Key Points
Question
Among critically ill patients with unhealthy alcohol use who are receiving mechanical ventilation, is high-dose baclofen effective in preventing agitation-related events?
Findings
In this randomized clinical trial that included 314 patients, the percentage of patients with an agitation-related event was 19.7% in the baclofen group and 29.7% in the placebo group, a difference that was statistically significant.
Meaning
Although high-dose baclofen significantly reduced agitation-related events, further research is needed to determine the possible role of baclofen for patients receiving mechanical ventilation with unhealthy alcohol use, considering the modest effect and the totality of findings for the secondary end points and adverse events.
Abstract
Importance
Unhealthy alcohol use can lead to agitation in the intensive care unit (ICU).
Objective
To assess whether high-dose baclofen reduces agitation-related events compared with placebo in patients with unhealthy alcohol use receiving mechanical ventilation.
Design, Settings, and Participants
This phase 3, double-blind, placebo-controlled, randomized clinical trial conducted in 18 ICUs in France recruited adults receiving mechanical ventilation who met criteria for unhealthy alcohol use. Patients were enrolled from June 2016 to February 2018; the last follow-up was in May 2019.
Interventions
Baclofen (n = 159), adjusted from 50 to 150 mg per day based on estimated glomerular filtration rate, or placebo (n = 155) during mechanical ventilation up to a maximum of 15 days before gradual dose reduction over 3 to 6 days.
Main Outcomes and Measures
The primary end point was the percentage of patients with at least 1 agitation-related event over the treatment period. Secondary outcomes included duration of mechanical ventilation, length of ICU stay, and 28-day mortality.
Results
Among 314 patients who were randomized (mean age, 57 years; 60 [17.2%] women), 313 (99.7%) completed the trial. There was a statistically significant decrease in the percentage of patients who experienced at least 1 agitation-related event in the baclofen group vs the placebo group (31 [19.7%] vs 46 [29.7%]; difference, −9.93% [95% CI, –19.45% to –0.42%]; adjusted odds ratio, 0.59 [95% CI, 0.35-0.99]). Of 18 prespecified secondary end points, 14 were not significantly different. Compared with the placebo group, the baclofen group had a significantly longer median length of mechanical ventilation (9 vs 8 days; difference, 2.00 [95% CI, 0.00-3.00]; hazard ratio [HR] for extubation, 0.76 [95% CI, 0.60-0.97]) and stay in the ICU (14 vs 11 days; difference, 2.00 [95% CI, 0.00-4.00]; HR for discharge, 0.70 [95% CI, 0.54-0.90]). At 28 days, there was no significant difference in mortality in the baclofen vs placebo group (25.3% vs 21.6%; adjusted odds ratio, 1.24 [95% CI, 0.72-2.13]). Delayed awakening (no eye opening at 72 hours after cessation of sedatives and analgesics) occurred in 14 patients (8.9%) in the baclofen group vs 3 (1.9%) in the placebo group.
Conclusions and Relevance
Among patients with unhealthy alcohol use receiving mechanical ventilation, treatment with high-dose baclofen, compared with placebo, resulted in a statistically significant reduction in agitation-related events. However, considering the modest effect and the totality of findings for the secondary end points and adverse events, further research is needed to determine the possible role of baclofen in this setting and to potentially optimize dosing.
Trial Registration
ClinicalTrials.gov Identifier: NCT02723383
This randomized clinical trial compares the effects of high-dose baclofen vs placebo on agitation-related events among adults in the ICU with unhealthy alcohol use receiving mechanical ventilation.
Introduction
Alcohol, considered the most commonly used psychoactive substance,1,2 was estimated to be responsible for 2.8 million deaths worldwide in 2016.3 In the US, an estimated 14.1 million adults have an alcohol use disorder.4 Unhealthy alcohol use, as defined by the US National Institutes of Health National Institute on Alcohol Abuse and Alcoholism (NIAAA),5,6 covers a wide spectrum, from risky use to alcohol abuse and dependence, with varying degrees of health risk. In the intensive care unit (ICU), patients with unhealthy alcohol use have higher risk of death and often experience agitation and/or delirium, which can compromise medical care and lead to long-term psychological sequelae.7,8
Guidelines from 2018 for the management of agitation and sedation in the ICU recommend against a pharmacological prevention strategy for agitation9 unless there is a specific risk factor.10 Although unhealthy alcohol use has been identified as a risk factor for agitation,7,11 there is no specific recommendation for the management of sedation in this high-risk population.
Baclofen, which acts as a γ-aminobutyric acid type B receptor agonist and may decrease or suppress alcohol craving in patients with alcohol use disorder,12 has, to our knowledge, never been assessed in a randomized clinical trial for prevention of agitation-related events. This randomized clinical trial tested the hypothesis that treatment of patients with unhealthy alcohol use receiving mechanical ventilation with high-dose baclofen could reduce agitation-related events compared with placebo.
Methods
Study Design, Setting, and Ethical Considerations
The protocol, developed and published by the study investigators,13 was approved by the ethics committee from Angers, France (Comité de Protection des Personnes Ouest II #2015/09), and was conducted in accordance with the Declaration of Helsinki. The study protocol and statistical analysis plan are available in Supplement 1. This was a multicenter double-blind, placebo-controlled, randomized clinical trial in 18 French medical and surgical ICUs at university and district hospitals. Written informed consent was sought for all patients prior to inclusion. However, if the patient lacked capacity to give consent, the ICU physician sought consent from a relative of the patient. If a relative was not available, research staff could proceed with emergency inclusion. As soon as patients regained capacity, a research technician or physician informed them about the trial and written consent was sought. If consent was withdrawn, treatment was stopped.
Participants
Adult patients aged 18 to 80 years requiring mechanical ventilation for an expected duration of 24 hours or more who met the NIAAA criteria for unhealthy alcohol use were eligible for inclusion. Unhealthy alcohol use was defined as consumption of more than 14 units per week for men and 7 units per week for women or men older than 65 years. One unit of alcohol corresponded to 1 drink containing approximately 12 to 14 g of pure alcohol, which is equivalent to 12 ounces of beer, 5 ounces wine, or 1.5 ounces of 80-proof liquor.14 Alcohol intake quantity was disclosed by the patient before intubation or, if this was not possible, by a relative or the medical record. Patients were excluded from enrollment if they received baclofen prior to ICU admission; had known hypersensitivity to baclofen; were pregnant; had a history of treatment-resistant epilepsy or epileptic seizure in past 6 months; had porphyria, celiac, or Parkinson disease; were admitted for burn treatment; had brain injury due to recent stroke or hemorrhage; had recent or former quadriplegia or paraplegia; had cardiac arrest before or after hospital admission; had a tracheotomy on ICU admission; had a hospital stay of at least 7 days prior to randomization; had mental impairment (ie, dementia, schizophrenia, bipolar disorder, severe depression); or had health care limitation due to poor prognosis. Patients were also excluded if enteral treatment was not accessible for more than 48 hours or if they did not have Social Security. To facilitate enrollment, eligibility criteria were modified after the first 5 patients were randomized. The upper age limit was increased from 70 to 80 years and the expected duration of mechanical ventilation was decreased from 48 to 24 hours.
Randomization and Blinding
Patients were randomized in a 1:1 ratio to receive treatment with baclofen or placebo by a remote system controlled by an independent research unit at the University Hospital of Nantes. Randomization was performed using a computer-generated random number with a block size of 4, stratified by center.
To try to ensure blinding, Nantes University Hospital pharmacy centrally prepared and labeled blister packs of drug capsules (50, 20, and 10 mg of baclofen compounded with lactose [POLPharma; Cooper]) and 3 matching placebos (lactose [Cooper]), which could not be distinguished. Patients, treating physicians, investigators, the trial statistician, and members of the data and safety monitoring board were blinded to trial group assignment.
Intervention
The trial design and algorithm for dose adjustment have been previously described in a preliminary pharmacokinetic study of high-dose baclofen for unhealthy alcohol users in the ICU; the algorithm is shown in eFigure 1 in Supplement 2.15,16 On day 1, patients received a single loading dose of baclofen or placebo, ranging from 50 to 150 mg, based on their estimated glomerular filtration rate (eGFR).17 From day 2 to 15, patients received 50 to 150 mg per day (based on eGFR), divided into 3 doses per day. If extubation or tracheotomy occurred before day 15, drug administration was stopped. If treatment was continued to day 15, the study drug was gradually reduced over 3 to 6 days based on the patient’s eGFR. In all cases, the treatment was discontinued on discharge from the ICU.
During the study period, a nursing-led sedation protocol was used in both groups to target and maintain a light level of sedation (ie, Richmond Agitation Sedation Scale score of −2 to +1), unless deep sedation was indicated for an acute medical condition.9 Agitation was determined by the Riker Sedation-Agitation Scale.8 The Clinical Institute Withdrawal Assessment of Alcohol Scale, Revised (CIWA-Ar) was used to detect withdrawal symptoms after extubation.18 When patients were capable of providing information, either prior to or after mechanical ventilation was stopped, they were asked to complete an NIAAA questionnaire about their alcohol use (eFigure 2 in Supplement 2).
Outcomes
The primary outcome was the occurrence of at least 1 agitation-related event over the treatment period, defined as unplanned extubation; pulling out lines, catheters, or drains; falling out of bed; fleeing the ICU (ie, leaving against the physician’s advice or without being seen); immobilization device removal; self-aggression; or aggression toward medical staff.
The secondary outcomes included the occurrence of at least 1 agitation-related event from day 1 to day 28, the number of adverse events from day 1 to 28, agitation requiring rapid administration of hypnotic or neuroleptic drug, extubation failure (defined as reintubation within 48 hours after extubation), need for tracheotomy, reintubation by day 28, ICU-acquired infection, cumulative doses of psychotropic drugs in the ICU, Riker Sedation-Agitation Scale score from day 1 to 28, CIWA-Ar score from day 1 to day 7 after extubation or tracheotomy, duration of ICU stay, duration of hospital stay, ICU mortality, hospital mortality, mortality from day 28 to 90, agitation and mortality in the ICU by day 28, duration of mechanical ventilation, and number of days alive without mechanical ventilation during the first 28 days.
Adverse Events and Adherence
According to prespecified rules,13 treatment could be temporarily discontinued if the eGFR fell below 15 mL/min/1.73 m2 without renal replacement therapy, aminotransferase enzyme level was more than 20 times greater than the reference range, or heart rate was less than 50/min. Permanent discontinuation of treatment was mandated if patients developed allergic symptoms or unilateral or bilateral mydriasis or had a seizure, stroke, heart rate less than 35/min, or delayed awakening (defined as no eye opening 72 hours after cessation of other sedatives and analgesics). The data and safety monitoring board reviewed adverse events up to 10 days after the end of treatment.
To determine the plasma concentration of baclofen, blood samples were drawn at 3 participating centers in a subset of patients 3 and 10 days after randomization (eFigure 1 in Supplement 2) and were analyzed by the pharmacology laboratory at Nantes University Hospital. Study protocol adherence, which was assessed daily until drug discontinuation, ranged from 0% to 100% and was defined by the ratio of the dose administered and the protocol-specified dose. In cases of temporary interruption (ie, enteral route unavailable or dispensing error), adherence was recorded as 0% for that day.
Sample Size
Sample size calculation was based on a previous cohort analysis19 that reported the incidence of agitation-related events in the ICU as 31% in low-risk alcohol users and 42% in unhealthy alcohol users. To detect a 15% reduction in agitation-related events in the baclofen group with an α risk of 5%, using a 2-sided test and 80% power, 314 patients needed to be included. A planned interim analysis after the first 157 patients were randomized indicated that an increase in the sample size was not required. The Friede and Kieser method enabled both the initial hypothesis and the risk of type I error to be maintained.20,21
Statistical Analysis
In the primary analysis, all patients were analyzed according to their randomization group using a center-adjusted logistic regression model, with center as the random effect. Missing data for the primary outcome were handled by multiple imputation methods (10 imputations; relative efficiency >99%). The relationships between several variables selected a priori (age, sex, body mass index, cirrhosis, guardianship, blood test results positive for alcohol on admission, other drug use, and Simplified Acute Physiology Score [SAPS] II) and the primary outcome as well as the treatment group were tested using χ2 or t tests. The final multiple imputation model was based on demographic data (sex, cirrhosis) and the SAPS II. Two sensitivity analyses were performed. The first considered death as a competing event for agitation in a time-to-event analysis using a subdistribution hazard regression model as a post hoc analysis (Fine-Gray analysis). Proportionality of the hazard assumption was tested using Schoenfeld residuals. In the second sensitivity analysis, all patients who died during the treatment period were considered to have had agitation. The per-protocol analysis excluded patients who withdrew consent, did not meet inclusion criteria, or received less than 100% of the protocol-specified dose.
Analyses of the secondary outcomes included all randomized patients and were center-adjusted. There was no imputation for missing data and 95% CIs were not adjusted for multiplicity. Because of the potential for type I error due to multiple comparisons, findings for secondary end points should be interpreted as exploratory. Normally distributed variables were expressed as mean and SD and nonnormal variables were presented as median and interquartile range. Categorical data, including mortality, were analyzed with logistic regression models. Longitudinal continuous data were analyzed with linear mixed-effects models for repeated measures, and assumptions of normality and homoscedasticity were tested. Time-to-event analyses were used considering death as a competing event (Fine-Gray regression model) for length of mechanical ventilation and length of stay in the ICU and the hospital. A center-adjusted Poisson regression model was applied to compare the number of agitation-related events between the groups. Data were analyzed with SAS software, version 9.4 (SAS Institute). All tests were 2-tailed, with significance defined as P <.05.
Results
Study Population
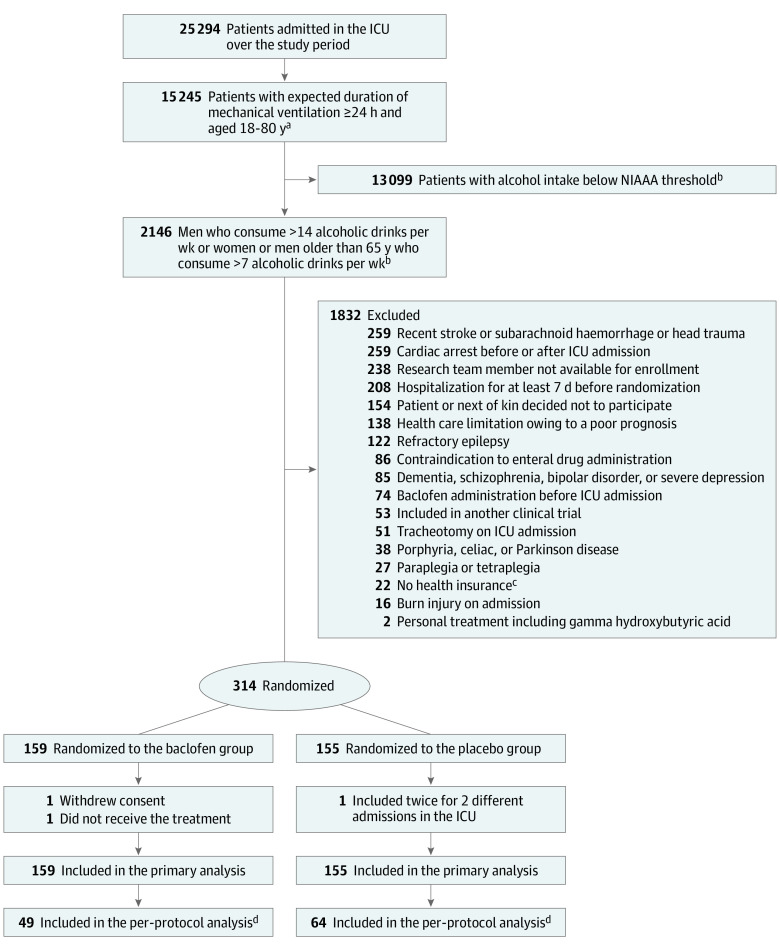
A total of 25 294 patients in the ICU were assessed for eligibility and, after exclusions, 314 were enrolled at 18 ICUs in France between June 2016 and February 2019. In the primary analysis, 159 patients were in the baclofen group and 155 were in the placebo group. After randomization, 1 patient withdrew consent, 1 did not receive the treatment because enteral route was unavailable, and 1 was enrolled twice for 2 different ICU admissions (Figure 1). Patient baseline characteristics at randomization were similar in the 2 treatment groups and are reported in Table 1. Of the 314 patients, 253 (80.6%) were men and 247 (78.6%) were admitted for an underlying medical condition. The mean (SD) SAPS II was 47.7 (16.9) and the median (interquartile range) alcohol intake was 6.0 (4.0-10.0) units per day among all patients.
Figure 1. Flow of Participants in a Study of the Effect of High-Dose Baclofen on Agitation-Related Events Among Patients With Unhealthy Alcohol Use Receiving Mechanical Ventilation.
aEligibility criteria were modified after the inclusion of the first 5 patients: the upper age limit was increased from 70 to 80 years and the expected duration of mechanical ventilation was decreased from 48 to 24 hours.
bThe National Institute on Alcohol Abuse and Alcoholism (NIAAA) threshold is defined as consumption of more than 14 units per week for men and 7 units per week for women or men older than 65 years. One alcoholic drink (unit) is defined as 1 drink that contains approximately 12 to 14 g of pure alcohol (12 ounces of beer, 5 ounces of wine, or 1.5 ounces of 80-proof liquor).
cAccording to French law.
dThe per-protocol analysis excluded patients who received less than 100% of the protocol-specified dose (ie, incomplete treatment): 1 patient who withdrew consent, 2 with overestimation of alcohol intake (ie, did not meet inclusion criteria), and 1 included twice for 2 different admissions in the intensive care unit (ICU).
Table 1. Characteristics of Participants in the Primary Analysis in a Study of the Effect of High-Dose Baclofen on Agitation-Related Events Among Patients With Unhealthy Alcohol Use Receiving Mechanical Ventilationa.
| Characteristic | No. (%) | |
|---|---|---|
| Baclofen (n = 159)b | Placebo (n = 155) | |
| Age, mean (SD), y | 57.4 (11.9) | 56.6 (11.1) |
| Men | 122/158 (77.2) | 131 (84.5) |
| Women | 36/158 (22.8) | 24 (15.5) |
| Body mass index, mean (SD) | 27.7 (7.6) | 26.7 (5.9) |
| Body mass index >30 | 50 (31.9) | 42 (27.1) |
| SAPS II, mean (SD)c | 49.0 (18.3) | 46.4 (15.4) |
| SOFA score, mean (SD)d | 8.3 (3.9) | 7.7 (3.9) |
| Medical history | n = 158 | |
| Diabetes | 23 (14.6) | 24 (15.5) |
| Cirrhosis | 26 (16.5) | 18 (11.6) |
| Child-Pugh B or C scoree | 13/26 (50.0) | 8/18 (44.4) |
| Prior upper gastrointestinal bleeding | 14/26 (53.8) | 9/18 (50.0) |
| Alcohol and substance use history | ||
| Active tobacco user | 99/158 (62.7) | 92 (59.4) |
| Pack-years, mean (SD)f | 35.3 (24.9) | 35.3 (17.2) |
| Units of alcohol intake per day, median (IQR)g | 5.0 (4.0-10.0) | 6.0 (4.0-10.0) |
| Prior alcohol withdrawal attemptsh | 29/158 (18.4) | 28 (18.1) |
| Positive alcohol blood test on admission | 21/156 (13.5) | 25 (16.1) |
| Alcohol abusei | 40/68 (58.8) | 51/80 (63.7) |
| Alcohol dependencei | 33/39 (84.6) | 31/49 (63.3) |
| Cannabis useh | 11/158 (7.0) | 7 (4.5) |
| Opioid dependenceh | 9/158 (5.7) | 7 (4.5) |
| Duration between hospital admission and ICU admission, median (IQR), d | 0.24 (0.01-1.01) | 0.26 (0.06-1.15) |
| Reason for hospital admission | ||
| Medical | 124/158 (78.5) | 123 (79.4) |
| Surgical | 34/158 (21.5) | 32 (20.6) |
| Domestic or public road accident related to alcohol intake | 19/34 (55.9) | 19/32 (59.4) |
| Mechanical ventilation on ICU admission | 69/158 (43.7) | 66 (42.6) |
| Mechanical ventilation within 24 h of ICU admission | 142/158 (89.9) | 133 (85.8) |
| Duration between ICU admission and intubation, median (IQR), d | 0.01 (0.0-0.17) | 0.01 (0.0-0.45) |
| Diagnosis in ICUj | n = 158 | |
| Acute respiratory distress syndrome | 36 (22.8) | 40 (25.8) |
| Septic shock | 34 (21.5) | 26 (16.8) |
| Acute respiratory failure | 18 (11.4) | 19 (12.3) |
| Altered mental statusk | 15 (9.5) | 13 (8.4) |
| Multiple trauma | 13 (8.2) | 16 (10.3) |
| Overdose or toxic ingestionl | 10 (6.3) | 4 (2.6) |
| Pancreatitis | 8 (5.1) | 7 (4.5) |
| Postoperative care | 7 (4.4) | 7 (4.5) |
| Delirium tremens | 4 (2.5) | 9 (5.8) |
| Otherm | 13 (8.2) | 14 (9.0) |
Abbreviations: IQR, interquartile range.
Details of missing data are shown in eTable 2 in Supplement 2.
One patient (of 159) in the baclofen group withdrew consent.
The Simplified Acute Physiology Score (SAPS) II ranges from 0 to 163, with higher scores indicating a greater severity of illness.22 It was calculated using age, type of admission (emergency surgery, elective surgery, medical patient), chronic diseases (acquired immunodeficiency syndrome, metastatic cancer, hematological malignancy), and the worst value for 12 physiological variables within the 24 hours after admission. SAPS II of 30 indicates 10% probability of death; 45, 34%; 50, 56%; and 80, 92%.
The Sequential Organ Failure Assessment (SOFA) score was used to assess the degree of dysfunction of 6 organ systems: respiratory, cardiovascular, coagulation, renal, neurologic, and hepatic. Each subscore ranges from 0 (healthy) to 4 (maximum severity of organ dysfunction). The overall score ranges from 0 to 24.23 An initial SOFA score up to 9 indicates a mortality of less than 33%.
Child-Pugh score is used to determine the prognosis of chronic liver disease and is calculated using 5 parameters: total bilirubin, serum albumin, prothrombin time, ascites, and hepatic encephalopathy. A score of 5 to 6 indicates Child-Pugh A (1-year survival, 100%); 7 to 9, Child-Pugh B (1-year survival, 80%); and 10 to 15, Child-Pugh C (1-year survival, 40%).24
Pack-years was calculated as packs smoked per day × years as a smoker.
Based on medical record or patient or next of kin declaration and was assessed according to the “standard” drink definition from the National Institute on Alcohol Abuse and Alcoholism (NIAAA; namely 1 unit contains approximately 14 g of alcohol).
Based on medical record or patient or next of kin declaration.
On ICU discharge, patients with a clear mental status (73 in the baclofen group and 85 in the placebo group) were subjected to the questionnaire of the Diagnostic and Statistical Manual of Mental Disorder, 4th Edition, adapted by the NIAAA, to detect alcohol use disorder (ie, alcohol abuse or dependence; eFigure 2 in Supplement 2).
Because 1 patient could have several diagnoses, only the main diagnosis that led to the admission in the ICU was noted, regardless of the indication for intubation.
Altered mental status was defined as Glasgow Coma Scale (GCS) score less than 15. The GCS score is the sum of scores for eye, verbal, and motor responses. The minimum score is 3, which indicates deep coma, and the maximum is 15, which indicates fully awake.
Alcohol or other drug overdose and self-poisoning with cardiotropic or psychotropic agents.
Other diagnoses in the intensive care unit (ICU) are listed in eTable 3 in Supplement 2.
Primary End Point
At the completion of the study, 77 patients had the primary outcome of at least 1 agitation-related event over the treatment period. In the primary analysis, the percentage of patients with at least 1 agitation-related event was significantly lower in the baclofen group than in the placebo group (31 patients [19.7%] vs 46 [29.7%]; difference, −9.93% [95% CI, –19.45% to –0.42%]; adjusted odds ratio [OR] after multiple imputation, 0.58 [95% CI, 0.34-0.98]). In a post hoc sensitivity analysis that considered mortality as a competing event for agitation, the decrease in agitation-related events remained significant for baclofen compared with placebo (adjusted subdistribution hazard ratio [HR], 0.62 [95% CI, 0.40-0.96]) (Figure 2A; eTable 4 in Supplement 2). In a second sensitivity analysis in which all patients who died during the treatment period were considered as having at least 1 agitation-related event, there was no significant difference between the groups for the primary outcome (absolute difference, –3.16% [95% CI, –14.21% to 7.90%]; adjusted OR, 0.88 [95% CI, 0.56-1.38]) (eTable 4 in Supplement 2).
Figure 2. Primary and Per-Protocol Analyses in a Study of the Effect of High-Dose Baclofen on Agitation-Related Events Among Patients With Unhealthy Alcohol Use Receiving Mechanical Ventilation.
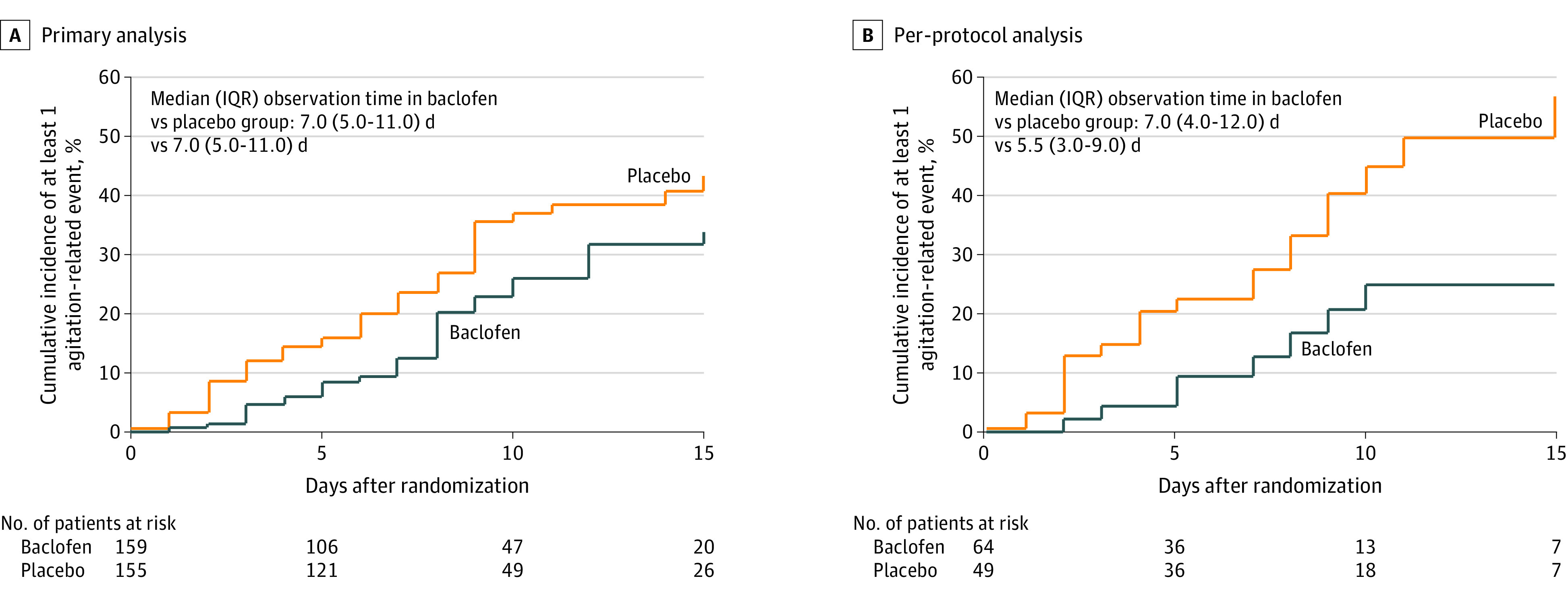
Trend lines were truncated when observations fell below 20%. Between-group differences were tested using Fine-Gray regression considering death as competing event for agitation. When baclofen was compared with placebo, the hazard ratio (HR) for agitation was 0.62 ([95% CI, 0.40-0.96]; P = .03) in the primary analysis and 0.36 ([95% CI, 0.17-0.79]; P = .01) in the per-protocol analysis. A hazard ratio less than 1 indicates that the probability of agitation was lower in the baclofen group at all times.
The per-protocol analysis excluded patients with less than 100% adherence, 1 patient with full withdrawal of consent, 2 with overestimation of alcohol intake (ie, they did not meet inclusion criteria), and 1 who was included twice for 2 different admissions in the ICU (eTable 4 in Supplement 2). In this analysis, the percentage of patients who had at least 1 agitation-related event over the treatment period was significantly lower in the baclofen group than in the placebo group (8 of 49 patients [16.3%] vs 22 of 64 [34.4%]; difference, –18.05% [95% CI, –33.62% to –2.48%]; adjusted OR, 0.37 [95% CI, 0.15-0.94]). This difference persisted in the per-protocol analysis when mortality was considered as a competing event for agitation (adjusted subdistribution HR, 0.36 [95% CI, 0.17-0.79]).
Secondary End Points
By day 28 after randomization, the percentage of patients with at least 1 agitation-related event did not differ significantly between treatment groups, occurring in 44 patients (27.8%) in the baclofen group and 54 (34.8%) in the placebo group (difference, –6.99% [95% CI, –17.24% to 3.30%]; adjusted OR, 0.72 [95% CI, 0.45-1.17]) (Table 2) However, the total number of agitation-related events by day 28 was significantly lower in patients randomized to the baclofen group vs the placebo group (70 vs 111 events; adjusted rate ratio, 0.63 [95% CI, 0.46-0.85]) (Table 2; eTable 4 in Supplement 2).
Table 2. Outcomes in a Study of the Effect of Baclofen on Agitation Events Among Patients With Alcohol Use Receiving Mechanical Ventilation.
| Outcome | Baclofen (n = 159) | Placebo (n = 155) | Absolute difference (95% CI), % | Odds ratio (95% CI)a | P value |
|---|---|---|---|---|---|
| Primary outcome | |||||
| Patients with ≥1 agitation-related event over the treatment period, No. (%)b | 31/157 (19.7) | 46 (29.7) | –9.93 (–19.45 to –0.42) | 0.58 (0.34 to 0.98)c | .04 |
| Secondary outcomes by day 28d | |||||
| Patients with ≥1 agitation-related event, No. (%) | 44/158 (27.8) | 54 (34.8) | –6.99 (–17.24 to 3.30) | 0.72 (0.45 to 1.17)c | .19 |
| Total agitation-related events | 70 | 111 | Rate ratio, 0.63 (0.46 to 0.85)e | .003 | |
| No. of rapid hypnotic or neuroleptic injections for agitation, mean (SD) | 3.2 (5.5) | 2.9 (5.1) | 0.22 (–1.27 to 0.84)f | .69 | |
| Sedative dose in the ICU, median (IQR), mg/d | |||||
| Propofol | 1172.50 (510.0 to 2292.9) | 1544.44 (918.3 to 2510.0) | –304.00 (–640.00 to 33.33)j,g | .08 | |
| Ketamine | 165.00 (106.6 to 200.0) | 150.00 (107.1 to 200.0) | 2.67 (–62.5 to 70.71)j,g | .94 | |
| Midazolam | 82.52 (36.7 to 156.8) | 81.08 (36.5 to 168.3) | 2.23 (–17.00 to 20.50)j,g | .82 | |
| Levomepromazine | 31.98 (19.8 to 53.4) | 45.15 (39.7 to 51.8) | –10.98 (–38.00 to 18.88)j,g | .52 | |
| Haloperidol | 2.00 (1.5 to 7.0) | 2.75 (1.1 to 5.5) | 0.32 (–2.00 to 3.00)j,g | .73 | |
| Clonidine | 0.69 (0.3 to 1.5) | 0.60 (0.3 to 0.8) | 0.12 (–0.42 to 1.14)j,g | .47 | |
| Dexmedetomidine | 0.51 (0.2 to 0.7) | 0.60 (0.3 to 1.6) | –0.12 (–1.09 to 0.43)j,g | .53 | |
| Sevoflurane inhalation time, h | 14.13 (11.5 to 16.8) | 17.67 (17.0 to 21.0) | –4.58 (–9.92 to 0.75)j,g | .22 | |
| Daily dose of analgesics in the ICU, median (IQR), mg/d | |||||
| Sufentanil | 0.20 (0.1 to 0.4) | 0.23 (0.1 to 0.4) | –0.01 (–0.07 to 0.04)j,g | .63 | |
| Fentanyl | 1.54 (0.7 to 2.7) | 2.55 (1.3 to 3.2) | –0.89 (–1.66 to –0.01)j,g | .06 | |
| Morphine | 16.67 (9.0 to 54.3) | 19.25 (9.0 to 36.7) | 1.50 (–4.17 to 9.08)j,g | .63 | |
| Remifentanil | 4.80 (3.0 to 5.9) | 3.93 (2.7 to 18.0) | –0.93 (–13.20 to 3.99)j,g | .92 | |
| Reintubation within 48 h of extubation, No. (%) | 11/155 (7.1) | 14/152 (9.0) | –2.11 (–8.24 to 4.00) | 0.76 (0.33 to 1.75)c | .52 |
| Tracheotomy, No. (%) | 7/158 (4.4) | 6 (3.9) | 0.55 (–3.86 to 4.98) | 1.16 (0.38 to 3.55)c | .80 |
| ICU-acquired infection, No. (%)h | 36/156 (23.1) | 32 (20.6) | 2.43 (–6.75 to 11.61) | 1.14 (0.66 to 1.96)c | .63 |
| Ventilator-free days, median (IQR)i | 14.0 (0.0 to 20.0) | 19.0 (0.0 to 24.0) | –2.00 (–4.00 to 0.00)j | .01k | |
| Length of mechanical ventilation, median (IQR), d | 9.0 (5.5 to 16.0) | 8.0 (4.0 to 13.0) | 2.00 (0.00 to 3.00)j | HR, 0.76 (0.60 to 0.97)l | .02 |
| Length of ICU stay, median (IQR), d | 14.0 (8.0 to 23.0) | 11.0 (7.0 to 18.0) | 2.00 (0.00 to 4.00)j | HR, 0.70 (0.54 to 0.90)l | .01 |
| Length of hospital stay, median (IQR), d | 22.0 (13.0 to 41.0) | 20.0 (14.0 to 38.0) | 0.00 (–4.00 to 3.00)j | HR, 0.81 (0.62 to 1.07)l | .14 |
| Agitation-related event or mortality, No. (%) | 66/154 (42.9) | 71/155 (45.8) | –5.12 (–16.34 to 6.10) | 0.82 (0.52 to 1.30)c | .39 |
| Death, No. (%)m | 39/154 (25.3) | 32/148 (20.6) | 3.70 (–5.84 to 13.25) | 1.24 (0.72 to 2.13)c | .44 |
Abbreviations: ICU, intensive care unit; IQR, interquartile range.
Unless otherwise noted. For all odds ratios and hazard ratios (HRs), the placebo group is used as the reference group.
Analysis included all randomized patients. Missing data (1 patient withdrew consent and 1 did not receive the treatment because the enteral route was unavailable) were handled by multiple imputation methods (eTable 2 in Supplement 2). Agitation-related events included pulling out lines, catheters, or drains; falling out of bed; leaving against the physician’s advice or without being seen; immobilization device removal; self-aggression; and aggression toward medical staff (eTable 5 in Supplement 2). An odds ratio less than 1 indicates that the risk of agitation was lower in the baclofen group than the placebo group. One patient was admitted to the ICU twice and included in the primary outcome analysis as 2 of the 155 placebo treatment periods.
Center-adjusted after logistic regression model (center as random effect).
For secondary outcomes, 95% CIs were not adjusted for multiplicity and therefore should not be used to infer definitive treatment effects. Other secondary outcomes are shown in eTable 4 in Supplement 2.
Center-adjusted after Poisson regression (center as random effect).
Center-adjusted mean difference after mixed linear regression analysis (center as random effect).
Groups were compared with a Wilcoxon test. This includes continuous and discontinuous infusion of sedatives or analgesics until day 28. The daily dose corresponds to the ratio of the total dose of each medication and the length of stay in the ICU in days. The repartition of sedatives and analgesics by day 28 is available in eTable 6 in Supplement 2.
Includes pneumonia, catheter infection, urinary infection, or bacteremia (eTable 4 in Supplement 2).
Calculated as the number of days between day 1 and 28 the patient was alive and not intubated. For patients who died between day 1 and 28, the ventilator-free days was null.
Differences in medians were estimated with the Hodges-Lehmann method.
For ventilator-free days, groups were compared using a Wilcoxon test.
Center-adjusted HR after subdistribution hazard regression analysis (Fine-Gray regression) considering death as a competing event of extubation (for length of mechanical ventilation) or discharge from the ICU (for length of ICU stay) or from the hospital (for length of hospital stay). The proportionality of the hazard assumption was checked using the scaled Schoenfeld residuals. An HR less than 1 meant that at all times the probability of extubation, discharge from the ICU, or discharge from the hospital was lower in the baclofen group. Data are censored on day 60 for ICU stay and on day 90 for hospital stay. P values for these parameters correspond to the analysis of the HR.
See eTable 4 in Supplement 2 for death at other time points.
In both treatment groups, propofol, midazolam, and sufentanil were the most commonly used sedative and analgesic drugs (eTable 6 in Supplement 2). There was no significant difference between the groups in the daily doses of sedative and analgesic medications used or in the number of rapid injections of hypnotic or neuroleptic medications by day 28 (Table 2). However, patients spent significantly more time with a Riker Sedation-Agitation Scale score between 1 and 3 (ie, deep sedation) by day 28 in the baclofen group than in the placebo group (mean of 7.0 vs 4.6 days; difference, 2.44 [95% CI, 1.08-3.80]) (eTable 4 in Supplement 2).
There was no significant difference between the groups in the prespecified secondary outcomes of reintubation, tracheotomy, and ICU-acquired infections. However, patients in the baclofen group had significantly fewer ventilator-free days than those in the placebo group (median of 14.0 vs 19.0 days; difference, –2.00 [95% CI, –4.00 to 0.00]) (Table 2). Compared with placebo, patients treated with baclofen had a significantly longer median duration of mechanical ventilation (9.0 vs 8.0 days; difference, 2.00 [95% CI, 0.00 to 3.00]; adjusted HR for extubation, 0.76 [95% CI, 0.60-0.97]) and stay in the ICU (14.0 vs 11.0 days; difference, 2.00 [95% CI, 0.00-4.00]; adjusted HR for ICU discharge, 0.70 [95% CI, 0.54-0.90]).
Over 7 days after extubation, CIWA-Ar scores did not differ significantly between the groups (eTable 4 in Supplement 2). By day 28, a total of 39 patients (25.3%) in the baclofen group and 32 (21.6%) in the placebo group died (difference, 3.70% [95% CI, –5.84% to 13.25%]; adjusted OR, 1.24 [95% CI, 0.72-2.13]) (Table 2). Median residual plasma concentration of baclofen was 425 ng/mL on day 3 (n = 33), with a maximum level of 966 ng/mL, and was 201 ng/mL on day 10 (n = 15), with a maximum level of 933 ng/mL (eTable 4 in Supplement 2).
Adherence and Adverse Events
Study participants received 92% of the total protocolized dose (Table 3). The percentage of patients with 100% adherence to the treatment protocol was 33.6% in the baclofen group and 44.3% in the placebo group. Adverse events requiring drug discontinuation occurred in 23 patients (14.6%) in the baclofen group and 7 (4.5%) in the placebo group. Delayed awakening that required discontinuation of the study drug occurred in 14 patients (8.9%) in the baclofen group and 3 (1.9%) in the placebo group. A complete list of adverse events is shown in eTable 7 and causes of death are shown in eTable 8 in Supplement 2.
Table 3. Adherence to Medication Protocol and Adverse Events in a Study of the Effect of High-Dose Baclofen on Agitation-Related Events Among Patients With Unhealthy Alcohol Use Receiving Mechanical Ventilation.
| Outcome | Baclofen (n = 159) | Placebo (n = 155) |
|---|---|---|
| Total protocolized doses given, mean (SD), % | 91 (13) | 92 (14) |
| Patients with 100% adherence to treatment protocol, No. (%)a | 49/146 (33.6) | 66/149 (44.3) |
| Treatment duration, median (IQR), d | 7.0 (5.0-13.0) | 8.0 (5.0-11.0) |
| Events requiring temporary stoppage of treatment drug, No. (%) | n = 158 | |
| eGFR<15 mL/min/1.73m2 without indication for RRTb | 15 (9.5) | 9 (5.8) |
| Enteral route unavailable | 7 (4.4) | 9 (5.8) |
| Dispensing error | 3 (1.9) | 4 (2.6) |
| Heart rate <50/min | 1 (0.6) | 0 |
| Patient oppositionc | 0 (0.0) | 2 (1.3) |
| Adverse events requiring drug discontinuation, No. (%)d | n = 158 | |
| Total | 23 (14.6) | 7 (4.5)e |
| Delayed awakeningf | 14 (8.9) | 3 (1.9) |
| Stroke | 4 (2.5) | 1 (0.6) |
| Seizure | 2 (1.3) | 4 (2.6) |
| Heart rate <35/min | 2 (1.3) | 0 |
| Reactive unilateral or bilateral mydriasis | 1 (0.6) | 0 |
Abbreviations: eGFR, estimated glomerular filtration rate; IQR, interquartile range; RRT, renal replacement therapy.
Adherence to the protocol was noted from 0% to 100% from day 1 until the definitive discontinuation of the treatment. It was defined each day by the ratio of the actual dose administered to the protocol-specified dose. Adherence is unknown for patients with missing data.
The equation in the Modification of Diet in Renal Disease study17 was used to determine the eGFR.
Patient refusing to take the study drug on 1 or more occasions.
Prespecified adverse events requiring premature definitive discontinuation of the study drug. Full description of adverse events is available in eTable 7 in Supplement 2.
One patient presented 2 reasons for premature discontinuation.
Defined as eyes not open 72 hours after sedation interruption.
Discussion
In this multicenter, double-blind, randomized clinical trial that enrolled patients with a history of unhealthy alcohol use who were receiving mechanical ventilation, the percentage of patients with the primary outcome of at least 1 agitation-related event was significantly lower in the baclofen group compared with the placebo group. However, the adverse event of delayed awakening, defined as no eye opening at 72 hours after cessation of sedatives and analgesics, was experienced more frequently in the baclofen group.
The difference of 9.93% for the primary end point of at least 1 agitation-related event was less than the 15% difference that the study was powered to detect. However, the total number of agitation-related events, a prespecified secondary outcome, was higher in the placebo group than in the baclofen group. This finding has clinical relevance because each episode of agitation may cause injury to the patient or medical staff and can hinder medical care.25 In addition to short-term complications,26 agitation may result in long-term consequences for patients.27 In this study, consistent with a previous report,28 the mortality rate of critically ill patients with unhealthy alcohol use was high. However, a post hoc sensitivity analysis that considered death as a competing risk of agitation supported the findings of the primary outcome.
Sedative infusion titrated based on the Richmond Agitation-Sedation Scale score, which is the current standard of care treatment algorithm to minimize duration of mechanical ventilation and length of stay in the ICU,29,30 was used to guide sedation dosing by all the participating centers except 1 (eTable 1 in Supplement 2). However, despite this standardized algorithm, patients who received placebo experienced 111 agitation-related events, suggesting that additional agitation prevention strategies may be beneficial for patients with unhealthy alcohol use requiring mechanical ventilation. The wide variety of sedatives and analgesics, often used in combination, may explain the lack of difference in the daily doses between the groups.
At the time of study initiation, the French drug agency supported the use of up to 300 mg of baclofen per day,31 whereas, as of 2017, baclofen dosing is now recommended not to exceed 80 mg per day.32 However, among the patients in the current study randomized to receive daily administration of 150 mg of baclofen (or equivalent based on eGFR) and who had study drug plasma levels checked all had baclofen levels below the toxic concentration of 1100 ng/mL.33
The deeper sedation experienced by patients randomized to receive baclofen compared with placebo may explain both the statistically significant decrease in the primary outcome of at least 1 agitation-related event and the significant increase in the prespecified secondary end points of duration of mechanical ventilation and of ICU stay.
Limitations
This study has several limitations. First, data were missing for the primary outcome in 2 patients, which was handled by multiple imputation. Second, because baclofen is a γ-aminobutyric acid agonist there is the possibility that it may increase delirium in critically ill patients, although a causal association has not been established. Third, use of the enteral route for baclofen administration resulted in the exclusion of 86 potentially eligible patients from this study because of uncertainty regarding the safety of administering baclofen intravenously. Fourth, this study included only patients who required mechanical ventilation. Whether baclofen can safely reduce agitation events in patients not receiving mechanical ventilation remains to be determined. Fifth, despite this study’s broad inclusion criteria, the patients enrolled were predominantly middle-aged men. Whether the results are applicable to women or to older or younger patients is uncertain. Sixth, patients were included on the basis of their reported alcohol intake, which may be inaccurate. Seventh, the management of alcohol-related agitation was not protocolized because there are no current recommendations in the ICU medical literature.34 Eighth, because delirium can present with 3 motor subtypes (hyperactive, hypoactive, and mixed), it would have been informative to assess delirium scales, such as the Confusion Assessment Method for the ICU or the Intensive Care Delirium Screening Checklist,35,36 to determine whether agitation corresponded to the hyperactive component of delirium.
Conclusions
Among patients with unhealthy alcohol use receiving mechanical ventilation, treatment with high-dose baclofen, compared with placebo, resulted in a statistically significant reduction in agitation-related events. However, considering the modest effect and the totality of findings for the secondary end points and adverse events, further research is needed to determine the possible role of baclofen in this setting and to potentially optimize dosing.
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Timeline and dose adjustment algorithm
eFigure 2. NIAAA questionnaire for the detection of Alcohol Use Disorder
eTable 1. Number of inclusions and institutional sedation scales for each center over the study period
eTable 2. Details of the missing data
eTable 3. Supplemental characteristics of the participants at baseline.
eTable 4. Supplemental analysis for the primary and other secondary outcomes
eTable 5. Details of agitation-related events
eTable 6. Sedative and analgesic drugs repartition between groups
eTable 7. Exhaustive list of adverse events
eTable 8. Causes of death by day 90
eReferences
Data sharing statement
References
- 1.Mackenbach JP, Kulhánová I, Bopp M, et al. Inequalities in alcohol-related mortality in 17 European countries: a retrospective analysis of mortality registers. PLoS Med. 2015;12(12):e1001909. doi: 10.1371/journal.pmed.1001909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spillane S, Shiels MS, Best AF, et al. Trends in alcohol-induced deaths in the United States, 2000-2016. JAMA Netw Open. 2020;3(2):e1921451-e1921451. doi: 10.1001/jamanetworkopen.2019.21451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.GBD 2016 Alcohol Collaborators Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. 2018;392(10152):1015-1035. doi: 10.1016/S0140-6736(18)31310-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.The National Survey on Drug Use and Health: 2019. Substance Abuse and Mental Health Services Administration Published September 11, 2020. Accessed November 23, 2020. https://www.samhsa.gov/data/report/dr-elinore-f-mccance-katz-webcast-slides-national-2019
- 5.NIAAA issues new clinician's guide for helping patients who drink too much. News release. National Institute on Alcohol Abuse and Alcoholism ; July 19, 2005. Accessed November 23, 2020. https://www.niaaa.nih.gov/news-events/news-releases/niaaa-issues-new-clinicians-guide-helping-patients-who-drink-too-much
- 6.Curry SJ, Krist AH, Owens DK, et al. ; US Preventive Services Task Force . Screening and behavioral counseling interventions to reduce unhealthy alcohol use in adolescents and adults: US Preventive Services Task Force recommendation statement. JAMA. 2018;320(18):1899-1909. doi: 10.1001/jama.2018.16789 [DOI] [PubMed] [Google Scholar]
- 7.Stewart D, Kinsella J, McPeake J, Quasim T, Puxty A. The influence of alcohol abuse on agitation, delirium and sedative requirements of patients admitted to a general intensive care unit. J Intensive Care Soc. 2019;20(3):208-215. doi: 10.1177/1751143718787748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pandharipande PP, Girard TD, Jackson JC, et al. ; BRAIN-ICU Study Investigators . Long-term cognitive impairment after critical illness. N Engl J Med. 2013;369(14):1306-1316. doi: 10.1056/NEJMoa1301372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical practice guidelines for the prevention and management of pain, agitation/sedation, delirium, immobility, and sleep disruption in adult patients in the ICU. Crit Care Med. 2018;46(9):e825-e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 10.van den Boogaard M, Slooter AJC, Brüggemann RJM, et al. ; REDUCE Study Investigators . Effect of haloperidol on survival among critically ill adults with a high risk of delirium: the REDUCE randomized clinical trial. JAMA. 2018;319(7):680-690. doi: 10.1001/jama.2018.0160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wassenaar A, van den Boogaard M, van Achterberg T, et al. Multinational development and validation of an early prediction model for delirium in ICU patients. Intensive Care Med. 2015;41(6):1048-1056. doi: 10.1007/s00134-015-3777-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Beaurepaire R Suppression of alcohol dependence using baclofen: a 2-year observational study of 100 patients. Front Psychiatry. 2012;3:103. doi: 10.3389/fpsyt.2012.00103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vourc’h M, Feuillet F, Mahe PJ, Sebille V, Asehnoune K; BACLOREA trial group . Baclofen to prevent agitation in alcohol-addicted patients in the ICU: study protocol for a randomised controlled trial. Trials. 2016;17(1):415. doi: 10.1186/s13063-016-1539-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saitz R Clinical practice. Unhealthy alcohol use. N Engl J Med. 2005;352(6):596-607. doi: 10.1056/NEJMcp042262 [DOI] [PubMed] [Google Scholar]
- 15.Vourc’h M, Dailly E, Hourmant Y, et al. Pharmacokinetic data on high dose baclofen administration in unhealthy alcohol user in the ICU. Data Brief. 2019;25:104231. doi: 10.1016/j.dib.2019.104231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vourc’h M, Dailly E, Hourmant Y, et al. Pharmacokinetics and toxicity of high-dose baclofen in ICU patients. Prog Neuropsychopharmacol Biol Psychiatry. 2019;92:450-456. doi: 10.1016/j.pnpbp.2019.02.016 [DOI] [PubMed] [Google Scholar]
- 17.Kuan Y, Hossain M, Surman J, El Nahas AM, Haylor J. GFR prediction using the MDRD and Cockcroft and Gault equations in patients with end-stage renal disease. Nephrol Dial Transplant. 2005;20(11):2394-2401. doi: 10.1093/ndt/gfi076 [DOI] [PubMed] [Google Scholar]
- 18.Schuckit MA Recognition and management of withdrawal delirium (delirium tremens). N Engl J Med. 2014;371(22):2109-2113. doi: 10.1056/NEJMra1407298 [DOI] [PubMed] [Google Scholar]
- 19.Vourc'h M, Bourdiol A, Thibault M, et al. Impact of alcohol use disorder and agitation-related adverse events on patient’s outcome in the ICU and the following one year after admission. Anaesth Crit Care Med J. 2020;5(1). doi: 10.23880/accmj-16000170 [DOI] [Google Scholar]
- 20.Friede T, Kieser M. Sample size recalculation in internal pilot study designs: a review. Biom J. 2006;48(4):537-555. doi: 10.1002/bimj.200510238 [DOI] [PubMed] [Google Scholar]
- 21.Friede T, Kieser M. Sample size recalculation for binary data in internal pilot study designs. Pharmaceutical Statistics. 2004;3(4):269-279. doi: 10.1002/pst.140. [DOI] [Google Scholar]
- 22.Le Gall JR, Lemeshow S, Saulnier F. A new Simplified Acute Physiology Score (SAPS II) based on a European/North American multicenter study. JAMA. 1993;270(24):2957-2963. doi: 10.1001/jama.1993.03510240069035 [DOI] [PubMed] [Google Scholar]
- 23.Ferreira FL, Bota DP, Bross A, Mélot C, Vincent JL. Serial evaluation of the SOFA score to predict outcome in critically ill patients. JAMA. 2001;286(14):1754-1758. doi: 10.1001/jama.286.14.1754 [DOI] [PubMed] [Google Scholar]
- 24.Wan S-Z, Nie Y, Zhang Y, Liu C, Zhu X. Assessing the prognostic performance of the Child-Pugh, model for end-stage liver disease, and albumin-bilirubin scores in patients with decompensated cirrhosis: a large Asian cohort from gastroenterology department. Dis Markers. Published online February 17, 2020. doi: 10.1155/2020/5193028 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 25.Siegel MD Management of agitation in the intensive care unit. Clin Chest Med. 2003;24(4):713-725. doi: 10.1016/S0272-5231(03)00104-7 [DOI] [PubMed] [Google Scholar]
- 26.Boulain T; Association des Réanimateurs du Centre-Ouest . Unplanned extubations in the adult intensive care unit: a prospective multicenter study. Am J Respir Crit Care Med. 1998;157(4 Pt 1):1131-1137. doi: 10.1164/ajrccm.157.4.9702083 [DOI] [PubMed] [Google Scholar]
- 27.Long AC, Kross EK, Davydow DS, Curtis JR. Posttraumatic stress disorder among survivors of critical illness: creation of a conceptual model addressing identification, prevention, and management. Intensive Care Med. 2014;40(6):820-829. doi: 10.1007/s00134-014-3306-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gacouin A, Tadie JM, Uhel F, et al. At-risk drinking is independently associated with ICU and one-year mortality in critically ill nontrauma patients. Crit Care Med. 2014;42(4):860-867. doi: 10.1097/CCM.0000000000000041 [DOI] [PubMed] [Google Scholar]
- 29.Kress JP, Pohlman AS, O’Connor MF, Hall JB. Daily interruption of sedative infusions in critically ill patients undergoing mechanical ventilation. N Engl J Med. 2000;342(20):1471-1477. doi: 10.1056/NEJM200005183422002 [DOI] [PubMed] [Google Scholar]
- 30.Girard TD, Kress JP, Fuchs BD, et al. Efficacy and safety of a paired sedation and ventilator weaning protocol for mechanically ventilated patients in intensive care (Awakening and Breathing Controlled trial): a randomised controlled trial. Lancet. 2008;371(9607):126-134. doi: 10.1016/S0140-6736(08)60105-1 [DOI] [PubMed] [Google Scholar]
- 31.Temporary recommendation of use for baclofen in alcohol withdrawal. Agence Nationale de Sécurité du Médicament et des Produits de Santé. Published March 14, 2014. Accessed November 26, 2020. https://www.ansm.sante.fr/S-informer/Points-d-information-Points-d-information/Une-recommandation-temporaire-d-utilisation-RTU-est-accordee-pour-le-baclofene-Point-d-information
- 32.Naudet F, Braillon A. Baclofen and alcohol in France. Lancet Psychiatry. 2018;5(12):961-962. doi: 10.1016/S2215-0366(18)30419-X [DOI] [PubMed] [Google Scholar]
- 33.Schulz M, Iwersen-Bergmann S, Andresen H, Schmoldt A. Therapeutic and toxic blood concentrations of nearly 1,000 drugs and other xenobiotics. Crit Care. 2012;16(4):R136. doi: 10.1186/cc11441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Awissi D-K, Lebrun G, Coursin DB, Riker RR, Skrobik Y. Alcohol withdrawal and delirium tremens in the critically ill: a systematic review and commentary. Intensive Care Med. 2013;39(1):16-30. doi: 10.1007/s00134-012-2758-y [DOI] [PubMed] [Google Scholar]
- 35.Girard TD, Exline MC, Carson SS, et al. ; MIND-USA Investigators . Haloperidol and ziprasidone for treatment of delirium in critical illness. N Engl J Med. 2018;379(26):2506-2516. doi: 10.1056/NEJMoa1808217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Riker RR, Fraser GL. The new practice guidelines for pain, agitation, and delirium. Am J Crit Care. 2013;22(2):153-157. doi: 10.4037/ajcc2013480 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Trial protocol and statistical analysis plan
eMethods
eFigure 1. Timeline and dose adjustment algorithm
eFigure 2. NIAAA questionnaire for the detection of Alcohol Use Disorder
eTable 1. Number of inclusions and institutional sedation scales for each center over the study period
eTable 2. Details of the missing data
eTable 3. Supplemental characteristics of the participants at baseline.
eTable 4. Supplemental analysis for the primary and other secondary outcomes
eTable 5. Details of agitation-related events
eTable 6. Sedative and analgesic drugs repartition between groups
eTable 7. Exhaustive list of adverse events
eTable 8. Causes of death by day 90
eReferences
Data sharing statement