Abstract
Sjögren’s syndrome (SS) is a common autoimmune disease characterized by lymphocytic infiltration and destruction of exocrine glands. The disease manifests primarily in the salivary and lacrimal glands, but other organs are also involved, leading to dry mouth, dry eyes, and other extra-glandular manifestations. Studying the disease in humans is entailed with many limitations and restrictions; therefore, the need for a proper mouse model is mandatory. SS mouse models are categorized, depending on the disease emergence into spontaneous or experimentally manipulated models. The usefulness of each mouse model varies depending on the SS features exhibited by that model; each SS model has advanced our understanding of the disease pathogenesis. In this review article, we list all the available murine models which have been used to study SS and we comment on the characteristics exhibited by each mouse model to assist scientists to select the appropriate model for their specific studies. We also recommend a murine strain that is the most relevant to the ideal SS model, based on our experience acquired during previous and current investigations.
Keywords: Sjögren’s syndrome, Autoimmunity, Salivary glands, Lacrimal glands
1. Introduction
Sjögren’s syndrome (SS) is a multisystem rheumatoid inflammatory disease that manifests primarily in exocrine glands and mainly affects middle-aged women.1, 2, 3 Its prevalence is variable among different populations ranging from 0.1 to 0.72%.4, 5, 6, 7, 8, 9 In addition to the glandular dysfunction, several extra-glandular manifestations associated with lymphocytic infiltration and B cells hyperactivity in other organs are present.10,11 Similar to other autoimmune diseases, SS can be found solitary (Primary SS) or accompanying other autoimmune diseases (Secondary SS), like Systemic Lupus Erythematosus (SLE) and Rheumatoid Arthritis (RA).3,12 Despite major differences in immune defence mechanisms, evolutionary distance, and living environment, murine models have been used extensively in biomedical research to avoid the ethical challenges in human research.13 Murine models have taken a large share, much more than other animal models, due to their availability as natural models and through genetic engineering.
SS usually develops as a result of triggering environmental factors in genetically susceptible individuals.14 However, the ambiguity involving the etiology of the disease and the initial pathological processes are due to the time gap between the onset of the disease and the emergence of clinical symptoms. In other words, to advance our understanding of these missing pathological steps, we must identify at-risk individuals and perform longitudinal studies involving several minor salivary gland biopsies and blood samples. However, such studies are challenging for several reasons. Firstly, our understating of SS genetics is still underway, unlike other autoimmune diseases, like SLE and RA.15 SS does not follow a simple Mendelian-like pattern and is a complex autoimmune disease, due to the polygenic inheritance.16 Therefore, identifying the susceptible individual is difficult. Secondly, the morbidity associated with longitudinal human studies involving the harvest of tissues and blood, has ethical and patient recruitment difficulties. Giving all the previous difficulties associated with human studies, an alternative animal option is strongly justified.
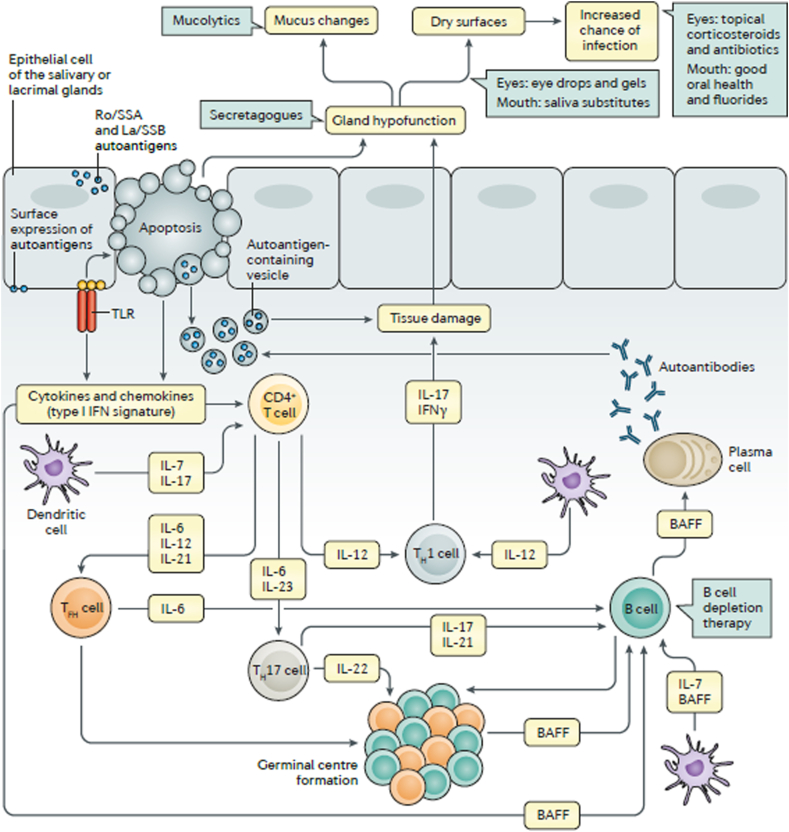
In general, SS is caused by genetic and epigenetic factors; the genetic analysis of SS patients provided important information about SS mechanism and the involved molecules, which includes human leukocyte antigen (HLA)-DR molecules, and genes of both innate and acquired immunity. Other reports suggest that viruses play an important role in SS pathogenesis, and that the viral provoke might occur years before SS evolution. It is believed that cycles of innate and acquired immune system activation cause lacrimal and salivary glandular damage and subsequent dysfunction. This damage is caused mainly by glandular and extraglandular lymphocytes, subsequently, cytokines and chemokines are released within the tissue, in addition to glandular epithelial cells and local vascular adhesive molecules. In Fig. 1,17 Brito-Zerón et al., 2016 summarize SS mechanism.
Fig. 1.
[from Brito-Zerón 17]: Pathogenesis of SS syndrome involves. Salivary gland epithelial cells (SGECs) express toll-like receptors (TLRs) which lead to autoantigens, chemokines and cytokines formation, apoptosis, then, SGECs hypofunction. SGECs secrete autoantigens that trigger immune cells. CD4+ T cells turn into follicular helper T (TFH) cells that enhance B cell survivability. B cell differentiation is promoted by its interaction with SGECs. Potential treatments are presented in green boxes. BAFF: B cell-activating factor, IFN: interferon, TH: T helper.
Mice have been an invaluable tool for studying SS etiopathogenesis and for drug testing due to their small size, easy breeding, and a relatively low maintenance cost in comparison to bigger animal models.18,19 Several spontaneous and engineered murine models have been used extensively and a substantial knowledge about SS etiopathogenesis has been generated. However, each of these models is unique but is inherently incapable of providing all answers to our SS investigations, due to genetic and phenotypic differences from humans.13 Theoretically speaking, an ideal murine model must display a range of characteristics, such as; etiology, etiopathology, clinical features, serology and immunobiology20; nonetheless, in reality, this ideal model, does not exist. However, some models display more of these characteristics than the others, which makes them better candidates for studying SS.
In this review, we list all the available murine models used as possible candidates for SS studies. We have also listed all the reported characteristics that these murine models exhibit to enable the researchers to decide which model will be more suitable for their intended use. Finally, we provide our opinion as to which model has recapitulated the key features of SS the best, based on our previous and current investigations.
1.1. The ideal murine model
SS patients display a panel of clinical and laboratory features that distinguish the disease from other autoimmune diseases, more specifically, other dry eyes/mouth conditions caused by certain factors. The clinical features are fundamental and necessary for the selection of a murine model, Table 1.21,22 Other laboratory characteristics, such as autoantibodies and certain blood cytokines, are also important; they represent key markers in diagnosing the disease and are utilized as key parameters for therapeutic success.
Table 1.
| Clinical features |
| Dry mouth. |
| Dry eyes. |
| Histopathology features |
| Lymphocytic infiltration in the lacrimal and/or salivary glands. |
| Destruction of the gland stroma and decreased acinar cell number. |
| Persistent lesion. |
| Serological features |
| Hypergammaglobulinemia |
| Anti-SSA |
| Anti-SSB |
| Anti-M3R (anti-muscarinic 3 receptor) |
| RF (rheumatoid factor). |
| Anti-CPP (anti-citrullinated cyclic peptide). |
| ACA (anti-centromere antibody). |
| AMA (anti-mitochondrial antibody. |
| Extra-glandular features |
| Multiple organs involvement including skin, respiratory system, liver, blood vessels etc. |
| Genetic features |
| Abnormal MHC I and II genes expression, especially HLA-DR and HLA-DQ. |
| Non-MHC-related genes: INF signaling pathway; B-cell activation and autoantibody production. |
2. Spontaneous mouse models
Genetic predisposition is strongly related to SS etiopathogenesis.1,19,23,24 The development of SS in spontaneous models involves several genes which gives us an invaluable tool to study the effect of these genes on the disease onset and progression; whereas it is challenging to perform such studies in SS patients. NOD mouse model and its derivatives are the best available models for studying SS pathogenesis and drug testing, in addition to several other models. Table 2 summarizes the different characteristics, autoantibodies, genetics and pathogenesis of the spontaneous mouse models.
Table 2.
Spontaneous mouse models of Sjögren’s Syndrome. Summary of the characteristics, autoantibodies, genetics, and pathogenesis reported in each mouse model.
| Spontaneous Mouse Model | Characteristics | Autoantibodies | Genetics & Pathogenesis | References |
|---|---|---|---|---|
| NOD Mice | - Lymphocytic infiltration of lacrimal, salivary glands and pancreas leading to sialadenitis, dry eye and xerostomia, insulitis and β-islet destruction | - anti-SSA/Ro, anti-SSB/La and anti-muscarinic receptor III | - Insulin-dependent diabetes loci 3 and 5 | 25–33 |
| NOD.B10.H2b Mice | - Lymphocytic infiltration of lacrimal, salivary glands | - dsDNA, ssDNA, and U1-snRNP68 in female mice | - MHC I–Ag7Idd1 susceptibility locus is replaced by the MHC I-Ab locus from C57BL/10 mice | 32,34,35 |
| C57BL/6.NODAec1Aec2 Mice | - Lymphocytic infiltration of salivary glands with decreased salivary flow rate - Dacryoadenitisin males only |
- ANAs - IgM against mM3R-transfected Flp-In CHO cell |
- Aec2 and Aec1 on chromosomes 1 and 3, respectively | 36 |
| NOD.IFN-γ−/− & NOD.IFN-γ R−/− Mice |
- No salivary function loss - Lymphocytic infiltration - Increase in acinar cells apoptosis, or abnormal salivary protein expression by 20 weeks of age |
- IFN-γ | 37 | |
| NOD.IL4−/− Mice | - Lymphocyte infiltration started at week 8 and was more severe at 20 weeks compared to NOD mice | - Absence of M3R autoantibodies | - IL-4 | 38 |
| NOD Igμ−/- Mice | - Salivary and lacrimal gland T cell infiltration at 8 weeks and normal salivary function | - Higher cysteine protease activity in acinar and ductal cells | 39 | |
| NZB/W F1 Mice | - Lymphocyte infiltration of lacrimal and salivary glands at week 16; more severe in lacrimal glands of old female mice | - Absence of autoantibodies, anti–SS–A and anti–SS–B | 40, 41 | |
| MRL/lpr Mice | - A complex of SLE, SS, and RA-like disease. - Lymphocyte infiltration of lacrimal and salivary glands - Submandibular gland is more affected than parotid and sublingual glands |
- Controversial reports of anti-SSA/Ro or anti SSB/La - Absence of anti–SS–A and anti–SS–B |
- IL-1β and TNF-α before and IL-6 after lesion appearance | 41, 42 |
| NFS/s1d Mice | - Spontaneous inflammatory change in the salivary and lacrimal glands in thymectomised mice 3 days after birth. - Females show more severe inflammation. |
- Anti-salivary duct autoantibodies - A 120-kDa autoantigen α-fodrin |
- Lymphocytic infiltration with CD3+ and CD4+ T cells with some CD8+ T cells and B220 B cells - Upregulated IL-1 beta, TNF-α, IL-2, IFN-γ, IL-6, IL-10, IL-12p40, and adhesion molecules, ICAM-1, LFA-1, CD44, Mel-14. |
45–47 |
| Aly/aly Mice | - Mononuclear cell infiltrate; mainly of CD4+ T cells infiltrate periductal areas of salivary and lacrimal glands, pancreas and lungs | - Mononuclear cell infiltrate; mainly of CD4+ T cells | 48 | |
| IQI/Jic Mice | - Lymphocyte infiltration in salivary and lacrimal glands starting at 4 weeks. - 80% of females at all ages showed sialadenitis that worsened after 6 months of age. Females show more severe symptoms compared to males. - At 8 weeks, B cells and CD4+ T cells infiltrate salivary and lacrimal glands, lungs, pancreas and kidneys. |
- MHC II+, CD11c+ and B7-2+ dendritic cells - CD4+ CD25+ Treg cells |
49–53 |
2.1. NOD mice
Non-obese diabetic (NOD) mouse is an inbred strain that was established by Makino et al., 38 years ago, from a cataract-prone sub-line of the outbred ICR mouse.25 NOD mice were originally used as an animal model for diabetes mellitus type 1, due to lymphocytic infiltration leading to insulitis and β-islet destruction. Around 70% of the female NOD mice and 20% of the males develop spontaneous diabetes by 13 weeks of age. This strain shares many similarities with diabetic patients, like weight loss, hyperglycemia, hypercholesterolemia, glycosuria, ketonuria, polyuria, polydipsia, and polyphagia. However, their use for Sjögren’s-like disease did not start until the late ‘90s. It is one of the most popular strains for studying SS due to several similarities it shares with SS patients, such as decreased glandular secretions and lymphocytic infiltration.26 Lymphocytic infiltration was evident in submandibular and lacrimal glands, starting at 12 weeks of age with a subsequent glandular dysfunction at 20 weeks.25 Successive studies have revealed that specific autoantibodies, like anti-SSA/Ro, anti-SSB/La and anti-muscarinic receptor III were elevated.27, 28, 29, 30, 31, 32 Genetic analysis of the involved insulin-dependent diabetes (idd) loci revealed that only idd3 and idd5 are linked to SS exocrinopathy in NOD mice, whereas, the rest are associated with diabetes type I only. Although the link between MHC-associated genes and SS-like disease is weak, several studies support that MHC-II genes are linked to defected central autoimmunity.33 Female NOD mice show submandibular glands sialoadenitis as early as 8 weeks of age. Lacrimal dysfunction and lymphocytic infiltration are evident in nearly 52% of female NOD mice (unpublished data). Dryness of the eyes is further complicated into thinning of the corneal epithelium in the mice with lacrimal involvement.123
2.2. NOD.B10.H2b mice
NOD.B10.H2b is a congenic line of the NOD strain where the MHC I–Ag7 Idd1 susceptibility locus is replaced by the MHC I-Ab locus from C57BL/10 mice: they are negative for NOD MHC class I and II antigens.34 These mice exhibit lymphocytic infiltration into the salivary and lacrimal glands the same way SS-like disease does in NOD mice32 but infiltration is less severe (unpublished data). Almost all the female mice showed submandibular infiltration by the age of 11 months in a varying degree of severity. Male mice showed less severe inflammation and only 9% were infiltration-free.34 They suffer from salivary gland dysfunction without the accompanying pancreas infiltration (insulitis) and the resulting severe diabetes32; however, our investigation did not reveal glandular dysfunction when the mice were followed up for 52 weeks (unpublished data). Serum analysis revealed the presence of anti-nuclear antibodies against: double-stranded DNA (dsDNA), single-stranded DNA (ssDNA), and U1-snRNP68 in female mice.35
2.3. C57BL/6.NODAec1Aec2
C57BL/6.NODAec1Aec2 mouse strain was established by Cha et al. in 2006119 and was further described by Nguyen et al.36 Successive studies have revealed that two genetic regions on chromosomes 1 and 3 termed Aec2 and Aec1, respectively, are sufficient to recapitulate SS-like disease in disease-free C57BL/6 mice.36 When this mouse strain was first established, it was verified for the suitability of using it as SS mouse model. The lymphocyte infiltration in the salivary gland was detected at 10 and 19 weeks in males and females, respectively. However, female mice showed more severe infiltration, and were larger at 22 weeks of age. Surprisingly, males exhibited a more severe form of dacryoadenitis while females showed none. Caspase-3 levels in the submandibular gland were elevated between 4 and 14 weeks of age, then decreased afterwards; however, the lacrimal glands showed an opposite trend. Serine protease level was detected at 10 weeks of age, and ANAs (Antinuclear antibodies) were evident as early as 5 weeks of age in males and 10 weeks in females. In addition, all mice examined were found to have positive IgM against mM3R-transfected Flp-In CHO cells in their sera. Upon examining salivary function, females and males lost 35–40% of salivary flow rates between 5-19 and 5–22 weeks of age, respectively.36
2.4. Other NOD derivative mice
A number of engineered NOD mice were established to verify the role/involvement of specific genes or proteins in the pathogenesis of SS.19 This list includes: NOD.IFN-γ−/−, NOD.IFN-γ R−/−, NOD.IL4−/−, NOD Igμ−/−.
Interestingly, NOD.IFN-γ−/− and NOD.IFN-γ R−/− mice showed no salivary function loss, lymphocytic infiltration, increase in acinar cells apoptosis, or abnormal salivary protein expression by 20 weeks of age. The previous findings emphasize on the pivotal role of IFN-γ in the pathogenesis of SS in NOD mice. Beyond 30 weeks of age, sparse leukocytes were found in the submandibular glands similar to the healthy control.37 Surprisingly, lymphocytic infiltration was apparent in lacrimal glands, particularly males, similar to that of NOD mice. Apoptosis signals in the submandibular and lacrimal glands using Caspase 3 and TUNEL assays, showed comparable level to the healthy control mice.
NOD.IL4−/− mouse model was created to study the role of IL-4 in the pathogenesis of SS. Upon examining the targeted tissue, several proinflammatory cytokines were found but not IL-4. Although the cytokine was not found at the time of examination, it was still important in the pathogenesis of SS. Lymphocytic inflammation was evident as early as 8 weeks, similar to NOD mice, but at 20 weeks the infiltration was more severe than that in NOD mice. Although the lymphocytic infiltration was severe, it did not lead to dry mouth. Assessment of apoptosis, measured by the level of positive acinar cells with TUNEL test, revealed a comparable level to NOD mice and higher than that of healthy mice. Serum analysis revealed the absence of M3R autoantibodies which might, partially, explain the absence of xerostomia despite the presence of lymphocytic inflammation. This mouse model provides a strong evidence that IL-4 does participate in the pathogenesis of SS regardless of its undetected level in the tissue.38
To study the role of B lymphocytes, Robinson established the NOD Igμ−/- mouse model that lacked functional B lymphocytes in their immune system.39 Although these mice exhibited salivary and lacrimal gland T cell infiltration at 8 weeks, they had normal salivary function. Older mice (>20 weeks) showed higher cysteine protease activity (apoptosis indicator in acinar and ductal cells) in comparison to their younger 8-week-old counterparts. This suggested the strong role of autoantibodies produced by B lymphocytes on the function of exocrine glands.39
2.5. NZB/W F1 (NZB/NZW F1) mice
The first spontaneous mouse model utilized in the study of SS was NZB/W F1.40 It was generated by crossing the first filial generation New Zealand black (NZB) with the New Zealand white (NZW). This model spontaneously develops SS and SLE disease characteristics. Both NZB and NZB/NZW F1 share similar disease characteristics while NZW does not. Both salivary and lacrimal glands showed lymphocytic infiltration by 16 weeks of age, but it was more intense in the lacrimal gland. Histological sections from both glands showed periductal and perivascular infiltration. Acinar cells showed a washed-out appearance while the connective tissue looked more edematous. However, cross sections of the gland showed well-preserved gland architecture. The severity of the mononuclear infiltration was influenced by the gland type, sex and age; where lacrimal glands of female older mice showed much more lesions compared to their counterparts. Although not all parotid glands showed lesions, they were found to be the most severely affected glands and the sublingual glands were the least affected. When the mononuclear infiltration was evident, not always, it did not exceed 1–2 small lesions.40 However, autoantibodies, anti–SS–A and anti–SS–B, were not detected in their serum.41
2.6. MRL/lpr (MRL/Mp-lpr) mice
MRL/lpr is a congenic strain that has obtained the lpr (lymphoproliferation) mutation from MRL/MpJ strain42. Mice with lpr mutation lack Fas which is a cell surface protein that transduces apoptosis. These mice develop immune complex disease with features of SLE, SS, and RA-like disease.41,42 Submandibular gland lesions were found at the age of two months.43 Over expression of IL-1β and TNF-α was evident before salivary gland lesions, whereas, IL-6 elevation was in accordance with the lesions.44 Hoffman et al., have conducted one of the earliest studies on the exocrine involvement of this strain. They have found that more mice showed infiltration in the submandibular gland than in the parotid or sublingual. Lacrimal glands involvement was present in almost all mice studied. No anti-SSA/Ro or anti SSB/La was detected in the sera of these mice, but later on, studies have found that some mice do develop these autoantibodies. The presence of autoantibodies was shown in MRL/lpr as a congenic mouse from the MRL strain. However, autoantibodies, anti–SS–A and anti–SS–B, were not detected in their serum.41
2.7. NFS/sld mice
NFS/s1d mouse model has an autosomal recessive mutation that arrests sublingual gland differentiation. Several cytokines, IL-1 beta, TNF-α, IL-2, IFN-γ, IL-6, IL-10, IL-12p40, and adhesion molecules, ICAM-1, LFA-1, CD44, Mel-14, were upregulated in this model.45 NFS/sld thymectomised mice develop spontaneous inflammatory changes in salivary and lacrimal glands 3 days after birth. No significant inflammation was found in other organs or in other non-thymectomised mice. Females were affected significantly higher and showed more lesions in their glands than males. The lymphocytic infiltration was more prominent in females and was composed mainly of CD3+ and CD4+ T cells with some CD8+ T cells and B220 B cells. Sera analysis revealed anti-salivary duct autoantibodies in mice with autoimmune lesions.46 A 120-kDa autoantigen α-fodrin was purified from the salivary glands of these mice, this autoantigen induces proliferation of T cells, in vitro. Neonatal immunization with this autoantigen prevented disease development.47
2.8. Aly/aly mice
Alymphoplaisa (aly/aly) mice has a spontaneous mutation that results in an absence of extrasplenic lymphoid tissues, including lymph nodes and Peyer’s patches,122 and show defects in both humoral and cellular immunity. Histopathological analysis revealed chronic inflammatory changes in exocrine organs, such as salivary glands, lacrimal glands, and pancreas of the homozygotes (aly/aly), but not the heterozygotes (aly/+). In these exocrine organs, mononuclear cells consisting mainly of CD4+ T cells, infiltrate periductal areas, and, in some cases, the cell infiltration extend to glandular lobules. The inflammatory changes in exocrine organs were transferred by a T cell-enriched fraction of spleen cells from homozygous animals. These results suggest that autoimmune mechanisms mediated by self-reactive T cells may be involved in the inflammatory lesions of various exocrine organs in the homozygous mice, although these mice show immunodeficiency. Inflammatory changes were observed in lungs of homozygotes. Since SS is characterized by diffuse lymphocyte infiltration in the periductal areas of lacrimal and salivary glands and is occasionally associated with pulmonary disease, aly/aly mice may serve as a unique spontaneous SS model.48
2.9. IQI/Jic mice
IQI/Jic is an inbred strain established from ICR mice.49 These mice produce antinucleolar autoantibodies in response to mercuric chloride exposure.50 Lymphocytic focal infiltration is evident in salivary and lacrimal glands of these mice. Females showed more involvement of the salivary glands than males; up to 80% of all females at all ages showed sialadenitis that worsened after 6 months of age. However, males showed slight lesions and the incidence increased with age.49 Infiltrating immune cells were detected at 4 weeks of age, involving MHC II+, CD11c+ and B7-2+ dendritic cells (DCs). At 8 weeks, infiltrating lymphocytes were seeded in submandibular glands of females and lacrimal glands of males. These lymphocytes were B cells and CD4+ T cells in similar ratios.51 In this strain, lymphocytic infiltration was found to involve multiple organs; lungs, pancreas and kidneys were infiltrated with CD4+ T-cells and B-cells at advanced ages, like SS patients.52 When these mice were subjected to neonatal thymectomy, severe lesions were found in the lacrimal glands suggesting a crucial rule of CD4+ CD25+ Treg cells.53
3. Transgenic mouse models
Table 3 summarizes the characteristics, autoantibodies, genetics, and pathogenesis reported in each transgenic mouse model.
Table 3.
Transgenic mouse models of Sjögren’s Syndrome. Summary of the characteristics, autoantibodies, genetics, and pathogenesis reported in each mouse model.
| Transgenic Mouse Model | Characteristics | Autoantibodies | Genetics & Pathogenesis | References |
|---|---|---|---|---|
| HTLV-1 Tax Transgenic Mice | - Lymphocytic infiltration of salivary and lacrimal glands. - Equal in male and female mice | - Disease severity corresponds to tax gene expression which increases with age | 20,54,55 | |
| IL-6 Transgenic Mice | - Rheumatoid, osteoporosis and psoriasis Larger spleen index and autoimmune-like lesions |
- Anti-mitochondrial antibodies | - Elevation of IL-6 | 20, 56-58 |
| IL-10 Transgenic Mice | - IL-10 - MHC II antigen |
59,60 | ||
| IL-12 Transgenic Mice | - Lymphocyte infiltration of salivary and lacrimal glands - Significantly low salivary flow rate, age-dependant in males, starting at 16 weeks but not in females (7–20 weeks) |
- ANAs - Insignificant high levels of SSA/Ro |
B220+ B cells and CD4+ T cells | 61–63 |
| IL-14α Transgenic Mice | - Lymphocyte infiltration of parotid gland and kidneys. - Lymphoma developed in aged mice similar to SS and SLE patients |
- Some mice had one or two autoantibodies elevated but the majority did not express any | - Higher IL-14 α transcripts - High IgM and IgG at 6 months - Significant IgA and IgG2a at 9 months |
20,63-68 |
| BAFF Transgenic Mice | - MZ-like B cells circulating in the blood, lymph nodes, and salivary glands. - Kidneys and salivary glands destruction due to lymphocytes infiltration |
- Excessive levels of autoantibodies | - High level of B cell activating factor | 69–72 |
| Id3 −/− Knockout Mice | - Severe lymphocyte infiltration in salivary and lacrimal glands at 2 months - Decreased salivary and tear flow rates in both males and female at 2–4 months - Occasional infiltration in lungs and kidneys at > 1 year |
- Autoantibodies at a significantly high frequency after one year of age | - T lymphocytes (CD4+ and CD8+) and B cells | 73–78 |
| TGF-β1 Knockout Mice | - Mice carrying a homozygous mutation for TGF-β1 die at around three weeks of age due to organ failure caused by Wasting Syndrome - Marked tissue infiltration necrosis of liver, heart, stomach, lung, pancreas, salivary glands and striated muscles at 1 week |
- Absence of TGF-β1 | 79–83 | |
| PI3K Knockout Mice | - Autoimmunity developed at 2 months and became profound at 4 months - Infiltration of salivary and lacrimal glands, liver, lungs and intestines |
- ANAs and Anti-SSA | - CD4+ T cells, some CD8+ T cells and B220+ B cells | 84–86 |
| TSP-1-Deficient Mice | - Mice were normal at birth but developed SS-like disease in lacrimal glands - Lymphocytes infiltration of lacrimal glands, apoptosis, abnormal tear formation, corneal deterioration, crusty eyes |
- Anti-SSA and anti-SSB autoantibodies | - CD4+ T lymphocytes secret IL-17 | 81, 87-90 |
| RbAp48 Transgenic Mice | - At 24 weeks of age exhibited autoimmune exocrinopathy similar to SS. - Lymphocytic infiltration in salivary and lacrimal glands at 30–50 weeks - Significant decrease in salivary and tear volumes at 30 weeks - Females are more affected at all ages |
- Anti-SSA/Ro, anti-SSB/La, and anti-α-fodrin (120-kD) autoantibodies | - Infiltrating CD4+ cells with some B220+, CD8+, and CD11+ cells - MHC II expression on exocrine epithelial cells |
19, 91-93 |
| ArKO Mice | - Spontaneously develop SS-like symptoms in both females and males - Salivary glands enlargement and massive lymphocytic infiltration |
- Anti-α-fodrin antibodies | - B220+ B cells | 94–96 |
| Opn Transgenic Mice | - Significant saliva loss by 16 weeks - Lymphocytic infiltration in 62% of submandibular glands, 50% in lacrimal glands, and 12.5% in both glands of female Opn Tg mice |
- OPN overexpression - Elevated IL-4, IL-6, IL-2 and TNF-α levels. |
97–99 | |
| CD25 KO (IL-2R⍺−/−) Mice | - Enlarged, red, inflamed lacrimal glands at 8 weeks - Atrophied glands at 16 weeks glands - Similar pathology in males and females - Glandular cell atrophy and fibrosis with lymphocyte infiltration; CD4+ and CD8+ cells, at all ages |
- Higher Th17 (TGF-β1, IL-17A, IL-23R, IL-21, CCL20, and Th1 (IFN-⍺, IL-2, IL-12, IL-12RB1, IL-18R, T-bet) cytokines | 100–103 |
3.1. HTLV-1 tax transgenic mice
Human T-cell leukemia virus 1 (HTLV-1) is a retrovirus involved in adult T-cell leukemia and in the pathogenesis of autoimmune diseases, such as; SS and RA.20,54 This model contains the HTLV-1 tax gene under the control of the viral long terminal repeat (LTR) which leads to a phenotype that involves exocrine glands.20,55 Diffuse and multifocal ductal epithelial cell proliferation is evident in these mice at an early age, which later intensifies leading to distortion of glandular architecture, particularly, of the submandibular and parotid glands.55 As the proliferation advances, lymphocytic infiltration starts to surround the enlarged epithelial masses, and later plasma cells appear.55 Lacrimal glands undergo epithelial cell proliferation that is not as severe as in salivary glands and is delayed. The severity of the glandular pathology corresponds to tax gene expression. Tax protein production increases with age and it is produced equally in males and females.55
3.2. IL-6 transgenic mice
Interleukin-6 (IL-6) is a cytokine that is originally known to be necessary for the maturation of B cells. Later, its multifunctionality was revealed to involve a critical role in hematopoiesis and immune response, particularly, the acute phase. Dysregulation of IL-6 leads to several autoimmune diseases, such as; rheumatoid, osteoporosis and psoriasis.56,57 The effect of IL-6 on the development of SS and other autoimmune diseases was studied by using transgenic hybrid mice for GVHD model with MHC class II disparity. These mice showed elevated IL-6. Systematic investigation of these mice showed a larger spleen index and autoimmune-like lesions that left the animal very weak. In addition, these mice showed elevated antimitochondrial antibodies. The previous findings strongly correlated with the elevated level of IL-6 and the progression of the autoimmune diseases.20,58
3.3. IL-10 transgenic mice
Interleukin-10 (IL-10) is known for the maturation and regulation of T and B cells, as well as enhancing MHC II antigen expression; therefore, an abnormal level of IL-10 might play a role in the pathogenesis of autoimmune diseases. Furthermore, IL-10 controls cytokine production by natural killer cells and immunoglobulins by B cells.59 IL-10 is generally considered the most important anti-inflammatory interleukin that prevents inflammation-mediated tissue damage.60 This mouse model was created by microinjection of IL-10 mouse cDNA in C57BL/6 fertilized eggs under the amylase promoter.120 This model showed epithelial apoptosis accompanied with glandular infiltration of Fas-ligand (FasL)+ CD4+ T cells, and less than 10% were CD8+. The glandular infiltration was evident as early in 8-week-old mice and increased in intensity as they aged. The glandular tissue stained positive for MHC class II I-AK. Clinically, the mice exhibited lower saliva and tear secretion than their control group at 8-week-old, and it continued to decline overtime. No differences were found between males and females.
3.4. IL-12 transgenic mice
IL-12 is a proinflammatory heterodimeric cytokine produced by Antigen presenting cells, B cells and phagocytic cells. It is responsible for the production of several cytokines, especially IFN-y.121 IL-12 acts as a growth factor for activated T and NK cells and is best known for inducting differentiation of CD4+ T lymphocytes from a Th0 to Th1 phenotype.20, 61, 62 This mouse model was created by Kimura et al., 2005 to investigate the effect of chronic exposure to IL-12 in murine thyroid glands. This strain was engineered to express IL-12 p70 under the transcriptional control of the thyroglobulin promoter.62 Stimulated salivary flow rate was measured in females and males, and was found statistically lower in IL-12 transgenic mice when compared to their wild type counterparts. However, this decrease was age-dependant in males; salivary flow rate was decreased at 16 weeks of age when compared to control mice, while females were not age dependant as they showed lower salivary flow rate at 7–20 weeks of age. Histological analysis of salivary and lacrimal glands reveled lymphocytic infiltration, composed mainly of B220+ B cells and CD4+ T cells. Strong correlation was found between the glandular hypofunction and lymphocytic infiltration in females. ANAs were found statistically higher in IL-12 transgenic mice when compared to control counterparts at 13, 32 and 36 weeks of age. However, when the level of SSA/Ro levels were investigated, higher values were found in the transgenic mice but not high enough to be significant at all measured time points.63
3.5. IL-14α transgenic mice
IL-14α is a cytokine that induces activated B cell proliferation, inhibits immunoglobulin secretion, and expands certain B cells subpopulations.64,65 Analysis of peripheral blood leukocytes IL-14α transcripts in primary and secondary SS patients were found higher than the age-, ethnic- and sex-matched controls.66 IL-14α transgenic mice were created to study the role of IL-14α, in vivo.67 Transgenic mice at different ages were examined and their sera were evaluated for immunoglobulins and autoantibodies production. By six months of age, these mice developed hypergammaglobulinemia including IgM and IgG. A significant increase in IgA and IgG2a levels was found in the serum by 9 months of age. Analysis of autoantibodies associated with SS and SLE-like IgG ANA, anti-dsDNA, anti-chromatin, anti-Ro, anti-La, anti-Sm, and anti-nRNP, showed some mice with one or two elevated autoantibodies but the majority of mice did not express any.63 Histological assessment of parotid glands and kidneys revealed lymphocytic infiltration and IgM deposition in the kidneys. Aged mice developed CD5+ B cell lymphoma, similar to what is found in some SS and SLE patients.20,68
3.6. BAFF transgenic mice
BAFF transgenic mice produce a very high level of B cell activating factor (BAFF). BAFF is involved in B-cell survival; however, excessive levels can lead to inability to respond to censoring death signals and escaping critical tolerance checkpoint.69,70 BAFF Tg mice exhibit enlarged marginal zone (MZ) B-cell compartment and show MZ-like B cells circulating in the blood, lymph nodes, and salivary glands. These mice are characterized by the presence of excessive levels of autoantibodies, leading to kidneys and salivary glands destruction, similar to SLE and SS patients, respectively.71 Previous features highlight a possible link between the active autoimmune cells and BAFF.71 BAFF Tg mouse tended to develop SS as they age. Older mice (>one year) exhibited larger submandibular glands, decreased saliva flow rates, as well as, submandibular gland destruction, due to severe lymphocytic infiltration.72 The disease severity was variable among these mice and was not affected by sex. No anti-SSA/Ro or anti/SSB/La autoantibodies were detected in their sera, regardless of disease severity. Cell population was composed of a larger proportion of B cells but it was variable in this strain72.
3.7. Id3 knockout (Id3−/−) mice
DNA binding inhibitors (1,2,3, and 4 (IDs)) are nuclear proteins, when present at high concentration, bind to basic Helix-loop-helix transcription factors (bHLH). bHLH is a family of proteins that control cell fate, differentiation and proliferation, forming ID-bHLH dimers, which lack the basic binding site, therefore, no interaction with DNA takes place.73,74 Id3 is important in the development of T lymphocytes75,76 and B lymphocytes.77 Id3 expression is high in proliferating cells but low in differentiating cells.77 Id3-null mice showed selective defects in humoral immunity because Id3 is necessary in BCR-mediated B lymphocytes proliferation.77 This phenotype was partially explained by the study of purified B cells, which showed that Id3 was required for BCR-mediated B-cell proliferation. In addition, another study showed that Id3 was required for optimal expression of IFN-γ.77 Id3-null mice recapitulated many of the primary SS symptoms that patients present.78 They secreted significantly less saliva and tears in both males and females, as early as 2–4 months of age. Both males and females were unable to keep their eyelids opened at around six months of age due to severe dryness. Histological assessment of salivary and lacrimal glands revealed lymphocytic infiltration at around two months of age which was more significant at six months. T lymphocytes (CD4+ and CD8+) and B cells were identified in the lymphocytic infiltrations. Serum analysis revealed the presence of autoantibodies at a significantly high frequency after one year of age but not before. The pancreas, kidneys, lungs, thyroid and liver did not display any gross abnormalities. However, histological assessment of some old animals (>one-year-old) showed occasional infiltration in lungs and kidneys.78
3.8. TGF-β1 knockout mice
Transforming Growth Factor-β1 (TGF-β1) a pleotropic cytokine secreted by T cells and is essential for immune homeostasis.79,80 It is responsible for innate and adaptive immune cell regulation and tolerance. In addition, it has a suppressive action on several immune cells, including T/B cells and macrophages. The immune system increases the release of TGF-β1 to protect and/or recover from autoimmune diseases.81 Mice carrying a homozygous mutation for TGF-β1 die at around 3 weeks after birth, due to organ failure caused by Wasting Syndrome. Upon organ examination, animals exhibited a marked tissue infiltration and necrosis.82 The infiltration involved many organs including; the liver, heart, stomach, lung, pancreas, salivary glands and striated muscles. Salivary glands showed slight to moderate multifocal infiltration in the periductal region.82 Inflammation was evident around one week after birth; however, it was variable among mice.83 These infiltrating cells were mainly lymphocytes and some plasm cells. As the infiltration proceeded, the acinar cells were the most affected; shrunk and atrophied.
3.9. PI3K knockout mice
Phosphoinositide 3-kinase (PI3K) is an enzyme activated by receptors for antigen, cytokines, costimulatory molecules, immunoglobulins, and chemoattractant. PI3K is responsible for immune cells proliferation and differentiation.84,85 In class 1A phosphoinositide-3-kinase deficient mice, autoimmunity developed around 2 months of age and became more profound at 4 months, in females and males equally.86 Large infiltration in the lacrimal glands was found in the periductal area with acinar cell destruction. Infiltration foci were composed mainly of CD4+ T, some CD8+ T and B220+ B cells. Other organs, such as; liver, lungs and intestines showed signs of infiltration with less penetration. Serum analysis revealed the presence of ANAs in almost two thirds of the mice. Anti-SSA titre was higher in phosphoinositide-3-kinase-deficient mice than controls and it tended to increase with age.86
3.10. TSP-1-deficient mice
Thrombospondin-1 (TSP-1) is largely responsible for the activation of the latent form of TGF-β1 extracellularly, in vivo.87 TGF-β1 is a bipolar cytokine which plays an important role in immunity development.88 Its overexpression is associated with fibrosis and exaggerated immune response; however, TGF-β1 is responsible for limiting innate and adaptive immune responses to reinstate immune homeostasis.81,88,89 Based on the previous information, the effect of TSP-1 on SS pathogenesis was investigated. When TSP-1-deficient mice were created, they appeared normal at birth, but later, they developed lacrimal glands SS-like disease. The lacrimal glands were invaded with lymphocytes that led to apoptosis, glandular deterioration and abnormal tear formation. These mice develop ocular surface abnormalities similar to SS patients, including corneal deterioration and crusty eyes. Serum analysis of TSP-1 null mice revealed the presence of anti-SSA and anti-SSB autoantibodies. CD4+ T lymphocytes were found to secret IL-17 which is strongly linked to chronic inflammation. Isolated antigen presenting cells were capable of activating T-lymphocytes to secrete IL-17, in vitro.90 Further studies are needed to investigate salivary glands changes in TSP-1 null mice.
3.11. RbAp48 transgenic mice
Retinoblastoma Associated Protein 48 (RbAp48), known as RBBP4, is a protein that interacts with multiple cellular proteins in cell growth and apoptosis.19,91 RbAp48 Transgenic mice were created by microinjection of gene fragments containing RbAp48 cDNA, regulated by salivary gland-specific promoter, into fertilized eggs from C57BL/6.92 Ovariectomized C57BL/6 mice showed enhanced salivary and lacrimal glands apoptosis via p53-mediated overexpression of RbAp48.92 Therefore, RbAp48 Transgenic mice are a useful strain to study the role of estrogen deficiency in autoimmunity. Mice examined at 24 weeks of age exhibited autoimmune exocrinopathy similar to SS patients; however, lymphocytic infiltration in salivary and lacrimal glands was more frequent at 30–50 weeks. Females displayed more severe form of the disease, at all ages. The majority of infiltrating cells were CD4+ with some B220+, CD8+, and CD11+. A significant decrease in saliva and tears volumes was evident, starting at 30 weeks of age. High levels of anti-SSA/Ro, anti-SSB/La, and anti-α-fodrin (120-kD) autoantibodies were detected in their sera.93 MHC II expression was evident on exocrine epithelial cells which enabled them to act as antigen presenting cells. Therefore, these cells might express exocrine antigens to CD4+ cells which could lead to the initiation of autoimmune reactions.93
3.12. Aromatase knockout (ArKO) mice
Aromatase is a cytochrome P450 responsible for estrogen biosynthesis.94 Estrogen receptors α- and β-knockout mouse models showed other abnormalities, like autoimmune nephritis but not SS; therefore, an animal model that lacks estrogen itself, not the receptors, was the model of choice.95 ArKO, mice lack the aromatase gene and consequently lack estrogen. Mice examined at 12–17 months old developed mild splenomegaly, bone marrow hypercellularity, and impaired renal function, due to chronic estrogen deficiency. Additionally, they spontaneously develop SS-like symptoms in both females and males. Gross examination of salivary glands showed enlargement and massive lymphocytic infiltration, mainly B220+ B cells, which led to destruction of acinar cells. Alpha-fodrin fragments were detected in the salivary gland, and anti-α-fodrin antibodies were found in their sera. These findings are important hallmarks of SS.96
3.13. Opn transgenic mice
Osteopontin (OPN) is a multifunctional protein involved in various physiological processes including immunity.124 T cells activation has been linked to high upregulation of OPN gene; therefore, various autoimmune diseases exhibited OPN overexpression including SS.97, 98, 99 In Opn Transgenic mice, OPN overexpression was achieved by the immunoglobulin enhancer/SV40 promoter which led to OPN overexpression in bone. These mice exhibited significant saliva loss by 16 weeks of age. Histological assessment of female Opn Transgenic mice revealed that 62% of submandibular gland, 50% of lacrimal gland, and 12.5% of both glands had lymphocytic infiltration.98 Immunohistochemical analysis of the submandibular glands showed higher staining for OPN in ductal cells and colocalization of OPN with lymphocytic infiltration. Serum analysis showed elevated IL-4, IL-6, IL-2, and TNF-α levels.
3.14. CD25 knockout (IL-2R⍺−/−) mice
CD25 is an interleukin 2 (IL-2) receptor alpha subunit (IL-2R⍺). IL-2 receptor is composed of IL-2R⍺ (CD122) and γ chains complexed with ⍺ subunit (CD25).100 Binding of IL-2 to its receptor leads to interruption of Th17 differentiation; therefore, in the absence of IL-2 receptor ⍺ subunit, favors a more differentiated Th17 production which leads to autoimmunity.100,101 This mouse model lacks the expression of IL-2R⍺ and exhibits multi-organ inflammatory condition in exocrine glands and gastrointestinal tract.102 Rahimy et al. ran an analytical study to investigate lacrimal glands of this mouse model.100 Excised lacrimal glands were enlarged, red, and inflamed at 8 weeks of age. Further histological analysis revealed acinar atrophy and fibrosis, and periductal fibrosis accompanied with severe lymphocytic infiltration. However, at 16 weeks, these glands became small and atrophied. Histological analysis revealed generalized disarrangement, acinar cells loss, and glandular atrophy.100 Cytometry analysis indicated the abundance of CD4+ and CD8+ cells, at all ages. Unlike other mouse models, CD25 KO displayed similar pathology in males and females. The main markers for lacrimal gland activity; peroxidase and EGF were evaluated. Peroxidase was never detected at any age, and EGF was very low. Young CD25 KO mice showed higher Th17 (TGF-β1, IL-17A, IL-23R, IL-21, CCL20, and Th1 (IFN-⍺, IL-2, IL-12, IL-12RB1, IL-18R, T-bet) cytokines.100,103 The cornea showed higher irregularities and the conjunctiva showed CD4+ and CD8+ infiltration similar to that of the lacrimal glands.103
4. Immunization mouse models
Table 4 summarizes the characteristics, autoantibodies, genetics, and pathogenesis reported of immunization mouse models.
Table 4.
Immunization mouse models of Sjögren’s Syndrome. Summary of the characteristics, autoantibodies, genetics, and pathogenesis reported in each mouse model.
| Immunization Mouse Model | Characteristics | Autoantibodies | Genetics & Pathogenesis | References |
|---|---|---|---|---|
| CA II Immunization | - Lymphocyte infiltration in salivary glands, pancreas and kidneys | 104–106 | ||
| M3R peptide immunization | - Low saliva secretion and significant lymphocytic infiltration in salivary glands | - Very high levels of M3R autoantibodies | - Mainly CD4+ with few B cells and IFN-γ and IL-17- secreting cells | 107,108 |
| Ro Immunization | - Lymphocytic infiltration in salivary glands - Low salivary flow rate |
- CD4+ (45%), CD8+ (18%) T lymphocytes and CD19+ (38%) B lymphocytes. | 109–112 |
4.1. CA II immunization
Carbonic Anhydrase (CA) is a basic zinc metalloenzyme with a wide distribution in the tissues where it regulates acid base status.104,105 Autoantibodies against CA were found in the sera of SS and SLE patients, and the titers correlated to the disease activity.105 Experimental sialadenitis was induced by immunizing PL/J (H-2u) mice with human CAII, intradermally. Immunized mice showed significant infiltration in salivary glands compared to untreated mice. The infiltration was observed around intercalated ducts and intralobular, causing acinar cell atrophy. Some mice showed lymphocytic infiltration in the pancreas and the kidneys. Mice bearing H-2s and H-2u were found susceptible to CAII immunization.106
4.2. M3R peptide immunization
M3 muscarinic acetylcholine Receptor (M3R) is expressed in exocrine glands, including salivary and lacrimal glands.107 It delivers the neural command from the parasympathetic system to initiate saliva and tears secretions. Several studies reported that 40% of SS patients has M3R reactive T cells in their blood, and 9–100% of these patients tested positive for M3R autoantibodies.107 Iizuka et al. have established M3R mouse model to study the effect of this receptor on the development of sialadenitis in the presence of autoantibodies against it. They injected fragments of murine M3R into M3R−/−, then the splenocytes were inoculated and injected into Rag1−/− mice (M3R−/−/Rag1−/−). Upon examination of M3R−/−/Rag1−/−, very high levels of M3R autoantibodies were found in the serum and saliva secretion was very low.108 Histological assessment revealed significant lymphocytic infiltration in the salivary glands, mainly CD4+ with fewer B cells, and IFN-γ and IL-17- secreting cells. In addition, few apoptotic cells were found in the salivary glands. The previous data strongly suggests that blocking M3R by autoantibodies is an important event in SS development.
4.3. Ro immunization
Autoantibodies against ribonucleoproteins SSA/Ro (Anti-SSA/Ro) and SSB/La (anti-SSB/La) existed in >75% of SS patients when measured via a sensitive technique.109 Their levels serve as a diagnostic marker for SS and other autoimmune diseases.110,111 Scofield et al. have tested the ability of short peptides from the 60-kDa Ro (or SSA) Ag immunization to induce SS-like disease in BALB/c mice. Immunized mice developed an immune reaction and produced antibodies against both Ro/La antigens, similar to SS patients. Upon histological examination, lymphocytic infiltration was found in the salivary glands, mainly, CD4+ (45%), CD8+ (18%) T lymphocytes and CD19+ (38%) B lymphocytes. Salivary flow was lower in the immunized mice not in the control.112
5. Infection mouse model
Table 5 summarizes the characteristics, autoantibodies, genetics, and pathogenesis reported in each infection mouse model.
Table 5.
Infection mouse models of Sjögren’s Syndrome. Summary of the characteristics, autoantibodies, genetics, and pathogenesis reported in each mouse model.
| Infection Mouse Models | Characteristics | Autoantibodies | Genetics & Pathogenesis | References |
|---|---|---|---|---|
| Cytomegalovirus C57B1/6 [B6]-+/+ Mice | - Extensive inflammatory cell infiltration detected in salivary glands at 28 days post-infection but not at 10 days | - Did not show high level of anti-Ro (SS–B), anti-La (SS-A) and rheumatoid factor | - Inflammatory response leading to cell death followed by regeneration | 14, 20, 113-118 |
| Cytomegalovirus Fas-deficient B6-lpr/lpr Mice | - Extensive inflammatory cell infiltration detected in salivary glands at 28 days post-infection but not at 10 days - Only salivary glands showed inflammation despite the absence of mCMV in the gland after 100 days |
- High level of anti-Ro (SS–B), anti-La (SS-A) and rheumatoid factor (RF) 100 days post-infection | - Inflammatory response leading to cell death followed by regeneration | 14, 20, 113-118 |
| Cytomegalovirus TNFRI-deficient B6-tnfrl−/− Mice | - Extensive inflammatory cell infiltration detected in salivary glands at 28 days post-infection but not at 10 days | - Inflammatory response leading to cell death followed by regeneration | 14, 20, 113-118 | |
| Cytomegalovirus B6-tnfr1−/−-lpr/lpr Mice | - Extensive inflammatory cell infiltration detected in salivary glands at 28 days post-infection but not at 10 days - Only salivary glands showed inflammation despite the absence of mCMV in the gland after 100 days |
- High levels of anti-Ro (SS–B), anti-La (SS-A) and rheumatoid factor (RF) 100 days post-infection | - Inflammatory response leading to cell death followed by regeneration | 14, 20, 113-118 |
5.1. Murine cytomegalovirus
Environmental factors have been documented as aggravating factors for autoimmune diseases in genetically-predisposed patients.14,20 Several viruses were found to be involved in the pathogenesis of SS, including Epstein-Bar (EBV), hepatitis C and cytomegalo virus (CMV).113 Despite the usefulness of murine models for studying the role of CMV in the etiopathogenesis of SS, the virus targets different cell types in humans and mice. Human CMV (HCMV) usually targets the ductal cells, while the murine CMV (mCMV) prefers the acinar cells, and it seems to induce an inflammatory response leading to cell death followed by regeneration.114, 115, 116, 117 Four mouse strains: C57B1/6 [B6]-+/+, Fas-deficient B6-lpr/lpr, TNFRI-deficient B6-tnfr1−/−, and B6-tnfr1−/−-lpr/lpr mice were found useful as they recapitulated specific phenotypes of SS-like disease when transfected with mCMV. At 28 days post-infection, extensive inflammatory cell infiltration was detected in the salivary glands of C57BL/6 [B6]-+/+, B6-tnfr1−/− and B6-lpr/lpr. However, at 10 days post infection, no inflammation was observed in C57BL/6 [B6]-+/+ and B6-tnfr1−/− which was the time at which the infectious mCMV was no longer detectable. On the other hand, in B6-lpr/lpr, only salivary glands showed inflammation despite the absence of mCMV in the gland after 100 days. B6-lpr/lpr infected mice showed high level of anti-Ro (SS–B), anti-La (SS-A) and rheumatoid factor (RF), 100 days post-infection while C57BL/6 [B6]-+/+ did not.118
6. Conclusion
After reviewing all the available data regarding the murine models for SS, we can conclude that NOD strain is the model that recapitulates the disease characteristics the best, and it is the most suitable for drug testing. NOD mice exhibit heterogenous clinical and laboratory features, comparable to that of SS patients. However, this should not lead us to abandon the other mouse models or neglect the important data that was generated. On the contrary, these data, often, supported and complemented to results obtained from NOD mice.
References
- 1.Bolstad A.I., Jonsson R. Genetic aspects of Sjogren’s syndrome. Arthritis Res. 2002;4(6):353–359. doi: 10.1186/ar599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fox R.I. Primary Sjogren syndrome: clinical and immunopathologic features. Semin Arthritis Rheum. 1984;14(2):77–105. doi: 10.1016/0049-0172(84)90001-5. [DOI] [PubMed] [Google Scholar]
- 3.Fox R.I. Sjogren’s syndrome. Lancet. 2005;366(9482):321–331. doi: 10.1016/S0140-6736(05)66990-5. [DOI] [PubMed] [Google Scholar]
- 4.Trontzas P.I., Andrianakos A.A. Sjogren’s syndrome: a population based study of prevalence in Greece. The ESORDIG study. Ann Rheum Dis. 2005;64(8):1240–1241. doi: 10.1136/ard.2004.031021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kabasakal Y. The prevalence of Sjogren’s syndrome in adult women. Scand J Rheumatol. 2006;35(5):379–383. doi: 10.1080/03009740600759704. [DOI] [PubMed] [Google Scholar]
- 6.Birlik M. Prevalence of primary Sjogren’s syndrome in Turkey: a population-based epidemiological study. Int J Clin Pract. 2009;63(6):954–961. doi: 10.1111/j.1742-1241.2008.01749.x. [DOI] [PubMed] [Google Scholar]
- 7.Goransson L.G. The point prevalence of clinically relevant primary Sjogren’s syndrome in two Norwegian counties. Scand J Rheumatol. 2011;40(3):221–224. doi: 10.3109/03009742.2010.536164. [DOI] [PubMed] [Google Scholar]
- 8.Maldini C. Epidemiology of primary Sjogren’s syndrome in a French multiracial/multiethnic area. Arthritis Care Res. 2014;66(3):454–463. doi: 10.1002/acr.22115. [DOI] [PubMed] [Google Scholar]
- 9.Bolstad A.I., Skarstein K. Epidemiology of Sjogren’s syndrome-from an oral perspective. Curr Oral Health Rep. 2016;3(4):328–336. doi: 10.1007/s40496-016-0112-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Generali E. Cutaneous and mucosal manifestations of Sjogren’s syndrome. Clin Rev Allergy Immunol. 2017;53(3):357–370. doi: 10.1007/s12016-017-8639-y. [DOI] [PubMed] [Google Scholar]
- 11.Liang M. Cardiac arrhythmias as the initial manifestation of adult primary Sjogren’s syndrome: a case report and literature review. Int J Rheum Dis. 2015;18(7):800–806. doi: 10.1111/1756-185X.12616. [DOI] [PubMed] [Google Scholar]
- 12.Kassan S.S., Moutsopoulos H.M. Clinical manifestations and early diagnosis of Sjogren syndrome. Arch Intern Med. 2004;164(12):1275–1284. doi: 10.1001/archinte.164.12.1275. [DOI] [PubMed] [Google Scholar]
- 13.Delaleu N. Sjogren’s syndrome: studying the disease in mice. Arthritis Res Ther. 2011;13(3):217. doi: 10.1186/ar3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wahren-Herlenius M., Dörner T. Immunopathogenic mechanisms of systemic autoimmune disease. Lancet. 2013;382(9894):819–831. doi: 10.1016/S0140-6736(13)60954-X. [DOI] [PubMed] [Google Scholar]
- 15.Lessard C.J. 2011. Genetics, Genomics, and Proteomics of Sjögren’s Syndrome; pp. 11–31. [Google Scholar]
- 16.Anaya J.M., Delgado-Vega A.M., Castiblanco J. Genetic basis of Sjogren’s syndrome. How strong is the evidence? Clin Dev Immunol. 2006;13(2-4):209–222. doi: 10.1080/17402520600876911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brito-Zerón P., Baldini C., Bootsma H. Sjögren syndrome. Nat Rev Dis Primers. 2016;7(2):16047. doi: 10.1038/nrdp.2016.47. [DOI] [PubMed] [Google Scholar]
- 18.Abbott A. Laboratory animals: the Renaissance rat. Nature. 2004;428(6982):464–466. doi: 10.1038/428464a. [DOI] [PubMed] [Google Scholar]
- 19.Park Y.S., Gauna A.E., Cha S. Mouse models of primary Sjogren’s syndrome. Curr Pharmaceut Des. 2015;21(18):2350–2364. doi: 10.2174/1381612821666150316120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lavoie T.N., Lee B.H., Nguyen C.Q. Current concepts: mouse models of Sjogren’s syndrome. J Biomed Biotechnol. 2011;2011 doi: 10.1155/2011/549107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soyfoo M.S., Steinfeld S., Delporte C. Usefulness of mouse models to study the pathogenesis of Sjogren’s syndrome. Oral Dis. 2007;13(4):366–375. doi: 10.1111/j.1601-0825.2007.01376.x. [DOI] [PubMed] [Google Scholar]
- 22.Nezos A., Mavragani C.P. Contribution of genetic factors to Sjogren’s syndrome and Sjogren’s syndrome related lymphomagenesis. J Immunol Res. 2015;2015 doi: 10.1155/2015/754825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bolstad A.I. HLA markers and clinical characteristics in Caucasians with primary Sjogren’s syndrome. J Rheumatol. 2001;28(7):1554–1562. [PubMed] [Google Scholar]
- 24.Perez P. Gene expression and chromosomal location for susceptibility to Sjogren’s syndrome. J Autoimmun. 2009;33(2):99–108. doi: 10.1016/j.jaut.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 25.Makino S. Breeding of a non-obese, diabetic strain of mice. Jikken Dobutsu. 1980;29(1):1–13. doi: 10.1538/expanim1978.29.1_1. [DOI] [PubMed] [Google Scholar]
- 26.Lee B.H., Tudares M.A., Nguyen C.Q. Sjogren’s syndrome: an old tale with a new twist. Arch Immunol Ther Exp. 2009;57(1):57–66. doi: 10.1007/s00005-009-0002-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miyagawa J. Ultrastructural and immunocytochemical aspects of lymphocytic submandibulitis in the non-obese diabetic (NOD) mouse. Virchows Arch B Cell Pathol Incl Mol Pathol. 1986;51(3):215–225. doi: 10.1007/BF02899031. [DOI] [PubMed] [Google Scholar]
- 28.Moore P.A. Histologic examination of the NOD-mouse lacrimal glands, a potential model for idiopathic autoimmune dacryoadenitis in Sjogren’s syndrome. Lab Anim Sci. 1996;46(1):125–128. [PubMed] [Google Scholar]
- 29.Hu Y. Functional changes in salivary glands of autoimmune disease-prone NOD mice. Am J Physiol. 1992;263(4 Pt 1):E607–E614. doi: 10.1152/ajpendo.1992.263.4.E607. [DOI] [PubMed] [Google Scholar]
- 30.Humphreys-Beher M.G. Animal models for autoimmune disease-associated xerostomia and xerophthalmia. Adv Dent Res. 1996;10(1):73–75. doi: 10.1177/08959374960100011501. [DOI] [PubMed] [Google Scholar]
- 31.Humphreys-Beher M.G. Characterization of antinuclear autoantibodies present in the serum from nonobese diabetic (NOD) mice. Clin Immunol Immunopathol. 1993;68(3):350–356. doi: 10.1006/clin.1993.1137. [DOI] [PubMed] [Google Scholar]
- 32.Robinson C.P. A novel NOD-derived murine model of primary Sjogren’s syndrome. Arthritis Rheum. 1998;41(1):150–156. doi: 10.1002/1529-0131(199801)41:1<150::AID-ART18>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 33.Aoki C.A. NOD mice and autoimmunity. Autoimmun Rev. 2005;4(6):373–379. doi: 10.1016/j.autrev.2005.02.002. [DOI] [PubMed] [Google Scholar]
- 34.Wicker L.S. Autoimmune syndromes in major histocompatibility complex (MHC) congenic strains of nonobese diabetic (NOD) mice. The NOD MHC is dominant for insulitis and cyclophosphamide-induced diabetes. J Exp Med. 1992;176(1):67–77. doi: 10.1084/jem.176.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kiripolsky J. Systemic manifestations of primary Sjogren’s syndrome in the NOD.B10Sn-H2(b)/J mouse model. Clin Immunol. 2017;183:225–232. doi: 10.1016/j.clim.2017.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen C. Sjogren’s syndrome-like disease of C57BL/6.NOD-Aec1 Aec2 mice: gender differences in keratoconjunctivitis sicca defined by a cross-over in the chromosome 3 Aec1 locus. Scand J Immunol. 2006;64(3):295–307. doi: 10.1111/j.1365-3083.2006.01828.x. [DOI] [PubMed] [Google Scholar]
- 37.Cha S. A dual role for interferon-gamma in the pathogenesis of Sjogren’s syndrome-like autoimmune exocrinopathy in the nonobese diabetic mouse. Scand J Immunol. 2004;60(6):552–565. doi: 10.1111/j.0300-9475.2004.01508.x. [DOI] [PubMed] [Google Scholar]
- 38.Brayer J.B. IL-4-dependent effector phase in autoimmune exocrinopathy as defined by the NOD.IL-4-gene knockout mouse model of Sjogren’s syndrome. Scand J Immunol. 2001;54(1-2):133–140. doi: 10.1046/j.1365-3083.2001.00958.x. [DOI] [PubMed] [Google Scholar]
- 39.Robinson C.P. Transfer of human serum IgG to nonobese diabetic Igmu null mice reveals a role for autoantibodies in the loss of secretory function of exocrine tissues in Sjogren’s syndrome. Proc Natl Acad Sci U S A. 1998;95(13):7538–7543. doi: 10.1073/pnas.95.13.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kessler H.S. A laboratory model for Sjogren’s syndrome. Am J Pathol. 1968;52(3):671–685. [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffman R.W. Sjogren’s syndrome in MRL/l and MRL/n mice. Arthritis Rheum. 1984;27(2):157–165. doi: 10.1002/art.1780270206. [DOI] [PubMed] [Google Scholar]
- 42.Watanabe-Fukunaga R. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356(6367):314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 43.Jonsson R. Sialadenitis in the MRL-l mouse: morphological and immunohistochemical characterization of resident and infiltrating cells. Immunology. 1987;60(4):611–616. [PMC free article] [PubMed] [Google Scholar]
- 44.Hayashi Y., Haneji N., Hamano H. Pathogenesis of Sjogren’s syndrome-like autoimmune lesions in MRL/lpr mice. Pathol Int. 1994;44(8):559–568. doi: 10.1111/j.1440-1827.1994.tb01716.x. [DOI] [PubMed] [Google Scholar]
- 45.Hayashi Y. Effector mechanism of experimental autoimmune sialadenitis in the mouse model for primary Sjogren’s syndrome. Cell Immunol. 1996;171(2):217–225. doi: 10.1006/cimm.1996.0196. [DOI] [PubMed] [Google Scholar]
- 46.Haneji N. A new animal model for primary Sjogren’s syndrome in NFS/sld mutant mice. J Immunol. 1994;153(6):2769–2777. [PubMed] [Google Scholar]
- 47.Haneji N. Identification of alpha-fodrin as a candidate autoantigen in primary Sjogren’s syndrome. Science. 1997;276(5312):604–607. doi: 10.1126/science.276.5312.604. [DOI] [PubMed] [Google Scholar]
- 48.Tsubata R. Autoimmune disease of exocrine organs in immunodeficient alymphoplasia mice: a spontaneous model for Sjogren’s syndrome. Eur J Immunol. 1996;26(11):2742–2748. doi: 10.1002/eji.1830261129. [DOI] [PubMed] [Google Scholar]
- 49.Saegusa J., Kubota H. Sialadenitis in IQI/Jic mice: a new animal model of Sjogren’s syndrome. J Vet Med Sci. 1997;59(10):897–903. doi: 10.1292/jvms.59.897. [DOI] [PubMed] [Google Scholar]
- 50.Saegusa J., Kiuchi Y., Itoh T. Antinucleolar autoantibody induced in mice by mercuric chloride--strain difference in susceptibility. Jikken Dobutsu. 1990;39(4):597–599. doi: 10.1538/expanim1978.39.4_597. [DOI] [PubMed] [Google Scholar]
- 51.Konno A. Presence of B7-2+ dendritic cells and expression of Th1 cytokines in the early development of sialodacryoadenitis in the IqI/Jic mouse model of primary Sjorgren’s syndrome. Autoimmunity. 2003;36(4):247–254. doi: 10.1080/0891693031000141077. [DOI] [PubMed] [Google Scholar]
- 52.Takada K. Spontaneous development of multiple glandular and extraglandular lesions in aged IQI/Jic mice: a model for primary Sjogren’s syndrome. Rheumatology. 2004;43(7):858–862. doi: 10.1093/rheumatology/keh209. [DOI] [PubMed] [Google Scholar]
- 53.Takada K., Takiguchi M., Inaba M. Different effects on the inflammatory lesions in the lacrimal and salivary glands after neonatal thymectomy in IQI/Jic mice, a model for Sjogren’s syndrome. J Vet Med Sci. 2005;67(9):955–957. doi: 10.1292/jvms.67.955. [DOI] [PubMed] [Google Scholar]
- 54.Zucker-Franklin D. Non-HIV retroviral associations with rheumatic disease. Curr Rheumatol Rep. 2000;2(2):156–162. doi: 10.1007/s11926-000-0056-0. [DOI] [PubMed] [Google Scholar]
- 55.Green J.E. Exocrinopathy resembling Sjogren’s syndrome in HTLV-1 tax transgenic mice. Nature. 1989;341(6237):72–74. doi: 10.1038/341072a0. [DOI] [PubMed] [Google Scholar]
- 56.Ishihara K., Hirano T. IL-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine Growth Factor Rev. 2002;13(4-5):357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 57.Park S.J. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 58.Kimura T. Induction of autoimmune disease by graft-versus-host reaction across MHC class II difference: modification of the lesions in IL-6 transgenic mice. Clin Exp Immunol. 1994;95(3):525–529. doi: 10.1111/j.1365-2249.1994.tb07030.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Spits H., de Waal Malefyt R. Functional characterization of human IL-10. Int Arch Allergy Immunol. 1992;99(1):8–15. doi: 10.1159/000236329. [DOI] [PubMed] [Google Scholar]
- 60.Mingomataj E.C., Bakiri A.H. Regulator versus effector paradigm: interleukin-10 as indicator of the Switching response. Clin Rev Allergy Immunol. 2016;50(1):97–113. doi: 10.1007/s12016-015-8514-7. [DOI] [PubMed] [Google Scholar]
- 61.Trinchieri G. Interleukin-12: a cytokine produced by antigen-presenting cells with immunoregulatory functions in the generation of T-helper cells type 1 and cytotoxic lymphocytes. Blood. 1994;84(12):4008–4027. [PubMed] [Google Scholar]
- 62.Kimura H. Interleukin (IL)-12-driven primary hypothyroidism: the contrasting roles of two Th1 cytokines (IL-12 and interferon-gamma) Endocrinology. 2005;146(8):3642–3651. doi: 10.1210/en.2005-0275. [DOI] [PubMed] [Google Scholar]
- 63.Vosters J.L. Interleukin-12 induces salivary gland dysfunction in transgenic mice, providing a new model of Sjogren’s syndrome. Arthritis Rheum. 2009;60(12):3633–3641. doi: 10.1002/art.24980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ambrus J.L., Jr., Fauci A.S. Human B lymphoma cell line producing B cell growth factor. J Clin Invest. 1985;75(2):732–739. doi: 10.1172/JCI111754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Ambrus J.L., Jr. Identification of a cDNA for a human high-molecular-weight B-cell growth factor. Proc Natl Acad Sci U S A. 1993;90(13):6330–6334. doi: 10.1073/pnas.90.13.6330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shen L. IL-14 alpha, the nexus for primary Sjogren’s disease in mice and humans. Clin Immunol. 2009;130(3):304–312. doi: 10.1016/j.clim.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 67.Alt E. Fibroblasts share mesenchymal phenotypes with stem cells, but lack their differentiation and colony-forming potential. Biol Cell. 2011;103(4):197–208. doi: 10.1042/BC20100117. [DOI] [PubMed] [Google Scholar]
- 68.Shen L. Development of autoimmunity in IL-14 alpha-transgenic mice. J Immunol. 2006;177(8):5676–5686. doi: 10.4049/jimmunol.177.8.5676. [DOI] [PubMed] [Google Scholar]
- 69.Ware C.F. Decoy receptors thwart B cells. Nature. 2000;404(6781):949–950. doi: 10.1038/35010263. [DOI] [PubMed] [Google Scholar]
- 70.Mackay F., Schneider P. Cracking the BAFF code. Nat Rev Immunol. 2009;9(7):491–502. doi: 10.1038/nri2572. [DOI] [PubMed] [Google Scholar]
- 71.Fletcher C.A. Development of nephritis but not sialadenitis in autoimmune-prone BAFF transgenic mice lacking marginal zone B cells. Eur J Immunol. 2006;36(9):2504–2514. doi: 10.1002/eji.200636270. [DOI] [PubMed] [Google Scholar]
- 72.Groom J. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109(1):59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Norton J.D. ID helix-loop-helix proteins in cell growth, differentiation and tumorigenesis. J Cell Sci. 2000;113(Pt 22):3897–3905. doi: 10.1242/jcs.113.22.3897. [DOI] [PubMed] [Google Scholar]
- 74.Lasorella A., Benezra R., Iavarone A. The ID proteins: master regulators of cancer stem cells and tumour aggressiveness. Nat Rev Canc. 2014;14(2):77–91. doi: 10.1038/nrc3638. [DOI] [PubMed] [Google Scholar]
- 75.Rivera R.R. Thymocyte selection is regulated by the helix-loop-helix inhibitor protein, Id3. Immunity. 2000;12(1):17–26. doi: 10.1016/s1074-7613(00)80155-7. [DOI] [PubMed] [Google Scholar]
- 76.Bain G. Regulation of the helix-loop-helix proteins, E2A and Id3, by the Ras-ERK MAPK cascade. Nat Immunol. 2001;2(2):165–171. doi: 10.1038/84273. [DOI] [PubMed] [Google Scholar]
- 77.Pan L. Impaired immune responses and B-cell proliferation in mice lacking the Id3 gene. Mol Cell Biol. 1999;19(9):5969–5980. doi: 10.1128/mcb.19.9.5969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li H., Dai M., Zhuang Y. A T cell intrinsic role of Id3 in a mouse model for primary Sjogren’s syndrome. Immunity. 2004;21(4):551–560. doi: 10.1016/j.immuni.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 79.Kelly A. Regulation of innate and adaptive immunity by TGFbeta. Adv Immunol. 2017;134:137–233. doi: 10.1016/bs.ai.2017.01.001. [DOI] [PubMed] [Google Scholar]
- 80.Sanjabi S., Oh S.A., Li M.O. Regulation of the immune response by TGF-beta: from conception to autoimmunity and infection. Cold Spring Harb Perspect Biol. 2017;9(6) doi: 10.1101/cshperspect.a022236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Prud’homme G.J., Piccirillo C.A. The inhibitory effects of transforming growth factor-beta-1 (TGF-beta1) in autoimmune diseases. J Autoimmun. 2000;14(1):23–42. doi: 10.1006/jaut.1999.0339. [DOI] [PubMed] [Google Scholar]
- 82.Shull M.M. Targeted disruption of the mouse transforming growth factor-beta 1 gene results in multifocal inflammatory disease. Nature. 1992;359(6397):693–699. doi: 10.1038/359693a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.McCartney-Francis N.L. Autoimmune Sjogren’s-like lesions in salivary glands of TGF-beta1-deficient mice are inhibited by adhesion-blocking peptides. J Immunol. 1996;157(3):1306–1312. [PubMed] [Google Scholar]
- 84.Fruman D.A., Cantley L.C. Phosphoinositide 3-kinase in immunological systems. Semin Immunol. 2002;14(1):7–18. doi: 10.1006/smim.2001.0337. [DOI] [PubMed] [Google Scholar]
- 85.Deane J.A. Enhanced T cell proliferation in mice lacking the p85beta subunit of phosphoinositide 3-kinase. J Immunol. 2004;172(11):6615–6625. doi: 10.4049/jimmunol.172.11.6615. [DOI] [PubMed] [Google Scholar]
- 86.Oak J.S. Sjogren’s syndrome-like disease in mice with T cells lacking class 1A phosphoinositide-3-kinase. Proc Natl Acad Sci U S A. 2006;103(45):16882–16887. doi: 10.1073/pnas.0607984103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Crawford S.E. Thrombospondin-1 is a major activator of TGF-beta1 in vivo. Cell. 1998;93(7):1159–1170. doi: 10.1016/s0092-8674(00)81460-9. [DOI] [PubMed] [Google Scholar]
- 88.Roescher N., Tak P.P., Illei G.G. Cytokines in Sjogren’s syndrome. Oral Dis. 2009;15(8):519–526. doi: 10.1111/j.1601-0825.2009.01582.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Wahl S.M. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19(1):55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 90.Turpie B. Sjogren’s syndrome-like ocular surface disease in thrombospondin-1 deficient mice. Am J Pathol. 2009;175(3):1136–1147. doi: 10.2353/ajpath.2009.081058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Qian Y.W., Lee E.Y. Dual retinoblastoma-binding proteins with properties related to a negative regulator of ras in yeast. J Biol Chem. 1995;270(43):25507–25513. doi: 10.1074/jbc.270.43.25507. [DOI] [PubMed] [Google Scholar]
- 92.Ishimaru N. Novel role for RbAp48 in tissue-specific, estrogen deficiency-dependent apoptosis in the exocrine glands. Mol Cell Biol. 2006;26(8):2924–2935. doi: 10.1128/MCB.26.8.2924-2935.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Ishimaru N. Expression of the retinoblastoma protein RbAp48 in exocrine glands leads to Sjogren’s syndrome-like autoimmune exocrinopathy. J Exp Med. 2008;205(12):2915–2927. doi: 10.1084/jem.20080174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Simpson E. Regulation of expression of the genes encoding steroidogenic enzymes in the ovary. J Steroid Biochem Mol Biol. 1992;41(3-8):409–413. doi: 10.1016/0960-0760(92)90366-q. [DOI] [PubMed] [Google Scholar]
- 95.Shim G.J. Disruption of the estrogen receptor beta gene in mice causes myeloproliferative disease resembling chronic myeloid leukemia with lymphoid blast crisis. Proc Natl Acad Sci U S A. 2003;100(11):6694–6699. doi: 10.1073/pnas.0731830100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Shim G.J. Aromatase-deficient mice spontaneously develop a lymphoproliferative autoimmune disease resembling Sjogren’s syndrome. Proc Natl Acad Sci U S A. 2004;101(34):12628–12633. doi: 10.1073/pnas.0405099101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rittling S.R., Singh R. Osteopontin in immune-mediated diseases. J Dent Res. 2015;94(12):1638–1645. doi: 10.1177/0022034515605270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Husain-Krautter S. The osteopontin transgenic mouse is a new model for Sjogren’s syndrome. Clin Immunol. 2015;157(1):30–42. doi: 10.1016/j.clim.2014.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sun W. [The function of osteopontin and other cytokines in the diagnosis of Sjoigren’s syndrome] Shang Hai Kou Qiang Yi Xue. 2013;22(3):322–325. [PubMed] [Google Scholar]
- 100.Rahimy E. Spontaneous autoimmune dacryoadenitis in aged CD25KO mice. Am J Pathol. 2010;177(2):744–753. doi: 10.2353/ajpath.2010.091116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Laurence A. Interleukin-2 signaling via STAT5 constrain T helper 17 cell generation. Immunity. 2007;26(3):371–381. doi: 10.1016/j.immuni.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 102.Pelegrino F.S. Deletion of interferon-gamma delays onset and severity of dacryoadenitis in CD25KO mice. Arthritis Res Ther. 2012;14(6):R234. doi: 10.1186/ar4077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.De Paiva C.S. Age-related T-cell cytokine profile parallels corneal disease severity in Sjogren’s syndrome-like keratoconjunctivitis sicca in CD25KO mice. Rheumatology. 2010;49(2):246–258. doi: 10.1093/rheumatology/kep357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Deutsch H.F. Carbonic anhydrases. Int J Biochem. 1987;19(2):101–113. doi: 10.1016/0020-711x(87)90320-x. [DOI] [PubMed] [Google Scholar]
- 105.Inagaki Y. A novel autoantibody reactive with carbonic anhydrase in sera from patients with systemic lupus erythematosus and Sjogren’s syndrome. J Dermatol Sci. 1991;2(3):147–154. doi: 10.1016/0923-1811(91)90060-b. [DOI] [PubMed] [Google Scholar]
- 106.Nishimori I. Induction of experimental autoimmune sialoadenitis by immunization of PL/J mice with carbonic anhydrase II. J Immunol. 1995;154(9):4865–4873. [PubMed] [Google Scholar]
- 107.Sumida T. The role of M3 muscarinic acetylcholine receptor reactive T cells in Sjogren’s syndrome: a critical review. J Autoimmun. 2014;51:44–50. doi: 10.1016/j.jaut.2013.12.012. [DOI] [PubMed] [Google Scholar]
- 108.Iizuka M. Pathogenic role of immune response to M3 muscarinic acetylcholine receptor in Sjogren’s syndrome-like sialoadenitis. J Autoimmun. 2010;35(4):383–389. doi: 10.1016/j.jaut.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 109.Harley J.B., Scofield R.H., Reichlin M. Anti-Ro in Sjogren’s syndrome and systemic lupus erythematosus. Rheum Dis Clin N Am. 1992;18(2):337–358. [PubMed] [Google Scholar]
- 110.Vitali C. Classification criteria for Sjogren’s syndrome: a revised version of the European criteria proposed by the American-European Consensus Group. Ann Rheum Dis. 2002;61(6):554–558. doi: 10.1136/ard.61.6.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Sankar V., Noll J.L., Brennan M.T. Diagnosis of Sjogren’s syndrome: American-European and the American College of Rheumatology classification criteria. Oral Maxillofac Surg Clin. 2014;26(1):13–22. doi: 10.1016/j.coms.2013.09.001. [DOI] [PubMed] [Google Scholar]
- 112.Scofield R.H. Immunization with short peptides from the 60-kDa Ro antigen recapitulates the serological and pathological findings as well as the salivary gland dysfunction of Sjogren’s syndrome. J Immunol. 2005;175(12):8409–8414. doi: 10.4049/jimmunol.175.12.8409. [DOI] [PubMed] [Google Scholar]
- 113.Igoe A., Scofield R.H. Autoimmunity and infection in Sjogren’s syndrome. Curr Opin Rheumatol. 2013;25(4):480–487. doi: 10.1097/BOR.0b013e32836200d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Lagenaur L.A. Structure and function of the murine cytomegalovirus sgg1 gene: a determinant of viral growth in salivary gland acinar cells. J Virol. 1994;68(12):7717–7727. doi: 10.1128/jvi.68.12.7717-7727.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.McCordock H.A., Smith M.G. The visceral lesions produced in mice by the salivary gland virus of mice. J Exp Med. 1936;63(3):303–310. doi: 10.1084/jem.63.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mims C.A., Gould J. Infection of salivary glands, kidneys, adrenals, ovaries and epithelia by murine cytomegalovirus. J Med Microbiol. 1979;12(1):113–122. doi: 10.1099/00222615-12-1-113. [DOI] [PubMed] [Google Scholar]
- 117.Cavanaugh V.J. Vigorous innate and virus-specific cytotoxic T-lymphocyte responses to murine cytomegalovirus in the submaxillary salivary gland. J Virol. 2003;77(3):1703–1717. doi: 10.1128/JVI.77.3.1703-1717.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Fleck M. Murine cytomegalovirus induces a Sjogren’s syndrome-like disease in C57Bl/6-lpr/lpr mice. Arthritis Rheum. 1998;41(12):2175–2184. doi: 10.1002/1529-0131(199812)41:12<2175::AID-ART12>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 119.Cha S. IDD3 and IDD5 alleles from nod mice mediate Sjogren’s syndrome-like autoimmunity. Adv Exp Med Biol. 2002;506:1035–1039. doi: 10.1007/978-1-4615-0717-8_146. [DOI] [PubMed] [Google Scholar]
- 120.Saito I. Fas ligand-mediated exocrinopathy resembling Sjogren’s syndrome in mice transgenic for IL-10. J Immunol. 1999;162:2488–2494. [PubMed] [Google Scholar]
- 121.Vignali D.A.A. IL-12 family cytokines: immunological playmakers. Nat Immunol. 2012;13(8):722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Macpherson A.J. The donor splice site mutation in NFkappaB-inducing kinase of alymphoplasia (aly/aly) mice. Immunogenetics. 2003;54:693–698. doi: 10.1007/s00251-002-0517-x. [DOI] [PubMed] [Google Scholar]
- 123.Abughanam G. Mesenchymal Stem Cells Extract (MSCsE)-based therapy alleviates xerostomia and keratoconjunctivitis sicca in Sjogren’s syndrome-like disease. Int J Mol Sci. 2019;20(19) doi: 10.3390/ijms20194750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Konno S. Role of osteo- pontin, a multifunctional protein, in allergy and asthma. Clin Exp Allergy. 2011;41(10):1360–1366. doi: 10.1111/j.1365-2222.2011.03775.x. [DOI] [PubMed] [Google Scholar]