Abstract
To evaluate the effectiveness of an integrated emergency department (ED)/hospital at home (HH) medical care model in mild COVID-19 pneumonia and evaluate baseline predictors of major outcomes and potential savings. Retrospective cohort study with patients evaluated for COVID-19 pneumonia in the ED, from March 3 to April 30, 2020. All of them were discharged home and controlled by HH. The main outcomes were ED revisit and the need for deferred hospital admission (protocol failure). Outcome predictors were analyzed by simple logistic regression model (OR; 95% CI). Potential savings of this medical care model were estimated. Of the 377 patients attended in the ED, 109 were identified as having mild pneumonia and were included in the ED/HH medical care model. Median age was 50.0 years, 52.3% were males and 57.8% had Charlson index ≥ 1. The median HH stay was 8 (IQR 3.7–11) days. COVID-19-related ED revisit was 19.2% (n = 21) within 6 days (IQR 3–12.5) after discharge from ED. Overall protocol failure (deferred hospital admission) was 6.4% (n = 7), without ICU admission. The ED/HH model provided potential cost savings of 77% compared to traditional stay, due to the costs of home care entails 23% of the expenses generated by a conventional hospital stay. 789 days of hospital stay were avoided by HH, rather than hospital admission. An innovative ED/HH model for selected patients with mild COVID-19 pneumonia is feasible, safe and effective. Less than 6.5% of patients requiring deferred hospital admission and potential savings were generated due to hospitalization.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11739-021-02661-8.
Keywords: COVID-19, Pneumonia, Hospital based home cares, Emergency department
Introduction
The clinical spectrum of pneumonia in SARS-CoV-2 disease (COVID-19) varies from mild episodes to severe cases of respiratory distress [1, 2]. Patients with moderate or severe disease are generally admitted to hospital for the high risk of complications [3–6].
However, mild cases are usually discharged and recover at home with symptomatic treatment and isolation [7]. There are few studies in the emergency department (ED) setting describing the clinical and evolutive characteristics of patients with SARS-CoV-2 pneumonia discharged home directly from the ED [8–11].
Hospitalization at home (HH) is a healthcare approach that provides active treatment by healthcare professionals in the patient’s home for a condition that would require hospital treatment [12–14]. HH allows providing care to patients with mild or moderate disease discharged from the ED, which generates a greater availability of hospital beds for more severe cases. This model of medical care integrates caregivers in the health care process, providing greater information and health care education, avoiding hospital visits, and reducing nosocomial infections [15].
Several data have been published regarding prognostic factors of poor outcome in hospitalized patients [16] but frequently without adjustment for confounders. To date, no series has evaluated independent prognostic factors of ED return in mild COVID-19 pneumonia after discharge.
Early identification in the ED of patients with COVID-19 disease who may develop critical illness, and therefore require hospital admission is of great importance, as it allows selecting patients who might benefit from an outpatient care model and thereby optimize the use of resources [17–19]. An integrated emergency—medical care at home system as an intermediate approach between outpatient control in primary care and hospital admission, is a new model for the management of cases of mild pneumonia in an overloaded health care system [20].
This study aims to evaluate the effectiveness of an integrated emergency department—hospital at home (ED/HH) medical care model in mild COVID-19 pneumonia and identify baseline predictors of major outcomes during follow-up according to the characteristics of the patients in the first visit to the ED, as well as estimate the potential savings of this medical care model.
Methods
Study design and patients
The study included a retrospective cohort of patients evaluated for COVID-19 pneumonia in the ED of a university hospital in Southeastern Spain from March 3 to April 30, 2020 according to the protocol for COVID-19 diagnosis and management established by the local Infections and Antibiotics commission. The Ethics Committee of the University General Hospital of Alicante, Spain, approved the study protocol. The research has been carried out following the general recommendations and, specifically, regarding the confidentiality of data collected in the Helsinki declaration of investigation.
Selection of participants
Those patients with diagnosis of COVID-19 were selected. For this, a positive test result for SARS-CoV-2 by reverse transcriptase–polymerase chain reaction (RT-PCR), mainly in oropharyngeal aspirates, was required. 109 patients (32.2%) over 14 years of age were identified in the ED as having mild pneumonia, without high comorbidity or immunosuppression and were included in the study (Fig. 1). The criteria for mild pneumonia included: mild unilobar or multilobar alveolar pneumonia (radiological opacities < 50% pulmonary area) without dyspnea, sat02 ≥ 95% (FiO2 0.21), PaO2:FiO2 > 300 and a respiratory rate < 20 rpm, normal glutamic oxaloacetic transaminase (GOT)/glutamic pyruvic transaminase (GPT) and lactate dehydrogenase (LDH) < 232 U/L, D-dimer < 1000 ng/ml, lymphocyte count > 1200 per mm3, and a normal 50 m walking test (pulse oximetry saturation: desaturation < 5 points, and > 93%).
Fig. 1.
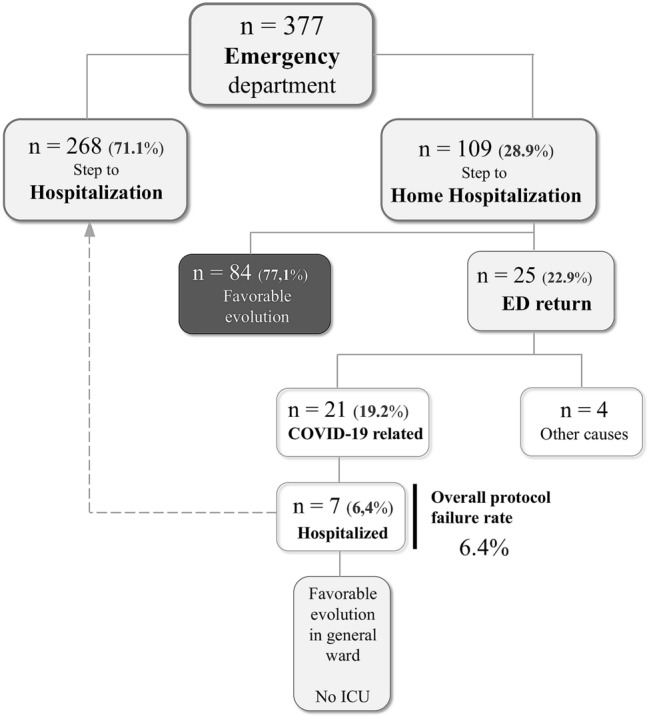
Flowchart of COVID-19 pneumonia cases evaluated in the hospital. ICU intensive care unit, ED emergency department
The exclusion criteria for the ED/HH care model were: the impossibility of oral intake or intolerance, any psychiatric condition, drug dependence or active alcoholism; the absence of adequate housing (optimal health conditions and with a telephone line); the impossibility of carrying out self-care, administration and monitoring of oral medication; being unable to perform simple techniques (blood glucose, temperature, pulse oximetry, among others); and residence outside the corresponding HH coverage area. All of them are circumstances that hinder optimal care at home.
Interventions
After discharge, the protocol established active HH tracing. All patients who were offered but declined HH care were admitted to acute hospital care. The emergency physician contacted the HH unit and informed of the ED discharge home. The clinical criteria for hospital at home admission were patients with unilateral or bilateral pneumonia of less than one field extension and without comorbidity, age below 60 years and without high-risk laboratory abnormalities (increased troponins, ferritin, D dimer and LDH). They were also defined as criteria for referral to hospital at home, patients with comorbidity (heart failure, stage III–IV chronic kidney disease, diabetes mellitus, moderate-severe chronic obstructive pulmonary disease or other severe comorbidities) without evidence of pneumonia on chest X-rays studied by an expert radiologist. In addition to the clinical criteria, the admission in hospital at home requires family support to maintain relative vigilance and good communication with the unit team.
Patients were evaluated by the HH physician within 24 h after discharge from the ED. The patient had subsequent direct one-on-one nursing supervision for a period of 24 h and intermittent nursing visits at least once daily. The HH physician made daily home visits, had access to blood tests and chest X-rays if they considered it necessary, and was available 24 h a day for urgent or emergency visits. Nursing and other care components, such as medical equipment, oxygen therapy, skilled therapies were provided at home, as well as pharmaceutical support including hydroxychloroquine (5 days) alone or associated with azithromycin or amoxicillin. Disease-specific HH clinical outcome evaluations and specific discharge criteria were developed and provided a pathway for care. Adherence to infection prevention and control recommendations during home isolation was checked (Supplementary-material 1). The HH physician followed the patient until they were considered stable enough for discharge, at which time care reverted to the primary care physician. In the case of rapid progression of respiratory failure through clinical evaluation, systemic inflammatory response or significant radiological progression, the patient was referred to the ED for reevaluation.
The study population was categorized into two subgroups: patients returning to the ED due to COVID-19 and those with a favorable outpatient evolution.
Variables and data collection
Exploratory variables
Demographic data as well as data related to signs and symptoms, underlying comorbidities, Charlson comorbidity index, usual medications, imaging, laboratory results and treatments received were collected from electronic medical records (Supplementary material 2).
Outcomes
(1) Return to the ED (95% confidence interval [CI]) and associated factors. (2) Deferred hospital admission requirement (protocol failure) (95% CI). (3) Potential ED/HH care model savings.
To calculate savings, the median HH patients stay and the total number of days of the cohort that did not require deferred hospital admission was considered. The daily saving (338.53 $/day) was calculated considering the difference with the average cost of a day of hospital admission in internal medicine (439.85 $/day) and hospital at home (101.32 $/day).
The days of home admission were calculated, and the savings generated compared to the same days in a conventional hospitalization were considered. Admissions to intensive care units were not taken into account, because there were no patients who required such assistance.
Statistical analysis
Categorical variables are expressed as frequencies and their percentages (%). Continuous variables are shown as median (interquartile range [IQR]). The measure of frequency used was the cumulative incidence for ED return of each of the exposure variables. An association study was made with the Chi square test and the magnitude of the association by a simple logistic regression model. Due to the impossibility of maintaining the ratio variable/event 1:10, to avoid risks of overfitting, the multivariate analysis was not established, as it was considered unstable. So, we focus on the univariate associations.
A p value < 0.050 was defined as being statistically significant. Analyses were performed using IBM SPSS Statistics v25 (Armonk, NY).
Results
Table 1 shows the general characteristics of the study population. The subjects were mainly young in age (median 50.0 years old (IQR 40.0–59.5)), with a low presence of comorbidities, low-grade inflammation and raised inflammatory markers and absence of lymphopenia. Two out of three presented bilateral pneumonia but showed radiological opacities that did not extensively involve the lungs. The 50-m walk test was only pathological in one patient who belonged to the medical team of the health area and requested ambulatory assistance. No patient was lost to follow-up.
Table 1.
General characteristics of the study population and risk factors associated with COVID-19 emergency department return
| Total population [n = 109] |
ED return due to COVID-19 [n = 21] n (%) |
ED no return [n = 84] n (%) |
OR (95%CI) | P value | |
|---|---|---|---|---|---|
| Demographics | |||||
| Age, years | 50 (40—59.5) | 52.0 (40.0–62.0) | 51.2 (42.0–58.2) | ||
| < 65 | 93 (85.3) | 18 (85.7) | 75 (89.2) | 1 (ref) | |
| ≥ 65 | 16 (14.7) | 3 (14.3) | 9 (10.7) | 0.96 (0.25–3.73) | .955 |
| Gender, % | |||||
| Male | 56 (52.3) | 8 (38.1) | 49 (58.3) | 1(ref) | |
| Female | 53(47.7) | 13 (61.9) | 35 (41.7) | 2.04 (0.77–5.42) | .152 |
| Long-term care resident | 0.9 | 0.0 | |||
| No | 108 (99.1) | 21(100) | 84 (100) | 1(ref) | |
| Yes | 1 (0.01) | 0(0.0) | 0 (0.0) | NC | - |
| Health professional % | |||||
| No | 91 (83.5) | 14 (66.7) | 74 (88.1) | 1(ref) | |
| Yes | 18 (16.5) | 7 (21.1) | 10 (11.9) | 3.50 (1.16–10.57) | .026 |
| Comorbidities | |||||
| Hypertension | |||||
| No | 89 (81.7) | 10 (47.6) | 56 (66.7) | 1(ref) | |
| Yes | 20 (18.3) | 3 (14.3) | 28 (33.3) | 0.69 (0.18–2.64) | .594 |
| Type 2 diabetes | |||||
| No | 100 (91.7) | 17 (81) | 83 (98.8) | 1(ref) | |
| Yes | 9 (8.3) | 4 (19.0) | 1 (1.2) | 3.91 (0.95–16.07) | .059 |
| Body mass index, kg/m2 | 26.4 (22.9–29.3) | 26.3 (24.5–30.4) | 25.2 (24.6–29.6) | ||
| Obesity | |||||
| No | 83 (76.1) | 15 (71.4) | 68 (80.1) | 1(ref) | |
| Yes | 26 (23.9) | 6 (28.6) | 16 (19.0) | 1.36 (0.47–3.96) | .573 |
| Cardiovascular disease | |||||
| No | 100 (91.7) | 18 (85.7) | 82 (97.6) | 1(ref) | |
| Yes | 9 (8.3) | 3 (14.2) | 2 (2.4) | 2.22 (0.51–9.74) | .289 |
| Smoke | |||||
| No | 94 (86.2) | 18 (85.7) | 76 (90.5) | 1(ref) | |
| Yes | 15 (13.9) | 3 (14.2) | 8 (9.5) | 1.04 (026–4.08) | .953 |
| Chronic respiratory disease | |||||
| No | 90 (82.6) | 18 (85.7) | 72 (85.7) | 1(ref) | |
| Yes | 19 (17.4) | 3 (14.3) | 12 (14.3) | 0.73 (0.19–2.78) | .643 |
| Immunosuppression | |||||
| No | 104 (95.4) | 19 (90.5) | 81 (96.4) | 1(ref) | |
| Yes | 5 (4.6) | 2 (9.5) | 3 (3.6)) | 2.98 (0.46–19.10) | .249 |
| Charlson comorbidity index | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | 1.0 (0.0–2.0) | ||
| Charlson index ≥ 1 | |||||
| No | 46 (42.2) | 6 (28.6) | 40 (47.6) | 1(ref) | |
| Yes | 63 (57.8) | 15 (71.4) | 44 (52.4) | 2.08 (0.74–5.87) | .165 |
| 10-year expected survival, %a | 95.8 (77.4–98.3) | 95.8 (83.7–98.1) | 94.6 (84.5 – 99.1) | ||
| > 96 | 40 (36.7) | 6 (28.6) | 34 (40.5) | 1(ref) | |
| < 96 | 66 (60.6) | 12 (57.1) | 50 (59.5) | 1.26 (0.43–3.67) | .673 |
| Clinical presentation | |||||
| Clinical duration, daysb | 7.00 (4.0–10.0) | 6.5 (3.2–9.5) | 7.8 (3.5 – 9.6) | ||
| > 7 | 60 (55.0) | 10 (47.6) | 50 (59.5) | ||
| < 7 | 48 (44.0) | 11 (52.4) | 34 (40.4) | 1.32 (0.49–3.48) | .580 |
| Fever (temperature > 37.8 ºC) | |||||
| No | 39 (35.8) | 10 (47.6) | 29 (34.5) | 1(ref) | |
| Yes | 70 (64.2) | 11 (52.3) | 55 (65.4) | 0.54 (0.21–1.42) | .212 |
| Dry cough | |||||
| No | 39 (35.8) | 8 (38.1) | 31 (36.9) | 1 (ref) | |
| Yes | 69 (63.9) | 13 (11.9) | 53 (63.1) | 0.90 (0.34–2.41) | .833 |
| Wet cough | |||||
| No | 92 (84.4) | 18 (85.7) | 74 (88) | 1 (ref) | |
| Yes | 17 (15.6) | 3 (14.3) | 10 (11.9) | 0.88 (0.23–3.39) | .854 |
| Dyspnea | |||||
| No | 64 (58.7) | 10 (47.6) | 54 (64.3) | 1 (ref) | |
| Yes | 45 (41.3) | 11 (52.4) | 30 (35.7) | 1.75 (0.67–4.55) | .254 |
| Diarrhea | |||||
| No | 87 (79.8) | 18 (85.7) | 69 (82.1) | 1 (ref) | |
| Yes | 22 (20.2) | 3 (14.2) | 15 (17.8) | 0.61 (0.16–2.27) | .457 |
| Confusion | |||||
| No | 109 (100) | 21 (100) | 84 (100) | 1 (ref) | |
| Yes | 0 (0.0) | 0 (0.0) | 0 (0.0) | NC | - |
| Fatigue | |||||
| No | 80 (73.3) | 16 (76.2) | 64 (76.1) | 1 (ref) | |
| Yes | 29 (26.6) | 5 (23.8) | 20 (23.8) | 0.83 (0.27–2.52) | .747 |
| Myalgias-arthralgias | |||||
| No | 60 (55) | 14 (66.7) | 46 (54.7) | 1 (ref) | |
| Yes | 49 (44.9) | 7 (33.3) | 38 (45.2) | 0.55 (0.20–1.48) | .237 |
| Anosmia-dysgeusia | |||||
| No | 92 (84.4) | 12 (57.1) | 80 (95.2) | 1 (ref) | |
| Yes | 17 (15.6) | 2 (9.5) | 4 (4.8) | 0.51 (0.11–2.43) | .401 |
| Initial assessment | |||||
| Room air oximetry, % | 97.0 (96.0–99.0) | 97.0 (95.5–99.5) | 98.0 (95.8 – 99.6) | ||
| > 97% | 48 (44.0) | 9 (42.8) | 39 (46.4) | 1 (ref) | |
| < 97% | 61 (55.9) | 12 (57.1) | 45 (53.6) | 1.06 (0.41–2.77) | .904 |
| PaO2/FiO2 | 448.0 (390.0–471.0) | 454.9 (397.5–478.4) | 458.9 (392.4 – 478.5) | ||
| > 448 | 73 (67) | 7 (33.3) | 66 (78.6) | 1 (ref) | |
| < 448 | 36 (33.0) | 5 (23.8) | 18 (21.4) | 0.62 (0.12–3.19) | .575 |
| Respiratory rate, breaths/min | 14.0 (12.0–16.0) | 15.0 (13.0–16.0) | 14.5 (12.4 – 15.3) | ||
| < 14 | 44 (40.4) | 5 (23.8) | 38 (45.2) | 1 (ref) | |
| > 14 | 65 (59.6) | 16 (76.2) | 46 (54.8) | 2.48 (0.83–7.38) | .102 |
| Systolic BP, mmHg | 137.5 (124.0–151.0) | 137.0 (122.0–141.5) | 132.3 (123.4 – 138.7) | ||
| > 137 | 55 (50.5) | 10 (47.6) | 45 (53.6) | 1 (ref) | |
| < 137 | 54 (49.5) | 11 (52.3) | 39 (46.4) | 1.10 (0.42–2.85) | .845 |
| Diastolic BP, mmHg | 87.0 (75.0–98.0) | 80.0 (71.5–84.5) | 78.2 (71.3 – 82.2) | ||
| > 87 | 51 (46.7) | 3 (14.2) | 48 (57.1) | 1 (ref) | |
| < 87 | 56 (51.3) | 18 (85.7) | 36 (42.9) | 7.57 (2.07–27.65) | .002 |
| Heart rate, bpm | 95 (86.0–103.0) | 90.0 (81.5–99.5) | 83 (81.2 – 102. 5) | ||
| > 95 | 52 (47.7) | 6 (28.6) | 46 (54.8) | 1 (ref) | |
| < 95 | 57 (52.2) | 15 (71.4) | 38 (45.2) | 1.79 (0.62–5.19) | .278 |
| Laboratory data | |||||
| eGFR, ml/min/m2 | 90.0 (83.6–90.0) | 90.0 (79.0–90.0) | 90.0 (82.4 – 90.09 | ||
| > 60 | 105 (96.3) | 19 (90.4) | 82 (97.6) | 1 (ref) | |
| < 60 | 4 (3.7) | 2 (9.5) | 2 (2.4) | 4.36 (0.57–33.01) | .153 |
| Creatine phosphokinase, U/L | 81.0 (55.0–121.0) | 82.0 (47.0–141.0) | 81.1 (60.2–140.4) | ||
| < 121 | 79 (72.4) | 14 (66.7) | 65 (77.4) | 1 (ref) | |
| > 121 | 28 (25.7) | 7 (33.3) | 19 (22.6) | 1.54 (0.55–4.34) | .407 |
| Troponin T, ng/L | 6.0 (5.0–7.0) | 5.5(5.0–12.5) | 5.2 (5.0 – 7.2) | ||
| < 7 | 77 (70.6) | 12 (4.8) | 65 (77.4) | 1 (ref) | |
| > 7 | 37 (33.9) | 8 (38.1) | 19 (22.6) | 1.26 (0.46–3.44) | .646 |
| Alanine aminotransferase, U/L | 21.0 (15.0–32.5) | 21.0 (13.0–36.5) | 16.6 (14.2 – 34.3) | ||
| < 30 | 78 (71.5) | 15 (71.4) | 53 (63) | 1 (ref) | |
| > 30 | 31 (28.4) | 6 (28.5) | 31 (36.9) | 1.01 (0.35–2.89) | .988 |
| Aspartate aminotransferase, U/L | 24.0 (19.0–30.0) | 24.0 (19.5–28.5) | 22 (19.8 – 27.4) | ||
| < 30 | 78 (71.6) | 17 (81) | 61 (72.6) | 1 (ref) | |
| > 30 | 31 (28.4) | 4 (19.0) | 23 (27.4) | 0.53 (0.16–1.72) | .294 |
| Brain natriuretic peptide, pg/mL | 36.5 (19.0–83.0) | 38.0 (23.0–64.2) | 35.2 (21.3 – 80.3) | ||
| < 83 | 75 (68.9) | 16 (76.2) | 59 (70.2) | 1 (ref) | |
| > 83 | 27 (24.8) | 4 (19.0) | 25 (29.8) | 0.64 (0.19–2.12) | .467 |
| C-reactive protein, mg/dL | 0.80 (0.19–3.16) | 0.5 (0.1–2.5) | 0.4 (0.2 – 0.8) | ||
| < 3 | 82 (75.2) | 17 (80.9) | 65 (77.4) | 1 (ref) | |
| > 3 | 27 (24.8) | 4 (19.0) | 19 (22.6) | 0.52 (0.11–2.53) | .420 |
| Procalcitonin, ng/mL | 0.040 (0.03–0.06) | 0.045 (0.03–0.07) | 0.04 (0.03 – 0.05) | ||
| < 0.06 | 36 (33.0) | 8 (38.1) | 28 (33.3) | 1 (ref) | |
| > 0.06 | 68 (62.3) | 12 (57.4) | 56 (66.7) | 0.75 (0.27–2.04) | .574 |
| Lactate dehydrogenase, U/L | 203.0 (172.0–232.0) | 201.0 (173.5–221.5) | 180.4 (173.4 -223.4) | ||
| < 232 | 79 (72.5) | 19 (90.5) | 60 (71.4) | 1 (ref) | |
| > 232 | 28 (25.7) | 2 (9.5) | 24 (28.6) | 0.24 (0.05–1.12) | .070 |
| D-dimers, ng/mL | 0.38 (0.23–0.61) | 0.34 (0.25–0.54) | 0.2 (0.1 – 0.5) | ||
| < 0.6 | 77 (70.1) | 17 (80.9) | 60 (71.4) | 1 (ref) | |
| > 0.6 | 28 (25.7) | 4 (19.0) | 24 (28.6) | 0.58 (0.17–1.92) | .381 |
| Leukocytes, per mm3 | 7000.0 (5505.0–8430.0) | 6460.0 (5040.0–8375.0) | 6890 (5038 – 7896.4) | ||
| < 8430 | 82 (75.2) | 17 (80.9) | 65 (77.4) | 1 (ref) | |
| > 8430 | 27 (24.8) | 4 (19.0) | 19 (22.6) | 0.66 (0.20–2.18) | .501 |
| Lymphocytes, per mm3 | 1750.0 (1210.0–2360.0) | 1500.0 (1075.0–1895.0) | 1345.2 (1080.2 -2320.4) | ||
| > 1210 | 82 (75.2) | 15 (71.4) | 67 (79.8) | 1 (ref) | |
| < 1210 | 27 (24.8) | 6 (28.6) | 17 (20.2) | 1.27 (0.44–3.71) | .654 |
| Ferritin, μg/L | 211.0 (106.2–456.7) | 220.5 (59.7–532.2) | 210.4 (140.5 -350.6 | ||
| < 450 | 32 (29.4) | 9 (42.9) | 23 (27.4) | 1 (ref) | |
| > 450 | 10 (9.2) | 3 (14.3) | 61 (72.6) | 1.09 (0.23–5.19) | .909 |
| Pneumonia features | |||||
| Bilateral pneumonia | |||||
| No | 40 (36.7) | 6 (28.5) | 34 (40.5) | 1 (ref) | |
| Yes | 69 (63.3) | 15 (71.4) | 50 (59.5) | 1.57 (0.55–4.45) | .392 |
| CURB65 score ≥ 1 | |||||
| No | 84 (77.1) | 16 (76.2) | 68 (80.9) | 1 (ref) | |
| Yes | 21 (19.3) | 5 (23.8) | 16 (19.0) | 1.32 (0.42–4.16) | .626 |
| Outpatient therapy | |||||
| Azithromycin | |||||
| No | 21 (19.3) | 5 (23.8) | 16 (19.0) | 1 (ref) | |
| Yes | 87 (79.8) | 16 (76.2) | 68 (80.9) | 0.72 (0.23–2.25) | .574 |
| Another antibiotic | |||||
| No | 74 (67.9) | 18 (85.7) | 56 (66.7) | ||
| Yes | 35 (32.1) | 3 (14.3) | 28 (33.3) | 0.28 (0.08–1.05) | .059 |
Data shown as median (p25-75), % (Risk Factor Present) unless otherwise specified; number of patients who returned / total number (%). “P” in bold, statistically significant differences. a10-year expected survival derived from Charlson comorbidity index score. bDays of symptoms before admission
Continuous covariates were categorized based on their median levels, except laboratory parameters that were categorized regarding their 75-percentiles, to show the impact of severe, extreme values in the outcomes – except for those in which severity is defined by lowest levels, such as lymphocyte counts, where 25-percentiles were used. For the following variables, standard categorizations were followed: age ≥ 65 years, Charlson comorbidity index ≥ 3, estimated glomerular filtration rate (eGFR, by CKD-EPI formula) < 60 ml/min/1.73 m2, and CURB65 ≥ 1
BP, blood pressure; CURB65, Severity Score for Community-Acquired Pneumonia; eGFR, estimated glomerular filtration rate (by CKD-EPI formula). Outpatient therapy also included hydroxychloroquine, amoxicillin, other antibiotics, without impact on return to emergency department (data not reported)
Out of 109 patients, 108 (99%) were actively treated with hydroxychloroquine associated with azithromycin in 87 (79.8%) and with amoxicillin in 50 (45.8%). Subcutaneous heparin was initiated in 18 patients (16.5%). All this as indicated by the hospital protocols, at the time of the study. The median HH stay was 8 (IQR 3.7–11) days; 44.4% of patients presented new symptoms: 24.2% cough, 18.2% diarrhea, 11.7% dyspnea and 8.1% pain.
Outcome: ED return
The ED COVID-19-related return rate was 19.2% (95% CI 12.9–27.6) (n = 21), due to general and respiratory symptoms, and occurred within first 6 days (IQR 3.0–12.5) after ED discharge. Of them, seven patients required hospital admission while 14 were discharged after ruling out complications. Four patients also returned due to unrelated causes.
The association between ED return and explanatory variables is shown in Table 1.
However, due to limited sample size, multivariable logistic regression model was not estimated to establish independent associations. The multivariate regression model could be unstable and generate risks of overfitting, due to the high number of variables and the limited number of events (21), losing the recommended 1:10 ratio.
Outcome: deferred hospital admission
The overall protocol failure rate was 6.4% (95% CI 3.1–12.6) (n = 7). Four men and three women required hospital admission related to COVID-19, having a median age of 55 years (IQR 46.0–57.0), a body mass index of 25.7 (IQR 23.1–28.7) and a Charlson index ≥ 1 in four patients. The reasons for the revisit were the worsening of dyspnea. They were subjected to other imaging tests (computer tomography) and finally were admitted for radiological and clinical worsening of their pneumonia. Respiratory failure was detected in four of them. Tocilizumab was used in two patients. The hospital stay ranged from 4 to 11 days, and no patient required admission to the intensive care unit (ICU). The median follow-up was 32 days (IQR 24–36).
Outcome: Potential ED/HH care model savings
The ED/HH model provided potential savings per mild COVID-19 pneumonia patient, not requiring hospital admission (102 patients). Savings could reach 338.53 $/day (internal medicine admission cost 439.85 $/day versus HH cost 101.32 $/day). Direct cost per day was reduced 77% through HH, due to the costs of home care entails 23% of the expenses generated by a conventional hospital stay. The total number of stay days in HH avoiding hospital admission was 789.
Discussion
The ED/HH care model allowed the outpatient management of 109 patients with mild COVID-19 pneumonia. The protocol for COVID-19 diagnosis and management established by the local Infections and Antibiotics commission is feasible and effective, with less than 20% of patients requiring a new ED visit, and less than 6.5% needing delayed hospital admission, providing an estimated potential savings of 77% of medical care per patient due to avoiding hospital admission.
To our knowledge, this is the first study of an ED/HH model that completely substitutes acute hospital care in patients with mild COVID-19 pneumonia. Scarce substitution models with this integrated ED/HH approach have been reported [14, 21]. The ED/HH model reported is unique in several differential aspects. Firstly, there are no studies analyzing the evolution of patients with COVID-19 pneumonia directly discharged home from the ED and referred to a home care service. Secondly, the selection of patient criteria for ED discharge showed to be adequate according to the low rates of complications and ED revisits, as well as the absence of adverse drug effects (hydroxychloroquine and/or azithromycin) and deferred hospital admissions less than 6.5%. Furthermore, none of the patients admitted to hospital presented severe disease requiring ICU admission or the use of non-invasive ventilation. Lastly, the present ED/HH model provided an intensive level of medical services not offered in many studies of HH care. The substantial medical supervision provided was appropriate because of the characteristics of the patients and the disease, as it provided timely care; that is not part of the management of standard community-based home care services [7, 22].
Health system designers should view provision of care in the site that will be most efficient in improving health in developing care delivery innovations. It is time to move beyond the limited home care-payment models and create sustainable new clinical models of care provided in home-based settings [7]. In this sense, the Alicante ED/HH model provided a potential to reduce direct costs due to admission by more than 75%.
Strategic tools to predict risk are necessary in patients with COVID-19 taking into account the limited health resources available against the SARS-CoV-2 pandemic [16, 23]. Nevertheless, predictors should also be assessed considering different clinical settings.
Despite the fact that some variables showed an association in the univariate model (p < 0.2), the limited number of events did not make it possible to estimate the association using the multivariate model. In the simple logistic regression model, low DBP, as an early marker of the increase in cardiac output or systolic volume or arterial stiffness in young adults [24], was associated with a 30-day ED revisit. In the multivariate analysis of several studies [26, 27], diastolic blood pressure on hospital admission constitutes poor prognosis independent factor in patients evaluated in the emergency department with pneumonia. It constitutes part of the variables of different pneumonia risk scales [28]; however, in our study, the association was not estimated using a multivariate model, due to limited sample.
It seems that COVID-19 involves the cardiovascular system in the early stages of the disease, complicating the clinical course by inflammatory response and microvascular-endothelial dysfunction. In this setting, monitoring DBP, in the ED visit could help stratify risk and potentially lead to earlier and more intensive therapy.
The meaning of high concentrations of LDH (within the reference range) as a protective factor in returning to the ED is uncertain, however, we cannot confirm that in mild pneumonia managed in an outpatient setting it does not behave as an independent marker of poor evolution [16, 28].
The main knowledge derived from the Alicante cohort for emergency area clinicians was that the factors classically related to worse outcomes in hospitalized patients with severe pneumonia, such as being a man, the presence of obesity, worse respiratory parameters and some analytical markers (lymphopenia, ferritin, d-dimer, T-troponin) [15], were not useful to predict the need for a new emergency consultation.
One of the limitations of this study was that it was a brief observational, retrospective, single-center study, and data collection was not systematized in advance.
One of the main limitations was the sample size, due to the low incidence of unfavorable events in patients who were admitted to the HH, a larger sample size would be necessary to establish significant associations.
Efforts were undertaken to capture and revise data by a clinical team with experience in COVID-19. As a non-randomized study conclusion cannot be drawn as to decision-making related to direct discharge of patients with COVID-19 pneumonia from the ED. The mild nature of the pneumonia presented, together with the clinical profile, made part of the study population candidates for follow-up at home by their general physician, without the need for HH support. Finally, the following considerations should also be taken into account: as this was a hospital-based cohort, predictors might not be applicable to outpatients with COVID-19; alternative models to conventional hospitalization must be adapted to the health care needs of each setting, and therefore, the sociodemographic and health circumstances of our health department may differ from those of other departments, and thus, caution should be taken when extrapolating our results.
In summary, our study shows that an innovative ED/HH model for selected patients with mild COVID-19 pneumonia is feasible and effective. As the burden of COVID-19 and the demand for acute hospital services increase, correct identification of cases with low probability of poor evolution in the ED may aid in delivering proper care, referral to HH care and optimization of the use of limited resources. These findings contribute to better defining the picture of mild outpatient COVID-19 pneumonia and support the reporting of ambulatory outcome rates in future research.
Supplementary Information
Below is the link to the electronic supplementary material.
Author contributions
PLL, OMP, EM, JG, JSP and DSI conceived and designed the study. PLL, BE, TG, SS, SG, ABP, FM and AGR supervised the conduct of the study and data collection. BP, FR, MA and IJ undertook recruitment of participating centres and patients and managed the data, including quality control. OMP, MA and JSP provided statistical advice on study design and analysed the data; PLL and OMP drafted the manuscript, and all authors substantially contributed to its revision. PLL takes responsibility for the paper as a whole.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Compliance with ethical standards
Conflict of interest
Without potential conflicts of interest for each author: PLL reports no conflict of interest, PLL reports no conflict of interest, OMP reports no conflict of interest. BE reports no conflict of interest, TG reports no conflict of interest, ABP reports no conflict of interest. SS reports no conflict of interest, FM reports no conflict of interest, AGR reports no conflict of interest, FR reports no conflict of interest, IJ reports no conflict of interest, SG reports no conflict of interest, BP reports no conflict of interest, EM reports no conflict of interest, JG reports no conflict of interest, DSI reports no conflict of interest, MA reports no conflict of interest, JSP reports no conflict of interest.
Human and animal rights statement
This work was approved by the Ethics Committe of the University General Hospital of Alicante (Spain). Under the exceptional circumstances generated by the COVID-19 pandemic, the urgent need to obtain feasible data related to this new disease, and the noninterventional and retrospective nature to the project, the requirement that written patient consent be obtained to be included in the study was waived. All patients were codified by investigators of the participating centers before entering their data into the general database, thereby ensuring patient anonymity to investigators analyzing the database. The project was carried out in strict compliance with the principles of the Declaration of Helsinki.
Informed consent
For this type of study, formal consent is not required.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Pere Llorens and Oscar Moreno-Pérez contributed to the manuscript equally and share first authorship.
References
- 1.Wang C, Horby PW, Hayden FG, Gao GF. A novel coronavirus outbreak of global health concern. Lancet. 2020;395:470–473. doi: 10.1016/S0140-6736(20)30185-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu N, Zhang D, Wang W, Li X, Yang B, Song J, et al. A Novel Coronavirus from patients with pneumonia in China, 2019. N Engl J Med. 2020;382:727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guan WJ, Ni ZY, Hu Y, Liang WH, Ou CQ, He JX, et al. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Song F, Shi N, Shan F, Zhang Z, Shen J, Lu H, et al. Emerging 2019 novel coronavirus (2019-nCoV) Pneumonia. Radiology. 2020;295:210–217. doi: 10.1148/radiol.2020200274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang F, Deng L, Zhang L, Cai Y, Cheung CW, Xia Z. Review of the clinical characteristics of coronavirus disease 2019 (COVID-19) J Gen Intern Med. 2020;35:1545–1549. doi: 10.1007/s11606-020-05762-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gandhi RT, Lynch JB, Del Rio C. Mild or moderate Covid-19. N Engl J Med. 2020;383:1757–1766. doi: 10.1056/NEJMcp2009249. [DOI] [PubMed] [Google Scholar]
- 8.López-Barbeito B, García-Martínez Am Coll-Vinent B, Arrate-Placer CF, Vargas CR, Sánchez, et al. Factores asociados a revisita en pacientes con diagnóstico de infección por SARS-CoV-2 dados de alta de un servicio de urgencias hospitalario. Emergencias. 2020;32:386–394. [PubMed] [Google Scholar]
- 9.Berdahl CT, Glennon NC, Henreid AJ, Torbati SS. The safety of home discharge for low-risk emergency department patients presenting with coronavirus-like symptoms during the COVID-19 pandemic: a retrospective cohort study. JACEP Open. 2020 doi: 10.1002/emp2.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hernández-Biette A, Sanz-Santos J, Boix-Palop L, Navarro Rolón A, Martínez-Palau M, De la Sierra IA. Factores de riesgo de rehospitalización en pacientes con COVID-19 leve tras el alta desde el servicio de urgencias. Emergencias. 2020;32:413–415. [PubMed] [Google Scholar]
- 11.Gil-Rodrigo A, Miró O, Piñera P, Burillo-Putze G, Jiménez S, Martín A, et al. Evaluación de las características clínicas y evolución de pacientes con COVID-19 a partir de una serie de 1000 pacientes atendidos en servicios de urgencias españoles. Emergencias. 2020;32:233–241. [PubMed] [Google Scholar]
- 12.Leff B, Soones T, DeCherrie L. The Hospital at Home program for older adults. JAMA Intern Med. 2016;176:1724. doi: 10.1001/jamainternmed.2016.6307. [DOI] [PubMed] [Google Scholar]
- 13.Arias-de la Torre J, Zioga EAM, Muñoz L, Estrada D, Espallargues M. Early-discharge and admission-avoidance hospital-at-home programs: outcomes and associated factors. Emergencias. 2019;31:440–512. [PubMed] [Google Scholar]
- 14.Conley J, O’Brien CW, Leff BA, Bolen S, Zulman D. Alternative strategies to inpatient hospitalization for acute medical conditions. JAMA Intern Med. 2016;176:1693. doi: 10.1001/jamainternmed.2016.5974. [DOI] [PubMed] [Google Scholar]
- 15.Leff B, Burton L, Mader SL, Naughton B, Burl J, Inouye SH, et al. Hospital at home: feasibility and outcomes of a program to provide hospital-level care at home for acutely ill older patients. Ann Intern Med. 2005;143:798–808. doi: 10.1007/s11606-018-4307-z. [DOI] [PubMed] [Google Scholar]
- 16.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395:1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Froes F, Pereira JG, Póvoa P. Outpatient management of community-acquired pneumonia. Curr Opin Infect Dis. 2018;31:170–176. doi: 10.1097/QCO.0000000000000435. [DOI] [PubMed] [Google Scholar]
- 18.Castro Delgado R, Arcos GP. Analysis of health response capacity as a key element in planning for epidemic emergencies. Emergencias. 2020;32:157–159. [PubMed] [Google Scholar]
- 19.Juan Torres-Macho J, Ryan P, Valencia J, Pérez-Butragueño M, Jiménez E, Fontán-Vela M, et al. The PANDEMYC score. An easily applicable and interpretable model for predicting mortality associated with COVID-19. J. Clin. Med. 2020;9:3066. doi: 10.3390/jcm9103066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corrado OJ. Hospital-at-home. Age Ageing. 2001;30(Suppl 3):11–14. doi: 10.1093/ageing/30.suppl_3.11. [DOI] [PubMed] [Google Scholar]
- 21.Freund Y, Philippon AL. Patients with infections in the emergency department: What should we look for? Emergencias. 2020;32:75–76. [PubMed] [Google Scholar]
- 22.Rivas-Clemente FPJ, Pérez-Baena S, Ochoa-Vilor S, Hurtado-Gallar J. Patient-initiated emergency department visits without primary care follow-up: frequency and characteristics. Emergencias. 2019;31:234–238. [PubMed] [Google Scholar]
- 23.Liang W, Liang H, Ou L, Chen B, Chen A, Li C, et al. Development and validation of a clinical risk score to predict the occurrence of critical illness in hospitalized patients with COVID-19. JAMA Intern Med. 2020;180(8):1–9. doi: 10.1001/jamainternmed.2020.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Strandberg TE, Pitkala K. What is the most important component of blood pressure: systolic, diastolic or pulse pressure? Curr Opin Nephrol Hypertens. 2003;12(3):293–329. doi: 10.1097/00041552-200305000-00011. [DOI] [PubMed] [Google Scholar]
- 25.Rider AC, Frazee BW. Community-acquired pneumonia. Emerg Med Clin North Am. 2018;36:665–683. doi: 10.1016/j.emc.2018.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lim WS, Van der Eerden MM, Laing R, Boersma WG, Karalus N, Town GI, et al. Defining community acquired pneumonia severity on presentation to hospital: an international derivation and validation study. Thorax. 2003;58:377–382. doi: 10.1136/thorax.58.5.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buising KL, Thursky KA, Black JF, MacgGregor L, Street AC, Kennedy MP, et al. A prospective comparison of severity scores for identifying patients with severe community acquired pneumonia: reconsidering what is meant by severe pneumonia. Thorax. 2006;61:419–424. doi: 10.1136/thx.2005.051326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Du R-H, Liang L-R, Yang C-Q, Wang W, Cao T-Z, Li M, et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: a prospective cohort study. Eur Respir J. 2020;55:2000524. doi: 10.1183/13993003.00524-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


