducted with DistillerSR software (Evidence Partners, ON, Canada) using standardised forms for title and abstract screening and for full-text review. Each level of review (see Fig. 1) was completed in duplicate by authors L.G.K., K.G.H., E.L.S., Y.Z., P.F.-L., C.A.P. and A.E.H., and any conflicts were resolved through discussion.
Figure 1. PRISMA flow chart.
At the title- and abstract-screening level, we included all human studies that related to any chemical exposure and TTP or fecundability and screened out editorials, opinion pieces, introductions to special sections or articles that described only lifestyle (e.g. caffeine, alcohol, illicit drugs, medication, stress) or clinical factors (e.g. semen parameters, IVF success, obesity). We obtained full-text reports for all titles that did not meet exclusion criteria or where there was any uncertainty about content. At the second (full text) level of screening, we included only original empirical research papers that considered TTP or fecundability as an outcome and examined exposure to persistent POPs, specifically: OC pesticides (DDT, DDE, aldrin, chlordane, chlordecone, dieldrin, endosulfan, endrin, heptaclor, hexachlorobenzene (HCB), HCH, lindane, mirex, toxaphene, PCP); BFRs (PBDEs, PBBs, hexabromocyclododecane (HBCDD)); PCBs (dioxins, furans); PFAS (PFOA, PFOS, perfluorononanoic acid (PFNA), perfluorohexane sulphonic acid (PFHxS)); other chemicals on the Stockholm Convention POPs list; and POPs in general. No a priori inclusion/exclusion criteria were applied according to how individuals were exposed (e.g. day-to-day activities or in the workplace) or according to study design (i.e. retrospective or prospective cohort, cross-sectional or case-control).
Data extraction
We created standardised forms for data extraction in DistillerSR, which were also completed in duplicate, and coding discrepancies were resolved through discussion. Where available, we reviewed prior articles that described study methods in greater detail. Review authors were not blind to article titles or authors. Once data had been extracted, characteristics of the papers reviewed were placed in Table III. Results of analyses for female and male chemical exposure were summarised in Tables IV and V, respectively. In some cases, data from the same study were analysed differently in separate publications. We elected to count only the primary paper as a distinct contribution to the literature, listing the results of the alternative or subgroup analyses underneath the main publication in Tables III and IV.
Table III.
Study characteristics.
| First author, year | Study name/acronym | Country | Period of exposure | Sample/study design | No. women | No. men | Measurement mode of chemical exposure |
AHRQ rating/additional quality points | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Serum | Plasma | Non-biological | ||||||||
| Bach, 2015a | ABC | Denmark | 2008–2013 | General/ retrospective | 1372 | 0 | PFAS: PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDA, PFUnA | Good/4 | ||
| Bach, 2015b | DNBC | Denmark | 1996–2002 | General/ retrospective | 1601 | 0 | PFAS: PFOA, PFOS | Good/4 | ||
| Fei, 2009 (smaller subset) | 1240 | 0 | PFAS: PFOA, PFOS | |||||||
| Bach, 2018 | Lifestyle During Pregnancy Study nested within the DNBC | Denmark | 1996–2002 | General/ retrospective | 1251 | 0 | PFAS: PFOS, PFOA, PFHxS, PFHpS, PFNA, PFDA | Good/4 | ||
| Buck Louis, 2009 | NYSACS | USA | 1996–1997 | Specializeda/ prospective | 83 | 0 | PCBs: total (76 congeners), oestrogenic, antioestrogenic | Good/4 | ||
| Buck Louis, 2013 | LIFE | USA | 2005–2009 | General/ prospective | 501 | 501 | PBB 153; OC pesticides: HCB, β HCH, γ-HCH, oxychlordane, trans-nonachlor, p,p´-DDT, o,p´-DDT, p,p´-DDE, mirex; PBDEs: BDE 17, 28, 47, 66, 85, 99, 100, 153, 154, 183; PCBs: PCB 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 114, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 201, 206, 209; PFAS: EtFOSAA, MeFOSAA, PFDeA, PFNA, PFOSA, PFOS, PFOA | Good/3.5 | ||
| Lum, 2017 (reanalysis adjusting for cycle length) | 501 | 0 | PFAS: PFDeA, PFNA, PFOA, PFOS, PFOSA, EtFOSAA, MeFOSAA | |||||||
| Hwang, 2019 (latent class reanalysis of joint male and female exposure) | 401 | 401 | PCB 28, 44, 49, 52, 66, 74, 87, 99, 101, 105, 110, 114, 118, 128, 138, 146, 149, 151, 153, 156, 157, 167, 170, 172, 177, 178, 180, 183, 187, 189, 194, 195, 196, 201, 206, 209 | |||||||
| Zhang, 2019 (weighted kernel machine regression reanalysis of joint male and female exposure) | 401 | 401 | PCB 28, 66, 74, 99, 105, 118, 138, 146, 153, 156, 170, 180, 187, 194, 196, 201, 206, 209 | |||||||
| Campagna, 2015 | Italy | 1946–1950 | Specializedb/ retrospective | 0 | 1223 | DDT | Poor/0 | |||
| Chevrier, 2013 | PELAGIE | France | 2002–2006 | General/ retrospective | 332 | 0 | PCBs: PCB 74, 99, 101, 118, 138, 153, 170, 180, 183, 187, 194, 203, ΣPCBs; Organochlorine pesticides: α-HCH, β-HCH, y-HCH, HCB, heptachlor, heptachlor epoxide, aldrin, dieldrin, α-endosulfan, β-endosulfan, p,p’-DDE, p,p’-DDT, o,p’-DDE, o,p’-DDT; PBDEs: BDE 28, 47, 99, 100, 153, 154, 183, 209; PBB 153; HBCDD | Good/4 | ||
| Cohn, 2011 | CHDS | USA | 1960–1963 | General/ retrospectivec | 289 | 0 | PCBs: PCB 66, 74, 99, 101, 105, 118, 138, 153, 156, 167, 170, 180, 183, 187, 201, 203 | Fair/2 | ||
| Gennings, 2013 (reanalysis of PCB mixtures using weighted quantile sum regression) | 289 | 0 | PCBs: PCB 56, 66, 74, 99, 101, 105, 118, 138, 146, 153, 156, 167, 170, 180, 183, 187, 201, 203 | |||||||
| Crawford, 2017 | Time to Conceive | USA | 2008–2009 | General/ prospective | 99 | 0 | PFAS: PFOA, PFOS, PFNA, PFHxS | Fair/3 | ||
| Eskenazi, 2010 | SWHS | Italy | 1976 | Specializedd/ retrospective | 278 | 0 | TCDD | Good/3 | ||
| Gao, 2016 | LWBC | China | 2010–2012 | General/ retrospective | 207 | 0 | PBDEs: BDE 28, 47, 85, 99, 100, 153, 154, 183, ΣPBDE (28, 47, 99, 100, 153) | Good/3 | ||
| Han, 2016 | Fisheater Family Health Study | USA | 1973–1991 | Specializeda/ retrospectivec | 151 | 0 | PCBs, p,p'-DDE | Poor/1 | ||
| Harley, 2008 | CHAMACOS | USA | 1999–2000 | Specializedb/ retrospective | 402 | 0 | p,p'-DDT, o,p'-DDT, p,p'-DDE | Good/4 | ||
| Harley, 2010 | CHAMACOS | USA | 1999–2000 | Specializedb/ retrospective | 223 | 0 | PBDEs: BDE 47, 99, 100, 153 | Good/4 | ||
| Jorgensen, 2014 | INUENDO | Greenland, Poland, Ukraine | 2002–2004 | General/ retrospective | 938 | 401 | PFAS: PFOS, PFOA, PFHxS, PFNA | Good/4 | ||
| Small, 2011 | USA | 1973–1974 | Specializedd/ retrospectivec | 194 | 0 | PBB 153 | Poor/1 | |||
| Snijder, 2011 | Generation R | Netherlands | 2002–2006 | General/ retrospective | 2774 | 2728 | Polychlorinated organic compounds, pesticides, flame retardants, any endocrine disruptors | Fair/2 | ||
| Velez, 2015 | MIREC | Canada | 2008–2011 | General/ retrospective | 1743 | 0 | PFAS: PFOS, PFOA, PFHxS | Good/3 | ||
| Vestergaard, 2012 | Danish First Pregnancy Planner Study | Denmark | 1992–1995 | General/ prospective | 222 | 0 | PFAS: PFOS, PFOA, PFHxS, PFNA, PFDA, MeFOSAA, EtFOSAA, PFOSA | Good/3 | ||
| Whitworth, 2016 | MoBa | Norway | 2003–2004 | General/ retrospective | 451 | 0 | PFAS: PFOSA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFHxS, PFHpS, PFOS | Good/4 | ||
| Whitworth, 2012 (dichotomous TTP outcome only) | 910 | 0 | PFAS: PFOS, PFOA | |||||||
| Ding, 2014 (reanalysis accounting for case-cohort sampling) | 910 | 0 | PFOA | |||||||
| Yang, 2008 | Yucheng | Taiwan | 1979 | Generald/ retrospective | 412 | 0 | PCBs, PCDFs | Fair/2 | ||
Anglers/fisheaters and spouses.
Pesticide applicators/farmworkers and spouses.
Prenatal exposure.
Accidental exposure.
ABC, Aarhus Birth Cohort; DNBC, Danish National Birth Cohort; NYSACS, New York State Angler Cohort Study; LIFE, Longitudinal Investigation of Fertility and the Environment; PELAGIE, Perturbateurs endocriniens: Étude Longitudinale sur les Anomalies de la Grossesse, l’Infertilité et l’Enfance; CHDS, Child Health and Development Studies; SWHS, Seveso Women’s Health Study; LWBC, Laizhou Wan Birth Cohort; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; MIREC, Maternal-Infant Research on Environmental Chemicals; MoBa, Norwegian Mother and Child Cohort Study
Table IV.
Fecundability odds ratios in women.
| First author, year | Study name/acronym | Unit of analysis | Organochlorine pesticides | Brominated flame retardants (PBDEs and PBBs) | Polychlorinated organic compounds (PCBs, dioxins, and furans) | PFAS | Covariates |
|---|---|---|---|---|---|---|---|
| Bach, 2015a | ABC | Quartiles (Q4 vs Q1) | Nulliparous PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDA, PFUnA: ns | Age, prepregnancy BMI, education | |||
| Continuous | Nulliparous PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDA, PFUnA: ns | ||||||
| Bach, 2015b | DNBC | Quartiles (Q4 vs Q1) | Nulliparous PFOS: FOR=0.79 [0.63, 0.99] | Age, prepregnancy BMI, SES, sample | |||
| Parous PFOA: 0.76 [0.61, 0.94] | |||||||
| Continuous (ln) | Nulliparous PFOS: FOR=0.78 [0.63, 0.97] | ||||||
| Parous PFOA: 0.72 [0.61, 0.87] | |||||||
| Fei, 2009 (smaller subset) | Quartiles (Q4 vs Q1) | Combined parity PFOA: FOR=0.60 [0.47, 0.76 and OR TTP >12 months=2.54 [1.47, 4.39]; PFOS: FOR=0.74 [0.58, 0.93 and OR TTP >12 months=1.77 [1.06, 2.95] | Maternal age, parity, prepregnancy BMI, SES, prepregnancy alcohol; paternal age, education | ||||
| Bach, 2018 | Lifestyle During Pregnancy Study nested within the DNBC | Quartiles (Q4 vs Q1) | Nulliparous PFHxS, PFHpS, PFOS, PFOA, PFNA, PFDA: ns | Age, prepregnancy BMI, SES | |||
| Parous PFOA: FOR=0.79 [0.75, 0.83]; PFOS: FOR=0.69 [0.66, 0.73]; PFHxS: FOR= 0.71 [0.53, 0.94]; PFHpS: FOR=0.79 [0.58, 1.09]; PFNA: FOR=0.97 [0.93, 1.01]; PFDA: FOR=0.85 [0.82, 0.89] | Age, prepregnancy BMI, SES, interpregnancy interval | ||||||
| Buck Louis, 2009 | NYSACS | Continuous (ln) | ΣPCBs: FOR=1.10 [1.00, 1.20]; ΣEstrogenic PCBs: FOR=0.32 [0.03, 3.89]; ΣAntioestrogenic PCBs: FOR=0.01 [<0.00, 1.99]; ΣOther PCBs: 1.44 [0.88, 2.37] | Serum lipids, frequency of intercourse, age, parity, smoking, alcohol | |||
| Buck Louis, 2013 | LIFE | Continuous (log_SD) | HCB, β-HCH, γ-HCH, oxychlordane, trans-nonachlor, p,p'-DDT, o,p'-DDT, p,p'-DDE, mirex: ns | PBB 153: ns; BDE 17, 28, 47, 66, 85, 99, 100, 153, 154, 183: ns | PCB 118: FOR=0.82 [0.68, 0.98]; PCB 167: FOR=0.79 [0.64, 0.97]; PCB 209: FOR=0.82 [0.68, 0.99] | Combined parity PFOSA: FOR=0.82 [0.71, 0.95] | Left truncation, sum of all other chemicals in class, age, prepregnancy BMI, serum cotinine, site, lipids (except in PFAS models) |
| Lum, 2017 (reanalysis adjusting for cycle length) | Tertiles (T3 vs T1) | Combined parity PFNA: OR=0.7 [0.3, 1.1] | Intercourse pattern, menstrual cycle length, age, prepregnancy BMI, smoking at enrolment, all other PFAS measured | ||||
| Chevrier, 2013 | PELAGIE | Tertiles (T3 vs T1) | β-HCH: FOR=0.61 [0.43, 0.86]; p,p'-DDE: FOR=0.60 [0.42, 0.84]; HCB: FOR=0.67 [0.48, 0.95] | BDE 209: ns | PCB 118: FOR=0.64 [0.45, 0.91]; PCB 138: FOR=0.57 [0.40, 0.81]; PCB 153: FOR=0.48 [0.33, 0.69]; PCB 170: FOR=0.64 [0.44, 0.91]; PCB 180: FOR=0.64 [0.44, 0.92]; PCB 187: 0.59 [0.40, 0.85]; ΣPCBs: FOR=0.46 [0.32, 0.66] | Age, prepregnancy BMI, smoking, oral contraceptive use, total lipids | |
| ≥LOD vs <LOD | Heptachlor epoxide: FOR=0.76 [0.58, 1.00] | PCB 183: FOR=0.69 [0.52, 0.94] | |||||
| Continuous (log10) | β-HCH: FOR=0.49 [0.29, 0.80]; p,p'-DDE: FOR=0.84 [0.71, 0.99] | PCB 118: FOR=0.42 [0.23, 0.75]; PCB 138: FOR=0.43 [0.22, 0.82]; PCB 153: FOR=0.37 [0.18, 0.74]; PCB 180: FOR=0.47 [0.23, 0.97]; PCB 187: FOR=0.63 [0.43, 0.91]; ΣPCBs: 0.39 [0.19, 0.78] | |||||
| Cohn, 2011 | CHDS | Continuous (per SD) | PCB 99: FOR=0.75 [0.62, 0.91]; PCB 105: FOR=1.21 [1.04, 1.41]; PCB 138: FOR=1.99 [1.45, 2.75]; PCB 156: FOR=0.83 [0.71, 0.97]; PCB 183: FOR=1.21 [0.99, 1.48]; PCB 187: FOR=0.44 [0.28, 0.69]; (ΣPCB 99, 156, 187)/(ΣPCB 105, 138, 183): FOR=0.80 [0.70, 0.92] | All other PCB congeners, if mother breastfed, maternal race/ethnicity, lipids | |||
| 75th vs. 25th percentile | PCB 99: FOR=0.69 [0.53, 0.89]; PCB 105: FOR=1.44 [1.09, 1.91]; PCB 138: FOR=2.14 [1.50, 3.05]; PCB 156: FOR=0.78 [0.63, 0.96]; PCB 183: FOR=1.27 [0.99, 1.63]; PCB 187: FOR=0.59 [0.44, 0.79]; (ΣPCB 99, 156, 187)/(ΣPCB 105, 138, 183): FOR=0.62 [0.47, 0.83] | ||||||
| Crawford, 2017 | Time to Conceive | Quartiles (Q4 vs Q1–3) | Combined parity PFOA, PFOS, PFNA, PFHxS: ns | Age, mean cycle length | |||
| Eskenazi, 2010 | SWHS | Continuous (log10) | Measured TCDD: FOR=0.75 [0.60, 0.95; Extrapolated TCDD: FOR=0.73 [0.58, 0.94] | Maternal and paternal age, | |||
| Quartiles (Q4 vs. Q1) | Measured TCDD: FOR=0.63 [0.42, 0.96] | parity, irregular menstrual cycle, OC use, smoking, history of gynaecologic or chronic health conditions | |||||
| Gao, 2016 | LWBC | Continuous (ln) then OR back-transformed | PBDE 28: OR TTP >12 months=1.34 [1.03, 1.76] | Maternal age, prepregnancy BMI, education, occupation, second-hand smoke, parity; paternal age, occupation, smoking, alcohol; family income | |||
| Han, 2016 | Fisheater Family Health Study | >7.4 μg/L vs <2.5 μg/L | p,p'-DDE: ns | PCB: FOR=0.42 [0.20, 0.88] | Age, cohort, education | ||
| Harley, 2008 | CHAMACOS | Continuous (log10) | p,p'-DDT, o,p'-DDT, p,p'-DDE: ns | Age, irregular menstrual cycle, hormonal contraceptive use, breastfeeding, history of gynaecologic conditions, caffeine, years in USA, maternal and paternal occupational pesticide exposure, home pesticide use, proximity of home to fields, trying to conceive | |||
| Harley, 2010 | CHAMACOS | Continuous (log10) | BDE 100: FOR=0.61 [0.42, 0.89]; BDE 153: FOR=0.52 [0.33, 0.81]; ΣPBDEs: FOR=0.68 [0.47, 0.98] | Age, years in USA, history of gynaecologic conditions, hormonal contraceptive use, breastfeeding, caffeine, pesticide, exposure | |||
| Jorgensen, 2014 | INUENDO | Tertiles (T3 vs T1) | Nullipaorus PFOA, PFOS, PFHxS, PFNA: ns | Age, smoking, prepregnancy BMI, gestational week of blood sampling (country for pooled analyses) | |||
| Combined parity PFNA Greenland: FOR=0.71 [0.54, 0.94] and OR TTP >12 months=2.15 [1.05, 4.42] | Age, smoking, parity, prepregnancy BMI, gestational week of blood sampling (country for pooled analyses) | ||||||
| Continuous (log10) | Nulliparous PFOA Ukraine: FOR=1.46 [1.02, 2.08]; PFOA pooled sample: FOR=1.31 [1.03, 1.68] | Age, smoking, prepregnancy BMI, gestational week of blood sampling (country for pooled analysis) | |||||
| Combined parity PFNA Greenland: FOR=0.72 [0.58, 0.89] and OR TTP >12 months=1.97 [1.22, 3.19]; PFNA pooled sample: FOR=0.80 [0.69, 0.94] and OR TTP >12 months=1.53 [1.08, 2.15] | Age, smoking, parity, prepregnancy BMI, gestational week of blood sampling (country for pooled analysis) | ||||||
| Small, 2011 | Tertiles (LOD and median split above LOD) | PBBs: ns | None | ||||
| Snijder, 2011 | Generation R | Occupational exposure (y/n) | Any pesticides, occupational: ns | Occupational: ns | Occupational: ns | Age | |
| Velez, 2015 | MIREC | Continuous (log_SD) | Combined parity PFOA: FOR=0.89 [0.83, 0.94], OR TTP >12 months=1.31 [1.11, 1.53]; PFOS: FOR=0.96 [0.91, 1.02], OR TTP >12 months=1.14 [0.98, 1.34]; PFHxS: FOR=0.91 [0.86, 0.97], OR TTP >12 months=1.27 [1.09, 1.48] | Age, prepregnancy BMI | |||
| Vestergaard, 2012 | Danish First Pregnancy Planner Study | Continuous (ln) | Nulliparous PFOS: FOR=1.39 [0.92, 2.09]; PFHxS: FOR=1.33 [1.01, 1.75]; EtFOSAA: FOR=0.79 [0.62, 1.00] | Female age, prepregnancy BMI, smoking, length of menstrual cycle, female diseases affecting fecundability, oligospermia | |||
| Whitworth, 2016 | MoBa | Interquartile range | Nulliparous PFOSA, PFOA, PFNA, PFDA, PFUnDA, PFDoDA, PFTrDA, PFHxS, PFHpS, PFOS: ns | Age, prepregnancy BMI | |||
| Whitworth, 2012 (dichoto-mous TTP outcome only) | Quartiles (Q4 vs Q1) | Nulliparous: ns | Age, prepregnancy BMI, prepregnancy alcohol (latter for PFOA only) | ||||
| Parous PFOA: OR TTP >12 months=2.1 [1.0, 4.4]; PFOS: OR TTP >12 months=2.1 [1.2, 3.9] | |||||||
| Yang, 2008 | Yucheng | Exposed vs unexposed | PCBs/PCDFs: FOR = 0.90 [0.80–1.00], OR TTP >12 months=2.34 [1.23, 4.59] | Maternal age, paternal age, frequency of intercourse |
ABC, Aarhus Birth Cohort; DNBC, Danish National Birth Cohort; NYSACS, New York State Angler Cohort Study; LIFE, Longitudinal Investigation of Fertility and the Environment; PELAGIE, Perturbateurs endocriniens: Étude Longitudinale sur les Anomalies de la Grossesse, l’Infertilité et l’Enfance; CHDS, Child Health and Development Studies; SWHS, Seveso Women’s Health Study; LWBC, Laizhou Wan Birth Cohort; CHAMACOS, Center for the Health Assessment of Mothers and Children of Salinas; MIREC, Maternal-Infant Research on Environmental Chemicals; MoBa, Norwegian Mother and Child Cohort Study.
Table V.
Fecundability odds ratios in men.
| First author, year | Study name/acronym | Unit of analysis | Organochlorine pesticides | Brominated flame retardants (PBDEs and PBBs) | Polychlorinated organic compounds (PCBs, dioxins, and furans) | PFAS | Covariates |
|---|---|---|---|---|---|---|---|
| Buck Louis, 2013 | LIFE | Continuous (log_SD) | p,p'-DDE: FOR=0.83 [0.70, 0.97] | PBB 153: ns; BDE 17, 28, 47, 66, 85, 99, 100, 153, 154, 183: ns | PCB 101: FOR=1.28 [1.09, 1.51]; PCB 138: FOR=0.71 [0.52, 0.98]; PCB 156: FOR=0.77 [0.62, 0.96]; PCB 157: FOR=0.83 [0.70, 0.97]; PCB 167: FOR=0.82 [0.70, 0.96]; PCB 170: FOR=0.74 [0.56, 0.98]; PCB 172: FOR=0.82 [0.68, 0.99]; PCB 180: FOR=0.81 [0.66, 1.00]; PCB 209: FOR=0.78 [0.65, 0.94] | Combined parity EtFOSAA, MeFOSAA, PFDeA, PFNA, PFOSA, PFOS, PFOA: ns | Left truncation, sum of all other chemicals in class, age, BMI, serum cotinine, site, lipids (except in PFAS models) |
| Campagna, 2015 | Occupational exposure (direct, indirect, unexposed) | DDT: ns | None | ||||
| Jorgensen, 2014 | INUENDO | Tertiles (T3 vs T1) | Combined parity PFOA, PFOS, PFHxS, PFNA: ns | Paternal and maternal age, paternal BMI | |||
| Continuous (log10) | Combined parity PFNA Greenland: FOR=0.70 [0.50, 0.99] | ||||||
| Snijder, 2011 | Generation R | Occupational exposure (y/n) | Any pesticides, occupational: ns | Occupational: ns | Occupational: ns | Age |
LIFE, Longitudinal Investigation of Fertility and the Environment.
Risk of bias assessment
We used the Newcastle-Ottawa Scale (NOS) (Stang, 2010; Wells et al., 2011; Zeng et al., 2015) to assess risk of bias in three domains: participant selection/exposure, comparability of groups and outcome assessment (see Table II). High-quality study characteristics (associated with low risk of bias) were awarded a star with a maximum of one star for each numbered item within each domain. Because selection/exposure item 4 (‘Demonstration that outcome, i.e. pregnancy, was not present at the start of the study’) was not relevant for retrospective studies enrolling participants in the prenatal period or later, we replaced this item with ‘Was exposure measured in women and men?’, giving a star to studies that measured exposures in both partners. We followed recommendations to convert the NOS score to Agency for Healthcare Research and Quality (AHRQ) standards of good, fair and poor (Singh et al., 2015). Specifically, good-quality studies were identified as those awarded 3–4 stars in the selection/exposure domain AND 1–2 stars in the comparability domain AND 2–3 stars in the outcome domain. Fair-quality studies were indicated by 2 stars for selection/exposure AND 1–2 stars for comparability AND 1–2 stars for outcome, whereas poor-quality studies scored 0–1 for selection/exposure OR 0 for comparability OR 0 for outcome.
Table II.
Assessment of risk of bias and study quality.
| Newcastle-Ottawa Scale domains | Criteria for higher quality |
|---|---|
| Selection | |
| Representativeness of exposed cohort or occupational group | Truly or somewhat representative of the population or occupational group* |
| Selection of the non-exposed cohort | Drawn from the same community or occupational group as the exposed cohort and over the same time period* |
| Adequacy of exposure measure | Independent, individual-level biological measure (e.g. blood, semen)* |
| Participants in whom exposure was measured | Women and men* |
| Comparability | |
| Comparability of cohorts on the basis of design (e.g. groups are matched on key variables) or control for confounders in analysis | Controlled for age* |
| Controlled for at least one additional factor (e.g. body mass index, socioeconomic status, race or lifetyle factors such as smoking)* | |
| Outcome | |
| Assessment of outcome | Independent, biological measure of pregnancy (e.g. home pregnancy test) OR medical record confirmation OR recruited when already pregnant (if retrospective design)* |
| Study design | Time to pregnancy measured prospectively OR recalled during pregnancy* |
| Completeness of outcome data | No missing outcome data OR complete follow-up (all subjects accounted for)* |
| Additional quality metrics | |
| 1. Was exposure measured between 12 months preconception and one month postpartum? | Yes |
| 2. Was exposure measured in blood? | Yes |
| 3. For chemicals other than PFAS: Were blood lipids controlled for either by adjusting the exposure measure or by adding blood lipids to the regression model? For PFAS: Was analysis conducted in nulliparous women? | Yes |
| 4. Were participants actively trying to conceive? | Yes |
| 5. Was pregnancy status assessed with daily pregnancy tests? | Yes |
Study characteristics that were used to convert NOS scores to AHRQ standards of good, fair and poor.
The NOS criteria apply generally to epidemiological studies. However, several issues are particularly relevant to TTP studies examining persistent chemical exposures that were not captured by the NOS. Thus, we devised five additional quality metrics to identify specific methodological features of POP and fecundability studies that distinguish those of highest quality: (i) POP exposure was measured between 12 months preconception and 1 month postpartum; (ii) POP exposure was measured in blood; (iii) analyses were adjusted for blood lipids (except for PFAS analyses) or stratified by parity (for PFAS analyses); (iv) participants were actively trying to conceive; and (v) pregnancy status was assessed daily (Table II).
Most POPs are lipophilic. Nevertheless, depending on the causal relation among chemicals, lipids and outcome, adjustment for serum lipid concentrations may not always be necessary. Because the true causal relation is rarely known, however, lipid adjustment is commonly practiced and we therefore awarded studies that did so an extra quality point (#3) (except for those of PFAS, as stated above and explained below). The two prevalent methods for lipid adjustment are standardisation (dividing the chemical concentration by the lipid concentration) or including the lipid concentration in the statistical model as a covariate. Simulations have shown covariate adjustment to be less prone to bias than standardisation, but have also shown the validity of both methods to be vulnerable to error in lipid measurement, which may occur if non-fasting serum samples are used (Schisterman et al., 2005).
As indicated above by the different criteria used to award the extra quality point #3 to analyses of PFAS, this group of chemicals has two features that distinguish it from other POPs and that require special consideration when examining associations between PFAS and TTP. PFAS are not lipophilic, so lipid adjustment is not necessary. In addition, serum PFAS concentrations generally decline during pregnancy (Kato et al., 2014; Pan et al., 2017) and are lower in parous compared to nulliparous women (Brantsæter et al., 2013), likely as a result of transferring PFAS to the foetus during gestation and breastfeeding (Mondal et al., 2014; Cariou et al., 2015; Motas Guzmàn et al., 2016). Causal diagrams have shown that the best way to avoid several potential sources of bias in analyses of PFAS and TTP is to restrict analyses to nulliparous women (Bach et al., 2018). Therefore, we awarded PFAS studies the extra quality point #3 if they stratified by parity or restricted to nulliparous women.
TTP studies are inherently limited. On the one hand, retrospective studies, often conducted among women participating in pregnancy cohorts, lack data from couples who failed to conceive and from pregnancies that resulted in early miscarriage, and may be subject to biased recall of TTP (although over the short term, recalled TTP has been shown to have reasonable validity (Zielhuis et al., 1992)). Additionally, retrospective studies measure exposure levels in samples collected after the outcome has occurred. In the case of non-persistent chemicals, where body burden can vary with day-to-day exposure, this is problematic, as urinary concentrations measured during pregnancy (often in a spot sample) may not accurately reflect preconception levels. For POPs, which are generally lipophilic and have half-lives of years, this is less concerning, although evidence suggests levels may vary due to physiologic changes across periconception, pregnancy and the postpartum period (Bloom et al., 2007, 2009; Buck Louis et al., 2019). While these studies have evaluated changes in absolute POP concentration over the course of pregnancy, they do not evaluate whether the rank order of POP concentration within their study populations varies. If the rank order is preserved, then results can be interpreted as associations between the relative concentrations of POPs and fecundity. As an example, although the absolute value of maternal blood lead concentration changes over the course of pregnancy, the intra-class correlations remain quite high, preserving the rank order and making interpretations viable. On the other hand, while prospective TTP studies are generalisable only to pregnancy planners, who likely differ from non-planners on a variety of factors, they are less likely to suffer other forms of bias and guarantee that exposure was measured prior to outcome. To indicate these benefits, we chose to award the NOS study design quality point to prospective studies or those that measured retrospective TTP during pregnancy, when recall may still be considered reliable. We also awarded one of our additional quality points (#4) exclusively to prospective studies.
Results
Our search returned 4573 potentially relevant journal articles, of which 3950 were removed at the title and abstract-screening level. Of the remaining 623 full-text articles assessed for eligibility, 595 met study exclusion criteria, ultimately yielding 28 articles from 19 different studies (Fig. 1). Of these, 21 papers contained original analyses and seven employed alternative sampling or analytic techniques to analyse data presented in the primary papers (Fei et al., 2009; Whitworth et al., 2012; Gennings et al., 2013; Ding et al., 2014; Lum et al., 2017; Hwang et al., 2019; Zhang et al., 2019). We present results from each alternative approach alongside the corresponding original analysis; together they are counted as one paper for the purposes of this systematic review. We excluded two publications that restated or summarised results that were already contained in the original articles. The reviewing team achieved good preliminary pairwise agreement in selecting articles for inclusion (weighted overall kappas for full-text review and for selection of chemicals = 0.73 and 0.98, respectively).
Description of studies
Characteristics of the 28 papers (21 primary, 7 alternative analyses) and 19 studies that provided results for this review are summarised in Table III. Studies were conducted in Europe (n = 9), North America (n = 7) and East Asia (n = 3). The California-based Center for the Health Assessment of Mothers and Children of Salinas (CHAMACOS) study (Harley et al., 2008, 2010) and the Danish National Birth Cohort (DNBC) (Bach et al., 2015b, 2018) each contributed two primary papers on different POPs. Twelve studies recruited participants from the general population and the remaining seven recruited from specialised populations, including communities of anglers and fish eaters (Buck Louis et al., 2009; Han et al., 2016), pesticide applicators (Campagna et al., 2015), farmworkers (Harley et al., 2008, 2010) and women who had been accidentally exposed through large-scale poisoning incidents (Yang et al., 2008; Eskenazi et al., 2010; Small et al., 2011). Four studies recruited during the preconception period and measured TTP prospectively (the New York State Angler Cohort Study (NYSACS, Buck Louis et al., 2009)), the LIFE study (Buck Louis et al., 2013; Lum et al., 2017), Time to Conceive (Crawford et al., 2017) and the Danish First Pregnancy Planner Study (Vestergaard et al., 2012)). Three of the retrospective TTP studies had data on the participants’ own prenatal exposure (Cohn et al., 2011; Small et al., 2011; Han et al., 2016). Among the three studies that did not have biological measures, exposure was assessed by means of employment records (Campagna et al., 2015), self-reported occupation linked to a job-exposure matrix (Snijder et al., 2011) and inclusion in a victims’ registry (Yang et al., 2008). Several papers analysed data from historic cohorts, the earliest of which assessed exposures between 1946 and 1950 (Campagna et al., 2015), while others focused on more contemporary cohorts, the most recent of which concluded exposure data collection between 2011 and 2013 (Bach et al., 2015a; Velez et al., 2015; Gao et al., 2016). Analytic sample sizes ranged from 83 (Buck Louis et al., 2009) to 2774 (Snijder et al., 2011) participants. While most analyses focused only on TTP in relation to the female partner’s exposure, three papers reported results for both female and male partners (Snijder et al., 2011; Buck Louis et al., 2013; Jorgensen et al., 2014), and one focused exclusively on males (Campagna et al., 2015).
A note on effect sizes
In most cases, studies reported effect sizes as fecundability odds ratios (FORs) with 95% confidence intervals (CIs), modelling TTP as discrete, time-to-event data in months or menstrual cycles. Analogous to a hazard ratio, a FOR represents the probability of conceiving in a specified time period (month or cycle), conditional on not having conceived in the prior time period, per unit of chemical exposure. However, because different units of exposure were used, it is difficult to compare FORs across studies. Researchers frequently examined chemical exposure as a continuous variable, either on the arithmetic or logarithmic scale, and two studies analysed log-transformed chemical concentrations and then rescaled by the standard deviation (SD) to estimate the FOR per SD increase in the log chemical concentration (Buck Louis et al., 2013; Velez et al., 2015). One analysis calculated the FOR based on an interquartile range difference (Whitworth et al., 2016) and another based on an SD difference (Cohn et al., 2011). Alternatively or in addition, researchers modelled exposures categorically, comparing the probability of conception in the sample-specific highest tertile (T3) or quartile (Q4) to the lowest (T1 or Q1) in those with exposure greater than or equal to the limit of detection (LOD) versus below the LOD, or in other categories based on the distribution of their exposure data (Tables IV and V). In studies with non-biological exposure assessment, such as accidental poisoning, the FOR represents the per-month/cycle probability of conception in the exposed group relative to a non-exposed comparison group. A number of papers reported both FORs and odds ratios (ORs) for infertility, defined as TTP >12 versus ≤12 months, and used logistic regression to calculate ORs per unit change in chemical exposure (Tables IV and V). In general, diminished fecundability, or longer TTP, is indicated by an FOR <1 (reduced probability of conception in a given month/cycle) or an OR for infertility >1 (greater odds of taking more than 12 months to conceive).
Associations of female exposure to organochlorine pesticides with TTP
Among the five papers that examined associations of female exposure to OC pesticides with TTP, one was conducted in a prospective study and the others were conducted in retrospective studies. One of the retrospective studies was intergenerational and consisted of women’s retrospective report of TTP within a long-term prospective study that began during or shortly after their mothers’ pregnancy. Mothers’ serum p,pʹ-DDE levels were extrapolated to approximate exposure during the time they were pregnant with their daughters, who eventually reported their own TTP (Han et al., 2016).
Evidence for no association with TTP
The prospective LIFE study measured chemical concentrations in preconception serum samples of 501 couples in Michigan and Texas who were discontinuing contraception. The female partners tracked their menstrual cycles, days of sexual intercourse and various other behaviours until pregnancy was achieved or 12 cycles had been completed (Buck Louis et al., 2013). Exposure to β-HCH, γ-HCH, oxychlordane, trans-nonachlor, p,pʹ-DDT, o,pʹ-DDT, p,pʹ-DDE or mirex was not associated with TTP in unadjusted models. HCB was associated with longer TTP (FOR = 0.87 (0.77, 0.998)), but when adjusted for relevant covariates, serum lipids and site, the point estimate approached the null. In an analysis of 402 participants from the CHAMACOS study, which recruited pregnant women from five prenatal clinics that serve a low-income, predominantly farmworker population in California, neither p,pʹ-DDT, o,pʹ-DDT nor p,pʹ-DDE measured in late second-trimester serum was associated with self-reported TTP when modelled continuously with covariate adjustment (Harley et al., 2008). Both the LIFE and CHAMACOS studies were rated ‘good’ on the NOS/AHRQ scale and met 3.5 and four of the five additional quality metrics, respectively. Prenatal exposure of 151 women to p,pʹ-DDE, extrapolated from measurements taken when their mothers participated in a cohort study of Michigan female anglers and fish eaters between 1973 and 1991 (in most cases postnatally), was not associated with self-reported TTP when they attempted to conceive decades later (Han et al., 2016). This analysis ranked ‘poor’ on the NOS/AHRQ scale and, among other limitations, did not include lipid adjustment. Finally, a large retrospective analysis among 2774 participants in the population-based Generation R Study, a multi-ethnic prospective cohort study in Rotterdam, the Netherlands, that recruited women in early pregnancy between 2002 and 2006, yielded no association between possible or probable occupational exposure to pesticides in the preconception window, calculated according to a job-exposure matrix, and TTP (Snijder et al., 2011). This study was rated ‘fair’ on the NOS/AHRQ scale and met two additional quality metrics but could not distinguish between OCs and other classes of pesticides.
Evidence for longer TTP
Seven out of 14 OC pesticides measured in the cord blood serum of 332 participants in the French Perturbateurs endocriniens: Étude Longitudinale sur les Anomalies de la Grossesse, l’Infertilité et l’Enfance (PELAGIE) study were detected in >10% of the samples and analysed in relation to TTP self-reported in the first half of pregnancy (Chevrier et al., 2013). β-HCH and p,pʹ-DDE were associated with longer TTP when the exposures were modelled both as log10-transformed variables (FOR = 0.49 (0.29, 0.80) and 0.84 (0.71, 0.99), respectively) and when categorised into tertiles (T3 vs. T1: FOR = 0.61 (0.43, 0.86) and 0.60 (0.42, 0.84), respectively). HCB was also associated with longer TTP in the tertile model (FOR = 0.67 (0.48, 0.95)), as was heptachlor epoxide when dichotomised at the LOD (FOR = 0.76 (0.58, 1.00)). This study was rated ‘good’ on the NOS/AHRQ scale and met all of the additional quality metrics except daily pregnancy tests.
Weight of evidence
The majority of studies indicate no association between female exposure to OC pesticides and TTP. Although the PELAGIE study reported an association between higher female OC pesticide concentration and longer TTP, chemical levels were measured in cord serum, which may not be an accurate proxy of maternal periconceptional exposure. While wet-weight cord and maternal blood concentrations of OC pesticides measured in close proximity are positively correlated (Dewan et al., 2013; Sexton et al., 2013), levels are generally lower in cord blood. Furthermore, as serum lipid levels increase across pregnancy, lipid-adjusted concentrations decrease (Vizcaino et al., 2014; Fisher et al., 2016), even when wet-weight concentrations increase (Adetona et al., 2013). The PELAGIE study adjusted for lipids, but did not account for length of gestation, which could confound the relation between OC pesticide concentration and TTP. Longer TTP is associated with preterm birth (Joffe and Li, 1994), which was not an exclusion criterion for the analysis. Results of this analysis may also be influenced by selection bias, as sampling is conditioned on cord blood availability (i.e. live birth), and longer TTP (Joffe and Li, 1994) and exposure to DDT (Eskenazi et al., 2009) and HCH (Pathak et al., 2010) are all associated with miscarriage. Two other studies of comparable quality found no associations for female OC pesticide exposure and TTP (Harley et al., 2008; Buck Louis et al., 2013), including the LIFE study, which assessed a similar array of chemicals in preconception serum samples.
Associations of female exposure to brominated flame retardants with TTP
Female exposure to BFRs, including PBBs and PBDEs, was analysed in six cohorts. While most studies assessed exposure during pregnancy and reported TTP retrospectively, the prospective LIFE study measured exposure to PBBs and PBDEs in preconception blood samples, while a study of female offspring of women exposed to high levels of PBBs through accidental contamination of agricultural products in Michigan assessed prenatal exposure and recalled TTP.
Evidence for no association with TTP
In 1973, a majority of Michigan residents were exposed to extremely high levels of PBB when Firemaster, a toxic flame retardant comprising 18 PBB congeners, was mistakenly sold to farmers throughout the state. Approximately 4000 residents were enrolled in long-term health monitoring, and they and their offspring continue to be followed. Small et al. (2011) analysed self-reported first-pregnancy TTP, dichotomised as ≤12 months versus >12 months, among daughters who had potential in utero PBB exposure. PBB concentrations around the time of the daughters’ conception were extrapolated by applying a decay model to levels in maternal serum samples collected shortly after the incident. No association was found between PBB levels and odds of TTP >12 months. This study received a ‘poor’ NOS/AHRQ rating, in part because the authors did not control for any covariates in their analysis, and only met one of the additional quality metrics. The prospective LIFE study, which measured concentrations of PBB 153 and 10 PBDE congeners in preconception serum, found no statistically significant associations with continuous TTP (Buck Louis et al., 2013). A similar result was found in the PELAGIE study, which measured PBB 153 and eight PBDE congeners, but only analysed BDE 209 in relation to recalled TTP, as the others were detectable in <10% of samples (Chevrier et al., 2013). Investigators from the Generation R Study also reported no association between occupational exposure to flame retardants and retrospective TTP (Snijder et al., 2011).
Evidence for longer TTP
The Chinese Laizhou Wan Birth Cohort (LWBC) includes women who were recruited when admitted for delivery from a county hospital in an area of Shandong Province that contains numerous BFR factories. In an analysis of data from 207 participants, BDE 28 was associated with greater odds of self-reported TTP >12 months (OR = 1.34 (1.03, 1.76)) (Gao et al., 2016). This study received a ‘good’ NOS/AHRQ rating and met three out of five additional quality metrics. In the Californian CHAMACOS study, late second-trimester serum levels of BDE 100 and 153 were associated with longer recalled TTP (FOR = 0.61 (0.42, 0.89) and 0.52 (0.33, 0.81), respectively) as was the sum of the four PBDE congeners (BDEs 47, 99, 100 and 153) that were detected in >75% of samples (FOR = 0.68 (0.47, 0.98)) (Harley et al., 2010).
Weight of evidence
Although the majority of female BFR exposure analyses reported null results, two high-quality papers reported positive associations between PBDEs and TTP. Both the Chinese LWBC and Californian CHAMACOS studies sampled populations that were exposed to high levels of BFRs relative to most of the other studies identified in the systematic review. The majority of PBDE production is not restricted in China, although levels have been declining since production and use of pentaBDEs (e.g. BDEs 85, 99, 100) was phased out in 2007. Nevertheless, they still remain elevated in Shandong Province, where the LWBC recruited participants (Ma et al., 2017). Between 1975, when California adopted Technical Bulletin 117, which required the use of chemical flame retardants in upholstered furniture, and 2003, when the state banned the manufacture and sale of pentaBDEs and octaBDEs (e.g. BDEs 196, 197, 203), levels of PBDEs in pregnant women were among the highest worldwide (Zota et al., 2011). CHAMACOS participants were recruited between 1999 and 2000. By contrast, the LIFE study enrolled participants between 2005 and 2009, following the 2004 nationwide US ban of both pentaBDEs and octaBDEs. Apart from the UK and Ireland, European countries never required furniture to be impregnated with chemical flame retardants, so levels in countries such as France and the Netherlands, where the PELAGIE and Generation R studies recruited, were never as high as China or the USA. Further research is needed to explore the potential dose-dependence of associations between BFRs and TTP, as there is no a priori reason to expect transportability of estimates given how tied to place and time POP exposure levels and their covariates of interest are, and to investigate effects of novel BFRs that have been introduced in the wake of PBDE regulations.
Associations of female exposure to polychlorinated organic compounds with TTP
Seven out of eight studies that measured female exposure to polychlorinated organic compounds, including PCBs, dioxins and furans, reported associations with TTP, although not in consistent directions. These included both prospective and retrospective studies in both general and specialised populations, including two studies with prenatal exposure data.
Evidence for no association with TTP
The only study to report no association between PCBs and TTP was the Generation R Study, which estimated exposure based on occupational exposure in the preconception period (Snijder et al., 2011).
Evidence for longer TTP
Both prospective TTP studies that assessed PCB exposure reported longer TTP for at least some PCB congeners. The NYSACS analysis included 83 women from the larger study who indicated upon enrolment that they were considering or undecided about future pregnancies and who, when re-contacted 5 years later, were discontinuing contraception during the substudy recruitment period. Blood samples were collected during the baseline visit, and women tracked their menstrual cycles, as well as their sexual intercourse and various other behaviours until they tested positive for pregnancy or completed 12 cycles without conceiving. A total of 76 PCB congeners were assayed and entered into models summed and in groups based on purported biologic activity: ‘estrogenic’, ‘antioestrogenic’ and ‘other’ (Buck Louis et al., 2009). The point estimates for the sums of both oestrogenic and antioestrogenic congeners indicated a non-significant association with longer TTP (FOR = 0.32 (0.03, 3.89) and 0.01 (<0.00, 1.99), respectively). The wide confidence intervals reflect the small sample size, the fact that the three biological groups were entered into the same model, and the inclusion of a number of additional covariates. This study received a ‘fair’ NOS/AHRQ rating but met four out of five additional quality criteria. The larger and similarly designed LIFE study assayed 36 PCB congeners, of which three were associated with longer TTP in covariate-adjusted models (FORPCB118 = 0.82 (0.68, 0.98), FORPCB167 = 0.79 (0.64, 0.79) and FORPCB209 = 0.82 (0.68. 0.99)), although two of them (PCBs 167 and 209) were detectable in only 25% and 23% of samples, respectively (Buck Louis et al., 2013).
Although the PELAGIE study enrolled women mid-pregnancy and measured chemicals in cord serum, the results were similar (Chevrier et al., 2013). PCBs 118, 138, 153, 170, 180, 183 and 187 were all associated with longer TTP when modelled continuously and/or categorically. The sum of all nine congeners that were detectable in >10% of samples was associated with longer TTP both in covariate-adjusted models that compared the highest to lowest tertiles and in models that examined continuous log10-transformed concentrations (FOR = 0.46 (0.32, 0.66) and 0.39 (0.19, 0.78), respectively).
Two historic cohorts with second-generation follow-up that assessed prenatal PCB exposure found associations with longer TTP in daughters. The Child Health and Development Studies (CHDS) analysis, which included 289 daughters born to women who had enrolled in the original study between 1960 and 1963 in Oakland, CA, assayed 16 PCB congeners in maternal serum collected 1–3 days postpartum (Cohn et al., 2011). Maternal PCBs 99, 156 and 187 were associated with longer retrospectively reported TTP in daughters in both continuous and categorical models jointly adjusted for all other PCBs, lipids and covariates. This study received an NOS/AHRQ rating of ‘fair’ and met two additional quality metrics. The CHDS data were reanalysed by Gennings et al. using non-linear weighted quantile sum regression to identify which congeners among the mixture of PCBs were most strongly associated with either longer or shorter TTP. They found that dioxin-like, antioestrogenic congeners (66, 74, 105, 118, 156 and 167) and two congeners not included in the original analysis and not classified according to proposed biological mechanism (56 and 146) were associated with longer TTP, as were the highly chlorinated PCBs (56, 101, 105, 146, 156, 167, 183 and 201) (Gennings et al., 2013).
In the other historic cohort, the Michigan Fisheater Family Health Study, in which PCB concentrations taken in serum samples collected at the time of initial enrolment were extrapolated to approximate exposure around the time of the daughters’ conception, the sum of PCB concentrations based on the Aroclor 1260 standard was divided into three categories (Han et al., 2016). Women with periconceptional exposure in both the middle and high concentration groups had longer recalled TTP compared to the referent group (FOR = 0.50 (0.36, 0.99) and 0.42 (0.20, 0.88), respectively).
Finally, two landmark studies of accidental exposure to polychlorinated organic compounds also reported longer TTP. The Seveso Women’s Health Study (SWHS) followed female residents of the region surrounding Seveso, Italy, where a chemical plant explosion in 1976 resulted in the highest exposure known in human residential populations to dioxin (2,3,7,8-tetrachlorodibenzo-p-dioxin, TCDD), the most toxic compound in the chemical class. Participants who were age 0–40 years at the time of the explosion and provided serum samples between 1976 and 1980 were recruited to a follow-up study in 1996. Among the 278 who delivered a live birth that was intentionally conceived in the interim, serum dioxin concentration was measured in archived samples and extrapolated to approximate exposure at the time of conception, an interval that averaged 9 years (Eskenazi et al., 2010). Concentrations shortly after the accident were associated with longer recalled TTP in both a covariate-adjusted continuous model and a model comparing the highest to lowest quartile (FOR = 0.75 (0.60, 0.95) and 0.63 (0.42, 0.96), respectively). Results were similar when extrapolated concentrations were used, although the 95% CI for the categorical model included the null value. This study received a ‘good’ NOS/AHRQ rating and met three additional quality metrics.
In 1979, an accidental poisoning incident occurred in central Taiwan, where more than 2000 people consumed cooking oil contaminated with PCBs which, when heated, produced toxic polychlorinated dibenzofurans (PCDFs) causing symptoms of ‘oil disease’ (‘Yu-cheng’ in Chinese). In a 2003 follow-up study, researchers analysed data from 186 married women from the Yucheng cohort who were age 25–45 years (infancy through 20 years old at the time of the accident) and had become pregnant after the period of exposure and 226 unexposed women who had lived near the registry members (Yang et al., 2008). Those in the Yucheng cohort took longer to conceive their first pregnancy than those in the reference cohort (FOR = 0.90 (0.80, 1.00)) and had higher odds of TTP >12 months (OR = 2.34 (1.23, 4.59)). This study received a ‘fair’ NOS/AHRQ rating and met two additional quality criteria.
Evidence for shorter TTP
While both the NYSACS and CHDS papers reported longer TTP for some PCB congeners, they found that others were associated with shorter TTP. Specifically, the sum of all 76 PCB congener concentrations measured in preconception serum in the NYSACS was negatively associated with TTP (FOR = 1.10 (1.00, 1.20)), driven by PCBs in the non-oestrogenic, non-antioestrogenic category (Buck Louis et al., 2009). In the CHDS, concentrations of PCBs 105, 138 and 183 in maternal postpartum serum were associated with shorter TTP in both continuous and categorical models jointly adjusted for all other PCBs, lipids and covariates (Cohn et al., 2011). In a non-linear weighted quantile sum mixture analyses of the CHDS data, the dioxin-like, antioestrogenic congeners (PCBs 66, 74, 105, 118, 156 and 167) were associated with shorter TTP (Gennings et al., 2013).
Weight of evidence
The nearly consistent results of seven out of eight studies of vastly different design that were conducted in disparate geographic locations and temporal periods, and in populations with widely varying ranges of exposure lend support to a link between female exposure to most polychlorinated organic compounds and reduced fecundability. Exceptions must be noted, however. Both the NYSACS and the CHDS reported that female concentrations of certain PCB congeners were associated with increased fecundability. Similarly, the LIFE study’s finding that PCB 101 was associated with shorter TTP in contrast to several other PCBs that were associated with longer TTP implies that the stereochemical differences among various congeners may cause them to act via different biological mechanisms, sometimes resulting in opposing effects.
Associations of female exposure to polyfluoroalkyl substances with TTP
Nearly every paper identified by this systematic review and published since 2012 has examined PFAS in relation to TTP, indicating the growing attention being paid to this class of POPs, the use of which is just beginning to be regulated. Five retrospective and three prospective studies analysed female exposure to these chemicals and TTP, yielding nine primary articles, as the DNBC and Norwegian Mother and Child Cohort Study (MoBa) studies each published two papers on PFAS.
Evidence for no association with TTP
Nearly all of the analyses that reported no associations between female PFAS exposure and TTP were conducted in nulliparous women, as recommended. In the Danish Aarhus Birth Cohort (ABC), 16 PFAS were measured in stored serum samples collected between 9 and 20 weeks gestation in 1372 nulliparous participants with planned pregnancies. Among the seven PFAS that had detectable levels in ≥50% of samples, none was associated with TTP (Bach et al., 2015a). In an analysis that included 1251 women with early pregnancy plasma PFAS measures and TTP information nested within the equally high-ranking DNBC, results were stratified by parity and no associations were observed among nulliparous women (Bach et al., 2018). The multisite INUENDO study reported no difference in TTP comparing the highest tertile to the lowest of four PFAS (PFOA, PFOS, PFHxS and PFNA) measured in prenatal serum samples in nulliparous women from Greenland, Poland and Ukraine, as well as all three countries combined (Jorgensen et al., 2014). In addition, an analysis of a subsample of nulliparous participants from the MoBa study that assessed fecundability in relation to 10 different PFAS (Whitworth et al., 2016) found no associations, replicating the results of a prior analysis of nulliparous women from the same cohort in which odds of TTP >12 months was used as an outcome (Whitworth et al., 2012). All four of these European studies received a ‘good’ NOS/AHRQ rating and met four out of five extra quality criteria.
The North Carolina Time to Conceive study recruited women attempting to become pregnant, collected preconception serum samples and had participants track their menstrual cycles, intercourse and other behaviours in a daily diary for up to 6 months (Crawford et al., 2017). Among 99 participants with serum PFAS measures, there was no association of PFOA, PFOS, PFNA or PFHxS with TTP in either cycle-specific or day-specific models. Following a test for effect modification by parity, which was not statistically significant, the authors chose not to stratify by or adjust for parity in their analysis.
Evidence for longer TTP
Two analyses of nulliparous women reported associations between PFAS and longer TTP. The Danish First Pregnancy Planner Study enrolled 430 couples trying to conceive and followed them for up to 6 months. Female participants provided preconception serum samples, 222 of which were available for chemical analysis (Vestergaard et al., 2012). Among the several PFAS and PFAS metabolites assayed, only log-transformed ethyl perfluorooctane sulphonamido acetic acid (EtFOSAA) was associated with longer TTP (FOR = 0.79 (0.62, 1.00)). In an analysis of two randomly selected subsamples from the DNBC (n = 440 and 1161), PFOS and PFOA were measured in mid-pregnancy plasma around the same time participants were asked to recall their TTP (Bach et al., 2015b). When pooled and stratified by parity, an association was observed between PFOS and longer TTP in both categorical (highest vs. lowest quartile) and continuous (ln-transformed) models among nulliparous women (FOR = 0.79 (0.63, 0.99) and 0.78 (0.63, 0.97), respectively). Results were similar for parous women. A prior unstratified analysis among 1240 participants in the same cohort that adjusted for parity found both reduced fecundability and increased odds of TTP >12 months when comparing highest to lowest quartiles of PFOA (FOR = 0.60 (0.47, 0.76), OR = 2.54 (1.47, 4.39)) and PFOS (FOR = 0.74 (0.58, 0.93), OR=.77 (1.06, 2.95)) (Fei et al., 2009).
Two studies that performed their analyses in samples that combined parous and nulliparous women and did not adjust for parity reported positive associations between PFAS concentrations and TTP. The prospective LIFE study measured several PFAS and metabolites in preconception serum; the only one associated with TTP was perfluorooctane sulphonamine (PFOSA) (FOR = 0.82 (0.71, 0.95)), although only 10% of samples had detectable levels (Buck Louis et al., 2013). A subsequent reanalysis of the same data that adjusted for cycle length found reduced odds of conception among those in the highest versus lowest tertile of PFNA exposure while controlling for six other PFAS (OR = 0.7 (0.3, 1.1)) (Lum et al., 2017). The retrospective Maternal-Infant Research on Environmental Chemicals (MIREC) study is a Canadian population-based study of women recruited in early pregnancy, at which time they provided plasma samples for measurement of PFOA, PFOS and PFHxS concentrations (Velez et al., 2015). In results that combined parous and nulliparous women and did not adjust for parity, all three PFAS were associated with reduced fecundability (FOR = 0.89 (0.83, 0.94), 0.96 (0.91, 1.02) and 0.91 (0.86, 0.97), respectively) and higher odds of TTP >12 months (OR = 1.31 (1.11, 1.53), 1.14 (0.98, 1.34) and 1.27 (1.09, 1.48), respectively). This study was ranked ‘good’ on the NOS/AHRQ scale and met three additional quality metrics.
Finally, Ding et al. conducted a reanalysis of the PFAS data in the MoBA Study to account for the case-cohort sampling design, which had not been accounted for in the earlier analyses (Whitworth et al., 2012, 2016). This failure time model analysis found a significant association of PFOA with longer TTP; however, the sample included both nulliparous and parous women (Ding et al., 2014). The earlier Whitworth paper similarly found a link between PFOA and greater odds of infertility, but the association only persisted in parous women following stratification.
Evidence for shorter TTP
While no associations with TTP among nulliparous women were found when PFAS were modelled categorically in the large multisite European INUENDO study that recruited pregnant women and their male partners at their first prenatal visit from hospitals and antenatal clinics throughout Greenland, in Warsaw, Poland and in Kharkiv, Ukraine, an association between PFOA and shorter TTP among nulliparous women was reported when the exposure was modelled continuously. In the Ukrainian subsample, a 10-fold increase in PFOA was associated with a 46% increase in fecundability (2%, 108%), and when participants from Greenland, Poland and Ukraine were combined, a 31% increase in fecundability was observed (3%, 68%) (Jorgensen et al., 2014).
Weight of evidence
Consistent null results from several high-quality studies that restricted their analyses to nulliparous women argue against any associations between PFAS and TTP. By contrast, two good studies did report associations of PFOS with longer TTP among nulliparous women, suggesting that the many members of the PFAS chemical class may not always act in concert. All but one of these studies used retrospective reports of TTP and measured PFAS in prenatal blood samples, yet the INUENDO study was the only one to adjust for gestational age at sampling even though PFAS levels are known to decline across pregnancy. Interestingly, the later of the two DNBC analyses reported discordant results between nulliparous and parous participants even though the authors adjusted for inter-pregnancy interval in the parous group, which theoretically should account for the gradual postnatal increase in PFAS following the decline during pregnancy and breastfeeding (Bach et al., 2018). This suggests that the issue of bias in PFAS analyses conducted among parous or combined parous/nulliparous study populations may not be easily resolved. Further research is needed to explore more of the hundreds of PFAS in production that are not included in these analyses and determine whether they fall into groups that share common biological mechanisms and may have similar associations with reproductive outcomes such as TTP.
Associations of male exposures with TTP
Only four studies examined male exposure to POPs and TTP, yielding too little evidence to draw conclusions. Results were not always consistent with those found in female partners, suggesting that POPs may have sex-specific effects on gametogenesis, an avenue for future research.
OC pesticides
Evidence supporting an association between exposure to OC pesticides and TTP is slim. In a study of an historic cohort of 1223 men who lived in Sardinia during the anti-malarial campaign between November 1946 and May 1950, exposure to DDT was examined in relation to TTP, measured as the time lag between the date of marriage and the ninth month before the birth date of the first child (Campagna et al., 2015). There was no statistically significant difference in fecundability between those exposed to DDT indirectly as bystanders (warehouse workers, drivers or inspectors in the campaign) or directly as pesticide applicators and those exposed at the background level in covariate-unadjusted analysis. Results were the same when DDT exposure was estimated based on the exact dates of starting and ending each job, the task performed and the concentration of DDT in the insecticide preparation. This study ranked ‘poor’ on the NOS/AHRQ scale and met none of the additional quality metrics. There was also no association between preconception occupational exposure to any pesticides and retrospectively-assessed TTP among 2728 male participants in the Generation R Study (Snijder et al., 2011).
By contrast, among 501 male participants in the prospective LIFE study, preconception exposure to p,pʹ-DDE measured in blood was associated with longer TTP (FOR = 0.83 (0.70, 0.97) in a covariate-adjusted model, although results were null for the other eight OC pesticides measured (Buck Louis et al., 2013).
Brominated flame retardants
In keeping with results for female participants, there were no associations between male preconception PBB 153 or PBDE exposure and prospectively measured TTP in the LIFE study (Buck Louis et al., 2013) or between male occupational exposure to flame retardants and retrospective TTP in the Generation R Study (Snijder et al., 2011).
Polychlorinated organic compounds
As with female participants, the Generation R Study reported no association between male estimated occupational PCB exposure and TTP (Snijder et al., 2011).
In the high-quality LIFE study, considerably more PCB congeners measured in male preconception serum were associated with longer TTP than in their female partners (PCBs 138, 156, 157, 167, 170, 172, 180 and 209). Several of these chemicals were detectable in <50% of samples, including PCBs 167 and 209, the only two with statistically significant results for both men and women. By contrast, PCB 101 was associated with shorter TTP (FOR = 1.28 (1.09, 1.51)), although it was detectable in only 38% of samples among males (Buck Louis et al., 2013). Two reanalyses of the LIFE Study data examined joint associations of male and female partners’ PCB exposure with risk of infertility (defined as TTP >12 months). Zhang et al. (2019) and Hwang et al. (2019) used different methods to examine joint exposure, but both found that summary measures of PCB exposure in both male and female partners were associated with couple infertility, with the male partners’ PCB concentrations contributing more to infertility risk than the female partners’.
Per- and PFAS
The prospective LIFE study measured seven PFAS and metabolites in 501 male partners and found no association between any of them and TTP in an analysis that combined parous and nulliparous couples (Buck Louis et al., 2013). The retrospective INUENDO study investigated 401 male partners’ exposure, also combining parous and nulliparous couples, and found no association between PFOA, PFOS, PFHxS or PFNA with TTP when comparing those in the highest tertiles of exposure to the lowest. When male PFAS concentrations were modelled continuously, however, a 10-fold increase in PFNA among Greenland participants was associated with a 30% decrease in fecundability (1%, 50%); there were no associations for other PFAS or for men from Poland or Ukraine (Jorgensen et al., 2014).
Discussion
The relationship between specific POPs and fecundability has been studied in a variety of settings; however, we are not aware of any prior systematic reviews that summarise across chemical classes. After conducting our exhaustive review of five major databases for English-language empirical studies published in the past 12 years, the combined evidence suggests that at least some of these chemicals may have negative effects on reproductive processes that contribute to delayed TTP (Table VI). Of note, our review lends support for adverse effects of female exposure to PCBs on fecundability, with some additional support for associations of female exposure to PBDEs and select PFAS with longer TTP. In contrast, the systematic review provided little or no support for associations between female exposure to OC pesticides and TTP. There were too few studies of male exposure to any of the POP groups and fecundability to draw conclusions, although there was suggestive evidence of a link between male PCB exposure and TTP in one high-quality prospective study.
Table VI.
Summary of the weight of evidence among papers reviewed on female exposures1 to persistent organic pollutants and fecundability.
| No association with TTP | Associated with longer TTP | |||
|---|---|---|---|---|
| Strength of evidence |
High |
Polychlorinated biphenyls (PCBs) 2 | √ | |
| Organochlorine (OC) pesticides | √ | |||
| Per- and polyfluoroalkyl substances (PFAS) 3 | √ | |||
|
Low |
Brominated flame retardants (BFRs) | √ | ||
There was too little evidence to draw conclusions for male exposures.
While most PCBs were associated with longer TTP, certain congeners were associated with shorter TTP.
Evidence was strongest among studies that were restricted to nulliparous women.
In addition to being an indicator of subfecundity that may warrant medical intervention such as IVF, delayed TTP is of public health importance because it may be a predictor of future health problems for both men and women. Men with poor semen quality, a contributing factor to couple subfecundity, are at elevated risk of non-reproductive cancers (Ku et al., 2015), cardiovascular disease (Eisenberg et al., 2015), diabetes (La Vignera et al., 2012), autoimmunity (Glazer et al., 2018; Brubaker et al., 2018), overall morbidity (Salonia et al., 2009; Eisenberg et al., 2015; Latif et al., 2017) and mortality (Groos et al., 2006; Jensen et al., 2009; Eisenberg et al., 2014). Women with conditions that contribute to infecundity such as polycystic ovarian syndrome (de Groot et al., 2011; Lim et al., 2019; Ramezani Tehrani et al., 2020), endometriosis (Tan et al., 2019) and premature ovarian failure (Muka et al., 2016; Honigberg et al., 2019; Zhu et al., 2019) as well as women who experience failed fertility therapy (Udell et al., 2017) are at increased risk of metabolic syndrome, cardiovascular disease, adverse birth outcomes and overall mortality. These associations likely are not causal, but result from common causes, which may include exposure to POPs.
Study limitations
While we found heterogeneity in the quality of the studies we reviewed on POPs and TTP, most ranked highly, with 11 out of 19 receiving a ‘good’ NOS/AHRQ rating and meeting 3 or 4 additional quality metrics. Nevertheless, all neglected to adjust for multiple comparisons, increasing the likelihood of Type I error (i.e. that a significant effect was erroneously reported), especially in analyses that examined chemicals from different classes, which are less likely to be highly correlated. Selection bias is of concern for retrospective studies, as they condition on both conception and continued pregnancy either until the time when biospecimens are collected or live birth in cases when exposure is measured in cord blood, when pregnancy duration/live birth may be associated with both the chemical being studied and TTP. Exposure measured in a spot blood sample may be a source of measurement error, as it may not represent exposure at the time of conception for POPs with short half-lives (e.g. BDE 209, which has a half-life of about 2 weeks).
Across studies, statistical models adjusted for different sets of covariates; while this may be due to varying sources of confounding in different populations (e.g. socioeconomic status may be related to POP exposure in some populations but not others), in some cases this was due to a lack of agreement on appropriate confounders. The INUENDO study was the only retrospective PFAS study to include gestational age at blood sampling, which is appropriate as concentrations decline across pregnancy. A recent study by Buck Louis et al. (2019) suggests that concentrations of other POPs may also vary across pregnancy, indicating that adjusting for gestational age at sampling may be good practice for all of these chemical classes.
Several PFAS studies combined parous and nulliparous women in their analyses, even though PFAS levels have been shown to decline with increasing parity, likely because women transfer PFAS to their foetuses/offspring during gestation and breastfeeding. Other POPs have also been shown to decrease with parity (Fisher et al., 2016), raising the question of whether all analyses of POPs and TTP should be conducted in nulliparous women. Because pregnancy involves the mobilisation and remodelling of adipose tissue and that redistribution may differ by overweight/obesity status (Straughen et al., 2013), BMI and gestational weight gain may affect prenatal blood concentrations of lipophilic POPs in retrospective studies.
Comparisons of results across studies were complicated, as studies measured different chemicals and congeners. While chemicals from the same class were generally measured similarly (e.g. gas chromatography-mass spectrometry for PCBs, PBBs, PBDEs and OCs; liquid chromatography-mass spectrometry for PFAS), details of the procedures varied by laboratory. The issue of whether these differences substantially impact results is directly addressed by Sholtz et al. (2011), who found that measurements of chlorinated pesticides and PCBs were able to be combined over a 13-year period without meaningful misclassification due to laboratory variation or time period. Moreover, exposures were modelled differently across the studies we reviewed, with some studies choosing to log-transform their data and/or categorise exposures using sample-specific cut-points, including the LOD, which also varied by laboratory. Each study population had a different exposure distribution, frequently dependent on both geography and time period. Finally, study populations were likely exposed to different mixtures of chemicals, and interactions among them may distort results when chemicals’ associations with TTP are assessed individually.
Future research
The results of this systematic review indicate several recommendations for future research. First, although prospective fecundability studies using daily pregnancy tests are expensive and logistically difficult and may not be generalisable to the broader population as they are restricted to pregnancy planners, they are optimal for accurate exposure and outcome assessment. Prospective studies measure the exposure prior to the outcome, strengthening the evidence for causation, and avoid both inaccuracies in outcome measurement due to recalled TTP and selection bias based on conception, pregnancy continuation or live birth. Second, when studying POPs with short half-lives, the body burden may be better captured with repeated biosamples across the preconception period, mitigating exposure misclassification. Third, in order to ensure that models include appropriate covariates (e.g. prepregnancy BMI) and results are analysed in appropriate subsamples (e.g. nulliparous women), further exploration of the metabolism and toxicokinetics of these chemicals is necessary, particularly during pregnancy. Fourth, studies need to consider POPs that are less commonly studied and those that are replacing chemicals that are being voluntarily phased out, regulated or banned, including new generations of BFRs and PFAS (Pellizzari et al., 2019).
With respect to data analysis, attention should be paid to distributions of chemicals, as variations across populations may complicate cross-study comparisons. Chemical mixtures analyses and analyses considering interactions between chemical exposures and non-chemical exposures (e.g. psychosocial measures, genotypes, diet and activity) are important to consider, although samples need to be large in order to achieve adequate statistical power. As a result, prospective multi-site studies that are designed so that pooled analyses may be conducted are ideal to clarify the complex effects of these chemicals on fecundability. Such studies would preclude difficulties caused by cross-study comparisons of studies using different confounders, means of exposure and outcome assessment and exposure distributions, and ensure adequate power to test for interactions.
Conclusions
While results were not consistent among the 28 articles from 19 different studies that we reviewed, at least some POPs appear to be associated with reduced fecundability. The evidence was strongest for female exposure to PCBs and weakest for OC pesticides, with equivocal findings for PBDEs and PFAS. Results for male exposure were limited and inconclusive. In terms of BFRs and PFAS in particular, more research needs to be done, as there are numerous members of these chemical classes that have not been studied in relation to TTP, including newer ‘replacement’ molecules that are being introduced as studies have shown adverse health outcomes associated with older ones. ‘Legacy’ chemicals such as DDT and PCBs, which have been regulated or banned in most parts of the world, still merit attention, as their persistence in the environment and the human body means that they will continue to affect human health for years to come. These health effects are not evenly distributed across populations, with countries in the Global South bearing a higher burden, as OC pesticides may still be used to combat tropical disease and industries such as e-waste recycling expose workers to PCBs, dioxins and furans, which also contaminate soil and groundwater (Chakraborty et al., 2018). Even within developed countries, risk is not equally shared, as disadvantaged communities are more likely to live near industrial and military sites that have contaminated the surrounding environment. In addition, individuals with lower income are less able to afford to replace older furniture and household goods that may contain PBDEs and PFAS. To the degree that these populations are at elevated risk of exposure, they may also be at risk for decreased fecundability, impacting not only their ability to reproduce, but also indicating increased likelihood of long-term health problems. Additional research is needed to understand how associations between POPs and TTP may vary by population, how POPs may interact with other chemical and non-chemical exposures, and how POPs are distributed in and metabolised by the human body, especially during the periconceptional and prenatal periods. The results of such studies should be summarised and disseminated to clinicians, who can then inform patients about the potential for POPs to interfere with reproductive function and provide advice about ways to reduce exposure.
Supplementary data
Supplementary data are available at Human Reproduction Update online.
Authors’ roles
Study conception and design: L.G.K., K.G.H., E.L.S., P.F.-L., C.A.P. and A.E.H.; search strategy design and execution: M.K.-F.; data extraction and interpretation: L.G.K., K.G.H., E.L.S., Y.Z., P.F.-L., C.A.P. and A.E.H.; drafting, critical revision and final approval of the manuscript: all authors.
Funding
Research reported in this publication was supported by the Environmental Influences on Child Health Outcomes (ECHO) program, Office of The Director, National Institutes of Health, under Award Number U2COD023375 (Coordinating Center), U24OD023382 (Data Analysis Center), UH3OD023244 (Hipwell/Keenan: A.E.H., M.K.-F.), UH3OD023305 (Trasande: L.G.K.), UH3OD023249 (Stanford/Clark/Porucznik: C.A.P.), UG3OD023356 (Eskenazi: K.G.H.) and UH3OD023289 (Ferrara: Y.Z.). In addition, L.G.K. was supported by NIH grant K99ES030403, Y.Z. was supported by grant K01DK120807, M.K.-F. was supported by grant R21AA025484, E.L.S. was supported by grant T32ES023772 (Factor-Litvak) and P.F.-L. was supported by grants P30ES009089, R01ES013543, R01ES014393 and R01ES08977.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Conflict of interest
None declared.
Supplementary Material
References
- Abdelouahab N, Ainmelk Y, Takser L. Polybrominated diphenyl ethers and sperm quality. Reprod Toxicol 2011;31:546–550. [DOI] [PubMed] [Google Scholar]
- Adetona O, Horton K, Sjodin A, Jones R, Hall DB, Aguillar-Villalobos M, Cassidy BE, Vena JE, Needham LL, Naeher LP. Concentrations of select persistent organic pollutants across pregnancy trimesters in maternal and in cord serum in Trujillo, Peru. Chemosphere 2013;91:1426–1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen J, Lambrecht L. Responses of rhesus-monkeys to polybrominated biphenyls. Toxicol Appl Pharmacol 1978;23:340–341. [Google Scholar]
- Agency for Toxic Substances and Disease Registry. Toxicological Profile for DDT, DDE, and DDD: draft for public comment. U.S. Centers for Disease Control and Prevention, 2019. Atlanta, GA. [PubMed]
- Bach C, Matthiesen B, Olsen J, Henriksen B. Conditioning on parity in studies of perfluoroalkyl acids and time to pregnancy: an example from the Danish National Birth Cohort. Environ Health Perspect 2018;126:117003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach CC, Bech BH, Nohr EA, Olsen J, Matthiesen NB, Bossi R, Uldbjerg N,, Bonefeld-Jorgensen EC, Henriksen TB. Serum perfluoroalkyl acids and time to pregnancy in nulliparous women. Environ Res 2015. a;142:535–541. [DOI] [PubMed] [Google Scholar]
- Bach CC, Liew Z, Bech BH, Nohr EA, Fei C, Bonefeld-Jorgensen EC, Henriksen TB, Olsen J. Perfluoroalkyl acids and time to pregnancy revisited: an update from the Danish National Birth Cohort. Environ Health 2015. b;14:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird DD, Wilcox AJ, Weinberg CR. Use of time to pregnancy to study environmental exposures. Am J Epidemiol 1986;124:470–480. [DOI] [PubMed] [Google Scholar]
- Bergeron JM, Crews D, McLachlan JA. PCBs as environmental estrogens: turtle sex determination as a biomarker of environmental contamination. Environ Health Perspect 1994;102:780–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Buck-Louis GM,, Schisterman EF, Kostyniak PJ, Vena JE. Changes in maternal serum chlorinated pesticide concentrations across critical windows of human reproduction and development. Environ Res 2009;109:93–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom MS, Buck Louis GM, Schisterman EF, Liu A, Kostyniak PJ. Maternal serum polychlorinated biphenyl concentrations across critical windows of human development. Environ Health Perspect 2007;115:1320–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brantsæter AL, Whitworth KW, Ydersbond TA, Haug LS, Haugen M, Knutsen HK, Thomsen C, Meltzer HM, Becher G, Sabaredzovic A et al. Determinants of plasma concentrations of perfluoroalkyl substances in pregnant Norwegian women. Environ Int 2013;54:74–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brubaker WD, Li S, Baker LC, Eisenberg ML. Increased risk of autoimmune disorders in infertile men: analysis of US claims data. Andrology 2018;6:94–98. [DOI] [PubMed] [Google Scholar]
- Buck Louis G, Cooney M, Peterson C. The ovarian dysgenesis syndrome. J Dev Orig Health Dis 2011;2:25–35. [Google Scholar]
- Buck Louis GM, Dmochowski J, Lynch C, Kostyniak P, McGuinness BM, Vena JE. Polychlorinated biphenyl serum concentrations, lifestyle and time-to-pregnancy. Hum Reprod 2009;24:451–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Sundaram R, Schisterman EF, Sweeney AM, Lynch CD, Gore-Langton RE, Maisog J, Kim S, Chen Z, Barr DB. Persistent environmental pollutants and couple fecundity: the LIFE study. Environ Health Perspect 2013;121:231–236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck Louis GM, Yeung E, Kannan K, Maisog J, Zhang C, Grantz KL, Sundaram R. Patterns and variability of endocrine-disrupting chemicals during pregnancy: implications for understanding the exposome of normal pregnancy. Epidemiology 2019;30 Suppl 2:S65–S–75.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campagna M, Satta G, Fadda D, Pili S, Cocco P. Male fertility following occupational exposure to dichlorodiphenyltrichloroethane (DDT). Environ Int 2015;77:42–47. [DOI] [PubMed] [Google Scholar]
- Cano-Sancho G, Ploteau S, Matta K, Adoamnei E, Louis GB, Mendiola J, Darai E, Squifflet J, Le Bizec B, Antignac JP. Human epidemiological evidence about the associations between exposure to organochlorine chemicals and endometriosis: Systematic review and meta-analysis. Environ Int 2019;123:209–223. [DOI] [PubMed] [Google Scholar]
- Cariou R, Veyrand B, Yamada A, Berrebi A, Zalko D, Durand S, Pollono C, Marchand P, Leblanc JC, Antignac JP et al. Perfluoroalkyl acid (PFAA) levels and profiles in breast milk, maternal and cord serum of French women and their newborns. Environ Int 2015;84:71–81. [DOI] [PubMed] [Google Scholar]
- Carlsen E, Giwercman A, Keiding N, Skakkebaek NE. Evidence for decreasing quality of semen during past 50 years. BMJ 1992;305:609–613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carson R. Silent Spring. Cambridge, MA: Houghton Mifflin, 1962. [Google Scholar]
- Chakraborty P, Selvaraj S, Nakamura M, Prithiviraj B, Cincinelli A, Bang JJ. PCBs and PCDD/Fs in soil from informal e-waste recycling sites and open dumpsites in India: levels, congener profiles and health risk assessment. Sci Total Environ 2018;621:930–938. [DOI] [PubMed] [Google Scholar]
- Chevrier C, Warembourg C, Gaudreau E, Monfort C, Le Blanc A, Guldner L, Cordier S. Organochlorine pesticides, polychlorinated biphenyls, seafood consumption, and time-to-pregnancy. Epidemiology 2013;24:251–260. [DOI] [PubMed] [Google Scholar]
- Choy JT, Eisenberg ML. Comprehensive men's health and male infertility. Transl Androl Urol 2020;9:S239–S243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Codru N, Schymura MJ, Negoita S, Rej R, Carpenter DO. Diabetes in relation to serum levels of polychlorinated biphenyls and chlorinated pesticides in adult Native Americans. Environ Health Perspect 2007;115:1442–1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohn BA, Cirillo PM, Sholtz RI, Ferrara A, Park JS, Schwingl PJ. Polychlorinated biphenyl (PCB) exposure in mothers and time to pregnancy in daughters. Reprod Toxicol 2011;31:290–296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connor K, Ramamoorthy K, Moore M, Mustain M, Chen I, Safe S, Zacharewski T, Gillesby B, Joyeux A, Balaguer P. Hydroxylated polychlorinated biphenyls (PCBs) as estrogens and antiestrogens: structure-activity relationships. Toxicol Appl Pharmacol 1997;145:111–123. [DOI] [PubMed] [Google Scholar]
- Crawford NM, Fenton SE, Strynar M, Hines EP, Pritchard DA, Steiner AZ. Effects of perfluorinated chemicals on thyroid function, markers of ovarian reserve, and natural fertility. Reprod Toxicol 2017;69:53–59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis SI, Blanck HM, Hertzberg VS, Tolbert PE, Rubin C, Cameron LL, Henderson AK, Marcus M. Menstrual function among women exposed to polybrominated biphenyls: a follow-up prevalence study. Environ Health 2005;4:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Groot PC, Dekkers OM, Romijn JA, Dieben SW, Helmerhorst FM. PCOS, coronary heart disease, stroke and the influence of obesity: a systematic review and meta-analysis. Hum Reprod Update 2011;17:495–500. [DOI] [PubMed] [Google Scholar]
- Dewan P, Jain V, Gupta P, Banerjee BD. Organochlorine pesticide residues in maternal blood, cord blood, placenta, and breastmilk and their relation to birth size. Chemosphere 2013;90:1704–1710. [DOI] [PubMed] [Google Scholar]
- Ding J, Zhou H, Liu Y, Cai J, Longnecker MP. Estimating effect of environmental contaminants on women's subfecundity for the MoBa study data with an outcome-dependent sampling scheme. Biostatistics 2014;15:636–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding N, Harlow SD, Randolph JF, Loch-Caruso R, SK Park. Perfluoroalkyl and polyfluoroalkyl substances (PFAS) and their effects on the ovary. Hum Reprod Update 2020;26:724–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Cullen MR, Galusha D, Lamb DJ, Lipshultz LI. Semen quality, infertility and mortality in the USA. Hum Reprod 2014;29:1567–1574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril 2015;103:66–71. [DOI] [PubMed] [Google Scholar]
- Ely DM, Hamilton BE. Trends in fertility and mother's age at first birth among rural and metropolitan counties: United States, 2007-2017. NCHS Data Brief 2018;323:1–8. [PubMed] [Google Scholar]
- Eskenazi B, Chevrier J, Rosas LG, Anderson HA, Bornman MS, Bouwman H, Chen A, Cohn BA, de Jager C, Henshel DS. , et al. The Pine River statement: human health consequences of DDT use. Environ Health Perspect 2009;117:1359–1367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eskenazi B, Warner M, Marks AR, Samuels S, Needham L, Brambilla P, Mocarelli P. Serum dioxin concentrations and time to pregnancy. Epidemiology 2010;21:224–231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fei C, McLaughlin JK, Lipworth L, Olsen J. Maternal levels of perfluorinated chemicals and subfecundity. Hum Reprod 2009;24:1200–1205. [DOI] [PubMed] [Google Scholar]
- Fisher M, Arbuckle TE, Liang CL, LeBlanc A, Gaudreau E, Foster WG, Haines D, Davis K, Fraser WD. Concentrations of persistent organic pollutants in maternal and cord blood from the maternal-infant research on environmental chemicals (MIREC) cohort study. Environ Health 2016;15:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galhardo A, Pinto-Gouveia J, Cunha M, Matos M. The impact of shame and self-judgment on psychopathology in infertile patients. Hum Reprod 2011;26:2408–2414. [DOI] [PubMed] [Google Scholar]
- Gao Y, Chen L, Wang C, Zhou Y, Wang Y, Zhang Y, Hu Y, Ji L, Shi R, Cui C et al. Exposure to polybrominated diphenyl ethers and female reproductive function: a study in the production area of Shandong, China. Sci Total Environ 2016;572:9–15. [DOI] [PubMed] [Google Scholar]
- Gennings C, Carrico C, Factor-Litvak P, Krigbaum N, Cirillo PM, Cohn BA. A cohort study evaluation of maternal PCB exposure related to time to pregnancy in daughters. Environ Health 2013;12:66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer CH, Tottenborg SS, Giwercman A, Brauner EV, Eisenberg ML, Vassard D, Magyari M, Pinborg A, Schmidt L, Bonde JP. Male factor infertility and risk of multiple sclerosis: a register-based cohort study. Mult Scler 2018;14:1835–1842. [DOI] [PubMed] [Google Scholar]
- Goncharov A, Rej R, Negoita S, Schymura M, Santiago-Rivera A, Morse G, Carpenter DO. Lower serum testosterone associated with elevated polychlorinated biphenyl concentrations in Native American men. Environ Health Perspect 2009;117:1454–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonsioroski A, Mourikes VE, Flaws JA. Endocrine disruptors in water and their effects on the reproductive system. Int J Mol Sci 2020;21: 1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore AC, Chappell VA, Fenton SE, Flaws JA, Nadal A, Prins GS, Toppari J, Zoeller RT. EDC-2: the endocrine society's second scientific statement on endocrine-disrupting chemicals. Endocr Rev 2015;36:E1–E150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groos S, Krause W, Mueller UO. Men with subnormal sperm counts live shorter lives. Soc Biol 2006;53:46–60. [DOI] [PubMed] [Google Scholar]
- Han L, Hsu WW, Todem D, Osuch J, Hungerink A, Karmaus W. In utero exposure to polychlorinated biphenyls is associated with decreased fecundability in daughters of Michigan female fisheaters: a cohort study. Environ Health 2016;15:92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hany J, Lilienthal H, Sarasin A, Roth-Harer A, Fastabend A, Dunemann L, Lichtensteiger W, Winneke G. Developmental exposure of rats to a reconstituted PCB mixture or aroclor 1254: effects on organ weights, aromatase activity, sex hormone levels, and sweet preference behavior. Toxicol Appl Pharmacol 1999;158:231–243. [DOI] [PubMed] [Google Scholar]
- Hardell L, van Bavel B, Lindström G, Björnfoth H, Orgum P, Carlberg M, Sörensen CS, Graflund M. Adipose tissue concentrations of p,p'-DDE and the risk for endometrial cancer. Gynecol Oncol 2004. a;95:706–711. [DOI] [PubMed] [Google Scholar]
- Hardell L, Van Bavel B, Lindstrom G, Carlberg M, Eriksson M, Dreifaldt AC, Wijkstrom H, Starkhammar H, Hallquist A, Kolmert T. Concentrations of polychlorinated biphenyls in blood and the risk for testicular cancer. Int J Androl 2004. b;27:282–290. [DOI] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Bradman A, Barr DB, Eskenazi B. DDT exposure, work in agriculture, and time to pregnancy among farmworkers in California. J Occup Environ Med 2008;50:1335–1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harley KG, Marks AR, Chevrier J, Bradman A, Sjodin A, Eskenazi B. PBDE concentrations in women's serum and fecundability. Environ Health Perspect 2010;118:699–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hipwell AE, Kahn LG, Factor-Litvak P, Porucznik CA, Siegel EL, Fichorova RN, Hamman RF, Klein-Fedyshin M, Harley KG. Exposure to non-persistent chemicals in consumer products and fecundability: a systematic review. Hum Reprod Update 2019;25:51–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honigberg MC, Zekavat SM, Aragam K, Finneran P, Klarin D, Bhatt DL, Januzzi JL Jr, Scott NS, Natarajan P. Association of premature natural and surgical menopause with incident cardiovascular disease [published online ahead of print November 18 2019]. JAMA. doi:10.1001/jama.2019.1919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howards PP, Terrell ML, Jacobson MH, Taylor KC, Kesner JS, Meadows JW, Spencer JB, Manatunga AK, Marcus M. Polybrominated biphenyl exposure and menstrual cycle function. Epidemiology 2019;30:687–694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang BS, Chen Z, MBL G, Albert PS. A Bayesian multi-dimensional couple-based latent risk model with an application to infertility. Biometrics 2019;75:315–325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang SS, Dukhovny D, Gopal D, Cabral H, Missmer S, Diop H, Declercq E, Stern JE. Health of infants after ART-treated, subfertile, and fertile deliveries. Pediatrics 2018;142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TK, Jacobsen R, Christensen K, Nielsen NC, Bostofte E. Good semen quality and life expectancy: a cohort study of 43,277 men. Am J Epidemiol 2009;170:559–565. [DOI] [PubMed] [Google Scholar]
- Joffe M. Time to pregnancy: a measure of reproductive function in either sex. Asclepios Project. Occup Environ Med 1997;54:289–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joffe M, Li Z. Association of time to pregnancy and the outcome of pregnancy. Fertil Steril 1994;62:71–75. [DOI] [PubMed] [Google Scholar]
- Johnson PI, Altshul L, Cramer DW, Missmer SA, Hauser R, Meeker JD. Serum and follicular fluid concentrations of polybrominated diphenyl ethers and in-vitro fertilization outcome. Environ Int 2012;45:9–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen KT, Specht IO, Lenters V, Bach CC, Rylander L, Jonsson BA, Lindh CH, Giwercman A, Heederik D, Toft G et al. Perfluoroalkyl substances and time to pregnancy in couples from Greenland, Poland and Ukraine. Environ Health 2014;13:116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn LG, Philippat C, Nakayama SF, Slama R, Trasande L. Endocrine-disrupting chemicals: implications for human health. Lancet Diabetes Endocrinol 2020;8:703–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato K, Wong LY, Chen A, Dunbar C, Webster GM, Lanphear BP, Calafat AM. Changes in serum concentrations of maternal poly- and perfluoroalkyl substances over the course of pregnancy and predictors of exposure in a multiethnic cohort of Cincinnati, Ohio pregnant women during 2003-2006. Environ Sci Technol 2014;48:9600–9608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaya H, Hany J, Fastabend A, Roth-Harer A, Winneke G, Lilienthal H. Effects of maternal exposure to a reconstituted mixture of polychlorinated biphenyls on sex-dependent behaviors and steroid hormone concentrations in rats: dose-response relationship. Toxicol Appl Pharmacol 2002;178:71–81. [DOI] [PubMed] [Google Scholar]
- Kelce WR, Stone CR, Laws SC, Gray LE, Kemppainen JA, Wilson EM. Persistent DDT metabolite p,p'-DDE is a potent androgen receptor antagonist. Nature 1995;375:581–585. [DOI] [PubMed] [Google Scholar]
- Kholkute S, Rodriguez J, Dukelow W. The effects of polybrominated biphenyls and perchlorinated terphenyls on in vitro fertilization in the mouse. Arch Environ Contamin Toxicol 1994;26:208–211. [DOI] [PubMed] [Google Scholar]
- Kleanthi GK, Lykeridou K, Protopapa E, Lazaris A. Mechanisms of actions and health effects of organochlorine substances: a review. Health Sci J 2008;2: 86–95. [Google Scholar]
- Klotz DM, Beckman BS, Hill SM, McLachlan JA, Walters MR, Arnold SF. Identification of environmental chemicals with estrogenic activity using a combination of in vitro assays. Environ Health Perspect 1996;104:1084–1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacevic R, Vojinovic-Miloradov M, Teodorovic I, Andric S. Effect of PCBs on androgen production by suspension of adult rat Leydig cells in vitro. J Steroid Biochem Mol Biol 1995;52:595–597. [DOI] [PubMed] [Google Scholar]
- Ku JY, Park NC, Jeon TG, Park HJ. Semen analysis in cancer patients referred for sperm cryopreservation before chemotherapy over a 15-year period in Korea. World J Mens Health 2015;33:8–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuiper GG, Lemmen JG, Carlsson B, Corton JC, Safe SH, van der Saag PT, van der Burg B, Gustafsson JA. Interaction of estrogenic chemicals and phytoestrogens with estrogen receptor beta. Endocrinology 1998;139:4252–4263. [DOI] [PubMed] [Google Scholar]
- Kuriyama SN, Talsness CE, Grote K, Chahoud I. Developmental exposure to low-dose PBDE-99: effects on male fertility and neurobehavior in rat offspring. Environ Health Perspect 2004;113:149–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- La Vignera S, Condorelli R, Vicari E, D'Agata R, Calogero AE. Diabetes mellitus and sperm parameters. J Androl 2012;33:145–153. [DOI] [PubMed] [Google Scholar]
- Latif T, Kold Jensen T, Mehlsen J, Holmboe SA, Brinth L, Pors K, Skouby SO, Jorgensen N, Lindahl-Jacobsen R. Semen quality as a predictor of subsequent morbidity: a Danish Cohort Study of 4,712 men with long-term follow-up. Am J Epidemiol 2017;186:910–917. [DOI] [PubMed] [Google Scholar]
- Levine H, Jorgensen N, Martino-Andrade A, Mendiola J, Weksler-Derri D, Mindlis I, Pinotti R, Swan SH. Temporal trends in sperm count: a systematic review and meta-regression analysis. Hum Reprod Update 2017;23:646–659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtensteiger W, Faass O, Ceccatelli R, Schlumpf M. Developmental exposure to PBDE 99 and PCB affects estrogen sensitivity of target genes in rat brain regions and female sexual behavior. Organohalogen compd 2004;66:3965–3970. [Google Scholar]
- Lim SS, Kakoly NS, Tan JWJ, Fitzgerald G, Bahri Khomami M, Joham AE, Cooray SD, Misso ML, Norman RJ, Harrison CL et al. Metabolic syndrome in polycystic ovary syndrome: a systematic review, meta-analysis and meta-regression. Obes Rev 2019;20:339–352. [DOI] [PubMed] [Google Scholar]
- Lopez-Espinosa MJ, Mondal D, Armstrong BG, Eskenazi B, Fletcher T. Perfluoroalkyl substances, sex hormones, and insulin-like growth factor-1 at 6-9 years of age: a cross-sectional analysis within the c8 health project. Environ Health Perspect 2016;124:1269–1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk BH, Loke AY. The impact of infertility on the psychological well-being, marital relationships, sexual relationships, and quality of life of couples: a systematic review. J Sex Marital Ther 2015;41:610–625. [DOI] [PubMed] [Google Scholar]
- Lum KJ, Sundaram R, Barr DB, Louis TA, Buck Louis GM. Perfluoroalkyl chemicals, menstrual cycle length, and fecundity: findings from a prospective pregnancy study. Epidemiology 2017;28:90–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma Y, Li P, Jin J, Wang Y, Wang Q. Current halogenated flame retardant concentrations in serum from residents of Shandong Province, China, and temporal changes in the concentrations. Environ Res 2017;155:116–122. [DOI] [PubMed] [Google Scholar]
- Makey CM, McClean MD, Braverman LE, Pearce EN, Sjödin A, Weinberg J, Webster TF. Polybrominated diphenyl ether exposure and reproductive hormones in North American men. Reprod Toxicol 2016;62:46–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGlynn KA, Quraishi SM, Graubard BI, Weber JP, Rubertone MV, Erickson RL. Persistent organochlorine pesticides and risk of testicular germ cell tumors. J Natl Cancer Inst 2008;100:663–671. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Hauser R. Exposure to polychlorinated biphenyls (PCBs) and male reproduction. Syst Biol Reprod Med 2010;56:122–131. [DOI] [PubMed] [Google Scholar]
- Meeker JD, Johnson PI, Camann D, Hauser R. Polybrominated diphenyl ether (PBDE) concentrations in house dust are related to hormone levels in men. Sci Total Environ 2009;407:3425–3429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009;6:e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moher D, Shamseer L, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mondal D, Weldon RH, Armstrong BG, Gibson LJ, Lopez-Espinosa MJ, Shin HM, Fletcher T. Breastfeeding: a potential excretion route for mothers and implications for infant exposure to perfluoroalkyl acids. Environ Health Perspect 2014;122:187–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan M, Deoraj A, Felty Q, Roy D. Environmental estrogen-like endocrine disrupting chemicals and breast cancer. Mol Cell Endocrinol 2017;457:89–102. [DOI] [PubMed] [Google Scholar]
- Motas Guzmàn M, Clementini C, Pérez-Cárceles MD, Jiménez Rejón S, Cascone A, Martellini T, Guerranti C, Cincinelli A. Perfluorinated carboxylic acids in human breast milk from Spain and estimation of infant's daily intake. Sci Total Environ 2016;544:595–600. [DOI] [PubMed] [Google Scholar]
- Muka T, Oliver-Williams C, Kunutsor S, Laven JS, Fauser BC, Chowdhury R, Kavousi M, Franco OH. Association of age at onset of menopause and time since onset of menopause with cardiovascular outcomes, intermediate vascular traits, and all-cause mortality: a systematic review and meta-analysis. JAMA Cardiol 2016;1:767–776. [DOI] [PubMed] [Google Scholar]
- Mumford SL, Kim S, Chen Z, Gore-Langton RE, Boyd Barr D, Buck Louis GM. Persistent organic pollutants and semen quality: The LIFE Study. Chemosphere 2015;135:427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Center for Health Statistics. Key Statistics from the National Survey of Family Growth: Impaired Fecundity. U.S. Centers for Disease Control and Prevention, 2020. Atlanta, GA. [Google Scholar]
- Newton J, Braselton W Jr, Lepper L, McCormack K, Hook J. Effects of polybrominated biphenyls on metabolism of testosterone by rat hepatic microsomes. Toxicol Appl Pharmacol 1982;63:142–149. [DOI] [PubMed] [Google Scholar]
- Pan Y, Zhu Y, Zheng T, Cui Q, Buka SL, Zhang B, Guo Y, Xia W, Yeung LW, Li Y et al. Novel chlorinated polyfluorinated ether sulfonates and legacy per-/polyfluoroalkyl substances: placental transfer and relationship with serum albumin and glomerular filtration rate. Environ Sci Technol 2017;51:634–644. [DOI] [PubMed] [Google Scholar]
- Pathak R, Mustafa M, Ahmed RS, Tripathi AK, Guleria K, Banerjee BD. Association between recurrent miscarriages and organochlorine pesticide levels. Clin Biochem 2010;43:131–135. [DOI] [PubMed] [Google Scholar]
- Pellizzari ED, Woodruff TJ, Boyles RR, Kannan K, Beamer PI, Buckley JP, Wang A, Zhu Y, Bennett DH. Identifying and prioritizing chemicals with uncertain burden of exposure: opportunities for biomonitoring and health-related research. Environ Health Perspect 2019;127:126001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen MS, Halling J, Jorgensen N, Nielsen F, Grandjean P, Jensen TK, Weihe P. Reproductive function in a population of young Faroese men with elevated exposure to polychlorinated biphenyls (PCBs) and perfluorinated alkylate substances (PFAS). Int J Environ Res Public Health 2018;15: 1880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porta M, Bosch de Basea M, Benavides FG, López T, Fernandez E, Marco E, Alguacil J, Grimalt JO, Puigdomènech E. Differences in serum concentrations of organochlorine compounds by occupational social class in pancreatic cancer. Environ Res 2008;108:370–379. [DOI] [PubMed] [Google Scholar]
- Qin J, Liu X, Sheng X, Wang H, Gao S. Assisted reproductive technology and the risk of pregnancy-related complications and adverse pregnancy outcomes in singleton pregnancies: a meta-analysis of cohort studies. Fertil Steril 2016;105:73–85.e1–6. [DOI] [PubMed] [Google Scholar]
- Ramezani Tehrani F, Amiri M, Behboudi-Gandevani S, Bidhendi-Yarandi R, Carmina E. Cardiovascular events among reproductive and menopausal age women with polycystic ovary syndrome: a systematic review and meta-analysis. Gynecol Endocrinol 2020;36:12–23. [DOI] [PubMed] [Google Scholar]
- Recio-Vega R, Ocampo-Gomez G, Borja-Aburto VH, Moran-Martinez J, Cebrian-Garcia ME. Organophosphorus pesticide exposure decreases sperm quality: association between sperm parameters and urinary pesticide levels. J Appl Toxicol 2008;28:674–680. [DOI] [PubMed] [Google Scholar]
- Robinson SL, Parikh T, Lin T, Bell EM, Heisler E, Park H, Kus C, Stern JE, Yeung EH. Infertility treatment and autism risk using the Modified Checklist for Autism in Toddlers (M-CHAT). Hum Reprod 2020;35:684–693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Safe SH, Zacharewski T. Organochlorine exposure and risk for breast cancer. Prog Clin Biol Res 1997;396:133–145. [PubMed] [Google Scholar]
- Salonia A, Matloob R, Gallina A, Abdollah F, Sacca A, Briganti A, Suardi N, Colombo R, Rocchini L, Guazzoni G. , et al. Are infertile men less healthy than fertile men? Results of a prospective case-control survey. Eur Urol 2009;56:1025–1031. [DOI] [PubMed] [Google Scholar]
- Schisterman EF, Whitcomb BW, Louis GM, Louis TA. Lipid adjustment in the analysis of environmental contaminants and human health risks. Environ Health Perspect 2005;113:853–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrader TJ, Cooke GM. Effects of Aroclors and individual PCB congeners on activation of the human androgen receptor in vitro. Reprod Toxicol 2003;17:15–23. [DOI] [PubMed] [Google Scholar]
- Senapati S. Infertility: a marker of future health risk in women? Fertil Steril 2018;110:783–789. [DOI] [PubMed] [Google Scholar]
- Sexton K, Salinas JJ, McDonald TJ, Gowen RM, Miller RP, McCormick JB, Fisher-Hoch SP. Biomarkers of maternal and fetal exposure to organochlorine pesticides measured in pregnant Hispanic women from Brownsville, Texas. Int J Environ Res Public Health 2013;10:237–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, Shekelle P, Stewart LA. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ 2015;350:g7647. [DOI] [PubMed] [Google Scholar]
- Sholtz RI, McLaughlin KR, Cirillo PM, Petreas M, Park JS, Wolff MS, Factor-Litvak P, Eskenazi B, Krigbaum N, Cohn BA. Assaying organochlorines in archived serum for a large, long-term cohort: implications of combining assay results from multiple laboratories over time. Environ Int 2011;37:709–714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Kaur J, Singh S, Thumburu K, Jaiswal N, Chauhan A, Agarwal A, Paul N, Sagwal S. Comparison of Newcastle Ottawa Scale (NOS) and Agency for Health Research and Quality (AHRQ) as Risk of Bias Assessment Tools for Cohort Studies: Filtering the Information Overload for Better Decisions. Abstracts of the 23rd Cochrane Colloquium. Vienna, Austria: John Wiley and Sons, 2015. [Google Scholar]
- Skakkebaek NE, Rajpert-De Meyts E, Main KM. Testicular dysgenesis syndrome: an increasingly common developmental disorder with environmental aspects. Hum Reprod 2001;16:972–978. [DOI] [PubMed] [Google Scholar]
- Small CM, Murray D, Terrell ML, Marcus M. Reproductive outcomes among women exposed to a brominated flame retardant in utero. Arch Environ Occup Health 2011;66:201–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smarr MM, Sapra KJ, Gemmill A, Kahn LG, Wise LA, Lynch CD, Factor-Litvak P, Mumford SL, Skakkebaek NE, Slama R et al. Is human fecundity changing? A discussion of research and data gaps precluding us from having an answer. Hum Reprod 2017;32:499–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snijder CA, Brouwers MM, Jaddoe VW, Hofman A, Roeleveld N, Burdorf A. Occupational exposure to endocrine disruptors and time to pregnancy among couples in a large birth cohort study: the Generation R Study. Fertil Steril 2011;95:2067–2072. [DOI] [PubMed] [Google Scholar]
- Straughen JK, Trudeau S, Misra VK. Changes in adipose tissue distribution during pregnancy in overweight and obese compared with normal weight women. Nutr Diabetes 2013;3:e84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunderam S, Kissin DM, Zhang Y, Folger SG, Boulet SL, Warner L, Callaghan WM, Barfield WD. Assisted reproductive technology surveillance—United States, 2016. MMWR Surveill Summ 2019;68:1–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swan SH, Elkin EP, Fenster L. The question of declining sperm density revisited: an analysis of 101 studies published 1934-1996. Environ Health Perspect 2000;108:961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talsness CE, Kuriyama SN, Sterner-Kock A, Schnitker P, Grande SW, Shakibaei M, Andrade A, Grote K, Chahoud I. In utero and lactational exposures to low doses of polybrominated diphenyl ether-47 alter the reproductive system and thyroid gland of female rat offspring. Environ Health Perspect 2007;116:308–314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan J, Taskin O, Iews M, Lee AJ, Kan A, Rowe T, Bedaiwy MA. Atherosclerotic cardiovascular disease in women with endometriosis: a systematic review of risk factors and prospects for early surveillance. Reprod Biomed Online 2019;39:1007–1016. [DOI] [PubMed] [Google Scholar]
- Tsai MS, Lin CY, Lin CC, Chen MH, Hsu SH, Chien KL, Sung FC, Chen PC, Su TC. Association between perfluoroalkyl substances and reproductive hormones in adolescents and young adults. Int J Hyg Environ Health 2015;218:437–443. [DOI] [PubMed] [Google Scholar]
- Udell JA, Lu H, Redelmeier DA. Failure of fertility therapy and subsequent adverse cardiovascular events. CMAJ 2017;189:E391–E397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- United Nations Environment Programme. All POPs Listed in the Stockholm Convention. Secretariat of the Stockholm Convention, 2008. Geneva, Switzerland.
- U.S. Environmental Protection Agency. Technical Fact Sheet - Polybrominated Diphenyl Ethers (PBDEs) United States Environmental Protection Agency Office of Land and Emergency Management, 2017. Washington, D.C.
- Velez MP, Arbuckle TE, Fraser WD. Maternal exposure to perfluorinated chemicals and reduced fecundity: the MIREC study. Hum Reprod 2015;30:701–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vested A, Giwercman A, Bonde JP, Toft G. Persistent organic pollutants and male reproductive health. Asian J Androl 2014;16:71–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vestergaard S, Nielsen F, Andersson AM, Hjollund NH, Grandjean P, Andersen HR, Jensen TK. Association between perfluorinated compounds and time to pregnancy in a prospective cohort of Danish couples attempting to conceive. Hum Reprod 2012;27:873–880. [DOI] [PubMed] [Google Scholar]
- Vizcaino E, Grimalt JO, Fernandez-Somoano A, Tardon A. Transport of persistent organic pollutants across the human placenta. Environ Int 2014;65:107–115. [DOI] [PubMed] [Google Scholar]
- Wattigney WA, Irvin-Barnwell E, Pavuk M, Ragin-Wilson A. Regional variation in human exposure to persistent organic pollutants in the United States, NHANES. J Environ Public Health 2015;2015:571839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wells G, SheaB, O’ConnellD, PetersonJ, WelchV, LososM, Tugwell P.. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-analysis. Ottawa, Ontario: The Ottawa Health Research Institute, 2011. [Google Scholar]
- Wen X, Xiong Y, Qu X, Jin L, Zhou C, Zhang M, Zhang Y. The risk of endometriosis after exposure to endocrine-disrupting chemicals: a meta-analysis of 30 epidemiology studies. Gynecol Endocrinol 2019;35:645–650. [DOI] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Baird DD, Becher G, Hoppin JA, Skjaerven R, Thomsen C, Eggesbo M, Travlos G, Wilson R et al. Perfluorinated compounds and subfecundity in pregnant women. Epidemiology 2012;23:257–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitworth KW, Haug LS, Sabaredzovic A, Eggesbo M, Longnecker MP. Brief report: plasma concentrations of perfluorooctane sulfonamide and time-to-pregnancy among primiparous women. Epidemiology 2016;27:712–715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielsoe M, Kern P, Bonefeld-Jorgensen EC. Serum levels of environmental pollutants is a risk factor for breast cancer in Inuit: a case control study. Environ Health 2017;16:56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff MS, Toniolo PG, Lee EW, Rivera M, Dubin N. Blood levels of organochlorine residues and risk of breast cancer. J Natl Cancer Inst 1993;85:648–652. [DOI] [PubMed] [Google Scholar]
- Wu AK, Elliott P, Katz PP, Smith JF. Time costs of fertility care: the hidden hardship of building a family. Fertil Steril 2013;99:2025–2030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang CY, Wang YJ, Chen PC, Tsai SJ, Guo YL. Exposure to a mixture of polychlorinated biphenyls and polychlorinated dibenzofurans resulted in a prolonged time to pregnancy in women. Environ Health Perspect 2008;116:599–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zegers-Hochschild F, Adamson GD, de Mouzon J, Ishihara O, Mansour R, Nygren K, Sullivan E, van der Poel S. The International Committee for Monitoring Assisted Reproductive Technology (ICMART) and the World Health Organization (WHO) revised glossary on ART terminology, 2009. Hum Reprod 2009;24:2683–2687. [DOI] [PubMed] [Google Scholar]
- Zeng X, ZhangY, KwongJ S, ZhangC, LiS, SunF, NiuY, Du L. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med 2015;8:2–10. [DOI] [PubMed] [Google Scholar]
- Zhang S, Tan R, Pan R, Xiong J, Tian Y, Wu J, Chen L. Association of perfluoroalkyl and polyfluoroalkyl substances with premature ovarian insufficiency in Chinese women. J Clin Endocrinol Metab 2018;103:2543–2551. [DOI] [PubMed] [Google Scholar]
- Zhang W, Chen Z, Liu A, Buck Louis GM. A weighted kernel machine regression approach to environmental pollutants and infertility. Stat Med 2019;38:809–827. [DOI] [PubMed] [Google Scholar]
- Zhu D, Chung HF, Dobson AJ, Pandeya N, Giles GG, Bruinsma F, Brunner EJ, Kuh D, Hardy R, Avis NE et al. Age at natural menopause and risk of incident cardiovascular disease: a pooled analysis of individual patient data. Lancet Public Health 2019;4:e553–e564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielhuis GA, Hulscher ME, Florack EI. Validity and reliability of a questionnaire on fecundability. Int J Epidemiol 1992;21:1151–1156. [DOI] [PubMed] [Google Scholar]
- Zota AR, Park JS, Wang Y, Petreas M, Zoeller RT, Woodruff TJ. Polybrominated diphenyl ethers, hydroxylated polybrominated diphenyl ethers, and measures of thyroid function in second trimester pregnant women in California. Environ Sci Technol 2011;45:7896–7905. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.