Abstract
Objective
To assess the impact of interhospital transfer on the interplay between functional outcome, mortality, reperfusion rates and workflow time metrics in patients undergoing endovascular thrombectomy (EVT) for acute ischaemic stroke due to large vessel occlusion (LVO) in the anterior cerebral circulation.
Design, setting and participants
This is an analysis of a prospective database of consecutive patients undergoing EVT for LVO presenting between January 2017 and December 2018 at a single Australian comprehensive stroke centre (CSC). Patients presented directly or were transferred to the CSC from 21 sites across New South Wales and the Australian Capital Territory.
Main outcome measures
The main outcome measures were rate of good 90-day functional outcome (modified Rankin Scale 0–2), successful reperfusion (Thrombolysis in Cerebral Infarction scale grade 2b or 3), symptomatic intracerebral haemorrhage (sICH) and 90-day mortality. Key workflow time metric milestones were examined.
Results
154 of 213 (72%) patients were interhospital transfers. There was no significant difference in baseline characteristics including age, National Institutes of Health Stroke Scale score, intravenous thrombolysis administration or procedure time between transferred and direct presenters (all p>0.05). Transferred patients had worse 90-day functional outcome (39.6% vs 61.0%, OR 0.42, 95% CI 0.23 to 0.78), higher mortality (25.3% vs 6.8%, OR 4.66, CI 1.59 to 13.70) and longer stroke onset to treatment (groin puncture) time (298 min vs 205 min, p<0.01). Successful reperfusion rates and sICH were similar between the cohorts (96.8% vs 98.3%, and 7.8% vs 3.4%).
Conclusion
Interhospital transfer is associated with longer stroke onset to treatment, worse 90-day functional outcome and higher mortality compared with patients presenting directly to the CSC.
Keywords: stroke, cerebrovascular disease, cerebrovascular
Introduction
Endovascular thrombectomy (EVT) is the standard of care for patients with acute ischaemic stroke (AIS) with large vessel occlusion (LVO) in the anterior cerebral circulation (ACC). Multicentre, randomised controlled trials have demonstrated the safety and efficacy of this procedure within 6 hours1–3 and in selected patients up to 24 hours from stroke onset according to advanced imaging findings.4 5 Shorter stroke onset to groin puncture time (defined as the start of treatment) and reperfusion has been associated with improved functional outcome. This effect diminishes over time.6
Primary stroke centres (PSC) are hospitals distributed across metropolitan and regional communities that offer dedicated stroke services, including intravenous thrombolysis (IVT) and stroke unit care.7 Comprehensive stroke centres (CSC) are large tertiary referral centres that have onsite 24-hour access to neurology, critical care and neurointerventional expertise, and provide additional specialised acute stroke services including EVT and neurosurgery. The current Australian model of stroke care expedites medical assessment and provides ready access to IVT at PSCs but requires transfer of patients eligible for EVT to a CSC.
Australia has a unique geography. It is the earth’s sixth largest land mass and has the lowest population density in the world. There is significant variation in the density of cities and remote areas. Delivery of EVT is complex and resource-intensive.8 Treatment at high-volume centres is associated with improved functional outcome and lower mortality. To achieve an adequate EVT case load and scalability, Australia has centralised CSCs to major cities. New South Wales is the most populous state and has four CSCs which provide 24/7 EVT service to all local health districts and specialty networks. Patients may present directly to a CSC for stroke treatment or undergo interhospital transfer to a CSC, travelling a range of distances to receive EVT depending on their primary location in a metropolitan, regional or remote setting.
An Australian study based in Victoria found no difference in 90-day functional outcomes between transferred and direct to CSC presenters undergoing EVT9; however, international registries have demonstrated poorer outcomes in transferred patients.10–12 New South Wales and other Australian states have different geographical challenges from Victoria. The impact of interhospital transfer on EVT workflow time metrics in Australia has not been previously studied.
The present study is a retrospective analysis of a prospectively collected cohort of consecutive patients undergoing EVT for AIS due to LVO in the ACC at Liverpool Hospital (LH), a single large CSC in Sydney, New South Wales, Australia, over a 2-year period. The study aim was to determine the impact of interhospital transfer on (1) the proportion of patients with good 90-day functional outcome (defined by a modified Rankin Scale (mRS) 0–2), (2) mortality and symptomatic intracranial haemorrhage (sICH) rates, (3) successful reperfusion (defined as Thrombolysis in Cerebral Infarction (TICI) scale grade ≥2 b), and (4) key workflow time metrics.
Methods
Study population
We analysed data from the prospective observational registry of consecutive patients undergoing EVT for AIS at LH, a single CSC in Sydney, between January 2017 and December 2018. Patients with an occlusion involving the ACC (internal carotid, anterior cerebral, middle cerebral M1, M2 branches or tandem lesions) demonstrated on CT angiography who underwent EVT were included. Patients with posterior circulation or distal middle cerebral artery (M3 or M4 branches) occlusions were excluded. The study population was treated in accordance with current Australian guidelines, which strongly recommend EVT for all patients with an occlusion involving the ACC within 6 hours and in selected patients between 6 and 24 hours of stroke onset depending on favourable advanced imaging findings.13
This registry represents a real-world data set. Patients were transported to the CSC via road ambulance, helicopter and/or aeroplane depending on the patient’s hospital location. In accordance with New South Wales health policy, transferred patients were repatriated to the referring hospital within 48 hours of EVT if clinically stable.
This research was completed without patient and public involvement.
Definitions and outcomes
Stroke onset was defined as the self-reported or witnessed time of onset of stroke symptoms. If this information could not be determined, then the time at which the patient was last known well was used.
EVT was performed under general anaesthesia in all cases using thrombectomy devices (Solitaire, Trevo or Catch Mini), aspiration catheter or both. No patients received intra-arterial thrombolytics.
Patients were categorised into direct or transfer depending on whether they initially ‘directly’ presented to the CSC or were ‘transferred’ from a PSC or regional hospital. For transferred patients the qualifying CT imaging was acquired prior to transport to the CSC. Repeat imaging prior to EVT was not routinely performed unless there was a significant clinical alteration.
Poststroke 90-day mRS score was captured by certified assessors either in person or by telephone. The mRS score was dichotomised into ‘good’ (mRS 0–2) and ‘poor’ (mRS 3–6) functional outcome groups. An mRS score of 0–2 was used as a measure of functional independence consistent with other studies.10 11 Successful reperfusion was defined as TICI scale grade ≥2 b. Safety outcomes were 90-day mortality rates and the rates of sICH within 24 hours, defined as neurological deterioration by ≥4 points on the National Institutes of Health Stroke Scale (NIHSS) compared with baseline NIHSS score and parenchymal haematoma type 2.14
Workflow time metrics assessed the time interval between stroke onset and arrival at the PSC and CSC and additional times to acquisition of CT brain imaging, administration of IVT, discussion with and acceptance by the CSC for EVT, groin puncture (as a measure of treatment onset), reperfusion, and procedure duration.
Statistical analysis
Statistical analyses were performed using SAS V.9.0. Baseline characteristics including age, gender, vascular risk factors, baseline NIHSS score and IVT treatment were assessed using standard descriptive statistics. Normality of continuous variables was determined with the Shapiro-Wilk test. Univariate relationship of baseline characteristics with interhospital transfer versus direct presentation to CSC status was assessed. The Pearson’s χ2 test was used for categorical variables and the Wilcoxon rank-sum test for non-normally distributed continuous variables. A two-tailed p value of <0.05 was the cut-off for statistical significance.
The dichotomised outcomes of functional independence, death, successful reperfusion and sICH were assessed with simple and multivariable logistic regression in the direct and transferred cohorts, as well as an analysis of a subgroup of transferred patients from PSCs within 30 km of the CSC. The multivariable model was adjusted for age, baseline NIHSS score, administration of IVT and stroke onset to groin puncture time. Ordinal logistic regression was used to analyse the change in 90-day mRS score. Relationship between interhospital transfer, baseline NIHSS score, age, stroke onset to groin puncture time, CT acquisition to groin puncture time and administration of IVT with good functional outcome were assessed in the total cohort and subgroup of patients with stroke onset to groin puncture <6 hours. Linear regression was used to assess the relationships between interhospital transfer status and workflow metrics. Listwise deletion was employed for analyses with missing data. All results were reported as OR with 95% CI.
Results
Two hundred and seventy patients underwent EVT in the designated time period. Thirty-seven patients were excluded due to posterior circulation (n=33), A3 (n=1) or M3 (n=3) occlusions. Twenty patients were excluded as they had achieved reperfusion at cerebral digital subtraction angiogram and did not require EVT. Two hundred and thirteen patients were included in the analysis. Of these, 154 (72.3%) were transferred from 21 sites (online supplementary appendix 1) and 59 (27.7%) presented directly to the CSC.
bmjno-2019-000030supp001.pdf (49.7KB, pdf)
Baseline characteristics
There was no significant difference in the baseline characteristics, vascular risk factor profile, IVT administration or LVO location between the two groups (table 1). The median baseline NIHSS score was 17 (transferred patients) and 18 (direct), consistent with moderate to severe AIS. A similar number received IVT (39.6% vs 35.6%). There was no difference in the number of patients with a baseline mRS score >2 between the groups (3.3% vs 3.4%). Only one transferred patient was reimaged on arrival at the CSC.
Table 1.
Baseline demographic and clinical characteristics of direct and transfer patient cohorts
| Baseline and clinical characteristics | |||
| Characteristics | Direct (n=59) | Transfer (n=154) | P value |
| Age (median, years) | 74 (35–96) | 76 (29–95) | 0.66 |
| Female | 26 (44.1%) | 74 (48.1%) | 0.60 |
| NIHSS score, median | 18 (1–34) | 17 (2–42) | 0.32 |
| Medical history | |||
| Hypertension | 40 (67.8%) | 108 (70.1%) | 0.74 |
| Ischaemic heart disease | 17 (28.8%) | 42 (27.3%) | 0.82 |
| Atrial fibrillation | 27 (45.8%) | 75 (48.7%) | 0.70 |
| Diabetes | 16 (27.1%) | 35 (22.7%) | 0.50 |
| Dyslipidaemia | 35 (59.3%) | 85 (55.2%) | 0.59 |
| Previous stroke | 13 (22.0%) | 25 (16.2%) | 0.32 |
| Smoking | 15 (25.4%) | 23 (14.9%) | 0.07 |
| Treatment | |||
| IVT | 21 (35.6%) | 61 (39.6%) | 0.59 |
| GA | 59 (100.0%) | 153 (99.4%) | 0.54 |
| Location of thrombus | 0.39 | ||
| M1 | 31 (52.5%) | 88 (57.1%) | |
| M2 | 14 (23.7%) | 23 (14.9%) | |
| ICA | 8 (13.6%) | 19 (12.3%) | |
| Tandem | 6 (10.2%) | 24 (15.6%) | |
| Baseline mRS score | 0.96 | ||
| 0–2 | 57 (96.6%) | 149 (96.8%) | |
| ≥3 | 2 (3.4%) | 5 (3.3%) | |
GA, general anaesthesia; ICA, internal carotid artery; IVT, intravenous thrombolysis; M1, segment 1 of the middle cerebral artery; M2, segment 2 of the middle cerebral artery; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; Tandem, cervical ICA and M1 or M2.
Clinical outcome
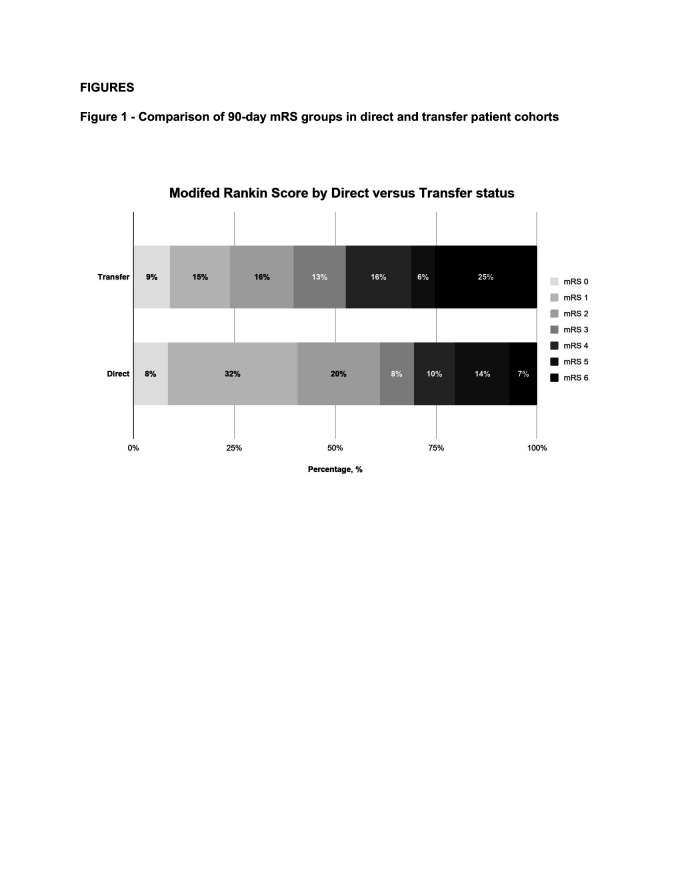
Transferred patients had lower rates of good functional outcome (39.6% vs 61.0%; OR 0.42, 95% CI 0.23 to 0.78) and higher mortality (25.3% vs 6.8%; OR 4.66, CI 1.59 to 13.70) than direct presenters at 90 days (table 2). There were similar findings in a subgroup analysis of patients transferred from PSCs within 30 km of the CSC (online supplementary appendix 2). There was a significant shift towards poorer 90-day mRS outcomes in transferred patients (common OR 2.33, CI 1.27 to 3.90; figure 1). There was no difference in the rate of successful reperfusion or sICH between directly presenting versus transferred patients (table 2).
Table 2.
Clinical outcomes of direct and transfer patient cohorts
| Clinical outcome | Direct (n=59) | Transfer (n=154) | OR | Adjusted OR* |
| mRS 0–2 | 36 (61.0%) | 61 (39.6%) | 0.42 (0.23–0.78) | 0.27 (0.12–0.60) |
| Death | 4 (6.8%) | 39 (25.3%) | 4.66 (1.59–13.70) | 7.41 (2.27–24.19) |
| TICI 2b/3 | 58 (98.3%) | 149 (96.8%) | 0.51 (0.06–4.49) | 0.56 (0.06–5.12) |
| sICH | 2 (3.4%) | 12 (7.8%) | 2.41 (0.52–11.1) | 2.32 (0.49–10.95) |
*Adjusted for age, baseline National Institutes of Health Stroke Scale score, administration of intravenous thrombolysis and stroke onset to groin puncture.
TICI 2b/3, Thrombolysis in Cerebral Infarction scale score of 2b or 3; mRS, modified Rankin Scale; sICH, symptomatic intracerebral haemorrhage.
Figure 1.
Comparison of 90-day modified Rankin Scale (mRS) groups in direct and transfer patient cohorts.
In the total cohort significant predictors of good functional outcome were direct presentation, lower baseline NIHSS score and younger age (table 3). In the subgroup of patients with stroke onset to groin puncture <6 hours, predictors of good functional outcome were direct presentation, lower baseline NIHSS score, younger age and reduction in the key time metrics of stroke onset and CT to groin puncture (table 3).
Table 3.
Associations with good functional outcome (mRS 0–2) following endovascular thrombectomy
| Characteristics | OR (total, n=213) (CI) | P value | OR (<6 hours, n=139) (CI) | P value |
| Interhospital transfer | 0.42 (0.23 to 0.78) | 0.006 | 0.36 (0.17 to 0.75) | 0.006 |
| Baseline NIHSS score | 0.86 (0.82 to 0.90) | <0.001 | 0.87 (0.82 to 0.92) | <0.001 |
| Age | 0.96 (0.94 to 0.98) | <0.001 | 0.97 (0.95 to 1.00) | 0.04 |
| Stroke onset to groin puncture, hours | 0.98 (0.94 to 1.02) | 0.36 | 0.57 (0.40 to 0.80) | 0.002 |
| CT to groin puncture, hours | 1.00 (0.92 to 1.09) | 0.95 | 0.48 (0.29 to 0.80) | 0.005 |
| IVT | 1.45 (0.83 to 2.53) | 0.19 |
‘<6 hours’ means less than 6 hours from stroke onset to groin puncture.
IVT, intravenous thrombolysis; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale score.
Workflow time metrics
Time of first hospital arrival to CT was significantly longer for transferred patients (30 min vs 24 min, p=0.03; table 4). In transferred patients, there was an increase in time from CT to discussion with the CSC (31 min vs 16 min, p=0.02), discussion with CSC to acceptance for EVT (12 min vs 9 min, p=0.05) and time of CT to groin puncture (144 min vs 86 min, p<0.01). Time of CSC arrival to groin puncture was shorter for transferred patients (16 min vs 109 min, p<0.01).
Table 4.
Comparison of workflow time metrics in direct and transfer patient cohorts
| Direct | Transfer | P value | |
| Workflow time metrics | |||
| Door of first hospital to CT | 24 (15–36) | 30 (20–50) | 0.03 |
| Door of first hospital to IVT | 61 (48–70) | 65 (52–86) | 0.09 |
| CT to discussion with CSC | 16 (3–30) | 31 (17–55) | 0.02 |
| Discussion with CSC to acceptance | 9 (6–14) | 12 (8–20) | 0.05 |
| PSC to CSC arrival | 156 (125–220) | ||
| CSC arrival to groin puncture | 109 (100–144) | 16 (13–24) | <0.001 |
| CT to groin puncture | 86 (67–105) | 144 (117–179) | 0.002 |
| First hospital arrival to groin puncture | 109 (100–144) | 172 (144–237) | 0.003 |
| Procedure time | 30 (23–47) | 33 (21–47) | 0.89 |
| Stroke onset to workflow epoch | |||
| Stroke onset to first hospital arrival | 100 (70–351) | 100 (60–340) | 0.87 |
| Stroke onset to CT | 120 (70–193) | 129 (85–330) | 0.34 |
| Stroke onset to first contact CSC | 141 (79–201) | 186 (118–435) | 0.12 |
| Stroke onset to acceptance by CSC | 154 (105–573) | 210 (135–473) | 0.28 |
| Stroke onset to IVT | 122 (110–150) | 141 (116–198) | 0.02 |
| Stroke onset to CSC arrival | 100 (70–351) | 276 (205–540) | <0.001 |
| Stroke onset to groin puncture | 205 (153–333) | 298 (222–556) | 0.01 |
| Stroke onset to reperfusion | 245 (198–357) | 348 (261–605) | 0.01 |
Times presented as median minutes with IQR.
CSC, comprehensive stroke centre; IVT, intravenous thrombolysis; PSC, primary stroke centre.
Time of stroke onset to administration of IVT and groin puncture was significantly longer for transferred patients (141 min vs 122 min, p=0.02; and 298 min vs 205 min, p=0.01, respectively; table 4). Stroke onset to reperfusion time was also longer in transferred patients (348 min vs 245 min, p=0.01). There was no significant difference in procedure time between the groups (33 min vs 30 min, p=0.89).
Discussion
In this study, 72% of patients with AIS were transferred from a PSC. Transferred patients had worse 90-day functional outcomes, including higher mortality, when compared with those directly presenting to the CSC despite similar baseline characteristics and adjustment for time to groin puncture. In patients with groin puncture <6 hours, time from stroke onset and CT to groin puncture significantly correlated with functional outcome. Interhospital transfer patients had longer workflow time metrics, including stroke onset and first hospital arrival to time of administration of IVT, CT to discussion with the CSC team, and CT to groin puncture and reperfusion times.
Poorer functional outcomes and increased mortality of transferred patients have been described in international studies.11 12 15 Studies have demonstrated that good outcome after EVT is time-dependent.6 We found that shorter stroke onset to treatment was a critical determinant of good functional outcome in patients with groin puncture <6 hours. This is concordant with findings from the Highly Effective Reperfusion Evaluated in Multiple Endovascular Stroke Trials Collaboration meta-analysis.6 The importance of expedited treatment in patients with groin puncture <6 hours may relate to the concept of fast and slow progression of infarction.16 Fast progressors experience rapid infarct growth with a limited window to reperfuse at-risk ischaemic tissue (penumbra). Slow progressors have a favourable collateral circulation and better tolerance of ischaemia. Slow progressors benefit from EVT due to eventual failure of collateral supply. Advanced imaging helps to identify slow progressors and is useful for patient selection for EVT in those presenting >6 hours from stroke onset.
Transferred patients had significantly longer stroke onset to groin puncture and reperfusion times, a finding also reported by international studies.10–12 17 In the present study, interhospital transfer was associated with delays in workflow, including increased times from CT to contact of and acceptance by the CSC. Earlier discussion and streamlined acceptance by the CSC represent a key opportunity for workflow improvement. Transferred patients experienced delays in CT to groin puncture time. While shorter CT to groin puncture has been associated with better functional outcomes,10 18 improvement in this workflow time metric poses a major challenge. Differences in hospital protocol and resourcing are likely to contribute to the disparity in workflow metrics. LH has a 24-hour onsite stroke neurology team. Directly presenting patients receive immediate stroke assessment with routine advanced imaging and early neurointerventional specialist input. In contrast, PSC sites are more likely to rely on remote neurology and radiology services.
Multiple strategies have been proposed to address unnecessary interhospital transfers and better identify patients with AIS at high risk of LVO and taking them directly to CSCs. Current prehospital clinical scales have low specificity in identifying LVO.19 Furthermore, prehospital bypass strategies are linked to delayed administration of IVT.20 Several countries have trialled mobile stroke units21 and telestroke22 services to expedite early specialist assessment and treatment. These approaches are expensive, limited in Australia and require substantial infrastructure, but may improve workflow metrics. At present, a centralised approach to EVT is the most feasible service delivery option.
Australia’s unique geography and population distribution affect the generalisability of findings from interstate or international sites. Optimal hospital transport strategy varies by region.23 24 Factors influencing this strategy include distance, ratio of PSC to CSC, and access to stroke physicians and advanced imaging. Contextualising these factors is critical to coordinating interhospital transfer. The percentage of transferred patients in our study was higher than reported in international cohorts.10 25 This reflects the relatively few CSCs in New South Wales which service 8 million people over an area of 80 million square kilometres. Most previous analyses of interhospital transfer have been in denser populations of smaller total area10 with access to more CSCs.11
There were several limitations to our study. It was based on a retrospective analysis of prospectively collected data from a single centre, which limits applicability to regions with different geography and population density. We did not analyse patient outcomes according to treatment during business hours versus after hours, which has been shown to influence workflow time metrics.9 Although there was no difference in baseline characteristics between transferred and direct presenter groups, we cannot exclude a (favourable or unfavourable) selection bias in transferred patients. Despite the possibility of a favourable selection bias, transferred patients still had a worse outcome. We did not assess the impact of advanced imaging including collateral circulation, which is a surrogate marker for prognosis and stroke severity. Repatriation to the referring hospital within 48 hours of EVT is a health policy directive for CSCs in New South Wales. This practice disrupts continuity of care and potentially introduces differences in postacute management that may influence outcome.
Conclusion
Interhospital transfer of patients with AIS for EVT is associated with delays in treatment from stroke onset, worse functional outcome and higher mortality. The majority of our patients were transferred. A correlation between stroke onset to groin puncture and good functional outcome was seen in patients presenting within 6 hours of stroke onset. Improvement in workflow time metrics should always be sought, although other factors may influence outcome.
Acknowledgments
We would like to thank Dr Wei Xuan, senior biostatistician, for supervision of statistical methods and results.
Footnotes
Contributors: LSE was the principal author of the manuscript. CB, DC, AM, NM, AC, JW and CC-S were involved in the conception, drafting and revision of the manuscript.
Funding: The authors have not declared a specific grant for this research from any funding agency in the public, commercial or not-for-profit sectors.
Competing interests: None declared.
Patient consent for publication: Not required.
Ethics approval: Approval to conduct this study was provided by our institution’s Human Research Ethics Committee.
Provenance and peer review: Not commissioned; externally peer reviewed.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
References
- 1.Berkhemer OA, Fransen PSS, Beumer D, et al. . A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. 10.1056/NEJMoa1411587 [DOI] [PubMed] [Google Scholar]
- 2.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. . Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–18. 10.1056/NEJMoa1414792 [DOI] [PubMed] [Google Scholar]
- 3.Goyal M, Demchuk AM, Menon BK, et al. . Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–30. 10.1056/NEJMoa1414905 [DOI] [PubMed] [Google Scholar]
- 4.Albers GW, Marks MP, Kemp S, et al. . Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018;378:708–18. 10.1056/NEJMoa1713973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nogueira RG, Jadhav AP, Haussen DC, et al. . Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018;378:11–21. 10.1056/NEJMoa1706442 [DOI] [PubMed] [Google Scholar]
- 6.Saver JL, Goyal M, van der Lugt A, et al. . Time to treatment with endovascular thrombectomy and outcomes from ischemic stroke: a meta-analysis. JAMA 2016;316:1279–88. 10.1001/jama.2016.13647 [DOI] [PubMed] [Google Scholar]
- 7.Stroke Foundation National acute stroke services framework, 2019. Available: https://strokefoundation.org.au/What-we-do/Treatment-programs/National-stroke-services-frameworks
- 8.English JD, Yavagal DR, Gupta R, et al. . Mechanical Thrombectomy-Ready comprehensive stroke center requirements and endovascular stroke systems of care: recommendations from the endovascular stroke standards Committee of the Society of vascular and Interventional neurology (SVIN). Interv Neurol 2015;4:138–50. 10.1159/000442715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Veenendaal P, Yan B, Churilov L, et al. . Endovascular clot retrieval by Hub-and-Spoke service delivery is feasible compared with Direct-to-Mothership. Cerebrovasc Dis 2018;46:172–7. 10.1159/000490421 [DOI] [PubMed] [Google Scholar]
- 10.Menon BK, Sajobi TT, Zhang Y, et al. . Analysis of workflow and time to treatment on thrombectomy outcome in the endovascular treatment for small core and proximal occlusion ischemic stroke (escape) randomized, controlled trial. Circulation 2016;133:2279–86. 10.1161/CIRCULATIONAHA.115.019983 [DOI] [PubMed] [Google Scholar]
- 11.Froehler MT, Saver JL, Zaidat OO, et al. . Interhospital transfer before thrombectomy is associated with delayed treatment and worse outcome in the STRATIS registry (systematic evaluation of patients treated with Neurothrombectomy devices for acute ischemic stroke). Circulation 2017;136:2311–21. 10.1161/CIRCULATIONAHA.117.028920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Venema E, Groot AE, Lingsma HF, et al. . Effect of interhospital transfer on endovascular treatment for acute ischemic stroke. Stroke 2019;50:923–30. 10.1161/STROKEAHA.118.024091 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stroke Foundation Clinical guidelines for stroke management, 2019. Available: https://informme.org.au/Guidelines/Clinical-Guidelines-for-Stroke-Management
- 14.Wahlgren N, Ahmed N, Dávalos A, et al. . Thrombolysis with alteplase for acute ischaemic stroke in the safe implementation of thrombolysis in Stroke-Monitoring study (SITS-MOST): an observational study. The Lancet 2007;369:275–82. 10.1016/S0140-6736(07)60149-4 [DOI] [PubMed] [Google Scholar]
- 15.Rinaldo L, Brinjikji W, McCutcheon BA, et al. . Hospital transfer associated with increased mortality after endovascular revascularization for acute ischemic stroke. J Neurointerv Surg 2017;9:1166–72. 10.1136/neurintsurg-2016-012824 [DOI] [PubMed] [Google Scholar]
- 16.Rocha M, Jovin TG. Fast versus slow progressors of infarct growth in large vessel occlusion stroke: clinical and research implications. Stroke 2017;48:2621–7. [DOI] [PubMed] [Google Scholar]
- 17.Shah S, Xian Y, Sheng S, et al. . Use, temporal trends, and outcomes of endovascular therapy after interhospital transfer in the United States. Circulation 2019;139:1568–77. 10.1161/CIRCULATIONAHA.118.036509 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun CH, Nogueira RG, Glenn BA, et al. . "Picture to puncture": a novel time metric to enhance outcomes in patients transferred for endovascular reperfusion in acute ischemic stroke. Circulation 2013;127:1139–48. [DOI] [PubMed] [Google Scholar]
- 19.Turc G, Maïer B, Naggara O, et al. . Clinical scales do not reliably identify acute ischemic stroke patients with large-artery occlusion. Stroke 2016;47:1466–72. 10.1161/STROKEAHA.116.013144 [DOI] [PubMed] [Google Scholar]
- 20.Prabhakaran S, Ward E, John S, et al. . Transfer delay is a major factor limiting the use of intra-arterial treatment in acute ischemic stroke. Stroke 2011;42:1626–30. 10.1161/STROKEAHA.110.609750 [DOI] [PubMed] [Google Scholar]
- 21.Fassbender K, Grotta JC, Walter S, et al. . Mobile stroke units for prehospital thrombolysis, triage, and beyond: benefits and challenges. The Lancet Neurology 2017;16:227–37. 10.1016/S1474-4422(17)30008-X [DOI] [PubMed] [Google Scholar]
- 22.López-Cancio E, Ribó M, Cardona P, et al. . Telestroke in Catalonia: increasing thrombolysis rate and avoiding interhospital transfers. Cerebrovasc Dis 2018;46:66–71. 10.1159/000492124 [DOI] [PubMed] [Google Scholar]
- 23.Schlemm E, Ebinger M, Nolte CH, et al. . Optimal transport destination for ischemic stroke patients with unknown vessel status: use of prehospital triage scores. Stroke 2017;48:2184–91. 10.1161/STROKEAHA.117.017281 [DOI] [PubMed] [Google Scholar]
- 24.Holodinsky JK, Williamson TS, Demchuk AM, et al. . Modeling stroke patient transport for all patients with suspected large-vessel occlusion. JAMA Neurol 2018;75:1477–86. 10.1001/jamaneurol.2018.2424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mulder MJHL, Jansen IGH, Goldhoorn R-JB, et al. . Time to endovascular treatment and outcome in acute ischemic stroke: Mr clean registry results. Circulation 2018;138:232–40. 10.1161/CIRCULATIONAHA.117.032600 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
bmjno-2019-000030supp001.pdf (49.7KB, pdf)