Key Points
Question
In adults with sepsis-induced respiratory or cardiovascular dysfunction, does treatment with vitamin C, thiamine, and hydrocortisone result in an increase in the number of days alive and free of mechanical ventilation and vasopressor use?
Findings
In this randomized clinical trial that included 501 patients, treatment with vitamin C, thiamine, and hydrocortisone compared with placebo resulted in a median of 25 vs 26 ventilator- and vasopressor-free days within the 30 days following randomization, a difference that was not statistically significant.
Meaning
Among critically ill patients with sepsis, treatment with vitamin C, thiamine, and hydrocortisone did not significantly improve ventilator- and vasopressor-free days, although, the trial was terminated early for administrative reasons and may have been underpowered to detect a clinically important difference.
Abstract
Importance
Sepsis is a common syndrome with substantial morbidity and mortality. A combination of vitamin C, thiamine, and corticosteroids has been proposed as a potential treatment for patients with sepsis.
Objective
To determine whether a combination of vitamin C, thiamine, and hydrocortisone every 6 hours increases ventilator- and vasopressor-free days compared with placebo in patients with sepsis.
Design, Setting, and Participants
Multicenter, randomized, double-blind, adaptive-sample-size, placebo-controlled trial conducted in adult patients with sepsis-induced respiratory and/or cardiovascular dysfunction. Participants were enrolled in the emergency departments or intensive care units at 43 hospitals in the United States between August 2018 and July 2019. After enrollment of 501 participants, funding was withheld, leading to an administrative termination of the trial. All study-related follow-up was completed by January 2020.
Interventions
Participants were randomized to receive intravenous vitamin C (1.5 g), thiamine (100 mg), and hydrocortisone (50 mg) every 6 hours (n = 252) or matching placebo (n = 249) for 96 hours or until discharge from the intensive care unit or death. Participants could be treated with open-label corticosteroids by the clinical team, with study hydrocortisone or matching placebo withheld if the total daily dose was greater or equal to the equivalent of 200 mg of hydrocortisone.
Main Outcomes and Measures
The primary outcome was the number of consecutive ventilator- and vasopressor-free days in the first 30 days following the day of randomization. The key secondary outcome was 30-day mortality.
Results
Among 501 participants randomized (median age, 62 [interquartile range {IQR}, 50-70] years; 46% female; 30% Black; median Acute Physiology and Chronic Health Evaluation II score, 27 [IQR, 20.8-33.0]; median Sequential Organ Failure Assessment score, 9 [IQR, 7-12]), all completed the trial. Open-label corticosteroids were prescribed to 33% and 32% of the intervention and control groups, respectively. Ventilator- and vasopressor-free days were a median of 25 days (IQR, 0-29 days) in the intervention group and 26 days (IQR, 0-28 days) in the placebo group, with a median difference of −1 day (95% CI, −4 to 2 days; P = .85). Thirty-day mortality was 22% in the intervention group and 24% in the placebo group.
Conclusions and Relevance
Among critically ill patients with sepsis, treatment with vitamin C, thiamine, and hydrocortisone, compared with placebo, did not significantly increase ventilator- and vasopressor-free days within 30 days. However, the trial was terminated early for administrative reasons and may have been underpowered to detect a clinically important difference.
Trial Registration
ClinicalTrials.gov Identifier: NCT03509350
This randomized trial compares the effect of vitamin C, thiamine, and hydrocortisone vs matching placebo on 30-day ventilator- and vasopressor-free days among patients with sepsis-induced respiratory and/or cardiovascular dysfunction.
Introduction
Sepsis is a common syndrome caused by infection leading to substantial morbidity and mortality.1 Of the estimated 1 750 000 cases of sepsis in the United States in 2014, approximately 55% required intensive care unit (ICU) admission and 20% to 30% died,2 making sepsis the third leading cause of hospital deaths, with an annual estimated cost of nearly $60 billion.3,4 Patients who survive are at risk of worse physical, emotional, and cognitive outcomes and reduced quality of life.5 Although early antibiotics, fluid resuscitation, hemodynamic support, and source control have been associated with improved patient outcomes,6 other sepsis interventions have not demonstrated consistent benefits.7
Recently, high-dose intravenous vitamin C has been proposed as a therapy for sepsis.8 There is biological plausibility for the value of vitamin C in sepsis,9 but the results of clinical studies have been inconsistent.10,11,12 The addition of thiamine and hydrocortisone to vitamin C has been suggested to be beneficial in preclinical and observational studies.13,14
The Vitamin C, Thiamine, and Steroids in Sepsis (VICTAS) trial was designed to test the hypothesis that combination therapy with vitamin C, thiamine, and hydrocortisone would improve clinically important outcomes in patients with sepsis-induced respiratory and/or circulatory failure.15
Methods
Trial Design and Oversight
The trial was a randomized, double-blind, adaptive-sample-size, placebo-controlled trial conducted at 43 hospitals in the United States. The protocol (Supplement 1) and statistical analysis plan (Supplement 2) have been previously published.15,16 The central institutional review board at Johns Hopkins University approved the trial protocol and consent documents for all sites. Participants or legally authorized representatives provided written informed consent prior to enrollment and randomization. An independent data and safety monitoring board conducted reviews of safety data and predicted probability of success during 4 predetermined interim analyses.
Trial Population
Patients aged 18 years or older with acute respiratory and/or cardiovascular dysfunction caused by suspected infection with planned ICU admission were eligible for enrollment. Infection was defined by the ordering of blood cultures and administration of at least 1 antimicrobial agent. Acute respiratory dysfunction was defined by an arterial partial pressure of oxygen/fraction of inspired oxygen (Fio2) of 300 or less or a pulse oximetry oxygen saturation/Fio2 of 315 or less and the need for (1) intubation and mechanical ventilation, (2) noninvasive positive pressure ventilation, or (3) high-flow nasal cannula at 40 L/min or higher with an Fio2 of at least 0.40. Cardiovascular dysfunction was defined as the need for any vasopressor for more than 1 hour to maintain a mean arterial pressure of 65 mm Hg or higher despite intravenous crystalloid fluid resuscitation of 1 L or more. There were no exclusions based on race or ethnicity. Race and ethnicity were considered possible effect modifiers and were systematically collected from the medical record using fixed categories. Full inclusion and exclusion criteria are detailed in Supplement 1.
Randomization
Patients were randomized in a 1:1 ratio to either the intervention group or the control group within 24 hours of qualifying organ dysfunction, and organ dysfunction was required to be ongoing at randomization. The randomization sequence was generated using statistical software and used permuted small blocks of size 2, 4, or 6, stratified by site. Study group randomization was operationalized via the use of presorted drug kits shipped to study sites from a centralized pharmacy and Vanderbilt University Medical Center, with participants assigned kits in predefined sequence. Randomization blinding was maintained by labeling study drug kits with numbers only. Investigational drug pharmacists were not blinded but were prohibited by protocol from revealing assignment to anyone.
Interventions
Participants received intravenous vitamin C (1.5 g), thiamine hydrochloride (100 mg), and hydrocortisone sodium succinate (50 mg) or matching placebos within 4 hours of randomization and then every 6 hours thereafter up to 96 hours, death, or discharge from the ICU, whichever occurred first. Participants could be treated with open-label corticosteroids by the clinical team: for daily doses of at least 200 mg of hydrocortisone (or equivalent), hydrocortisone or matching placebo was withheld by the investigational pharmacy.
All other management of participants was at the discretion of the clinical team. A point-of-care device approved for use in the setting of high plasma concentrations of vitamin C or a central laboratory device not affected by vitamin C were used for glucose measurements through 24 hours following the last dose of study drug.17
Outcomes
The primary outcome measure was ventilator- and vasopressor-free days (VVFDs) in the first 30 days following randomization (day 0). Participants who died were assigned 0 VVFDs. A “last status carried forward” approach was used in the calculation of VVFDs for participants discharged from the hospital before day 30.
The key secondary outcome was mortality within 30 days of randomization. A patient discharged alive was considered to have survived to day 30. Exploratory outcomes to support efficacy included ICU mortality, ICU and hospital lengths of stay, ICU delirium- and coma-free days, kidney replacement therapy–free days at day 30, and change between prerandomization and day 4 Sequential Organ Failure Assessment (SOFA) score (range, 0 [best] to 28 [worst]).18 Safety end points included the prespecified potentially associated adverse events of nephrolithiasis, hemolysis, hypersensitivity reactions, and injection site reactions. At discharge or at 30 days, whichever occurred sooner, participants were invited to participate in a 180-day telephone follow-up. For patients who died between discharge and 180 days, date of death was obtained at this follow-up.
Statistical Analysis
In the absence of phase 2 data at the time of trial design, an adaptive approach was used. The trial was designed to detect a difference in the primary end point of 1.5 VVFDs with recruitment of up to 2000 patients. These calculations were based on a mortality rate of 25% and the proposed end point of 1.5 VVFDs was based on a vote from the executive committee as meaningful (Supplement 3) while allowing early stoppage if a large effect on mortality was observed.15,16 At each planned interim analysis, all monitored and unmonitored data for participants with day 30 observations were used, and bayesian predictive distributions were used to impute the outcomes of participants with incomplete data. Together, these data were used to compute a predictive probability of success on the mortality end point when 200, 300, and 400 participants were enrolled. Predefined stopping rules for efficacy, but not futility, were in place for these early interim analyses. Beyond N = 400, interim analyses were planned at N = 500, N = 1000, and N = 1500, at which both efficacy and futility were to be assessed using the VVFD outcome (Supplement 3).
The success thresholds at each interim were calibrated to control the 1-sided type I error rate at 2.5% (Supplement 2).16 If a possible 20% mortality benefit were real, which is lower than the absolute risk reduction in mortality seen in a prior study,14 the trial was very likely (approximately 97% chance) to stop at or before 400 patients with a power of approximately 99%.15
Categorical variables are reported using frequencies and percentages. Continuous variables are reported as means with standard deviations and medians with interquartile ranges (IQRs). In the primary analysis, patients were analyzed according to their randomization group. Given the approach to calculating VVFDs, the primary outcome was available for all participants. The VVFDs are described using medians and IQRs; bivariable differences between groups are reported with 95% confidence intervals and compared using the Wilcoxon rank sum test. To account for the multiple interim analyses, the critical P value was set to a 1-sided threshold of P = .022. Mortality is described using frequencies and percentages. A gatekeeping strategy was used to decide whether to formally test for a treatment effect on mortality. Under this strategy, the mortality end point would be formally tested using a χ2 test with a 1-sided α = .024 if a difference in the primary end point of VVFDs was observed, but not otherwise. After completion of the study but before conducting the final statistical analysis, a post hoc comparison of survival between groups using a Cox proportional hazards model was added to the analysis plan. The proportionality assumption was tested using the Schoenfeld test. All models were summarized with hazard ratios assessing the risk of death following randomization.
Three sensitivity analyses were conducted to explore possible sources of bias that may inform interpretation of the main analysis. These prespecified analyses used a 2-sided P = .05 and included (1) a per-protocol analysis among participants who received at least 4 doses of the assigned study treatment without any major protocol deviations; (2) an analysis of participants for whom day 30 outcomes were directly observed; and (3) an analysis that excluded participants in the placebo group who received open-label steroids.
In an adjusted analysis, generalized linear mixed models were used to estimate the conditional effects of treatment, with site adjusted as a random-effect variable. Generalized estimating equations were used to estimate the marginal effect of study interventions. A proportional odds model was specified for VVFDs. Mortality was modeled assuming a logit link function. A full model was prespecified to include age, body mass index, Acute Physiology and Chronic Health Evaluation (APACHE) II score, race, sex, need for ventilation or vasopressors at baseline, whether sepsis was the primary reason for admission, whether a patient was admitted via the emergency department, and source of infection. Post hoc, body mass index was replaced with weight. No variable selection techniques were used. Multiple imputation based on predictive mean matching was used to overcome any missingness in covariates; missing VVFDs were estimated based on last value carried forward or, for the sensitivity analysis, observed outcomes only. Restricted cubic splines were used to address nonlinearities in the association between continuous variables and observed outcomes. To determine whether subgroup analyses were warranted, the interaction between treatment group assignment and baseline features of participants were evaluated. This was done by fitting the main covariate-adjusted model including all main effects, then individually testing for the interaction between each covariate and the treatment indicator using P ≤ .20. Interaction terms reaching this threshold were assumed to indicate possible effect modification and, thus, the need to explore for differential treatment effects within subgroups. Also, in a post hoc exploratory analysis, we used a graphical approach to further evaluate the possibility that time to treatment modifies the treatment effect.
Prespecified exploratory end points were compared by treatment group in a similar manner with the primary and secondary end points. All exploratory analyses used a 2-sided critical P = .05 without adjustment for multiplicity. All analyses, including generation of the randomization sequence, used R version 3.4.3 (R Foundation for Statistical Computing).
Early Trial Termination
The interim analyses planned for N = 500 proceeded as expected. After 501 participants were enrolled, additional funding for the trial was withheld due to a change in the funder’s priorities, and no further enrollments occurred, leading to administrative termination of the trial prior to meeting any prespecified stopping criterion. Aside from the knowledge that the trial had not yet stopped at an earlier interim analysis, the funder had no information regarding unblinded trial results at the time funding was terminated, which occurred on October 15, 2019. In a post hoc conditional analysis at the time of the N = 500 analysis, the predictive probability of reaching the protocol-specified criteria to declare efficacy on VVFD at N = 2000 was computed using Markov chain Monte Carlo methods. From the posterior distributions of the bayesian analysis model, we performed multiple imputation of the VVFD outcomes for all participants with unknown outcomes. This imputation resulted in 10 000 imputed data sets of size n = 2000, each of which was assessed for meeting the final analysis success threshold (Wilcoxon P < .022).
Results
Enrollment and Patient Characteristics
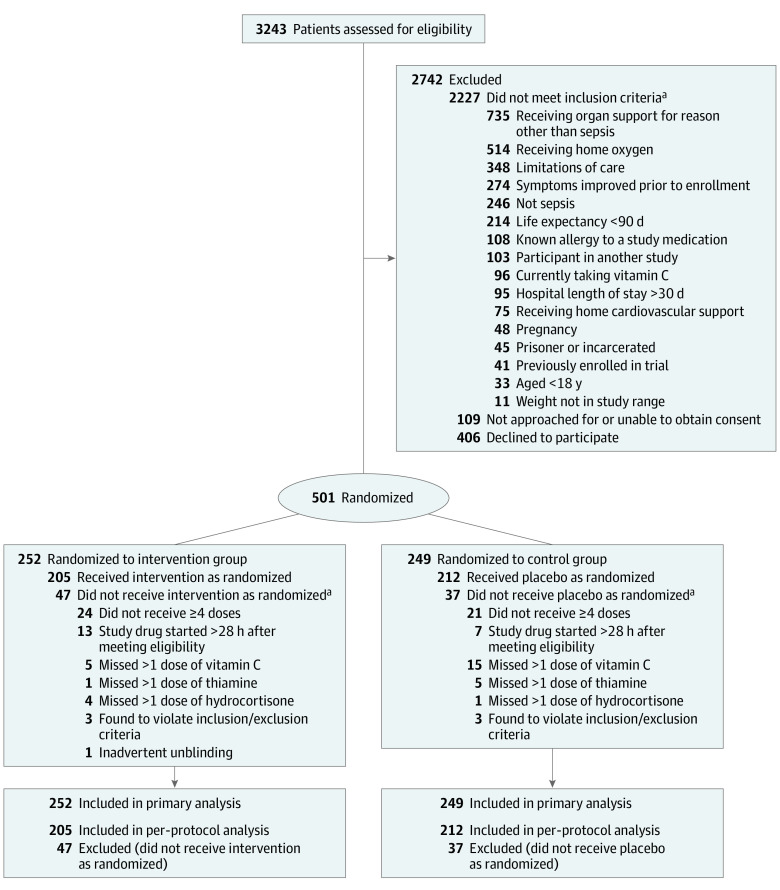
Of 3243 patients screened at 43 hospitals between August 2018 and July 2019, 501 were enrolled (Figure 1). The most common reasons for exclusion were need for organ support for a diagnosis other than sepsis (n = 735), home oxygen use (n = 514), patient refusal (n = 406), and limitations in care (n = 346). The median age of enrolled patients was 62 years, 30% were Black, and 46% were female (Table 1). Patients qualified for enrollment based on a need for both ventilatory and vasopressor support (41%), ventilatory support alone (21%), or vasopressor support alone (38%). The median baseline APACHE II and SOFA scores were 27.0 (IQR, 20.8-33.0) and 9.0 (IQR, 7.0-12.0), respectively. Following randomization, 252 were assigned to the intervention group and 249 to the control group. The median time between the onset of qualifying organ dysfunction and first dose of study drug was 14.7 hours (IQR, 7.9-20.9 hours). Thirty-three percent of patients in the intervention group and 32% of control patients received clinician-prescribed corticosteroids at a dose of at least 200 mg of hydrocortisone daily equivalent.
Figure 1. Participant Flow in the Vitamin C, Thiamine, and Steroids in Sepsis Trial.
aCategories are not mutually exclusive; participants could have more than 1 reason.
Table 1. Participant Characteristics.
| Characteristics | Main analysis | Per-protocol analysis | ||
|---|---|---|---|---|
| Intervention group (n = 252) | Control group (n = 249) | Intervention group (n = 205) | Control group (n = 212) | |
| Age, median (IQR), y | 62 (51-69) | 61 (50-72) | 62 (52-70) | 62 (49-72) |
| Sex, No. (%) | ||||
| Male | 139 (55.2) | 134 (53.8) | 117 (57.1) | 112 (52.8) |
| Female | 113 (44.8) | 115 (46.2) | 88 (42.9) | 100 (47.2) |
| Race, No./total (%) | ||||
| White | 149/232 (64.2) | 135/232 (58.2) | 121/188 (64.4) | 116/196 (59.2) |
| Black | 70/232 (30.2) | 80/232 (34.5) | 58/188 (30.9) | 69/196 (35.2) |
| Hispanic or Latino | 32/243 (13.2) | 24/239 (10.0) | 23/197 (11.7) | 21/202 (10.4) |
| Othera | 13/232 (5.6) | 17/232 (7.3) | 9/188 (4.8) | 11/196 (5.6) |
| Weight, median (IQR), kg | 80 (68-96) | 80 (67-97) | 78 (68-94) | 80 (68-98) |
| Diabetes, No. (%) | 85 (33.7) | 77 (30.9) | 67 (32.7) | 66 (31.1) |
| Cardiovascular disease, No. (%) | 129 (50.9) | 126 (50.6) | 106 (51.7) | 108 (50.9) |
| Respiratory disease, No. (%) | 55 (21.8) | 56 (22.5) | 44 (21.5) | 48 (22.6) |
| Current cancer, No. (%) | 41 (16.3) | 55 (22.1) | 38 (18.5) | 44 (20.8) |
| Neurological disease, No. (%) | 46 (18.3) | 48 (19.3) | 37 (18.0) | 42 (19.8) |
| Organ support at enrollment, No. (%) | ||||
| Vasopressor | 93 (36.9) | 97/248 (39.1) | 82 (40.0) | 84 (39.6) |
| Ventilator | 49 (19.4) | 54/248 (21.8) | 42 (20.5) | 47 (22.2) |
| Both | 110 (43.7) | 97/248 (39.1) | 81 (39.5) | 81 (38.2) |
| Type of ventilator support No./total No. ventilated (%) | 8 | |||
| Intubation | 110/158 (69.6) | 96/151 (63.6) | 88/122 (72.1) | 84/128 (65.6) |
| CPAP or BPAP | 29/158 (18.4) | 26/151 (17.2) | 22/122 (18.0) | 21/128 (16.4) |
| High-flow oxygen ≥40 L/minb | 19/158 (12.0) | 29/151 (19.2) | 12/122 (9.8) | 23/128 (18.0) |
| Intensive care unit admission source, No. (%) | ||||
| Emergency department | 169 (67.1) | 184 (73.9) | 134 (65.4) | 158 (74.5) |
| Hospital floor | 47 (18.7) | 40 (16.1) | 40 (19.5) | 35 (16.5) |
| Step-down unit | 9 (3.6) | 8 (3.2) | 7 (3.4) | 8 (3.8) |
| Intermediate care | 3 (1.2) | 1 (0.4) | 2 (1.0) | 1 (0.5) |
| Otherc | 24 (9.5) | 16 (6.4) | 22 (10.7) | 10 (4.7) |
| Admission reason, No. (%) | ||||
| Sepsis | 180 (71.4) | 176 (70.7) | 146 (71.2) | 151 (71.2) |
| Other medical | 63 (25.0) | 67 (26.9) | 50 (24.4) | 55 (25.9) |
| Other surgical | 9 (3.6) | 6 (2.4) | 9 (4.4) | 6 (2.8) |
| Clinical values, median (IQR) | ||||
| Heart rate, /min | 100 (84-115) | 94 (78-110) | 102 (83-115) | 94 (77-110) |
| Systolic blood pressure, mm Hg | 103 (90-120) | 103 (91-118) | 103 (90-119) | 104 (92-118) |
| Diastolic blood pressure, mm Hg | 58 (49-68) | 56 (51-66) | 58 (49-67) | 56 (51-66) |
| Mean arterial pressure, mm Hg | 72 (64-82) | 71 (65-79) [n = 248] | 72 (64-81) | 71 (66-80) [n = 211] |
| Respiratory rate, /min | 22 (18-28) | 22 (18-27) [n = 247] | 21 (18-26) | 22 (18-27) [n = 210] |
| Temperature, °C | 37 (36-38) [n = 248] | 37 (37-38) [n = 247] | 37 (36-38) [n = 201] | 37 (36-38) [n = 211] |
| White blood cell count, ×109/L | 14 (7-22) [n = 249] | 12 (6-19) [n = 247] | 14 (7-21) [n = 202] | 13 (6-19) [n = 210] |
| Lactic acid, mmol/L | 3 (2-5) [n = 203] | 3 (2-5) [n = 190] | 2 (2-4) [n = 168] | 3 (2-4) [n = 164] |
| APACHE II scored | 27 (22-33) [n = 239] | 27 (19-33) [n = 233] | 27 (22-33) [n = 194] | 27 (19-33) [n = 198] |
| SOFA scoree | 9 (7-12) | 9 (6-11) | 9 (7-12) | 8 (6-11) |
| Time to treatment, h | 15 (8-22) [n = 246] | 14 (8-20) [n = 244] | 15 (8-21) | 14 (8-20) |
| Infection source, No./total (%) | ||||
| Lung | 76/205 (37.1) | 81/204 (39.7) | 64/169 (37.9) | 69/175 (39.4) |
| Urinary tract | 27/205 (13.2) | 43/204 (21.1) | 23/169 (13.6) | 37/175 (21.1) |
| Intra-abdominal | 37/205 (18.0) | 24/204 (11.8) | 28/169 (16.6) | 22/175 (12.6) |
| Blood/vascular access | 37/205 (18.0) | 22/204 (10.8) | 32/169 (18.9) | 20/175 (11.4) |
| Skin or soft tissues | 11/205 (5.4) | 18/204 (8.8) | 9/169 (5.3) | 15/175 (8.6) |
| Central nervous system | 5/205 (2.4) | 1/204 (0.5) | 2/169 (1.2) | 1/175 (0.6) |
| Bone/joint | 2/205 (1.0) | 2/204 (1.0) | 2/169 (1.2) | 2/175 (1.1) |
| Other | 9/205 (4.4) | 11/204 (5.4) | 8/169 (4.7) | 7/175 (4.0) |
| Confirmed unknown | 1/205 (0.5) | 2/204 (1.0) | 1/169 (0.6) | 2/175 (1.1) |
Abbreviations: BPAP, bilevel positive airway pressure; CPAP, continuous positive airway pressure; IQR, interquartile range.
Other race included American Indian/Alaskan Native, Asian, Native Hawaiian/other Pacific Islander, mixed race, and race reported as other.
Patients receiving high-velocity nasal insufflation (Vapotherm) could be enrolled if receiving ≥30 L/min.
Other sources of admission included transfer from an outside hospital or health care facility (n = 27) and admission from a local operating room, intensive care unit, or emergency department (n = 13).
The Acute Physiology and Chronic Health Evaluation (APACHE) II score ranges from 0 to 71, with higher scores indicating greater risk of hospital death. A score of 25 indicates a mortality probability of approximately 50%.
The Sequential Organ Failure Assessment (SOFA) score ranges from 0 to 24, with higher scores indicating greater severity of organ dysfunction. SOFA scores between 7 and 9 are associated with a 40% to 50% mortality risk.
Baseline and clinical characteristics of enrolled patients are provided in Table 1; 67% vs 73% of patients admitted from the emergency department were randomized to the intervention group vs the control group, respectively. Severity of illness, comorbidities, source of infection, and type of infecting organism were similar between groups. Protocol adherence was high, with only 20 participants missing more than 1 dose of vitamin C or matching placebo, 6 missing more than 1 dose of thiamine or matching placebo, and 3 missing more than 1 dose of hydrocortisone or matching placebo.
Primary and Key Secondary Outcomes
There was no statistically significant difference between the intervention and control groups in VVFDs (25 days [IQR, 0-29 days] vs 26 days [IQR, 0-28 days], respectively). The median difference in VVFDs between the intervention and control groups was −1 day (95% CI, −4 to 2 days; P = .85). In both the intervention and control groups, the majority of patients with 0 VVFDs died (71% and 70%, respectively). Thirty-day all-cause mortality was 22% in the intervention group vs 24% in the control group. The adjusted analyses are given in Section 2.5 of Supplement 4. The proportional odds model for VVFDs did not reveal a statistical difference between the intervention and control groups (P = .16; model R2 = 0.32; model C statistic = 0.73). Requiring ventilatory support at enrollment was significantly associated with higher odds of 30-day mortality compared with not requiring ventilatory support at enrollment (odds ratio, 2.54; 95% CI, 1.33-5.38).
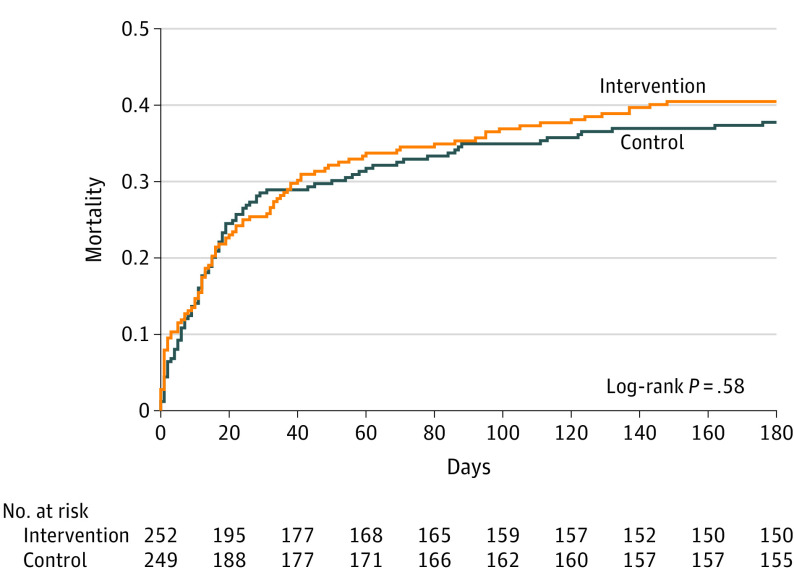
Long-term Outcome
At 180 days, mortality in the intervention and control groups was 40.5% vs 37.8% (difference, 2.7%; 95% CI, −11.3% to 5.8%) (Figure 2). The Cox proportional hazard model yielded an unadjusted hazard ratio for death in the intervention group of 1.08 at 180 days (95% CI, 0.82-1.43).
Figure 2. Survival in the Intervention and Control Groups at 180 Days.
Cumulative deaths for the 180-day follow-up period are shown. Median time to death in the intervention group was 16 (interquartile range, 4-41) days and in the control group was 15 (interquartile range, 6-29) days. The proportionality P = .81, suggesting that the proportionality assumption was met.
Differential Treatment Effects and Exploratory Outcomes
Prespecified exploratory end points in Table 2 showed no statistically significant difference between groups in bivariable comparisons of change in SOFA score to day 4, length of ICU stay, length of hospital stay, delirium-/coma-free days, or kidney replacement therapy–free days. Post hoc, weight replaced body mass index as the selected covariate and differential treatment effects by weight were explored. The VVFD model yielded an interaction between treatment and weight (P = .02) (Table 14 in Supplement 4). The nonlinear association between weight and outcomes for the treatment groups is shown in Section 2.5.5 in Supplement 4. In the per-protocol analysis, a similar interaction between treatment and weight was observed (P = .001) (Table 44 and Section 3.6.5 in Supplement 4). Treatment response was not significantly associated with any prespecified variable, including time to treatment (Table 14 in Supplement 4). The distribution of outcomes within quartile of time to treatment stratified by treatment group is shown in Section 4 of Supplement 4. Overall, 64 participants in the intervention group and 69 participants in the control group were treated within 8 hours, with median VVFDs of 28 (IQR, 24-29) and 27 (0-29), and there was no significant interaction between time to treatment and group assignment (P = .27).
Table 2. Exploratory Outcomes in the Main and Per-Protocol Analyses.
| Outcomes | Intervention group | Control group | Difference (95% CI) | P value |
|---|---|---|---|---|
| Main analysis | ||||
| Total No. | 252 | 249 | ||
| Mortality before ICU discharge, No. (%) | 52 (20.6) | 49 (19.7) | 0.9 (−8.0 to 6.1) | .79 |
| Mortality at 180 d, No. (%) | 102 (40.5) | 94 (37.8) | 2.7 (−11.3 to 5.8) | .53 |
| Change in SOFA score, median (IQR)a | 5 (3-7) | 5 (2-7) | 0.0 (−1.0 to 0.0) | .10 |
| Length of ICU stay, median (IQR), d | 4 (2-8) [n = 250] | 4 (2-8) [n = 245] | 0.0 (−2.0 to 1.0) | .82 |
| Length of hospital stay, median (IQR), d | 10 (6-17) [n = 250] | 9 (5-17) [n = 246] | 1.0 (−3.0 to 2.0) | .66 |
| Coma-/delirium-free days, median (IQR)b | 4 (2-5) [n = 237] | 4 (2-5) [n = 241] | 0.0 (0.0 to 1.0) | .45 |
| Kidney replacement therapy–free days, median (IQR) | 30 (0-30) | 30 (0-30) [n = 247] | 0.0 (0.0 to 0.0) | .58 |
| Per-protocol analysis | ||||
| Total No. | 205 | 212 | ||
| Mortality before ICU discharge, No. (%) | 34 (16.6) | 36 (17.0) | 0.4 (−6.8 to 7.6) | .91 |
| Mortality at 180 d, No. (%) | 81 (39.5) | 78 (36.8) | 2.7 (−12.0 to 6.6) | .57 |
| Change in SOFA score, median (IQR)a | 5 (3-7) | 5 (2-7) | 0.0 (−1.0 to 0.0) | .07 |
| Length of ICU stay, median (IQR), d | 4 (2-9) [n = 204] | 4 (2-8) [n = 210] | 0.0 (−1.0 to 1.0) | .52 |
| Length of hospital stay, median (IQR), d | 11 (6-19) [n = 204] | 10 (6-18) [n = 210] | 1.0 (−3.0 to 2.0) | .48 |
| Coma-/delirium-free days, median (IQR)b | 4 (2-5) [n = 200] | 4 (2-5) [n = 209] | 0.0 (0.0 to 1.0) | .52 |
| Kidney replacement therapy–free days, median (IQR) | 30 (13-30) | 30 (7-30) [n = 211] | 0.0 (0.0 to 0.0) | .78 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
The Sequential Organ Failure Assessment (SOFA) score can change by up to 24 points. An increasing SOFA score is associated with increasing mortality.
Coma- and delirium-free days are based on the daily Confusion Assessment Method for the ICU (CAM-ICU).19 The CAM-ICU is scored as either positive or negative based on agitation. If the Richmond Agitation Scale was −4 or −5 or the CAM-ICU was automatically scored positive, the participant was noted as experiencing coma or delirium on that day.
Safety and Adverse Event Outcomes
There were no reported serious adverse events in the study. There were 2 adverse events (hemorrhagic shock and worsening kidney function) in the intervention group assessed as potentially related to study participation.
Sensitivity Analysis
Of the 501 enrolled patients, 83 were excluded because of protocol deviations, leaving 417 in the per-protocol analysis set. The median number of VVFDs for patients in the per-protocol analysis was 26 (IQR, 0-29) in the intervention group vs 26 (IQR, 0-28) in the control group, with a median group difference of 0 VVFDs (95% CI, −3.00 to 2.00) and an odds ratio of 1.09 (95% CI, 0.78-1.53) (Table 35 in Supplement 4). At 30 days, 18% of patients in the per-protocol intervention group and 22% in the control group had died. Adjusted analyses, both conditional and marginal, were similar to unadjusted analyses, although the odds of 30-day mortality among participants with urinary tract infections was significantly lower than in patients with other types of infection (odds ratio, 0.31; 95% CI, 0.12-0.81) (Table 17 in Supplement 4). No association between 180-day mortality and study group was observed (intervention, 40%; control, 37%; difference, 3%; 95% CI, −12% to 7%). The unadjusted Cox proportional hazard ratio for 180-day death was 1.07 (95% CI, 0.78-1.46). The main findings were unchanged when restricting analyses to the 458 patients for whom outcomes were fully observed (Section 2.6 in Supplement 4) and when restricting analyses to the 402 patients who did not receive open-label steroids (Section 2.6.14 in Supplement 4).
Conditional Power Analysis Due to Administrative Early Trial Termination
At the time of the N = 500 analysis, the predictive probability that the trial would meet the protocol-specified criteria to declare efficacy on VVFDs at N = 2000 was 30.7%. At that time, there was about a 0.3-day difference in mean VVFDs between groups.
Discussion
In this randomized clinical trial conducted with an adaptive design and terminated early for administrative reasons, treatment with vitamin C, thiamine, and hydrocortisone did not significantly increase VVFDs over 30 days compared with placebo. This held true for all adjusted and per-protocol analyses for the primary, key secondary, and exploratory outcomes. However, due to the early termination, the study may have been underpowered to detect a clinically important difference in VVFDs.
Patients enrolled in each group were well matched and protocol adherence was high. Importantly, observed mortality in the control group was in the range used to conduct power calculations for the study.
This study’s results are concordant with prior clinical trials of vitamin C in sepsis. In patients with septic shock, the ACTS trial found that treatment with vitamin C, thiamine, and hydrocortisone did not reduce SOFA score or mortality,20 while the VITAMINS trial found that the combination did not increase days alive and free of vasopressors.10 Of note, all patients in the control group in the VITAMINS trial were treated with corticosteroids, compared with 33% of the control group in this trial. Comparison between this trial and the CITRIS-ALI study is less straightforward.18 In CITRIS-ALI, a higher dosage of intravenous vitamin C (50 mg/kg every 6 hours) was given to patients with acute lung injury in the setting of sepsis. Although the CITRIS-ALI study, similar to VICTAS, did not show differences in organ dysfunction scores over time, the CITRIS-ALI trial did show that a decrease in mortality in patients treated with vitamin C remains plausible. It is important to note that mortality in CITRIS-ALI was 1 of 46 secondary outcome measures, and no adjustment was made for multiple comparisons in the analysis18 It is possible that differences in study populations, differences in ancillary therapies such as corticosteroids and thiamine, and the higher dose of vitamin C used in CITRIS-ALI contributed to this different outcome.11,21,22
Limitations
This trial has several limitations. First, it was administratively stopped due to a lack of funding and, thus, may have been underpowered to show clinically meaningful differences in VVFD. Second, a fixed dose of vitamin C was tested; a higher dose or dosing based on plasma vitamin C concentrations might yield different results. Third, clinicians were allowed to administer corticosteroids at their discretion. If corticosteroids have a beneficial effect on VVFD, this would bias the trial results toward the null, although a post hoc sensitivity analysis demonstrated similar findings in patients not receiving open-label corticosteroids. Fourth, enrollment was limited to patients with sepsis-induced cardiovascular or respiratory failure; inclusion of patients with different types of organ dysfunction may have yielded different results. Fifth, the median time to receipt of intervention was 14.7 hours (IQR, 7.9-20.9 hours), and while this timing was earlier than in the VITAMINS trial, it remains unknown if still earlier administration could improve outcomes. In this study, treatment response was not associated with time to treatment, and there was no interaction between time and group assignment.
Conclusions
Among critically ill patients with sepsis, treatment with vitamin C, thiamine, and hydrocortisone, compared with placebo, did not significantly increase ventilator- and vasopressor-free days within 30 days. However, the trial was terminated early for administrative reasons and may have been underpowered to detect a clinically important difference.
Trial Protocol
Statistical Analysis Plan
Adaptive Design Report
VICTAS Statistical Analysis
VICTAS Collaborators
Data Sharing Statement
Section Editor: Christopher Seymour, MD, Associate Editor, JAMA (christopher.seymour@jamanetwork.org).
References
- 1.Singer M, Deutschman CS, Seymour CW, et al. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016;315(8):801-810. doi: 10.1001/jama.2016.0287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rhee C, Dantes R, Epstein L, et al. ; CDC Prevention Epicenter Program . Incidence and trends of sepsis in US hospitals using clinical vs claims data, 2009-2014. JAMA. 2017;318(13):1241-1249. doi: 10.1001/jama.2017.13836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Torio CM, Moore BJ. National Inpatient Hospital Costs: The Most Expensive Conditions by Payer, 2013 Agency for Healthcare Research and Quality; 2016. Healthcare Cost and Utilization Project Statistical Brief No. 204. [PubMed]
- 4.Buchman TG, Simpson SQ, Sciarretta KL, et al. Sepsis among Medicare beneficiaries, 1: the burdens of sepsis, 2012-2018. Crit Care Med. 2020;48(3):276-288. doi: 10.1097/CCM.0000000000004224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yende S, Austin S, Rhodes A, et al. Long-term quality of life among survivors of severe sepsis: analyses of two international trials. Crit Care Med. 2016;44(8):1461-1467. doi: 10.1097/CCM.0000000000001658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rhodes A, Evans LE, Alhazzani W, et al. Surviving Sepsis Campaign: international guidelines for management of sepsis and septic shock: 2016. Crit Care Med. 2017;45(3):486-552. doi: 10.1097/CCM.0000000000002255 [DOI] [PubMed] [Google Scholar]
- 7.Marshall JC Why have clinical trials in sepsis failed? Trends Mol Med. 2014;20(4):195-203. doi: 10.1016/j.molmed.2014.01.007 [DOI] [PubMed] [Google Scholar]
- 8.Spoelstra-de Man AME, Elbers PWG, Oudemans-Van Straaten HM. Vitamin C: should we supplement? Curr Opin Crit Care. 2018;24(4):248-255. doi: 10.1097/MCC.0000000000000510 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wilson JX Evaluation of vitamin C for adjuvant sepsis therapy. Antioxid Redox Signal. 2013;19(17):2129-2140. doi: 10.1089/ars.2013.5401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fujii T, Luethi N, Young PJ, et al. ; VITAMINS Trial Investigators . Effect of vitamin C, hydrocortisone, and thiamine vs hydrocortisone alone on time alive and free of vasopressor support among patients with septic shock: the VITAMINS randomized clinical trial. JAMA. 2020;323(5):423-431. doi: 10.1001/jama.2019.22176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fowler AA III, Truwit JD, Hite RD, et al. Effect of vitamin C infusion on organ failure and biomarkers of inflammation and vascular injury in patients with sepsis and severe acute respiratory failure: the CITRIS-ALI randomized clinical trial. JAMA. 2019;322(13):1261-1270. doi: 10.1001/jama.2019.11825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Putzu A, Daems AM, Lopez-Delgado JC, Giordano VF, Landoni G. The effect of vitamin C on clinical outcome in critically ill patients: a systematic review with meta-analysis of randomized controlled trials. Crit Care Med. 2019;47(6):774-783. doi: 10.1097/CCM.0000000000003700 [DOI] [PubMed] [Google Scholar]
- 13.Barabutis N, Khangoora V, Marik PE, Catravas JD. Hydrocortisone and ascorbic acid synergistically prevent and repair lipopolysaccharide-induced pulmonary endothelial barrier dysfunction. Chest. 2017;152(5):954-962. doi: 10.1016/j.chest.2017.07.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: a retrospective before-after study. Chest. 2017;151(6):1229-1238. doi: 10.1016/j.chest.2016.11.036 [DOI] [PubMed] [Google Scholar]
- 15.Hager DN, Hooper MH, Bernard GR, et al. The Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) protocol: a prospective, multi-center, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials. 2019;20(1):197. doi: 10.1186/s13063-019-3254-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lindsell CJ, McGlothlin A, Nwosu S, et al. Update to the Vitamin C, Thiamine and Steroids in Sepsis (VICTAS) protocol: statistical analysis plan for a prospective, multicenter, double-blind, adaptive sample size, randomized, placebo-controlled, clinical trial. Trials. 2019;20(1):670. doi: 10.1186/s13063-019-3775-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hager DN, Martin GS, Sevransky JE, Hooper MH. Glucometry when using vitamin C in sepsis: a note of caution. Chest. 2018;154(1):228-229. doi: 10.1016/j.chest.2018.03.018 [DOI] [PubMed] [Google Scholar]
- 18.Vincent JL, Moreno R, Takala J, et al. ; Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine . The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996;22(7):707-710. doi: 10.1007/BF01709751 [DOI] [PubMed] [Google Scholar]
- 19.Ely EW, Margolin R, Francis J, et al. Evaluation of delirium in critically ill patients: validation of the Confusion Assessment Method for the Intensive Care Unit (CAM-ICU). Crit Care Med. 2001;29(7):1370-1379. doi: 10.1097/00003246-200107000-00012 [DOI] [PubMed] [Google Scholar]
- 20.Moskowitz A, Huang DT, Hou PC, et al. ; ACTS Clinical Trial Investigators . Effect of ascorbic acid, corticosteroids, and thiamine on organ injury in septic shock: the ACTS randomized clinical trial. JAMA. 2020;324(7):642-650. doi: 10.1001/jama.2020.11946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Grooth HJ, Elbers PWG, Vincent JL. Vitamin C for sepsis and acute respiratory failure [letter]. JAMA. 2020;323(8):792. doi: 10.1001/jama.2019.21981 [DOI] [PubMed] [Google Scholar]
- 22.Fowler AA III, Fisher BJ, Kashiouris MG. Vitamin C for sepsis and acute respiratory failure [letter reply]. JAMA. 2020;323(8):792-793. doi: 10.1001/jama.2019.21987 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial Protocol
Statistical Analysis Plan
Adaptive Design Report
VICTAS Statistical Analysis
VICTAS Collaborators
Data Sharing Statement