Abstract
Background: The purpose of our study was to characterize clinical features among brain metastasis (BM) patients who were long term survivors (LTS). Methods: We reviewed a registry of BM patients referred to our multidisciplinary BM clinic between 2006 and 2014 and identified 97 who lived ≥ 3 years following BM diagnosis. The clinical and treatment characteristics were obtained from a prospectively maintained database, and additional information was obtained through review of electronic medical records and radiologic images. Survival analyses were performed using the Kaplan-Meier method. Results: Median follow up for LTS was 67 months (range 36–181). Median age was 54 years, 65% had single BM, 39% had stable extracranial disease at the time of BM treatment, and brain was the first site of metastasis in 76%. Targetable mutations were present in 39% of patients and 66% received treatment with targeted-, hormonal-, or immuno-therapy. Brain surgery at the time of diagnosis was performed in 40% and stereotactic radiosurgery (SRS) or whole brain radiotherapy (alone or combination) in 52% and 56%, respectively. Following initial BM treatment, 5-year intracranial disease-free survival was 39%, and the cumulative incidence of symptomatic radio-necrosis was 16%. Five and ten-year overall survival was 72% and 26%, respectively. Conclusion: Most LTS were younger than 60 years old and had a single BM. Many received treatment with surgery or targeted, immune, or hormonal therapy.
Keywords: brain metastasis, long-term survivors, molecular characteristics, systemic therapy, targeted therapy, immunotherapy
1. Introduction
Brain metastases (BM) affect approximately 30% of cancer patients and are especially common in lung, breast, kidney, gastrointestinal cancers, and melanoma [1,2]. The incidence of BM is increasing, due in part to improved neuroimaging and longer overall survival among cancer patients due to improved systemic therapies [3]. Historically, whole-brain radiotherapy (WBRT) was the principal management for all but single BM, irrespective of disease burden [4]. Today, stereotactic radiosurgery (SRS) for oligometastatic BM is the standard of care, in part because it results in lower rates of neuro-cognitive toxicity compared to WBRT and improved local control, albeit with worse distant brain control [5,6,7]. Furthermore, newer systemic therapies with improved central nervous system activity are increasingly used in the management of BM [8,9,10].
Few published reports have described the characteristics of BM patients who were long-term survivors (LTS) [11,12,13,14,15,16,17]. The purpose of our study was to characterize clinical features among BM patients who were LTS patients treated within our institution.
2. Methods and Materials
2.1. Patient Selection
With institutional ethics committee approval (University Health Network, protocol number 18-5741.1), we identified 97 LTS (≥3 years) from a prospectively maintained registry of patients treated for BM at Princess Margaret Cancer Center between January 2006 and December 2014. This registry includes all patients referred to our multidisciplinary BM clinic, which focuses on patients eligible for SRS. All patient, disease, and treatment-related information was retrieved from the database and, when required, verified through the evaluation of electronic medical records and radiologic images.
2.2. Treatment and Follow-up Protocols
Given the prolonged study period, management algorithms evolved over time. Treatment decisions were guided by multi-disciplinary discussions involving radiation oncologists, neurosurgeons, and medical oncologists. Our current treatment protocols for BM prescribe stereotactic radiosurgery (SRS) for limited numbers of metastases (<10) in patients with good performance status (PS). Many patients in our total cohort (56%) treated during an earlier era received WBRT alone or in combination with SRS or surgery. Likewise, some patients were diagnosed during an era that preceded molecular profiling and the availability of targeted agents or immunotherapy. Surgery was generally recommended for patients with larger or symptomatic metastases. Following treatment completion, patients were followed clinically and radiologically with magnetic resonance imaging (MRI) brain or contrast-enhanced computed tomography (CT) every 3 months for the first 2 years and, if stable, every 6–12 months subsequently. During follow up, all patients were reviewed by a multi-disciplinary team to determine disease control status and toxicity, and to formulate salvage treatment strategies when required.
2.3. Statistical Analysis
Analyses were performed using the Statistical Package for the Social Sciences (SPSS V21). For survival analysis, the Kaplan Meier product-limit method was used, starting with the date of the radiological diagnosis of BM. Log-rank test and Cox regression tests were used for univariate and multivariate analyses, respectively, to find features with a significant impact on survival. A two-sided “p-value” of <0.05 was considered to be statistically significant.
We designated extracranial disease or primary disease as “controlled” if there was no measurable disease progression 3 months prior to or following BM diagnosis. When the interval between diagnosis of primary malignancy and BM was ≤3 months, we classified BM as having been diagnosed at disease presentation. The administration of systemic therapy with respect to BM management was divided into three categories: before (>3 months prior to diagnosis), concurrently (+/− 3 months from diagnosis of BM), or later (>3 months).
3. Results
3.1. Survival
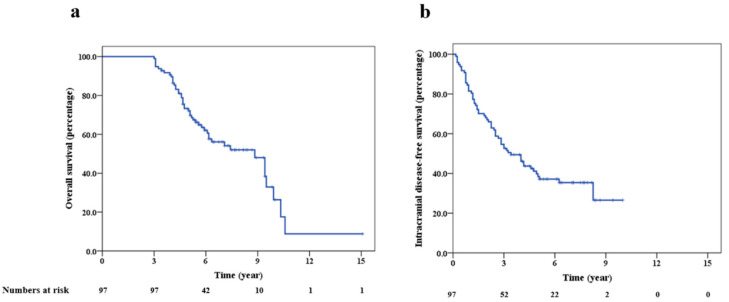
Among the 97 patients identified within our prospective database who survived greater than 3 years following their diagnosis of BM and were thus identified as LTS, median follow up was 67 months (range 36–181 months). Five-year and ten-year overall survival (OS) in the LTS cohort was 72% and 26%, respectively (Figure 1a), and median survival was 106 months (95% confidence interval, 79–133 months). At the time of last analysis, 42 patients were alive without active disease (intracranial or extracranial), eight were alive with progressive disease (in two cases intracranial), and 47 had died. Of patients who died, 25 had an MRI brain within three months of their deaths, which in 16 (64%), showed no active or progressive intracranial disease. We also investigated whether OS among LTS correlated with the era during which patients initially presented and found no difference (Supplementary Figure S1).
Figure 1.
Kaplan Meier survival plots showing overall survival (a) and intracranial disease-free survival (b) for long-term survivors.
3.2. Patient Clinical and Treatment Features
Demographic, disease, and treatment characteristics of LTS are summarized in Table 1. Median age was 54 years. The performance status at the time of BM diagnosis was eastern cooperative oncology group (ECOG) 0 or 1 in 75% of patients. The median number of BM at diagnosis was 1 (range 1 to 12). The most common primary cancers were lung (52%), breast (25%), thyroid (7%), and cutaneous melanoma (5%). At BM diagnosis, in 39% of patients, there was no evidence of active extracranial disease. In 76% of patients, BM was present at the time when metastatic disease was first diagnosed.
Table 1.
Details of various patient, disease, and treatment characteristics in the long-term survivors.
| Feature | Long-Term Survivors (n = 97) |
|---|---|
| Age | |
| <60 years | 69 (71%) |
| 60 years or more | 28 (29%) |
| Gender | |
| Male | 23 (24%) |
| Female | 74 (76%) |
| ECOG (during diagnosis of BM) | |
| 0–1 | 73 (75%) |
| 2 or more | 13 (14%) |
| Unknown | 11 (11%) |
| Number of intracranial lesions (at time of BM diagnosis) | |
| Single | 63 (65%) |
| Multiple | 34 (35%) |
| Primary | |
| Lung | 51 (52%) |
| Breast | 24 (25%) |
| Thyroid | 6 (7%) |
| Melanoma (skin) | 5 (5%) |
| Kidney | 3 (3%) |
| Unknown primary | 3 (3%) |
| Gynecological malignancies | 2 (2%) |
| Male genitourinary | 1 (1%) |
| Gastrointestinal | 1 (1%) |
| Head neck | 1 (1%) |
| Extracranial disease status (at the time of BM diagnosis) | |
| Controlled | 38 (39%) |
| Uncontrolled | 59 (61%) |
| Interval from cancer diagnosis to detection of BM | |
| Median (range) | 15 (0–266) months |
| First site of metastasis | |
| Brain | 74 (76%) |
| Others | 23 (24%) |
| Molecular characteristics | |
| Targetable mutations | |
| Yes | 38 (39%) |
| No | 51 (53%) |
| Unknown | 8 (8%) |
| Hormonal receptors (among patients with breast cancer) | |
| Yes | 16 (67%) |
| No | 8 (33%) |
| Unknown | 8 (8%) |
| Systemic therapy a | |
| Targeted therapy/Hormonal therapy/Immune therapy | 64 (66%) |
| None of the above/Unknown | 33 (34%) |
| Stereotactic radiosurgery a | |
| Yes | 50 (52%) |
| No | 47 (48%) |
| Whole brain radiotherapy a | |
| Yes | 54 (56%) |
| No | 43 (44%) |
| Surgery a | |
| Yes | 39 (40%) |
| No | 58 (60%) |
ECOG: Eastern cooperative oncology group; BM: Brain metastasis. before, during, or after diagnosis of brain metastasis. a intracranial treatment at the time of initial diagnosis of brain metastasis.
Targetable mutations and hormonal receptor positivity (among breast cancer patients) were seen in 39% and 67%, respectively. Table 2 describes the frequency of targetable mutations among non-small cell lung cancer, breast cancer, and melanomas. Epidermal growth factor receptor (EGFR) mutation and anaplastic lymphoma kinase (ALK) rearrangement were seen in 41% and 8% of patients with lung cancer, respectively. Among breast cancer patients, hormone receptor positive (HR)+/human epidermal growth factor receptor 2 (Her 2)+, HR+/Her2-, HR-/Her2+, and triple negative subtype was present in 25%, 42%, 25%, and 8%, respectively. Targeted therapy, hormonal therapy, or immunotherapy was administered to 64 (66%) of patients (alone or combined with chemotherapy in 16 and 48 patients, respectively) at some time-point following their cancer diagnosis. Of patients receiving targeted, immuno-, or hormonal therapy, treatments were initiated greater than 3 months before the diagnosis of BM, at the time of BM diagnosis, or 3 months after BM diagnosis in 41%, 47%, and 12% of patients, respectively. The details of systemic therapy administered at some point to LTS patients are described in Supplementary Table S1. Initial BM treatments are summarized in Table 3. Forty percent of patients underwent surgical resection of their BM at diagnosis, and SRS and WBRT was administered to 52% and 56%, respectively (alone or in combination). Seven patients did not receive any form of brain radiation; five patients were treated with systemic therapy alone, and two underwent surgery alone.
Table 2.
Frequency of commonly detected driver mutations between lung, breast, and melanoma primaries for the long-term survivors.
| Molecular Characteristics | Long-Term Survivors |
|---|---|
| Lung primary | (n = 51) |
| EGFR mutation | 21 (41%) |
| ALK rearrangement | 4 (8%) |
| EGFR/ALK- | 21 (41%) |
| Unknown | 5 (10%) |
| Breast primary | (n = 24) |
| ER/PR +/Her 2+ | 6 (25%) |
| ER/PR+/Her 2- | 10 (42%) |
| ER/PR-/Her 2+ | 6 (25%) |
| Triple-negative | 2 (8%) |
| Unknown | 0 (0%) |
| Melanoma | (n = 5) |
| BRAF+ | 0 (0%) |
| BRAF- | 2 (40%) |
| Unknown | 3 (60%) |
EGFR: Epidermal growth factor receptor; ALK: Anaplastic lymphoma kinase; ER: Estrogen receptor; PR: Progesterone receptor; Her 2: Human epidermal growth factor receptor 2.
Table 3.
Details of intracranial treatment during the initial diagnosis of brain metastases for the long-term survivors.
| Intracranial Treatment | Long-Term Survivors (n = 97) |
|---|---|
| SRS alone | 29 (30%) |
| Surgery alone | 2 (2%) |
| WBRT alone | 15 (15%) |
| Surgery with SRS | 7 (7%) |
| Surgery with WBRT | 25 (26%) |
| SRS and WBRT | 9 (9%) |
| Surgery with SRS and WBRT | 5 (5%) |
| Systemic therapy alone | 5 (5%) |
SRS: Stereotactic radiosurgery; WBRT: Whole brain radiotherapy.
3.3. Intracranial Outcomes
Median radiologic follow up was 65 months (range 14–168 months). Intracranial disease-free survival (iDFS) at 3 and 5 years was 53% and 39%, respectively (Figure 1b). Sixty-two patients (64%) had radiological evidence of intracranial progression following their initial treatment; median time to recurrence was 24 months (range 2–99 months). Forty-four percent of patients had a single episode of intracranial failure following their initial treatment; the median number of failures was two (range 1–6). As the time of intracranial progression following their initial BM treatment, SRS, WBRT, surgery, and partial brain radiation was administered to 46, 18, 17, and 9 patients, respectively (alone or in combination). The five-year OS for patients who experienced intracranial progression after their first treatment for BM was 68%, compared to 80% for patients who did not (p = 0.22).
3.4. Long-Term Toxicity
Fifteen patients developed symptomatic radio-necrosis (RN). The 2-year and 5-year cumulative incidences of RN was 10% and 16%, respectively. Nine patients had RN after the first course of treatment (two received SRS alone and seven had a combination of surgery/SRS with WBRT) and the remaining six developed RN following subsequent salvage treatments. Nine patients with RN were treated with steroids alone and six required surgery; two out of 15 patients with RN suffered worsening of pre-existing neurologic deficits during follow up. One patient developed severe dementia following a second course of WBRT.
4. Discussion
Brain metastases are generally associated with a dismal prognosis [2,3], but some patients have prolonged survival. Several prognostic indices have been developed to stratify patients and predict outcomes, including the graded prognostic assessment (GPA) [18,19,20]. The best surviving groups in recently reported disease-specific GPAs for lung adenocarcinoma, breast adenocarcinoma, and melanoma patients had median OS estimates of 47, 25, and 34 months, respectively [21,22,23]. These updated GPA classifications estimate markedly improved OS compared to the preceding models and incorporate molecular classifications. Generally speaking, specific molecular subtypes susceptible to targeted agents have the best outcomes.
Several previous reports have described patients who survived greater than two–three years following BM diagnosis11–17 (Table 4). In most studies, rates of OS among LTS were relatively stable during the three to five years following BM diagnosis. Furthermore, Kotecha et al. reported that 41% of patients who survived at least five years were alive at 10 years [17]. In our study, 42 patients were alive and without active disease at their last follow up.
Table 4.
Selected studies reporting long term survivors of brain metastases.
| Study | Cut-off for LTS | No of Patients | Percentage of Patients | Most Common Primary Sites | Patients Receiving WBRT | Patients Receiving SRS | Patients Undergoing Surgery | Crude rate Neurologic Death | Comments/Prognostic Factors |
|---|---|---|---|---|---|---|---|---|---|
| Hall et al. (2000) [11] | 2 years | 51 | 6.9% (2 years) |
1. NSCLC 2. Breast 3. Melanoma/ renal cell carcinoma |
98% | 8% | 57% | 22% | 1. Ovarian carcinoma patients had the highest rate of survival among cohort. 2. Single lesion, surgery, and WBRT were factors associated with LTS. |
| Lutterbach et al. (2002) [12] | 2 years | 2 years-48 3 years-25 5 years-12 |
2.8% (3 years) |
1. NSCLC 2. Breast |
98% | 0% | 73% | NA | 1. For patients with single lesion, radiation boost was considered following WBRT. 2. Survival was best patients with a single brain metastasis |
| Kondziolka et al. (2005) [13] | 4 years | 44 | 6.5% a (4 years) |
1. Lung 2. Breast 3. Kidney |
86% | 100% | NA b | 4% | Compared with a cohort of patients surviving < 3 months, LTS had better KPS, fewer metastases, and a lower extracranial disease burden. |
| Chao et al. (2006) [14] | 5 years | 32 | 2.5% (5 years) |
1. NSCLC 2. Breast 3. Melanoma |
66% | 28% | 69% | 0% (for 10-year survivors) |
1. Prognostic factors were compared to patients surviving <5 years. 2. Female gender, RPA class 1, surgery, and SRS were associated with better survival. |
| Niemiec et al. (2011) [15] | 3 years | 19 | 2% c | NSCLC | 79% | 32% | 53% | 33% | 1. Compared with control group of patients with brain metastases from lung cancer. 2. Female sex, RPA class 1, adenocarcinoma, control of primary tumour and no extracranial metastasis was associated with LTS. |
| Enders et al. (2016) [16] | 2 years | 21 | 18% | NSCLC | 81% | 0% | 100% | NR | Surgery for primary tumour was the only significant factor associated with LTS. |
| Kotecha et al. (2016) [17] | 10 years | 5 years-56 10 yrs-23 |
1.2% (10 years) |
1. NSCLC 2. Melanoma 3. Breast/unknown primary |
52% | 30% | 70% | 0% (for 10-year survivors) |
Female gender, single brain metastasis and SRS were associated with better overall survival |
| Current study (2020) | 3 years | 3 years-97 5 years-64 |
16% (5 years) |
1. NSCLC 2. Breast 3. Thyroid |
56% | 52% | 40% | 36% d | 71% of the LTS were <60 years, 65% had single BM at the time of diagnosis, 76% had brain as the first site of metastatic disease, 39% had targetable mutations, 66% received targeted/hormonal and immunotherapies. |
LTS: Long-term survivors; WBRT: Whole brain radiotherapy; SRS: Stereotactic radiosurgery; HR: Hormonal receptor; NSCLC: Non-small cell lung carcinoma: Brain metastasis; NA: Not available; OS: Overall survival. a The cohort was chosen from a selected group of patients who had undergone SRS treatment. b the number of patients who had surgery at the time of presentation of brain metastasis is unknown. c approximate value according to the authors. d. Defined as any radiologically active CNS disease within three months before death.
Intracranial disease burden is an important prognostic factor for BM patients [11,12,13,17]. In our cohort, 65% of LTS had a single metastasis. Kotecha et al. reported that 87% of 10-year survivors had a single BM at diagnosis, compared to 42% who survived less than 5 years [17]. These results confirm the relevance of this variable in prognostic indices like the GPA and score index for radiosurgery (SIR) [24,25].
The impact of treatment modality on survival for LTS is uncertain. Hall et al. reported that surgery and WBRT were associated with LTS [11]. In a series reported by Enders et al., the frequency of LTS among surgical patients was also relatively high (18%) [16]. Likewise, in our series, 40% of patients underwent surgery. This result, however, may be affected by selection bias insofar as the determination of surgical candidacy reflects pre-surgical performance status.
Kotecha et al. noted that SRS has a positive impact on survival from BM compared to WBRT. In our series, 50% of LTS underwent treatment with SRS. However, treatment with WBRT did not correlate with decreased rates of survival. Investigating the role of WBRT in LTS is important, given its potential to cause cognitive toxicity. In general, for patients with a limited number of BM, SRS has become the preferred modality because it results in improved neurocognitive outcomes compared to WBRT [5,6,7,26]. In particular, SRS may be more appropriate for patients predicted to be LTS, given that the potential for late toxicities from WBRT.
Finally, we report the molecular characteristics and use of specific systemic therapy (targeted, hormonal, or immunotherapy) in our LTS cohort. In addition to the well-documented activity of tyrosine kinase inhibitors against BM [27], a recent phase 2 study with 94 patients of melanoma demonstrated >50% response in nonirradiated BM treated with nivolumab and ipilimumab. In a meta-analysis of 1132 patients with melanoma BM, Rulli et al. reported that combination immunotherapy resulted in improved survival compared to mono-immunotherapy and combination targeted therapies [28]. In our study, among 89 LTS with known molecular features, 54% had a drug-targetable mutation or hormone receptor positivity and 66% received targeted, hormonal, or immunomodulating therapies. This may reflect, in part, the CNS activity of some targeted agents. The details of individual systemic agents and the timing of therapy in relation to the diagnosis of BM have been described in Supplementary Table S1. The most commonly used targeted agents included gefitinib and erlotinib in lung cancer and trastuzumab in breast cancer. Tamoxifen was the most commonly used hormonal agent. Given the small number of patients with primary cancers containing specific molecular features, we did not examine the effect of specific systemic agents on survival.
Strength and Limitations
Our study represents one of the largest and most well-characterized exclusively LTS cohorts. However, the patients included in this analysis have different cancers with diverse molecular features, which makes all but general conclusions difficult. Our study would be strengthened by the inclusion of a control group of patients from our database with median survival. With that we would have had the ability to compare that group’s clinical and treatment-related characteristics with that of the LTS cohort. Without the inclusion of a control group, it is difficult to conclude the characteristics that are specifically associated with LTS. Furthermore, a dedicated evaluation of long-term neuro-cognitive complications in LTS, functionality, occupational rehabilitation and the impact of different treatment modalities, would have been informative. In addition, patients included in this registry were referred to our BM clinic and as such had a high proportion of patients who were considered to have a favorable prognosis, including patients with a single brain metastasis.
5. Conclusions
The majority of long-term BM survivors in our cohort were younger than 60 years of age and had a single BM at diagnosis. Many patients were treated with surgical resection or targeted, hormonal, or immune modulating therapies. LTS represent a distinct subset of BM patients for whom treatment is more than palliative and for whom the long-term side effects and risks of treatment should be carefully considered.
Supplementary Materials
The following are available online at https://www.mdpi.com/1718-7729/28/1/54/s1, Figure S1: Kaplan Meier survival plot showing a comparison of overall survival for patients diagnosed with brain metastasis during 2003–2010 and 2011–2014. Table S1: Systemic therapies used for long-term survivors. Table S2: Correlation of different factors with intracranial disease-free survival and overall survival for the long-term survivors.
Author Contributions
Conceptualization, A.D. (Archya Dasgupta), D.B.S.; methodology, A.D. (Archya Dasgupta), J.C., J.W., B.-A.M., N.L., D.S.T., M.v.P., A.D. (Andrei Damyanovich), R.H., C.C., M.B., P.K., G.Z., A.B., F.Y.M., D.B.S.; formal analysis, A.D. (Archya Dasgupta), J.C., J.W., B.-A.M., N.L., D.S.T., M.v.P., A.D. (Andrei Damyanovich), R.H., C.C., M.B., P.K., G.Z., A.B., T.C., F.Y.M., D.B.S.; investigation, A.D. (Archya Dasgupta), D.B.S.; resources, A.D. (Archya Dasgupta), B.-A.M., N.L., D.S.T., M.B., P.K., G.Z., A.B., F.Y.M., D.B.S.; writing—original draft preparation, A.D. (Archya Dasgupta), J.C., J.W., B.-A.M., N.L., D.S.T., M.v.P., A.D. (Andrei Damyanovich), R.H., C.C., M.B., P.K., G.Z., A.B., F.Y.M., D.B.S.; writing—review and editing, A.D. (Archya Dasgupta), J.C., J.W., B.-A.M., N.L., D.S.T., M.v.P., A.D. (Andrei Damyanovich), R.H., C.C., M.B., P.K., G.Z., A.B., F.Y.M., D.B.S.; project administration, D.B.S. All authors have read and agreed to the published version of the manuscript.
Funding
The Robert and Andree Rheaume Fitzhenry Brain Metastases Program. The funding agency had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report; and in the decision to submit the article for publication.
Conflicts of Interest
Authors declare no conflict of interest.
Footnotes
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Barnholtz-Sloan J.S., Sloan A.E., Davis F.G., Vigneau F.D., Lai P., Sawaya R.E. Incidence Proportions of Brain Metastases in Patients Diagnosed (1973 to 2001) in the Metropolitan Detroit Cancer Surveillance System. J. Clin. Oncol. 2004;22:2865–2872. doi: 10.1200/JCO.2004.12.149. [DOI] [PubMed] [Google Scholar]
- 2.Cagney D.N., Martin A.M., Catalano P.J., Redig A.J., Lin N.U., Lee E.Q., Wen P.Y., Dunn I.F., Bi W.L., Weiss S.E., et al. Incidence and prognosis of patients with brain metastases at diagnosis of systemic malignancy: A population-based study. Neuro Oncol. 2017;19:1511–1521. doi: 10.1093/neuonc/nox077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arvold N.D., Lee E.Q., Mehta M.P., Margolin K., Alexander B.M., Lin N.U., Anders C.K., Soffietti R., Camidge D.R., Vogelbaum M.A., et al. Updates in the management of brain metastases. Neuro Oncol. 2016;18:1043–1065. doi: 10.1093/neuonc/now127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brown P.D., Ahluwalia M.S., Khan O.H., Asher A.L., Wefel J.S., Gondi V. Whole-Brain Radiotherapy for Brain Metastases: Evolution or Revolution? J. Clin. Oncol. 2018;36:483–491. doi: 10.1200/JCO.2017.75.9589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brown P.D., Jaeckle K., Ballman K.V., Farace E., Cerhan J.H., Anderson S.K., Carrero X.W., Barker F.G., Deming R., Burri S.H., et al. Effect of radiosurgery alone vs radiosurgery with whole brain radiation therapy on cognitive function in patients with 1 to 3 brain metastases: A randomized clinical trial. JAMA. 2016;316:401–409. doi: 10.1001/jama.2016.9839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown P.D., Ballman K.V., Cerhan J.H., Anderson S.K., Carrero X.W., Whitton A.C., Greenspoon J., Parney I.F., Laack N.N.I., Ashman J.B., et al. Postoperative stereotactic radiosurgery compared with whole brain radiotherapy for resected metastatic brain disease (NCCTG N107C/CEC·3): A multicentre, randomised, controlled, phase 3 trial. Lancet Oncol. 2017;18:1049–1060. doi: 10.1016/S1470-2045(17)30441-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sahgal A., Aoyama H., Kocher M., Neupane B., Collette S., Tago M., Shaw P., Beyene J., Chang E.L. Phase 3 trials of stereotactic radiosurgery with or without whole-brain radiation therapy for 1 to 4 brain metastases: Individual patient data meta-analysis. Int. J. Radiat. Oncol. Biol. Phys. 2015;91:710–717. doi: 10.1016/j.ijrobp.2014.10.024. [DOI] [PubMed] [Google Scholar]
- 8.Lin N.U., Diéras V., Paul D., Lossignol D., Christodoulou C., Stemmler H.-J., Roche H., Liu C.M.H., Greil R., Ciruelos E., et al. Multicenter Phase II Study of Lapatinib in Patients with Brain Metastases from HER2-Positive Breast Cancer. Clin. Cancer Res. 2009;15:1452–1459. doi: 10.1158/1078-0432.CCR-08-1080. [DOI] [PubMed] [Google Scholar]
- 9.Doherty M.K., Korpanty G.J., Tomasini P., Alizadeh M., Jao K., Labbé C., Mascaux C.M., Martin P., Kamel-Reid S., Tsao M.-S., et al. Treatment options for patients with brain metastases from EGFR/ALK -driven lung cancer. Radiother. Oncol. 2017;123:195–202. doi: 10.1016/j.radonc.2017.03.007. [DOI] [PubMed] [Google Scholar]
- 10.Tawbi H.A., Forsyth P.A., Algazi A.P., Hamid O., Hodi F.S., Moschos S., Khushalani N.I., Lewis K., Lao C.D., Postow M.A., et al. Combined Nivolumab and Ipilimumab in Melanoma Metastatic to the Brain. N. Engl. J. Med. 2018;379:722–730. doi: 10.1056/NEJMoa1805453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hall W.A., Djalilian H.R., Nussbaum E.S., Cho K.H. Long-term survival with metastatic cancer to the brain. Med. Oncol. 2000;17:279–286. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 12.Lutterbach J., Bartelt S., Ostertag C. Long-term survival in patients with brain metastases. J. Cancer Res. Clin. Oncol. 2002;128:417–425. doi: 10.1007/s00432-002-0354-1. [DOI] [PubMed] [Google Scholar]
- 13.Kondziolka D., Martin J.J., Flickinger J.C., Friedland D.M., Brufsky A.M., Baar J., Agarwala S., Kirkwood J.M., Lunsford L.D. Long-term survivors after gamma knife radiosurgery for brain metastases. Cancer. 2005;104:2784–2791. doi: 10.1002/cncr.21545. [DOI] [PubMed] [Google Scholar]
- 14.Chao S.T., Barnett G.H., Liu S.W., Reuther A.M., Toms S.A., Vogelbaum M.A., Videtic G.M., Suh J.H. Five-year survivors of brain metastases: A single-institution report of 32 patients. Int. J. Radiat. Oncol. Biol. Phys. 2006;66:801–809. doi: 10.1016/j.ijrobp.2006.05.015. [DOI] [PubMed] [Google Scholar]
- 15.Niemiec M., Glogowski M.W., Tyc-Szczepaniak D., Wierzchowski M., Kepka L. Characteristics of long-term survivors of brain metastases from lung cancer. Rep. Pract. Oncol. Radiother. 2011;16:49–53. doi: 10.1016/j.rpor.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Enders F., Geisenberger C., Jungk C., Bermejo J.L., Warta R., Von Deimling A., Herold-Mende C., Unterberg A. Prognostic factors and long-term survival in surgically treated brain metastases from non-small cell lung cancer. Clin. Neurol. Neurosurg. 2016;142:72–80. doi: 10.1016/j.clineuro.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 17.Kotecha R., Vogel S., Suh J.H., Barnett G.H., Murphy E.S., Reddy C.A., Parsons M., Vogelbaum M.A., Angelov L., Mohammadi A.M., et al. A cure is possible: A study of 10-year survivors of brain metastases. J. Neuro Oncol. 2016;129:545–555. doi: 10.1007/s11060-016-2208-8. [DOI] [PubMed] [Google Scholar]
- 18.Gaspar L., Scott C., Rotman M., Asbell S., Phillips T., Wasserman T., McKenna W., Byhardt R. Recursive partitioning analysis (RPA) of prognostic factors in three radiation therapy oncology group (RTOG) brain metastases trials. Int. J. Radiat. Oncol. Biol. Phys. 1997;37:745–751. doi: 10.1016/S0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 19.Sperduto P.W., Berkey B., Gaspar L.E., Mehta M., Curran W. A New Prognostic Index and Comparison to Three Other Indices for Patients with Brain Metastases: An Analysis of 1,960 Patients in the RTOG Database. Int. J. Radiat. Oncol. Biol. Phys. 2008;70:510–514. doi: 10.1016/j.ijrobp.2007.06.074. [DOI] [PubMed] [Google Scholar]
- 20.Nieder C., Mehta M.P. Prognostic indices for brain metastases—Usefulness and challenges. Radiat. Oncol. 2009;4:10. doi: 10.1186/1748-717X-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sperduto P.W., Yang T.J., Beal K., Pan H., Brown P.D., Bangdiwala A., Shanley R., Yeh N., Gaspar L.E., Braunstein S., et al. Estimating survival in patients with lung cancer and brain metastases: An update of the graded prognostic assessment for lung cancer using molecular markers (Lung-molGPA) JAMA Oncol. 2017;3:827–831. doi: 10.1001/jamaoncol.2016.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., Sneed P.K., Chao S.T., Weil R.J., Suh J., et al. Effect of Tumor Subtype on Survival and the Graded Prognostic Assessment for Patients with Breast Cancer and Brain Metastases. Int. J. Radiat. Oncol. Biol. Phys. 2012;82:2111–2117. doi: 10.1016/j.ijrobp.2011.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sperduto P.W., Jiang W., Brown P.D., Braunstein S., Sneed P., Wattson D.A., Shih H.A., Bangdiwala A., Shanley R., Lockney N.A., et al. Estimating Survival in Melanoma Patients with Brain Metastases: An Update of the Graded Prognostic Assessment for Melanoma Using Molecular Markers (Melanoma-molGPA) Int. J. Radiat. Oncol. Biol. Phys. 2017;99:812–816. doi: 10.1016/j.ijrobp.2017.06.2454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sperduto P.W., Kased N., Roberge D., Xu Z., Shanley R., Luo X., Sneed P.K., Chao S.T., Weil R.J., Suh J., et al. Summary Report on the Graded Prognostic Assessment: An Accurate and Facile Diagnosis-Specific Tool to Estimate Survival for Patients with Brain Metastases. J. Clin. Oncol. 2012;30:419–425. doi: 10.1200/JCO.2011.38.0527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weltman E., Salvajoli J.V., Brandt R.A., Hanriot R.D.M., Prisco F.E., Cruz J.C., Borges S.R.D.O., Wajsbrot D.B. Radiosurgery for brain metastases: A score index for predicting prognosis. Int. J. Radiat. Oncol. Biol. Phys. 2000;46:1155–1161. doi: 10.1016/S0360-3016(99)00549-0. [DOI] [PubMed] [Google Scholar]
- 26.Yamamoto M., Serizawa T., Shuto T., Akabane A., Higuchi Y., Kawagishi J., Yamanaka K., Sato Y., Jokura H., Yomo S., et al. Stereotactic radiosurgery for patients with multiple brain metastases (JLGK0901): A multi-institutional prospective observational study. Lancet Oncol. 2014;15:387–395. doi: 10.1016/S1470-2045(14)70061-0. [DOI] [PubMed] [Google Scholar]
- 27.Park S., Kim H.-T., Lee D., Kim K., Kim S.-W., Suh C., Lee J. Efficacy of epidermal growth factor receptor tyrosine kinase inhibitors for brain metastasis in non-small cell lung cancer patients harboring either exon 19 or 21 mutation. Lung Cancer. 2012;77:556–560. doi: 10.1016/j.lungcan.2012.05.092. [DOI] [PubMed] [Google Scholar]
- 28.Rulli E., Legramandi L., Salvati L., Mandala M. The impact of targeted therapies and immunotherapy in melanoma brain metastases: A systematic review and meta-analysis. Cancer. 2019;125:3776–3789. doi: 10.1002/cncr.32375. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.