Abstract
Objective:
Psychotropic medication use in youth with eating disorders (EDs) is poorly understood despite high co-occurrence of psychiatric disorders. This study examined characteristics associated with medication use in treatment-seeking youth with EDs.
Method:
Youth up to age 18 reported on medication use when presenting to an academic medical center outpatient ED service in the United States. Data presented were collected between 1998–2015.
Results:
The sample (N = 604) was predominantly female (90.6%) with a mean age of 15.3 years (SD = 2.3). Approximately one-third (30%, n =173) were taking psychotropic medications (40%, n =70, were taking multiple medications). Antidepressant use was most common (26%, n =152), followed by atypical antipsychotics (8%, n = 43). Adjusting for co-occurring psychiatric disorders, non-Hispanic Whites who had received prior treatment (psychotherapy, hospitalization) were significantly more likely to be using medication. Longer illness duration and prior treatment were associated with greater antidepressant use. For atypical antipsychotics, prior hospitalization was associated with greater use.
Conclusions:
Findings confirm moderate psychotropic medication use among young patients with EDs despite a lack of clarity regarding optimally effective pharmacologic interventions in this population. Pharmacological trials examining the efficacy of medications for young patients with EDs are warranted to inform future prescribing practice.
Keywords: psychotropic medication, eating disorders, anorexia nervosa, bulimia nervosa, adolescents
Eating disorders (EDs) treatment requires multidisciplinary coordination of medical, nutritional, and psychological care (Watson & Bulik, 2013). To date, less is known about prevalence and patterns of psychotropic medication use among children and adolescents. Despite the relatively high rate of co-occurring psychiatric disorders (e.g., depressive and anxiety disorders; obsessive-compulsive disorder) in youth with EDs (55–88%) (Swanson et al., 2011), psychotropic medication use in this population has rarely been investigated. One study of medication use among youth with a range of psychiatric disorders in the United States (U.S.) found that use was the third highest for those with EDs (19%), preceded only by attention deficit hyperactivity disorder (31%), and mood disorders (20%) (Merikangas et al., 2013). Given seemingly extensive medication use and the lack of significant empirical data supporting medication use if prescribed for EDs in youth, patterns of use in this population warrant attention.
Results from pharmacological treatment studies for youth with EDs have been discouraging or inconclusive (Couturier & Lock, 2007; Couturier et al., 2019). While there are several randomized controlled trials of psychological treatments (c.f., Eisler et al., 2007; Le Grange et al., 2007; Le Grange et al., 2015; Lock et al., 2010; Schmidt et al., 2007), there have been relatively few randomized controlled psychopharmacological trials in this population (Goldberg et al., 1980; Hagman et al., 2011; Kafantaris et al., 2011), with promising but less rigorous data from non-randomized trials (Spettigue et al., 2018) or case series (Dennis et al., 2006; Mehler et al., 2001; Pisano et al., 2014).
Atypical antipsychotics have demonstrated some positive effects in adults with anorexia nervosa (AN) for facilitating weight gain (Attia et al., 2019; Attia et al., 2011; Bissada et al., 2008) and decreasing ED cognitions (Attia et al., 2011; Bissada et al., 2008). However, results in adolescent AN trials have been mixed, with some studies showing benefits in weight gain (Spettigue et al., 2018) and others finding no significant effect on weight or ED symptoms (Hagman et al., 2011; Kafantaris et al., 2011; Kishi et al., 2012; Lebow et al., 2013). Research examining antidepressants in weight-restored adult samples with AN has demonstrated limited efficacy as an adjunct to outpatient therapy (Brambilla et al., 2007; Holtkamp et al., 2005) or in reducing relapse in adults with AN (Walsh et al., 2006). The American Psychiatric Association (APA) recommends delaying the prescription of antidepressants in adults with AN until weight restoration has been achieved (APA, 2006). Data on youth with AN are even less robust, with limited studies demonstrating effectiveness for antidepressants.
Fluoxetine has been approved by the U.S. Food and Drug Administration (FDA) and the United Kingdom National Institute for Clinical Excellence (NICE) for adults with bulimia nervosa (BN) (Crow, 2019), and there is preliminary support for its use as an adjunctive treatment for youth with BN (Kotler et al., 2003; www.nice.org.uk). For adults with binge eating disorder (BED), FDA-approved lisdexamfetamine (i.e., Vyvanse) may reduce binge eating behavior (see Crow [2019] for review), but to date, no large-scale trials with this medication have been conducted in youth with BED.
Other medication classes have been examined in adult samples (e.g., anticonvulsants) but have yet to be tested for evidence to support their use in youth with EDs. Providers also prescribe psychotropic medication (e.g., stimulants; mood stabilizers) to treat co-occurring psychiatric disorders in these youth (Swanson et al., 2011). The use of chart review methodology without structured interviews in a majority of research to date has provided limited systematic information on co-occurring psychiatric diagnoses in this patient population. In addition, recent data in the U.S. were not focused on youth specifically and could not provide more detailed information by diagnosis (e.g., BN) or gender (Mizusaki et al., 2018).
The primary aim of this study was to describe the prevalence and patterns of psychotropic medication use in a large treatment-seeking sample of youth presenting with EDs to a specialist outpatient service at an academic medical center in the U.S. The secondary aim was to examine child and family characteristics associated with psychotropic medication use. Finally, changes in psychotropic medication use patterns were examined over the 17-year study period.
Methods
Study population
Participants were youth up to age 18 who presented with their caregiver(s) to The University of Chicago Eating Disorders Program for diagnostic evaluation. The sample was comprised of consecutive cases between October 1998 and June 2015, and included youth who were being evaluated for randomized controlled trials. ED diagnoses prior to 2013 that followed DSM-IV criteria were converted to meet DSM-5 equivalency for the current study (see Vo et al., [2017] for methodological detail).
Of 733 participants approached, 669 (91.2%) youth and their caregiver(s) provided written informed assent/consent. Participants who did not meet criteria for an ED (n = 36) were excluded. Participants from one pilot trial (n = 29) for whom detailed medication data were not available were also excluded. Finally, for patients who presented to our program twice (n = 5), only data from their first presentation was included in this analysis. All protocols were approved by The University of Chicago and University of California, San Francisco Institutional Review Boards.
Setting
During the time of recruitment, this program specialized in primarily outpatient treatment for youth and young adults, using predominantly Family-Based Treatment. Patients or their caregiver(s) contacted the program directly to schedule an assessment, provided by a licensed practitioner representing a variety of mental health disciplines (psychology, psychiatry, and social work).
Clinical assessment
Clinicians systematically collected demographic and clinical information during the intake interview with youth and their caregiver(s), including reported medication use. Participants’ height and weight were measured (without shoes in light indoor clothing) on a stadiometer and regularly recalibrated scale, respectively. Percent median body mass index (% mBMI) was calculated using the 50th BMI percentile according to Centers for Disease Control (CDC) norms for age and gender (CDC, 2002). Underweight was conservatively defined as ˂ 5th BMI-for-age percentile.
Assessment of medication use.
Caregivers completed a paper-and-pencil questionnaire about their child’s psychotropic medication use (medication name and dosage). Medication use was then confirmed by the clinician during an initial clinical interview with the child and their caregiver(s). Characteristics of providers who prescribed the medication(s) (e.g., specialty) and the setting in which it was prescribed (e.g., level of care) were not collected. Duration of pharmacotherapy and indication were also not systematically recorded due to lack of collateral information from the prescribing provider. Medications were then coded into their respective classes.
Diagnostic Interviews.
Eating Disorder Examination (EDE, version 12.0).
The EDE is a diagnostic interview that has demonstrated good reliability and validity (Cooper et al., 1989; Rizvi et al., 2000; Rosen et al., 1990). Its global score was used to provide a measure of ED psychopathology (Fairburn & Cooper, 1993) and to approximate updated DSM-5 ED diagnoses (Vo et al., 2017). Youth were classified into groups of those meeting criteria for AN, Atypical AN, BN, subsyndromal BN, Avoidant/Restrictive Food Intake Disorder (ARFID), BED, BED of low frequency and/or limited duration, or “other” Other Specified Feeding or Eating Disorders (OSFED).
Co-occurring Psychopathology.
A semi-structured diagnostic interview was used to assess for current Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision psychiatric disorders (the Kiddie Schedule for Affective Disorders and Schizophrenia for School-Age Children [K-SADS] prior to 2013, and the Mini International Neuropsychiatric Interview for Children and Adolescents [MINI-Kid] after 2013, both of which have adequate psychometric properties in youth; Kaufman et al., 1997; Sheehan et al., 2010). Co-occurring psychiatric diagnoses were collapsed into mood disorders (i.e., major depressive disorder, bipolar 1 disorder, bipolar 2 disorder, dysthymic disorder, cyclothymia, and/or depressive disorder not otherwise specified), anxiety disorders (i.e., obsessive-compulsive disorder, panic disorder, specific phobia, post-traumatic stress disorder, social anxiety disorder, generalized anxiety disorder, separation anxiety disorder, and/or anxiety disorder not otherwise specified), and psychotic disorders broadly, including mood disorder with psychotic symptoms.
Data Analyses
SPSS 26 was used for statistical analysis. Descriptive statistics examined demographic and clinical characteristics, including rates of psychotropic medication use. Separate logistic regressions were used to evaluate demographic and clinical characteristics that were associated with 1) any psychotropic medication use, adjusting for presence of any co-occurring psychiatric diagnosis in order to specify current medication use for ED treatment, 2) antidepressant medication use, adjusting for co-occurring mood disorder and/or anxiety disorder, and 3) antipsychotic medication use, adjusting for relevant diagnosis—psychotic disorder, bipolar disorder, and/or major depressive disorder. Independent variables included age, gender, race/ethnicity, family income (divided by 10,000), ED diagnosis (AN [reference category], Atypical AN, BN, OSFED BN, and other), duration since ED onset (years), ED psychopathology (EDE global score), underweight status (i.e., ˂ 5th BMI-for-age percentile), purging frequency (weekly combined average frequency of vomiting, laxative use, and diuretic use over the past three months), previous outpatient psychotherapy, and previous inpatient hospitalization. In order to provide a more robust test of medication use patterns, independent variables significantly associated with the dependent variable in the initial model (p < .05) were entered into a final multinomial logistic regression. Analyses examining the association between underweight status and psychotropic medication use were also conducted with a cut-off more consistent with AN (%mBMI ˂ 87), with no changes in the pattern of results. We also examined rates of psychotropic medication usage during the study period, evaluating overall medication use and medication use by class across time in three blocks of approximately five years each (i.e., T1: 1998–2003; T2: 2004–2009; T3: 2010–2015).
Results
Descriptive Characteristics
The majority of youth (N = 604) were female (90.6%, n = 541) with a mean age of 15.3 years (SD = 2.3, range: [6,18]). Diagnoses included AN (32.6%, n = 197), Atypical AN (16.9%, n = 102), BN (27.6%, n = 167), subsyndromal BN (10.4%, n = 63), BED (1.3%, n = 8), ARFID (0.5%, n = 3), BED of low frequency and/or limited duration (0.5%, n = 3), Purging Disorder (2.0%, n = 12), and “other” OSFED (8.1%, n = 49). Youth with BN and subsyndromal BN were older in age, with a longer duration of illness, higher % mBMIs, and more severe ED psychopathology than youth with AN or Atypical AN. Youth with AN also had a significantly lower number of co-occurring psychiatric disorders than all other diagnostic groups except youth with Atypical AN (see Table 1 for additional differences by ED diagnosis).
Table 1.
Characteristics of sample by eating disorder diagnosis.
|
M (SD), or n (%) |
F or X2 | p-value | ||||||
|---|---|---|---|---|---|---|---|---|
| Overall | AN (n = 197) |
Atypical AN (n = 105) |
BN (n = 166) |
OSFED BN (n = 63) |
Other (n = 73) |
|||
| Age (yrs) | 15.33 (2.24) | 14.94 (2.23) | 14.66 (2.53) | 16.29 (1.53) | 16.05 (1.62) | 14.51 (2.74) | 17.06 | .001 |
| Duration of illness (mo) | 17.24 (17.98) | 13.91 (15.70) | 13.54 (12.56) | 26.74 (20.70) | 23.69 (20.18) | 23.69 (20.18) | 9.37 | .001 |
| %mBMI | 102.48 (33.51) | 80.99 (10.24) | 94.87 (11.90) | 112.57 (24.21) | 111.97 (22.06) | 139.38 (63.04) | 71.15 | .001 |
| EDE score | 2.64 (1.80) | 1.97 (2.18) | 2.36 (1.42) | 3.57 (1.27) | 3.19 (1.27) | 1.97 (1.45) | 24.09 | .001 |
| Prior hospitalization | 156 (30.1%) | 69 (35.9%) | 33 (13.3%) | 23 (24.0%) | 15 (24.2%) | 16 (23.2%) | 7.92 | .10 |
| Prior outpatient therapy | 380 (65.1%) | 131 (68.2%) | 68 (68.7%) | 110 (67.9%) | 33 (53.2%) | 38 (55.1%) | 8.85 | .07 |
| Co-occurring disorders | 288 (53.5%) | 71 (37.2%) | 52 (54.2%) | 93 (73.2%) | 31 (54.4%) | 41 (61.2%) | 41.97 | .001 |
| Number per patient | 0.84 (0.99) | 0.56 (0.86) | 0.84 (1.01) | 1.16 (0.94) | 1.00 (1.28) | 0.94 (1.28) | 8.12 | .001 |
| Mood disorder | 205 (36.6%) | 42 (22.1%) | 39 (41.1%) | 74 (48.4%) | 25 (43.9%) | 25 (38.5%) | 28.53 | .001 |
| Anxiety disorder | 126 (22.5%) | 43 (22.6%) | 20 (21.1%) | 39 (25.5%) | 11 (19.3%) | 13 (22.5%) | 1.47 | .83 |
| Psychotic disorder | 2 (0.4%) | 0 (0.0%) | 0 (0.0%) | 1 (0.7%) | 1 (1.8%) | 0 (0.0%) | 4.76 | .31 |
| DBD | 36 (6.4%) | 3 (1.6%) | 2 (2.1%) | 17 (11.1%) | 5 (8.8%) | 9 (13.8%) | 22.42 | .001 |
| ADHD | 26 (4.6%) | 3 (1.6%) | 1 (1.1%) | 12 (7.8%) | 4 (7.0%) | 6 (9.2%) | 14.15 | .007 |
Note: Percentage reflects the proportion of youth within the ED diagnostic category who have the presence of the characteristic (e.g., co-occurring mood disorder). Abbreviations: percent median Body Mass Index (%mBMI), Disruptive Behavior Disorder (DBD), Attention-Deficit/Hyperactivity Disorder (ADHD), Eating Disorders Examination (EDE).
The sample included predominantly Whites (87.8%, n = 526), as well as Blacks/African Americans (7.0%, n = 42), Asians (2.8%, n = 17), American Indians or Alaska Natives (0.8%, n = 5), and mixed-race youth (1.5%, n = 9). Latinos comprised 14.9% (n = 89) of the sample. Mean family income was U.S. $113,088 (SD = 99,401). Level of parental education was high, with 42.7% (n = 200) having earned a master’s or doctorate degree, 37.6% (n = 176) with a bachelor’s degree, and 19.7% (n = 96) with at least a high school degree.
Medication Use
Nearly one-third of participants were taking at least one psychotropic medication (29.8%, n = 173). Medication rates by psychotropic class indicated that antidepressant drugs were the most common (26.4%, n = 150), followed by atypical antipsychotics (7.5%, n = 43). Of those taking medication, 40.5% (n = 70) received concomitant medications (two: n = 59, 10.2%; three: n = 11, 1.9%). Of those who received concomitant medications, 16.7% (n = 11) received medications within the same class—mostly two antidepressants (n = 9). Medications were frequently prescribed to an individual who did not meet current DSM-IV criteria for the indicated psychiatric disorder[s]) across all drug classes, including antidepressants (40.8%, n = 62), antipsychotics (48.8%, n = 20), mood stabilizers (57.1%, n = 4), anxiolytics (64.7%, n = 11), ADHD medications (60.0%, n = 9), mood stabilizers (90.0%, n = 9), and anticonvulsants (75.0%, n = 3). Table 2 (see also supplemental Appendix A) provides additional information about psychotropic medication use.
Table 2.
Psychotropic medications by class and subclass
| Class | n (%) |
|---|---|
| Antidepressant | 150 (25.9%) |
| SSRI | 141 (24.4%) |
| SNRI | 10 (1.7%) |
| SARI | 4 (0.7%) |
| Aminoketone (buproprion) | 2 (0.3%) |
| Atypical Antipsychotic | 43 (7.5%) |
| Anxiolytic | 17 (2.9%) |
| Benzodiazepine | 14 (2.4%) |
| Spiro compound (buspirone) | 3 (0.5%) |
| ADHD | 16 (2.8%) |
| Stimulant | 12 (2.1%) |
| Non-stimulant | 5 (0.9%) |
| Anticonvulsants | 7 (1.2%) |
| Mood Stabilizer | 4 (0.7%) |
Usage over time
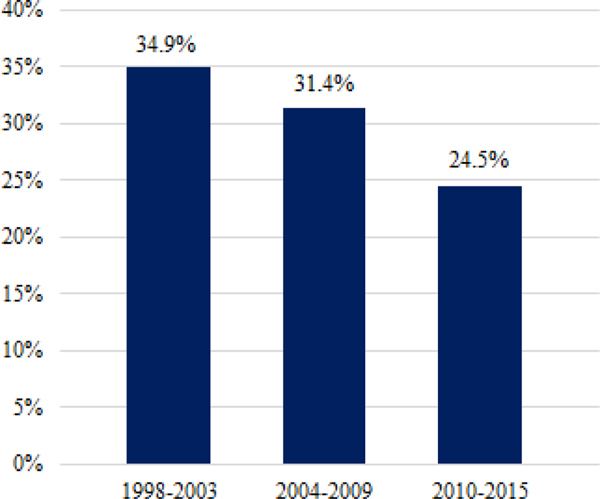
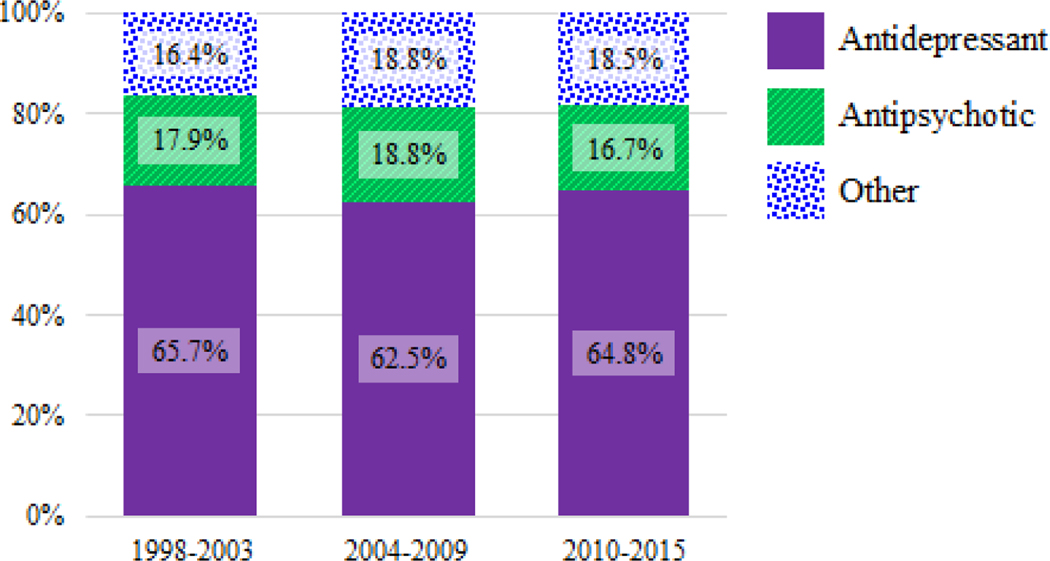
Overall medication use decreased over time from about one-third to one-quarter of youth from the first (1998–2003) to the last time period (2010–2015) (see Figure 1). Within total medication use, the proportion of antidepressant, atypical antipsychotic, and other medication use was relatively stable over time—with about two-thirds of medications prescribed being antidepressants, and the remaining third being about evenly split between atypical antipsychotics and other medications within each time period (see Figure 2).
Figure 1.
Percentage of youth taking any psychotropic medication by year.
Figure 2.
Percentage of all psychotropic medications used by class and year.
Correlates of Psychotropic Medication Use
Any psychotropic medication.
Co-occurring psychiatric disorder was significantly associated with any psychotropic medication use in the initial bivariate model (B = 0.85, SE = 0.20, Wald χ2 = 18.43, p < .001, OR [95% CI] = 2.34 [1.59,3.45]). Adjusting for co-occurring disorder, there were significant initial effects of race (racial/ethnic minority vs. non-Hispanic White: B = −0.47, SE = 0.23, Wald χ2 = 4.38, p < .001, OR [95% CI] = 2.42 [1.63,3.57]), prior inpatient hospitalization (B = 1.92, SE = 0.23, Wald χ2 = 71.45, p < .001, OR [95% CI] = 6.79 [4.36,10.59]), previous outpatient therapy (B = 1.55, SE = 0.26, Wald χ2 = 36.99, p < .001, OR [95% CI] = 4.71 [2.86,7.68]), and ED diagnosis (BN vs. AN: B = −0.70, SE = 0.27, Wald χ2 = 6.69, p = .01, OR [95% CI] = 0.50 [0.47,1.41]) on overall psychotropic medication use. In the multinomial model adjusting for co-occurring disorder (B = 1.11, SE = 0.24, Wald χ2 = 21.21, p < .001, OR [95% CI] = 3.04 [1.89,4.87]), youth who had received prior outpatient psychotherapy were over three times more likely to be using psychotropic medication (B = 1.26, SE = 0.28, Wald χ2 = 20.07, p < .001, OR [95% CI] = 3.52 [2.03,6.12]), and those who had received inpatient treatment were six times more likely to be using psychotropic medication (B = 1.79, SE = 0.24, Wald χ2 = 55.84, p < .001, OR [95% CI] = 6.01 [3.76,9.62]). Non-Hispanic White youth were almost two times more likely to receive psychotropic medications compared to racial/ethnic minorities (B = −0.57, SE = 0.28, Wald χ2 = 4.19, p = .04, OR [95% CI] = 0.57 [0.33,0.98]). ED diagnosis (ps > .47) was not significantly associated with medication use in the multinomial model.
Antidepressant medication.
Co-occurring mood or anxiety disorder (p < .001) was significantly associated with antidepressant medication use in the initial bivariate model (B = 0.78, SE = 0.20, Wald χ2 = 15.45, p < .001, OR [95% CI] = 2.18 [1.48,3.22]). Adjusting for these variables, there were significant initial effects of duration since ED onset (B = 0.19, SE = 0.08, Wald χ2 = 6.28, p = .01, OR [95% CI] = 1.21 [1.04,1.41]), purging frequency (B = 0.03, SE = 0.01, Wald χ2 = 4.17, p = .04, OR [95% CI] = 1.03 [1.00,1.05]), prior inpatient hospitalization (B = 1.67, SE = 0.23, Wald χ2 = 54.61, p < .001, OR [95% CI] = 5.31 [3.41,8.26]), and prior outpatient therapy on antidepressant medication use (B = 1.47, SE = 0.27, Wald χ2 = 29.82, p < .001, OR [95% CI] = 4.33 [2.56,7.33]). In the multinomial model adjusting for co-occurring mood/anxiety disorder (B = 0.93, SE = 0.27, Wald χ2 = 12.22, p < .001, OR [95% CI] = 2.54 [1.51,4.28]), youth with a longer duration since ED onset were more likely to be using antidepressants (B = 0.19, SE = 0.08, Wald χ2 = 5.33, p = .02, OR [95% CI] = 1.21 [1.03,1.43]); every additional year in duration since ED onset illness was associated with a 1.2-fold increase in antidepressant use. Those who had received outpatient psychotherapy were 2.5 times more likely to be using antidepressants (B = 0.94, SE = 0.34, Wald χ2 = 7.46, p = .006, OR [95% CI] = 2.57 [1.31,5.05]), and those who had received inpatient treatment were almost five times more likely to be using antidepressants (B = 1.57, SE = 0.27, Wald χ2 = 33.31, p < .001, OR [95% CI] = 4.82 [2.82,8.21]).
Atypical antipsychotic medication.
Relevant co-occurring disorder (i.e., psychotic disorder, bipolar disorder, or major depressive disorder) was significantly associated with atypical antipsychotic use in the initial bivariate model (B = 1.05, SE = 0.32, Wald χ2 = 10.20, p = .001, OR [95% CI] = 2.84 [1.50,5.41]). Adjusting for co-occurring diagnosis, there were significant initial effects of gender (B = 1.39, SE = 0.41, Wald χ2 = 11.56, p = .001, OR [95% CI] = 4.03 [1.80,8.98]), income (B = −0.10, SE = 0.04, Wald χ2 = 4.77, p = .03, OR [95% CI] = 0.91 [0.84,0.99]), ED diagnosis (BN vs. AN: B = −1.63, SE = 0.58, Wald χ2 = 7.81, p = .005, OR [95% CI] = 0.20 [0.06,0.62]), prior inpatient hospitalization (B = 1.70, SE = 0.35, Wald χ2 = 23.19, p < .001, OR [95% CI] = 5.49 [12.74,10.97]), and prior outpatient psychotherapy (B = 1.38, SE = 0.49, Wald χ2 = 7.99, p = .005, OR [95% CI] = 3.97 [1.53,10.32]) on psychotropic medication use. In the multinomial model adjusting for relevant co-occurring disorder (B = 1.51, SE = 0.57, Wald χ2 = 7.08, p = .008, OR [95% CI] = 4.50 [1.49,13.65]), higher family income was associated with lesser use of atypical antipsychotics (B = −0.17, SE = 0.05, Wald χ2 = 10.17, p = .001, OR [95% CI] = 0.85 [0.76,0.94]), such that for every $10,000 increase in income, youth were 1.2 times less likely to be using atypical antipsychotics. Compared to youth with AN, youth with atypical AN were 1.5 times less likely to be using atypical antipsychotics (B = 2.04, SE = 0.68, Wald χ2 = 8.89, p = .003, OR [95% CI] = 0.63 [2.01,29.34]) and those “other” diagnoses were 5 times more likely to be using atypical antipsychotics (B = 1.66, SE = 0.76, Wald χ2 = 4.75, p = .03, OR [95% CI] = 5.25 [1.18,23.37]). Youth who had received inpatient treatment were almost 4 times more likely to be using atypical antipsychotics (B = 1.34, SE = 0.58, Wald χ2 = 5.35, p = .02, OR [95% CI] = 3.84 [1.23,11.98]) compared to those with no prior inpatient treatment history. Gender and prior outpatient therapy were not significantly associated with atypical antipsychotic use (ps ≥ .10).
Discussion
Nearly one-third (29.8%) of youth presenting for evaluation to a specialist outpatient ED program were using psychotropic medication at intake, most commonly antidepressants (26.4%), followed by atypical antipsychotics (7.5%). The medication use rate in this sample was slightly lower than another report of a sample including children and young adults presenting for ED treatment in the U.S. (45.3%; Mizusaki et al., 2018), but double that of youth with any psychiatric disorder (14%) in a population-based sample (Merikangas et al., 2013) and triple that of treatment-seeking youth who presented to ED programs in the United Kingdom (Gowers et al., 2010). The use of two or more medications was highly common (40%), comparable to other studies of youth (31%; Garner et al., 2016). Of note, prior work suggests that medication use may differ according to ED treatment setting, as lower rates were shown upon entry to adolescent-medicine based hospital and outpatient programs in the United Kingdom (8%) (Gowers et al., 2010) and U.S. (12%) (Monge et al., 2015), whereas higher rates were found in an academic medical center (45%; Mizusaki et al., 2018) and residential ED programs in the U.S. (79%; Garner et al., 2016).
Our findings demonstrate overall decreasing rates in medication use in young patients with EDs from 1998 to 2015, in contrast with evidence suggesting the opposite pattern in adults with AN (Fazeli et al., 2012). Despite the black-box warning issued by the U.S. Food and Drug Administration (FDA) in 2004 indicating that antidepressants were associated with an increased risk of suicidal thinking, feeling, and behavior in young people, the proportion of antidepressants prescribed did not change in the period before to the period after 2004. Indeed, the proportion of medications by class remained stable over time, despite overall medication use trending downward. Nevertheless, it is possible that this FDA warning impacted attitudes in prescribing for youth, contributing to decreased overall use.
Several factors were associated with increased likelihood of overall medication use, with co-occurring psychiatric disorders and previous treatment (i.e., outpatient psychotherapy, inpatient hospitalization) appearing most robust. Non-Hispanic White race was also associated with greater overall medication use, compared to racial/ethnic minorities. Co-occurring mood and/or anxiety disorder, longer duration since ED onset, and previous general psychological treatment were all associated with greater antidepressant use. Despite the fact that there is an FDA-approved medication for BN in adults, youth with BN were no more likely to be using antidepressant medication compared to youth with other ED diagnoses. Furthermore, severity of ED psychopathology was not associated with medication use. Taken together, this reflects a potential tendency for antidepressants to be prescribed to youth regardless of ED diagnosis or severity and primarily for mood disorders, or possibly mood symptoms either independent of, or secondary to, malnutrition. Although mood disorders were significantly associated with antidepressant medication use, two-fifths of youth were taking an antidepressant “off-label” (i.e., without U.S. FDA-approved labeling for an indication, dose, or age group). Further, other medications had even higher rates of off-label use, ranging between 50–90% across medication classes.
Co-occurring psychiatric disorder and inpatient treatment was also associated with greater likelihood of atypical antipsychotic medication use. In contrast to antidepressants, ED diagnosis was associated with atypical antipsychotic use, such that compared to youth with AN, youth with atypical AN had significantly lesser use and youth with “other ED” diagnoses had significantly greater use. This could perhaps reflect practitioners’ hesitancy to prescribe atypical antipsychotics in youth, especially those with disorders that have greater medical risk, such as EDs. However, it is surprising given somewhat promising results from case series of atypical antipsychotics in AN and our findings that those with atypical AN (i.e., at higher weight) were less likely than those with AN to be taking atypical antipsychotics. Further, higher family income was associated with lesser use of atypical antipsychotics.
No other clinical factors were associated with medication use, across medication types. For example, youth who were underweight were overall no less likely to be using medication. While this is consistent with previous research (Monge et al., 2015), it is nevertheless surprising given the lack of evidence for the efficacy of medications in the context of underweight patients with AN (Claudino et al., 2006) and recommendations to delay the prescription of antidepressants until weight restoration has been achieved (APA, 2006). In addition, research indicates that co-occurring depressive symptoms in youth with AN improve over time within psychotherapy (Accurso et al., 2014), so providers might be advised to delay prescribing antidepressants until psychotherapy is underway and patients have at least partially restored weight, if depressive symptoms persist. Even so, it is possible that antidepressants were prescribed for psychiatric symptoms prior to the onset of the ED and subsequent weight loss.
In alignment with prior research finding greater medication use in White compared to Black and Latino youth (Leslie et al., 2003; Monge et al., 2015), in our sample, Non-Hispanic Whites were almost two times more likely to receive psychotropic medications compared to racial/ethnic minorities. We also found that lower family income was associated with significantly greater use of atypical antipsychotics, which contradicts the findings of another study across psychiatric disorders (Leslie et al., 2003). While speculative, this may reflect greater willingness in lower-income families to use potentially less expensive options than non-pharmacological intervention (e.g., more extensive psychotherapy that insurance may not substantiate), or greater provider reliance on the use of more intensive medication in families with fewer resources. However, few other demographic differences emerged. Age was not associated with medication use, which is consistent with research in a slightly older sample (Monge et al., 2015). Consistent with atypical antipsychotics being twice as common overall in boys than girls in the U.S. (Cooper et al., 2006), there were initial effects of gender, with boys being four times more likely to be using atypical antipsychotics. However, gender was not significantly associated with atypical antipsychotic use in the multinomial model.
Study strengths include the relatively large sample of youth with a broad range of ED diagnoses and the use of semi-structured diagnostic interviews to determine ED and co-occurring psychiatric diagnoses. However, several limitations should be noted. First, medication use in youth with EDs could not be directly compared to youth with other mental health disorders within the same practice setting. Other research indicates that rates of medication use in this study are comparable to those for youth with ADHD (31%), but higher than those for community samples of youth with a mood disorder (20%), anxiety disorder (12%), or disruptive behavior disorders (19%) (Merikangas et al., 2013). Second, prescribing provider (e.g., specialty), service setting characteristics (e.g., level of care), and indication(s) (e.g., atypical antipsychotics for weight gain versus management of psychotic symptoms) were unknown, which would have informed medication practices. Further, primary indication (e.g., ED versus co-occurring disorder) and start date/duration for prior mental health treatments (i.e., medication or therapy) were not assessed, which may have also impacted medication use patterns. For example, if an antidepressant medication were prescribed in the last year for major depressive disorder, the youth might not have met criteria for a depressive disorder at assessment, complicating the interpretation of off-label uses and correlates of medication use, particularly given that neither patients nor caregivers reported on medications’ perceived effectiveness. Furthermore, findings from this Midwestern region may not generalize to youth in other geographical areas. Given that the initial assessment of EDs in the current study was made according to DSM-IV criteria, we did not specifically assess for ARFID; while a small number of cases were identified as meeting DSM-5 criteria for ARFID in the current sample, this disorder (and relative medication use, given rates of comorbid psychiatric disorders in this population; Keery et al., 2019) was likely underrepresented. Finally, insurance information was not available, which has been found to be associated with medication use (Zito et al., 2005). However, family income was examined and may be related to insurance status.
These data suggest a relatively high perceived need for pharmacological treatment of psychiatric symptoms in youth with EDs among prescribers and/or these youth and their families. Antidepressants were the most common, which is not surprising given that more than a third of youth in this sample had a mood disorder, even though co-occurring mood symptoms in youth with AN do improve at times within psychotherapy without psychopharmacological intervention (Accurso et al., 2014; Trainor et al., 2020). Furthermore, relatively few clinical factors associated with medication use were identified in this study. The high rate of co-occurring psychiatric disorders and substantial distress with which these youth typically present pose a dilemma for the medical provider, particularly for those who lack ED expertise. Since the evidence base for pharmacological treatment of pediatric EDs is not robust at this time (Couturier et al., 2019), a focus on pharmacological trials that may determine the efficacy of psychotropic medication on both ED symptomology and weight gain (as indicated), and symptoms associated with comorbid psychopathology is warranted. Delineating the specific use of medications for either ED pathology, comorbid psychopathology (e.g., depression; anxiety) or their combination would greatly inform future prescribing practices. In advance of these trials, caution in prescribing practices might be specifically advised, given the potential impact of certain psychotropic medications on the metabolic and endocrine systems of developing youth (De Hert et al., 2011).
This is the first study to examine clinical characteristics associated with medication use in youth with EDs across the DSM-5 diagnostic spectrum. Additionally, this is the first study to examine psychotropic medication use in a large sample of youth seeking psychological treatment for EDs in the U.S., and as such, may highlight differences compared to prescription practices in other countries. Overall findings indicating relatively common medication use in this population highlights an urgent need for future medication trials that can evaluate pharmacological impact in treating both EDs or co-occurring symptoms in youth with EDs.
Supplementary Material
Highlights:
One-third of youth with EDs reported actively using psychotropic medication, most commonly antidepressants, followed by atypical antipsychotics. Overall rates of medication use decreased from 1998 to 2015, but the relative proportion of medication classes remained relatively stable.
Youth with co-occurring psychiatric disorders, previous outpatient psychotherapy, or history of general inpatient hospitalization were more likely to be using medications.
Certain characteristics were associated with medication use, including lower family income (greater likelihood of atypical antipsychotic use) and racial/ethnic minority status (lesser likelihood of antidepressant use).
Acknowledgments
Disclosure of Funding
This work was supported by grants K23 MH120347, T32-MH082761, and T32-MH018261 from the National Institute of Mental Health and K23-DK105234 from National Institute of Diabetes and Digestive and Kidney Diseases. Dr. Le Grange has received royalties from Guilford Press and Routledge and payments from the Training Institute for Child and Adolescent Eating Disorders, LLC. Dr. Goldschmidt has received royalties from Routledge and provides consultation for Sunovian.
Footnotes
Conflicts of Interest
Drs. Gorrell, Lebow, Kinasz, Mitchell, and Accurso report no biomedical financial interests or potential conflicts of interest.
References
- Accurso EC, Ciao AC, Fitzsimmons-Craft EE, Lock JD, & Le Grange D. (2014). Is weight gain really a catalyst for broader recovery?: The impact of weight gain on psychological symptoms in the treatment of adolescent anorexia nervosa. Behaviour Research and Therapy, 56, 1–6. doi: 10.1016/j.brat.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- APA Practice Guidelines for the Treatment of Psychiatric Disorders: Quick Reference Guidelines. (2006). doi: 10.1176/appi.books.9780890423370 [DOI] [Google Scholar]
- Attia E, Steinglass JE, Walsh BT, Wang Y, Wu P, Schreyer C, … Marcus MD (2019). Olanzapine Versus Placebo in Adult Outpatients With Anorexia Nervosa: A Randomized Clinical Trial. American Journal of Psychiatry, 176(6), 449–456. doi: 10.1176/appi.ajp.2018.18101125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attia E, Kaplan AS, Walsh BT, Gershkovich M, Yilmaz Z, Musante D, & Wang Y. (2011). Olanzapine versus placebo for out-patients with anorexia nervosa. Psychological Medicine, 41(10), 2177–2182. doi: 10.1017/s0033291711000390 [DOI] [PubMed] [Google Scholar]
- Bissada H, Tasca GA, Barber AM, & Bradwejn J. (2008). Olanzapine in the Treatment of Low Body Weight and Obsessive Thinking in Women With Anorexia Nervosa: A Randomized, Double-Blind, Placebo-Controlled Trial. American Journal of Psychiatry, 165(10), 1281–1288. doi: 10.1176/appi.ajp.2008.07121900 [DOI] [PubMed] [Google Scholar]
- Brambilla F, Garcia CS, Fassino S, Daga GA, Favaro A, Santonastaso P, … Monteleone P. (2007). Olanzapine therapy in anorexia nervosa: psychobiological effects. International Clinical Psychopharmacology, 22(4), 197–204. doi: 10.1097/yic.0b013e328080ca31 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention Growth Charts. (2002). Advanced Pediatric Assessment. doi: 10.1891/9780826150219.ap02 [DOI] [Google Scholar]
- Claudino AM, Silva de Lima M, Hay PP, Bacaltchuk J, Schmidt UU, & Treasure J. (2006). Antidepressants for anorexia nervosa. Cochrane Database of Systematic Reviews. doi: 10.1002/14651858.cd004365.pub2 [DOI] [PubMed] [Google Scholar]
- Cooper Z, Cooper PJ, & Fairburn CG (1989). The Validity of the Eating Disorder Examination and its Subscales. British Journal of Psychiatry, 154(6), 807–812. doi: 10.1192/bjp.154.6.807 [DOI] [PubMed] [Google Scholar]
- Cooper WO, Arbogast PG, Ding H, Hickson GB, Fuchs DC, & Ray WA (2006). Trends in Prescribing of Antipsychotic Medications for US Children. Ambulatory Pediatrics, 6(2), 79–83. doi: 10.1016/j.ambp.2005.11.002 [DOI] [PubMed] [Google Scholar]
- Couturier J, & Lock J. (2007). A review of medication use for children and adolescents with eating disorders. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 16(4), 173. PMID: 18392170 [PMC free article] [PubMed] [Google Scholar]
- Couturier J, Isserlin L, Spettigue W, & Norris M. (2019). Psychotropic Medication for Children and Adolescents with Eating Disorders. Child and Adolescent Psychiatric Clinics of North America, 28(4), 583–592. doi: 10.1016/j.chc.2019.05.005 [DOI] [PubMed] [Google Scholar]
- Crow SJ (2019). Pharmacologic Treatment of Eating Disorders. Psychiatric Clinics of North America, 42(2), 253–262. doi: 10.1016/j.psc.2019.01.007 [DOI] [PubMed] [Google Scholar]
- De Hert M, Dobbelaere M, Sheridan EM, Cohen D, & Correll CU (2011). Metabolic and endocrine adverse effects of second-generation antipsychotics in children and adolescents: A systematic review of randomized, placebo controlled trials and guidelines for clinical practice. European Psychiatry, 26(3), 144–158. doi: 10.1016/j.eurpsy.2010.09.011 [DOI] [PubMed] [Google Scholar]
- Dennis K, Le Grange D, & Bremer J. (2006). Olanzapine use in adolescent anorexia nervosa. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 11(2), e53–e56. doi: 10.1007/bf03327760 [DOI] [PubMed] [Google Scholar]
- Eisler I, Simic M, Russell GFM, & Dare C. (2007). A randomised controlled treatment trial of two forms of family therapy in adolescent anorexia nervosa: a five-year follow-up. Journal of Child Psychology and Psychiatry, 48(6), 552–560. doi: 10.1111/j.1469-7610.2007.01726.x [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Cooper Z. The eating disorder examination (12.0D) In: Fairburn CG, Wilson GT, eds. Binge eating: Nature, assessment and treatment. New York, NY: Guilford Press, 1993: 317–360. [Google Scholar]
- Fazeli PK, Calder GL, Miller KK, Misra M, Lawson EA, Meenaghan E, … Klibanski A. (2012). Psychotropic medication use in anorexia nervosa between 1997 and 2009. International Journal of Eating Disorders, 45(8), 970–976. doi: 10.1002/eat.22037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fluoxetine in the Treatment of Bulimia Nervosa. (1992). Archives of General Psychiatry, 49(2), 139. doi: 10.1001/archpsyc.1992.01820020059008 [DOI] [PubMed] [Google Scholar]
- Garner DM, Anderson ML, Keiper CD, Whynott R, & Parker L. (2016). Psychotropic medications in adult and adolescent eating disorders: clinical practice versus evidence-based recommendations. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 21(3), 395–402. doi: 10.1007/s40519-016-0253-0 [DOI] [PubMed] [Google Scholar]
- Goldberg SC, Halmi KA, Eckert ED, Casper RC, & Davis JM (1980). Cyproheptadine in Anorexia Nervosa. British Journal of Psychiatry, 134(1), 67–70. doi: 10.1192/bjp.134.1.67 [DOI] [PubMed] [Google Scholar]
- Gowers S, Claxton M, Rowlands L, Inbasagaran A, Wood D, Yi I, … Ayton A. (2010). Drug Prescribing in Child and Adolescent Eating Disorder Services. Child and Adolescent Mental Health, 15(1), 18–22. doi: 10.1111/j.1475-3588.2009.00535.x [DOI] [PubMed] [Google Scholar]
- Hagman J, Gralla J, Sigel E, Ellert S, Dodge M, Gardner R, … Wamboldt MZ (2011). A Double-Blind, Placebo-Controlled Study of Risperidone for the Treatment of Adolescents and Young Adults with Anorexia Nervosa: A Pilot Study. Journal of the American Academy of Child & Adolescent Psychiatry, 50(9), 915–924. doi: 10.1016/j.jaac.2011.06.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holtkamp K, Konrad K, Kaiser N, Ploenes Y, Heussen N, Grzella I, & Herpertz-Dahlmann B. (2005). A retrospective study of SSRI treatment in adolescent anorexia nervosa: insufficient evidence for efficacy. Journal of Psychiatric Research, 39(3), 303–310. doi: 10.1016/j.jpsychires.2004.08.001 [DOI] [PubMed] [Google Scholar]
- Kafantaris V, Leigh E, Hertz S, Berest A, Schebendach J, Sterling WM, … Malhotra AK (2011). A Placebo-Controlled Pilot Study of Adjunctive Olanzapine for Adolescents with Anorexia Nervosa. Journal of Child and Adolescent Psychopharmacology, 21(3), 207–212. doi: 10.1089/cap.2010.0139 [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Flynn C, Moreci P, … Ryan N. (1997). Schedule for Affective Disorders and Schizophrenia for School-Age Children-Present and Lifetime Version (K-SADS-PL): Initial Reliability and Validity Data. Journal of the American Academy of Child & Adolescent Psychiatry, 36(7), 980–988. doi: 10.1097/00004583-199707000-00021 [DOI] [PubMed] [Google Scholar]
- Keery H, LeMay-Russell S, Barnes TL, Eckhardt S, Peterson CB, Lesser J, … Le Grange D. (2019). Attributes of children and adolescents with avoidant/restrictive food intake disorder. Journal of Eating Disorders, 7(1). doi: 10.1186/s40337-019-0261-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishi T, Kafantaris V, Sunday S, Sheridan EM, & Correll CU (2012). Are Antipsychotics Effective for the Treatment of Anorexia Nervosa? The Journal of Clinical Psychiatry, 73(06), e757–e766. doi: 10.4088/jcp.12r07691 [DOI] [PubMed] [Google Scholar]
- Kotler LA, Devlin MJ, Davies M, & Walsh BT (2003). An Open Trial of Fluoxetine for Adolescents with Bulimia Nervosa. Journal of Child and Adolescent Psychopharmacology, 13(3), 329–335. doi: 10.1089/104454603322572660 [DOI] [PubMed] [Google Scholar]
- Lebow J, Sim LA, Erwin PJ, & Murad MH (2012). The effect of atypical antipsychotic medications in individuals with anorexia nervosa: A systematic review and meta-analysis. International Journal of Eating Disorders, 46(4), 332–339. doi: 10.1002/eat.22059 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Crosby RD, Rathouz PJ, & Leventhal BL (2007). A Randomized Controlled Comparison of Family-Based Treatment and Supportive Psychotherapy for Adolescent Bulimia Nervosa. Archives of General Psychiatry, 64(9), 1049. doi: 10.1001/archpsyc.64.9.1049 [DOI] [PubMed] [Google Scholar]
- Le Grange D, Lock J, Agras WS, Bryson SW, & Jo B. (2015). Randomized Clinical Trial of Family-Based Treatment and Cognitive-Behavioral Therapy for Adolescent Bulimia Nervosa. Journal of the American Academy of Child & Adolescent Psychiatry, 54(11), 886–894.e2. doi: 10.1016/j.jaac.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leslie LK, Weckerly J, Landsverk J, Hough RL, Hurlburt MS, & Wood PA (2003). Racial/Ethnic Differences in the Use of Psychotropic Medication in High-Risk Children and Adolescents. Journal of the American Academy of Child & Adolescent Psychiatry, 42(12), 1433–1442. doi: 10.1097/00004583-200312000-00010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Fitzpatrick KK, Jo B, Accurso E, … Stainer M. (2015). Can adaptive treatment improve outcomes in family-based therapy for adolescents with anorexia nervosa? Feasibility and treatment effects of a multi-site treatment study. Behaviour Research and Therapy, 73, 90–95. doi: 10.1016/j.brat.2015.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lock J, Le Grange D, Agras WS, Moye A, Bryson SW, & Jo B. (2010). Randomized Clinical Trial Comparing Family-Based Treatment With Adolescent-Focused Individual Therapy for Adolescents With Anorexia Nervosa. Archives of General Psychiatry, 67(10), 1025. doi: 10.1001/archgenpsychiatry.2010.128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mehler C, Wewetzer C, Schulze U, Warnke A, Theisen F, & Dittmann RW (2001). Olanzapine in children and adolescents with chronic anorexia nervosa. A study of five cases. European Child & Adolescent Psychiatry, 10(2), 151–157. doi: 10.1007/s007870170039 [DOI] [PubMed] [Google Scholar]
- Merikangas KR, He J, Rapoport J, Vitiello B, & Olfson M. (2013). Medication Use in US Youth With Mental Disorders. JAMA Pediatrics, 167(2), 141. doi: 10.1001/jamapediatrics.2013.431 [DOI] [PubMed] [Google Scholar]
- Mizusaki K, Gih D, LaRosa C, Richmond R, & Rienecke RD (2018). Psychotropic usage by patients presenting to an academic eating disorders program. Eating and Weight Disorders - Studies on Anorexia, Bulimia and Obesity, 23(6), 769–774. doi: 10.1007/s40519-018-0520-3 [DOI] [PubMed] [Google Scholar]
- Monge MC, Forman SF, McKenzie NM, Rosen DS, Mammel KA, Callahan ST, … Woods ER (2015). Use of Psychopharmacologic Medications in Adolescents With Restrictive Eating Disorders: Analysis of Data From the National Eating Disorder Quality Improvement Collaborative. Journal of Adolescent Health, 57(1), 66–72. doi: 10.1016/j.jadohealth.2015.03.021 [DOI] [PubMed] [Google Scholar]
- Moore JK, Watson HJ, Harper E, McCormack J, & Nguyen T. (2013). Psychotropic drug prescribing in an Australian specialist child and adolescent eating disorder service: a retrospective study. Journal of Eating Disorders, 1(1), 27. doi: 10.1186/2050-2974-1-27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- The National Institute for Health and Care Excellence. Eating disorders: Core interventions in the treatment and management of anorexia nervosa, bulimia nervosa and related eating disorders. NICE clinical guideline 9 Available at: www.nice.org.uk/CG9. Accessed March 2020. [Google Scholar]
- Pisano S, Catone G, Pascotto A, & Gritti A. (2014). Second Generation Antipsychotics in Adolescent Anorexia Nervosa: A New Hypothesis of Eligibility Criteria. Journal of Child and Adolescent Psychopharmacology, 24(5), 293–295. doi: 10.1089/cap.2013.0124 [DOI] [PubMed] [Google Scholar]
- Rizvi SL, Peterson CB, Crow SJ, & Agras WS (2000). Test‐retest reliability of the eating disorder examination. International Journal of Eating Disorders, 28(3), 311–316. doi: [DOI] [PubMed] [Google Scholar]
- Rosen JC, Vara L, Wendt S, & Leitenberg H. (1990). Validity studies of the eating disorder examination. International Journal of Eating Disorders, 9(5), 519–528. doi. [DOI] [Google Scholar]
- Schmidt U, Lee S, Beecham J, Perkins S, Treasure J, Yi I, … Eisler I. (2007). A Randomized Controlled Trial of Family Therapy and Cognitive Behavior Therapy Guided Self-Care for Adolescents With Bulimia Nervosa and Related Disorders. American Journal of Psychiatry, 164(4), 591–598. doi: 10.1176/ajp.2007.164.4.591 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Sheehan KH, Shytle RD, Bannon Y, Janavs J, Rogers JE, … Wilkinson B. (2010). Mini International Neuropsychiatric Interview for Children and Adolescents. PsycTESTS Dataset. doi: 10.1037/t29452-000 [DOI] [PubMed] [Google Scholar]
- Spettigue W, Norris ML, Maras D, Obeid N, Feder S, Harrison ME, ... & Buchholz A. (2018). Evaluation of the effectiveness and safety of olanzapine as an adjunctive treatment for anorexia nervosa in adolescents: an open-label trial. Journal of the Canadian Academy of Child and Adolescent Psychiatry, 27(3), 197. PMID: 30038658 [PMC free article] [PubMed] [Google Scholar]
- Swanson SA, Crow SJ, Le Grange D, Swendsen J, & Merikangas KR (2011). Prevalence and correlates of eating disorders in adolescents: Results from the national comorbidity survey replication adolescent supplement. Archives of general psychiatry, 68(7), 714–723. doi: 10.1001/archgenpsychiatry.2011.22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainor C, Gorrell S, Hughes EK, Sawyer SM, Burton C, & Grange DL (2020). Family‐based treatment for adolescent anorexia nervosa: What happens to rates of comorbid diagnoses? European Eating Disorders Review. doi: 10.1002/erv.2725 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vo M, Accurso EC, Goldschmidt AB, & Le Grange D. (2017). The Impact of DSM-5 on Eating Disorder Diagnoses. International Journal of Eating Disorders, 50(5), 578–581. doi: 10.1002/eat.22628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh BT, Kaplan AS, Attia E, Olmsted M, Parides M, Carter JC, … Rockert W. (2006). Fluoxetine After Weight Restoration in Anorexia Nervosa. JAMA, 295(22). doi: 10.1001/jama.295.22.2605 [DOI] [PubMed] [Google Scholar]
- Watson HJ & Bulik CM (2013) Update on the treatment of anorexia nervosa: Review of clinical trials, practice guidelines and emerging interventions. Psychological Medicine; 43(12):2477–500. doi: 10.1017/S0033291712002620. [DOI] [PubMed] [Google Scholar]
- Zito JM, Safer DJ, Zuckerman IH, Gardner JF, & Soeken K. (2005). Effect of Medicaid Eligibility Category on Racial Disparities in the Use of Psychotropic Medications Among Youths. Psychiatric Services, 56(2), 157–163. doi: 10.1176/appi.ps.56.2.157 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.