Abstract
Purpose
We aimed to investigate the relationship between the serum anion gap (AG) and all-cause mortality in patients with acute pancreatitis (AP) in intensive care units (ICUs).
Patients and Methods
In this retrospective cohort analysis, data of patients with AP were extracted from the Medical Information Mart for Intensive Care database (version III). We collected the maximum serum AG value within the first 24 hours of ICU admission. The main outcome was 90-day all-cause mortality. A multivariate Cox proportional hazard regression model was used to examine the association between the serum AG and mortality. The restricted cubic spline curve was used to confirm a non-linear relationship between serum AG values and mortality.
Results
Of the 279 patients included in the study, 87 (31.18%) died. The serum AG value was positively associated with 90-day all-cause mortality (hazard ratio [HR] 1.08, 95% confidence interval [CI] 1.02–1.14), after adjusting for age, sex, alcohol consumption, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score. There was a non-linear relationship between serum AG values and mortality after adjusting for potential confounders. We used a two-piecewise regression model to obtain a threshold inflection point value of 13.8 mmol/L. The HR and the 95% CI on the left inflection point were 0. 82 (0.61–1.09; p = 0.1719), and on the right inflection point were 1.15 (1.08–1.23; p < 0.0001).
Conclusion
The relationship between all-cause mortality in patients with acute pancreatitis and serum AG values was non-linear. All-cause mortality and serum AG values were positively correlated when the serum AG value was >13.8 mmol/L.
Keywords: anion gap, all-cause mortality, acute pancreatitis, MIMIC III database
Introduction
Acute pancreatitis (AP) is an acute inflammatory disease that can result in multiple system dysfunction.1 Studies have reported mortality rates for mild AP to range from <1% to 5%.2,3 However, 20% of patients with AP may develop severe pancreatitis, which has a reported mortality rate of 30% due to complications such as pancreatic necrosis and organ failure.2 An acid-base balance is the basis for maintaining cell metabolism and physiological function.4 The serum anion gap (AG) is a commonly used indicator to evaluate acid-base balance, which helps to identify the types of acidosis and the causes of acid-base imbalance.5 At present, the serum AG value is usually calculated based on the concentration of sodium, chlorine, potassium, and bicarbonate in serum.6 Albumin carries most of the negative charge in the body, so hypoproteinemia can lead to a reduction in the serum AG.7,8 The serum AG is commonly used in clinical practice as an important biochemical indicator for disease diagnosis or prognosis.9,10 The initial serum AG in adult patients in the intensive care unit (ICU) has been reported to be a sensitive and specific tool for predicting prognosis or death.11,12 Moreover, it has been used to predict mortality in children in the ICU.13 However, the relationship between serum AG and all-cause mortality in patients with AP has not been studied. In this study, we aimed to investigate the relationship between serum AG values and 90-day all-cause mortality in patients with AP in the ICU.
Patients and Methods
Data Source
This study was a retrospective cohort analysis. Data concerning patients with AP were retrieved from the Medical Information Mart for Intensive Care version III (MIMIC-III), which is a large, single-center, public database of critical care. This database includes >40,000 ICU inpatients who had been admitted to Beth Israel Deaconess Medical Center in Boston from 2001 to 2012.14 To access the database, we applied the Protecting Human Research Participants test (no. 36,208,651). The International Classification of Diseases, Ninth Revision (ICD-9) code for the diagnosis of AP was 5770. Diagnostic criteria for AP are based primarily on conformance with the 2012 Atlanta Consensus.15 Patients with recurrent AP were evaluated only at the first admission. We extracted clinical indicators such as demographic characteristics, ICD-9 codes, physiological indicators, and laboratory tests. Data were obtained through the Structured Query Language (SQL) performed in the MIMIC-III database. This study was approved by the Institutional Review Boards of Beth Israel Deaconess Medical Center (Boston, MA) and the Massachusetts Institute of Technology (Cambridge, MA), and the requirement for patient informed consent was waived due to the study design.
Baseline characteristics, collected within 24 hours of admission, included sex, age, medical history, the sequential organ failure assessment (SOFA) score, and the systemic inflammatory response system (SIRS) score for patients with AP who had been admitted to the ICU. Other clinical indicators included mean arterial pressure (BP), heart rate, body temperature, white blood cell count (WBC), and blood urea nitrogen (BUN), creatinine, pulse oxygen saturation (SpO2), and maximum values of serum AG within 24 hours of ICU admission. When the above indicators had multiple results within 24 hours, we took only the worst value. The study endpoint was all-cause mortality within 90 days from the date of ICU admission.
Study Population
Data concerning 904 patients (aged ≥ 18 years) with AP according to the ICD-9 code 5770 were retrieved. Only patients at the first admission and with a first diagnosis of AP were included. Patients with an ICU length of stay <24 hours or >90 days; those with immune dysfunctions such as tumors, metastatic tumors, or acquired immune deficiency syndrome (AIDS); and those with incomplete (>10% of values missing), incorrect, or uninterpretable data were excluded.
Statistical Analysis
In this study, data were expressed as mean (SD) or median (min, max; skewed distribution) for continuous variables and percentages (%) for categorical variables. A one-way analysis of variance (ANOVA) test (normal distribution) or Kruskal–Wallis H-test (skewed distribution) and chi-squared test or Fisher’s exact test (categorical variables) were used to detect the differences among different serum AG tertiles. We constructed three distinct models using Cox proportional hazards regression models to investigate the association between the serum AG value and mortality: the crude model, in which no covariates were adjusted; model 1, in which only age and sex were adjusted; and model 2, in which model-1 and other potential confounders were adjusted. We selected background variables based on existing literature and clinical judgment. We also adjusted for features such that if the influence of a confounder changed by >10%, it was incorporated into the adjusted model.16 To test the robustness of our results, we performed sensitivity analysis. We converted the serum AG value into a categorical by tertiles, to verify the results of serum AG as a continuous variable and to study the possibility of nonlinearity. Because Cox proportional hazard regression model-based methods may not always be capable of dealing with non-linear models, non-linearity between the serum AG value and mortality was addressed using a Cox proportional hazard regression model with restricted cubic spline and smooth curve fitting. If nonlinearity was detected, we first calculated the threshold inflection point using a recursive algorithm. We then constructed a two-piecewise regression model on both sides of the inflection point. Statistical software packages R (http://www.R-project.org, The R Foundation, Vienna, Austria) and Empower Stats (http://www. empowerstats.com, X&Y Solutions, Inc, Boston, MA, USA) were used for all statistical analyses in this study. A p-value <0.05 was considered statistically significant.
Patient and Public Involvement
No patients actively participated in this study; however, data concerning patients with AP were retrieved from the Medical Information Mart for Intensive Care version III (MIMIC-III), which is a large, single-center, public database of critical care.
Results
Participant Selection
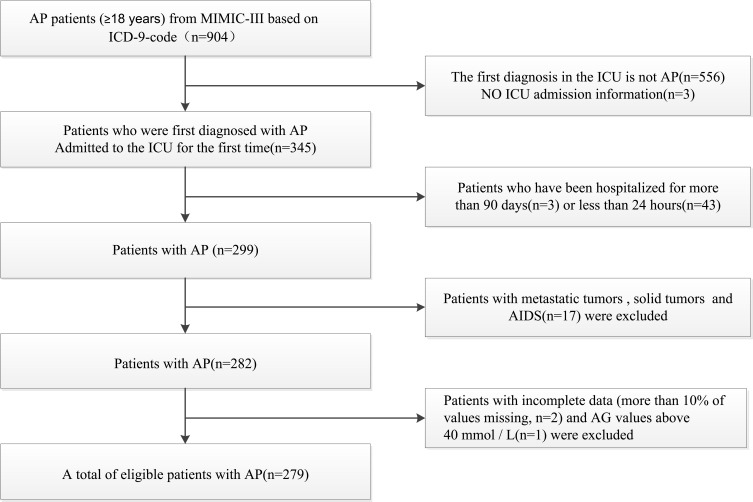
Data concerning 904 patients (aged ≥ 18 years) with AP were retrieved according to the ICD-9 code. We excluded patients whose first diagnosis was not AP (n = 556) and those who lacked ICU admission information (n = 3). Patients who had been admitted to the ICU for >90 days (n = 3) or <24 hours (n = 7) were excluded. Patients with solid tumors (n = 8), metastatic tumors (n = 3), or AIDS (n = 6) were excluded. We also excluded patients with missing AG values (n = 2) and those with AG values >40 mmol/L (n = 1). In total, 279 patients were included in the study (Figure 1).
Figure 1.
Flowchart of subject screening.
Abbreviations: AP, acute pancreatitis; MIMIC-III, Medical Information Mart for Intensive Care version III; ICU, intensive care unit.
Population and Baseline Characteristics
Participants were stratified into tertiles (T1, T2, and T3) according the maximum serum AG value within the first 24 hours of ICU admission (Table 1). Of 279 eligible patients with AP whose data were extracted from the MIMIC-III database (women, n = 125; men, n = 154), 87 (31.18%) participants died. Patients with high serum AG values (>=17.0 mmol/L) were more likely to have accompanying renal failure. The SOFA scores along with WBC, creatinine, BUN, glucose, and lactic acid values were significantly higher in those with high serum AG values than in those with low serum AG values.
Table 1.
Participants’ Baseline Characteristics
| Characteristics | AG Tertiles (mmol/L) | p-value | ||
|---|---|---|---|---|
| T1: <13.00 | T2: 14.00 −16.00 | T3: ≥ 17.00 | ||
| (n = 65) | (n = 102) | (n = 112) | ||
| Demographics | ||||
| Age, years, (SD) | 58.66 (16.76) | 61.77 (17.01) | 58.88 (15.62) | 0.345 |
| Sex, n (%) | 0.060 | |||
| Male | 30 (46.15) | 53 (51.96) | 71 (63.39) | |
| Female | 35 (53.85) | 49 (48.04) | 41 (36.61) | |
| Clinical features, n (%) | ||||
| Congestive heart failure | 0.464 | |||
| No | 54 (83.08) | 77 (75.49) | 90 (80.36) | |
| Yes | 11 (16.92) | 25 (24.51) | 22 (19.64) | |
| Hypertension | 0.128 | |||
| No | 36 (55.38) | 42 (41.18) | 46 (41.07) | |
| Yes | 29 (44.62) | 60 (58.82) | 66 (58.93) | |
| COPD | 0.069 | |||
| No | 52 (80.00) | 88 (86.27) | 103 (91.96) | |
| Yes | 13 (20.00) | 14 (13.73) | 9 (8.04) | |
| Diabetes mellitus | 0.084 | |||
| No | 52 (80.00) | 73 (71.57) | 72 (64.29) | |
| Yes | 13 (20.00) | 29 (28.43) | 40 (35.71) | |
| Renal failure | <0.001 | |||
| No | 64 (98.46) | 95 (93.14) | 91 (81.25) | |
| Yes | 1 (1.54) | 7 (6.86) | 21 (18.75) | |
| Liver disease | 0.446 | |||
| No | 53 (81.54) | 87 (85.29) | 88 (78.57) | |
| Yes | 12 (18.46) | 15 (14.71) | 24 (21.43) | |
| Obesity | 0.184 | |||
| No | 57 (87.69) | 96 (94.12) | 106 (94.64) | |
| Yes | 8 (12.31) | 6 (5.88) | 6 (5.36) | |
| Alcohol consumption | 0.109 | |||
| No | 50 (76.92) | 75 (73.53) | 71 (63.39) | |
| Yes | 15 (23.08) | 27 (26.47) | 41 (36.61) | |
| Biomarkers mean (SD)/median (min-max) | ||||
| Albumin, g/L | 2.65 (0.56) | 2.85 (0.58) | 3.05 (0.69) | 0.002 |
| Lactic acid, mmol/L | 1.53 (0.76) | 1.94 (1.31) | 2.78 (1.82) | <0.001 |
| Glucose, mg/dL | 158.48 (59.54) | 166.45 (73.65) | 201.91 (102.56) | <0.001 |
| WBC, 109/L | 13.89 (5.58) | 16.77 (7.21) | 15.28 (7.02) | 0.028 |
| SpO2, % | 91.64 (4.39) | 91.16 (3.51) | 92.26 (3.04) | 0.084 |
| Mean BP, mmHg | 64.20 (14.33) | 68.60 (16.72) | 66.67 (15.04) | 0.209 |
| BUN, mmol/L | 15.00 (3.00–64.00) | 19.00 (5.00–91.00) | 30.00 (3.00–128.00) | <0.001 |
| Creatinine, mg/dL | 0.80 (0.20–2.10) | 0.90 (0.40–3.40) | 1.60 (0.50–7.00) | <0.001 |
| eGFR, mL/min/1.73 m2 | 67.19 (3.04–199.12) | 70.55 (6.59–200.64) | 58.17 (5.44–227.57) | 0.954 |
| SIRS score | 3.23 (0.75) | 3.14 (0.91) | 3.16 (0.81) | 0.774 |
| SOFA score | 3.00 (0.00–11.00) | 3.00 (0.00–11.00) | 4.00 (0.00–18.00) | <0.001 |
| Death, n (%) | 0.052 | |||
| No | 47 (72.31) | 77 (75.49) | 68 (60.71) | |
| Yes | 18 (27.69) | 25 (24.51) | 44 (39.29) | |
Abbreviations: SD, standard deviation; BP, blood pressure (arterial); BUN, blood urea nitrogen; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; max, maximum; min, minimum; SIRS, systemic inflammatory response system; SOFA, sequential organ failure assessment; SpO2, pulse oxygen saturation; WBC, white blood cell count.
Univariate Analysis
Table 2 shows the results of univariate analysis that indicated congestive heart failure (p = 0.0443), renal failure (p = 0.0019), BUN (p < 0.0001), serum AG (p = 0.0017), and creatinine (p < 0.0001) values, age (p < 0.0001), and SOFA scores (p < 0.0001) positively correlated with mortality. Moreover, mean arterial blood pressure (p < 0.0001) negatively correlated with mortality (Table 2).
Table 2.
Factors Correlated to Mortality in Acute Pancreatitis Using Univariate Analysis
| Variables | Statistics | OR (95% CI) | p-value |
|---|---|---|---|
| Demographics | |||
| Age, years | 59.62 ± 16.54 | 1.04 (1.02, 1.06) | <0.0001 |
| Sex n (%) | |||
| Male | 154 (55.20) | 1.0 | |
| Female | 125 (44.80) | 0.98 (0.64–1.50) | 0.9345 |
| Clinical features, n (%) | |||
| Congestive heart failure | |||
| No | 221 (79.2) | 1.0 | |
| Yes | 58 (20.8) | 1.61 (1.01–2.56) | 0.0443 |
| Hypertension | |||
| No | 124 (44.44) | 1.0 | |
| Yes | 155 (55.56) | 1.12 (0.73–1.71) | 0.6177 |
| COPD | |||
| No | 243 (87.1) | 1.0 | |
| Yes | 36 (12.9) | 1.51 (0.86–2.64) | 0.1470 |
| Diabetes mellitus | |||
| No | 197 (70.61) | 1.0 | |
| Yes | 82 (29.39) | 0.83 (0.50–1.39) | 0.4820 |
| Renal failure | |||
| No | 250 (81.72) | 1.0 | |
| Yes | 29 (18.28) | 2.59 (1.42–4.74) | 0.0019 |
| Liver disease | |||
| No | 228 (92.83) | 1.0 | |
| Yes | 51 (7.17) | 1.17 (0.68–2.19) | 0.6179 |
| Obesity | |||
| No | 259 (92.83) | 1.0 | |
| Yes | 20 (7.17) | 0.46 (0.11–1.86) | 0.2761 |
| Alcohol consumption | |||
| No | 196 (70.25) | 1.0 | |
| Yes | 83 (29.75) | 0.58 (0.33–1.04) | 0.0590 |
| Biomarkers mean (SD)/median (min-max) | |||
| Albumin, g/L | 2.90 ± 0.64 | 0.84 (0.58–1.20) | 0.3322 |
| Lactic acid, mmol/L | 2.34 ± 2.14 | 1.04 (0.93–1.16) | 0.5003 |
| WBC, 109/L | 15.50 ± 6.84 | 1.03 (1.00–1.06) | 0.0915 |
| SpO2 vital, % | 91.73 ± 3.59 | 0.97 (0.92–1.03) | 0.3263 |
| BUN, mmol/L | 21 (12.75–32.25) | 1.02 (1.01–1.03) | <0.0001 |
| Serum AG, mmol/L | 16.39 ± 4.47 | 1.07 (1.02–1.11) | 0.0017 |
| eGFR, mL/min/1.73 m2 | 71.33 ± 44.47 | 1.00 (0.99–1.00) | 0.2687 |
| Creatinine, mg/dL | 1 (0.7–1.7) | 1.35 (1.18–1.56) | <0.0001 |
| Glucose, mg/dL | 178.71 ± 85.48 | 1.00 (1.00–1.00) | 0.4734 |
| Mean BP, mmHg | 66.84 ± 15.53 | 0.97 (0.95–0.98) | <0.0001 |
| SIRS score | 3.15 ± 0.87 | 1.05 (0.83–1.33) | 0.6810 |
| SOFA score | 4 (2–6) | 1.18 (1.12–1.25) | <0.0001 |
Abbreviations: AG, anion gap; OR, odds ratio; CI, confidence interval; BP, blood pressure (arterial); BUN, blood urea nitrogen; WBC, white blood cell count; COPD, chronic obstructive pulmonary disease; eGFR, estimated glomerular filtration rate; min, minute; SD, standard deviation; SIRS, systemic inflammatory response system; SOFA, sequential organ failure assessment; SpO2, pulse oxygen saturation.
Relationship Between Serum AG Values and All-Cause Mortality Using Different Models
A multivariate Cox proportional hazards regression model was used to evaluate the association between the serum AG value and 90-day all-cause mortality. Crude, minimally adjusted, and fully adjusted models are shown in Table 3. In the crude model, the serum AG was found to be positively correlated with mortality (hazard ratio [HR] 1.07, 95% confidence interval [CI] 1.02–1.11, p = 0.0017). In model 1, which was adjusted for age and sex, the serum AG also showed a positive correlation with mortality (HR 1.08, 95% CI 1.03–1.12, p = 0.0004). In model 2, which was adjusted for age, sex, alcohol consumption, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score, no obvious changes were observed (HR 1.08, 95% CI 1.02–1.14, p = 0.0091). Next, tests for trend were conducted with multivariate proportional hazards regression models by entering the median value of each serum AG tertile as a continuous variable in the models. Patients with a lower serum AG value (<13 mmol/L) were included in the reference group. In model 1, high serum tertile (AG ≥17 mmol/L) was associated with an increased risk of all-cause mortality (HR 1.95, 95% CI 1.11–3.42) after adjusting for age and sex. In model 2, after adjusting for age, sex, alcohol consumption, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score, high serum AG value (≥17 mmol/L) remained positively associated with 90-day all-cause mortality, compared with a low serum AG tertile (HR 1.88, 95% CI 1.04–3.41); the p-for-trend was 0.0182.
Table 3.
The Relationship Between the Serum AG and All-Cause Mortality in Patients with AP in Different Models
| Variable | Crude Model HR (95% CI) | p-value | Model 1 HR (95% CI) | p-value | Model 2 HR (95% CI) | p-value |
|---|---|---|---|---|---|---|
| Serum AG | 1.07 (1.02–1.11) | 0.0017 | 1.08 (1.03–1.12) | 0.0004 | 1.08 (1.02–1.14) | 0.0091 |
| Serum AG tertiles | ||||||
| T1 | 1.0 (Ref) | 1.0 (Ref) | 1.0 (Ref) | |||
| T2 | 0.91 (0.50–1.69) | 0.7731 | 0.79 (0.42–1.48) | 0.4542 | 0.72 (0.38–1.38) | 0.2564 |
| T3 | 1.82 (1.05–3.17) | 0.0332 | 1.95 (1.11–3.42) | 0.0207 | 1.88 (1.04–3.41) | 0.0370 |
| p-for-trend | 0.0131 | 0.0069 | 0.0182 | |||
Notes: Crude model: No covariates were adjusted. Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, alcohol abuse, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score.
Abbreviations: AG, anion gap; AP, acute pancreatitis; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazards ratio; SOFA, sequential organ failure assessment; Ref, reference.
Analyses of Non-Linear Relationships
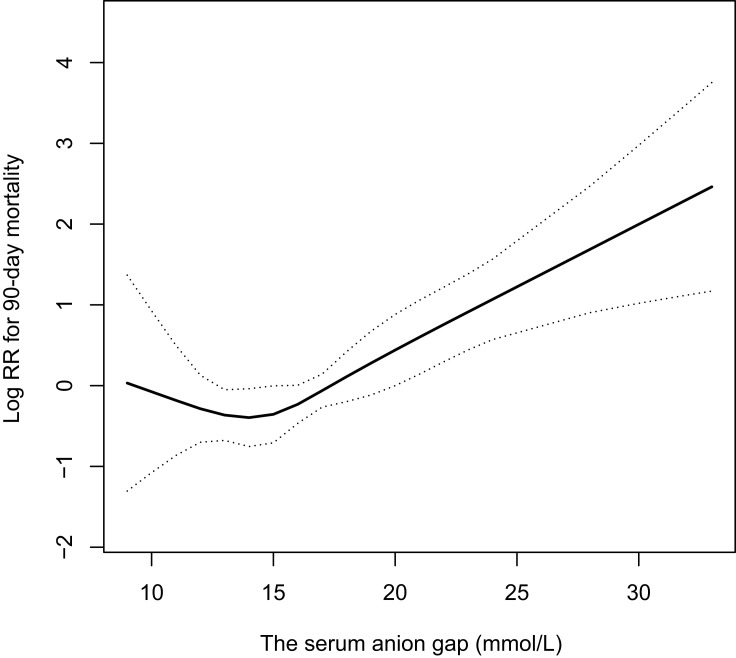
We found a non-linear relationship between the maximum serum AG within 24 hours of ICU admission and all-cause mortality after adjusting for age, sex, alcohol consumption, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score (Figure 2). We calculated that the inflection point was 13. 8 mmol/L using a two-piecewise regression model (Table 4). There was no significant correlation to the left of the inflection point (HR [95% CI] 0.82 ([0.61–1.09]), p = 0.1719; however, there was a positive correlation between the serum AG value and all-cause mortality to the right of the threshold inflection point (HR 1.15, 95% CI 1.08–1.23, p < 0.0001).
Figure 2.
The relationship between serum AG values and all-cause mortality in patients with AP. There was a nonlinear relationship between serum AG values and all-cause mortality in patients with AP after adjusting for age, sex, alcohol abuse, congestive heart failure, diabetes, hypertension, eGFR, albumin, and SOFA score. Solid rad line indicates the cubic spline functions between variables. Imaginary lines represent the 95% of confidence interval from the fit.
Abbreviations: AG, anion gap; AP, acute pancreatitis.
Table 4.
Threshold Effect Analysis of the Relationship Between the Serum AG and Death Using a Two-Piecewise Regression Model
| Serum AG Inflection Point | HR (95% CIs) | p-value |
|---|---|---|
| <13.8 | 0.82 (0.61, 1.09) | 0.1719 |
| ≥13.8 | 1.15 (1.08, 1.23) | <0.0001 |
| P for log likelihood ratio test | 0.046 |
Note: Adjusted for age, sex, alcohol abuse, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score.
Abbreviations: AG, anion gap; CI, confidence interval; eGFR, estimated glomerular filtration rate; HR, hazard ratio; SOFA, sequential organ failure assessment.
Discussion
In this study, we investigated the association between serum AG value and all-cause mortality in critically ill patients with AP. First, the serum AG value was associated with mortality in patients with AP after adjusting for age, sex, alcohol abuse, congestive heart failure, diabetes mellitus, hypertension, eGFR, albumin, and the SOFA score. Second, there was a non-linear relationship between serum AG values and death from all causes in patients with AP, and we calculated a threshold inflection point of 13.8mmol/L, using a two-segment linear regression model. To the right of the threshold, the number of all-cause deaths increased with an increase in the serum AG value, which was positively correlated. However, the trend was the opposite to the left of the threshold, although it was not significant. This may have something to do with our insufficient sample size.
The serum AG has been reported to be associated with death and complications in a variety of diseases. Cheng et al,17 found that high serum AG values in patients with acute kidney injury had higher all-cause mortality at 30 days, 90 days, and 365 days, HR (95% CI) were: 1.54 (1.33–1.75), 1.55, (1.38–1.73), and 1.46 (1.31–1.60) respectively. Abramowitz et al,18 found that the serum AG value was associated with mortality, the Relative Hazard (95% CI) was 1.20 (1.03–1.41), and higher levels of serum AG were present in patients with impaired renal function. A retrospective analysis of 440 participants data suggested that adjusted serum anion gap is an independent risk factor for all -cause mortality in advanced CKD patients, with HR (95% CI) 2.968 (1.143–7.708).19 The relationship between serum AG and mortality has been reported not only in adults but also in children. Kim and his team20 reported that corrected AG in children at ICU admission was associated with mortality, with odds ratio (95% CI) 1.110 (1.06–1.17). Cheng et al,17 and Tang et al,21 confirmed a U-shaped relationship between serum AG levels and all-cause mortality in critically ill patients with acute kidney injury and patients with congestive heart failure, respectively. Our findings are consistent with previous studies. We found a non-linear relationship between the serum AG and mortality, after adjusting for serum albumin level, eGFR and other confounding factors, and we further identified a threshold inflection point of the impact of AG on all-cause mortality in AP. As reported, the main component of the serum AG is the sum of the anionic charges on circulating proteins,22 changes in serum albumin concentration levels will cause changes in the serum AG. After adjusting for covariates including serum albumin, the serum AG was still associated with all-cause mortality in critical patients with AP. The serum AG value, which is a low-cost and easily available biomarker, may facilitate clinical assessment of the mortality risk for patients with AP.
Study Strengths and Limitations
Our study had the following strengths. First, both a Cox proportional hazard regression model and the restricted cubic spline curve were used to study the association between serum AG and 90-day all-cause mortality in patients with AP. Second, this was an observational study. Potential confounders were unavoidable; therefore, we combined existing literature, clinical judgment, and statistical adjustments to minimize the effect of confounders.
Our study also had some limitations. First, although we adjusted for certain factors, our results may have been influenced by other unknown factors. Second, only adults were included in our study; therefore, the relationship between serum AG levels and outcomes in children with AP could not be determined.
Conclusions
The relationship between the serum AG and all-cause mortality in patients with AP was non-linear after adjustment for potential confounders. Large prospective studies are needed to further confirm our results.
Acknowledgments
We wish to thank Dr. Jie Liu of the Department of Vascular and Endovascular Surgery, Chinese PLA General Hospital, for his contribution to the study design, and for his advice and comments regarding the manuscript. We would also like to thank Editage (www.editage.cn) for English language editing.
Funding Statement
This study was supported by the Scientific Research Foundation of Changde (grant number: 2017so317).
Data Sharing Statement
The clinical data of this study were derived from MIMIC-III (v. 1.4). This is an open and free database, and researchers must complete an online course at the National Institutes of Health, known as Protecting Human Research Participants, before they can apply for permission to access it.
Disclosure
The authors declare that there are no conflicts of interest regarding the publication of this paper.
References
- 1.Agarwal N, Pitchumoni CS. Acute pancreatitis: a multisystem disease. Gastroenterologist. 1993;1(2):115–128. [PubMed] [Google Scholar]
- 2.Wu BU, Johannes RS, Sun X, Tabak Y, Conwell DL, Banks PA. The early prediction of mortality in acute pancreatitis: a large population-based study. GUT. 2008;57(12):1698–1703. doi: 10.1136/gut.2008.152702 [DOI] [PubMed] [Google Scholar]
- 3.Dervenis C, Johnson CD, Bassi C, et al. Diagnosis, objective assessment of severity, and management of acute pancreatitis. Santorini consensus conference. Int J Pancreatol. 1999;25(3):195–210. doi: 10.1007/BF02925968 [DOI] [PubMed] [Google Scholar]
- 4.Rosner MH, Perazella MA, Choi MJ. American Society of Nephrology quiz and questionnaire 2014: acid-base and electrolyte disorders. Clin J Am Soc Nephrol. 2015;10(3):530–539. doi: 10.2215/CJN.10911114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kraut JA, Madias NE. Serum anion gap: its uses and limitations in clinical medicine. Clin J Am Society Nephrol. 2007;2(1):162–174. doi: 10.2215/CJN.03020906 [DOI] [PubMed] [Google Scholar]
- 6.Co I, Gunnerson K. Emergency department management of acute kidney injury, electrolyte abnormalities, and renal replacement therapy in the critically ill. Emerg Med Clin North Am. 2019;37(3):459–471. doi: 10.1016/j.emc.2019.04.006 [DOI] [PubMed] [Google Scholar]
- 7.Figge J, Jabor A, Kazda A, Fencl V. Anion gap and hypoalbuminemia. Crit Care Med. 1998;26(11):1807–1810. doi: 10.1097/00003246-199811000-00019 [DOI] [PubMed] [Google Scholar]
- 8.Nanji AA, Campbell DJ, Pudek MR. Decreased anion gap associated with hypoalbuminemia and polyclonal gammopathy. JAMA. 1981;246(8):859–860. doi: 10.1001/jama.1981.03320080045027 [DOI] [PubMed] [Google Scholar]
- 9.Park M, Jung SJ, Yoon S, Yun JM, Yoon HJ. Association between the markers of metabolic acid load and higher all-cause and cardiovascular mortality in a general population with preserved renal function. Hypertension Research. 2015;38(6):433–438. doi: 10.1038/hr.2015.23 [DOI] [PubMed] [Google Scholar]
- 10.Abramowitz MK, Hostetter TH, Melamed ML. Lower serum bicarbonate and a higher anion gap are associated with lower cardiorespiratory fitness in young adults. Kidney Int. 2012;81(10):1033–1042. doi: 10.1038/ki.2011.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imran MN, Leng PH, Yang S, Kurup A, Eng P. Early predictors of mortality in pneumococcal bacteraemia. Ann Academy Med Singapore. 2005;34(7):426–431. [PubMed] [Google Scholar]
- 12.Sahu A, Cooper HA, Panza JA. The initial anion gap is a predictor of mortality in acute myocardial infarction. Coron Artery Dis. 2006;17(5):409–412. doi: 10.1097/00019501-200608000-00002 [DOI] [PubMed] [Google Scholar]
- 13.Kim MJ, Kim YH, Sol IS, et al. Serum anion gap at admission as a predictor of mortality in the pediatric intensive care unit. Sci Rep. 2017;7(1):1456. doi: 10.1038/s41598-017-01681-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Johnson AE, Pollard TJ, Shen L, et al. MIMIC-III, a freely accessible critical care database. Scientific Data. 2016;3:160035. doi: 10.1038/sdata.2016.35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Banks PA, Bollen TL, Dervenis C, et al. Classification of acute pancreatitis–2012: revision of the Atlanta classification and definitions by international consensus. GUT. 2013;62(1):102–111. doi: 10.1136/gutjnl-2012-302779 [DOI] [PubMed] [Google Scholar]
- 16.Agoritsas T, Merglen A, Shah ND, O’Donnell M, Guyatt GH. Adjusted analyses in studies addressing therapy and harm: users’ guides to the medical literature. JAMA. 2017;317(7):748–759. doi: 10.1001/jama.2016.20029 [DOI] [PubMed] [Google Scholar]
- 17.Cheng B, Li D, Gong Y, Ying B, Wang B. Serum anion gap predicts All-Cause mortality in critically ill patients with acute kidney injury: analysis of the MIMIC-III database. Dis Markers. 2020;2020:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abramowitz MK, Hostetter TH, Melamed ML. The serum anion gap is altered in early kidney disease and associates with mortality. Kidney Int. 2012;82(6):701–709. doi: 10.1038/ki.2012.196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SW, Kim S, Na KY, et al. Serum anion gap predicts All-Cause mortality in patients with advanced chronic kidney disease: a retrospective analysis of a randomized controlled study. PLoS One. 2016;11(6):e0156381. doi: 10.1371/journal.pone.0156381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim MJ, Kim YH, Sol IS, et al. Serum anion gap at admission as a predictor of mortality in the pediatric intensive care unit. Sci Rep. 2017;7:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang Y, Lin W, Zha L, et al. Serum anion gap is associated with All-Cause mortality among critically ill patients with congestive heart failure. Dis Markers. 2020;2020:1–10. doi: 10.1155/2020/8833637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Feldman M, Soni N, Dickson B. Influence of hypoalbuminemia or hyperalbuminemia on the serum anion gap. J Lab Clin Med. 2005;146(6):317–320. doi: 10.1016/j.lab.2005.07.008 [DOI] [PubMed] [Google Scholar]